Specifics of Cryopreservation of Hydrogel Biopolymer Scaffolds with Encapsulated Mesenchymal Stem Cells
Abstract
:1. Introduction
- (1)
- (2)
- The correct microenvironment acting as an artificial cellular niche that can be constructed by, for example, scaffolds or the use of bioink for bioprinting. It should be noted that the “correct microenvironment” corresponding to the niche concept involves not just one that allows for the retention of cell viability, but also enables the initiation of various cellular events depending on signals coming from the scaffold, both mechanical and biochemical [4,5].
- Preparatory stage—replacing the growth medium with a cryopreservation medium containing the cryoprotector, followed by incubation at positive temperatures (to allow penetration of the structure with the cryoprotector before freezing);
- Freezing stage—at this stage, the choice of freezing speed (vitrification or gradual decrease in temperature) is critical;
- Cryostorage stage—the choice of an optimal temperature regimen for this stage and its stability can determine the maximum duration of successful cryostorage;
- Thawing stage—it is important to ensure thawing of the sample without allowing recrystallization of the liquid. This stage also includes the subsequent washing of the cryopreservative from the sample;
- Acclimatization stage—not used in all protocols, this stage includes incubation of the product in order to restore the functional activity of its cellular component [21].
2. Materials and Methods
2.1. Obtaining the ASC Cell Culture
2.2. Formation of Scaffolds with Encapsulated ASCs
2.3. Cryopreservation and Scaffold Recovery Procedures
2.4. Comparative Characteristics of the Scaffold Cellular Component before and after Cryopreservation
2.4.1. Microscopic Assessment of Cell State in Scaffold Structure
2.4.2. Evaluation of the Number and Viability of Cells in the Scaffold Structure
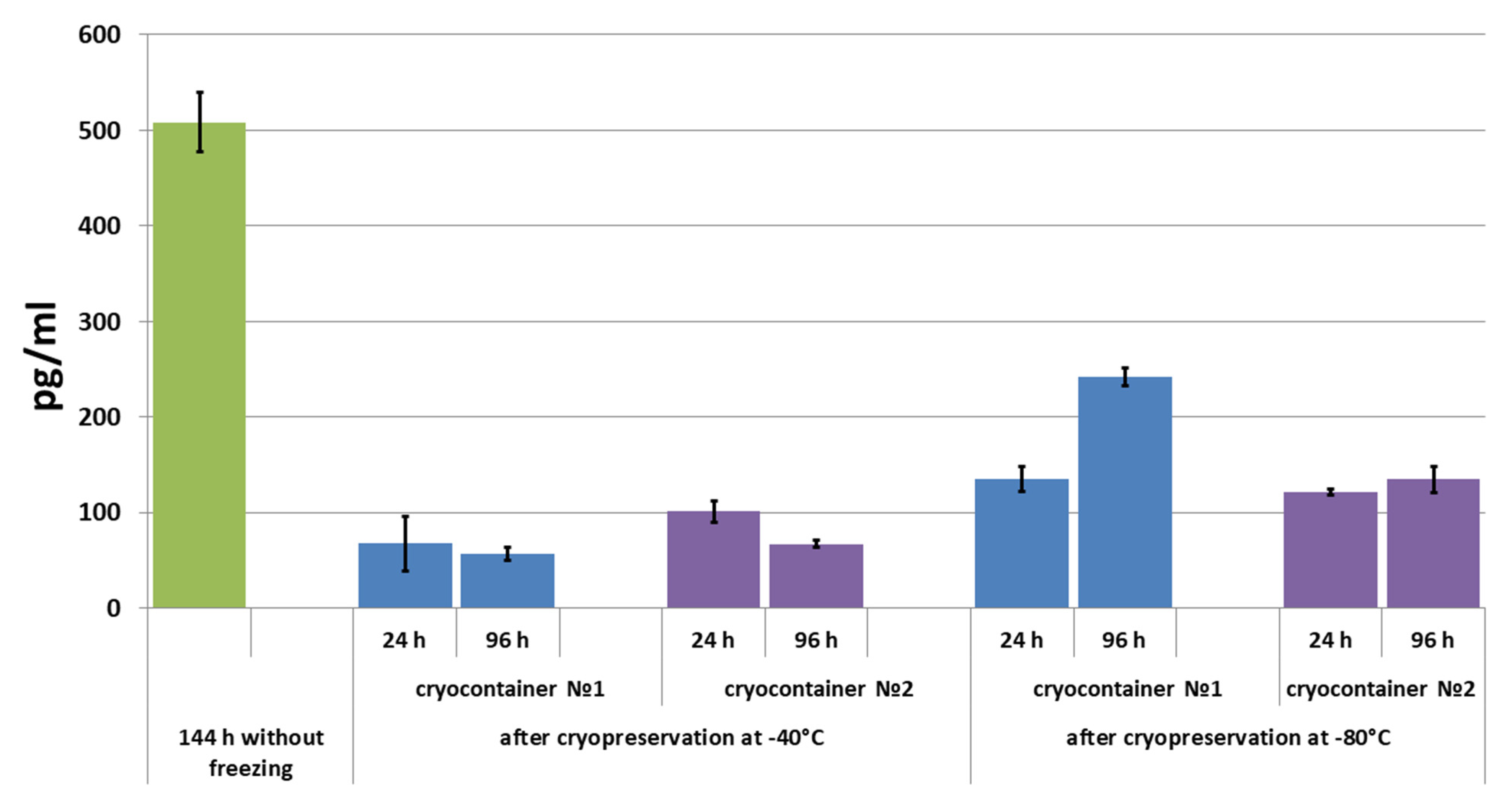
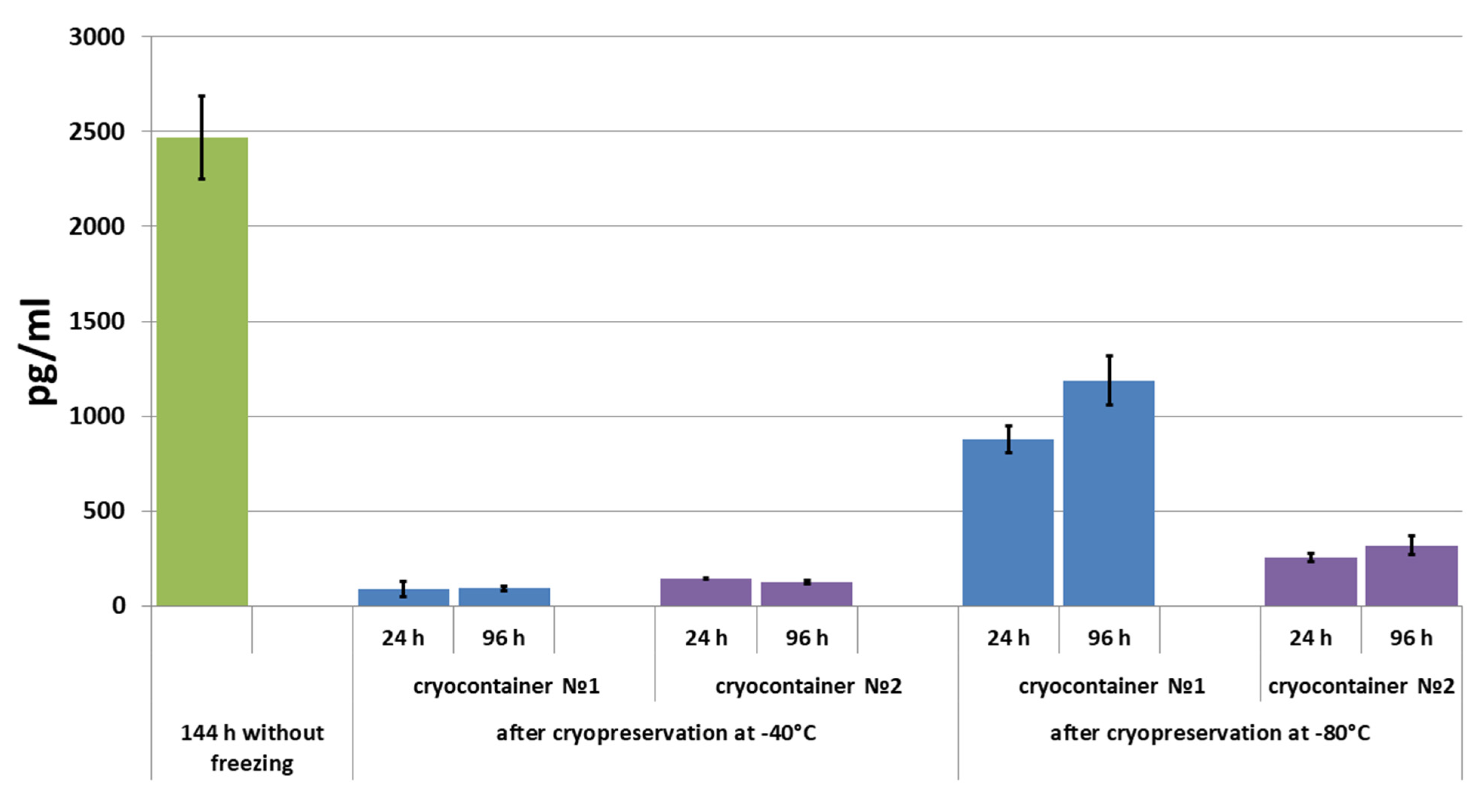
2.4.3. Assessment of Secretory Activity of ASCs in Scaffolds
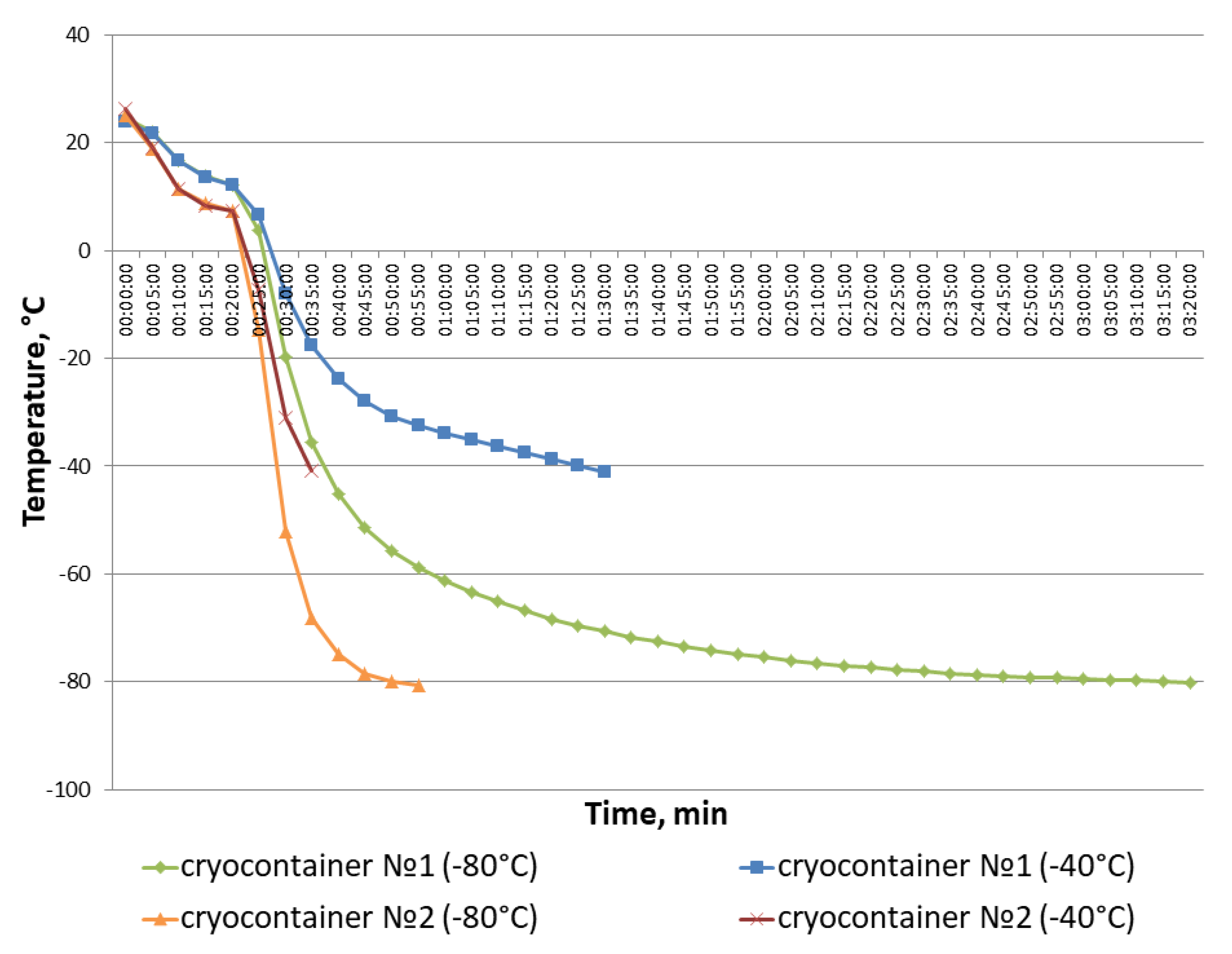
2.5. Estimation of Cooling Rate in Cryocontainers during Freezing
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Williams, D.F. Definitions of Biomaterials for the Twenty-First Century. In Proceedings of the Consensus Conference, Chengdu, China, 11–12 June 2018; ISBN 9780128182918. [Google Scholar]
- Del Bakhshayesh, A.R.; Asadi, N.; Alihemmati, A.; Tayefi Nasrabadi, H.; Montaseri, A.; Davaran, S.; Saghati, S.; Akbarzadeh, A.; Abedelahi, A. An Overview of Advanced Biocompatible and Biomimetic Materials for Creation of Replacement Structures in the Musculoskeletal Systems: Focusing on Cartilage Tissue Engineering. J. Biol. Eng. 2019, 13, 85. [Google Scholar] [CrossRef]
- Biru, E.I.; Necolau, M.I.; Zainea, A.; Iovu, H. Graphene Oxide–Protein-Based Scaffolds for Tissue Engineering: Recent Advances and Applications. Polymers 2022, 14, 1032. [Google Scholar] [CrossRef] [PubMed]
- Smith, Q.; Gerecht, S. Extracellular Matrix Regulation of Stem Cell Fate. Curr. Stem Cell Rep. 2018, 4, 13–21. [Google Scholar] [CrossRef]
- Nie, Y.; Zhang, S.; Liu, N.; Li, Z. Extracellular Matrix Enhances Therapeutic Effects of Stem Cells in Regenerative Medicine. In Composition and Function of the Extracellular Matrix in the Human Body; IntechOpen: London, UK, 2016; pp. 323–340. [Google Scholar]
- Mansbridge, J. Skin Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2008, 19, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Roux, S.; Bodivit, G.; Bartis, W.; Lebouvier, A.; Chevallier, N.; Fialaire-Legendre, A.; Bierling, P.; Rouard, H. In Vitro Characterization of Patches of Human Mesenchymal Stromal Cells. Tissue Eng. Part A 2015, 21, 417–425. [Google Scholar] [CrossRef]
- Krishna, L.; Dhamodaran, K.; Jayadev, C.; Chatterjee, K.; Shetty, R.; Khora, S.S.; Das, D. Nanostructured Scaffold as a Determinant of Stem Cell Fate. Stem Cell Res. Ther. 2016, 7, 188. [Google Scholar] [CrossRef] [PubMed]
- Akhmanova, M.; Osidak, E.; Domogatsky, S.; Rodin, S.; Domogatskaya, A. Physical, Spatial, and Molecular Aspects of Extracellular Matrix of in Vivo Niches and Artificial Scaffolds Relevant to Stem Cells Research. Stem Cells Int. 2015, 2015, 167025. [Google Scholar] [CrossRef]
- Roberts, K.J.; Kershner, A.M.; Beachy, P.A. The Stromal Niche for Epithelial Stem Cells: A Template for Regeneration and a Brake on Malignancy. Cancer Cell 2017, 32, 404–410. [Google Scholar] [CrossRef]
- Sharma, M.; Ross, C.; Srivastava, S. Ally to Adversary: Mesenchymal Stem Cells and Their Transformation in Leukaemia. Cancer Cell Int. 2019, 19, 139. [Google Scholar] [CrossRef]
- Bissoyi, A.; Pramanik, K.; Panda, N.N.; Sarangi, S.K. Cryopreservation of HMSCs Seeded Silk Nanofibers Based Tissue Engineered Constructs. Cryobiology 2014, 68, 332–342. [Google Scholar] [CrossRef]
- Arutyunyan, I.; Elchaninov, A.; Sukhikh, G.; Fatkhudinov, T. Cryopreservation of Tissue-Engineered Scaffold-Based Constructs: From Concept to Reality. Stem Cell Rev. Rep. 2022, 18, 1234–1252. [Google Scholar] [CrossRef] [PubMed]
- Mutsenko, V.; Knaack, S.; Lauterboeck, L.; Tarusin, D.; Sydykov, B.; Cabiscol, R.; Ivnev, D.; Belikan, J.; Beck, A.; Dipresa, D.; et al. Effect of “in Air” Freezing on Post-Thaw Recovery of Callithrix Jacchus Mesenchymal Stromal Cells and Properties of 3D Collagen-Hydroxyapatite Scaffolds. Cryobiology 2020, 92, 215–230. [Google Scholar] [CrossRef]
- Kofron, M.D.; Opsitnick, N.C.; Attawia, M.A.; Laurencin, C.T. Cryopreservation of Tissue Engineered Constructs for Bone. J. Orthop. Res. 2003, 21, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, H.; Ehashi, T.; Ohshima, N.; Jagawa, A. Cryopreservation of Fibroblasts Immobilized within a Porous Scaffold: Effects of Preculture and Collagen Coating of Scaffold on Performance of Three-Dimensional Cryopreservation. Artif. Organs 2010, 34, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Pravdyuk, A.I.; Petrenko, Y.A.; Fuller, B.J.; Petrenko, A.Y. Cryopreservation of Alginate Encapsulated Mesenchymal Stromal Cells. Cryobiology 2013, 66, 215–222. [Google Scholar] [CrossRef]
- Diaz-Dussan, D.; Peng, Y.-Y.Y.; Sengupta, J.; Zabludowski, R.; Adam, M.K.; Acker, J.P.; Ben, R.N.; Kumar, P.; Narain, R. Trehalose-Based Polyethers for Cryopreservation and Three-Dimensional Cell Scaffolds. Biomacromolecules 2020, 21, 1264–1273. [Google Scholar] [CrossRef]
- Costa, P.F.; Dias, A.F.; Reis, R.L.; Gomes, M.E. Cryopreservation of Cell/Scaffold Tissue-Engineered Constructs. Tissue Eng. Part C Methods 2012, 18, 852–858. [Google Scholar] [CrossRef]
- Batnyam, O.; Suye, S.I.; Fujita, S. Direct Cryopreservation of Adherent Cells on an Elastic Nanofiber Sheet Featuring a Low Glass-Transition Temperature. RSC Adv. 2017, 7, 51264–51271. [Google Scholar] [CrossRef]
- Linkova, D.D.; Rubtsova, Y.P.; Egorikhina, M.N. Cryostorage of Mesenchymal Stem Cells and Biomedical Cell-Based Products. Cells 2022, 11, 2691. [Google Scholar] [CrossRef]
- Hunt, C. Cryopreservation: Vitrification and Controlled Rate Cooling. Methods Mol. Biol. 2017, 1590, 41–77. [Google Scholar] [CrossRef]
- Katsen-Globa, A.; Meiser, I.; Petrenko, Y.A.; Ivanov, R.V.; Lozinsky, V.I.; Zimmermann, H.; Petrenko, A.Y. Towards Ready-to-Use 3-D Scaffolds for Regenerative Medicine: Adhesion-Based Cryopreservation of Human Mesenchymal Stem Cells Attached and Spread within Alginate-Gelatin Cryogel Scaffolds. J. Mater. Sci. Mater. Med. 2014, 25, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Nagao, M.; Sengupta, J.; Diaz-Dussan, D.; Adam, M.; Wu, M.; Acker, J.; Ben, R.; Ishihara, K.; Zeng, H.; Miura, Y.; et al. Synthesis of Highly Biocompatible and Temperature-Responsive Physical Gels for Cryopreservation and 3D Cell Culture. ACS Appl. Bio Mater. 2018, 1, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.H.; Kim, U.; Lee, S.G.; Ryu, B.; Kim, J.; Igor, A.; Kim, J.S.; Jung, C.R.; Park, J.H.; Kim, C.Y. Vitrification for Cryopreservation of 2D and 3D Stem Cells Culture Using High Concentration of Cryoprotective Agents. BMC Biotechnol. 2020, 20, 45. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, Y.A.; Petrenko, A.Y.; Martin, I.; Wendt, D. Perfusion Bioreactor-Based Cryopreservation of 3D Human Mesenchymal Stromal Cell Tissue Grafts. Cryobiology 2017, 76, 150–153. [Google Scholar] [CrossRef]
- Council of Europe. 2.7.29. Nucleated Cell Count and Viability. In The European Pharmacopoeia, 9.0 ed.; EDQM: Strasbourg, France, 2017; pp. 3983–4016. [Google Scholar]
- Guadix, J.A.; López-Beas, J.; Clares, B.; Soriano-Ruiz, J.L.; Zugaza, J.L.; Gálvez-Martín, P. Principal Criteria for Evaluating the Quality, Safety and Efficacy of HMSC-Based Products in Clinical Practice: Current Approaches and Challenges. Pharmaceutics 2019, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Cagol, N.; Bonani, W.; Maniglio, D.; Migliaresi, C.; Motta, A. Effect of Cryopreservation on Cell-Laden Hydrogels: Comparison of Different Cryoprotectants. Tissue Eng. Part C Methods 2018, 24, 20–31. [Google Scholar] [CrossRef]
- Egorikhina, M.N.; Rubtsova, Y.P.; Charykova, I.N.; Bugrova, M.L.; Bronnikova, I.I.; Mukhina, P.A.; Sosnina, L.N.; Aleynik, D.Y. Biopolymer Hydrogel Scaffold as an Artificial Cell Niche for Mesenchymal Stem Cells. Polymers 2020, 12, 2550. [Google Scholar] [CrossRef]
- Egorikhina, M.N.; Rubtsova, Y.P.; Aleynik, D.Y. Long-Term Cryostorage of Mesenchymal Stem Cell-Containing Hybrid Hydrogel Scaffolds Based on Fibrin and Collagen. Gels 2020, 6, 44. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Egorikhina, M.N.; Aleynik, D.Y.; Rubtsova, Y.P.; Levin, G.Y.; Charykova, I.N.; Semenycheva, L.L.; Bugrova, M.L.; Zakharychev, E.A. Hydrogel Scaffolds Based on Blood Plasma Cryoprecipitate and Collagen Derived from Various Sources: Structural, Mechanical and Biological Characteristics. Bioact. Mater. 2019, 4, 334–345. [Google Scholar] [CrossRef]
- Bahsoun, S.; Coopman, K.; Akam, E.C. Quantitative Assessment of the Impact of Cryopreservation on Human Bone Marrow-Derived Mesenchymal Stem Cells: Up to 24 h Post-Thaw and Beyond. Stem Cell Res. Ther. 2020, 11, 540. [Google Scholar] [CrossRef] [PubMed]
- Bahsoun, S.; Coopman, K.; Akam, E.C. The Impact of Cryopreservation on Bone Marrow-Derived Mesenchymal Stem Cells: A Systematic Review. J. Transl. Med. 2019, 17, 397. [Google Scholar] [CrossRef] [PubMed]
- Heng, B.C. Effect of Rho-Associated Kinase (ROCK) Inhibitor Y-27632 on the Post-Thaw Viability of Cryopreserved Human Bone Marrow-Derived Mesenchymal Stem Cells. Tissue Cell 2009, 41, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Antebi, B.; Asher, A.M.; Rodriguez, L.A.; Moore, R.K.; Mohammadipoor, A.; Cancio, L.C. Cryopreserved Mesenchymal Stem Cells Regain Functional Potency Following a 24-h Acclimation Period. J. Transl. Med. 2019, 17, 297. [Google Scholar] [CrossRef] [PubMed]
- Baust, J.G.; Gao, D.; Baust, J.M. Cryopreservation: An Emerging Paradigm Change. Organogenesis 2009, 5, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Curtis, L.A.; Janowska-Wieczorek, A.; McGann, L.E.; Elliott, J.A.W. Mesenchymal Stromal Cells Derived from Various Tissues: Biological, Clinical and Cryopreservation Aspects. Cryobiology 2015, 71, 181–197. [Google Scholar] [CrossRef]
- Qiu, G.; Zheng, G.; Ge, M.; Wang, J.; Huang, R.; Shu, Q.; Xu, J. Functional Proteins of Mesenchymal Stem Cell-Derived Extracellular Vesicles. Stem Cell Res. Ther. 2019, 10, 359. [Google Scholar] [CrossRef]
- Han, Y.; Yang, J.; Fang, J.; Zhou, Y.; Candi, E.; Wang, J.; Hua, D.; Shao, C.; Shi, Y. The Secretion Profile of Mesenchymal Stem Cells and Potential Applications in Treating Human Diseases. Signal Transduct. Target. Ther. 2022, 7, 92. [Google Scholar] [CrossRef]
- Wang, M.; Crisostomo, P.R.; Herring, C.; Meldrum, K.K.; Meldrum, D.R. Human Progenitor Cells from Bone Marrow or Adipose Tissue Produce VEGF, HGF, and IGF-I in Response to TNF by a P38 MAPK-Dependent Mechanism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R880–R884. [Google Scholar] [CrossRef]
- Heo, J.S.; Choi, Y.; Kim, H.S.; Kim, H.O. Comparison of Molecular Profiles of Human Mesenchymal Stem Cells Derived from Bone Marrow, Umbilical Cord Blood, Placenta and Adipose Tissue. Int. J. Mol. Med. 2016, 37, 115–125. [Google Scholar] [CrossRef]
- Kilroy, G.E.; Foster, S.J.; Wu, X.; Ruiz, J.; Sherwood, S.; Heifetz, A.; Ludlow, J.W.; Stricker, D.M.; Potiny, S.; Green, P.; et al. Cytokine Profile of Human Adipose-Derived Stem Cells: Expression of Angiogenic, Hematopoietic, and pro-Inflammatory Factors. J. Cell. Physiol. 2007, 212, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Banas, A.; Teratani, T.; Yamamoto, Y.; Tokuhara, M.; Takeshita, F.; Osaki, M.; Kawamata, M.; Kato, T.; Okochi, H.; Ochiya, T. IFATS Collection: In Vivo Therapeutic Potential of Human Adipose Tissue Mesenchymal Stem Cells After Transplantation into Mice with Liver Injury. Stem Cells 2008, 26, 2705–2712. [Google Scholar] [CrossRef]
- Ventura, C.; Cantoni, S.; Bianchi, F.; Lionetti, V.; Cavallini, C.; Scarlata, I.; Foroni, L.; Maioli, M.; Bonsi, L.; Alviano, F.; et al. Hyaluronan Mixed Esters of Butyric and Retinoic Acid Drive Cardiac and Endothelial Fate in Term Placenta Human Mesenchymal Stem Cells and Enhance Cardiac Repair in Infarcted Rat Hearts. J. Biol. Chem. 2007, 282, 14243–14252. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Xia, Y.; Kim, W.S.; Kim, M.H.; Kim, T.H.; Kim, K.J.; Park, B.S.; Sung, J.H. Hypoxia-Enhanced Wound-Healing Function of Adipose-Derived Stem Cells: Increase in Stem Cell Proliferation and up-Regulation of VEGF and BFGF. Wound Repair Regen. 2009, 17, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. The Role of Vascular Endothelial Growth Factor in Pathological Angiogenesis. Breast Cancer Res. Treat. 1995, 36, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Lin, X.; Pan, Y.; Zheng, X.; Shi, L.; Zhang, Y.; Ma, L.; Gao, C.; Zhang, S. A Collagen Scaffold Loaded with Human Umbilical Cord-Derived Mesenchymal Stem Cells Facilitates Endometrial Regeneration and Restores Fertility. Acta Biomater. 2019, 92, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Tomanek, R.J.; Schatteman, G.C. Angiogenesis: New Insights and Therapeutic Potential. Anat. Rec. 2000, 261, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.P. The Role of Vascular Endothelial Growth Factor in Angiogenesis. Acta Haematol. 2001, 106, 148–156. [Google Scholar] [CrossRef]
- Zhivodernikov, I.V.; Ratushnyy, A.Y.; Buravkova, L.B. Secretory Activity of Mesenchymal Stromal Cells with Different Degree of Commitment under Conditions of Simulated Microgravity. Bull. Exp. Biol. Med. 2021, 170, 560–564. [Google Scholar] [CrossRef]
- Chou, L.Y.; Ho, C.T.; Hung, S.C. Paracrine Senescence of Mesenchymal Stromal Cells Involves Inflammatory Cytokines and the NF-ΚB Pathway. Cells 2022, 11, 3324. [Google Scholar] [CrossRef]
- Hou, Y.; Ryu, C.H.; Jun, J.A.; Kim, S.M.; Jeong, C.H.; Jeun, S.S. IL-8 Enhances the Angiogenic Potential of Human Bone Marrow Mesenchymal Stem Cells by Increasing Vascular Endothelial Growth Factor. Cell Biol. Int. 2014, 38, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, A.; Grimm, D.; Sahana, J.; Bauer, J.; Kröger, M.; Infanger, M.; Magnusson, N.E. Key Proteins Involved in Spheroid Formation and Angiogenesis in Endothelial Cells After Long-Term Exposure to Simulated Microgravity. Cell. Physiol. Biochem. 2018, 45, 429–445. [Google Scholar] [CrossRef] [PubMed]
- Rennekampff, H.-O.; Hansbrough, J.F.; Kiessig, V.; Doré, C.; Sticherling, M.; Schröder, J.-M. Bioactive Interleukin-8 Is Expressed in Wounds and Enhances Wound Healing. J. Surg. Res. 2000, 93, 41–54. [Google Scholar] [CrossRef]
- Kubo, K.; Kuroyanagi, Y. The Possibility of Long-Term Cryopreservation of Cultured Dermal Substitute. Artif. Organs 2005, 29, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Jang, T.H.; Park, S.C.; Yang, J.H.; Kim, J.Y.; Seok, J.H.; Park, U.S.; Choi, C.W.; Lee, S.R.; Han, J. Cryopreservation and Its Clinical Applications. Integr. Med. Res. 2017, 6, 12–18. [Google Scholar] [CrossRef]
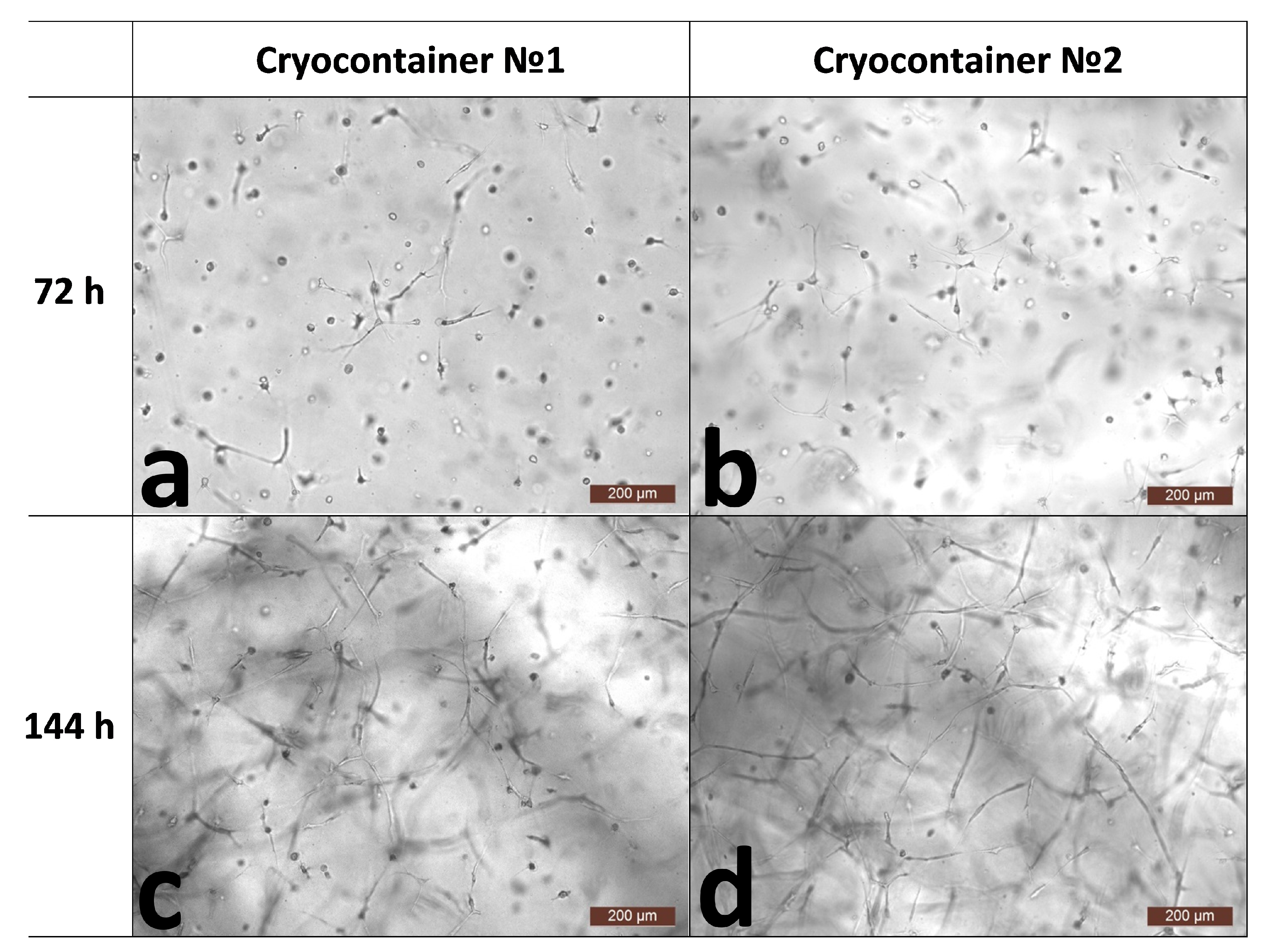
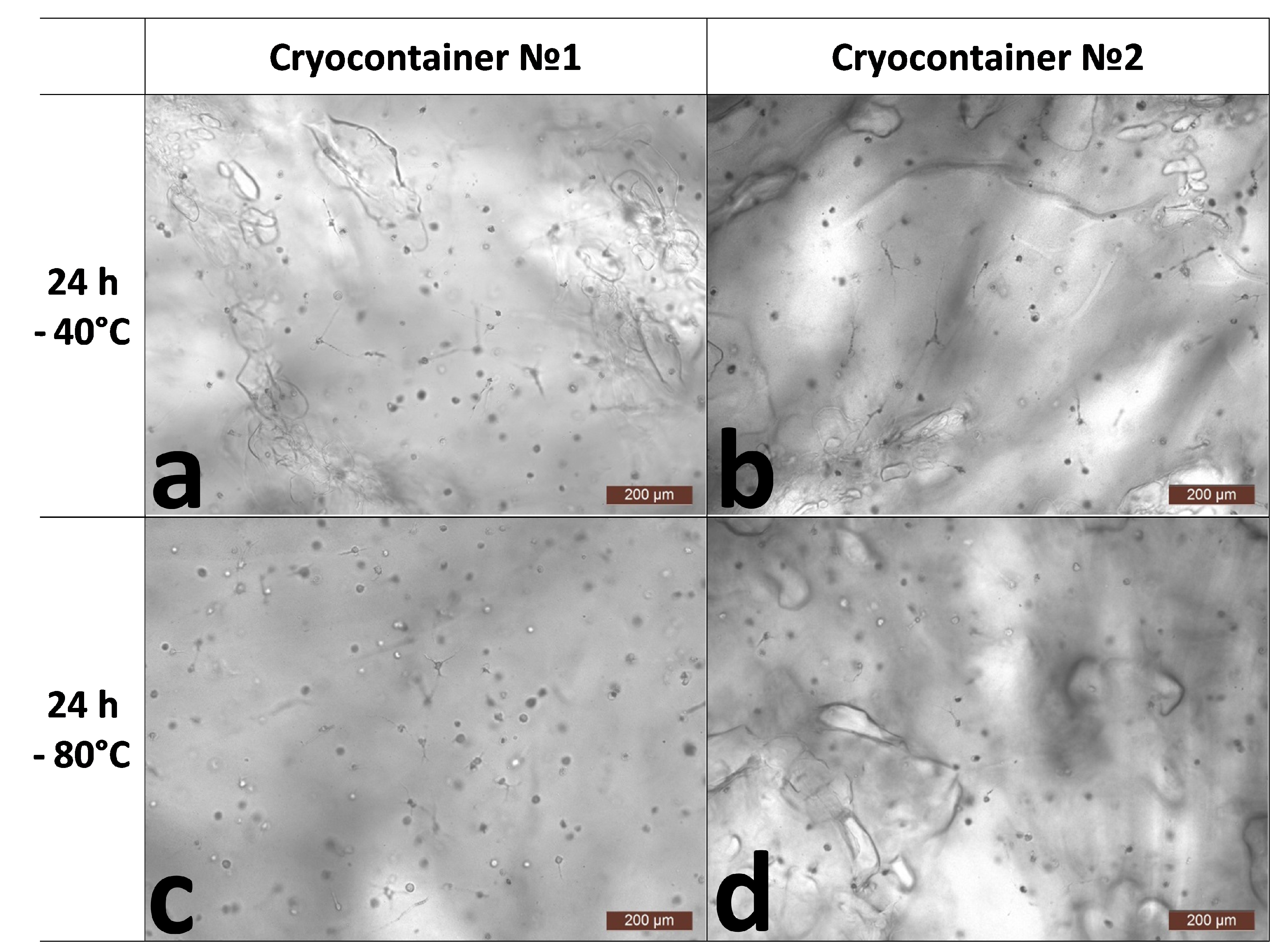


| Cryocontainer | Without Freezing | After Freezing | |||||
| 72 h | 144 h | −40 °C | −80 °C | ||||
| 24 h | 96 h | 24 h | 96 h | ||||
| No. 1 | Total Number of Cell Nuclei per 1 mm3 Scaffold | 404.16 ±11.19 | 643.65 ±18.33 ** | 526.28 ±9.45 ** ●●► | 444.82 ±13.66 * ●● | 524.09 ±15.50 ** ●●► | 722.48 ±26.16 ** ● |
| Percent of Nuclei of Dead Cells per 1 mm3 Scaffold (%) | 0.16 ±0.04 | 0.35 ±0.04 | 4.88 ±0.69 | 5.45 ±0.52 | 3.57 ±0.53 | 3.79 ±0.41 | |
| No. 2 | Total Number of Cell Nuclei per 1 mm3 Scaffold | 333.80 ±8.42 | 610.22 ±16.00 ** | 549.64 ±15.12 ** ●●► | 400.44 ±8.95 ** ●● | 511.09 ±12.34 ** ● | 525.40 ±12.70 ** ● |
| Percent of Nuclei of Dead Cells per 1 mm3 Scaffold (%) | 0.11 ±0.03 | 0.28 ±0.04 | 3.18 ±0.34 | 12.69 ±1.54 | 3.26 ±0.48 | 10.56 ±1.13 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egorikhina, M.N.; Rubtsova, Y.P.; Linkova, D.D.; Charykova, I.N.; Farafontova, E.A.; Aleinik, D.Y. Specifics of Cryopreservation of Hydrogel Biopolymer Scaffolds with Encapsulated Mesenchymal Stem Cells. Polymers 2024, 16, 247. https://doi.org/10.3390/polym16020247
Egorikhina MN, Rubtsova YP, Linkova DD, Charykova IN, Farafontova EA, Aleinik DY. Specifics of Cryopreservation of Hydrogel Biopolymer Scaffolds with Encapsulated Mesenchymal Stem Cells. Polymers. 2024; 16(2):247. https://doi.org/10.3390/polym16020247
Chicago/Turabian StyleEgorikhina, Marfa N., Yulia P. Rubtsova, Daria D. Linkova, Irina N. Charykova, Ekaterina A. Farafontova, and Diana Ya. Aleinik. 2024. "Specifics of Cryopreservation of Hydrogel Biopolymer Scaffolds with Encapsulated Mesenchymal Stem Cells" Polymers 16, no. 2: 247. https://doi.org/10.3390/polym16020247
APA StyleEgorikhina, M. N., Rubtsova, Y. P., Linkova, D. D., Charykova, I. N., Farafontova, E. A., & Aleinik, D. Y. (2024). Specifics of Cryopreservation of Hydrogel Biopolymer Scaffolds with Encapsulated Mesenchymal Stem Cells. Polymers, 16(2), 247. https://doi.org/10.3390/polym16020247








