Influence of Novel SrTiO3/MnO2 Hybrid Nanoparticles on Poly(methyl methacrylate) Thermal and Mechanical Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Samples
- Particles were placed in a liquid part containing MMA, and mixed on a magnetic stirrer, followed by ultrasonic homogenization.
- The liquid part with STO/MnO2 nanoparticles was mixed with powder in a volumetric ratio of 35/100, in accordance with the manufacturer’s instructions.
- The mixture was poured into a mold and left for 24 h at room temperature.
2.3. Characterization of Samples
3. Results and Discussion
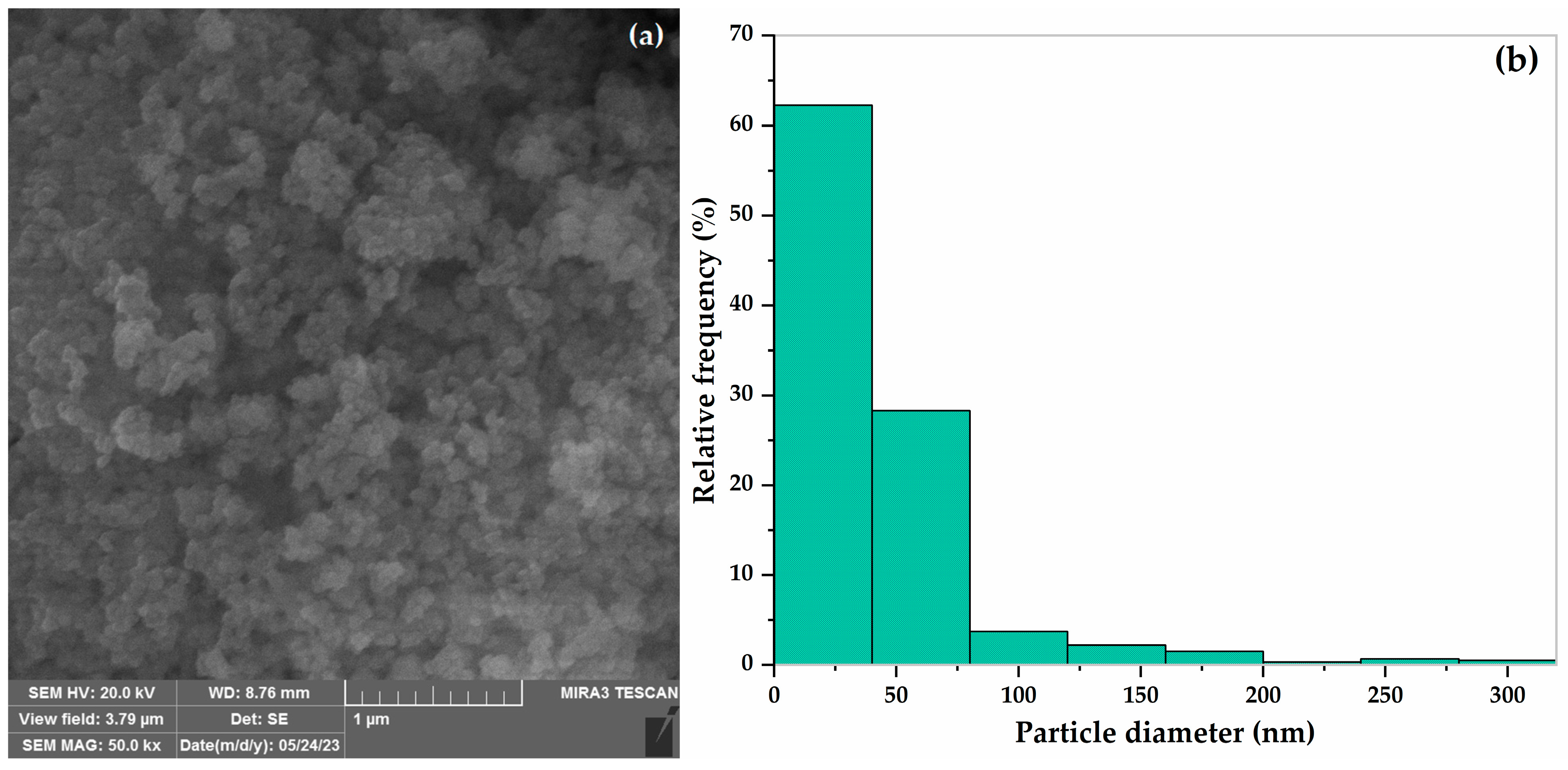
3.1. FESEM of STO/MnO2 Nanoparticles
3.2. XRD of STO/MnO2 Nanoparticles
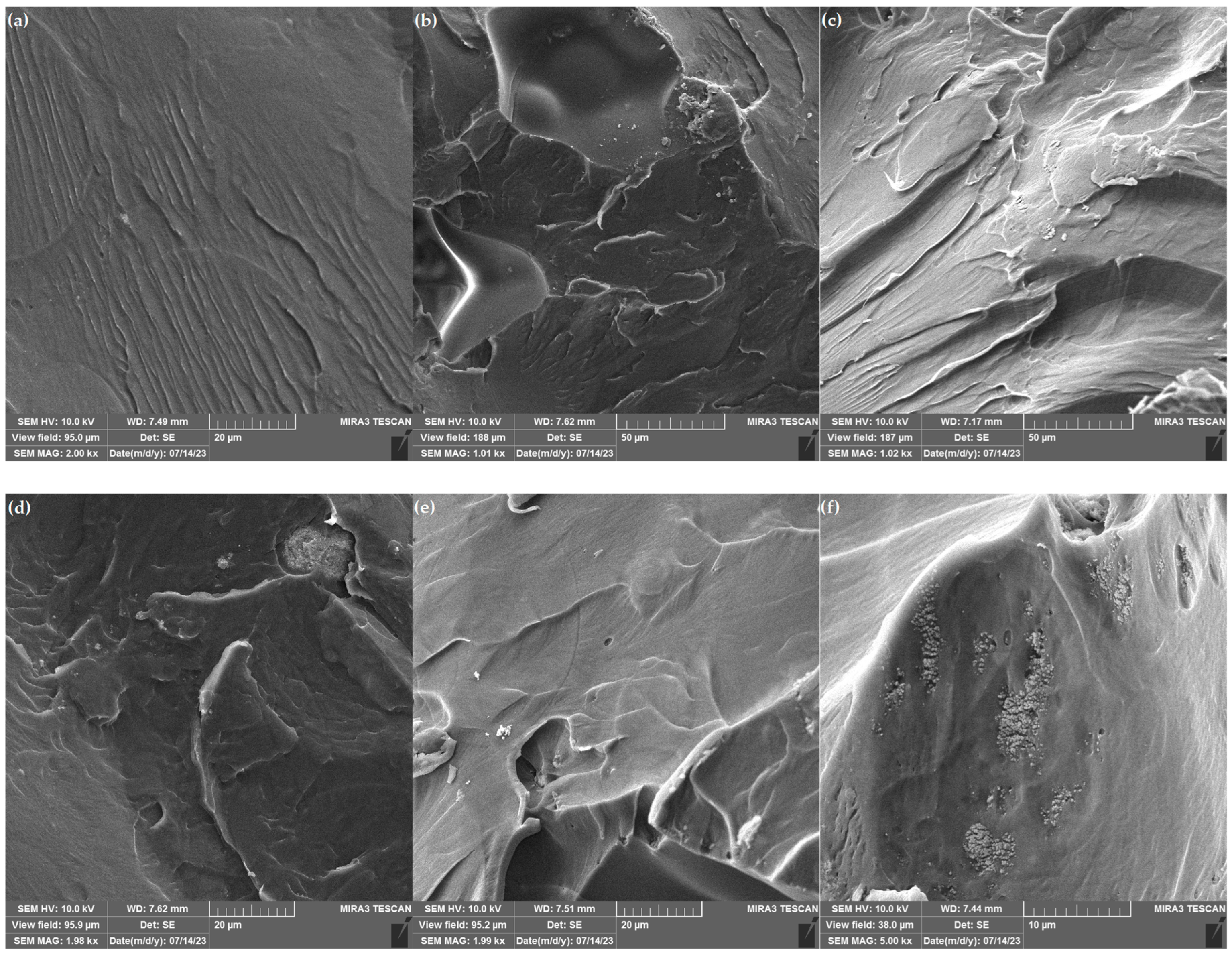
3.3. FESEM of PMMA and Composites
- Crack Bridging: Particles can bridge microcracks that form within the composite matrix. As a crack attempts to propagate, it encounters bridges, which resist further crack growth. The bridging mechanism increases the energy required for crack propagation enhancing the composite’s fracture resistance.
- Crack Deflection: Particles can cause cracks to change direction when they encounter the particle-matrix interface. This deflection reduces the effective crack length, increasing the composite’s resistance to fracture and improving its toughness.
- Crack tip pinning: As the crack propagates and encounters a pinning point, it experiences local resistance. This resistance arises due to the additional energy required to deform the material or overcome the obstacles presented by the pinning point. The energy required to deform the material around the pinning point, or to move the crack past the obstacle, is dissipated as heat. This energy dissipation contributes to the overall toughness of the material.
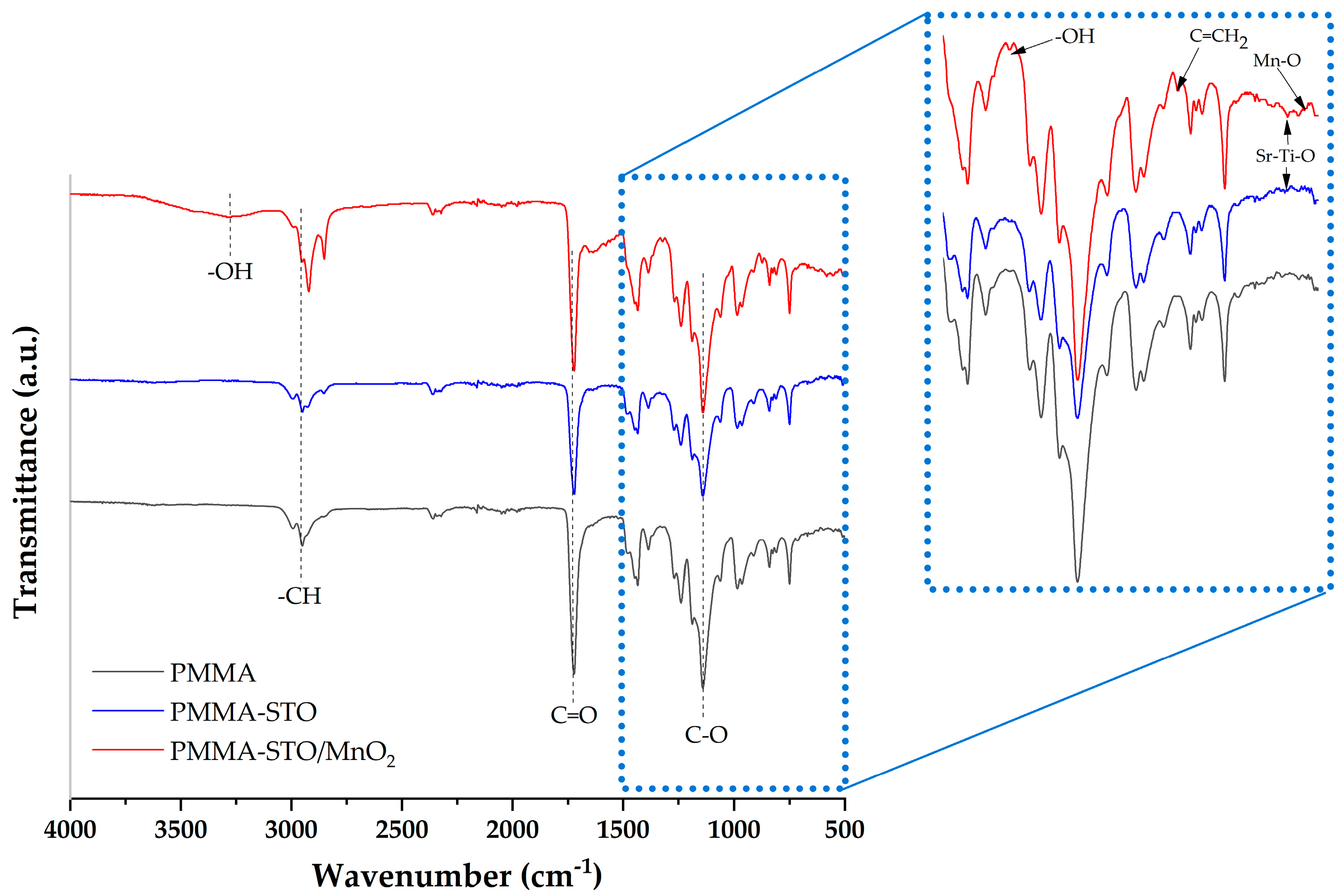
3.4. FTIR of PMMA and Composites
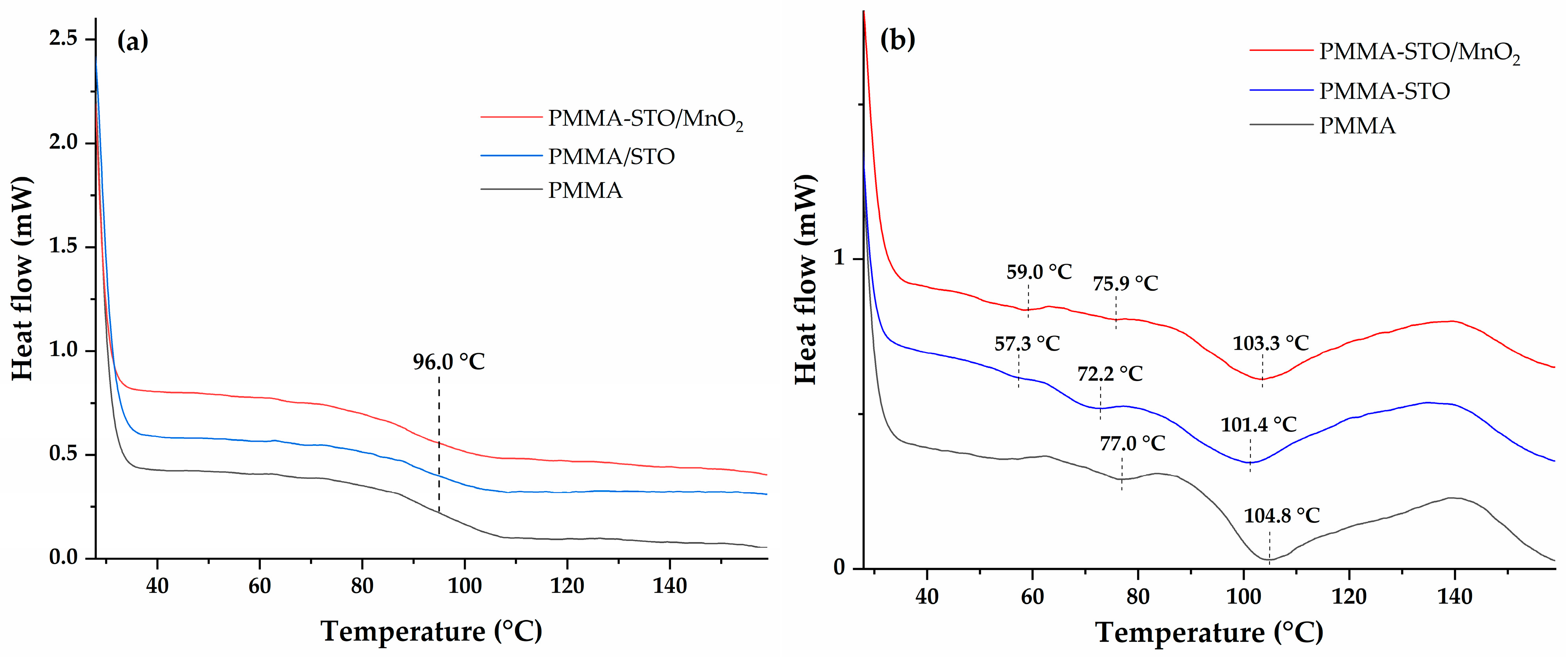
3.5. Differential Scanning Calorimetry (DSC)
3.6. Mechanical Properties
- Initial Crack Formation Phase: In this first phase, energy is absorbed as the crack initially forms within the material. It encompasses both the elastic response of the material and any minimal plastic deformation. For fully cured epoxy resins, this phase is characterized by relatively low energy absorption. The maximum load (Fmax) is achieved at the conclusion of this phase, and the energy absorbed up to this point is denoted as Efmax.
- Crack Propagation and Material Deterioration Phase: The second phase commences with the formation of the crack and extends until the material eventually fails or ruptures. During this phase, there is a notable degradation of mechanical properties. The total absorbed energy (Etot) encompasses all the energy absorbed from the start of the controlled energy impact test until the load drops below zero, marking the conclusion of the test.
- Brittle Failure: This type of failure is characteristic of materials like ceramics and rigid polymer structures, such as cross-linked polymers forming 3D covalently bonded networks. Brittle failure is characterized by minimal or no plastic deformation, rapid fracture propagation, and a low Etot value.
- Brittle-Ductile Fracture: In this scenario, there is a limited degree of plastic deformation that occurs just before the material breaks. It represents an intermediate stage between brittle and ductile behaviors.
- Ductile-Brittle Failure: Materials exhibiting this type of failure have the capacity for plastic deformation and can absorb more energy during impact compared to brittle materials. They undergo some plastic deformation before ultimately fracturing.
- Ductile fracture: The fourth type of failure is characterized by substantial plastic deformations occurring before fracture. Materials that exhibit this behavior absorb a significant amount of energy during the impact, resulting in a high Etot value.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rokaya, D.; Srimaneepong, V.; Sapkota, J.; Qin, J.; Siraleartmukul, K.; Siriwongrungson, V. Polymeric Materials and Films in Dentistry: An Overview. J. Adv. Res. 2018, 14, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yao, Y.; Tang, W.; Han, D.; Zhang, L.; Zhao, K.; Wang, S.; Meng, Y. Design of Dental Implants at Materials Level: An Overview. J. Biomed. Mater. Res. 2020, 108, 1634–1661. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.; Hotza, D.; Fredel, M.C.; Cruz, A.; Volpato, C.A.M. Materials and Manufacturing Techniques for Polymeric and Ceramic Scaffolds Used in Implant Dentistry. J. Compos. Sci. 2021, 5, 78. [Google Scholar] [CrossRef]
- Pituru, S.M.; Greabu, M.; Totan, A.; Imre, M.; Pantea, M.; Spinu, T.; Tancu, A.M.C.; Popoviciu, N.O.; Stanescu, I.-I.; Ionescu, E. A Review on the Biocompatibility of PMMA-Based Dental Materials for Interim Prosthetic Restorations with a Glimpse into Their Modern Manufacturing Techniques. Materials 2020, 13, 2894. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.S. Prosthodontic Applications of Polymethyl Methacrylate (PMMA): An Update. Polymers 2020, 12, 2299. [Google Scholar] [CrossRef] [PubMed]
- Abad-Coronel, C.; Bravo, M.; Tello, S.; Cornejo, E.; Paredes, Y.; Paltan, C.A.; Fajardo, J.I. Fracture Resistance Comparative Analysis of Milled-Derived vs. 3D-Printed CAD/CAM Materials for Single-Unit Restorations. Polymers 2023, 15, 3773. [Google Scholar] [CrossRef]
- Kaur, H.; Thakur, A. Applications of Poly(Methyl Methacrylate) Polymer in Dentistry: A Review. Mater. Today Proc. 2022, 50, 1619–1625. [Google Scholar] [CrossRef]
- Al-Dwairi, Z.N.; Tahboub, K.Y.; Baba, N.Z.; Goodacre, C.J. A Comparison of the Flexural and Impact Strengths and Flexural Modulus of CAD/CAM and Conventional Heat-Cured Polymethyl Methacrylate (PMMA). J. Prosthodont. 2020, 29, 341–349. [Google Scholar] [CrossRef]
- Karaman, T.; Eser, B.; Altintas, E.; Atala, M.H. Evaluation of the Effects of Finish Line Type and Width on the Fracture Strength of Provisional Crowns. Odontology 2021, 109, 76–81. [Google Scholar] [CrossRef]
- Alqutaibi, A.Y.; Baik, A.; Almuzaini, S.A.; Farghal, A.E.; Alnazzawi, A.A.; Borzangy, S.; Aboalrejal, A.N.; AbdElaziz, M.H.; Mahmoud, I.I.; Zafar, M.S. Polymeric Denture Base Materials: A Review. Polymers 2023, 15, 3258. [Google Scholar] [CrossRef]
- Al-Thobity, A.M. The Impact of Polymerization Technique and Glass-Fiber Reinforcement on the Flexural Properties of Denture Base Resin Material. Eur. J. Dent. 2020, 14, 092–099. [Google Scholar] [CrossRef] [PubMed]
- Yerliyurt, K.; Taşdelen, T.B.; Eğri, Ö.; Eğri, S. Flexural Properties of Heat-Polymerized PMMA Denture Base Resins Reinforced with Fibers with Different Characteristics. Polymers 2023, 15, 3211. [Google Scholar] [CrossRef]
- Ali Sabri, B.; Satgunam, M.; Abreeza, N.M.; NAbed, A. A Review on Enhancements of PMMA Denture Base Material with Different Nano-Fillers. Cogent Eng. 2021, 8, 1875968. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. PMMA-Based Nanocomposites for Odontology Applications: A State-of-the-Art. Int. J. Mol. Sci. 2022, 23, 10288. [Google Scholar] [CrossRef] [PubMed]
- Tham, W.L.; Chow, W.S.; Mohd Ishak, Z.A. Effects of Titanate Coupling Agent on the Mechanical, Thermal, and Morphological Properties of Poly(Methyl Methacrylate)/Hydroxyapatite Denture Base Composites. J. Compos. Mater. 2011, 45, 2335–2345. [Google Scholar] [CrossRef]
- Sosiati, H.; Rizky, A.M.; Latief, A.L.M.; Adi, R.K.; Hamdan, S. The Mechanical and Physical Properties of Microcrystalline Cellulose (MCC)/Sisal/PMMA Hybrid Composites for Dental Applications. Mater. Res. Express 2023, 10, 035301. [Google Scholar] [CrossRef]
- Ai, M.; Du, Z.; Zhu, S.; Geng, H.; Zhang, X.; Cai, Q.; Yang, X. Composite Resin Reinforced with Silver Nanoparticles–Laden Hydroxyapatite Nanowires for Dental Application. Dent. Mater. 2017, 33, 12–22. [Google Scholar] [CrossRef]
- Jiangkongkho, P.; Arksornnukit, M.; Takahashi, H. The Synthesis, Modification, and Application of Nanosilica in Polymethyl Methacrylate Denture Base. Dent. Mater. J. 2018, 37, 582–591. [Google Scholar] [CrossRef]
- Alhavaz, A.; Rezaei Dastjerdi, M.; Ghasemi, A.; Ghasemi, A.; Alizadeh Sahraei, A. Effect of Untreated Zirconium Oxide Nanofiller on the Flexural Strength and Surface Hardness of Autopolymerized Interim Fixed Restoration Resins. J. Esthet. Restor. Dent. 2017, 29, 264–269. [Google Scholar] [CrossRef]
- Gad, M.M.; Abualsaud, R. Behavior of PMMA Denture Base Materials Containing Titanium Dioxide Nanoparticles: A Literature Review. Int. J. Biomater. 2019, 2019, 6190610. [Google Scholar] [CrossRef]
- Malisic, V.; Gajic, V.; Porobic, S.; Pataric, A.; Putic, S.; Vujcic, I. The Effect of Gamma Irradiation on the Synthesis, Microbiological Sterility, and Improvement of Properties of PMMA-Al2O3 Composite Used in Dental Prosthesis Manufacturing. Radiat. Phys. Chem. 2023, 207, 110846. [Google Scholar] [CrossRef]
- Sharifianjazi, F.; Pakseresht, A.H.; Shahedi Asl, M.; Esmaeilkhanian, A.; Nargesi Khoramabadi, H.; Jang, H.W.; Shokouhimehr, M. Hydroxyapatite Consolidated by Zirconia: Applications for Dental Implant. J. Compos. Compd. 2020, 2, 26–34. [Google Scholar] [CrossRef]
- Passaro, J.; Bifulco, A.; Calabrese, E.; Imparato, C.; Raimondo, M.; Pantani, R.; Aronne, A.; Guadagno, L. Hybrid Hemp Particles as Functional Fillers for the Manufacturing of Hydrophobic and Anti-Icing Epoxy Composite Coatings. ACS Omega 2023, 8, 23596–23606. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, M.; Bagheri, R.; Khaledi, A.A.R. Effects of Aluminum Oxide Addition on the Flexural Strength, Surface Hardness, and Roughness of Heat-Polymerized Acrylic Resin. J. Dent. Sci. 2012, 7, 238–244. [Google Scholar] [CrossRef]
- Hata, K.; Ikeda, H.; Nagamatsu, Y.; Masaki, C.; Hosokawa, R.; Shimizu, H. Dental Poly(Methyl Methacrylate)-Based Resin Containing a Nanoporous Silica Filler. J. Funct. Biomater. 2022, 13, 32. [Google Scholar] [CrossRef]
- Alhotan, A.; Yates, J.; Zidan, S.; Haider, J.; Silikas, N. Flexural Strength and Hardness of Filler-Reinforced PMMA Targeted for Denture Base Application. Materials 2021, 14, 2659. [Google Scholar] [CrossRef]
- Gad, M.; Rahoma, A.; Al-Thobity, A.M.; ArRejaie, A. Influence of Incorporation of ZrO2 Nanoparticles on the Repair Strength of Polymethyl Methacrylate Denture Bases. Int. J. Nanomed. 2016, 11, 5633–5643. [Google Scholar] [CrossRef]
- Naznin, H.; Mallik, A.K.; Hossain, K.S.; Shahruzzaman, M.; Haque, P.; Rahman, M.M. Enhancement of Thermal and Mechanical Properties of PMMA Composites by Incorporating Mesoporous Micro-Silica and GO. Results Mater. 2021, 11, 100203. [Google Scholar] [CrossRef]
- Chen, S.-G.; Yang, J.; Jia, Y.-G.; Lu, B.; Ren, L. TiO2 and PEEK Reinforced 3D Printing PMMA Composite Resin for Dental Denture Base Applications. Nanomaterials 2019, 9, 1049. [Google Scholar] [CrossRef]
- Alqahtani, M. Mechanical Properties Enhancement of Self-Cured PMMA Reinforced with Zirconia and Boron Nitride Nanopowders for High-Performance Dental Materials. J. Mech. Behav. Biomed. Mater. 2020, 110, 103937. [Google Scholar] [CrossRef]
- Alqahtani, M. Effect of Hexagonal Boron Nitride Nanopowder Reinforcement and Mixing Methods on Physical and Mechanical Properties of Self-Cured PMMA for Dental Applications. Materials 2020, 13, 2323. [Google Scholar] [CrossRef]
- Nabhan, A.; Taha, M.; Ghazaly, N.M. Filler Loading Effect of Al2O3/TiO2 Nanoparticles on Physical and Mechanical Characteristics of Dental Base Composite (PMMA). Polym. Test. 2023, 117, 107848. [Google Scholar] [CrossRef]
- Wu, J.; Wang, H.; Mao, L.; Aliha, M.R.M. Composition Optimization of PMMA Base Denture Reinforced with Different Percentages of Nano Hydroxyapatite and Alumina Particles to Obtain Highest KIc and KIIc Values Using Hybrid IWO/PSO Algorithm. Theor. Appl. Fract. Mech. 2023, 128, 104090. [Google Scholar] [CrossRef]
- Beketova, A.; Theocharidou, A.; Tsamesidis, I.; Rigos, A.E.; Pouroutzidou, G.K.; Tzanakakis, E.-G.C.; Kourtidou, D.; Liverani, L.; Ospina, M.A.; Anastasiou, A.; et al. Sol–Gel Synthesis and Characterization of YSZ Nanofillers for Dental Cements at Different Temperatures. Dent. J. 2021, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- Kacem, K.; Casanova-Chafer, J.; Ameur, S.; Nsib, M.F.; Llobet, E. Gas Sensing Properties of Graphene Oxide Loaded with SrTiO3 Nanoparticles. J. Alloys Compd. 2023, 941, 169011. [Google Scholar] [CrossRef]
- Shi, X.-L.; Wu, H.; Liu, Q.; Zhou, W.; Lu, S.; Shao, Z.; Dargusch, M.; Chen, Z.-G. SrTiO3-Based Thermoelectrics: Progress and Challenges. Nano Energy 2020, 78, 105195. [Google Scholar] [CrossRef]
- Xian, T.; Yang, H.; Di, L.; Ma, J.; Zhang, H.; Dai, J. Photocatalytic Reduction Synthesis of SrTiO3-Graphene Nanocomposites and Their Enhanced Photocatalytic Activity. Nanoscale Res. Lett. 2014, 9, 327. [Google Scholar] [CrossRef]
- Escobar, A.; Muzzio, N.; Angelomé, P.C.; Bordoni, A.V.; Martínez, A.; Bindini, E.; Coy, E.; Andreozzi, P.; Grzelczak, M.; Moya, S.E. Strontium Titanate (SrTiO3) Mesoporous Coatings for Enhanced Strontium Delivery and Osseointegration on Bone Implants. Adv. Eng. Mater. 2019, 21, 1801210. [Google Scholar] [CrossRef]
- Sahoo, S.; Sinha, A.; Das, M. Synthesis, Characterization and in Vitro Biocompatibility Study of Strontium Titanate Ceramic: A Potential Biomaterial. J. Mech. Behav. Biomed. Mater. 2020, 102, 103494. [Google Scholar] [CrossRef]
- Qiao, H.; Zhang, C.; Dang, X.; Yang, H.; Wang, Y.; Chen, Y.; Ma, L.; Han, S.; Lin, H.; Zhang, X.; et al. Gallium Loading into a Polydopamine-Functionalised SrTiO3 Nanotube with Combined Osteoinductive and Antimicrobial Activities. Ceram. Int. 2019, 45, 22183–22195. [Google Scholar] [CrossRef]
- Si, Y.; Liu, H.; Yu, H.; Jiang, X.; Sun, D. A Heterogeneous TiO2/SrTiO3 Coating on Titanium Alloy with Excellent Photocatalytic Antibacterial, Osteogenesis and Tribocorrosion Properties. Surf. Coat. Technol. 2022, 431, 128008. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Takai, O.; Saito, N. Optical and Mechanical Properties of Transparent SrTiO 3 Thin Films Deposited by ECR Ion Beam Sputter Deposition. Phys. Status Solidi A 2013, 210, 311–319. [Google Scholar] [CrossRef]
- Davoglio, R.A.; Cabello, G.; Marco, J.F.; Biaggio, S.R. Synthesis and Characterization of α-MnO2 Nanoneedles for Electrochemical Supercapacitors. Electrochim. Acta 2018, 261, 428–435. [Google Scholar] [CrossRef]
- Mnyipika, S.H.; Munonde, T.S.; Nomngongo, P.N. MnO2@Reduced Graphene Oxide Nanocomposite-Based Electrochemical Sensor for the Simultaneous Determination of Trace Cd(II), Zn(II) and Cu(II) in Water Samples. Membranes 2021, 11, 517. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zheng, H.; Zhou, L.; Cheng, F.; Liu, Z.; Zhang, H.; Zhang, Q. Injectable Redox and Light Responsive MnO2 Hybrid Hydrogel for Simultaneous Melanoma Therapy and Multidrug-Resistant Bacteria-Infected Wound Healing. Biomaterials 2020, 260, 120314. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Abdolmaleki, A.; Tabebordbar, H. Production of PVC/α-MnO2 -KH550 Nanocomposite Films: Morphology, Thermal, Mechanical and Pb (II) Adsorption Properties. Eur. Polym. J. 2016, 78, 141–152. [Google Scholar] [CrossRef]
- Liang, Z.; Wang, H.; Zhang, K.; Ma, G.; Zhu, L.; Zhou, L.; Yan, B. Oxygen-Defective MnO2/ZIF-8 Nanorods with Enhanced Antibacterial Activity under Solar Light. Chem. Eng. J. 2022, 428, 131349. [Google Scholar] [CrossRef]
- Shaheen, S.; Iqbal, A.; Ikram, M.; Ul-Ain, K.; Naz, S.; Ul-Hamid, A.; Shahzadi, A.; Haider, A.; Nabgan, W.; Haider, J. Effective Disposal of Methylene Blue and Bactericidal Benefits of Using GO-Doped MnO2 Nanorods Synthesized through One-Pot Synthesis. ACS Omega 2021, 6, 24866–24878. [Google Scholar] [CrossRef]
- Balguri, P.K.; Thumu, U.; DG, H.S.; Penki, T.R.; Chilumala, I. Manganese Dioxide Nanostructures Reinforced Epoxy Nanocomposites: A Study of Mechanical Properties. Polym.-Plast. Technol. Mater. 2022, 61, 441–460. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, L.; Chen, K.; Ma, Y.; Peng, Q.; Ji, H.; Qiu, J. Flexible Textured MnO2 Nanorods/PVDF Hybrid Films with Superior Piezoelectric Performance for Energy Harvesting Application. Compos. Sci. Technol. 2020, 199, 108330. [Google Scholar] [CrossRef]
- Da Silva, L.F.; Avansi, W.; Moreira, M.L.; Mesquita, A.; Maia, L.J.Q.; Andrés, J.; Longo, E.; Mastelaro, V.R. Relationship between Crystal Shape, Photoluminescence, and Local Structure in SrTiO3 Synthesized by Microwave-Assisted Hydrothermal Method. J. Nanomater. 2012, 2012, 890397. [Google Scholar] [CrossRef]
- Le, M.-V.; Vo, N.-Q.-D.; Le, Q.-C.; Tran, V.A.; Phan, T.-Q.-P.; Huang, C.-W.; Nguyen, V.-H. Manipulating the Structure and Characterization of Sr1−xLaxTiO3 Nanocubes toward the Photodegradation of 2-Naphthol under Artificial Solar Light. Catalysts 2021, 11, 564. [Google Scholar] [CrossRef]
- Abdul-Hamead, A.A.; Othman, F.M.; Fakhri, M.A. Preparation of MgO–MnO2 Nanocomposite Particles for Cholesterol Sensors. J. Mater. Sci. Mater. Electron. 2021, 32, 15523–15532. [Google Scholar] [CrossRef]
- Kalubarme, R.S.; Cho, M.-S.; Yun, K.-S.; Kim, T.-S.; Park, C.-J. Catalytic Characteristics of MnO2 Nanostructures for the O2 Reduction Process. Nanotechnology 2011, 22, 395402. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Wang, X.; Wang, Y.; Hu, J. Textural and Capacitive Characteristics of MnO2 Nanocrystals Derived from a Novel Solid-Reaction Route. Electrochim. Acta 2009, 54, 1021–1026. [Google Scholar] [CrossRef]
- Saputra, E.; Muhammad, S.; Sun, H.; Ang, H.-M.; Tadé, M.O.; Wang, S. Manganese Oxides at Different Oxidation States for Heterogeneous Activation of Peroxymonosulfate for Phenol Degradation in Aqueous Solutions. Appl. Catal. B Environ. 2013, 142–143, 729–735. [Google Scholar] [CrossRef]
- Qu, D. The Study of the Proton Diffusion Process in the Porous MnO2 Electrode. Electrochim. Acta 2004, 49, 657–665. [Google Scholar] [CrossRef]
- Lu, P.; Hu, X.; Li, Y.; Zhang, M.; Liu, X.; He, Y.; Dong, F.; Fub, M.; Zhang, Z. One-step preparation of a novel SrCO3/g-C3N4 nano-composite and its application in selective adsorption of crystal violet. RSC Adv. 2018, 8, 6315. [Google Scholar] [CrossRef]
- Shokrieh, M.M.; Ghoreishi, S.M.; Esmkhani, M. Toughening Mechanisms of Nanoparticle-Reinforced Polymers. In Toughening Mechanisms in Composite Materials; Elsevier: Amsterdam, The Netherlands, 2015; pp. 295–320. ISBN 978-1-78242-279-2. [Google Scholar]
- Demirci, M.T.; Tarakçıoğlu, N.; Avcı, A.; Akdemir, A.; Demirci, İ. Fracture Toughness (Mode I) Characterization of SiO2 Nanoparticle Filled Basalt/Epoxy Filament Wound Composite Ring with Split-Disk Test Method. Compos. Part B Eng. 2017, 119, 114–124. [Google Scholar] [CrossRef]
- Tian, K.V.; Yang, B.; Yue, Y.; Bowron, D.T.; Mayers, J.; Donnan, R.S.; Dobó-Nagy, C.; Nicholson, J.W.; Fang, D.-C.; Greer, A.L.; et al. Atomic and Vibrational Origins of Mechanical Toughness in Bioactive Cement during Setting. Nat. Commun. 2015, 6, 8631. [Google Scholar] [CrossRef]
- Stajcic, I.; Veljkovic, F.; Petrovic, M.; Veličkovic, S.; Radojevic, V.; Vlahović, B.; Stajcic, A. Impact- and Thermal-Resistant Epoxy Resin Toughened with Acacia Honey. Polymers 2023, 15, 2261. [Google Scholar] [CrossRef] [PubMed]
- Quaresimin, M.; Salviato, M.; Zappalorto, M. Toughening Mechanisms in Nanoparticle Polymer Composites. In Toughening Mechanisms in Composite Materials; Elsevier: Amsterdam, The Netherlands, 2015; pp. 113–133. ISBN 978-1-78242-279-2. [Google Scholar]
- Rahmani, H.; Eslami-Farsani, R.; Ebrahimnezhad-Khaljiri, H. High Velocity Impact Response of Aluminum- Carbon Fibers-Epoxy Laminated Composites Toughened by Nano Silica and Zirconia. Fibers Polym. 2020, 21, 170–178. [Google Scholar] [CrossRef]
- Yousefi, F.; Mousavi, S.B.; Heris, S.Z.; Naghash-Hamed, S. UV-Shielding Properties of a Cost-Effective Hybrid PMMA-Based Thin Film Coatings Using TiO2 and ZnO Nanoparticles: A Comprehensive Evaluation. Sci. Rep. 2023, 13, 7116. [Google Scholar] [CrossRef] [PubMed]
- Abozaid, R.M.; Lazarević, Z.Ž.; Radović, I.; Gilić, M.; Šević, D.; Rabasović, M.S.; Radojević, V. Optical Properties and Fluorescence of Quantum Dots CdSe/ZnS-PMMA Composite Films with Interface Modifications. Opt. Mater. 2019, 92, 405–410. [Google Scholar] [CrossRef]
- Ramesh, S.; Leen, K.H.; Kumutha, K.; Arof, A.K. FTIR Studies of PVC/PMMA Blend Based Polymer Electrolytes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 66, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.N.; Saini, P.; Gupta, D.; Choudhary, V. Electrical and Mechanical Properties of PMMA/Reduced Graphene Oxide Nanocomposites Prepared via in Situ Polymerization. J. Mater. Sci. 2013, 48, 6223–6232. [Google Scholar] [CrossRef]
- ElFaham, M.M.; Mostafa, A.M.; Nasr, G.M. Unmanned Aerial Vehicle (UAV) Manufacturing Materials: Synthesis, Spectroscopic Characterization and Dynamic Mechanical Analysis (DMA). J. Mol. Struct. 2020, 1201, 127211. [Google Scholar] [CrossRef]
- Youssef, A.M.; Farag, H.K.; El-Kheshen, A.; Hammad, F.F. Synthesis of Nano-Structured Strontium Titanate by Sol-Gel and Solid State Routes. Silicon 2018, 10, 1225–1230. [Google Scholar] [CrossRef]
- Xie, T.; Wang, Y.; Liu, C.; Xu, L. New Insights into Sensitization Mechanism of the Doped Ce (IV) into Strontium Titanate. Materials 2018, 11, 646. [Google Scholar] [CrossRef]
- Kumar, Y.; Chopra, S.; Gupta, A.; Kumar, Y.; Uke, S.J.; Mardikar, S.P. Low temperature synthesis of MnO2 nanostructures for supercapacitor application. Mater. Sci. Energy Technol. 2020, 3, 566–574. [Google Scholar] [CrossRef]
- Alla, R.; Raghavendra, K.; Vyas, R.; Konakanchi, A. Conventional and contemporary polymers for the fabrication of denture prosthesis: Part I–overview, composition and properties. Int. J. Appl. Dent. Sci. 2015, 1, 82–89. [Google Scholar]
- Chrysafi, I.; Kontonasaki, E.; Anastasiou, A.D.; Patsiaoura, D.; Papadopoulou, L.; Vourlias, G.; Vouvoudi, E.; Bikiaris, D. Mechanical and Thermal Properties of PMMA Resin Composites for Interim Fixed Prostheses Reinforced with Calcium β-Pyrophosphate. J. Mech. Behav. Biomed. Mater. 2020, 112, 104094. [Google Scholar] [CrossRef] [PubMed]
- Elmadani, A.A.; Radović, I.; Tomić, N.Z.; Petrović, M.; Stojanović, D.B.; Heinemann, R.J.; Radojević, V. Hybrid Denture Acrylic Composites with Nanozirconia and Electrospun Polystyrene Fibers. PLoS ONE 2019, 14, e0226528. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, T.D.; Hawley, M.E. Chapter 5—Statistical Methods. In Introduction to Environmental Forensics, 2nd ed.; Academic Press: New York, NY, USA, 2007; pp. 129–183. ISBN 9780123695222. [Google Scholar] [CrossRef]


| PMMA | PMMA-STO | PMMA-STO/MnO2 | |
|---|---|---|---|
| Modulus of elasticity, MPa | 720.6 ± 22.13 | 1006.0 ± 15.37 | 1037.3 ± 24.63 |
| Tensile strength, MPa | 23.1 ± 1.22 | 29.9 ± 1.86 | 30.0 ± 1.41 |
| Hardness, MPa | 14.8 ± 0.43 | 19.2 ± 1.82 | 28.1 ± 2.23 |
| Etot, J | 1.1 ± 0.16 | 1.9 ± 0.14 | 2.7 ± 0.21 |
| PMMA-STO * | PMMA-STO/MnO2 ‡ | |||
|---|---|---|---|---|
| t-Value | p-Value | t-Value | p-Value | |
| Modulus of elasticity | 18.3465 | <0.0001 | 16.5664 | <0.0001 |
| Tensile strength | 5.2949 | 0.0061 | 6.4097 | 0.0030 |
| Hardness | 4.0752 | 0.0152 | 10.1433 | 0.0005 |
| Etot | 6.5175 | 0.0029 | 10.4970 | 0.0005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhmali, H.T.; Stajcic, I.; Stajcic, A.; Pesic, I.; Jovanovic, M.; Petrovic, M.; Radojevic, V. Influence of Novel SrTiO3/MnO2 Hybrid Nanoparticles on Poly(methyl methacrylate) Thermal and Mechanical Behavior. Polymers 2024, 16, 278. https://doi.org/10.3390/polym16020278
Elhmali HT, Stajcic I, Stajcic A, Pesic I, Jovanovic M, Petrovic M, Radojevic V. Influence of Novel SrTiO3/MnO2 Hybrid Nanoparticles on Poly(methyl methacrylate) Thermal and Mechanical Behavior. Polymers. 2024; 16(2):278. https://doi.org/10.3390/polym16020278
Chicago/Turabian StyleElhmali, Houda Taher, Ivana Stajcic, Aleksandar Stajcic, Ivan Pesic, Marija Jovanovic, Milos Petrovic, and Vesna Radojevic. 2024. "Influence of Novel SrTiO3/MnO2 Hybrid Nanoparticles on Poly(methyl methacrylate) Thermal and Mechanical Behavior" Polymers 16, no. 2: 278. https://doi.org/10.3390/polym16020278
APA StyleElhmali, H. T., Stajcic, I., Stajcic, A., Pesic, I., Jovanovic, M., Petrovic, M., & Radojevic, V. (2024). Influence of Novel SrTiO3/MnO2 Hybrid Nanoparticles on Poly(methyl methacrylate) Thermal and Mechanical Behavior. Polymers, 16(2), 278. https://doi.org/10.3390/polym16020278






