Study of Mechanical and Thermal Properties of Environmentally Friendly Composites from Beer Bagasse
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
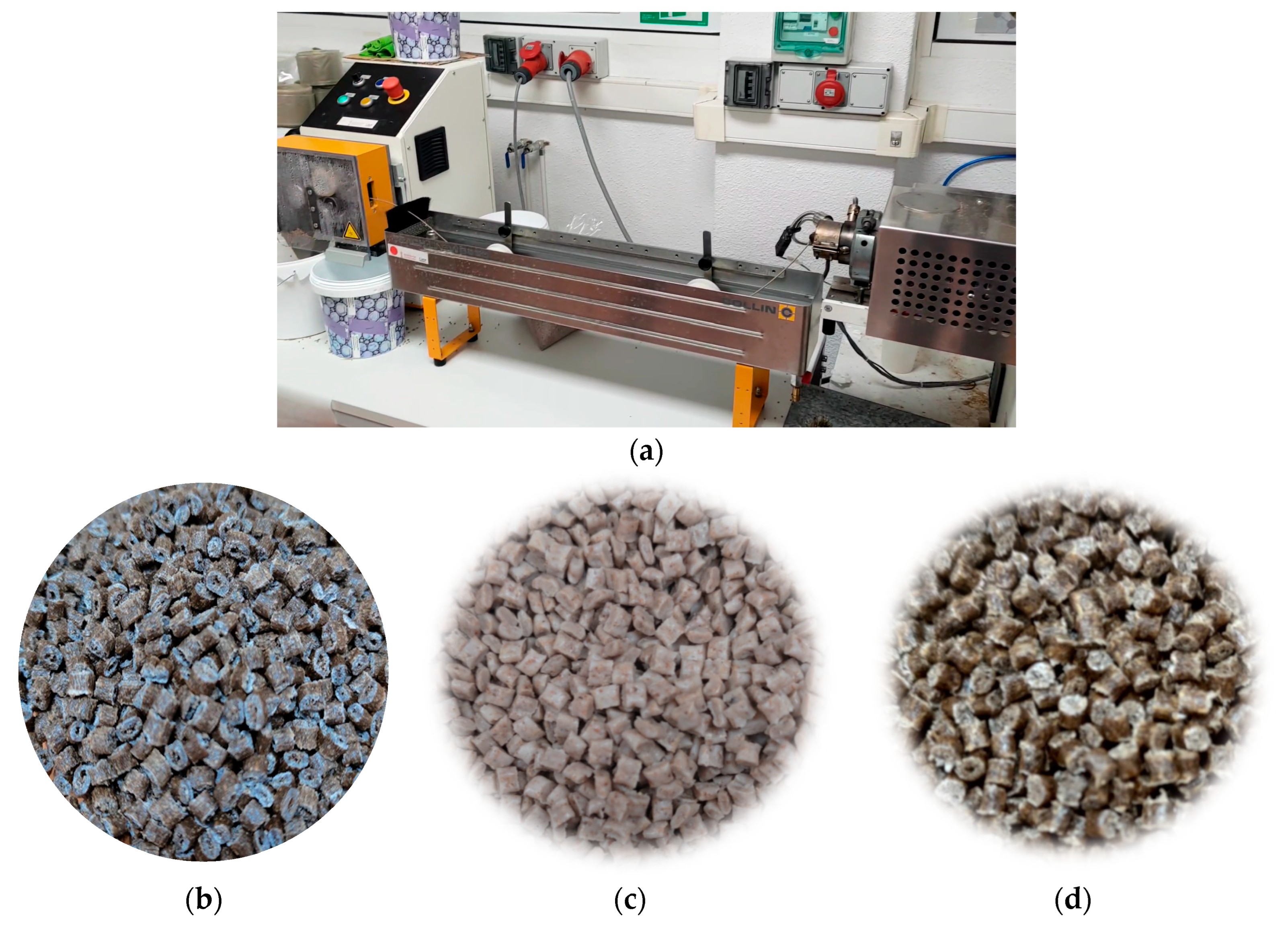
2.2. Biocomposite Preparation
2.2.1. Drying
2.2.2. Extrusion Compounding Process
2.2.3. Injection Moulding Process
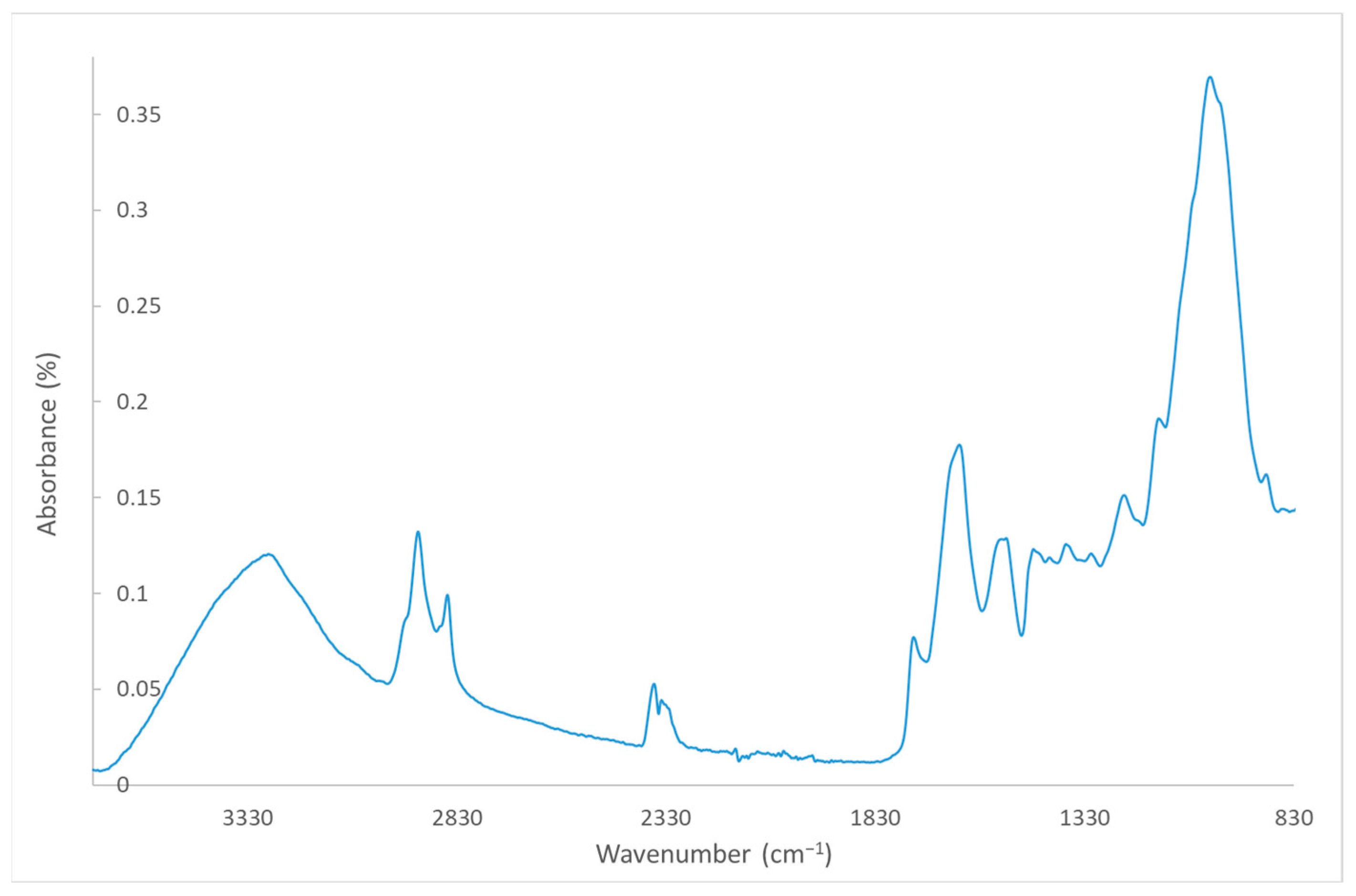
2.3. Biocomposite Characterisation
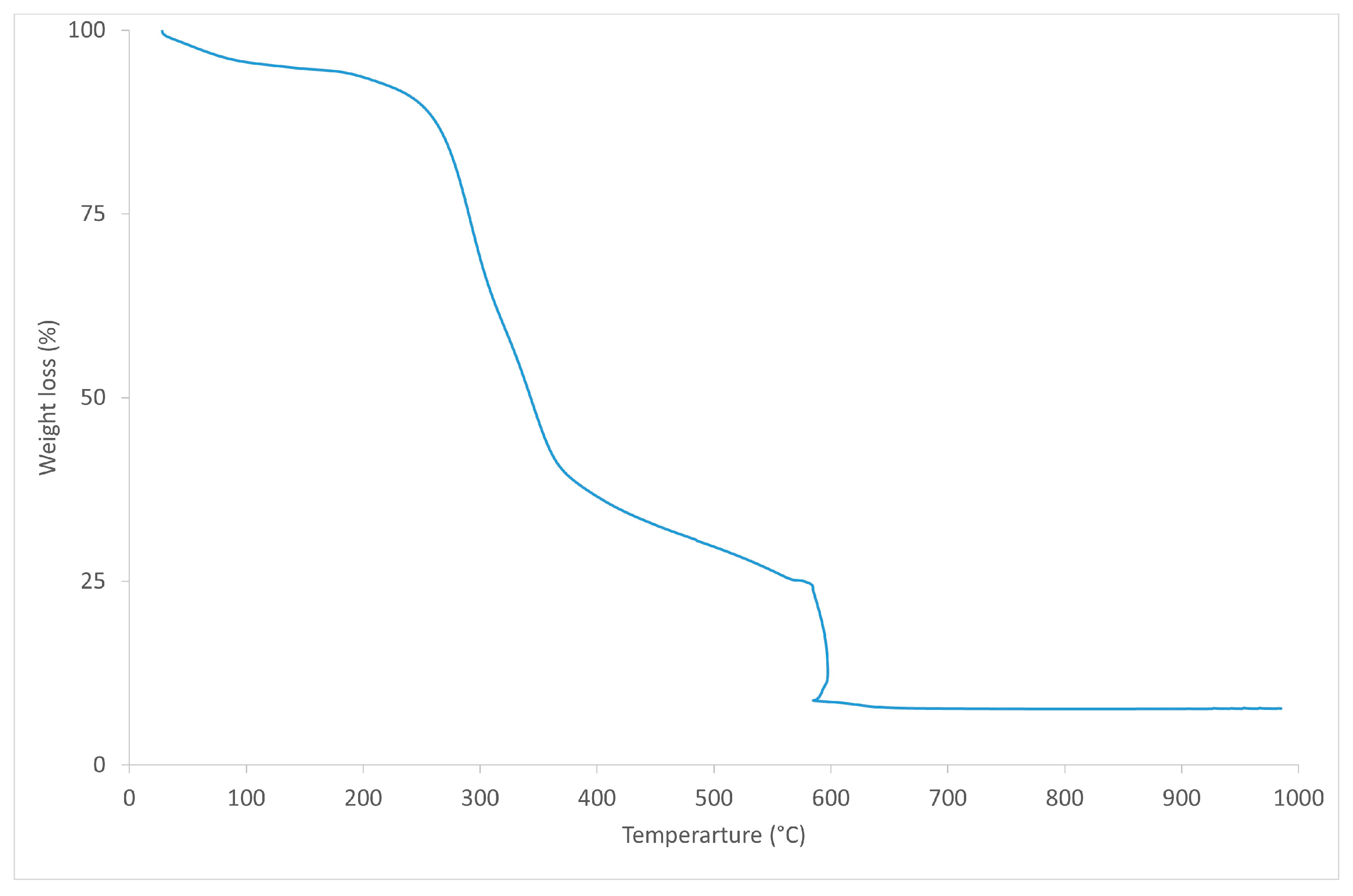
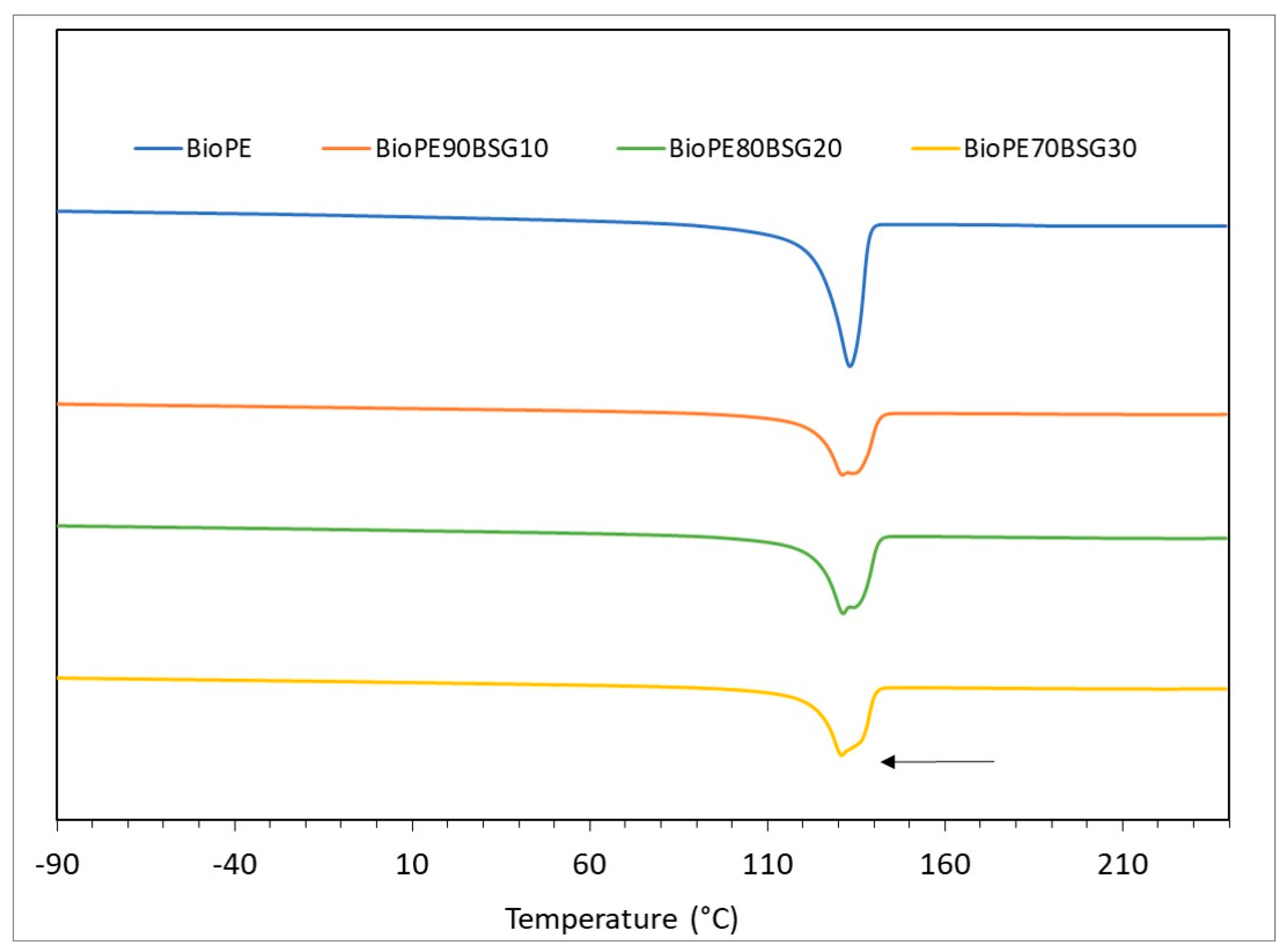
2.3.1. Thermal Characterisation
2.3.2. Rheological Characterisation
2.3.3. Mechanical Characterisation
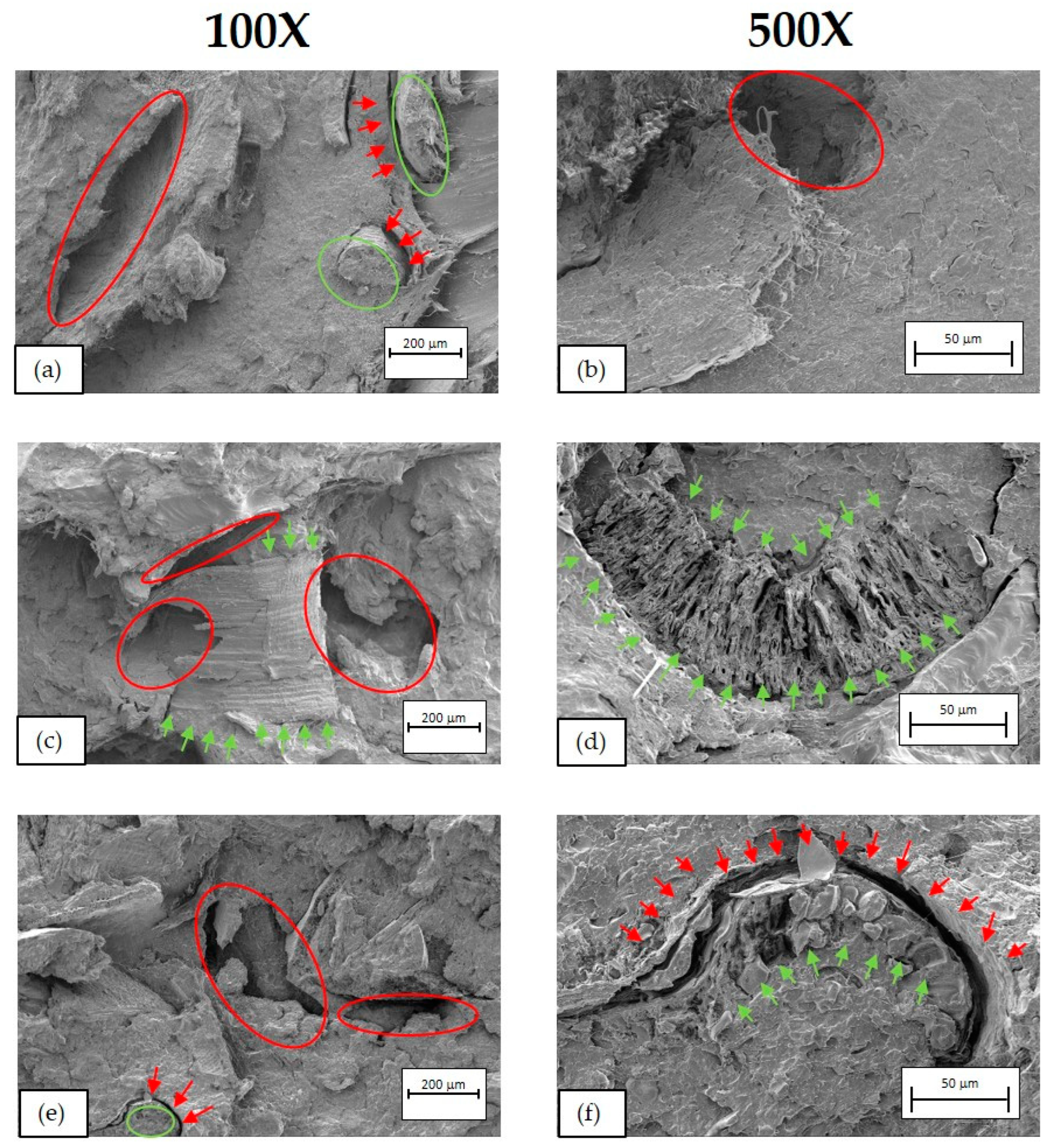
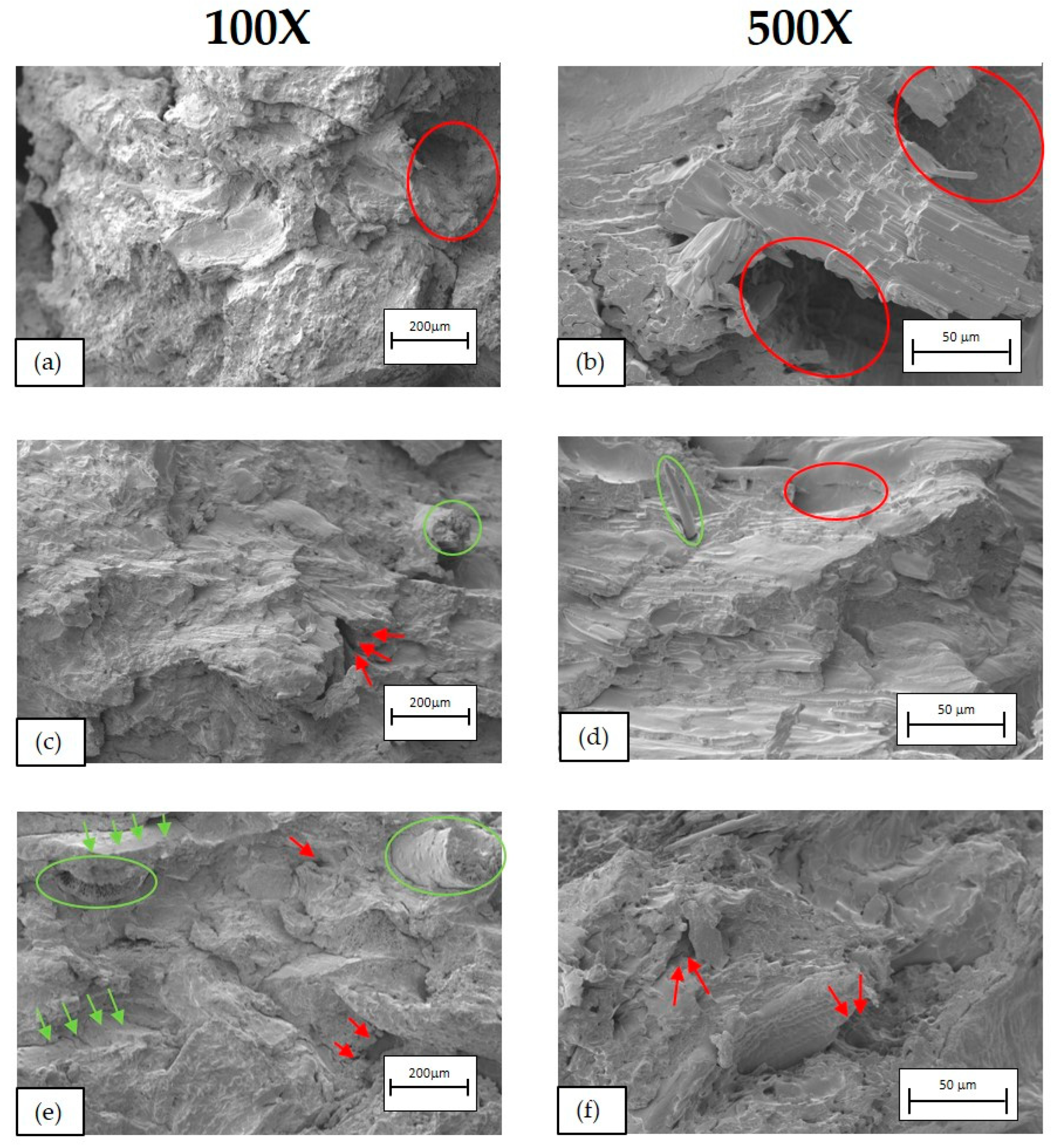
2.3.4. Morphological Characterisation
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Zheng, J.; Suh, S. Strategies to Reduce the Global Carbon Footprint of Plastics. Nat. Clim. Chang. 2019, 9, 374–378. [Google Scholar] [CrossRef]
- Lau, W.W.Y.; Shiran, Y.; Bailey, R.M.; Cook, E.; Stuchtey, M.R.; Koskella, J.; Velis, C.A.; Godfrey, L.; Boucher, J.; Murphy, M.B.; et al. Evaluating Scenarios toward Zero Plastic Pollution. Science 2020, 369, 1455–1461. [Google Scholar] [CrossRef]
- Atiwesh, G.; Mikhael, A.; Parrish, C.C.; Banoub, J.; Le, T.A.T. Environmental impact of bioplastic use: A review. Heliyon 2021, 7, e07918. [Google Scholar] [CrossRef]
- Chaudhary, A.K.; Singh, V.; Tewari, M. Utilization of Waste Agriculture Byproduct to Enhance the Economy of Farmers. Indian Res. J. Ext. Edu. Special Issue 2012, 1, 89–92. [Google Scholar]
- Tasdemir, M. Polypropylene/olive pit & almond shell polymer composites: Wear and friction. IOP Conf. Ser. Mater. Sci. Eng. 2017, 204, 012015. [Google Scholar] [CrossRef]
- Dolza, C.; Fages, E.; Gonga, E.; Gomez-Caturla, J.; Balart, R.; Quiles-Carrillo, L. Development and Characterization of Environmentally Friendly Wood Plastic Composites from Biobased Polyethylene and Short Natural Fibers Processed by Injection Moulding. Polymers 2021, 13, 1692. [Google Scholar] [CrossRef]
- Jorda-Reolid, M.; Gomez-Caturla, J.; Ivorra-Martinez, J.; Stefani, P.M.; Rojas-Lema, S.; Quiles-Carrillo, L. Upgrading Argan Shell Wastes in Wood Plastic Composites with Biobased Polyethylene Matrix and Different Compatibilizers. Polymers 2021, 13, 922. [Google Scholar] [CrossRef]
- Ivorra-Martinez, J.; Manuel-Mañogil, J.; Boronat, T.; Sanchez-Nacher, L.; Balart, R.; Quiles-Carrillo, L. Development and Characterization of Sustainable Composites from Bacterial Polyester Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) and Almond Shell Flour by Reactive Extrusion with Oligomers of Lactic Acid. Polymers 2020, 12, 1097. [Google Scholar] [CrossRef]
- Elfaleh, I.; Abbassi, F.; Habibi, M.; Ahmad, F.; Guedri, M.; Nasri, M.; Garnier, C. A comprehensive review of natural fibers and their composites: An eco-friendly alternative to conventional materials. Results Eng. 2023, 19. [Google Scholar] [CrossRef]
- Mylsamy, B.; Shanmugam, S.K.M.; Aruchamy, K.; Palanisamy, S.; Nagarajan, R.; Ayrilmiş, N. A Review on Natural Fiber Composites: Polymer Matrices, Fiber Surface Treatments, Fabrication Methods, Properties, and Applications. Polym. Eng. Sci. 2024, 64, 2345–2373. [Google Scholar] [CrossRef]
- Ghasem, J.M. Economic Model Assessment of Wood—Polymer Composites Production Fromagricultural Wastes. Ann. Biol. Res. 2013, 4, 169–174. [Google Scholar]
- Ramos, M.; Dominici, F.; De Luzi, F.; Jiménez, A.; Garrigós, M.C.; Torre, L.; Puglia, D. Effect of Almond Shell Waste on Physicochemical Properties of Polyester-Based Biocomposites. Polymers 2020, 12, 835. [Google Scholar] [CrossRef]
- Álvarez-Chávez, C.R.; Sánchez-Acosta, D.; Encinas-Encinas, J.C.; Esquer, J.; Quintana-Owen, P.; Madera-Santana, T.J. Characterization of Extruded Poly(Lactic Acid)/Pecan Nutshell Biocomposites. Int. J. Polym. Sci. 2017, 2017, 1–12. [Google Scholar] [CrossRef]
- Żelaziński, T.; Ekielski, A.; Durczak, K.; Morawska, M. Study on Biodegradable Materials From Thermoplastic Starch With the Addition of Nuts Shell. INMATEH—Agric. Eng. 2023, 70, 201–210. [Google Scholar] [CrossRef]
- García, A.I.; García, A.M.; Bou, S.F. Study of the Influence of the Almond Shell Variety Onthe Mechanical Properties of Starch-Basedpolymer Biocomposites. Polymers 2020, 12, 2049. [Google Scholar] [CrossRef]
- Laaziz, S.A.; Raji, M.; Hilali, E.M.; Essabir, H.; Rodrigue, D.; Bouhfid, R.; Qaiss, A.e.K. Bio-composites based on polylactic acid and argan nut shell: Production and properties. Int. J. Biol. Macromol. 2017, 104, 30–42. [Google Scholar] [CrossRef]
- Essabir, H.; Bensalah, M.O.; Rodrigue, D.; Bouhfid, R.; Qaiss, A.e.K. Biocomposites based on Argan nut shell and a polymer matrix: Effect of filler content and coupling agent. Carbohydr. Polym. 2016, 143, 70–83. [Google Scholar] [CrossRef]
- Mello, L.R.P.F.; Vergílio, R.M.; Mali, S. Caracterização Química e Funcional Do Resíduo Fibroso Da Indústria Cervejeira. BBR—Biochem. Biotechnol. Rep. 2013, 2, 191–194. [Google Scholar] [CrossRef]
- Panzarini, N.H.; Rabbers, A.; da Trindade, J.L.F.; Matos, E.; Canteri, M.H.G.; Bittencourt, J.V.M. Elaboração de Bolo de Mel Enriquecido Com Fibras Do Bagaço Da Indústria Cervejeira. Rev. Bras. de Tecnol. Agroindustrial 2014, 8, 1154–1164. [Google Scholar] [CrossRef][Green Version]
- Lamas, D.L.; Gende, L.B. Valorisation of Brewers’ Spent Grain for the Development of Novel Beverage and Food Products. Appl. Food Res. 2023, 3. [Google Scholar] [CrossRef]
- Castro-Criado, D.; Abdullah, J.A.A.; Romero, A.; Jiménez-Rosado, M. Stabilization and Valorization of Beer Bagasse to Obtain Bioplastics. Polymers 2023, 15, 1877. [Google Scholar] [CrossRef]
- Carichino, S.; Scanferla, D.; Fico, D.; Rizzo, D.; Ferrari, F.; Jordá-Reolid, M.; Martínez-García, A.; Corcione, C.E. Poly-Lactic Acid-Bagasse Based Bio-Composite for Additive Manufacturing. Polymers 2023, 15, 4323. [Google Scholar] [CrossRef]
- Chiparus, O.I. Bagasse Fiber for Production of Nonwoven Materials. Ph.D. Thesis, Louisiana State University and Agricultural & Mechanical College, Baton Rouge, LA, USA, May 2004. [Google Scholar]
- Aliyu, S.; Bala, M. Brewer’s Spent Grain: A Review of Its Potentials and Applications. African J. Biotechnol. 2011, 10, 324–331. [Google Scholar] [CrossRef]
- Socaci, S.A.; Fărcaş, A.C.; Diaconeasa, Z.M.; Vodnar, D.C.; Rusu, B.; Tofană, M. Influence of the Extraction Solvent on Phenolic Content, Antioxidant, Antimicrobial and Antimutagenic Activities of Brewers’ Spent Grain. J. Cereal Sci. 2018, 80, 180–187. [Google Scholar] [CrossRef]
- McCarthy, A.L.; O’Callaghan, Y.C.; Piggott, C.O.; FitzGerald, R.J.; O’Brien, N.M. Brewers’ Spent Grain; Bioactivity of Phenolic Component, Its Role in Animal Nutrition and Potential for Incorporation in Functional Foods: A Review. Proc. Nutr. Soc. 2013, 72, 117–125. [Google Scholar] [CrossRef]
- Circsyst-Circular Systemic Solutions. Available online: https://circsyst.eu/ (accessed on 20 August 2024).
- Shi, Y.; Yan, C.; Zhou, Y.; Wu, J.; Wang, Y.; Yu, S.; Chen, Y. Chapter 2—Polymer Materials for Additive Manufacturing—Powder Materials. In Materials for Additive Manufacturing; Academic Press: Cambridge, MA, USA, 2021; pp. 9–189. ISBN 978-0-12-819302-0. [Google Scholar]
- Neto, J.S.S.; de Queiroz, H.F.M.; Aguiar, R.A.A.; Banea, M.D. A Review on the Thermal Characterisation of Natural and Hybrid Fiber Composites. Polymers 2021, 13, 4425. [Google Scholar] [CrossRef]
- POLYBONDTM 3200. Available online: https://siigroup.com/products/polybond-3200 (accessed on 4 October 2024).
- POLYBONDTM 3009. Available online: https://siigroup.com/products/polybond-3009 (accessed on 4 October 2024).
- Kim, H.S.; Lee, B.H.; Choi, S.W.; Kim, S.; Kim, H.J. The Effect of Types of Maleic Anhydride-Grafted Polypropylene (MAPP) on the Interfacial Adhesion Properties of Bio-Flour-Filled Polypropylene Composites. Compos. Part A Appl. Sci. Manuf. 2007, 38, 1473–1482. [Google Scholar] [CrossRef]
- XIBONDTM 250 (Viscosity Modifier)—Technical Datasheet. Available online: https://polymer-additives.specialchem.com/product/a-aurorium-xibond-250 (accessed on 4 October 2024).
- Li, Q.; Matuana, L.M. Effectiveness of Maleated and Acrylic Acid-Functionalized Polyolefin Coupling Agents for HDPE-Wood-Flour Composites. J. Thermoplast. Compos. Mater. 2003, 16, 551–564. [Google Scholar] [CrossRef]
- UNE EN 16087-2; Soil Improvers and Growing Media—Determination of the Aerobic Biological Activity—Part 2: Self Heating Test for Compost. Asociación Española de Normalización: Madrid, Spain, 2012.
- UNE EN ISO 527-1:2020; Plastics—Determination of Tensile Properties—Part 1: General Principles. Asociación Española de Normalización: Madrid, Spain, 2020.
- UNE EN ISO 179-1:2010; Plastics—Determination of Charpy Impact Properties—Part 1: Non-Instrumented Impact Test. Española de Normalización: Madrid, Spain, 2010.
- UNE EN ISO 868-1:1997; Packaging Materials and Systems for Medical Devices Which Are to Be Sterilized. Part 1: General Requirements and Test Method. Asociación Española de Normalización: Madrid, Spain, 1997.
- Jawaid, M.; Abdul Khalil, H.P.S. Cellulosic/Synthetic Fibre Reinforced Polymer Hybrid Composites: A Review. Carbohydr. Polym. 2011, 86, 1–18. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, J.; Liang, X.; Huang, Z.; Li, J.; Peng, S. Crystallization Behaviors Regulations and Mechanical Performances Enhancement Approaches of Polylactic Acid (PLA) Biodegradable Materials Modified by Organic Nucleating Agents. Int. J. Biol. Macromol. 2023, 233, 123581. [Google Scholar] [CrossRef]






| Biocomposite Name | Polymer Used | Polymer Percentage (wt%) | BSG Percentage (wt%) | Compatibilising Additive | Additive Quantity (phr) |
|---|---|---|---|---|---|
| BioPE90BSG10 | BioPE | 90 | 10 | POLYBOND 3009 | 3 |
| BioPE80BSG20 | 80 | 20 | |||
| BioPE70BSG30 | 70 | 30 | |||
| PLA90BSG10 | PLA | 90 | 10 | XIBOND 250 | |
| PLA80BSG20 | 80 | 20 | |||
| PLA70BSG30 | 70 | 30 | |||
| PP90BSG10 | PP | 90 | 10 | POLYBOND 3200 | |
| PP80BSG20 | 80 | 20 | |||
| PP70BSG30 | 70 | 30 |
| Temperatures (°C) | Speed (rpm) | ||||||
|---|---|---|---|---|---|---|---|
| Zone 1 (°C) | Zone 2 (°C) | Zone 3 (°C) | Zone 4 (°C) | Zone 5 (°C) | Zone 6 (°C) | ||
| BioPE biocomposites | 40 | 75 | 185 | 195 | 190 | 190 | 75 |
| PLA biocomposites | 40 | 65 | 180 | 185 | 190 | 190 | 65 |
| PP biocomposites | 50 | 65 | 185 | 205 | 220 | 225 | 65 |
| Name | Temperature Profile (°C) | Injection Speed (rpm) | Compaction Time (s) | Compaction Pressure (bar) | Cool-down Time (s) | Loading Speed (s) |
|---|---|---|---|---|---|---|
| BioPE biocomposites | 200-200-190-180-35 | 60 | 10 | 400 | 40 | 70 |
| PLA biocomposites | 190-190-180-170-35 | 60 | 20 | 350 | 50 | 70 |
| PP biocomposites | 195-195-185-180-35 | 70 | 10 | 300 | 40 | 80 |
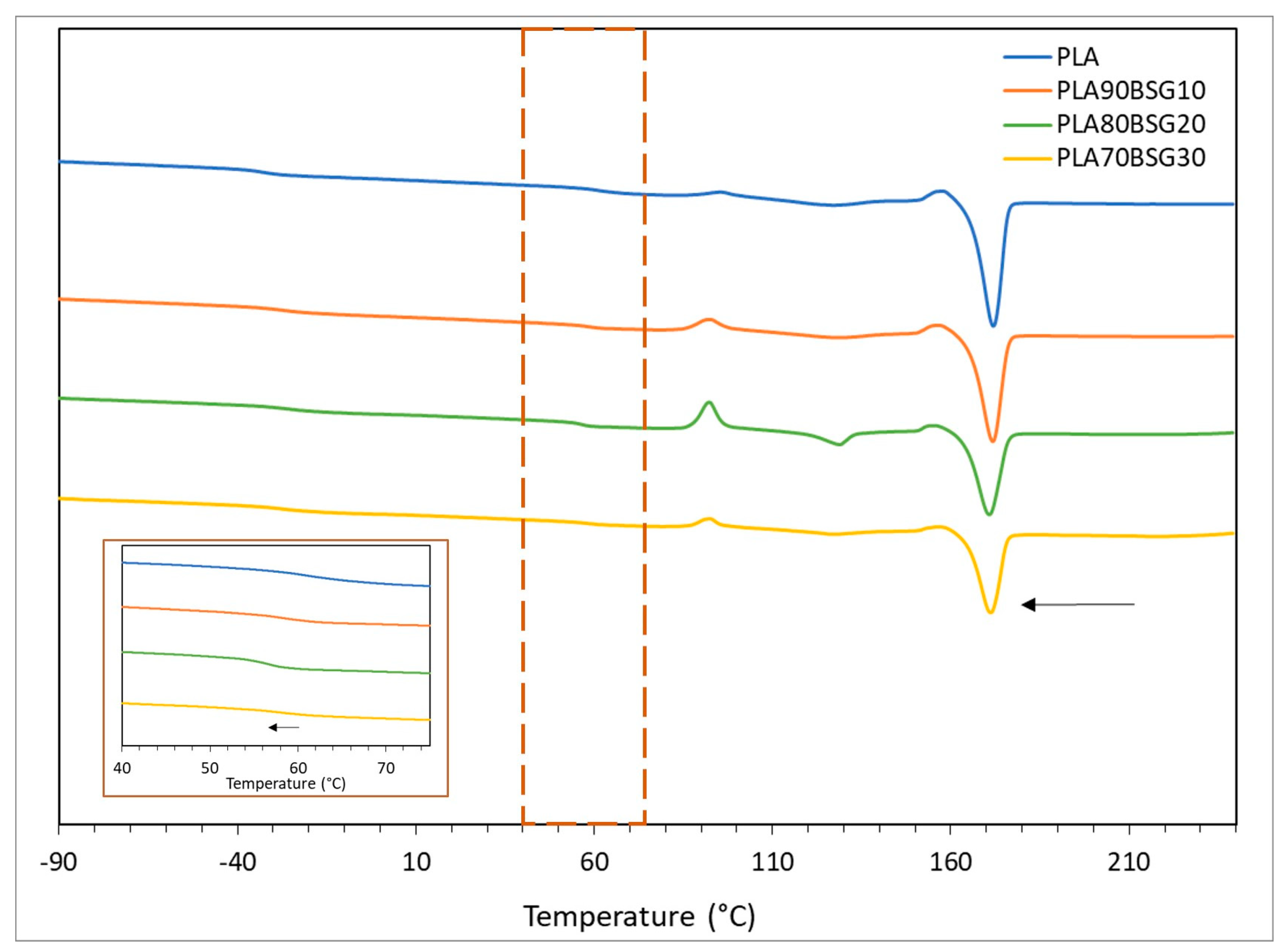
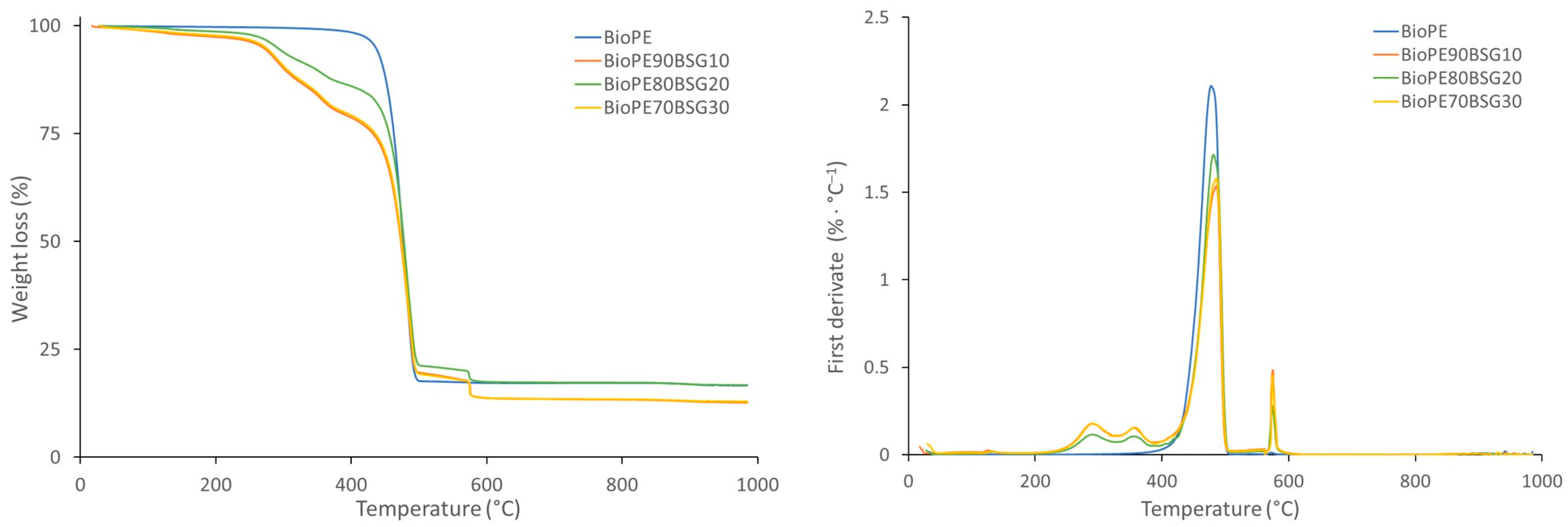
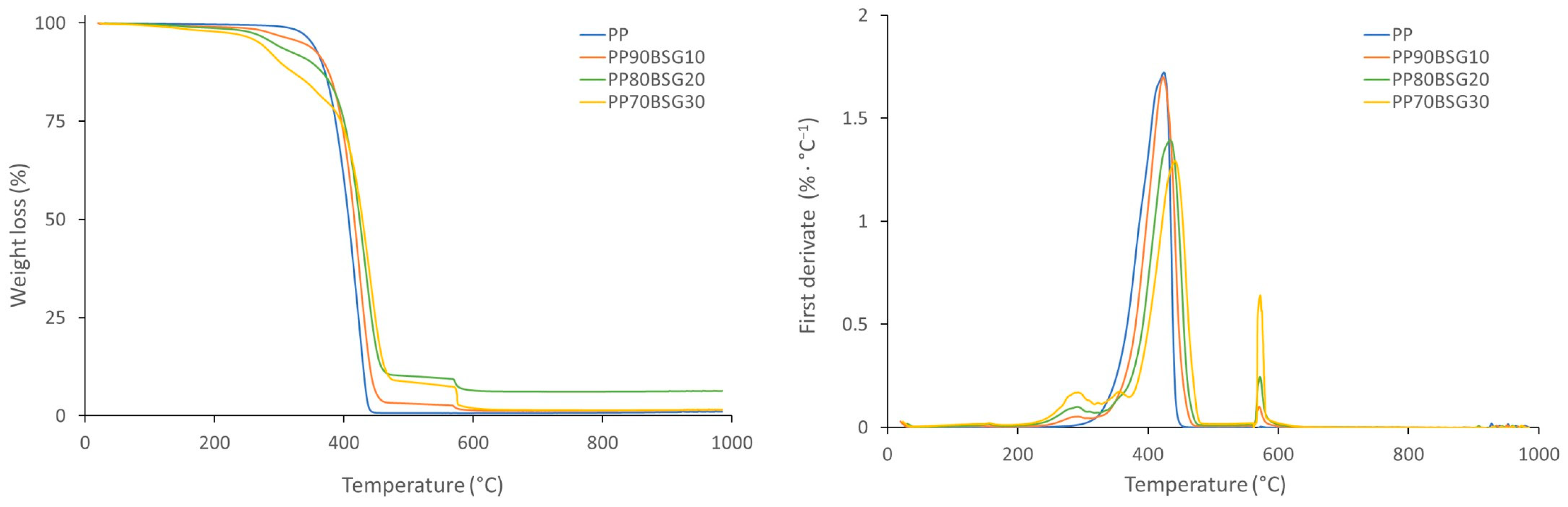
| Materials | Tg (°C) | Tm (°C) | Tonset (°C) | Tdmax (°C) | |
|---|---|---|---|---|---|
| BioPE biocomposites | BioPE HD4527 | - | 132.94 ± 0.31 | 451.82 ± 1.65 | 478.36 ± 0.57 |
| BioPE90BSG10 | - | 131.25 ± 0.14 | 263.81 ± 0.63 | 491.6 ± 4.68 | |
| BioPE80BSG20 | - | 131.39 ± 0.04 | 262.56 ± 0.30 | 480.60 ± 0.23 | |
| BioPE70BSG30 | - | 131.09 ± 0.14 | 264.60 ± 0.53 | 486.14 ± 0.98 | |
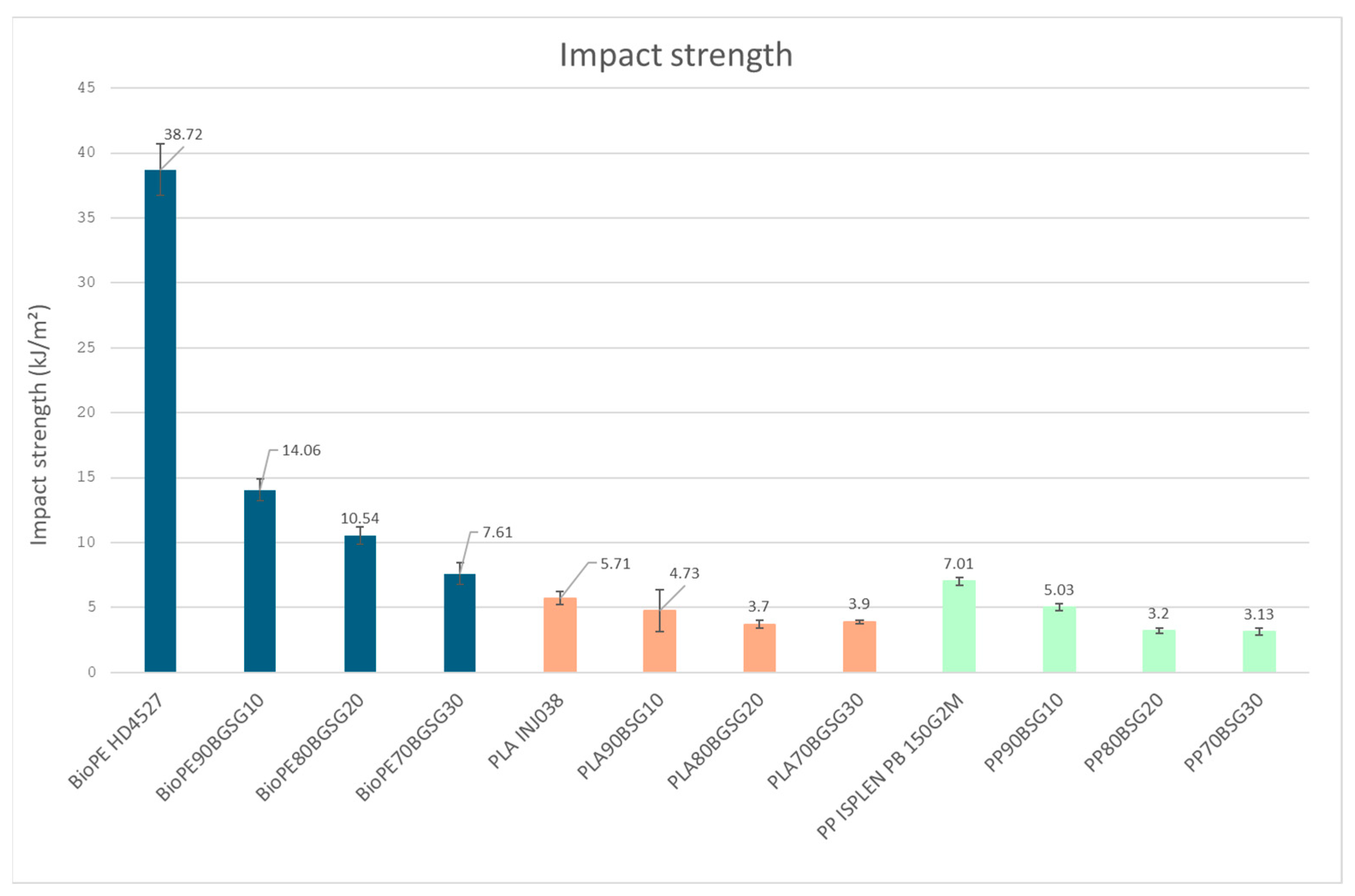
| PLA biocomposites | PLA INJ038 | 62.08 ± 0.61 | 171.99 ± 0.21 | 346.56 ± 2.11 | 368.90 ± 0.27 |
| PLA90BSG10 | 57.22 ± 0.67 | 171.71 ± 0.04 | 340.70 ± 1.99 | 367.34 ± 0.06 | |
| PLA80BSG20 | 57.50 ± 1.65 | 170.79 ±0.13 | 338.33 ± 1.35 | 368.35 ± 0.53 | |
| PLA70BSG30 | 58.33 ± 0.56 | 171.10 ± 0.05 | 261.2 ± 0.75 | 398.17 ± 4.61 | |
| PP biocomposites | PP ISPLEN PB 150G2M | - | 165.31 ± 0.63 | 366.19 ± 3.41 | 423.86 ± 3.00 |
| PP90BSG10 | - | 164.3 ± 0.24 | 379.57 ± 1.16 | 431.94 ± 5.82 | |
| PP80BSG20 | - | 163.58 ± 0.14 | 260.16 ± 3.25 | 433.41 ± 2.31 | |
| PP70BSG30 | - | 163.59 ± 0.09 | 259.54 ± 0.12 | 437.65 ± 4.08 |
| Materials | MFI (g/10 min) | |
|---|---|---|
| BioPE biocomposites | BioPE HD4527 | 15.7 ± 0.108 |
| BioPE90BSG10 | 11.1 ± 0.188 | |
| BioPE80BSG20 | 8.81 ± 0.069 | |
| BioPE70BSG30 | 7.67 ± 0.036 | |
| PLA biocomposites | PLA INJ038 | 33.4 ± 7.04 |
| PLA90BSG10 | 24.3 ± 0.490 | |
| PLA80BSG20 | 20.2 ± 0.13 | |
| PLA70BSG30 | 18.4 ± 0.42 | |
| PP biocomposites | PP ISPLEN PB 150G2M | 5.99 ± 0.03 |
| PP90BSG10 | 7.38 ± 0.14 | |
| PP80BSG20 | 6.35 ± 0.40 | |
| PP70BSG30 | 5.24 ± 0.26 |
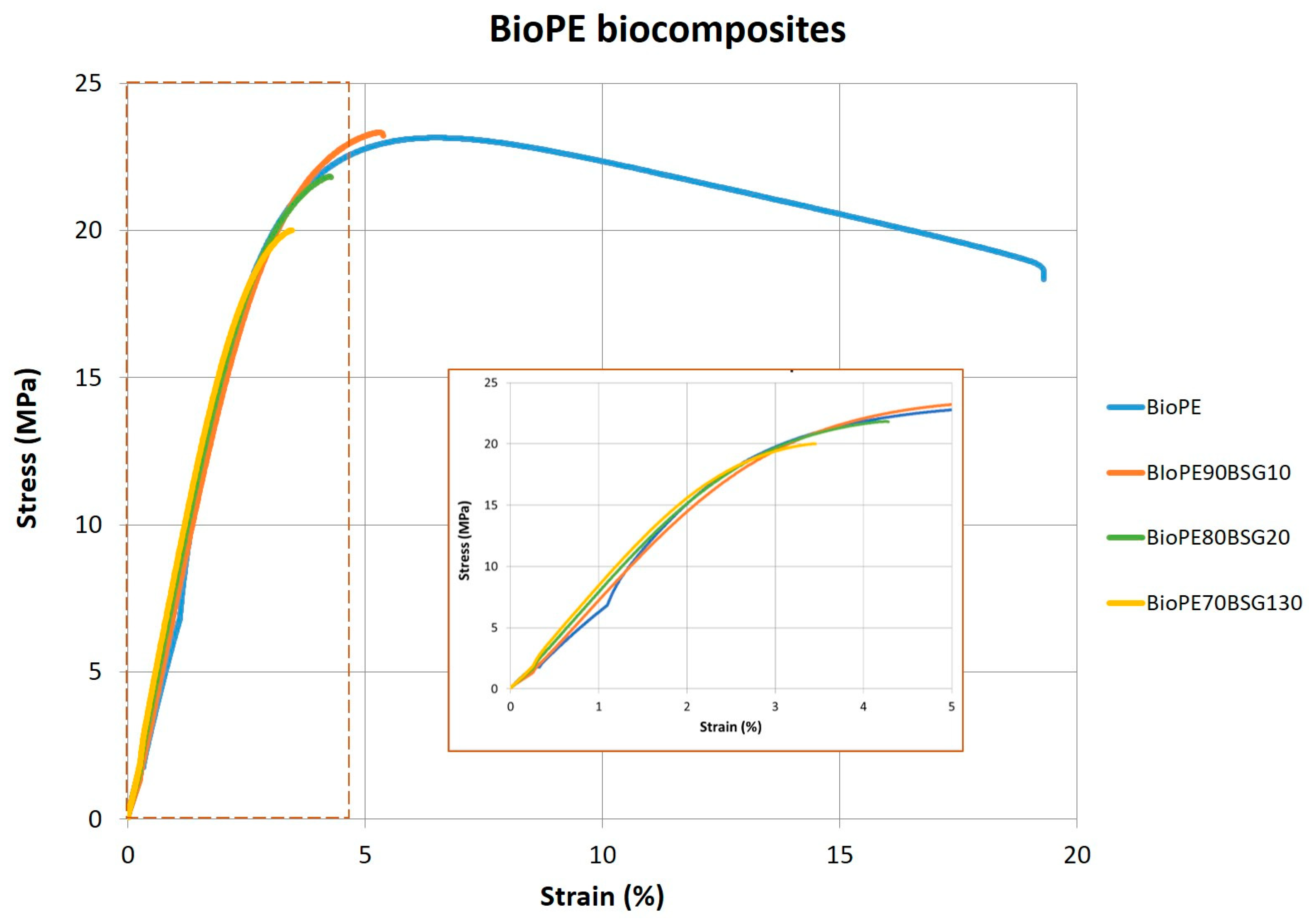
| Tensile Test Results | Hardness Shore D | ||||
|---|---|---|---|---|---|
| Et (MPa) | σm (MPa) | εm (%) | |||
| BioPE biocomposites | BioPE HD4527 | 592 ± 48.4 | 23.0 ± 0.212 | 6.5 ± 0.047 | 62 ± 2 |
| BioPE90BSG10 | 608 ± 87.1 | 24.1 ± 0.429 | 4.9 ± 0.29 | 76 ± 1 | |
| BioPE80BSG20 | 630 ± 30.6 | 21.7 ± 0.406 | 4.2 ± 0.20 | 74 ± 1 | |
| BioPE70BSG30 | 680 ± 17.4 | 19.9 ± 0.238 | 3.5 ± 0.068 | 73 ± 1 | |
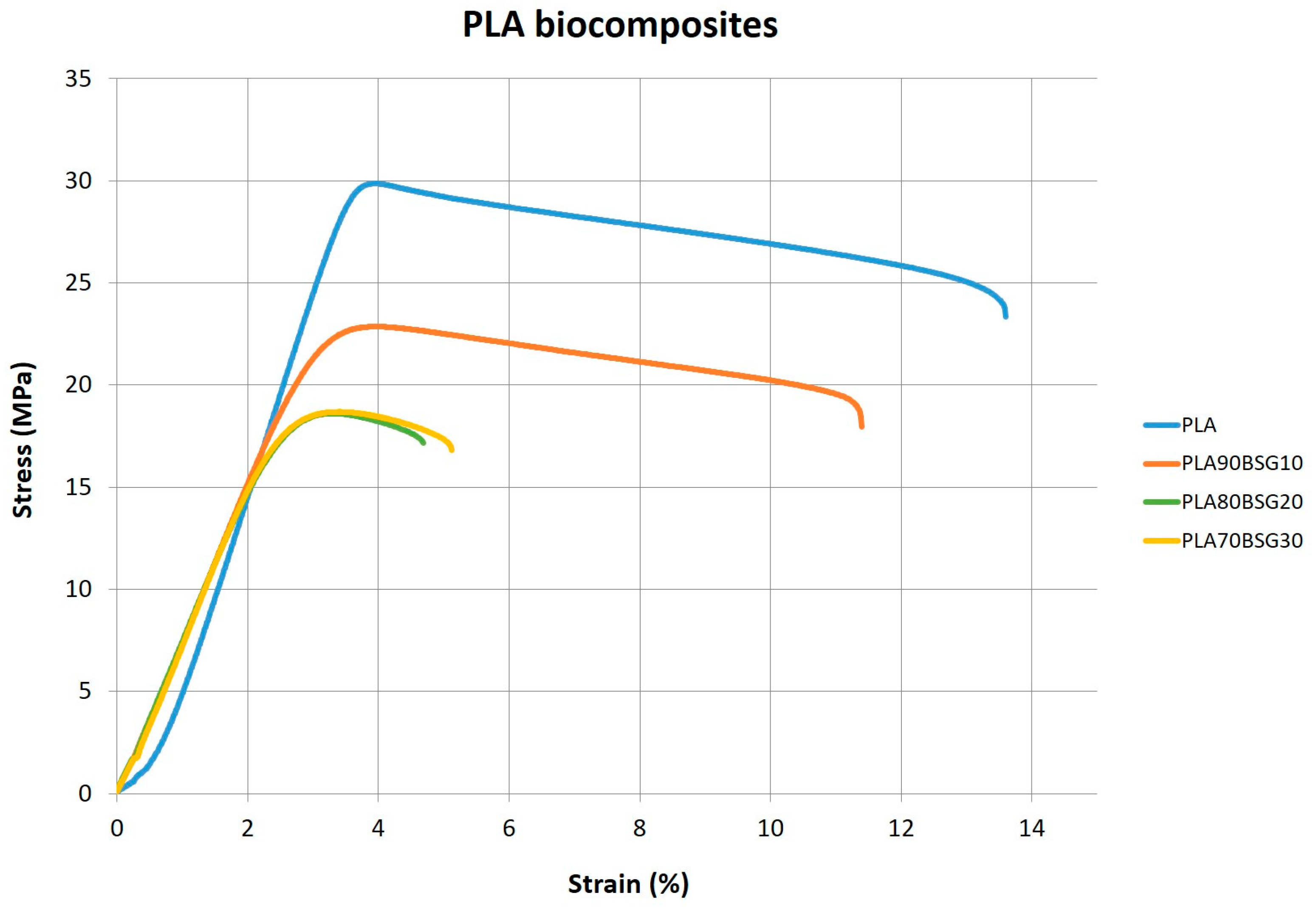
| PLA biocomposites | PLA INJ038 | 825 ± 97,4 | 30.4 ± 0.607 | 3.3 ± 0.13 | 96 ± 1 |
| PLA90BSG10 | 621 ± 30.8 | 23.2 ± 0.0961 | 4.0 ± 0.037 | 79 ± 1 | |
| PLA80BSG20 | 644 ± 6.39 | 18.6 ± 0.423 | 3.3 ± 0.061 | 78 ± 0 | |
| PLA70BSG30 | 635 ± 9.49 | 18.6 ± 0.467 | 3.4 ± 0.051 | 78 ± 1 | |
| PP biocomposites | PP ISPLEN PB 150G2M | 343 ± 27.9 | 21.4 ± 0.434 | 8.1 ± 0.062 | 75 ± 1 |
| PP90BSG10 | 614 ± 6.78 | 22.8 ± 0.206 | 7.2 ± 0.041 | 78 ± 1 | |
| PP80BSG20 | 636 ± 7.69 | 21.8 ± 0.157 | 6.0 ± 0.036 | 80 ± 1 | |
| PP70BSG30 | 677 ± 8.01 | 20.2 ± 0.213 | 4.7 ± 0.041 | 82 ± 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jordá-Reolid, M.; Martínez-García, A.; Ibáñez-García, A.; León-Cabezas, M.Á.; Galvañ-Gisbert, J. Study of Mechanical and Thermal Properties of Environmentally Friendly Composites from Beer Bagasse. Polymers 2024, 16, 2916. https://doi.org/10.3390/polym16202916
Jordá-Reolid M, Martínez-García A, Ibáñez-García A, León-Cabezas MÁ, Galvañ-Gisbert J. Study of Mechanical and Thermal Properties of Environmentally Friendly Composites from Beer Bagasse. Polymers. 2024; 16(20):2916. https://doi.org/10.3390/polym16202916
Chicago/Turabian StyleJordá-Reolid, María, Asunción Martínez-García, Ana Ibáñez-García, Miguel Ángel León-Cabezas, and Josefa Galvañ-Gisbert. 2024. "Study of Mechanical and Thermal Properties of Environmentally Friendly Composites from Beer Bagasse" Polymers 16, no. 20: 2916. https://doi.org/10.3390/polym16202916
APA StyleJordá-Reolid, M., Martínez-García, A., Ibáñez-García, A., León-Cabezas, M. Á., & Galvañ-Gisbert, J. (2024). Study of Mechanical and Thermal Properties of Environmentally Friendly Composites from Beer Bagasse. Polymers, 16(20), 2916. https://doi.org/10.3390/polym16202916







