Comparison of Two Methods for Measuring the Temperature Dependence of H2 Permeation Parameters in Nitrile Butadiene Rubber Polymer Composites Blended with Fillers: The Volumetric Analysis Method and the Differential Pressure Method
Abstract
:1. Introduction
2. Experiments and Analyses
2.1. High-Pressure Hydrogen Exposure of the Specimens in the Volumetric Analysis Method
2.2. H2 Emissions Measured by the Volumetric Analysis Method
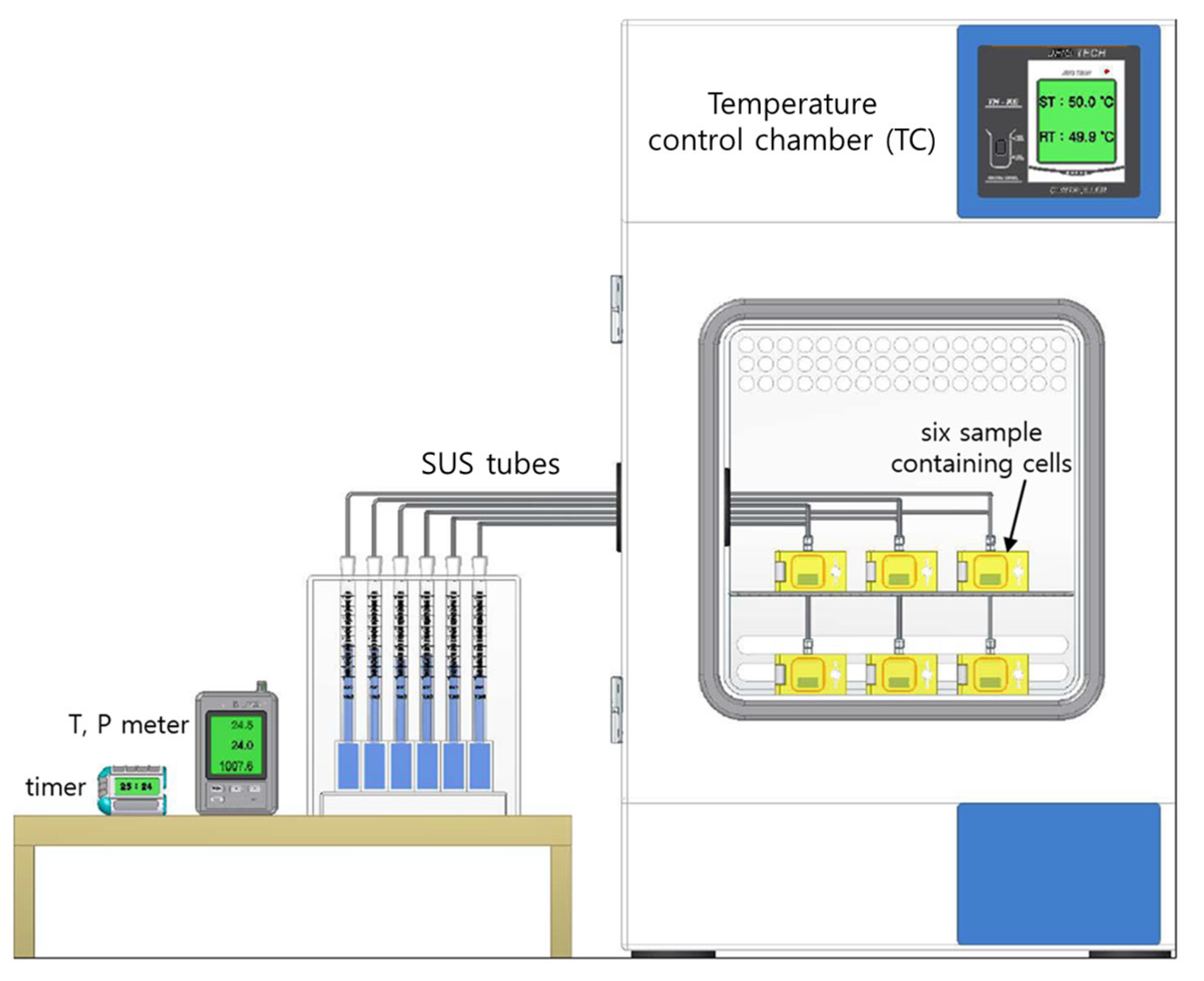
2.3. Temperature-Controlled System in the Volumetric Analysis Method
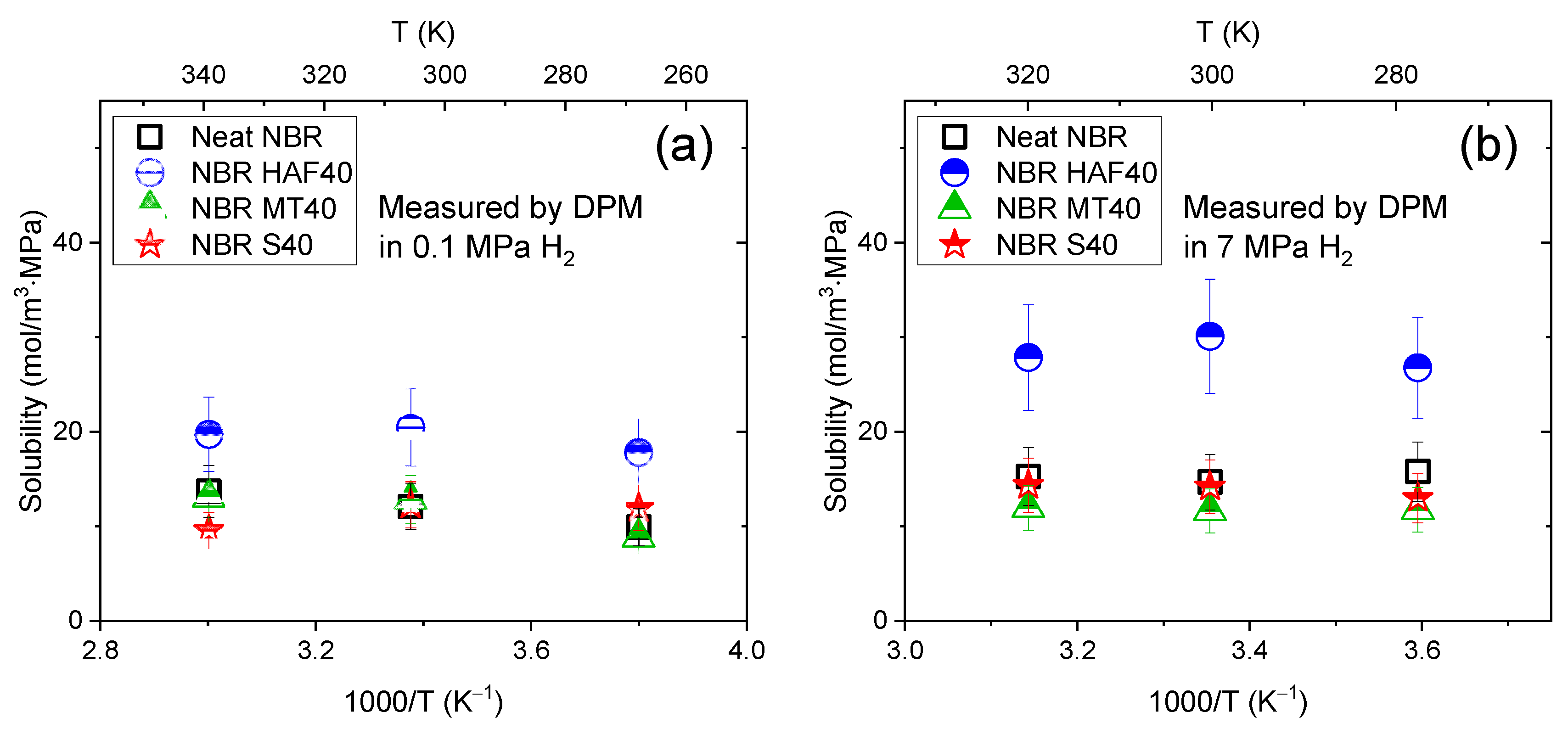
2.4. Analysis of the Hydrogen Uptake and Diffusivity for the Volumetric Analysis Method
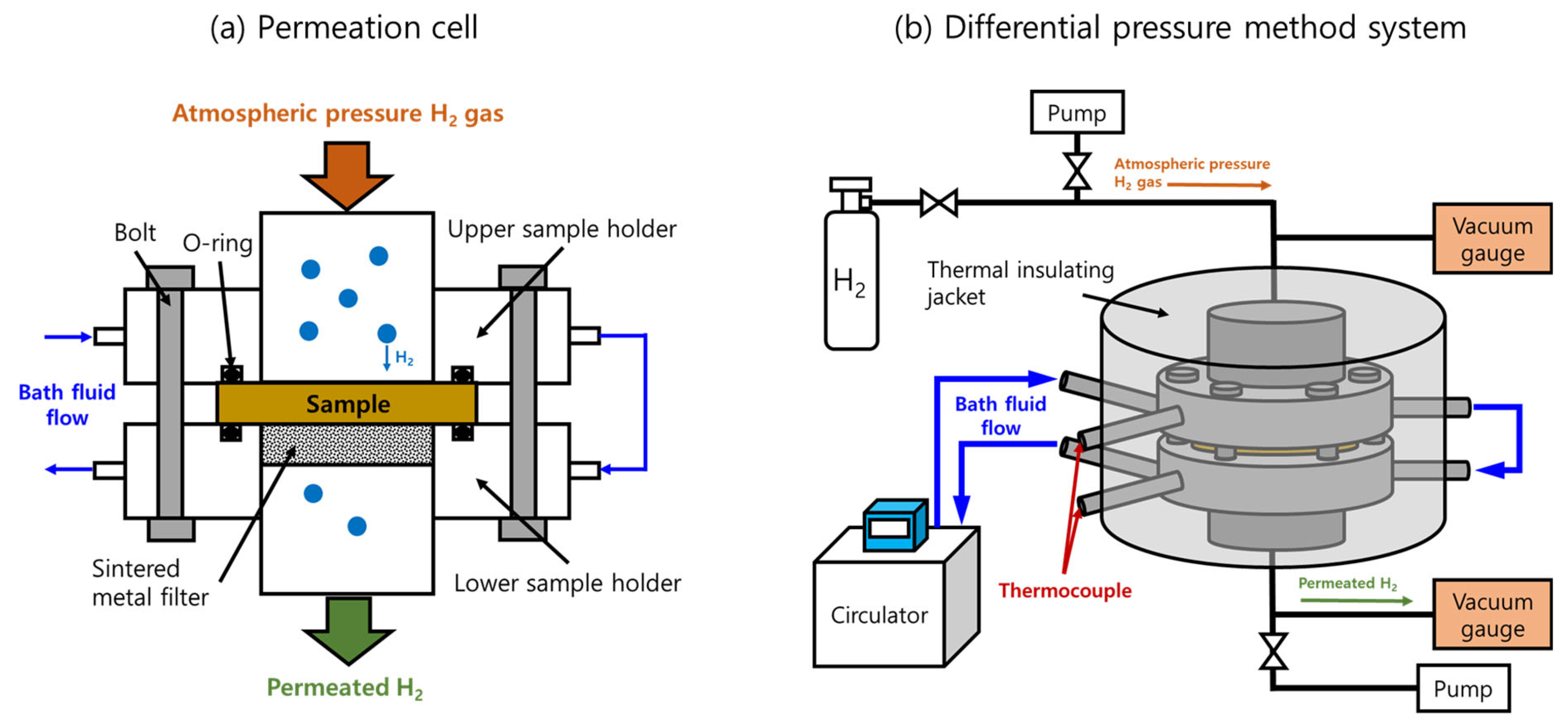
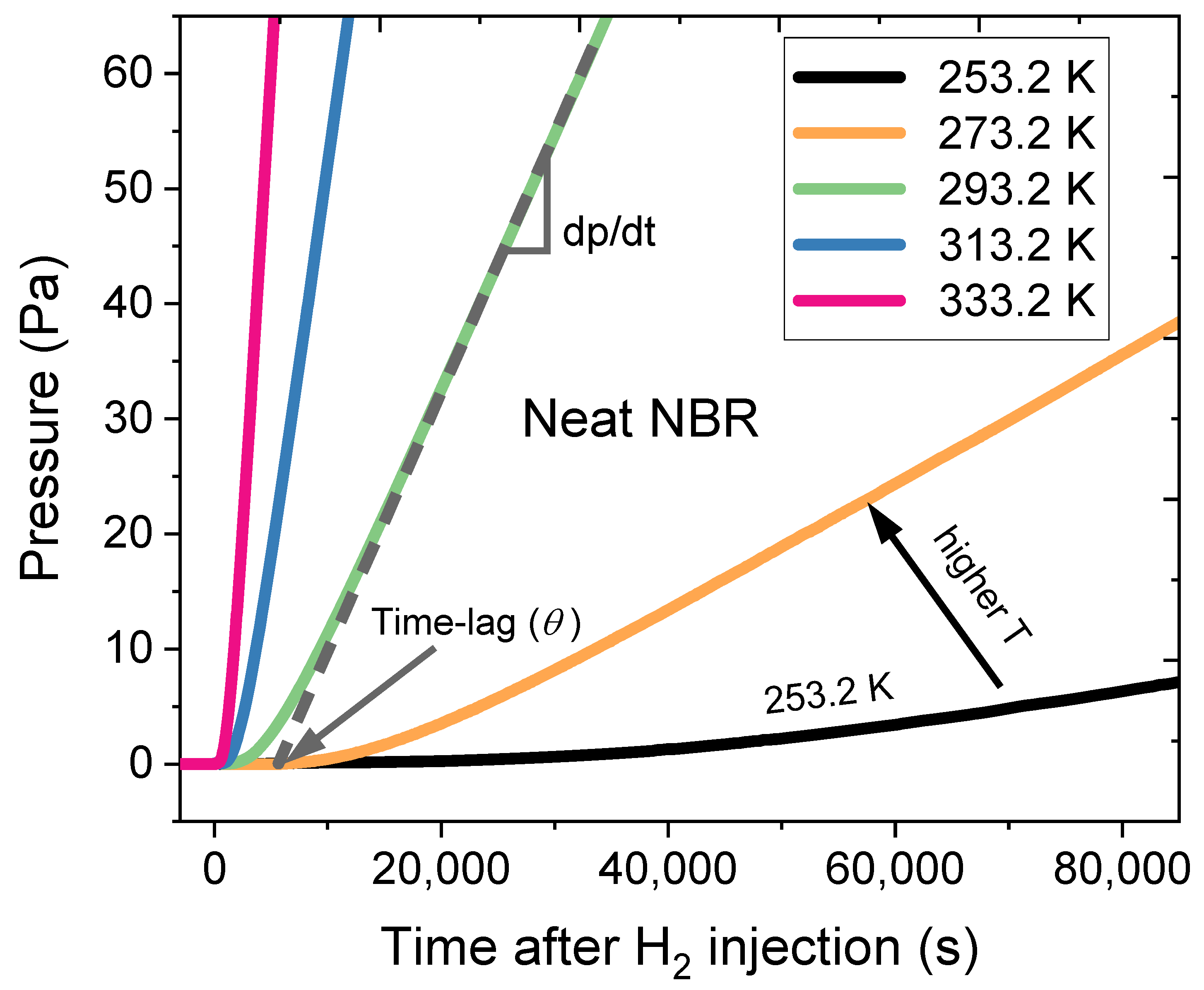
2.5. Permeation Cell and Temperature-Controlled System in the Differential Pressure Method
2.6. Dynamic Mechanical Analysis
3. Results and Discussion
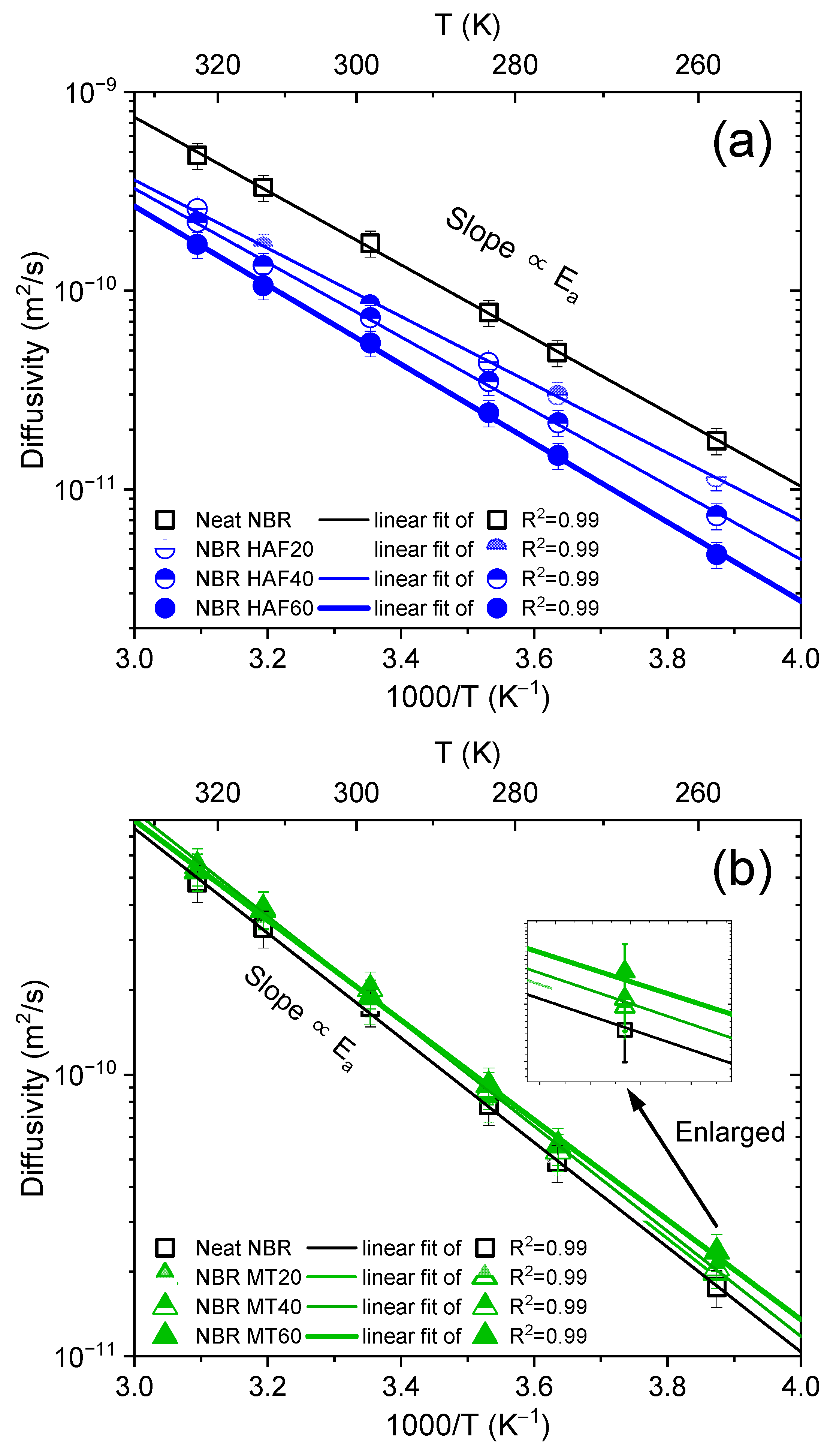
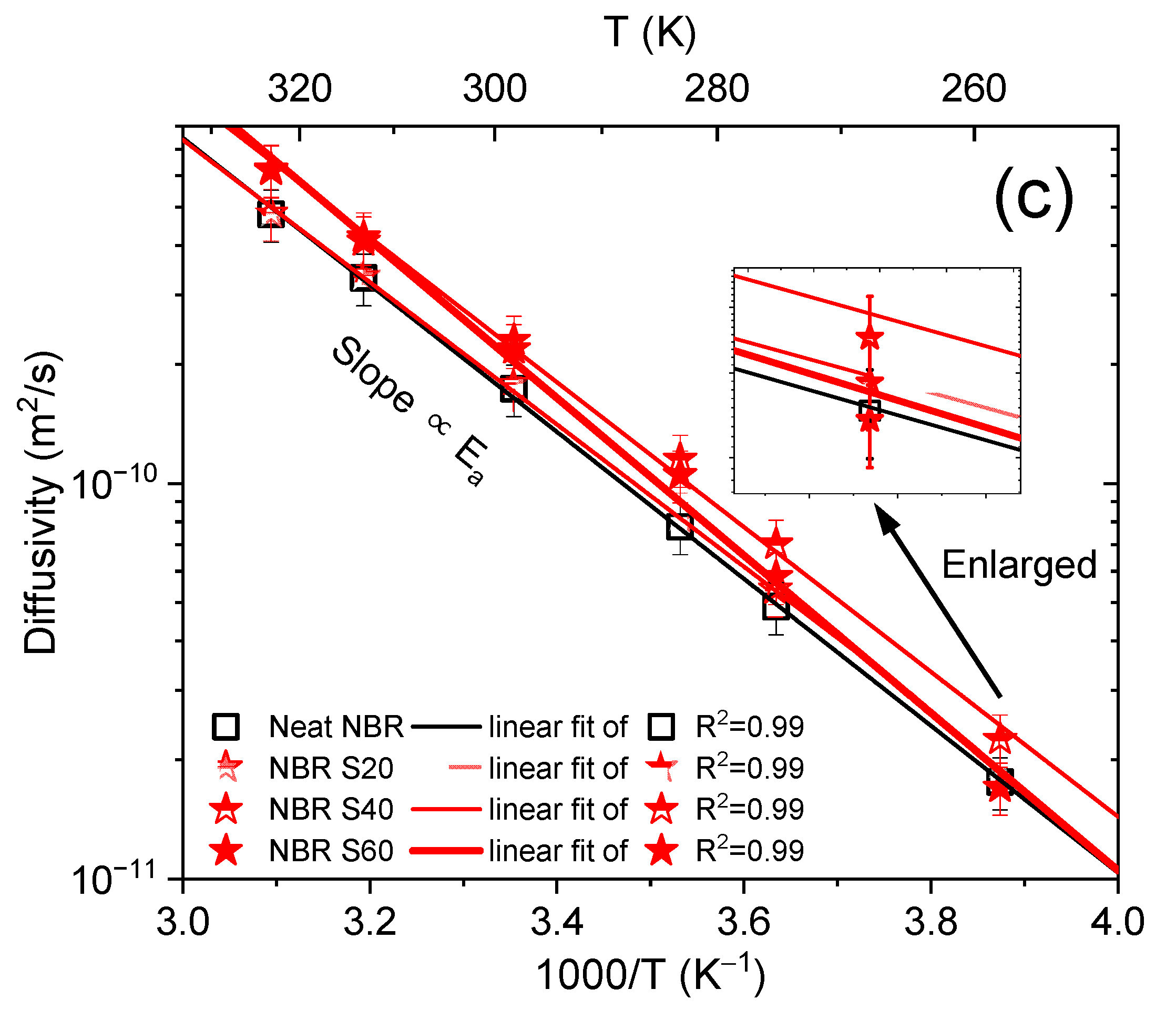
3.1. Temperature Dependence and Activation Energy of H2 Diffusivity in the Volumetric Analysis Method
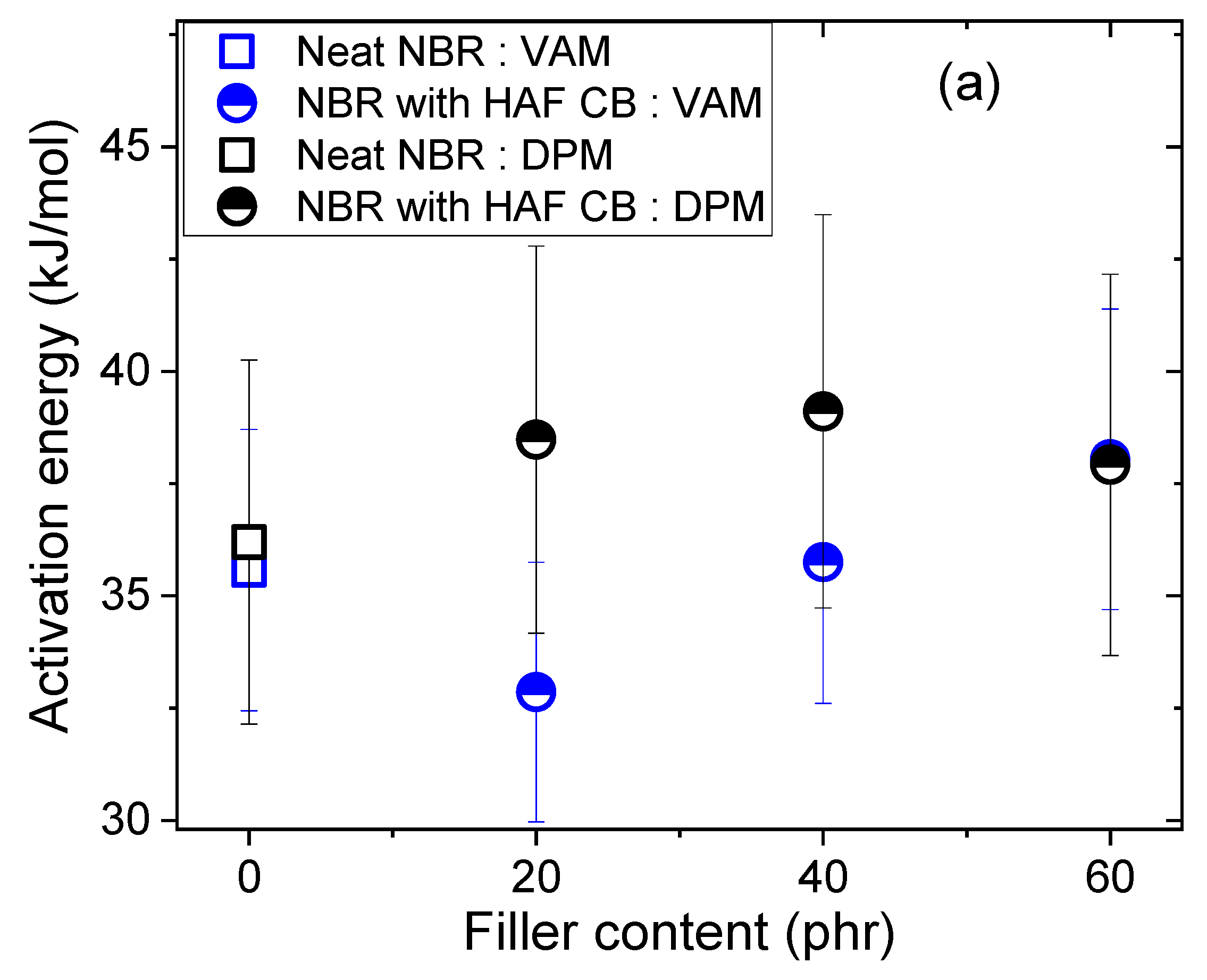
- NBR with HAF CB series: slope = (0.13 ± 0.01) kJ/(mol·phr)
- NBR with MT CB series: slope = (−0.04 ± 0.02) kJ/(mol·phr)
- NBR with silica series: slope = (0.09 ± 0.04) kJ/(mol·phr)
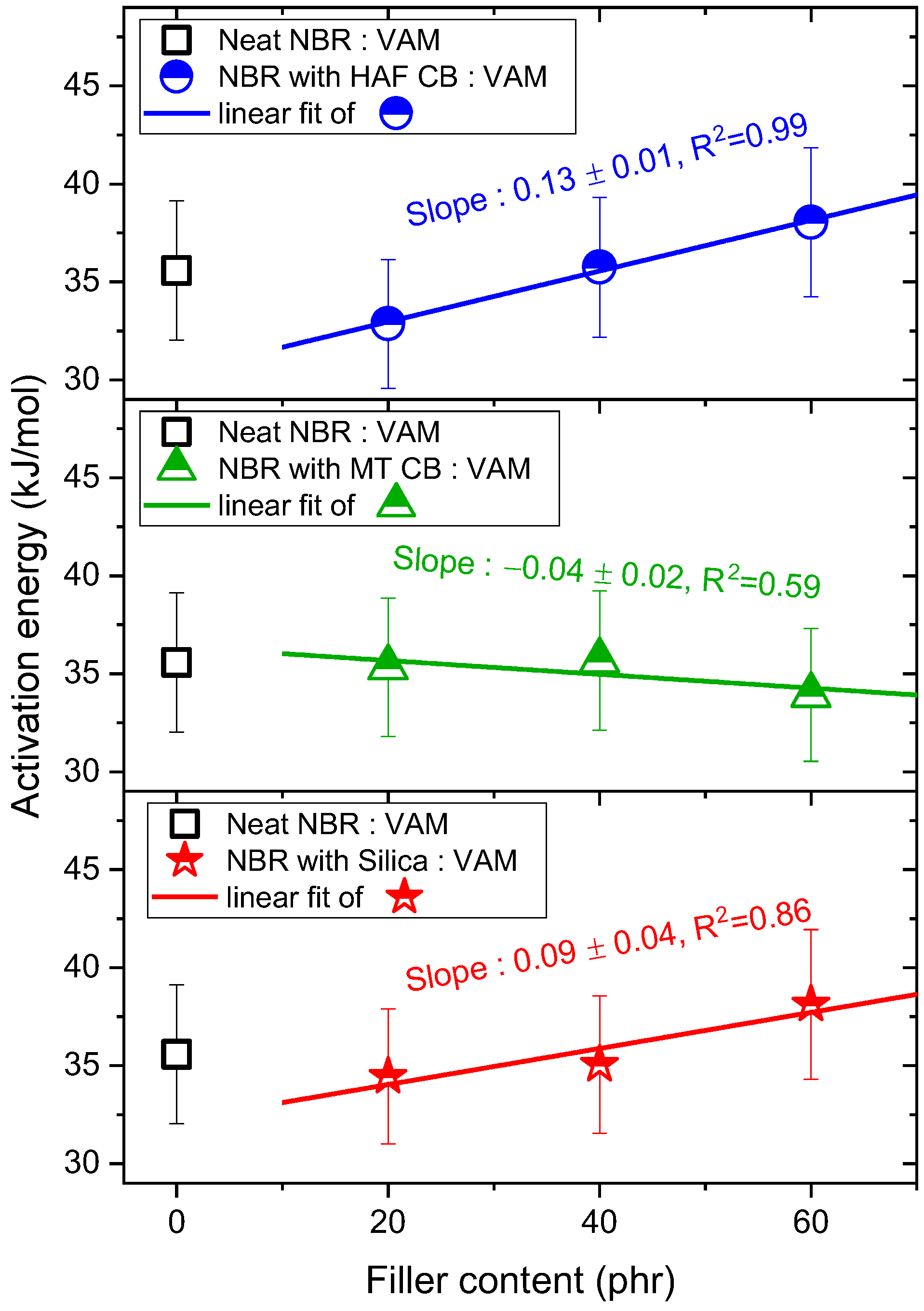
- NBR with HAF CB series: slope = (0.18 ± 0.05) °C/phr
- NBR with MT CB series: slope = (−0.07 ± 0.03) °C/phr
- NBR with silica series: slope = (0.10 ± 0.01) °C/phr

3.2. Comparison of the Activation Energies from the Volumetric Analysis Method and the Differential Pressure Method
4. Uncertainty Analyses
4.1. Uncertainty Analysis of Diffusivity in the Volumetric Analysis Method
4.2. Uncertainty Analysis of Permeability in the Differential Pressure Method
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Storey, R.F.; Mauritz, K.A.; Cox, B.D. Diffusion of various dialkyl phthalate plasticizers in PVC. Macromolecules 1989, 22, 289–294. [Google Scholar] [CrossRef]
- Bernardo, G. Diffusivity of alcohols in amorphous polystyrene. J. Appl. Polym. Sci. 2013, 127, 1803–1811. [Google Scholar] [CrossRef]
- Fang, X.; Vitrac, O. Predicting diffusion coefficients of chemicals in and through packaging materials. Crit. Rev. Food Sci. Nutr. 2015, 57, 275–312. [Google Scholar] [CrossRef]
- Bandehali, S.; Sanaeepur, H.; Amooghin, A.E.; Shirazian, S.; Ramakrishna, S. Biodegradable polymers for membrane separation. Sep. Purif. Technol. 2021, 269, 118731. [Google Scholar] [CrossRef]
- Gugliuzza, A.; Drioli, E. A review on membrane engineering for innovation in wearable fabrics and protective textiles. J. Membr. Sci. 2013, 446, 350–375. [Google Scholar] [CrossRef]
- Visco, A.; Scolaro, C.; Torrisi, A.; Torrisi, L. Diffusion of nitrogen gas through polyethylene based films. Polym. Cryst. 2021, 4, e10207. [Google Scholar] [CrossRef]
- Jung, J.K.; Baek, U.B.; Lee, S.H.; Choi, M.C.; Bae, J.W. Hydrogen gas permeation in peroxide-crosslinked ethylene propylene diene monomer polymer composites with carbon black and silica fillers. J. Polym. Sci. 2023, 61, 460–471. [Google Scholar] [CrossRef]
- Simmons, K.L.; Kuang, W.; Burton, S.D.; Arey, B.W.; Shin, Y.; Menon, N.C.; Smith, D. H-Mat hydrogen compatibility of polymers and elastomers. Int. J. Hydrogen Energy 2021, 46, 12300–12310. [Google Scholar] [CrossRef]
- Rattanasom, N.; Prasertsri, S. Mechanical properties, gas permeability and cut growth behaviour of natural rubber vulcanizates: Influence of clay types and clay/carbon black ratios. Polym. Test. 2012, 31, 645–653. [Google Scholar] [CrossRef]
- Yamabe, J.; Nishimura, S. Influence of carbon black on decompression failure and hydrogen permeation properties of filled ethylene-propylene–diene–methylene rubbers exposed to high-pressure hydrogen gas. J. Appl. Polym. Sci. 2011, 122, 3172–3187. [Google Scholar] [CrossRef]
- Fujiwara, H.; Ono, H.; Nishimura, S. Effects of fillers on the hydrogen uptake and volume expansion of acrylonitrile butadiene rubber composites exposed to high pressure hydrogen: -Property of polymeric materials for high pressure hydrogen devices (3). Int. J. Hydrogen Energy 2022, 47, 4725–4740. [Google Scholar] [CrossRef]
- Barth, R.R.; Simmons, K.L.; Marchi, C.W.S. Polymers for Hydrogen Infrastructure and Vehicle Fuel Systems; Sandia National Labaratories: Livermore, CA, USA, 2013. [Google Scholar]
- Fujiwara, H.; Ono, H.; Nishimura, S. Degradation behavior of acrylonitrile butadiene rubber after cyclic high-pressure hydrogen exposure. Int. J. Hydrogen Energy 2015, 40, 2025–2034. [Google Scholar] [CrossRef]
- Nihmath, A.; Ramesan, M.T. Hydroxyapatite as a potential nanofiller in technologically useful chlorinated acrylonitrile butadiene rubber. Polym. Test. 2020, 91, 106837. [Google Scholar] [CrossRef]
- Van Amerongen, G. The effect of fillers on the permeability of rubber to gases. Rubber Chem. Technol. 1955, 28, 821–832. [Google Scholar] [CrossRef]
- Welle, F. Diffusion coefficients and activation energies of diffusion of organic molecules in polystyrene below and above glass transition temperature. Polymers 2021, 13, 1317. [Google Scholar] [CrossRef]
- Jung, J.K.; Lee, C.H.; Son, M.S.; Lee, J.H.; Baek, U.B.; Chung, K.S.; Choi, M.C.; Bae, J.W. Filler effects on H2 diffusion behavior in nitrile butadiene rubber blended with carbon black and silica fillers of different concentrations. Polymers 2022, 14, 700. [Google Scholar] [CrossRef]
- Jung, J.K.; Kim, I.G.; Jeon, S.K.; Kim, K.-T.; Baek, U.B.; Nahm, S.H. Volumetric analysis technique for analyzing the transport properties of hydrogen gas in cylindrical-shaped rubbery polymers. Polym. Test. 2021, 99, 107147. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Oxford University Press: Oxford, UK, 1975. [Google Scholar]
- Demarez, A.; Hock, A.G.; Meunier, F.A. Diffusion of hydrogen in mild steel. Acta Metall. 1954, 2, 214–223. [Google Scholar] [CrossRef]
- Nelder, J.A.; Mead, R. A simplex method for function minimization. Comput. J. 1965, 7, 308–313. [Google Scholar] [CrossRef]
- Jung, J.K.; Kim, I.G.; Kim, K.T.; Ryu, K.S.; Chung, K.S. Evaluation techniques of hydrogen permeation in sealing rubber materials. Polym. Test. 2021, 93, 107016. [Google Scholar] [CrossRef]
- ISO 15105-1:2007; Plastics—Film and Sheeting. International Organization for Standardization: Geneva, Switzerland, 2007.
- Laidler, K.J. The development of the Arrhenius equation. J. Chem. Educ. 1984, 61, 494. [Google Scholar] [CrossRef]
- Kirchheim, R.; Huang, X.Y. A relationship between prefactor and activation energy for diffusion. Phys. Status Solidi 1987, 144, 253–257. [Google Scholar] [CrossRef]
- Bahadori, L.; Chakrabarti, M.H.; Manan, N.S.A.; Hashim, M.A.; Mjalli, F.S.; AlNashef, I.M.; Brandon, N. The effect of temperature on kinetics and diffusion coefficients of metallocene derivatives in polyol-based deep eutectic solvents. PLoS ONE 2015, 10, e0144235. [Google Scholar] [CrossRef]
- Litvinov, V.M.; Orza, R.A.; Klüppel, M.; van Duin, M.; Magusin, P.C.M.M. Rubber–filler interactions and network structure in relation to stress–strain behavior of vulcanized, carbon black filled EPDM. Macromolecules 2011, 44, 4887–4900. [Google Scholar] [CrossRef]
- Zadrapa, P.; Malac, J.; Konecny, P. Filler and mobility of rubber matrix molecules. Polym. Bull. 2011, 67, 927–936. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, S. Estimation and modeling of pressure-dependent gas diffusion coefficient for coal: A fractal theory-based approach. Fuel 2019, 253, 588–606. [Google Scholar] [CrossRef]
- Jung, J.K.; Baek, U.B.; Nahm, S.H.; Chung, K.S. Hydrogen sorption and desorption properties in rubbery polymer. Mater. Chem. Phys. 2022, 279, 125745. [Google Scholar] [CrossRef]
- JCGM 100:2008 GUM 1995 with Minor Corrections; Evaluation of Measurement Data—Guide to the Expression of Uncertainty in Measurement. Joint Committee for Guides in Metrology: Sèvres, France, 2008.



| Uncertainty Factor | Relative Value (%) |
|---|---|
| Repeated measurements | 3.5 |
| Accuracy of the electronic balance | 0.1 |
| Change in the sample volume | 1.4 |
| Standard deviation between the data and Equation (4) | 1.7 |
| Accuracy of the graduated cylinder | 0.3 |
| Resolution of the graduated cylinder | 0.2 |
| Accuracy of the manometer | 0.6 |
| Variations in the temperature/pressure | 0.2 |
| Combined standard uncertainty, uc | 4.2 |
| Coverage factor, k | 2.1 |
| Expanded uncertainty, U = kuc | 8.8 |
| Uncertainty Factor | Relative Value (%) |
|---|---|
| Repeated measurements | 2.9 |
| Resolution of the thermocouple | 0.01 |
| Accuracy of the thermocouple | 0.03 |
| Accuracy of the vacuum gauge | 0.1 |
| Volume of the permeated side in the cell | 0.1 |
| Standard deviation between the data and the dp/dt in Equation (5) | 0.01 |
| Thickness measurement for the sample | 0.8 |
| Maximum change in the sample thickness | 1.2 |
| Variation in the permeation area contacting H2 | 2.9 |
| Combined standard uncertainty, uc | 4.3 |
| Coverage factor, k | 2.6 |
| Expanded uncertainty, U = kuc | 11.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.H.; Kim, Y.W.; Kim, D.J.; Chung, N.K.; Jung, J.K. Comparison of Two Methods for Measuring the Temperature Dependence of H2 Permeation Parameters in Nitrile Butadiene Rubber Polymer Composites Blended with Fillers: The Volumetric Analysis Method and the Differential Pressure Method. Polymers 2024, 16, 280. https://doi.org/10.3390/polym16020280
Lee JH, Kim YW, Kim DJ, Chung NK, Jung JK. Comparison of Two Methods for Measuring the Temperature Dependence of H2 Permeation Parameters in Nitrile Butadiene Rubber Polymer Composites Blended with Fillers: The Volumetric Analysis Method and the Differential Pressure Method. Polymers. 2024; 16(2):280. https://doi.org/10.3390/polym16020280
Chicago/Turabian StyleLee, Ji Hun, Ye Won Kim, Do Jung Kim, Nak Kwan Chung, and Jae Kap Jung. 2024. "Comparison of Two Methods for Measuring the Temperature Dependence of H2 Permeation Parameters in Nitrile Butadiene Rubber Polymer Composites Blended with Fillers: The Volumetric Analysis Method and the Differential Pressure Method" Polymers 16, no. 2: 280. https://doi.org/10.3390/polym16020280
APA StyleLee, J. H., Kim, Y. W., Kim, D. J., Chung, N. K., & Jung, J. K. (2024). Comparison of Two Methods for Measuring the Temperature Dependence of H2 Permeation Parameters in Nitrile Butadiene Rubber Polymer Composites Blended with Fillers: The Volumetric Analysis Method and the Differential Pressure Method. Polymers, 16(2), 280. https://doi.org/10.3390/polym16020280






