Abstract
Phenylethynyl-terminated imide (PETI) oligomers are highly valued for their diverse applications in films, moldings, adhesives, and composite material matrices. PETIs can be synthesized at varying molecular weights, enabling the fine-tuning of their properties to meet specific application requirements. Upon thermal curing, these oligomers form super-rigid network structures that enhance solvent resistance, increase glass-transition temperatures, and improve elastic moduli. Their low molecular weights and melt viscosities further facilitate processing, making them particularly suitable for composites and adhesive bonding. This review examines recent advancements in developing ultra-high-temperature PETIs, focusing on their structure–processing–properties relationships. It begins with an overview of the historical background and key physicochemical characteristics of PETIs, followed by a detailed discussion of PETIs synthesized from monomers featuring noncoplanar configurations (including kink and cardo structures), fluorinated groups, flexible linkages, and liquid crystalline mesogenic structures. The review concludes by addressing current challenges in this research field and exploring potential future directions.
1. Introduction
Thermosetting polyimides (PIs) based on phenylethynyl-terminated imide (PETI) oligomers are among the most critical high-performance resin matrices for high-temperature aerospace and aircraft applications [,,]. Upon curing, PI resins form highly rigid network structures through the additional reaction of phenylethynyl groups, imparting exceptional thermo-oxidative stability, mechanical strength, and resistance to heat and radiation [,,].
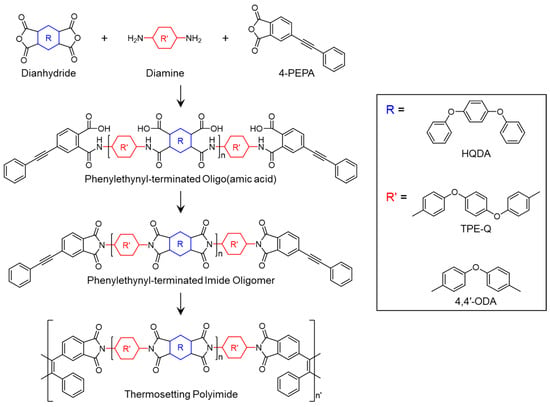
PETIs are commonly synthesized through a conventional two-step procedure and subsequently converted into thermosetting polyimides through thermal curing at approximately 370 °C for 1–2 h [,,]. The synthesis involves the reaction of a dianhydride, diamine monomers, and 4-phenylethynyl phthalic anhydride (PEPA) as an end-capping agent. Consequently, the chemical structures of the dianhydride and diamine monomers, along with the molecular weight of the PETIs, can be adjusted to tailor their properties, enabling the optimization of performance for specific applications [,,,,,]. Due to their exceptional properties, PETIs with diverse structures have been widely studied.
The PETI-5, derived from 3,3′,4,4′-biphenyltetracarboxylic dianhydride (s-BPDA) and developed at NASA Langley Research Center (LaRC), with a targeted molecular weight of 5000 g mol−1, demonstrates an excellent combination of high fracture toughness, heat resistance, and processability []. It exhibits a minimum melt viscosity of 1000 Pa∙s at 371 °C and a cured glass transition temperature (Tg) of 270 °C. Studies by Scola et al. revealed that substituting s-BPDA units with 4,4′-(hexafluoroisopropylidene)-diphthalic anhydride (6FDA) units significantly reduces the melt viscosity with minimal impact on Tg []. The 6FDA-based PETI exhibited a minimum melt viscosity of 48 Pa∙s at 337 °C and a cured Tg of 268 °C [,]. Around the year 2000, Connell et al. [] introduced PETI-298, PETI-330, and PETI-375, synthesized using symmetric or unsymmetric biphenyl dianhydride. These resins exhibited a distinct Tg of 298 °C, 330 °C, and 375 °C, respectively, making them suitable for applications across various service temperatures. Each resin demonstrated low viscosity and excellent melt stability at 280 °C, facilitating resin transfer molding (RTM) processing into composites with robust mechanical properties [,]. Recently, the use of unsymmetric dianhydrides in RTM resin development has increased. Substituting symmetric BPDA units with asymmetric BPDA units enhanced the cured Tg and improved the processability of PI resins [,]. The asymmetric structure of a-BPDA needs more space for molecular motion, requiring more energy, which leads to an increase in the glass transition temperature (Tg). At the same time, this asymmetric and non-planar structure hinder close chain packing, thereby improving processability [,,]. Further advancements include developing PI resins with low melting temperatures using 2,3,3′,4′-oxydiphthalic anhydride (a-ODPA) and 2,3,3′,4′-benzophenone anhydride (a-BTDA) as precursors, with phenylethynyl groups as terminal moieties [,,]. This innovation has significantly advanced research in this field.
The ongoing development of PETI resins with high Tg and low melt viscosities remains a critical focus area. These resins are preferred for their ability to combine desirable properties, including exceptional fracture toughness and resistance to microcracking. This review summarizes recent advances in PETI resins, emphasizing molecular design and the relationships between structure–processing–properties. The insights provided aim to pave the way for further research into high-temperature composite resins, contributing to advancements in the field.
2. PETI Resins with Intrinsic Structure
2.1. Isomeric Structure
Isomers are molecules that share the same chemical formula but exhibit different properties due to variations in the arrangement of atoms or their spatial orientation, and they are classified into two main types: structural isomers and stereoisomers. Structural isomers differ in their bonding and arrangement of atoms, while stereoisomers differ in the spatial orientation of their atoms. Consequently, isomers with the same molecular formula can possess distinct physical and chemical properties. These characteristics have led to extensive studies on polyimides synthesized using isomeric dianhydrides or diamines [,,,]. Generally, using isomeric monomers results in a less ordered and more flexible three-dimensional structure [], enabling the fabrication of processable polyimides without significant loss of thermal stability []. For instance, a PETI based on 2,3,3′,4′-biphenyltetracarboxylic dianhydride (a-BPDA) exhibits a higher Tg than one based on 3,3′,4,4′-biphenyltetracarboxylic dianhydride (s-BPDA). This difference is attributed to the asymmetric structure of a-BPDA, which suppresses internal rotation around the biphenyl linkage compared to s-BPDA. The a-BPDA structure requires a larger sweep volume for crankshaft motion, necessitating more energy (i.e., higher temperature), thereby resulting in a higher Tg. Conversely, isomer-based PETIs such as a-BPDA exhibit enhanced processability. For example, TriA-PI, a PETI based on a-BPDA, exhibited improved solubility and reduced viscosity compared to a PETI based on s-BPDA due to the asymmetric and non-planar structure of a-BPDA [,]. The characteristic charge transfer complex (CTC) in imides is formed through π-electron conjugation in polyimides, where the nitrogen atom acts as the electron donor and the carboxyl group of the dianhydride functions as the electron acceptor [,]. This results in strong inter- and intrachain interactions []. Therefore, the introduction of isomeric structures into the molecular backbone can disrupt the chain packing due to the asymmetric nature of the isomers, reducing the CTC formation and molecular interactions, which ultimately enhance processability []. This section discusses PETIs derived from isomeric monomers. The chemical structures used in the studies presented in this section are shown in Scheme 1.
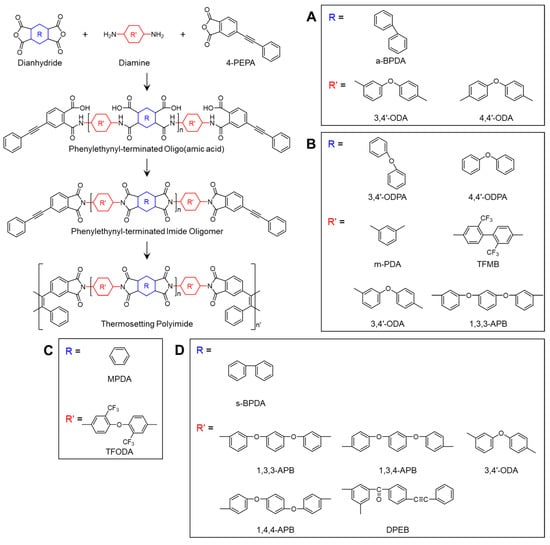
Scheme 1.
Chemical structures of PETIs with isomeric configurations: (A) Oligo-n []; (B) PI-n []; (C) Oligo-n []; (D) Pn and PETI-RTM [].
Yokota et al. [] reported the development of Triple-A PI (Tri-A PI), an amorphous, asymmetric, addition-type PETI based on a-BPDA, 3,4′-ODA, and 4,4′-ODA. Specifically, Oligo-1.5 (a-BPDA/3,4′-ODA;4,4′-ODA (50:50)/PEPA, n = 1.5) demonstrated high solubility in DMAc and exhibited an improved dynamic melt viscosity of 200 Poise at 300 °C. Oligo-10 (n = 10) also showed high solubility in DMAc but exhibited a dynamic melt viscosity of 20,000 Poise at 365 °C. The complex viscosity curves are shown in Figure 1A. Furthermore, while PETI-5 based on s-BPDA exhibited a Tg of 270 °C, the a-BPDA-based Oligo-1.5 and Oligo-10 exhibited higher Tg values of 341 °C and 308 °C, respectively, after curing at 370 °C for 1 h. The thermal properties of PETI oligomers and cured resins are summarized in Table 1. Also, the TGA curve in air atmosphere can be seen in Figure 2A.
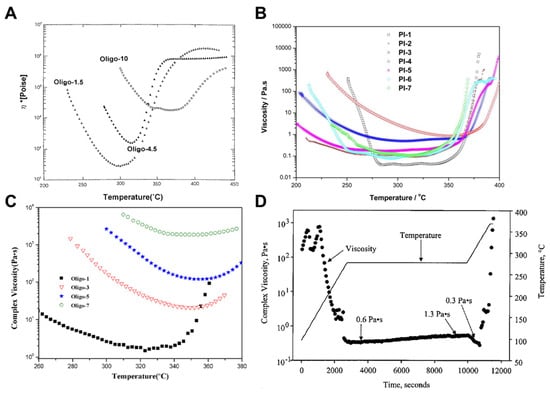
Figure 1.
Complex viscosity curves: (A) Oligo-n (reprinted with permission from [], Copyright 2001, SAGE Publications); (B) PI-n (reprinted with permission from [], Copyright 2015, SAGE Publications); (C) Oligo-n (reprinted with permission from [], Copyright 2018, Wiley); (D) P4 (reprinted with permission from [], Copyright 2002, SAGE Publications).

Table 1.
Thermal properties of PETIs with isomeric structures.
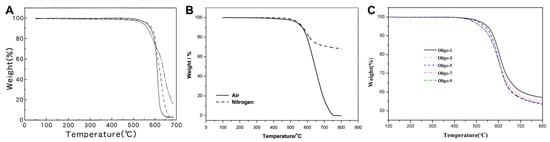
Figure 2.
TGA curves: (A) The fully cured Oligo-n and polyimide in air; Oligo-1.5 (— · —), Oligo-10 (– – –), PI (a-BPDA/3,4′-ODA; 4,4′-ODA) (50:50) (——) (reprinted with permission from [], Copyright 2001, SAGE Publications); (B) Cured PI-6 in air and in nitrogen (reprinted with permission from [], Copyright 2015, SAGE Publications); (C) The fully cured Oligo-n films in nitrogen (reprinted with permission from [], Copyright 2018, Wiley).
Wang et al. [] reported the synthesis and characterization of isomeric PETIs derived from 3,4′-ODPA, an isomer of 4,4′-ODPA. PI-6 (3,4′-ODPA/TFMB;1,3,3-APB (50:50)/PEPA) exhibited a minimum melt viscosity of 0.08 Pa·s at 295 °C, whereas PI-7 (4,4′-ODPA/TFMB;1,3,3-APB (50:50)/PEPA) demonstrated a minimum melt viscosity of 0.11 Pa·s at 312 °C. This difference was attributed to the asymmetric structure of 3,4′-ODPA, which reduces chain linearity and intermolecular bonding. PI-6 maintained a viscosity below 1 Pa·s even after being held at 250 °C for 2 h, indicating its suitability for RTM processing at this temperature, whereas PI-7 exhibited higher viscosity at 250 °C, making it unsuitable for injection molding. The complex viscosity curves are shown in Figure 1B. The Tg of the cured PI-6 and PI-7, measured by DSC and DMA, were similar, at 304 °C and 292 °C, respectively, and 352 °C and 356 °C. Both cured PI thermosets demonstrated high thermal stability, with a 5% weight loss temperature exceeding 540 °C. The TGA curves are shown in Figure 2B. After curing, PI-6 exhibited a tensile strength of 45 MPa and an elongation of 3.38%, while PI-7 showed a tensile strength of 66 MPa and an elongation of 1.86%, suggesting that the 4,4′-ODPA-based cured resins had higher stiffness. The thermal properties of PETI oligomers and cured resins are summarized in Table 1.
Fang et al. [] were the first to report the development of a PETI based on MPDA, an isomer of PMDA. Oligo-5 (MPDA/TFODA/PEPA, n = 5) was completely soluble in both high-boiling-point solvents such as NMP and low-boiling-point solvents, including THF, 1,4-dioxane, and acetone, whereas Oligo-PMDA (PMDA/TFODA/PEPA, n = 5) was insoluble in low-boiling-point solvents. Furthermore, Oligo-5 exhibited a minimum complex melt viscosity of 120 Pa·s at 354 °C, while Oligo-PMDA did not melt. These characteristics were attributed to the bent structure of MPDA, which suppresses molecular aggregation and increases free volume. The complex viscosity curves are shown in Figure 1C. After curing, Oligo-5 demonstrated excellent thermal stability with a high Tg of 373 °C and a Td5% of 539 °C, attributed to the rigidity of MPDA. These results are presented in Table 1 and Figure 2C. It also exhibited outstanding mechanical properties, including a tensile strength of 80 MPa, a tensile modulus of 1.9 GPa, and an elongation of 7.1%.
Smith et al. [], at NASA Langley Research Center, reported PETIs synthesized using s-BPDA and the isomeric diamines 1,3,3-APB, 1,3,4-APB, and 1,4,4-APB. Before curing, the Tg of PETI-RTM (s-BPDA/1,3,3-APB; 3,4′-ODA (75:25)/PEPA) and P4 (s-BPDA/1,3,4-APB; 3,4′-ODA (75:25)) were 132 °C and 139 °C, respectively. After curing, the Tg values increased to 258 °C for PETI-RTM and 298 °C for P4, while P5 (s-BPDA/1,4,4-APB; 3,4′-ODA (75:25)/PEPA) did not exhibit a Tg in the DSC analysis. These results suggest that Tg increases with the rigidity of the APB isomer (1,4,4-APB > 1,3,4-APB > 1,3,3-APB). The thermal properties of PETI oligomers and cured resins are summarized in Table 1. The melt viscosities of PETIs based on 1,3,3-APB and 1,3,4-APB showed no significant difference; however, the melt viscosity of P5, based on 1,4,4-APB, was five times higher than that of P4, based on 1,3,4-APB. The complex viscosity curves are shown in Figure 1D.
2.2. Noncoplanar Structures (Kink, Spiro, and Cardo Structures)
2.2.1. Kink Structure
The kink structure can be described as a crank and twisted non-planar configuration [], which distorts polymer chains, reducing packing density and increasing chain flexibility []. This distortion partially hinders the formation of charge transfer complexes (CTCs) within or between molecules, allowing for the formation of colorless polyimide films with high transparency and improved solubility []. Similar strategies involving kink structures in PETIs have been explored. The chemical structures used in the study presented in this section are shown in Scheme 2.
Scheme 2.
Chemical structures of PETIs with kinked configurations: PETI, PE-3F, and PE-6F oligoimides [].
Scola et al. [] reported PETIs featuring kink structures based on 3FDA. PE-3F-PETI-5K (3FDA/3,4′-ODA; APB (85:15)/PEPA) and PETI-5K (s-BPDA/3,4′-ODA; APB (85:15)/PEPA) exhibited Tg of 218 °C and 225 °C, respectively. Upon curing, the Tg values increased to 272 °C and 270 °C, which are not significantly different from those of the uncured PETIs. However, while PE-3F-PETI-5K did not display a melt temperature (Tm), PETI-5K showed a Tm of 349 °C, suggesting that the PETI based on 3FDA possesses a less ordered structure with greater molecular freedom and weaker intermolecular interactions, resulting in an amorphous structure. The thermal properties of PETI oligomers and cured resins are summarized in Table 2. The calculated dihedral angles at the 1,1′ positions of 3FDA and s-BPDA were 84.7° and 42.6°, respectively, indicating a larger degree of out-of-plane twisting for 3FDA and leading to greater intermolecular distances in PE-3F-PETI-5K. At 310 °C, PETI-5K exhibited an initial viscosity of 20,759 Pa·s, which increased to 412,205 Pa·s after 60 min of isothermal treatment at the same temperature. By contrast, PE-3F-PETI-5K showed a significantly lower initial viscosity of 321 Pa·s, rising only to 527 Pa·s after identical treatment, indicating much higher melt stability. The complex viscosity curves are shown in Figure 3A. The difference in melt stability is associated with the electronic environment around the phenylethynyl end groups. The curing reaction of phenylethynyl occurs through thermally induced free radical initiation and propagation. Since radicals can be destabilized by the inductive effect of electron-withdrawing groups, these groups hinder the initiation step of ethynyl bond cleavage. Consequently, at 310 °C, the 3FDA and 6FDA units significantly slow down the crosslinking reaction due to the suppression of initial bond cleavage and radical formation. As a result, PE-3F-PETI-5K exhibits higher melt stability, with a reduced crosslinking rate at the minimum melt viscosity temperature, due to the stabilization of the phenylethynyl end groups. Additionally, the TGA curves in nitrogen atmosphere are presented in Figure 3B.

Table 2.
Thermal properties of PETIs with kink structures.
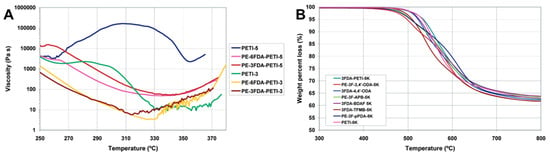
Figure 3.
(A) Viscosity curves of PE-3F oligoimides; (B) TGA curves of PE-3F oligoimides in nitrogen (reprinted with permission from [], Copyright 2003, American Chemical Society).
2.2.2. Cardo Structure
The term “cardo”, derived from Latin meaning hinge or ring, refers to a structure in which a cyclic unit is integrated into the main polymer chain [,]. This structure is similar to a spiro configuration; however, unlike spiro, the cardo structure features only one ring connected to the main chain through a bridging unit or central group. By contrast, the spiro structure consists of two rings that are connected orthogonally through a single common atom []. Due to its twisted configuration, the cardo structure hinders chain rotation and loosens chain packing, leading to increased free volume [,,]. Introducing a cardo group into the polymer backbone is a proven method for enhancing solubility []. Additionally, the rigid nature of the cardo structure contributes to a high Tg and excellent thermal stability []. This study presents PETIs with cardo structures. The chemical structures used in the studies presented in this section are shown in Scheme 3.
Scheme 3.
Chemical structures of PETIs with cardo configurations: (A) o-BAFL-n []; (B) Oligo-n []; (C) PI oligomer/Cardo-HPI blends []; (D) B-O-n, F-O-n, B-T-n, F-T-n, B-P-n, and F-P-n [].
Yokota et al. [] reported on PETIs based on BAFL, incorporating fluorenylidene groups with bulky and rigid cardo structures. The PETI designated as o-BAFL-0 (Tri-A) (a-BPDA/4,4′-ODA/PEPA) exhibited 20 wt.% solubility in NMP, with gelation occurring at a 30 wt.% NMP solution. By contrast, o-BAFL-50 (a-BPDA/BAFL; 4,4′-ODA (50:50)/PEPA) demonstrated enhanced solubility, showing 40 wt.% solubility in NMP at room temperature and maintaining a homogeneous solution even after two months in a 30 wt.% NMP solution, attributed to the introduction of BAFL. The Tg values and minimum melt viscosity of the uncured PETIs increased with the content of the rigid BAFL. o-BAFL-0 exhibited a Tg of 218 °C and a minimum melt viscosity of 81 Pa·s at 344 °C, while o-BAFL-50 had a Tg of 273 °C and a minimum melt viscosity of 1810 Pa·s at 349 °C. The complex viscosity curves are shown in Figure 4A. After curing, o-BAFL-0 showed a Tg of 340 °C and a Td5% of 556 °C under argon, whereas o-BAFL-50 exhibited a Tg of 362 °C and a Td5% of 561 °C. Thermal properties of PETI oligomers and cured resins are summarized in Table 3. The mechanical properties of the cured resin films revealed that o-BAFL-0 and o-BAFL-50 had tensile moduli of 2.55 GPa and 2.65 GPa, strengths at break of 118 MPa and 112 MPa, and elongations at break of 15.5% and 6.9%, respectively. These trends were attributed to the increased rigidity of the fluorene groups.
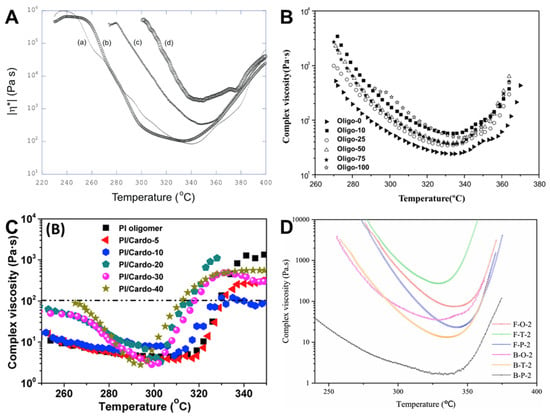
Figure 4.
Complex viscosity curves: (A) TriA-PI and o-BAFL series; ((a) TriA-PI (o-BAFL-0), (b) o-BAFL-10, (c) o-BAFL-25, (d) o-BAFL-50) (reprinted with permission from [], Copyright 2006, SAGE Publications); (B) Oligo-n (reprinted with permission from [], Copyright 2018, Springer); (C) PI oligomer/Cardo-HPI blends (reprinted with permission from [], Copyright 2019, Elsevier); (D) F-O-2, F-T-2, F-P-2, B-O-2, B-T-2, and B-P-2 (reprinted with permission from [], Copyright 2023, Elsevier).

Table 3.
Thermal properties of PETIs with cardo structures.
Fang et al. [] introduced PETIs incorporating DAPI, a rigid structure featuring four side groups: three methyl groups and one phenyl group, compared to BAFL, which contains two phenyl groups. Oligo-0 (m-TDPA/4,4′-ODA/PEPA) was highly soluble in high-boiling-point solvents such as DMAc and NMP but only partially soluble in low-boiling-point solvents like THF, CHCl3, and 1,4-dioxane. By contrast, Oligo-25 (m-TDPA/DAPI; 4,4′-ODA (25:75)/PEPA) was soluble in both high- and low-boiling-point solvents, attributed to the asymmetric and non-planar structure of DAPI. The minimum melt viscosity of Oligo-0 was 24.1 Pa·s at 334 °C, whereas Oligo-25 exhibited a higher viscosity of 34.7 Pa·s at 331 °C. Despite this, both minimum melt viscosities were below 60 Pa·s around 330 °C, indicating the difference was insignificant. The complex viscosity curves are shown in Figure 4B. The Tg value of Oligo-0 was 190 °C, whereas Oligo-25 showed an increased Tg of 205 °C, reflecting the rise in DAPI content. The cured resins of Oligo-0 and Oligo-25 exhibited Tg of 304 °C and 322 °C, respectively. The thermal properties of PETI oligomers and cured resins are summarized in Table 3. However, their Td5% values were 523 °C and 497 °C, indicating a trend of decreased thermal stability with increased DAPI content due to the degradation of aliphatic rings. The TGA curves are shown in Figure 5A. In terms of mechanical properties, Oligo-0 demonstrated a modulus of 1.8 GPa, strength of 90 MPa, and elongation at break of 10.7%, while Oligo-25 exhibited a higher modulus of 2.2 GPa but lower strength of 69 MPa and elongation of 4.8%.
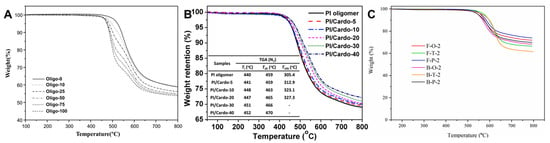
Figure 5.
TGA curves: (A) Oligo series in nitrogen (reprinted with permission from [], Copyright 2018, Springer); (B) Cured PI/Cardo-HPI thermosets in nitrogen (reprinted with permission from [], Copyright 2019, Elsevier); (C) Thermosetting polyimides in nitrogen (reprinted with permission from [], Copyright 2023, Elsevier).
Zhang et al. [] reported the development of new crosslinked polyimide thermosets by blending BisAHPF-based 9,9-bis(3-(3-phenylethynyl)phthalimide-4-hydroxyphenyl)fluorene (Cardo-HPI) as a diluent into PETIs. Compared to the pristine PETI (PI oligomer), the Tg of PI/Cardo-40, with Cardo-HPI content increased to 40 wt.%, decreased from 193 °C to 162 °C, indicating that Cardo-HPI functions as a low molecular weight plasticizer. The introduction of Cardo-HPI also elevated the phenylethynyl concentration per unit mass of the blend, prompting an earlier onset of the crosslinking reaction. The minimum melt viscosity of Cardo-HPI was 1.65 Pa·s at 268 °C, lower than that of the PI oligomer, which was 3.8 Pa·s at 306 °C. This reduction was attributed to the low molecular weight and increased free volume induced by the fluorene group. Consequently, the minimum melt viscosity of PI/Cardo-40 decreased to 2.7 Pa·s at 294 °C. The complex viscosity curves are shown in Figure 4C. However, the blend exhibited higher viscosity in the high-temperature region than the PI oligomer, likely due to the earlier onset of crosslinking and the thermal rearrangement of the ortho-hydroxy imide unit into a rigid benzoxazole group. After curing, the Tg, crosslinking density, CTE, and Td5% of the PI oligomer were 264 °C, 1.6 × 103 mol/cm3, 37.3 ppm/°C, and 459 °C, respectively. By contrast, PI/Cardo-40 exhibited a Tg of 403 °C, crosslinking density of 27.1 × 103 mol/cm3, CTE of 28.1 ppm/°C, and Td5% of 470 °C. These improvements were attributed to the uniform dispersion and rigid structure of Cardo-HPI. The TGA curves are shown in Figure 5B and thermal properties of PETI oligomers and cured resins are summarized in Table 3.
Wang et al. [] reported PETIs based on BPAF, a fluorene-containing dianhydride monomer. The B-O-2 (3,4′-BPDA/4,4′-ODA/PEPA) exhibited a Tg of 181 °C, while F-O-2 (BPAF/4,4′-ODA/PEPA) showed a higher Tg of 213 °C, attributed to the more bent and rigid structure of BPAF. F-O-2 was soluble in various organic solvents at room temperature, whereas B-O-2 was only partially soluble upon heating due to the cardo structure of BPAF disrupting the compact chain packing. The minimum melt viscosity of F-O-2 was 74 Pa·s at 339 °C, whereas B-O-2 exhibited a lower value of 35 Pa·s at 328 °C, attributed to the higher molecular weight and rigid, sterically hindered structure of BPAF. The thermal properties of PETI oligomers are summarized in Table 3. And the complex viscosity curves are shown in Figure 4D. After curing, B-O-2 exhibited a Tg of 383 °C and a Td5% of 547 °C, while F-O-2 showed a higher Tg of 408 °C and Td5% of 559 °C, indicating enhanced thermal stability due to the rigidity of BPAF. The TGA curves are shown in Figure 5C and thermal properties of cured resins are summarized in Table 3. BPAF-based polyimides exhibited lower tensile properties compared to previously reported PETI-5 and TriA-PI (tensile strength >100 MPa and elongation at break >13%) due to their high crosslinking density. However, cured F-O-2 demonstrated superior flexural strength of 166 ± 7 MPa compared to 132 ± 5 MPa for cured B-O-2, reflecting better flexural properties owing to the higher chain stiffness of BPAF-based polyimides compared to 3,4′-BPDA-based polyimides.
2.2.3. Asymmetric Structure
Introducing bulky substituent pendants into monomers can synthesize polymers with asymmetric and non-planar structures, reducing intramolecular and intermolecular packing, decreasing melt viscosity and enhancing solubility [,]. Consequently, irregular and bulky substituent monomers can improve the processability of PETIs without significantly compromising backbone rigidity []. Studies on PETIs derived from asymmetric monomers are presented. The chemical structures used in the studies presented in this section are shown in Scheme 4.
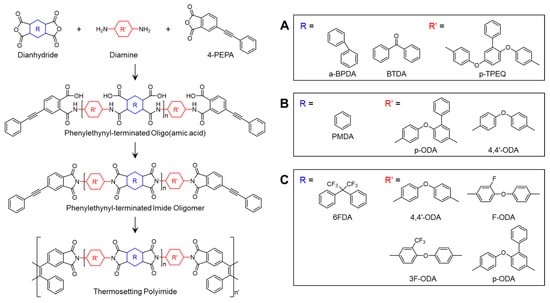
Scheme 4.
Chemical structures of PETIs with asymmetric configurations: (A) Oligo-s–a1–9 and Oligo-bt–a1–9 []; (B) PMDA/p-ODA/PEPA imide oligomer []; (C) PETI-H, PETI-F, PETI-3F, and PETI-P [].
Chen et al. [] reported PETIs based on the irregular and bulky substituted monomer p-TPEQ. The Tg of Oligo-s–a1 (s-BPDA/p-TPEQ/PEPA, n = 1) and Oligo-s–a4 (n = 4) were 161.5 °C and 190 °C, respectively. Both PETIs exhibited solubility in DMF at 1 wt.%, while in NMP, Oligo-s–a1 was soluble at 5 wt.% and Oligo-s–a4 at 1 wt.%. The low minimum melt viscosities of Oligo-s–a1 and Oligo-s–a4 were 5 Pa·s at 260 °C and 93 Pa·s at 343 °C, respectively, due to the pendant phenyl groups and diether linkages of p-TPEQ. The complex viscosity curves are shown in Figure 6A. After curing, the Tg and Td5% values were 305 °C and 506 °C for Oligo-s–a1, and 272 °C and 530 °C for Oligo-s–a4. The thermal properties of PETIs are shown in Table 4. Notably, cured Oligo-s–a4 displayed excellent mechanical properties with a strength of 140 MPa, a modulus of 3.4 GPa, and an elongation of 8.8%.
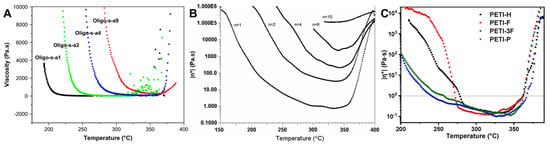
Figure 6.
Complex viscosity curves: (A) Oligo-s–a1–9 (reprinted with permission from [], Copyright 2006, Elsevier); (B) Uncured PMDA/p-ODA/PEPA imide oligomers (n = 1–10) (reprinted with permission from [], Copyright 2012, Elsevier); (C) PETI-H, PETI-F, PETI-3F, and PETI-P (reprinted with permission from [], Copyright 2023, Elsevier).

Table 4.
Thermal properties of PETIs with asymmetric structures.
Yokota et al. [] reported on PETIs based on the asymmetric monomer p-ODA. The PETI derived from PMDA/4,4′-ODA/PEPA was insoluble in NMP and had no observable Tg below 370 °C, while the PETI derived from PMDA/p-ODA/PEPA (n = 1) exhibited over 33 wt.% solubility in NMP, a Tg of 152 °C, and a minimum melt viscosity of 1 Pa·s. By contrast, the PMDA/p-ODA/PEPA (n = 4) exhibited similar solubility, with a Tg of 226 °C and a minimum melt viscosity of 208 Pa·s. The complex viscosity curves are shown in Figure 6B and the thermal properties of PETI oligomers are summarized in Table 4. These findings suggest that PMDA/p-ODA/PEPA-based PETIs possess superior processability compared to conventional PMDA-based PETIs, likely due to the random head-to-tail, head-to-head, and tail-to-tail repeating units that disrupt intermolecular interactions within planar and rigid phenyl-pyromellitimide-phenyl structures. The pendant phenyl group in p-ODA restricts the rotation of the ether bond in 4,4′-ODA and limits both intra- and intermolecular interactions due to its bulky and non-planar structure. The cured resins demonstrated excellent thermal stability, with Tg and Td5% values of 356 °C and 528 °C for PMDA/p-ODA/PEPA (n = 1) and 346 °C and 539 °C for PMDA/p-ODA/PEPA (n = 4), along with average elongation at break (εb,ave) values of 9.6% and 15.7%, respectively. The thermal properties of cured resins are summarized in Table 4. The high εb,ave values indicate that the curing reaction mechanism of PEPA was primarily a chain extension rather than cyclization or crosslinking.
Yang et al. [] studied PETIs based on 6FDA and asymmetric 4,4′-diaminodiphenyl ether substituted with fluorine (F-ODA), trifluoromethyl group (3F-ODA), or phenyl group (p-ODA), employing an expanded set of diamines. Among these, PETI-P (6FDA/p-ODA/PEPA) did not exhibit melt behavior. The Tm of PETI-H (6FDA/4,4′-ODA/PEPA), PETI-F (6FDA/F-ODA/PEPA), and PETI-3F (6FDA/3F-ODA/PEPA) decreased in the order PETI-H > PETI-F > PETI-3F. As the volume of the pendant group increased, close packing and intermolecular interactions were reduced, leading to decreased crystallinity and Tm. However, PETI-P exhibited a higher Tg (140 °C) than PETI-3F (133 °C) due to the larger phenyl group, which caused increased steric hindrance and hindered internal rotation of the backbone. The thermal properties of PETI oligomers and cured resins are summarized in Table 4. PETI-H exhibited partial solubility in most organic solvents due to its symmetric backbone, whereas PETI-3F and PETI-P were soluble in both high-boiling solvents like NMP and low-boiling solvents such as THF and dioxane, attributed to reduced intermolecular interactions and close packing from the introduction of asymmetric units. Consequently, the temperature scale |η*| < 1 Pa·s (T|η*| ≤ 1 Pa·s) was broader for the amorphous forms with lower Tm, with PETI-3F ranging from 241°C to 366 °C and PETI-P from 257 °C to 363 °C, compared to PETI-H (279–361 °C) and PETI-F (275–361 °C), which exhibited lower minimum melt viscosities. The fractional free volume (FFV) of the PETIs increased in the order PETI-P < PETI-H < PETI-F < PETI-3F, influenced by the space-filling effect of the phenyl group within the interstitial chain space. The melt stability of PETI-H, PETI-F, PETI-3F, and PETI-P, evaluated at 280 °C for 4 h, were in the range of 0.36–26.3, 0.20–1.62, 0.36–3.74, and 0.49–323 Pa·s, respectively. The complex viscosity curves are shown in Figure 6C. PETI-H and PETI-P exhibited relatively low melt stability, possibly due to the rigid chains. After curing, PETI-H had the highest Tg of 463 °C, while PETI-P had the lowest Tg of 429 °C, likely due to differences in the chain packing. All cured resins demonstrated excellent thermal stability, with Td5% values exceeding 553 °C. The TGA curves are shown in Figure 7.
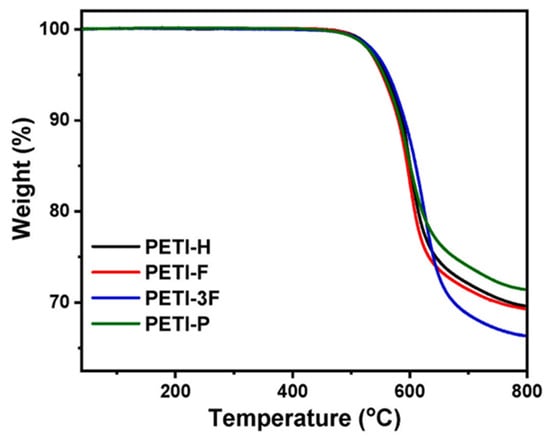
Figure 7.
TGA curves: Cured PETI-H, PETI-F, PETI-3F, and PETI-P resins (reprinted with permission from [], Copyright 2023, Elsevier).
2.3. Fluorinated Units in Main Chains
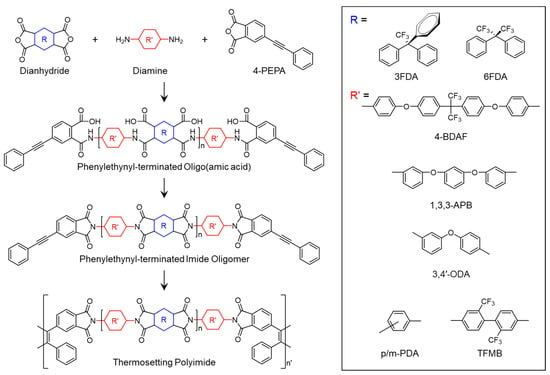
Introducing bulky substituents such as trifluoromethyl groups (–CF3) into polymer backbones significantly influences the polymers’ physical properties by increasing packing distance and free volume, which reduces intermolecular interactions and results in lower melt viscosity and improved solubility [,,]. This section presents PETIs with fluorinated units in their main chains. The chemical structures used in the studies presented in this section are shown in Scheme 5.
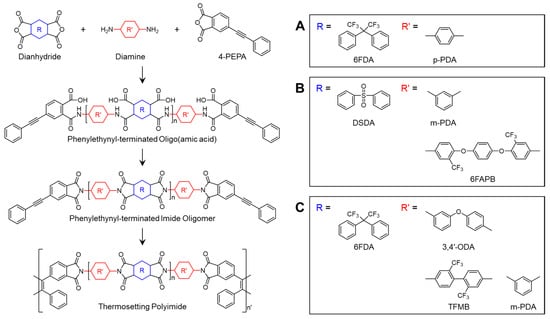
Scheme 5.
Chemical structures of PETIs with fluorinated units in main chains: (A) AFR-PEPA-N []; (B) PI-n []; (C) PETI-O, PETI-F, and PETI-P [].
Morgan et al. [] reported PETIs based on 6FDA incorporating the (–C(CF3)2–) group. They synthesized AFR-PEPA-2 (6FDA/p-PDA/PEPA, n = 2) and AFR-PEPA-8 (n = 8), with Tg values of 194 °C and 260 °C, respectively. AFR-PEPA-2 exhibited a Tm of 323 °C, whereas AFR-PEPA-8 showed no Tm. Both PETIs displayed excellent thermal stability, with Td5% of 566 °C for AFR-PEPA-2 and 557 °C for AFR-PEPA-8, exceeding the Td5% values of traditional PETI-5 and 6FDA-modified PETI by over 50 °C. The thermal properties of PETI oligomers and cured resins are summarized in Table 5. Comparatively, PETI-5, with molecular weights of 1250, 2500, and 5000 g/mol, exhibited minimum complex melt viscosities of 5 Pa·s at 335 °C, 90 Pa·s at 335 °C, and 1000 Pa·s at 370 °C, respectively. AFR-PEPA-2, with a calculated number-average molecular weight (Mn) of 1601 g/mol, demonstrated a melt viscosity of 10 Pa·s at 340 °C, while AFR-PEPA-8, with an Mn of 4699 g/mol, exhibited a melt viscosity of 227 Pa·s at 371 °C, values similar to or lower than those of PETI-5. The reduced melt viscosity of AFR-PEPA-2 and AFR-PEPA-8 was attributed to weaker intermolecular interactions and increased backbone flexibility due to the bulky –CF3 groups in the 6FDA structure. Upon curing at 360 °C, AFR-PEPA-2 showed a Tg of 370 °C, a Young’s modulus of 3.67 GPa, a tensile strength of 50.2 MPa, and a failure strain of 1.5%. When cured at 390 °C, these values improved to a Tg of 382 °C, a Young’s modulus of 3.35 GPa, a tensile strength of 63.9 MPa, and a failure strain of 2.2%, indicating excellent mechanical properties, likely due to the elimination of network defects from unreacted groups.

Table 5.
Thermal properties of PETIs with fluorinated units in main chains.
Fan et al. [] reported PETIs based on the 6FAPB diamine containing trifluoromethyl groups (–CF3). The synthesized PETIs were designated as PI-1 (DSDA/m-PDA/PEPA), PI-2 (DSDA/m-PDA;6FAPB(75:25)/PEPA), and PI-6 (DSDA/6FAPB/PEPA). PI-1 was soluble only in highly polar solvents such as NMP and DMF, while PI-6 exhibited solubility in less polar solvents like DMSO, THF, and even non-boiling solvents such as acetone and chloroform. The minimum melt viscosity of PI-1 was 4545 Pa·s at 361 °C, whereas PI-6, which contains a flexible ether linkage and bulky trifluoromethyl substituents, demonstrated a significantly lower minimum melt viscosity of 128 Pa·s at 318 °C. The complex viscosity curves are shown in Figure 8A. This reduction in viscosity was attributed to the flexible ether bridge structure and the bulky trifluoromethyl groups, which disrupt compact chain packing and reduce polymer density. The Tg of PI-2 and PI-6 were 207 °C and 199 °C, respectively. Upon curing, PI-2 exhibited a Tg of 305 °C, as measured by DSC, while the Tg of cured PI-6 was unobservable. As the mole fraction of 6FAPB increased, the Tg of the PETIs decreased. The Td5% of cured PI-2 and PI-6 were 530 °C and 533 °C, respectively, demonstrating excellent thermal stability. The high thermal stability of 6FAPB, despite its flexible structure, is likely related to the presence of trifluoromethyl groups. The thermal properties of PETI oligomers and cured resins are summarized in Table 5.
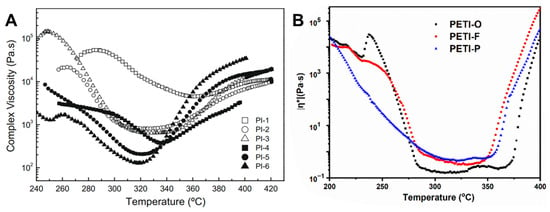
Figure 8.
Complex viscosity curves: (A) PI-n (reprinted with permission from [], Copyright 2006, Elsevier); (B) PETI-O, PETI-F, and PETI-P (reprinted with permission from [], Copyright 2021, MDPI).
Yang et al. [] reported on PETIs based on 6FDA containing the (–C(CF3)2–) group, in combination with 3,4′-ODA, m-PDA, and TFDB (substituted with –CF3 groups). The Tg of PETI-O (6FDA/3,4′-ODA/PEPA), PETI-F (6FDA/TFDB/PEPA), and PETI-P (6FDA/m-PDA/PEPA) were 157 °C, 170 °C, and 158 °C, respectively. PETI-F had the highest Tg, likely due to the rigidity and linearity introduced by the –CF3 substituted biphenyl structure. PETI-O and PETI-F exhibited clear melting endotherms, whereas PETI-P did not exhibit any melting behavior. The thermal properties of PETI oligomers and cured resins are summarized in Table 5. PETI-F showed unique crystalline properties, with PETI-O displaying clear diffraction peaks and PETI-P exhibiting a broad amorphous peak in wide-angle X-ray diffraction (WXRD) patterns, suggesting that the bent chain structure introduced by m-PDA reduced chain packing and crystallinity. The minimum melt viscosities of PETI-O, PETI-F, and PETI-P were 0.15 Pa·s at 309 °C, 0.31 Pa·s at 331 °C, and 0.45 Pa·s at 323 °C, respectively. The lower melt viscosity of PETI-F compared to PETI-P was attributed to the noncoplanar twisted biphenyl structure and increased free volume due to the bulky –CF3 groups. The complex viscosity curves are shown in Figure 8B. Melt stability was assessed by monitoring melt viscosity variation over 2 h at 280 °C, revealing values of 0.52–2.17 Pa·s for PETI-O, 2.41–38.9 Pa·s for PETI-F, and 1.52–19.1 Pa·s for PETI-P. These results indicate that the –CF3 group enhanced the electron-withdrawing ability of the PETI backbone, thereby increasing curing reactivity, while the –O– group decreased this ability, leading to higher curing reactivity. The Td5% of cured PETI-O, PETI-F, and PETI-P were 558 °C, 578 °C, and 577 °C, respectively, demonstrating excellent thermal stability. The TGA curves are shown in Figure 9. All cured resins also exhibited good mechanical properties, with tensile strengths ranging from 50.8 to 66.2 MPa, tensile moduli from 1.96 to 2.40 GPa, and failure strains from 2.5% to 3.4%.
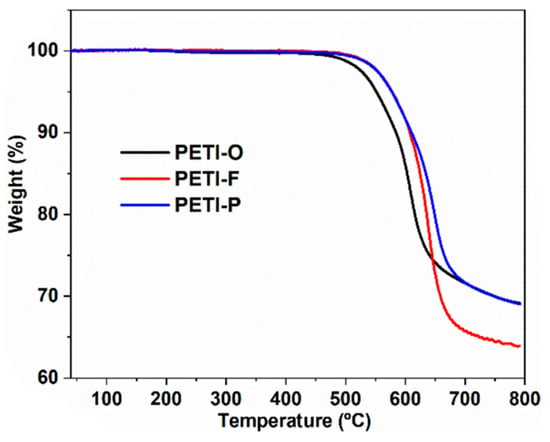
Figure 9.
TGA curves: Cured PETI-O, PETI-F, and PETI-P in nitrogen (reprinted with permission from [], Copyright 2021, MDPI).
2.4. Flexible Linkages in Main Chains
Aromatic polyimides are generally known for their poor processability due to a rigid polymer backbone and strong intermolecular interactions []. The introduction of flexible units, such as ether (–O–) or thioether linkages (–S–), into the polymer backbone imparts flexibility to the polymer chains, which can lower the Tm and melt viscosity or enhance the solubility of the polyimides, thereby improving their processability []. This section reviews several studies on phenylethynyl-terminated imide oligomers incorporating flexible units. The chemical structures used in the studies presented in this section are shown in Scheme 6.
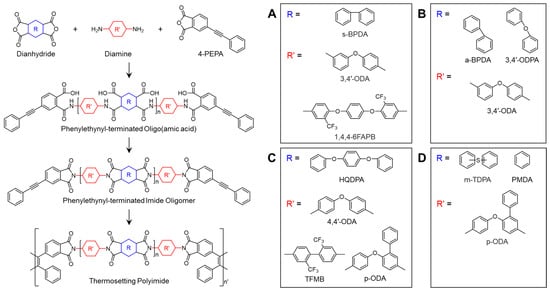
Scheme 6.
Chemical structures of PETIs with flexible units in main chains: (A) PI-n []; (B) Oligo-n []; (C) o-O-n, o-T-n, and o-p-n []; (D) Oligo-n [].
Yang et al. [] reported a new series of PETIs based on 1,4,4-6FAPB and 3,4′-ODA diamines containing flexible ether groups. The synthesized PETIs were designated as PI-2 (s-BPDA/1,4,4-6FAPB; 3,4′-ODA (50:50)/PEPA, Mn = 1250), PI-4 (Mn = 2500), PI-5 (Mn = 5000), and PI-6 (Mn = 10,000). The Tm of PI-2, PI-4, PI-5, and PI-6 were 250 °C, 290 °C, 300 °C, and 325 °C, respectively, with minimum melt viscosities of 0.6 Pa·s (at 299 °C), 11.2 Pa·s (at 315 °C), 750 Pa·s (at 306 °C), and 934 Pa·s (at 332 °C), respectively, indicating that viscosity increases with molecular weight. The complex viscosity curves are shown in Figure 10A. Upon maintaining the temperature at 300 °C for 2 h, the melt viscosity of PI-4 increased from 17.1 to 10,350 Pa·s, while PI-2 exhibited a range from 0.4 to 1391 Pa·s, suggesting that melt stability is closely related to molecular weight and chemical structure. The Tg of PI-2, PI-4, PI-5, and PI-6 were 124 °C, 180 °C, 209 °C, and 238 °C, respectively, showing a trend similar to the melt viscosity. However, Tg values measured by DSC and DMA after curing increased as the molecular weight decreased, attributed to higher crosslinking density in lower molecular weight samples. The thermal properties of PETI oligomers and cured resins are summarized in Table 6. The Td5% were 570 °C, 578 °C, 603 °C, and 599 °C, respectively, indicating excellent thermal stability. The mechanical properties of the cured polyimides exhibited tensile strengths ranging from 34.5 to 114.3 MPa and elongation at break between 2.5% and 13.8%. As the Mn increased from 1250 to 5000, both tensile strength and elongation at break improved. However, beyond an Mn of 5000, no further enhancement in mechanical properties was observed.
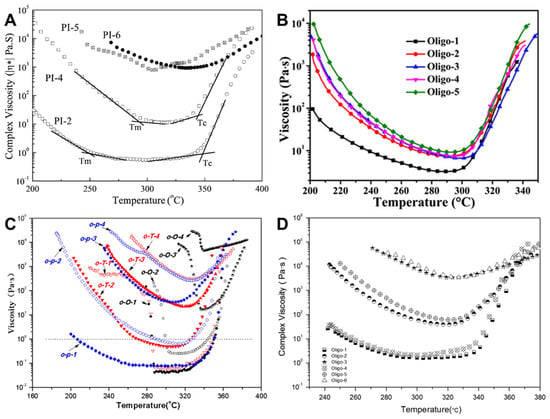
Figure 10.
Complex viscosity curves: (A) PI-n (reprinted with permission from [], Copyright 2007, Elsevier); (B) Oligo-n (reprinted with permission from [], Copyright 2018, Springer); (C) o-O-n, o-T-n, and o-p-n (reprinted with permission from [], Copyright 2019, SAGE Publications); (D) Oligo-n (reprinted with permission from [], Copyright 2016, SAGE Publications).

Table 6.
Thermal properties of PETIs with flexible units in main chains.
Hu et al. [] reported PETIs based on a-ODPA containing flexible ether units, a-BPDA dianhydrides, and 3,4′-ODA diamine. The synthesized oligomers, designated as Oligo-1 (a-ODPA/3,4′-ODA/PEPA), Oligo-4 (a-ODPA; a-BPDA (20:80)/3,4′-ODA/PEPA), and Oligo-5 (a-BPDA/3,4′-ODA/PEPA), exhibited Tg of 185 °C, 192 °C, and 201 °C, respectively. After curing, the Tg values measured by DMA were 317 °C for Oligo-1, 334 °C for Oligo-4, and 338 °C for Oligo-5, attributed to PEPA crosslinking. The Tg values before and after curing increased with higher a-BPDA content. The thermal properties of PETI oligomers and cured resins are summarized in Table 6. All uncured PETIs were highly soluble in strongly polar solvents such as NMP, DMF, DMAc, and DMSO due to the flexible ether linkages and the asymmetric structure of the monomers within the chains. The minimum melt viscosities of Oligo-1, Oligo-4, and Oligo-5 were reported as 3.2 Pa·s (at 290 °C), 7.4 Pa·s (at 296 °C), and 9.2 Pa·s (at 292 °C), respectively. The complex viscosity curves are shown in Figure 10B. PETIs with a higher a-ODPA content exhibited lower minimum melt viscosities and greater melt viscosity stability, enhancing their processability. The tensile strengths of all cured resins were close to or above 100 MPa, with strains ranging from 10.3% to 13.8%. A comparison of the CTE values for Oligo-4 and Oligo-5 films revealed that Oligo-4 exhibited higher CTE values in both the glassy and rubbery states, attributed to the reduced in-plane orientation due to increased ether linkages in the main chain. The Td5% in both nitrogen and oxygen atmospheres were above 556 °C and 558 °C, respectively, indicating excellent thermal stability. The TGA curves are shown in Figure 11A.
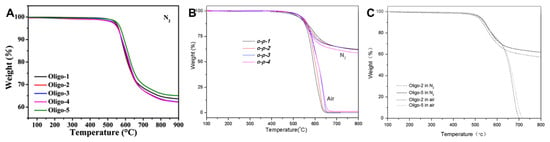
Figure 11.
TGA curves: (A) Oligo-n (reprinted with permission from [], Copyright 2018, Springer); (B) o-p-n (reprinted with permission from [], Copyright 2019, SAGE Publications); (C) Oligo-2 and Oligo-5 (reprinted with permission from [], Copyright 2016, SAGE Publications).
Wang et al. [] reported a new series of PETIs synthesized from HQDPA dianhydride with flexible ether groups and diamines 4,4′-ODA, TFDB, and p-ODA. The PETIs were designated as follows: o-O-1 (HQDPA/4,4′-ODA/PEPA, Mn = 750), o-T-1 (HQDPA/TFDB/PEPA, Mn = 750), o-p-1 (HQDPA/p-ODA/PEPA, Mn = 750), o-p-2 (HQDPA/p-ODA/PEPA, Mn = 1250), o-p-3 (HQDPA/p-ODA/PEPA, Mn = 2500), and o-p-4 (HQDPA/p-ODA/PEPA, Mn = 5000). An XRD analysis indicated that the PETIs in the p-ODA series were amorphous, showing no melting peaks in the DSC. The minimum melt viscosities of o-O-1, o-T-1, and o-p-1 were 0.04 Pa·s at 288 °C, 0.06 Pa·s at 288 °C, and 0.04 Pa·s at 296 °C, respectively. The complex viscosity curves are shown in Figure 10C. The melt viscosity variations at 280 °C over 2 h were 34.8–54.8 Pa·s, 81.9–170 Pa·s, and 0.11–0.39 Pa·s, respectively, suggesting that the asymmetric structure and irregular arrangement, caused by the bulky pendant phenyl groups of p-ODA, reduced intra- and intermolecular interactions. The Tg of the p-ODA series ranged from 118 °C to 202 °C, increasing with molecular weight. However, the Tg of the cured PETIs, measured by DSC, decreased with increasing Mn due to lower crosslinking density at higher molecular weights. The series demonstrated excellent thermal stability, with Td5% above 522 °C. The TGA curves are shown in Figure 11B. The Tg of the cured o-p-1 resin, measured by DMA, was 355 °C, which exceeded those of PETI-298 and PETI-330. The thermal properties of PETI oligomers and cured resins are summarized in Table 6. The p-ODA-based cured resins exhibited superior mechanical properties, with tensile strengths ranging from 51 to 95 MPa, moduli of 2.5 to 2.6 GPa, and elongations between 2% and 8%.
Fang et al. [] reported the synthesis of PETIs based on m-TDPA, which features a flexible thioether linkage (–S–) and p-ODA, known for its bulky substituents. The PETIs synthesized included Oligo-2 (m-TDPA/p-ODA/PEPA, Mn = 2500) and Oligo-5 (m-TDPA;PMDA (50:50)/p-ODA/PEPA, Mn = 2500), both of which exhibited high solubility in organic solvents at a 20 wt.% solid content, not only in polar aprotic solvents but in THF and DMSO. This high solubility was attributed to the flexible thioether linkage in m-TDPA, which lowers the rotational energy barrier of the polymer main chain, along with the asymmetric structures of 3,4′-TDPA and 3,3′-TDPA that disrupt molecular chain regularity, thereby reducing intermolecular interactions and chain packing density. The minimum melt viscosity of Oligo-5 was 61.5 Pa·s at 321 °C, higher than that of Oligo-2, which was 38.4 Pa·s at 319 °C. The complex viscosity curves are shown in Figure 10D. The increase in viscosity with higher PMDA content was due to the symmetry and planarity of PMDA. All synthesized m-TDPA-based PETIs exhibited lower melt viscosities than PMDA/p-ODA and Tri-A PI oligomers, highlighting the chain flexibility imparted by the thioether linkage. The Tg of Oligo-2 and Oligo-5 were reported as 205 °C and 211 °C, respectively, with cured Tg values measured by DMA being 302 °C and 320 °C, respectively. The higher Tg of Oligo-5 was due to the rigid PMDA structure. After curing, the Tg decreased with increasing Mn, attributed to reduced crosslinking density at higher molecular weights. The thermal properties of PETI oligomers and cured resins are summarized in Table 6. The thermal stability of the cured PETIs was excellent, with Td5% of 509 °C for Oligo-2 and 521 °C for Oligo-5. The TGA curves are shown in Figure 11C. Notably, most samples exhibited higher decomposition temperatures in air compared to nitrogen, likely due to differing degradation mechanisms of the thioether linkage. The mechanical properties of Oligo-2 included a modulus of 2.9 GPa, a tensile strength of 52 MPa, and elongation of 2.2%, while Oligo-5 exhibited a modulus of 3.0 GPa, a tensile strength of 77 MPa, and elongation of 3.5%. The introduction of the rigid PMDA structure slightly enhanced the mechanical properties of the cured resins.
2.5. Inorganic Hybrid Structure
Polyhedral oligomeric silsesquioxane (POSS) is characterized by a polyhedral structure consisting of a nanoscale inorganic cage made of silicon and oxygen atoms. These hybrid materials combine an inorganic core with an organic periphery, including organic substituents, making them versatile for various applications []. Incorporating POSS into materials significantly enhances properties such as wear resistance, thermal stability, resistance to oxidation and ultraviolet radiation, and hydrophobicity []. However, the large size of POSS units in copolymers can disrupt the chain packing density and reduce CTC interactions, increasing free volume, which may decrease polymer density and viscoelastic properties []. The chemical structures used in the studies presented in this section are shown in Scheme 7.
Conversely, carboranes, which are carbon–boron cluster compounds with a dodecahedral structure (o-, m-, p-C2B10H12), exhibit exceptional thermal stability and hydrophobicity due to their unique cage structure and high boron content [,]. When incorporated into a polymer matrix, carborane structures can significantly enhance the thermal stability of the polymer, providing advantages over other boron-containing compounds.
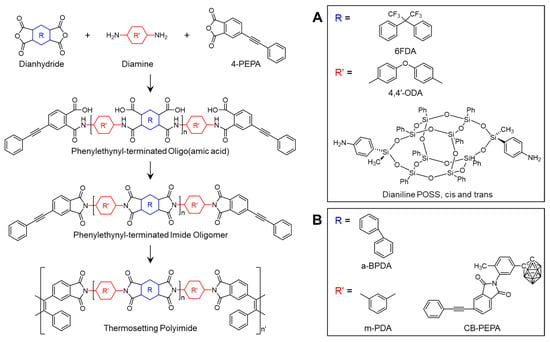
Scheme 7.
Chemical structures of PETIs with inorganic hybrid configurations: (A) 0, 1, 2, 3 cis- and/or trans-POSS (reproduced with permission from [], Copyright 2013, American Chemical Society); (B) PI-mPDA/CB-PEPA blend series (reproduced with permission from [], Copyright 2017, SAGE Publications).
Yandek et al. [] reported on a series of polyimides based on bis(4-anilinylmethylsilyloxy)octaphenylsilsesquioxane (dianiline POSS), including both cis and trans isomers of POSS dianiline. The synthesized PETIs were designated as 0 POSS (6FDA/4,4′-ODA/PEPA), 1 cis- and/or trans-POSS (6FDA/4,4′-ODA;cis- and/or trans-POSS(80:20)/PEPA), and 3 cis- and/or trans-POSS (6FDA/4,4′-ODA;cis- and/or trans-POSS(60:40)/PEPA). When comparing the viscosity of these PETIs at 250 °C under varying shear rates, it was found that most POSS-incorporated PETIs exhibited significantly lower zero-shear viscosity (η0) compared to 0 POSS, attributed to the large POSS molecules disrupting compact chain packing and charge transfer complex (CTC) interactions. CTC interactions are characteristic of imides and occur between the electron-rich nitrogen atoms (donors) and the electron-deficient carbonyl groups (acceptors), occasionally behaving as physical cross-links. Thus, as chain packing and CTC interactions decrease, the melt viscosity decreases. However, 1 trans-POSS exhibited behavior similar to 0 POSS, and 3 cis-POSS had the highest η0, indicating that the POSS isomer type influenced the packing and orientation of the PETI chains, thereby affecting their response to shear stress. The complex viscosity curves are shown in Figure 12A. After curing, 1 POSS demonstrated 28–38% less water absorption than 0 POSS, and 3 POSS showed 67–70% lower saturated water absorption, indicating superior moisture resistance. Consequently, the Tg of the saturated 3 cis/trans-POSS polyimide was 10 °C higher than that of 0 POSS. In terms of thermal stability, 0 POSS had the highest Td5% at 508 °C, while POSS incorporation decreased thermal stability by 10–15 °C. Despite this, the 3 POSS polyimide exhibited the highest char yield due to the increased concentration of reactive intermediates from phenyl hydrogen during pyrolysis, promoting additional crosslinking. Moreover, in air, the POSS cages oxidized to form silicon dioxide (SiO2), which helped suppress mass loss during the initial stages of decomposition. The TGA curves are shown in Figure 13A and the Td5% values of cured resins are summarized in Table 7.
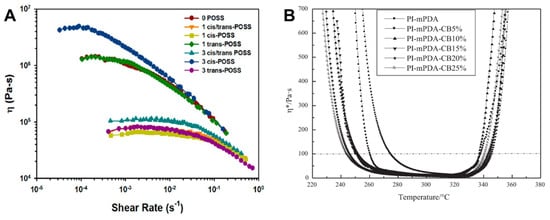
Figure 12.
Viscosity curves: (A) Steady shear viscosity dependence on shear rate for uncured oligomides at 250 °C (reprinted with permission from [], Copyright 2013, American Chemical Society); (B) PI-mPDA/CB-PEPA blend series (reprinted with permission from [], Copyright 2017, SAGE Publications).
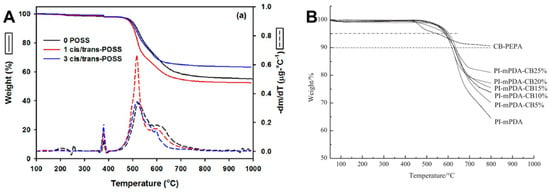
Figure 13.
TGA curves: (A) Mass loss profiles [solid lines] and their first derivatives [dashed lines] for all oligomers in nitrogen (reprinted with permission from [], Copyright 2013, American Chemical Society); (B) Cured PI-mPDA/CB-PEPA blend series in nitrogen (reprinted with permission from [], Copyright 2017, SAGE Publications).

Table 7.
Thermal properties of PETIs with inorganic hybrid structures.
Chen et al. [] investigated the effects of blending PETIs with an imide compound diluent (CB-PEPA) containing the icosahedral carborane, 1-(3-amino-4-tolyl)-1,2-dicarba-dodecaborane (CBNH). The base PETI, PETI-mPDA (a-BPDA/m-PDA/PEPA), exhibited a minimum melt viscosity of 12.2 Pa·s at 325 °C and maintained a melt viscosity below 100 Pa·s within the temperature range of 275–346 °C. By contrast, PI-mPDA-CB25%, with 20 wt.% CB-PEPA added showed a lower minimum melt viscosity of 6.7 Pa·s at 317 °C and a broader processing window, maintaining a melt viscosity below 100 Pa·s over the range of 243–342 °C. The complex viscosity curves are shown in Figure 12B. The cured resins of PI-mPDA and PI-mPDA-CB25% exhibited Td5% values of 585 °C and 586 °C, respectively, with char yields of 64.5% and 81.0% at 800 °C, attributed to the incorporation of the carborane cage. Notably, adding 5 wt.% CB-PEPA increased the Td5% to 604 °C, although further increases in CB-PEPA content led to a decrease in Td5%, likely due to the rapid weight loss of CB-PEPA observed around 480 °C. The TGA curves are shown in Figure 13B and Td5% values of cured resins are summarized in Table 7.
2.6. Liquid Crystalline Mesogenic Structure
Liquid crystalline polymers (LCPs) exhibit superior mechanical and thermal properties due to the alignment of polymer chains driven by intermolecular interactions []. By optimizing the curing temperature within the liquid crystalline behavior range, PETIs can be developed to retain liquid crystalline textures at room temperature, enhancing intermolecular interactions through aligned polymer chains. This alignment can improve heat resistance, thermal conductivity, and mechanical properties. However, the application of this approach to imide oligomers has rarely been explored. Herein, we introduce a PETI that leverages liquid crystallinity for improved performance. The chemical structures used in the study presented in this section are shown in Scheme 8.
Scheme 8.
Chemical structures of PETIs with liquid crystalline mesogenic configurations: LC-PI series (reprinted with permission from [], Copyright 2021, American Chemical Society).
Gu et al. [] reported a series of phenylethynyl-terminated liquid crystalline polyimides (LC-PIs) that exhibit liquid crystalline behavior. The synthesized LC-PIs were designated as LC-PII (HQDA/4,4′-ODA/PEPA), LC-PIII (HQDA/4,4′-ODA;TPE-Q(75:25)/PEPA), LC-PIIII (HQDA/4,4′-ODA;TPE-Q(50:50)/PEPA), LC-PIIV (HQDA/4,4′-ODA;TPE-Q(25:75)/PEPA), and LC-PIV (HQDA/TPE-Q/PEPA). A DSC analysis of the precursor preLC-PIIV showed endothermic peaks at 272 °C and 388 °C, indicating the liquid crystalline range. Within this temperature range, bright yellow dots were observed under POM. XRD spectra displayed a strong peak at 18°, confirming that the liquid crystalline state persisted at room temperature due to crosslinking of the phenylethynyl groups. The cured LC-PIIV resin exhibited the best mechanical properties, with a tensile strength of 119.0 MPa, an elongation at break of 50.3%, a modulus of 2.1 GPa, and a toughness of 55.4 MJ/m3. These superior properties were attributed to the microstructural alignment of polymer chains within the liquid crystalline range and the close packing of the chains. Due to the closely packed structure, where molecular chains maintain a microscopically ordered arrangement, the phthalimide mesomorphic units present in the main chain exhibit strong interactions with each other, thereby reinforcing intermolecular interactions between the molecular chains. This promotes stress resistance and enhances the thermal and mechanical properties. The Tg of LC-PIIV was 329.3 °C, and the Td5% was 478.7 °C, demonstrating excellent thermal stability. The TGA curves are shown in Figure 14 and the thermal properties of PETI oligomers and cured resins are summarized in Table 8.
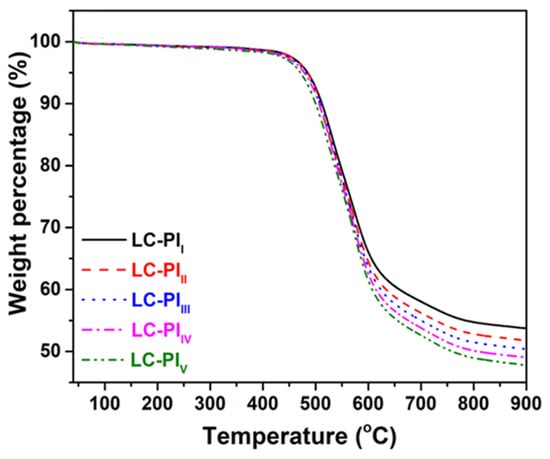
Figure 14.
TGA curves: LC-PI series (reprinted with permission from [], Copyright 2021, American Chemical Society).

Table 8.
Thermal properties of PETIs with liquid crystalline mesogenic structures.
3. Conclusions and Perspectives
This review has highlighted significant advancements in the development of ultra-high-temperature PETI oligomers, emphasizing the complex interactions between their molecular structures, processing conditions, and resulting material properties. The incorporation of monomers with noncoplanar configurations, such as kink, and cardo structures, alongside the inclusion of fluorinated groups, flexible linkages, and liquid crystalline mesogenic structures, has enabled the tailoring of PETI properties to meet the demanding requirements of advanced applications. These structural modifications have enhanced the thermal stability, glass transition temperatures, and solvent resistance of PETIs while optimizing processing characteristics, including reduced melt viscosity and improved processability.
Despite these advancements, several challenges remain. The synthesis of PETIs with precisely controlled molecular weights and narrow polydispersity indices remains complex, necessitating further refinement of polymerization techniques. Additionally, the relationship between molecular architecture and macroscopic properties, particularly for ultra-high-temperature applications, requires deeper investigation. Developing predictive models that accurately correlate molecular structure with performance metrics is crucial for further progress.
Future research should focus on exploring novel monomeric structures and polymerization strategies to enhance the thermal, mechanical, and electrical properties of PETIs. Expanding the scope of applications by integrating PETIs into multifunctional composites and hybrid materials could unlock new potentials, particularly in aerospace, electronics, and high-performance coatings. Moreover, addressing the environmental impact and sustainability of PETI production and processing is essential to align this research with global trends toward greener and more sustainable material solutions. By addressing these challenges, the field of PETIs is poised to significantly contribute to the next generation of high-performance materials.
Funding
This research was funded by Agency for Defense Development, grant number UI230010TD.
Acknowledgments
This work was supported by Agency for Defense Development of the Korean Government (grant No. UI230010TD).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hergenrother, P.M.; Smith, J.G. Chemistry and Properties of Imide Oligomers End-Capped with Phenylethynylphthalic Anhydrides. Polymer 1994, 35, 4857–4864. [Google Scholar] [CrossRef]
- Connell, J.W.; Smith, J.G.; Hergenrother, P.M. Oligomers and Polymers Containing Phenylethynyl Groups. J. Macromol. Sci. Part C Polym. Rev. 2000, 40, 207–230. [Google Scholar] [CrossRef]
- Yokota, R.; Yamamoto, S.; Yano, S.; Sawaguchi, T.; Hasegawa, M.; Yamaguchi, H.; Ozawa, H.; Sato, R. Molecular Design of Heat Resistant Polyimides Having Excellent Processability and High Glass Transition Temperature. High Perform. Polym. 2001, 13, S61–S72. [Google Scholar] [CrossRef]
- Cho, D.; Drzal, L.T. Characterization, Properties, and Processing of LaRCTM PETI-5 as a High-Temperature Sizing Material. I. FTIR Studies on Imidization and Phenylethynyl End-Group Reaction Behavior. J. Appl. Polym. Sci. 2000, 76, 190–200. [Google Scholar] [CrossRef]
- Fang, X.; Xie, X.; Simone, C.D.; Stevens, M.P.; Scola, D.A. A Solid-State 13C NMR Study of the Cure of 13C-Labeled Phenylethynyl End-Capped Polyimides. Macromolecules 2000, 33, 1671–1681. [Google Scholar] [CrossRef]
- Fang, X.; Rogers, D.F.; Scola, D.A.; Stevens, M.P. A Study of the Thermal Cure of a Phenylethynyl-Terminated Imide Model Compound and a Phenylethynyl-Terminated Imide Oligomer (PETI-5). J. Polym. Sci. Part A Polym. Chem. 1998, 36, 371–504. [Google Scholar] [CrossRef]
- Smith, J.G.; Connell, J.W.; Hergenrother, P.M. The Effect of Phenylethynyl Terminated Imide Oligomer Molecular Weight on the Properties of Composites. J. Compos. Mater. 2000, 34, 614–628. [Google Scholar] [CrossRef]
- Meng, X.; Zheng, Y.; Yan, J.; Li, Y.; Wang, Z.; Li, G. 2,3,3′,4′-Oxydiphthalic Dianhydride-Based Phenylethynyl-Terminated Imide Oligomers for Low-Temperature Resin Transfer Molding Applications. High Perform. Polym. 2016, 28, 962–970. [Google Scholar] [CrossRef]
- Meng, X.; Wen, Y.; Wang, X.; Shen, D.; Yan, J.; Wang, Z. High Performance Imide Oligomers and Thermosets Derived from 9,9-Bis(3,4-Dicarboxyphenyl)Fluorene Dianhydride. Polymer 2023, 281, 126086. [Google Scholar] [CrossRef]
- Miyauchi, M.; Ishida, Y.; Ogasawara, T.; Yokota, R. Synthesis and Characterization of Soluble Phenylethynyl-Terminated Imide Oligomers Derived from Pyromellitic Dianhydride and 2-Phenyl-4,4′-Diaminodiphenyl Ether. React. Funct. Polym. 2013, 73, 340–345. [Google Scholar] [CrossRef]
- Zuo, H.; Chen, J.; Hu, A.; Fan, L.; Yang, S. Meltable Phenylethynyl-Capped Oligoimide Resins Derived from 1,4-Bis(4-Amino-2-Trifluoromethylphenoxy)Benzene and 3,4′-Oxydianiline. Eur. Polym. J. 2007, 43, 3892–3903. [Google Scholar] [CrossRef]
- Wang, W.; Chen, G.; Fang, X. Phenylethynyl-Terminated Oligoimides with Ultra-Low Melt Viscosity Derived from 1,4-Bis(3,4-Dicarboxy Phenoxy)Benzene Dianhydride. High Perform. Polym. 2019, 31, 580–589. [Google Scholar] [CrossRef]
- Sun, L.; Wang, W.; Chen, G.; Fang, X. Highly Soluble Phenylethynyl-Endcapped Imide Oligomers Derived from Thioetherdiphthalic Anhydride Isomers. High Perform. Polym. 2017, 29, 289–297. [Google Scholar] [CrossRef]
- Simone, C.D.; Scola, D.A. Phenylethynyl End-Capped Polyimides Derived from 4,4′-(2,2,2-Trifluoro-1-Phenylethylidene)Diphthalic Anhydride, 4,4′-(Hexafluoroisopropylidene)Diphthalic Anhydride, and 3,3′,4,4′-Biphenylene Dianhydride: Structure−Viscosity Relationship. Macromolecules 2003, 36, 6780–6790. [Google Scholar] [CrossRef]
- Adamczak, A.D.; Spriggs, A.A.; Fitch, D.M.; Awad, W.; Wilkie, C.A.; Grunlan, J.C. Thermal Degradation of High-Temperature Fluorinated Polyimide and Its Carbon Fiber Composite. J. Appl. Polym. Sci. 2010, 115, 2254–2261. [Google Scholar] [CrossRef]
- Li, Y.; Murphy, L.A.; Lincoln, J.E.; Morgan, R.J. Phenylethynyl End-Capped Fluorinated Imide Oligomer AFR-PEPA-N: Morphology and Processibility Characteristics. Macromol. Mater. Eng. 2007, 292, 78–84. [Google Scholar] [CrossRef]
- Smith, J.G.; Connell, J.W.; Hergenrother, P.M.; Ford, L.A.; Criss, J.M. Transfer Molding Imide Resins Based on 2,3,3′,4′-Biphenyltetracarboxylic Dianhydride. Macromol. Symp. 2003, 199, 401–418. [Google Scholar] [CrossRef]
- Connell, J.W.; Smith, J.G.; Hergenrother, P.M.; Criss, J.M. High Temperature Transfer Molding Resins: Laminate Properties of PETI-298 and PETI-330. High Perform. Polym. 2003, 15, 375–394. [Google Scholar] [CrossRef]
- Connell, J.W.; Smith, J.G., Jr.; Hergenrother, P.M. High Temperature Transfer Molding Resins: Preliminary Composite Properties of PETI-375. In Proceedings of the SAMPE 2004 Symposium and Exhibition, Long Beach, CA, USA, 16–20 May 2004. [Google Scholar]
- Ogasawara, T.; Ishikawa, T.; Yokota, R.; Ozawa, H.; Taguchi, M.; Sato, R.; Shigenari, Y.; Miyagawa, K. Processing and Properties of Carbon Fiber Reinforced Triple-A Polyimide (Tri-A PI) Matrix Composites. Adv. Compos. Mater. 2002, 11, 277–286. [Google Scholar] [CrossRef]
- Li, M.; Liu, X.Y.; Qin, J.Q.; Gu, Y. Molecular Dynamics Simulation on Glass Transition Temperature of Isomeric Polyimide. Express Polym. Lett. 2009, 3, 665–675. [Google Scholar] [CrossRef]
- Chuang, K.C.; Criss, J.M.; Mintz, E.A. Polyimides Based on Asymmetric Dianhydrides (II) (a-BPDA vs a-BTDA) for Resin Transfer Molding (RTM); E-17516; NASA Glenn Research Center: Cleveland, OH, USA, 2010. [Google Scholar]
- Chuang, K.C. Low Melt Viscosity Imide Resins for Resin Transfer Molding. In Innovations in Materials Manufacturing, Fabrication, and Environmental Safety; CRC Press: Boca Raton, FL, USA, 2010; pp. 649–650. [Google Scholar]
- Ding, M. Isomeric Polyimides. Prog. Polym. Sci. 2007, 32, 623–668. [Google Scholar] [CrossRef]
- Fang, X.; Yang, Z.; Zhang, S.; Gao, L.; Ding, M. Polyimides Derived from Mellophanic Dianhydride. Macromolecules 2002, 35, 8708–8717. [Google Scholar] [CrossRef]
- Hasegaw, M.; Sensui, N.; Shindo, Y.; Yokota, R. Structure and Properties of Novel Asymmetric Biphenyl Type Polyimides. Homo-and Copolymers and Blends. Macromolecules 1999, 32, 387–396. [Google Scholar] [CrossRef]
- Li, Q.; Fang, X.; Wang, Z.; Gao, L.; Ding, M. Polyimides from Isomeric Oxydiphthalic Anhydrides. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 3249–3260. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Effects of Meta and Para Diamines on the Properties of Polyetherimide Nanocomposite Films Prepared by the Sol-Gel Process. J. Appl. Polym. Sci. 2007, 105, 1093–1100. [Google Scholar] [CrossRef]
- Pinson, D.M.; Yandek, G.R.; Haddad, T.S.; Horstman, E.M.; Mabry, J.M. Thermosetting Poly(Imide Silsesquioxane)s Featuring Reduced Moisture Affinity and Improved Processability. Macromolecules 2013, 46, 7363–7377. [Google Scholar] [CrossRef]
- Su, C.; Liu, P.; Yue, J.; Huan, H.; Yang, Z.; Yang, K.; Guo, H.; Zhao, J. High-Transparency and Colorless Polyimide Film Prepared by Inhibiting the Formation of Chromophores. Polymers 2022, 14, 4242. [Google Scholar] [CrossRef]
- Abe, A.; Nakano, T.; Yamashita, W.; Fukukawa, K.; Okazaki, M.; Tamai, S. Theoretical and Experimental Studies on the Mechanism of Coloration of Polyimides. ChemPhysChem 2011, 12, 1367–1377. [Google Scholar] [CrossRef]
- Meng, X.; Yan, J.; Fan, W.; Liu, J.; Wang, Z.; Li, G. Thermosetting Polyimides and Composites Based on Highly Soluble Phenylethynyl-Terminated Isoimide Oligomers. RSC Adv. 2014, 4, 37458–37469. [Google Scholar] [CrossRef]
- Li, H.; Wang, W.; Chen, G.; Chen, X.; Li, Y.; Zhou, H.; Fang, X. Highly Soluble Phenylethynyl-Terminated Imides Derived from Mellophanic Dianhydride (MPDA). Polym. Adv. Technol. 2018, 29, 2797–2805. [Google Scholar] [CrossRef]
- Smith, J.G.; Connell, J.W.; Hergenrother, P.M.; Criss, J.M. Resin Transfer Moldable Phenylethynyl Containing Imide Oligomers. J. Compos. Mater. 2002, 36, 2255–2265. [Google Scholar] [CrossRef]
- Liaw, D.J.; Wang, K.L.; Huang, Y.C.; Lee, K.R.; Lai, J.Y.; Ha, C.S. Advanced Polyimide Materials: Syntheses, Physical Properties and Applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Seo, C.H.; Lim, S.W.; Min, H.J.; Kim, J.H.; Kim, J.H. Preparation of Semi-Alicyclic Homo-and Blended Polyimide Membranes Using Alicyclic Dianhydrides with Kink Structures and Their Gas Separation Properties. J. Ind. Eng. Chem. 2022, 114, 347–360. [Google Scholar] [CrossRef]
- Damaceanu, M.D.; Constantin, C.P.; Nicolescu, A.; Bruma, M.; Belomoina, N.; Begunov, R.S. Highly Transparent and Hydrophobic Fluorinated Polyimide Films with Ortho-Kink Structure. Eur. Polym. J. 2014, 50, 200–213. [Google Scholar] [CrossRef]
- Kazama, S.; Teramoto, T.; Haraya, K. Carbon Dioxide and Nitrogen Transport Properties of Bis(Phenyl)Fluorene-Based Cardo Polymer Membranes. J. Memb. Sci. 2002, 207, 91–104. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kazama, S.; Inoue, K.; Toyama, T.; Nagai, Y.; Haraya, K.; Mohamed, H.F.M.; Orouke, B.E.; Oshima, N.; Kinomura, A.; et al. Positron Annihilation in Cardo-Based Polymer Membranes. J. Phys. Chem. B 2014, 118, 6007–6014. [Google Scholar] [CrossRef]
- Tong, X.; Wang, S.; Dai, J.; Wang, S.; Zhang, K.; Zhao, X.; Wang, D.; Chen, C. The Effect of Chain Rigidity and Microstructure on Gas Separation Performance of the Cardo-Based Polyimides. Polymer 2022, 254, 125046. [Google Scholar] [CrossRef]
- Carta, M.; Croad, M.; Jansen, J.C.; Bernardo, P.; Clarizia, G.; McKeown, N.B. Synthesis of Cardo-Polymers Using Tröger’s Base Formation. Polym. Chem. 2014, 5, 5255–5261. [Google Scholar] [CrossRef]
- Hu, Z.; Li, S.; Zhang, C. Synthesis and Properties of Polyamide–Imides Containing Fluorenyl Cardo Structure. J. Appl. Polym. Sci. 2007, 106, 2494–2501. [Google Scholar] [CrossRef]
- Li, X.; Zhang, P.; Dong, J.; Gan, F.; Zhao, X.; Zhang, Q. Preparation of Low-κ Polyimide Resin with Outstanding Stability of Dielectric Properties versus Temperature by Adding a Reactive Cardo-Containing Diluent. Compos. Part B Eng. 2019, 177, 107401. [Google Scholar] [CrossRef]
- Ishida, Y.; Ogasawara, T.; Yokota, R. Development of Highly Soluble Addition-Type Imide Oligomers for Matrix of Carbon Fiber Composite (I): Imide Oligomers Based on Asymmetric Biphenyltetracarboxylic Dianhydride and 9,9-Bis(4-Aminophenyl) Fluorene. High Perform. Polym. 2006, 18, 727–737. [Google Scholar] [CrossRef]
- Li, H.; Wang, W.; Chen, G.; Liu, Y.; Fang, X. Highly Soluble Phenylethynyl Terminated Oligoimides Derived from 5(6)-Amino-1-(4-Aminophenyl)-1,3,3-Trimethylindane, 4,4′-Oxydianiline and Mixed Thioetherdiphthalic Anhydride Isomers. J. Polym. Res. 2018, 25, 1–9. [Google Scholar] [CrossRef]
- Hong, W.; Yuan, L.; Yang, S. High Temperature Phenylethynyl-Terminated Imide Oligomers Derived from Asymmetric Diphenyl Ether Diamines for Resin Transfer Molding. Polymer 2023, 269, 125635. [Google Scholar] [CrossRef]
- Miyauchi, M.; Ishida, Y.; Ogasawara, T.; Yokota, R. Highly Soluble Phenylethynyl-Terminated Imide Oligomers Based on KAPTON-Type Backbone Structures for Carbon Fiber-Reinforced Composites with High Heat Resistance. Polym. J. 2013, 45, 594–600. [Google Scholar] [CrossRef]
- Rao, X.; Zhou, H.; Dang, G.; Chen, C.; Wu, Z. New Kinds of Phenylethynyl-Terminated Polyimide Oligomers with Low Viscosity and Good Hydrolytic Stability. Polymer 2006, 47, 6091–6098. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, P.; Yao, H.; Guan, S. Synthesis and Characterization of Fluorinated Polyimide Oligomers Terminated with a Phenylethynyl Group. React. Funct. Polym. 2012, 72, 621–626. [Google Scholar] [CrossRef]
- Sun, H.; Huo, H.; Nie, H.; Yang, S.; Fan, L. Phenylethynyl Terminated Oligoimides Derived from 3,3′,4,4′-Diphenylsulfonetetracarboxylic Dianhydride and Their Adhesive Properties. Eur. Polym. J. 2009, 45, 1169–1178. [Google Scholar] [CrossRef]
- Hong, W.; Yuan, L.; Ma, Y.; Cui, C.; Zhang, H.; Yang, S.; Sun, W.H. Resin Transfer Moldable Fluorinated Phenylethynyl-terminated Imide Oligomers with High Tg: Structure–Melt Stability Relationship. Polymers 2021, 13, 903. [Google Scholar] [CrossRef]
- Yu, P.; Wang, Y.; Yu, J.; Zhu, J.; Hu, Z. Influence of Different Ratios of A-ODPA/a-BPDA on the Properties of Phenylethynyl Terminated Polyimide. J. Polym. Res. 2018, 25, 1–11. [Google Scholar] [CrossRef]
- Wright, M.E.; Schorzman, D.A.; Feher, F.J.; Jin, R.Z. Synthesis and Thermal Curing of Aryl-Ethynyl-Terminated CoPOSS Imide Oligomers: New Inorganic/Organic Hybrid Resins. Chem. Mater. 2003, 15, 264–268. [Google Scholar] [CrossRef]
- Seurer, B.; Vij, V.; Haddad, T.; Mabry, J.M.; Lee, A. Thermal Transitions and Reaction Kinetics of Polyhederal Silsesquioxane Containing Phenylethynylphthalimides. Macromolecules 2010, 43, 9337–9347. [Google Scholar] [CrossRef]
- Yue, J.; Li, Y.; Li, H.; Zhao, Y.; Zhao, C.; Wang, X. Thermal Curing of Novel Carborane-Containing Phenylethynyl Terminated Imide Oligomers. RSC Adv. 2015, 5, 98010–98019. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, J.; Chen, G. Synthesis and Characterization of a Carborane-Containing Monofunctional Imide Monomer as a Modifier for Imide Oligomer. High Perform. Polym. 2018, 30, 812–820. [Google Scholar] [CrossRef]
- Olamilekan, A.I.; Yeo, H. Thermal Conducting Thermosets Driven by Molecular Structurally Enhanced Mesogen Interactions. ACS Appl. Polym. Mater. 2021, 3, 4147–4155. [Google Scholar] [CrossRef]
- Ruan, K.; Guo, Y.; Gu, J. Liquid Crystalline Polyimide Films with High Intrinsic Thermal Conductivities and Robust Toughness. Macromolecules 2021, 54, 4934–4944. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).