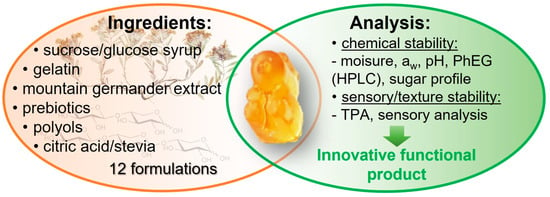
Physicochemical and Sensory Stability Evaluation of Gummy Candies Fortified with Mountain Germander Extract and Prebiotics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Mountain Germander Extract
2.2.2. Production of Gummy Candy
Preparation of Gelatin Solution
Preparation of Citric Acid and Stevia Solution
Preparation of Sugar Syrup
Preparation of Gummy Candy Mass and Formation of Candies
2.2.3. Stability Study
2.2.4. Determination of Dry Matter
2.2.5. Determination of Water Activity
2.2.6. Determination of pH Value
2.2.7. HPLC Analyses
Sample Preparation
Analysis of Polyphenols by Reversed-Phase HPLC
Analysis of Sugars by Ligand-Exchange HPLC
2.2.8. Texture Analysis
2.2.9. Color Measurement
2.2.10. Sensory Evaluation
2.2.11. Statistical Analysis
3. Results and Discussion
3.1. Visual Appearance of the Candy
3.2. Dry Matter Content, Water Activity and pH Value
3.3. Content of Phenylethanoid Glycosides
3.4. Color
3.5. Sugar Profile and Content
3.6. Texture Analysis
3.7. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dille, M.J.; Hattrem, M.N.; Draget, K.I. Bioactively Filled Gelatin Gels; Challenges and Opportunities. Food Hydrocoll. 2018, 76, 17–29. [Google Scholar] [CrossRef]
- Pickford, E.F.; Jardine, N.J. Functional Confectionery. In Functional Foods—Concepts to Products; Gibson, G.R., Williams, C.M., Eds.; Woodhead Publishing: Cambridge, UK, 2000; pp. 259–286. ISBN 9780849308512. [Google Scholar]
- Food and Beverage Insider. Chewy Candy Sales Continue to Climb (Bradford, K.). Available online: https://www.foodbeverageinsider.com/confectionery/chewy-candy-sales-continue-to-climb (accessed on 2 November 2023).
- Mandura, A.; Šeremet, D.; Ščetar, M.; Vojvodić Cebin, A.; Belščak-Cvitanović, A.; Komes, D. Physicochemical, Bioactive, and Sensory Assessment of White Tea-Based Candies During 4-Months Storage. J. Food Process. Preserv. 2020, 44, e14628. [Google Scholar] [CrossRef]
- Gunes, R.; Palabiyik, I.; Konar, N.; Toker, O.S. Soft Confectionery Products: Quality Parameters, Interactions with Processing and Ingredients. Food Chem. 2022, 385, 132735. [Google Scholar] [CrossRef]
- Baziwane, D.; He, Q. Gelatin: The Paramount Food Additive. Food Rev. Int. 2003, 19, 423–435. [Google Scholar] [CrossRef]
- Dille, M.J.; Haug, I.J.; Draget, K.I. Gelatin and Collagen. In Handbook of Hydrocolloids, 3rd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Cambridge, UK, 2021; pp. 1073–1097. ISBN 978-1-84569-587-3. [Google Scholar]
- Konar, N.; Gunes, R.; Palabiyik, I.; Toker, O.S. Health Conscious Consumers and Sugar Confectionery: Present Aspects and Projections. Trends Food Sci. Technol. 2022, 123, 57–68. [Google Scholar] [CrossRef]
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and Gellatine. Annu. Rev. Food Sci. Technol. 2015, 6, 527–557. [Google Scholar] [CrossRef] [PubMed]
- Rawson, E.S.; Miles, M.P.; Larson-Meyer, D.E. Dietary Supplements for Health, Adaptation, and Recovery in Athletes. Int. J. Sport Nutr. Exerc. 2018, 28, 188–199. [Google Scholar] [CrossRef]
- Periche, A.; Heredia, A.; Escriche, A.; Andrés, A.; Castelló, M.L. Optical, Mechanical and Sensory Properties of Based-Isomaltulose Gummy Confections. Food Biosci. 2014, 7, 37–44. [Google Scholar] [CrossRef]
- Mandura Jarić, A.; Čikoš, A.; Pocrnić, M.; Aladić, K.; Jokić, S.; Šeremet, D.; Vojvodić Cebin, A.; Komes, D. Teucrium montanum L.—Unrecognized Source of Phenylethanoid Glycosides: Green Extraction Approach and Elucidation of Phenolic Compounds via NMR and UHPLC-HR MS/MS. Antioxidants 2023, 12, 1903. [Google Scholar] [CrossRef]
- Nastić, N.; Švarc-Gajić, J.; Delerue-Matos, C.; Morais, S.; Barroso, M.F.; Moreira, M.M. Subcritical Water Extraction of Antioxidants from Mountain Germander (Teucrium montanum L.). J. Supercrit. Fluids 2018, 138, 200–206. [Google Scholar] [CrossRef]
- Stanković, M.S.; Niciforović, M.; Topuzović, M.; Solujić, S. Total Phenolic Content, Flavonoid Concentrations and Antioxidant Activity, of the Whole Plant and Plant Parts Extracts from Teucrium montanum L. var. Montanum, F. Supinum (L.) Reichenb. Biotechnol. Biotechnol. Equip. 2011, 25, 2222–2227. [Google Scholar] [CrossRef]
- Šeremet, D.; Vugrinec, K.; Petrović, P.; Butorac, A.; Kuzmić, S.; Vojvodić Cebin, A.; Mandura, A.; Lovrić, M.; Pjanović, R.; Komes, D. Formulation and Characterization of Liposomal Encapsulated Systems of Bioactive Ingredients from Traditional Plant Mountain Germander (Teucrium montanum L.) for the Incorporation into Coffee Drinks. Food Chem. 2022, 370, 131257. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.Y.; Li, M.; Lin, T.; Qiu, Y.; Zhu, Y.T.; Li, X.L.; Tao, W.D.; Wang, P.; Ren, X.X.; Chen, L.P. A Review on the Structure and Pharmacological Activity of Phenylethanoid Glycosides. Eur. J. Med. Chem. 2021, 209, 112563. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, M.; Ghaani, M.R.; Bayazeid, O. Phenylethanoid Glycosides as a Possible COVID-19 Protease Inhibitor: A Virtual Screening Approach. J. Mol. Model. 2021, 27, 341. [Google Scholar] [CrossRef] [PubMed]
- Cheohen, C.F.A.R.; Esteves, M.E.A.; da Fonseca, T.S.; Leal, C.M.; Assis, F.L.F.; Campos, M.F.; Rebelo, R.S.; Allonso, D.; Leitão, G.G.; da Silva, M.L.; et al. In silico Screening of Phenylethanoid Glycosides, a Class of Pharmacologically Active Compounds as Natural Inhibitors of SARS-CoV-2 Proteases. Comput. Struct. Biotechnol. J. 2023, 21, 1461–1472. [Google Scholar] [CrossRef]
- Zumbé, A.; Lee, A.; Storey, D. Polyols in Confectionery: The Route to Sugar-Free, Reduced Sugar and Reduced Calorie Confectionery. Br. J. Nutr. 2001, 81, S31–S45. [Google Scholar] [CrossRef]
- Livesey, G. Health Potential of Polyols as Sugar Replacers, with Emphasis on Low Glycaemic Properties. Nutr. Res. Rev. 2003, 16, 163–191. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Feng, J.; Regenstein, J.; Lv, Y.; Li, J. Confectionery gels: Effects of Low Calorie Sweeteners on the Rheological Properties and Microstructure of Fish Gelatin. Food Hydrocoll. 2017, 67, 157–165. [Google Scholar] [CrossRef]
- Franck, A. Food Applications of Prebiotics. In Handbook of Prebiotics; Gibson, G.R., Roberfroid, M.B., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 437–448. ISBN 978-0-8493-8171-3. [Google Scholar]
- Gok, S.; Toker, O.S.; Palabiyik, I.; Konar, N. Usage Possibility of Mannitol and Soluble Wheat Fiber in Low Calorie Gummy Candies. LWT-Food Sci. Technol. 2020, 128, 109531. [Google Scholar] [CrossRef]
- Lele, V.; Ruzauskas, M.; Zavistanaviciute, P.; Laurusiene, R.; Rimene, G.; Kiudulaite, D.; Tomkeviciute, J.; Nemeikstyte, J.; Stankevicius, R.; Bartkiene, E. Development and Characterization of the Gummy–Supplements, Enriched with Probiotics and Prebiotics. CyTA-J. Food 2018, 16, 580–587. [Google Scholar] [CrossRef]
- Amorim, C.; Silvero, S.C.; Prather, K.L.J.; Rodrigues, L.R. From Lignocellulosic Residues to Market: Production and Commercial Potential of Xylooligosaccharides. Biotechnol. Adv. 2019, 15, 107397. [Google Scholar] [CrossRef] [PubMed]
- Palaniappan, A.; Antony, U.; Emmambux, M.N. Current Status of Xylooligosaccharides: Production, Characterization, Health Benefits and Food Application. Trends Food Sci. Technol. 2021, 111, 506–519. [Google Scholar] [CrossRef]
- Shandong Longlive Biotechnology Co., Ltd. Available online: http://www.longlivegroup.com/col.jsp?id=104 (accessed on 2 November 2023).
- Vojvodić Cebin, A.; Ralet, M.C.; Vigouroux, J.; Karača, S.; Martinić, A.; Komes, D.; Bonnin, E. Valorisation of Walnut Shell and Pea Pod as Novel Sources for the Production of Xylooligosaccharides. Carbohydr. Polym. 2021, 263, 117932. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.K.; Jayapal, N.; Jayaram, C.; Roy, S.; Kolte, A.P.; Senani, S.; Sridhar, M. Xylooligosaccharides as Prebiotics from Agricultural By-Products: Production and Applications. Bioact. Carbohydr. Diet. Fibre 2015, 5, 62–71. [Google Scholar] [CrossRef]
- Cruz-Guerrero, A.; Gómez-Ruiz, L.; Guzmán-Rodríguez, F. Xylooligosaccharides. In Handbook of Food Bioactive Ingredients: Properties and Appplications; Jafari, S.M., Rashidinejad, A., Simal-Ganara, J., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2023; pp. 1243–1270. ISBN 978-3-031-28109-9. [Google Scholar]
- Seifert, S.; Watzl, B. Prebiotics and the Immune System: Review of Experimental and Human Data. In Handbook of Prebiotics; Gibson, G.R., Roberfroid, M.B., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 143–162. ISBN 978-0-8493-8171-3. [Google Scholar]
- Van der Abbeele, P.; Duysburgh, C.; Jiang, T.A.; Rebaza, M.; Pinheiro, I.; Marzorati, M. A Combination of Xylooligosaccharides and a Polyphenol Blend Affect Microbial Composition and Activity in the Distal Colon Exerting Immunomodulating Properties on Human Cells. J. Funct. Foods 2018, 47, 163–171. [Google Scholar] [CrossRef]
- Padmore, J.M. Animal Feed—AOAC official method 930.15—Moisture in Animal Feed. In Official Methods of Analysis, 15th ed.; Helrich, K., Ed.; AOAC International: Arlington, VA, USA, 1990; Volume 1, pp. 69–70. ISBN 0-935584-42-0. [Google Scholar]
- Delgado, P.; Bañón, S. Effects of Replacing Starch by Inulin on Physicochemical, Texture and Sensory Characteristics of Gummy Jellies. CyTA-J. Food 2017, 16, 1. [Google Scholar] [CrossRef]
- Ergun, R.; Lietha, R.; Hartel, R.W. Moisture and Shelf Life in Sugar Confections. Crit. Rev. Food Sci. Nutr. 2010, 50, 162–192. [Google Scholar] [CrossRef]
- Efe, N.; Dawson, P. A Review: Sugar-based Confectionery and the Importance of Ingredients. Eur. J. Agric. Food Sci. 2022, 4, 1–8. [Google Scholar] [CrossRef]
- Plotnikova, V.; Zharkova, I.M.; Magomedov, G.O.; Magomedov, M.G.; Khvostov, A.A.; Miroshnichenko, E.N. Forecasting and Quality Control of Confectionery Products with the Use of “Water Activity” Indicator. IOP Conf. Ser. Earth Environ. Sci. 2021, 640, 062003IOP. [Google Scholar] [CrossRef]
- Burey, P.; Bhandari, B.R.; Rutgers, R.P.G.; Halley, P.J.; Torley, P.J. Confectionery gels: A Review on Formulation, Rheological and Structural Aspects. Int. J. Food Prop. 2009, 12, 176–210. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, S.; Zhao, Y.; Zhu, G. Echinacoside: A Promising Active Natural Products and Pharmacological Agents. Pharmacol. Res. 2023, 197, 106951. [Google Scholar] [CrossRef]
- FDA-U.S. Food and Drug Administration. FDA, Serving Size Updates on the New Nutrition Facts Label. Available online: https://www.fda.gov/food/new-nutrition-facts-label/serving-size-updates-new-nutrition-facts-label (accessed on 18 June 2023).
- Mokrzycki, W.S.; Tatol, M. Colour Difference ΔE—A Survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Stokes, M.; Fairchild, M.D.; Berns, R.S. Precision Requirements for Digital Color Reproduction. ACM Trans. Graph. 1992, 11, 406–422. [Google Scholar] [CrossRef]
- EFSA Nutrition Claims. Available online: https://food.ec.europa.eu/safety/labelling-and-nutrition/nutrition-and-health-claims/nutrition-claims_en (accessed on 8 November 2023).
- Finegold, S.M.; Li, Z.; Summanen, P.H.; Downes, J.; Thames, G.; Corbett, K.; Dowd, S.; Krakde, M.; Heberde, D. Xylooligosaccharide Increases Bifidobacteria but not Lactobacilli in Human Gut Microbiota. Food Funct. 2014, 5, 436–445. [Google Scholar] [CrossRef] [PubMed]
- EFSA, Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific opinion on dietary reference values for carbohydrates and dietary fibre. EFSA J. 2010, 8, 1462. [Google Scholar] [CrossRef]
- Lee, W.; Spiekerman, C.; Heima, M.; Eggertsson, H.; Ferretti, G.; Milgrom, P.; Nelson, S. The Effectiveness of Xylitol in a School-based Cluster-randomized Clinical Trial. Caries Res. 2015, 49, 41–49. [Google Scholar] [CrossRef]
- Ly, K.A.; Riedy, C.A.; Milgrom, P.; Rothen, M.; Roberts, M.C.; Zhou, L. Xylitol Gummy Bear Snacks: A School-based Randomized Clinical Trial. BMC Oral Health 2008, 8, 20. [Google Scholar] [CrossRef]
- Wang, R.; Hartel, R.W. Confectionery gels: Gelling Behavior and Gel Properties of Gelatin in Concentrated Sugar Solutions. Food Hydrocoll. 2022, 124 Pt A, 107132. [Google Scholar] [CrossRef]
- Szczesniak, A.S. Texture is a Sensory Property. Food Qual. Prefer. 2002, 13, 215–225. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Introducing Inulin-type Fructans. Br. J. Nutr. 2005, 93, S13–S25. [Google Scholar] [CrossRef]
- Avallone, P.R.; Romano, M.; Sarrica, A.; Delmonte, M.; Pasquino, R.; Grizzuti, N. Effect of Sugars on Gelation Kinetics of Gelatin Gels. Fluids 2022, 7, 163. [Google Scholar] [CrossRef]
- DeMars, L.L.; Ziegler, G.R. Texture and Structure of Gelatin/Pectin-based Gummy Confections. Food Hydrocoll. 2001, 15, 643–653. [Google Scholar] [CrossRef]
- Ersoy, E.; Süvari, G.; Ercan, S.; Özkan, E.E.; Karahan, S.; Tuncay, E.A.; Cantürk, Y.Y.; Kara, E.M.; Zengin, G.; Boğa, M. Towards a Better Understanding of Commonly Used Medicinal Plants from Turkiye: Detailed Phytochemical Screening and Biological Activity Studies of Two Teucrium L. Species with in vitro and in silico Approach. J. Ethnopharmacol. 2023, 312, 116482. [Google Scholar] [CrossRef] [PubMed]
- Jurišić Grubešić, R.; Kremer, D.; Vladimir-Knežević, S.; Vuković Rodríguez, J. Analysis of Polyphenols, Phytosterols, and Bitter Principles in Teucrium L. Species. Cent. Eur. J. Biol. 2012, 7, 542–550. [Google Scholar] [CrossRef]
- Malakov, P.Y.; Papanov, G.Y.; Boneva, I.M. Neo-clerodane Diterpenoids from Teucrium montanum. Phytochemistry 1992, 31, 4029–4030. [Google Scholar] [CrossRef]

| Ingredient (g) | Formulations | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HSb | LSb | NSb | HSinulin | LSinulin | NSinulin | HSFOS | LSFOS | NSFOS | HSXOS | LSXOS | NSXOS | |
| Gelatin | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Extract (gelatin) | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 |
| Extract (syrup) | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 |
| Sucrose | 150 | 75 | - | 100 | 25 | 100 | 25 | - | 100 | 25 | - | |
| Glucose syrup | 75 | 75 | - | 75 | 75 | - | 75 | 75 | - | 75 | 75 | - |
| Xylitol | - | 75 | 75 | - | 75 | 75 | - | 75 | 75 | - | 75 | 75 |
| Maltitol | - | - | 150 | - | - | 100 | - | - | 100 | - | - | 100 |
| Inulin | - | - | - | 50 | 50 | 50 | - | - | - | - | - | - |
| FOS | - | - | - | - | - | - | 50 | 50 | 50 | - | - | - |
| XOS | - | - | - | - | - | - | - | - | - | 50 | 50 | 50 |
| Citric acid | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Stevia | - | - | - | - | 0.3 | 0.3 | - | 0.3 | 0.3 | - | 0.3 | 0.3 |
| Sample | % Dry Matter | aw | pH | |||
|---|---|---|---|---|---|---|
| 0 Months | 2 Months | 0 Months | 2 Months | 0 Months | 2 Months | |
| HSb | 71.35 ± 0.47 | 71.97 ± 0.03 | 0.756 ± 0.001 a | 0.770 ± 0.003 a | 3.71 ± 0.05 | 3.68 ± 0.00 |
| LSb | 69.74 ± 0.73 | 69.73 ± 0.16 | 0.763 ± 0.002 | 0.765 ± 0.000 | 3.85 ± 0.01 | 3.86 ± 0.00 |
| NSb | 75.72 ± 0.14 | 75.96 ± 1.97 | 0.717 ± 0.004 | 0.725 ± 0.000 | 3.80 ± 0.01 | 3.83 ± 0.00 |
| HSinulin | 71.41 ± 0.40 | 70.85 ± 0.02 | 0.792 ± 0.002 | 0.792 ± 0.004 | 3.76 ± 0.07 | 3.76 ± 0.09 |
| LSinulin | 69.85 ± 0.64 | 69.91 ± 0.03 | 0.780 ± 0.009 | 0.788 ± 0.001 | 3.84 ± 0.01 | 3.88 ± 0.02 |
| NSinulin | 75.29 ± 0.24 b | 75.71 ± 0.34 b | 0.791 ± 0.009 c | 0.752 ± 0.001 c | 3.54 ± 0.07 | 3.56 ± 0.05 |
| HSFOS | 72.89 ± 0.94 | 72.73 ± 0.20 | 0.793 ± 0.002 d | 0.770 ± 0.001 d | 3.76 ± 0.04 | 3.75 ± 0.05 |
| LSFOS | 72.07 ± 0.56 | 72.40 ± 0.13 | 0.767 ± 0.005 | 0.759 ± 0.001 | 3.70 ± 0.02 e | 3.76 ± 0.03 e |
| NSFOS | 74.54 ± 0.19 | 75.16 ± 0.11 | 0.751 ± 0.003 f | 0.739 ± 0.001 f | 3.78 ± 0.07 | 3.77 ± 0.04 |
| HSXOS | 70.17 ± 0.47 | 70.74 ± 0.20 | 0.766 ± 0.003 g | 0.776 ± 0.001 g | 3.83 ± 0.00 | 3.81 ± 0.00 |
| LSXOS | 70.40 ± 0.18 | 70.91 ± 0.85 | 0.732 ± 0.000 h | 0.739 ± 0.001 h | 3.91 ± 0.00 | 3.89 ± 0.00 |
| NSXOS | 74.65 ± 0.14 i | 75.62 ± 0.03 i | 0.687 ± 0.001 j | 0.703 ± 0.002 j | 3.78 ± 0.01 | 3.79 ± 0.01 |
| Echinacoside μg/g DM | Verbascoside μg/g DM | Other Phenylethanoid Glycosides μg/g DM * | ||||
|---|---|---|---|---|---|---|
| 0 Months | 2 Months | 0 Months | 2 Months | 0 Months | 2 Months | |
| HSb | 929.82 ± 85.79 a | 765.67 ± 55.73 a | 127.75 ± 10.28 b | 106.76 ± 7.32 b | 813.69 ± 70.93 c | 645.68 ± 42.21 c |
| LSb | 915.75 ± 29.87 | 807.59 ± 25.37 | 127.93 ± 5.45 | 110.98 ± 3.28 | 822.62 ± 39.84 d | 677.27 ± 18.29 d |
| NSb | 899.19 ± 55.16 | 907.20 ± 14.44 | 123.49 ± 6.51 | 123.68 ± 1.47 | 787.76 ± 44.82 | 755.87 ± 9.84 |
| HSinulin | 877.97 ± 16.11 | 822.56 ± 31.06 | 120.54 ± 2.40 | 113.85 ± 4.24 | 691.16 ± 116.65 | 683.17 ± 24.94 |
| LSinulin | 824.93 ± 42.64 | 823.56 ± 37.50 | 121.00 ± 8.71 | 114.54 ± 5.05 | 782.23 ± 52.27 | 701.48 ± 28.09 |
| NSinulin | 705.86 ± 19.80 e | 654.00 ± 0.41 e | 96.48 ± 3.29 f | 87.13 ± 0.42 f | 617.46 ± 14.10 g | 539.07 ± 5.85 g |
| HSFOS | 781.03 ± 25.09 | 800.94 ± 53.66 | 109.40 ± 2.20 | 112.76 ± 7.66 | 689.46 ± 18.46 | 674.96 ± 42.20 |
| LSFOS | 743.12 ± 18.37 | 733.91 ± 52.17 | 103.18 ± 3.62 | 101.33 ± 7.90 | 662.77 ± 29.51 | 619.74 ± 44.11 |
| NSFOS | 676.89 ± 97.21 | 666.85 ± 24.22 | 101.64 ± 12.80 | 90.86 ± 4.03 | 592.56 ± 87.41 | 563.48 ± 23.70 |
| HSXOS | 815.42 ± 72.38 | 876.30 ± 21.19 | 113.38 ± 9.39 | 124.33 ± 3.51 | 735.09 ± 52.67 | 765.84 ± 17.03 |
| LSXOS | 854.74 ± 28.02 | 852.91 ± 29.81 | 120.89 ± 4.44 | 120.50 ± 4.61 | 773.12 ± 27.89 | 749.81 ± 26.02 |
| NSXOS | 744.20 ± 14.25 | 747.81 ± 7.06 | 103.52 ± 2.74 | 103.49 ± 1.18 | 668.47 ± 13.79 | 648.60 ± 5.78 |
| 0 Months | 2 Months | ||||||
|---|---|---|---|---|---|---|---|
| Sample | L* | a* | b* | L* | a* | b* | ΔE |
| HSb | 29.80 ± 0.22 | 3.38 ± 0.39 | 13.66 ± 0.36 a | 29.59 ± 0.34 | 3.34 ± 0.47 | 8.20 ± 0.47 a | 5.50 ± 0.45 |
| LSb | 28.01 ± 0.62 | 4.33 ± 0.24 | 16.37 ± 0.97 | 26.18 ± 0.43 | 3.77 ± 0.34 | 12.89 ± 1.28 | 4.09 ± 1.00 |
| NSb | 32.40 ± 0.15 b | 3.46 ± 0.02 | 9.00 ± 0.04 | 43.38 ± 2.83 b | 2.55 ± 0.39 | 6.78 ± 1.00 | 11.28 ± 2.86 |
| HSinulin | 37.34 ± 0.09 c | 1.97 ± 0.19 | 12.47 ± 0.69 | 34.08 ± 0.56 c | 2.71 ± 0.11 | 11.68 ± 0.41 | 3.45 ± 0.65 |
| LSinulin | 26.69 ± 0.72 | 3.67 ± 0.15 | 12.14 ± 0.68 | 26.54 ± 1.67 | 4.03 ± 0.28 | 11.73 ± 1.65 | 2.26 ± 0.89 |
| NSinulin | 34.88 ± 0.21 d | 1.72 ± 0.06 e | 9.72 ± 0.22 | 33.49 ± 0.41 d | 2.60 ± 0.19 e | 9.36 ± 0.66 | 1.83 ± 0.32 |
| HSFOS | 29.36 ± 0.43 e | 4.35 ± 0.14 | 13.86 ± 0.34 | 26.32 ± 0.70 e | 4.51 ± 0.39 | 11.02 ± 0.96 | 4.35 ± 0.25 |
| LSFOS | 27.29 ± 0.42 | 4.23 ± 0.21 | 17.69 ± 1.10 f | 26.82 ± 1.28 | 4.11 ± 0.38 | 12.43 ± 1.43 f | 5.45 ± 1.44 |
| NSFOS | 31.09 ± 0.39 | 3.70 ± 0.19 | 13.54 ± 0.95 | 30.38 ± 1.37 | 2.89 ± 0.47 | 11.32 ± 1.18 | 3.00 ± 0.77 |
| HSXOS | 29.32 ± 0.39 g | 5.31 ± 0.19 | 12.95 ± 0.41 h | 26.58 ± 0.73 g | 5.79 ± 0.48 | 7.84 ± 0.79 h | 5.94 ± 0.27 |
| LSXOS | 28.61 ± 1.22 i | 4.60 ± 0.65 j | 12.74 ± 1.83 | 23.23 ± 1.21 i | 6.90 ± 0.46 j | 12.60 ± 1.10 | 5.96 ± 1.26 |
| NSXOS | 29.36 ± 0.16 k | 4.59 ± 0.16 | 12.55 ± 0.35 l | 28.26 ± 0.91 k | 4.93 ± 0.16 | 9.05 ± 0.27 l | 3.80 ± 0.23 |
| Ingredient | Sucrose (%) | Maltose (%) | Glucose (%) | Fructose (%) | Xylose (%) |
|---|---|---|---|---|---|
| Glucose syrup | n.d. | 11.04 | 15.36 | n.d. | n.d. |
| Inulin | 6.59 | n.d. | n.d. | n.d. | n.d. |
| FOS | n.d. | n.d. | 4.20 | n.d. | |
| XOS | n.d. | n.d. | n.d. | n.d. | n.d. |
| Sample/sugar | Suc+Malt (% dmb) | Glc (% dmb) | Fru (% dmb) | Xylitol (% dmb) | Maltitol (% dmb) |
| 0 months | |||||
| HSb | 65.85 ± 1.12 | 6.69 ± 0.71 | 1.14 ± 0.07 j | n.d. | n.d. |
| LSb | 33.93 ± 0.47 a | 6.16 ± 0.22 | n.d. k | 32.93 ± 0.00 | n.d. |
| NSb | n.d. | n.d. | n.d. | 37.50 ± 0.13 p | 48.53 ± 0.22 r |
| HSinulin | 43.38 ± 1.02 | 6.54 ± 0.39 f | 1.58 ± 0.16 | n.d. | n.d. |
| LSinulin | 15.19 ± 0.06 b | 5.61 ± 0.07 g | n.d.l | 33.34 ± 0.09 | n.d. |
| Nsinulin | 1.30 ± 0.25 c | n.d. | n.d. | 31.69 ± 5.78 | 34.07 ± 1.53 s |
| HSFOS | 42.78 ± 0.83 | 10.40 ± 0.10h | 3.17 ± 0.17 m | n.d. | n.d. |
| LSFOS | 14.27 ± 0.54 d | 9.68 ± 0.03 | 2.23 ± 0.25 | 33.53 ± 0.70 | n.d. |
| NSFOS | 0.79 ± 0.04 | n.d. | n.d. n | 30.66 ± 2.43 | 38.17 ± 1.69 |
| HSXOS | 50.33 ± 0.23 | 5.70 ± 0.35 | n.d. o | n.d. | n.d. |
| LSXOS | 16.24 ± 0.19 e | 5.65 ± 0.09 i | n.d. | 32.86 ± 0.15 | n.d. |
| NSXOS | n.d. | n.d. | n.d. | 28.48 ± 2.04 | 38.12 ± 1.12 |
| 2 months | |||||
| HSb | 59.53 ± 2.86 | 6.34 ± 0.98 | 1.36 ± 0.02 j | n.d. | n.d. |
| LSb | 31.65 ± 0.39 a | 5.40 ± 0.41 | 0.24 ± 0.01 k | 32.27 ± 0.45 | n.d. |
| NSb | n.d. | n.d. | n.d. | 36.46 ± 0.12 p | 45.69 ± 0.33 r |
| HSinulin | 42.53 ± 0.55 | 5.48 ± 0.20 f | 1.15 ± 0.03 | n.d. | n.d. |
| LSinulin | 13.91 ± 0.05 b | 4.87 ± 0.03 g | 0.24 ± 0.00 l | 33.34 ± 1.21 | n.d. |
| Nsinulin | ≤0.5c | n.d. | n.d. | 29.93 ± 0.16 | 37.75 ± 0.24 s |
| HSFOS | 40.02 ± 0.34 | 8.59 ± 0.34 h | 2.41 ± 0.04 m | n.d. | n.d. |
| LSFOS | 12.74 ± 0.35 d | 8.90 ± 0.82 | 2.00 ± 0.10 | 32.68 ± 0.34 | n.d. |
| NSFOS | 0.33 ± 0.00 | n.d. | 0.82 ± 0.03 n | 30.21 ± 0.48 | 38.54 ± 0.82 |
| HSXOS | 49.46 ± 1.27 | 5.95 ± 0.12 | 0.82 ± 0.15 o | n.d. | n.d. |
| LSXOS | 13.59 ± 0.21e | 4.33 ± 0.06 i | n.d. | 32.59 ± 0.51 | n.d. |
| NSXOS | n.d. | n.d. | n.d. | 30.43 ± 0.60 | 37.17 ± 0.16 |
| Sample | Hardness (g) | Resilience (g) | Cohesiveness (g) | Springiness (g) | Chewiness (g) | |
|---|---|---|---|---|---|---|
| 0 months | HSb | 558.81 ± 1.86 | 92.13 ± 3.09 | 0.78 ± 0.01 f | 68.18 ± 2.27 | 650.44 ± 73.12 |
| LSb | 486.81 ± 30.77 a | 87.25 ± 2.16 | 0.78 ± 0.00 | 63.64 ± 0.00 | 525.91 ± 10.17 | |
| NSb | 591.76 ± 26.38 b | 68.54 ± 7.03 c | 0.67 ± 0.03 g | 56.06 ± 3.47 i | 372.28 ± 46.36 l | |
| HSinulin | 569.80 ± 43.70 | 54.98 ± 0.12 | 0.62 ± 0.01 | 56.06 ± 1.31 | 386.86 ± 24.17 | |
| LSinulin | 564.94 ± 17.18 | 79.45 ± 1.06 d | 0.70 ± 0.00 | 58.33 ± 1.31 | 435.67 ± 6.25 | |
| NSinulin | 697.52 ± 107.85 | 77.70 ± 1.28 | 0.71 ± 0.02 | 60.61 ± 1.31 | 481.49 ± 37.27 m | |
| HSFOS | 640.03 ± 46.74 | 85.25 ± 1.93 | 0.76 ± 0.02 | 64.39 ± 1.31 | 558.72 ± 48.24 | |
| LSFOS | 593.23 ± 44.93 | 84.10 ± 2.24 | 0.74 ± 0.00 h | 61.36 ± 0.00 j | 491.43 ± 22.75 n | |
| NSFOS | 624.36 ± 100.72 | 90.20 ± 0.27 | 0.74 ± 0.00 | 63.64 ± 2.27 | 527.47 ± 33.92 | |
| HSXOS | 495.69 ± 33.19 | 87.75 ± 1.88 | 0.75 ± 0.01 | 65.15 ± 1.31 | 512.03 ± 9.21 | |
| LSXOS | 554.52 ± 10.67 | 86.29 ± 1.95 | 0.74 ± 0.01 | 64.39 ± 1.31 k | 504.49 ± 11.02 | |
| NSXOS | 593.34 ± 25.65 | 89.51 ± 0.09 e | 0.72 ± 0.01 | 63.64 ± 0.00 | 502.03 ± 11.61 | |
| Commercial product | 721.49 ± 13.74 | 66.73 ± 1.50 | 0.73 ± 0.00 | 68.18 ± 2.27 | 605.45 ± 28.41 | |
| 2 months | HSb | 721.82 ± 91.14 | 83.67 ± 2.53 | 0.82 ± 0.02 f | 65.15 ± 1.31 | 616.15 ± 91.69 |
| LSb | 566.43 ± 20.58 a | 84.31 ± 1.35 | 0.78 ± 0.01 | 64.39 ± 1.31 | 497.34 ± 33.25 | |
| NSb | 486.07 ± 20.48 b | 90.15 ± 1.10 c | 0.76 ± 0.01 g | 65.91 ± 0.00 i | 545.47 ± 17.34 l | |
| HSinulin | 618.22 ± 20.29 | 59.28 ± 3.64 | 0.63 ± 0.04 | 56.06 ± 2.62 | 376.70 ± 54.99 | |
| LSinulin | 570.95 ± 26.75 | 83.02 ± 1.43 d | 0.72 ± 0.02 | 61.36 ± 2.27 | 472.46 ± 34.73 | |
| NSinulin | 686.95 ± 73.78 | 73.77 ± 3.18 | 0.68 ± 0.07 | 57.58 ± 7.31 | 387.47 ± 3.89 m | |
| HSFOS | 642.56 ± 51.92 | 90.28 ± 5.34 | 0.76 ± 0.02 | 65.15 ± 2.62 | 569.23 ± 64.34 | |
| LSFOS | 579.51 ± 43.80 | 88.81 ± 0.54 | 0.76 ± 0.01 h | 65.15 ± 1.31 j | 542.02 ± 15.42 n | |
| NSFOS | 620.88 ± 51.13 | 85.85 ± 1.94 | 0.74 ± 0.01 | 62.12 ± 1.31 | 518.85 ± 52.78 | |
| HSXOS | 552.45 ± 12.20 | 83.30 ± 3.85 | 0.75 ± 0.00 | 62.88 ± 1.31 | 467.80 ± 10.71 | |
| LSXOS | 566.00 ± 25.12 | 85.61 ± 1.21 | 0.73 ± 0.01 | 62.12 ± 1.31 k | 478.18 ± 22.52 | |
| NSXOS | 592.82 ± 14.95 | 83.35 ± 1.23 e | 0.72 ± 0.01 | 62.12 ± 1.31 | 491.01 ± 29.31 |
| Hardness | Resilience | Cohesiveness | Springiness | Chewiness | Sensory Hardness | ||
|---|---|---|---|---|---|---|---|
| 0 months | Hardness | 1 | −0.141 | −0.232 | −0.209 | −0.075 | 0.467 |
| Resilience | 1 | 0.919 ** | 0.882 ** | 0.819 ** | 0.624 * | ||
| Cohesiveness | 1 | 0.893 ** | 0.877 ** | −0.688 * | |||
| Springiness | 1 | 0.950 ** | −0.505 | ||||
| Chewiness | 1 | −0.521 | |||||
| Sensory hardness | 1 | ||||||
| 2 months | Hardness | 1 | −0.315 | −0.002 | −0.278 | 0.055 | −0.125 |
| Resilience | 1 | 0.798 ** | 0.899 ** | 0.783 ** | 0.043 | ||
| Cohesiveness | 1 | 0.918 ** | 0.900 ** | −0.010 | |||
| Springiness | 1 | 0.909 ** | 0.023 | ||||
| Chewiness | 1 | −0.031 | |||||
| Sensory hardness | 1 |
| Sample/Parameter | Transparency | Sweetness | Bitterness | Hardness | General Acceptance |
|---|---|---|---|---|---|
| 0 months | |||||
| HS_b | 8.50 ± 0.67 a | 5.80 ± 0.75 | 4.30 ± 1.42 | 4.90 ± 0.83 | 7.50 ± 0.92 |
| LS_b | 8.30 ± 0.46 | 5.20 ± 0.75 | 5.10 ± 1.45 | 4.40 ± 0.80 | 7.20 ± 0.75 |
| NS_b | 3.70 ± 0.64 b | 6.20 ± 0.98 | 4.30 ± 1.62 | 6.00 ± 0.77 | 6.10 ± 1.04 m |
| HS_inulin | 1.60 ± 0.49 | 5.00 ± 1.00 | 4.10 ± 1.37 | 6.20 ± 1.54 | 6.00 ± 1.00 |
| LS_inulin | 2.30 ± 0.46 | 4.80 ± 0.87 | 4.50 ± 1.12 | 5.00 ± 0.63 | 6.10 ± 0.54 |
| NS_inulin | 2.50 ± 0.50 c | 5.60 ± 1.02 i | 4.30 ± 1.10 j | 6.00 ± 1.90 | 6.00 ± 0.89 n |
| HS_FOS | 6.70 ± 1.00 | 5.30 ± 1.00 | 4.30 ± 1.49 | 5.70 ± 0.64 | 6.10 ± 0.54 o |
| LS_FOS | 6.80 ± 0.98 d | 5.70 ± 0.78 | 4.30 ± 1.19 | 5.20 ± 0.98 | 6.70 ± 0.90 |
| NS_FOS | 7.20 ± 0.40 e | 6.20 ± 0.60 | 3.90 ± 1.30 | 5.00 ± 0.45 | 5.60 ± 1.20 |
| HS_XOS | 5.80 ± 1.08 f | 5.10 ± 0.94 | 4.90 ± 1.58 k | 5.70 ± 0.78 l | 6.40 ± 1.02 |
| LS_XOS | 8.10 ± 0.54 g | 5.20 ± 1.08 | 4.40 ± 1.11 | 4.90 ± 0.94 | 6.00 ± 0.89 p |
| NS_XOS | 6.90 ± 0.70 h | 6.20 ± 0.75 | 4.40 ± 1.43 | 5.80 ± 0.75 | 6.00 ± 0.63 |
| 2 months | |||||
| HS_b | 7.10 ± 0.54 a | n.d. | n.d. | n.d. | n.d. |
| LS_b | 8.60 ± 0.49 | 5.80 ± 0.75 | 4.00 ± 1.34 | 4.90 ± 1.04 | 7.60 ± 0.66 |
| NS_b | 1.10 ± 0.30 b | 6.00 ± 0.89 | 3.30 ± 1.00 | 5.90 ± 0.83 | 3.10 ± 1.81 m |
| HS_inulin | 1.80 ± 0.75 | n.d. | n.d. | n.d. | n.d. |
| LS_inulin | 2.30 ± 0.78 | n.d. | n.d. | n.d. | n.d. |
| NS_inulin | 1.10 ± 0.30 c | 6.56 ± 0.83 i | 2.30 ± 1.10 j | 5.40 ± 1.67 | 3.00 ± 1.18 n |
| HS_FOS | 7.60 ± 0.49 | 4.90 ± 0.54 | 4.00 ± 1.00 | 6.50 ± 1.20 | 7.10 ± 0.70 o |
| LS_FOS | 8.20 ± 0.75 d | 5.50 ± 0.67 | 3.30 ± 1.00 | 4.40 ± 1.50 | 7.10 ± 0.83 |
| NS_FOS | 3.80 ± 1.08 e | 5.60 ± 0.66 | 3.30 ± 1.25 | 5.20 ± 1.33 | 6.00 ± 0.77 |
| HS_XOS | 7.50 ± 0.81 f | 5.20 ± 0.60 | 3.30 ± 0.78 k | 7.20 ± 1.08 l | 6.70 ± 0.64 |
| LS_XOS | 8.70 ± 0.46 g | 5.10 ± 1.41 | 3.60 ± 0.66 | 5.70 ± 1.00 | 6.30 ± 0.64 p |
| NS_XOS | 5.30 ± 1.27 h | 5.70 ± 0.46 | 3.50 ± 0.92 | 5.60 ± 1.20 | 6.60 ± 0.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vojvodić Cebin, A.; Bunić, M.; Mandura Jarić, A.; Šeremet, D.; Komes, D. Physicochemical and Sensory Stability Evaluation of Gummy Candies Fortified with Mountain Germander Extract and Prebiotics. Polymers 2024, 16, 259. https://doi.org/10.3390/polym16020259
Vojvodić Cebin A, Bunić M, Mandura Jarić A, Šeremet D, Komes D. Physicochemical and Sensory Stability Evaluation of Gummy Candies Fortified with Mountain Germander Extract and Prebiotics. Polymers. 2024; 16(2):259. https://doi.org/10.3390/polym16020259
Chicago/Turabian StyleVojvodić Cebin, Aleksandra, Magdalena Bunić, Ana Mandura Jarić, Danijela Šeremet, and Draženka Komes. 2024. "Physicochemical and Sensory Stability Evaluation of Gummy Candies Fortified with Mountain Germander Extract and Prebiotics" Polymers 16, no. 2: 259. https://doi.org/10.3390/polym16020259
APA StyleVojvodić Cebin, A., Bunić, M., Mandura Jarić, A., Šeremet, D., & Komes, D. (2024). Physicochemical and Sensory Stability Evaluation of Gummy Candies Fortified with Mountain Germander Extract and Prebiotics. Polymers, 16(2), 259. https://doi.org/10.3390/polym16020259