Facile Preparation of Polyacrylonitrile-Based Activated Carbon Fiber Felts for Effective Adsorption of Dipropyl Sulfide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
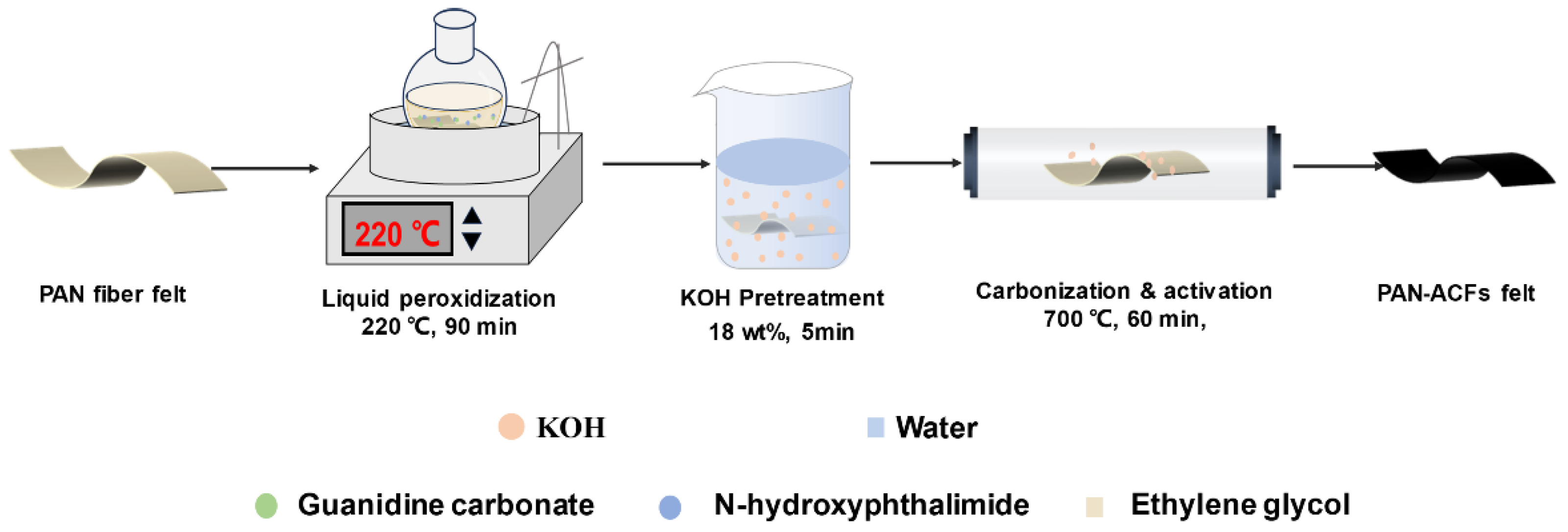
2.2. Preparation of the ACF Felt
2.3. Characterization
2.4. Adsorption Experiments
2.5. Adsorption Kinetics Analysis
2.6. Adsorption Equilibrium Isotherm
2.7. Adsorption Thermodynamic
3. Results and Discussions
3.1. Morphologies of the PAN Fiber Felt and ACF Felt
3.2. Evolution of the Microstructures of Fibers
3.3. Surface Chemical Properties
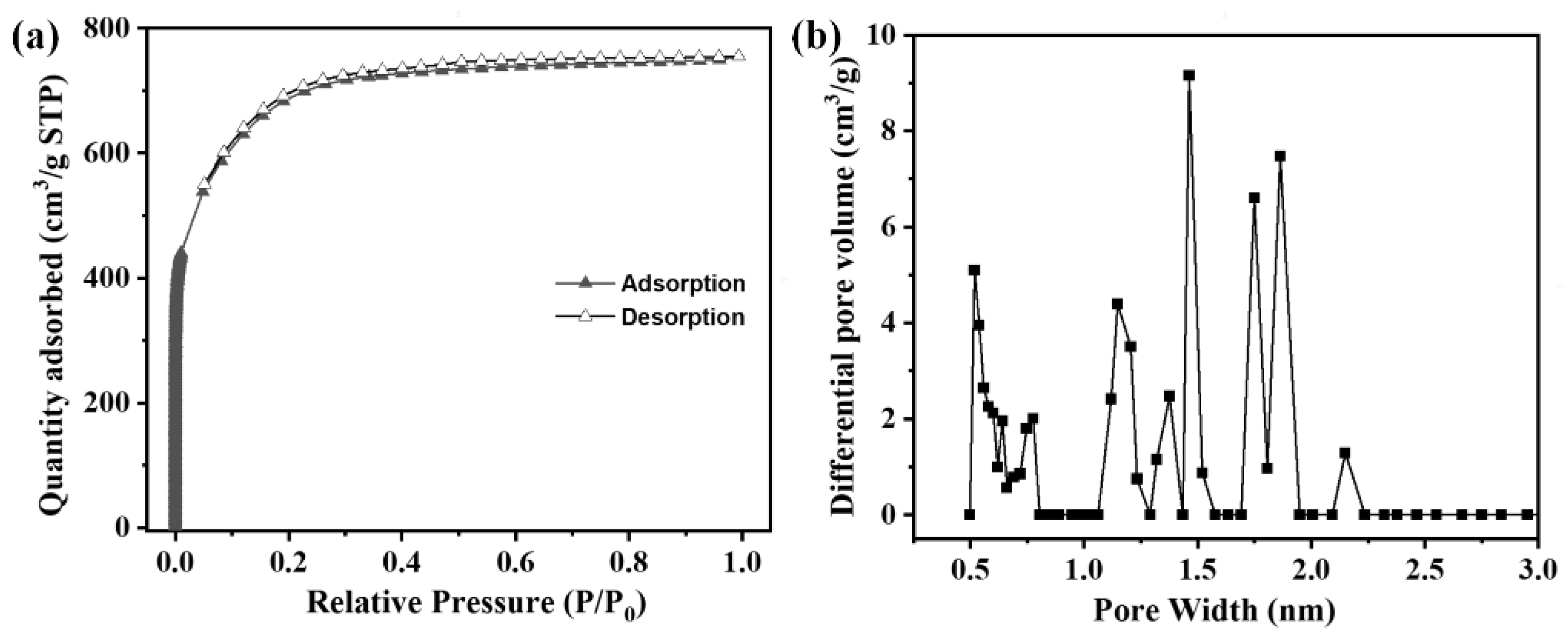
3.4. Specific Surface Area and Pore Structure Analysis
3.5. Adsorption Properties of the ACF Felt towards DPS
3.6. Adsorption Kinetic Analysis
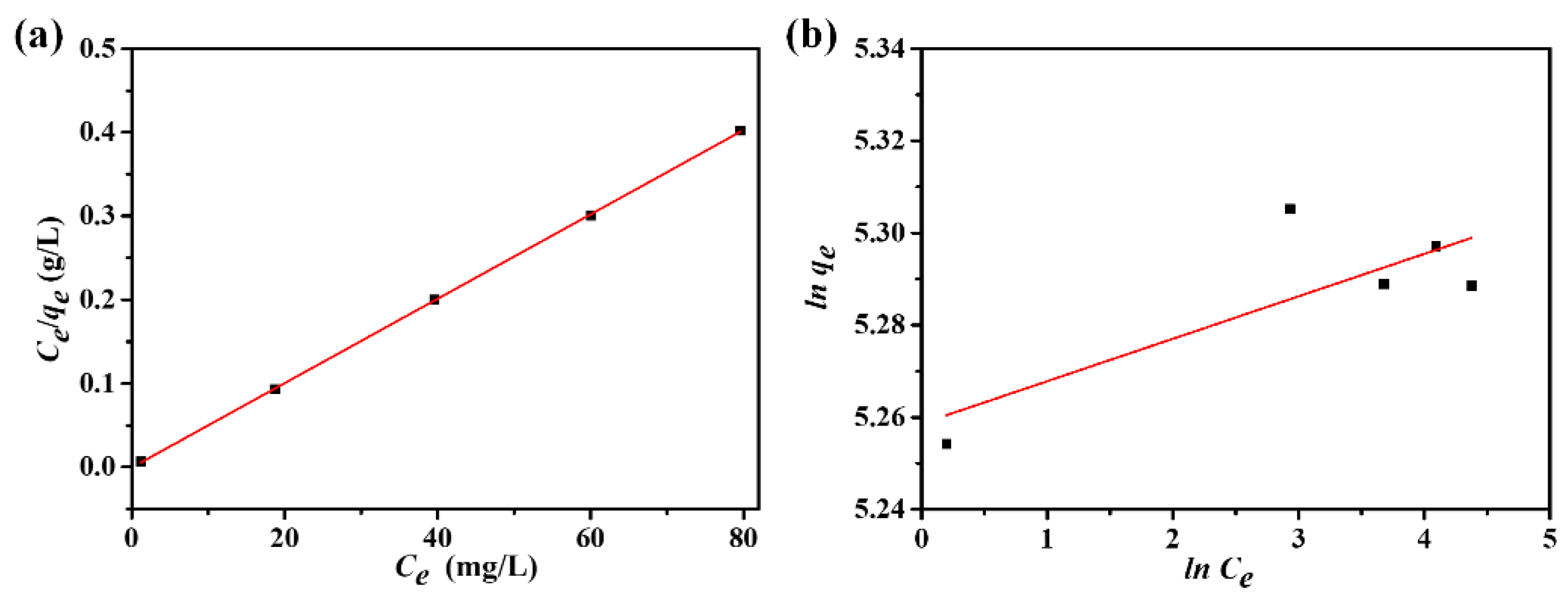
3.7. Adsorption Equilibrium Analysis
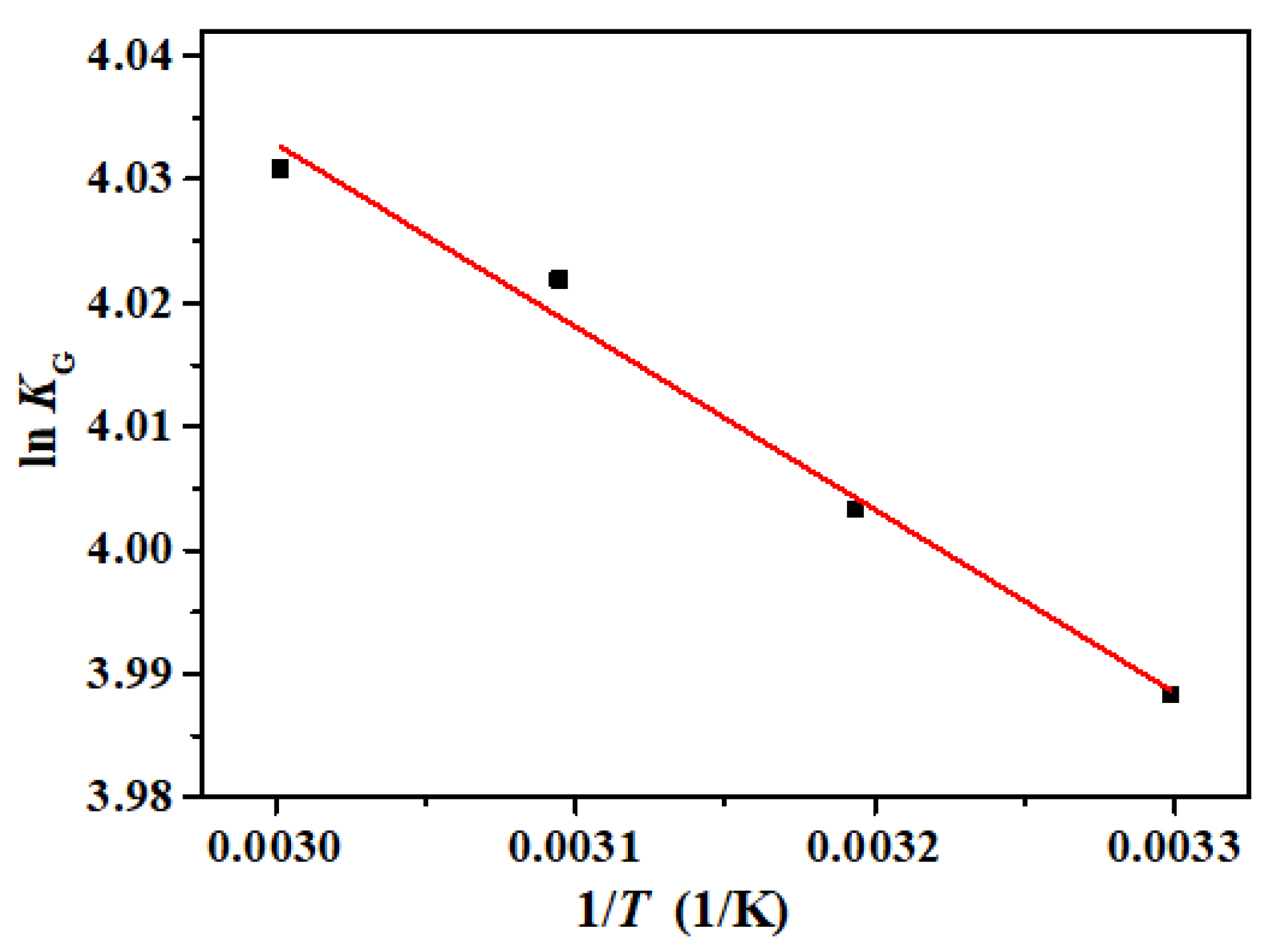
3.8. Adsorption Thermodynamics Analysis
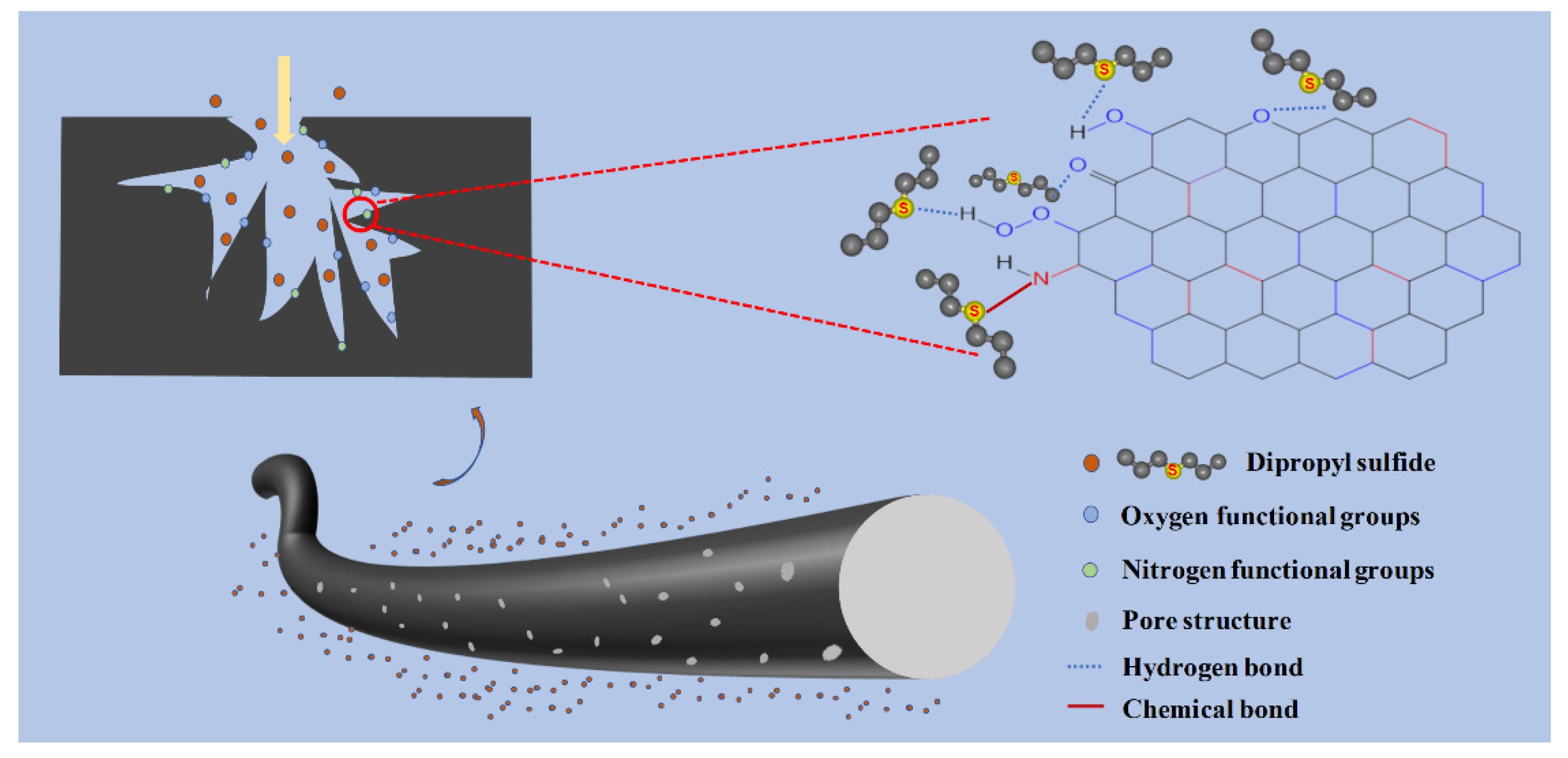
3.9. Adsorption Mechanism of DPS towards ACFs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Razavi, S.M.; Salamati, P.; Saghafinia, M.; Abdollahi, M. A review on delayed toxic effects of sulfur mustard in Iranian veterans. Daru-J. Pharm. Sci. 2012, 20, 51. [Google Scholar] [CrossRef] [PubMed]
- Eckert, W.G. Mass deaths by gas or chemical poisoning. A historical perspective. Am. J. Forensic Med. Pathol. 1991, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Gai, W.-m.; Cheng, W.-y.; Deng, Y.-f. Hazardous chemical leakage accidents and emergency evacuation response from 2009 to 2018 in China: A review. Saf. Sci. 2021, 135, 105101. [Google Scholar] [CrossRef]
- Bhuiyan, M.A.R.; Wang, L.; Shaid, A.; Shanks, R.A.; Ding, J. Advances and applications of chemical protective clothing system. J. Ind. Text. 2018, 49, 97–138. [Google Scholar] [CrossRef]
- Fichtner, S.; Hofmann, J.; Moeller, A.; Schrage, C.; Giebelhausen, J.M.; Boehringer, B.; Glaeser, R. Decomposition of 2-chloroethylethylsulfide on copper oxides to detoxify polymer-based spherical activated carbons from chemical warfare agents. J. Hazard. Mater. 2013, 262, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Couzon, N.; Dhainaut, J.; Campagne, C.; Royer, S.; Loiseau, T.; Volkringer, C. Porous textile composites (PTCs) for the removal and the decomposition of chemical warfare agents (CWAs)—A review. Coord. Chem. Rev. 2022, 467, 214598. [Google Scholar] [CrossRef]
- Modak, A.; Bhanja, P.; Selvaraj, M.; Bhaumik, A. Functionalized porous organic materials as efficient media for the adsorptive removal of Hg(ii) ions. Environ. Sci. Nano 2020, 7, 2887–2923. [Google Scholar] [CrossRef]
- Gopinath, K.P.; Vo, D.-V.N.; Gnana Prakash, D.; Adithya Joseph, A.; Viswanathan, S.; Arun, J. Environmental applications of carbon-based materials: A review. Environ. Chem. Lett. 2021, 19, 557–582. [Google Scholar] [CrossRef]
- Guidotti, M.; Evangelisti, C.; Rossodivita, A.; Ranghieri, M.C. Nano-structured Solids and Heterogeneous Catalysts for the Selective Decontamination of Chemical Warfare Agents. Detect. Chem. Biol. Radiol. Nucl. Agents Prev. Terror. 2014, 1, 275–284. [Google Scholar] [CrossRef]
- Jang, Y.J.; Kim, K.; Tsay, O.G.; Atwood, D.A.; Churchill, D.G. Update 1 of: Destruction and Detection of Chemical Warfare Agents. Chem. Rev. 2015, 115, PR1–PR76. [Google Scholar] [CrossRef]
- Yu, H.; Son, Y.R.; Yoo, H.; Cha, H.G.; Lee, H.; Kim, H.S. Chitosan-Derived Porous Activated Carbon for the Removal of the Chemical Warfare Agent Simulant Dimethyl Methylphosphonate. Nanomaterials 2019, 9, 1703. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Singh, V.V.; Tripathi, N.K.; Sathe, M.; Verma, V.; Sharma, S.P.; Tomar, L.N.S.; Chaturvedi, A.; Yadav, S.S.; Thakare, V.B.; et al. Chemical Protection Studies of Activated Carbon Spheres based Permeable Protective Clothing Against Sulfur Mustard, a Chemical Warfare Agent. Def. Sci. J. 2019, 69, 577–584. [Google Scholar] [CrossRef]
- Xu, D.X.; Li, C.; Yang, L.; Zhu, W.C.; Huang, B.D.; Zhang, C.; Shao, T. Detection of sulfur mustard simulants using the microwave atmospheric pressure plasma optical emission spectroscopy method. Front. Chem. 2023, 11, 1173870. [Google Scholar] [CrossRef] [PubMed]
- Graichen, A.M.; Vachet, R.W. Using Metal Complex Ion-Molecule Reactions in a Miniature Rectilinear Ion Trap Mass Spectrometer to Detect Chemical Warfare Agents. J. Am. Soc. Mass Spectrom. 2013, 24, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, T.; Ren, J.; Li, J.; Ge, J.; Shan, H.; Ji, T.; Xu, M.; Liu, Q. Nano-silver functionalized spherical activated carbon with enhanced dipropyl sulfide adsorption capacity and antibacterial properties. RSC Adv. 2022, 12, 9933–9943. [Google Scholar] [CrossRef]
- Hassan, M.F.; Sabri, M.A.; Fazal, H.; Hafeez, A.; Shezad, N.; Hussain, M. Recent trends in activated carbon fibers production from various precursors and applications—A comparative review. J. Anal. Appl. Pyrolysis 2020, 145, 104715. [Google Scholar] [CrossRef]
- Komatsubara, Y.; Ida, S.; Fujitsu, H.; Mochida, I. Catalytic activity of PAN-based active carbon fibre (PAN-ACF) activated with sulphuric acid for reduction of nitric oxide with ammonia. Fuel 1984, 63, 1738–1742. [Google Scholar] [CrossRef]
- Yusof, N.; Ismail, A.F. Post spinning and pyrolysis processes of polyacrylonitrile (PAN)-based carbon fiber and activated carbon fiber: A review. J. Anal. Appl. Pyrolysis 2012, 93, 1–13. [Google Scholar] [CrossRef]
- Golkarnarenji, G.; Naebe, M.; Badii, K.; Milani, A.S.; Jazar, R.N.; Khayyam, H. A machine learning case study with limited data for prediction of carbon fiber mechanical properties. Comput. Ind. 2019, 105, 123–132. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, L.-Z.; Song, L.; Wang, Y.; Li, S.; Shi, Y. Research on Improving the Pre-Oxidation Process of the Excellent Mechanical Strength Carbon Fiber. Macromol. Chem. Phys. 2023, 224, 2300114. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W. Preparation and characterization of activated carbon fibers from liquefied poplar bark. Mater. Lett. 2013, 112, 26–28. [Google Scholar] [CrossRef]
- Li, K.; Li, K.; Wang, C.; Ning, P.; Sun, X.; Song, X.; Wang, Y. Preparation of polyacrylonitrile-based activated carbon fiber for CS2 adsorption. Res. Chem. Intermed. 2020, 46, 3459–3476. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, G. Preparation and characterization of activated carbon fibers from liquefied wood by KOH activation. Holzforschung 2016, 70, 195–202. [Google Scholar] [CrossRef]
- Gong, Y.; Li, D.; Luo, C.; Fu, Q.; Pan, C. Highly porous graphitic biomass carbon as advanced electrode materials for supercapacitors. Green Chem. 2017, 19, 4132–4140. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, W.; An, Z.; Kong, S.; Chen, D. Adsorption of anionic dye onto magnetic Fe3O4/CeO2 nanocomposite: Equilibrium, kinetics, and thermodynamics. Adsorpt. Sci. Technol. 2019, 37, 185–204. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.; Wang, M.; Zhang, Q.; Ji, T.; Chen, T.; Yu, D. Adsorption of methylene blue from aqueous solution onto viscose-based activated carbon fiber felts: Kinetics and equilibrium studies. Adsorpt. Sci. Technol. 2019, 37, 256–264. [Google Scholar] [CrossRef]
- Vyawahare, P.; Tun, H.; Vaughn, M.W.; Chen, C. From Langmuir Isotherm to Brunauer-Emmett-Teller Isotherm. AIChE J. 2021, 68, 59482–59485. [Google Scholar] [CrossRef]
- Stromer, B.S.; Woodbury, B.; Williams, C.F. Tylosin sorption to diatomaceous earth described by Langmuir isotherm and Freundlich isotherm models. Chemosphere 2018, 193, 912–920. [Google Scholar] [CrossRef]
- Fegade, U.; Jethave, G.; Su, K.-Y.; Huang, W.-R.; Wu, R.-J. An multifunction Zn0.3Mn0.4O4 nanospheres for carbon dioxide reduction to methane via photocatalysis and reused after five cycles for phosphate adsorption. J. Environ. Chem. Eng. 2018, 6, 1918–1925. [Google Scholar] [CrossRef]
- Yağmur, H.K.; Kaya, İ. Synthesis and characterization of magnetic ZnCl2-activated carbon produced from coconut shell for the adsorption of methylene blue. J. Mol. Struct. 2021, 1232, 130017. [Google Scholar] [CrossRef]
- Yoshikawa, Y.; Teshima, K.; Futamura, R.; Tanaka, H.; Kaneko, K. Structural mechanism of reactivation with steam of pitch-based activated carbon fibers. J. Colloid Interface Sci. 2020, 578, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Liu, Y.; Zeng, G.; Xu, W.; Zheng, B.; Tan, X.; Wang, S. Adsorption of hexavalent chromium by polyacrylonitrile (PAN)-based activated carbon fibers from aqueous solution. Rsc. Adv. 2015, 5, 25389–25397. [Google Scholar] [CrossRef]
- Kumamaru, F.; Kajiyama, T.; Takayanagi, M. Formation of single crystals of poly(acrylonitrile) during the process of solution polymerization. J. Cryst. Growth 1980, 48, 202–209. [Google Scholar] [CrossRef]
- Su, Y.J.; Ko, T.H.; Lin, J.H. Preparation of ultra-thin PAN-based activated carbon fibers with physical activation. J. Appl. Polym. Sci. 2008, 108, 3610–3617. [Google Scholar] [CrossRef]
- Kim, M.; Kim, J. Redox deposition of birnessite-type manganese oxide on silicon carbide microspheres for use as supercapacitor electrodes. Acs. Appl. Mater. Interfaces 2014, 6, 9036–9045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xue, C.-H.; Ma, H.-R.; Ding, Y.-R.; Jia, S.-T. Fabrication of PAN Electrospun Nanofibers Modified by Tannin for Effective Removal of Trace Cr(III) in Organic Complex from Wastewater. Polymers 2020, 12, 210. [Google Scholar] [CrossRef]
- Chirayath, V.A.; Callewaert, V.; Fairchild, A.J.; Chrysler, M.D.; Gladen, R.W.; McDonald, A.D.; Imam, S.K.; Shastry, K.; Koymen, A.R.; Saniz, R.; et al. Auger electron emission initiated by the creation of valence-band holes in graphene by positron annihilation. Nat. Commun. 2017, 8, 16116. [Google Scholar] [CrossRef]
- Yue, Z.R.; Jiang, W.; Wang, L.; Gardner, S.D.; Pittman, C.U. Surface characterization of electrochemically oxidized carbon fibers. Carbon 1999, 37, 1785–1796. [Google Scholar] [CrossRef]
- Xie, Y.; Sherwood, P.M.A. X-ray photoelectron-spectroscopic studies of carbon fiber surfaces. 11. Differences in the surface chemistry and bulk structure of different carbon fibers based on poly(acrylonitrile) and pitch and comparison with various graphite samples. Chem. Mater. 1990, 2, 293–299. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, J.; Zhao, G. Study on the structure evolution and temperature zone regulation mechanism of polyacrylonitrile fibers during pre-oxidation process. Iran. Polym. J. 2023, 32, 1511–1522. [Google Scholar] [CrossRef]
- Pacheco, F.G.; Cotta, A.A.C.; Gorgulho, H.F.; Santos, A.P.; Macedo, W.A.A.; Furtado, C.A. Comparative temporal analysis of multiwalled carbon nanotube oxidation reactions: Evaluating chemical modifications on true nanotube surface. Appl. Surf. Sci. 2015, 357, 1015–1023. [Google Scholar] [CrossRef]
- Nordfors, D.; Nilsson, A.; M»rtensson, N.; Svensson, S.; Gelius, U.; Ågren, H. X-ray excited photoelectron spectra of free molecules containing oxygen. J. Electron Spectrosc. Relat. Phenom. 1991, 56, 117–164. [Google Scholar] [CrossRef]
- Piazuelo, R.; Rodríguez-Castellón, E.; Jiménez-Jiménez, J.; Jiménez-López, A.; Benavente, J. Chemical surface and electrochemical characterization of zirconium phosphate heterostructures. Microporous Mesoporous Mater. 2008, 115, 23–32. [Google Scholar] [CrossRef]
- Bae, J.; Hong, J.-Y. Fabrication of nitrogen-doped porous carbon nanofibers for heavy metal ions removal. Carbon Lett. 2021, 31, 1339–1347. [Google Scholar] [CrossRef]
- Kim, B.-H. Supercapacitive properties of nanoporous carbon nanofibers developed from polyacrylonitrile and tetraethyl orthosilicate. J. Electroanal. Chem. 2014, 734, 84–89. [Google Scholar] [CrossRef]
- Weidenthaler, C.; Lu, A.-H.; Schmidt, W.; Schüth, F. X-ray photoelectron spectroscopic studies of PAN-based ordered mesoporous carbons (OMC). Microporous Mesoporous Mater. 2006, 88, 238–243. [Google Scholar] [CrossRef]
- Kim, M.-A.; Jang, D.; Tejima, S.; Cruz-Silva, R.; Joh, H.-I.; Kim, H.C.; Lee, S.; Endo, M. Strengthened PAN-based carbon fibers obtained by slow heating rate carbonization. Sci. Rep. 2016, 6, 22988. [Google Scholar] [CrossRef]
- Yue, Z.R.; Benak, K.R.; Wang, J.W.; Mangun, C.L.; Economy, J. Elucidating the porous and chemical structures of ZnCl2 activated polyacrylonitrile on a fiberglass substrate. J. Mater. Chem. 2005, 15, 3142–3148. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, C.; Wang, Q.; Li, S.; Zhao, X.; Luo, H. Preparation and electrochemical properties of self-doped nitrogen porous carbon derived from Zn-MOFs. Carbon Lett. 2020, 30, 315–324. [Google Scholar] [CrossRef]
- Lin, G.; Ma, R.; Zhou, Y.; Liu, Q.; Dong, X.; Wang, J. KOH activation of biomass-derived nitrogen-doped carbons for supercapacitor and electrocatalytic oxygen reduction. Electrochim. Acta 2018, 261, 49–57. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Kierzek, K.; Machnikowski, J.; Béguin, F. Relationship between the nanoporous texture of activated carbons and their capacitance properties in different electrolytes. Carbon 2006, 44, 2498–2507. [Google Scholar] [CrossRef]
- Yang, B.; Wang, C.; Ji, X.; Yue, X.; Lv, G.; Wang, M. Investigation on water vapor adsorption performance of carbon based composite adsorption material ACF-silica sol-LiCl. Microporous Mesoporous Mater. 2022, 344, 112205. [Google Scholar] [CrossRef]
- Illingworth, J.M.; Rand, B.; Williams, P.T. Understanding the mechanism of two-step, pyrolysis-alkali chemical activation of fibrous biomass for the production of activated carbon fibre matting. Fuel Process. Technol. 2022, 235, 107348. [Google Scholar] [CrossRef]
- Bai, X.; Lin, J.; Zhang, Z.; Zhan, Y. Immobilization of lead, copper, cadmium, nickel, and zinc in sediment by red mud: Adsorption characteristics, mechanism, and effect of dosage on immobilization efficiency. Environ. Sci. Pollut. Res. 2022, 29, 51793–51814. [Google Scholar] [CrossRef]
- Liu, D.; Tang, Y.; Li, J.; Hao, Z.; Zhu, J.; Wei, J.; Liu, C.; Dong, L.; Jia, B.; Chen, G. Eupatorium adenophorum derived adsorbent by hydrothermal-assisted HNO3 modification and application to Pb2+ adsorption. J. Environ. Chem. Eng. 2021, 9, 105972. [Google Scholar] [CrossRef]
- Tabrizi, S.H.; Tanhaei, B.; Ayati, A.; Ranjbari, S. Substantial improvement in the adsorption behavior of montmorillonite toward Tartrazine through hexadecylamine impregnation. Environ. Res. 2022, 204, 111965. [Google Scholar] [CrossRef] [PubMed]
- Karimi, F.; Ayati, A.; Tanhaei, B.; Sanati, A.L.; Afshar, S.; Kardan, A.; Dabirifar, Z.; Karaman, C. Removal of metal ions using a new magnetic chitosan nano-bio-adsorbent; A powerful approach in water treatment. Environ. Res. 2022, 203, 111753. [Google Scholar] [CrossRef] [PubMed]
- Lafdani, E.K.; Laurén, A.; Cvetkovic, J.; Pumpanen, J.; Saarela, T.; Palviainen, M. Nitrogen Recovery from Clear-Cut Forest Runoff Using Biochar: Adsorption–Desorption Dynamics Affected by Water Nitrogen Concentration. Water Air Soil Pollut. 2021, 232, 432. [Google Scholar] [CrossRef]
- Zhu, L.; Yao, Y.; Chen, D.; Lan, P. The effective removal of Pb2+ by activated carbon fibers modified by lcysteine: Exploration of kinetics, thermodynamics and mechanism. RSC Adv. 2022, 12, 20062–20073. [Google Scholar] [CrossRef]
- Pang, T.; Aye Chan, T.S.; Jande, Y.A.C.; Shen, J. Removal of fluoride from water using activated carbon fibres modified with zirconium by a drop-coating method. Chemosphere 2020, 255, 126950. [Google Scholar] [CrossRef]
- Wang, L.; Huang, Z.; Zhang, M.; Chai, B. Adsorption of methylene blue from aqueous solution on modified ACFs by chemical vapor deposition. Chem. Eng. J. 2012, 189–190, 168–174. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Wang, A. Fast removal of methylene blue from aqueous solution by adsorption onto chitosan-g-poly (acrylic acid)/attapulgite composite. Desalination 2011, 266, 33–39. [Google Scholar] [CrossRef]
- Li, Y.; Du, Q.; Liu, T.; Peng, X.; Wang, U.; Sun, J.; Wang, Y.; Wu, S.; Wang, Z.; Xia, Y. Comparative study of methylene blue dye adsorption onto activated carbon, graphene oxide, and carbon nanotubes. Chem. Eng. Res. Des. Trans. Inst 2013, 91, 361–368. [Google Scholar] [CrossRef]
- Weng, M.; Zhu, Q.; Wang, A.; Liu, G.; Huang, H. A new type of SnO2@β-Fe(Zr)OOH hollow nanosphere as a bifunctional adsorbent for removing nitrate from water: Kinetics, isotherm, and thermodynamic studies. J. Mater. Sci. 2020, 55, 15797–15812. [Google Scholar] [CrossRef]
- Chu, X.; Guo, P.; Pei, Y.; Yan, S.; Hu, H.; Qiao, M.; Fan, K.; Zong, B.; Zhang, X. Adsorption and Thermal Reaction of Dipropyl Sulfide on Skeletal Ni Adsorbents. J. Phys. Chem. C 2007, 111, 17535–17540. [Google Scholar] [CrossRef]
- Zhu, L.L.; Shen, D.K.; Luo, K.H. A critical review on VOCs adsorption by different porous materials: Species, mechanisms and modification methods. J. Hazard. Mater. 2020, 389, 122102. [Google Scholar] [CrossRef]
- Figueiredo, J.L. Functionalization of porous carbons for catalytic applications. Cheminform 2013, 1, 9351–9364. [Google Scholar] [CrossRef]





| C0 | qe exp (mg/g) | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | ||||
|---|---|---|---|---|---|---|---|
| k1 (1/min) | qe cal (mg/g) | R2 | k2 (g·mg−1·min−1) | qe cal (mg/g) | R2 | ||
| 40 | 191.75 | 2.87 × 10−2 | 193.01 | 0.9359 | 3.37 × 10−4 | 197.63 | 0.9997 |
| 60 | 202.38 | 1.90 × 10−2 | 86.19 | 0.8557 | 3.23 × 10−4 | 207.90 | 0.9998 |
| 80 | 199.50 | 2.61 × 10−2 | 264.05 | 0.9978 | 3.00 × 10−4 | 205.76 | 0.9998 |
| 100 | 200.75 | 2.78 × 10−2 | 240.71 | 0.9983 | 3.47 × 10−4 | 206.17 | 0.9999 |
| C0 | The First Stage | The Second Stage | ||||
|---|---|---|---|---|---|---|
| Kint [mg/(g∙min1/2)] | C | R2 | Kint [mg/(g∙min1/2)] | C | R2 | |
| 40 | 5.50 | 107.30 | 0.9852 | 0.26 | 185.71 | 0.8798 |
| 60 | 5.68 | 114.12 | 0.9733 | 0.36 | 193.63 | 0.8274 |
| 80 | 5.14 | 116.74 | 0.9924 | 0.55 | 186.72 | 0.9308 |
| 100 | 4.92 | 122.86 | 0.9801 | 0.50 | 189.27 | 0.9066 |
| Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|
| qm (mg/g) | KL (L/mg) | R2 | KF (mg/g) | 1/n | R2 |
| 198.41 | 0.957 | 0.9999 | 252 | 9.21 × 10−3 | 0.5203 |
| ΔS (J∙mol−1∙K−1) | ΔH (kJ/mol) | ΔG (kJ/mol) | |||
|---|---|---|---|---|---|
| 30 °C | 40 °C | 50 °C | 60 °C | ||
| 37.22 | 1.23 | −10.05 | −10.42 | −10.81 | −11.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; He, Y.; Hu, S.; Ge, J.; Chen, T.; Shan, H.; Ji, T.; Yu, D.; Liu, Q. Facile Preparation of Polyacrylonitrile-Based Activated Carbon Fiber Felts for Effective Adsorption of Dipropyl Sulfide. Polymers 2024, 16, 252. https://doi.org/10.3390/polym16020252
Zhang T, He Y, Hu S, Ge J, Chen T, Shan H, Ji T, Yu D, Liu Q. Facile Preparation of Polyacrylonitrile-Based Activated Carbon Fiber Felts for Effective Adsorption of Dipropyl Sulfide. Polymers. 2024; 16(2):252. https://doi.org/10.3390/polym16020252
Chicago/Turabian StyleZhang, Tianhao, Yafang He, Shiqi Hu, Jianlong Ge, Tianye Chen, Haoru Shan, Tao Ji, Decheng Yu, and Qixia Liu. 2024. "Facile Preparation of Polyacrylonitrile-Based Activated Carbon Fiber Felts for Effective Adsorption of Dipropyl Sulfide" Polymers 16, no. 2: 252. https://doi.org/10.3390/polym16020252
APA StyleZhang, T., He, Y., Hu, S., Ge, J., Chen, T., Shan, H., Ji, T., Yu, D., & Liu, Q. (2024). Facile Preparation of Polyacrylonitrile-Based Activated Carbon Fiber Felts for Effective Adsorption of Dipropyl Sulfide. Polymers, 16(2), 252. https://doi.org/10.3390/polym16020252






