Grafting of Lactic Acid and ε-Caprolactone onto Alpha-Cellulose and Sugarcane Bagasse Cellulose: Evaluation of Mechanical Properties in Polylactic Acid Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Modification of α-Cel or FBA-Cel by Lactic Acid
2.3. Surface Grafting of α-Cel and FBA-Cel Modified via In Situ Polymerization
2.4. The Polymerization Procedure of ε-CL Initiated with Sn(Oct)2 for α-Cel or FBA-Cel
2.5. Manufacture of the Composite Material of PLA and Modified or Unmodified α-Cel and FBA-Cel
2.6. Characterization
2.6.1. Scanning Electron Microscopy (SEM)
2.6.2. Fourier Transform Infrared (FTIR) Spectroscopy by Attenuated Total Reflectance (ATR)
2.6.3. Mechanical Properties
2.6.4. Differential Scanning Calorimetry (DSC)
2.6.5. X-Ray Photoelectron Spectroscopy (XPS) Measurements
3. Results and Discussion
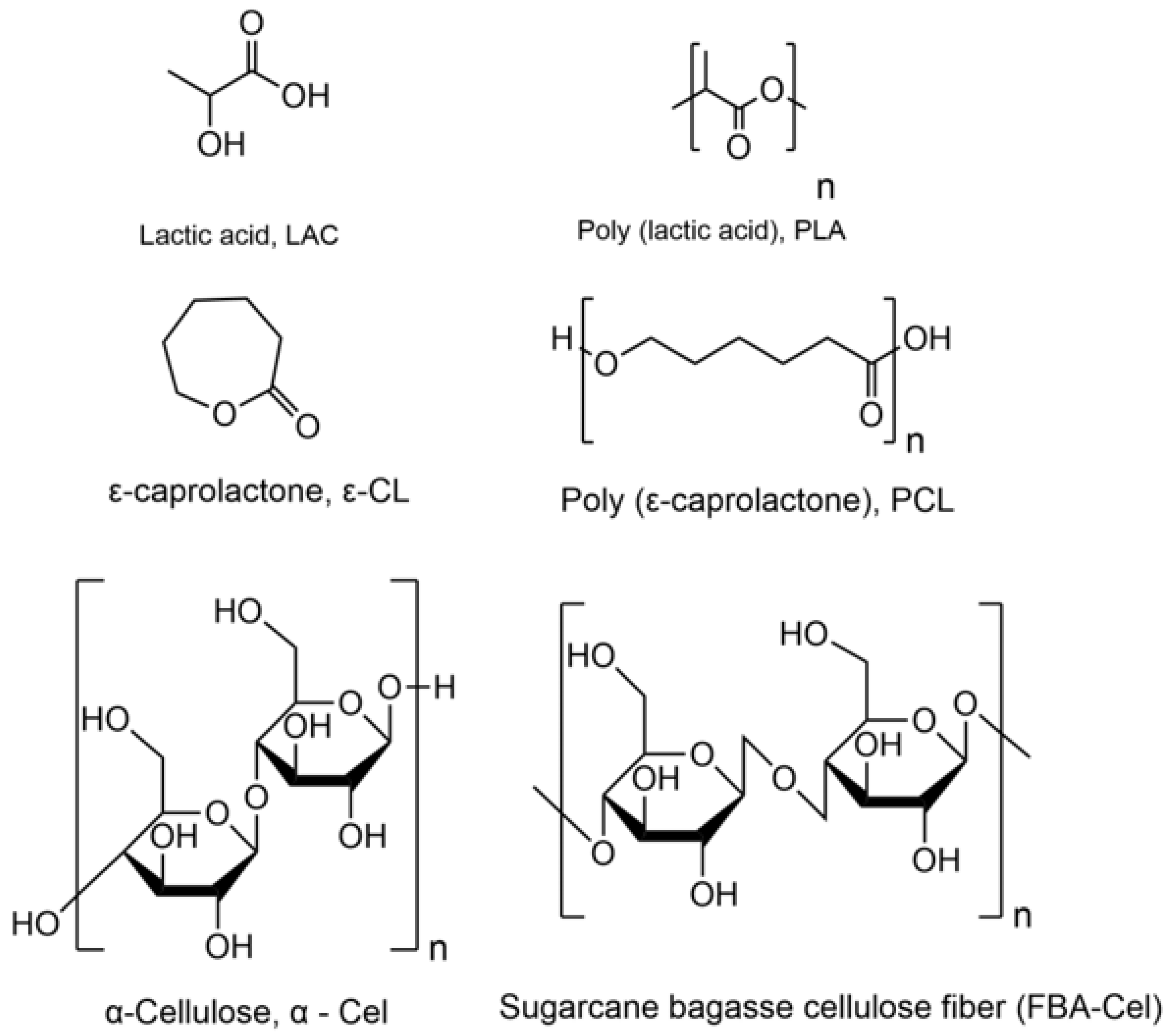
3.1. Reaction Scheme and Morphology of Grafted Fibers
3.2. Structural Changes Evaluated by FTIR
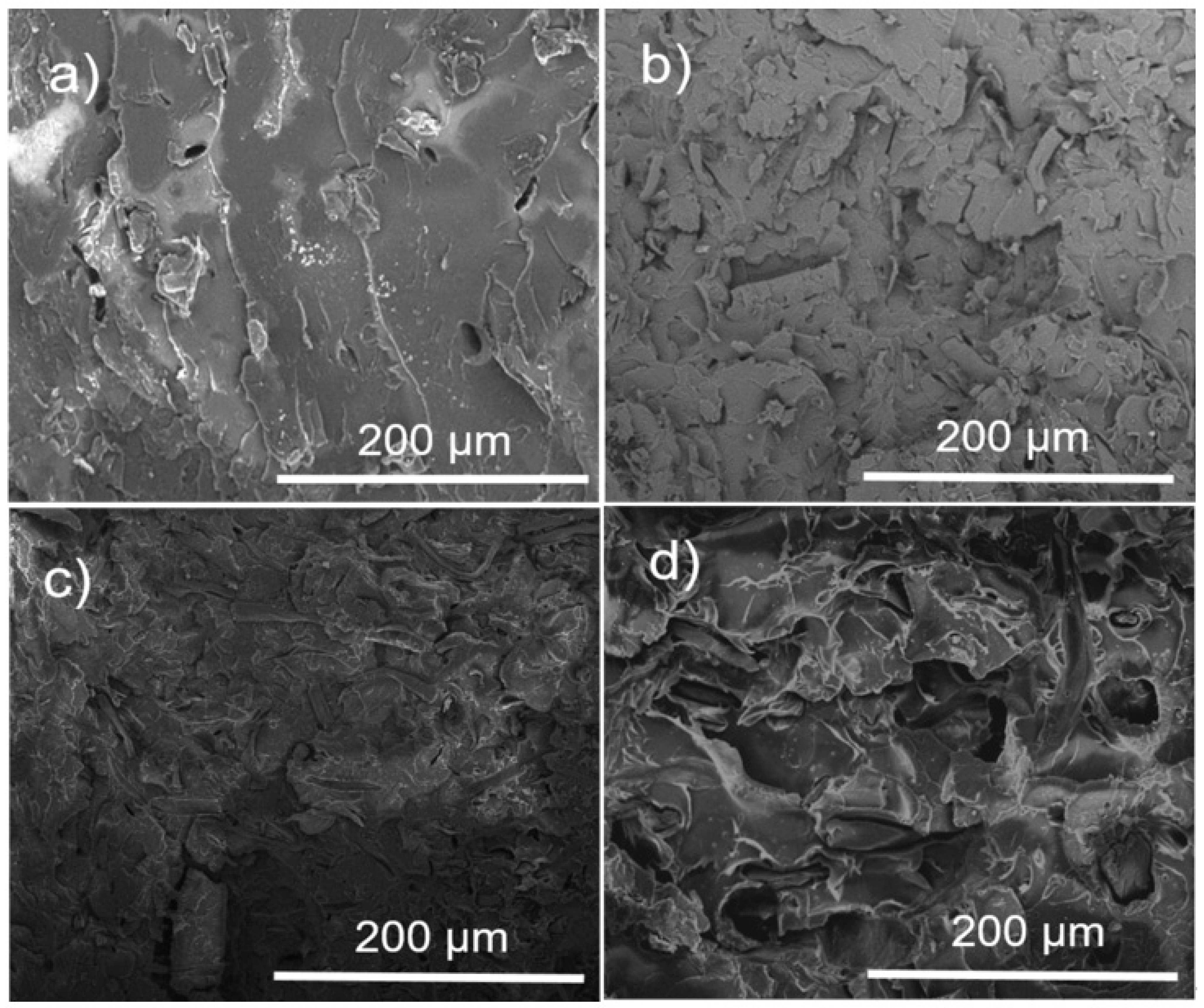
3.3. Composite Morphology Evaluated by SEM
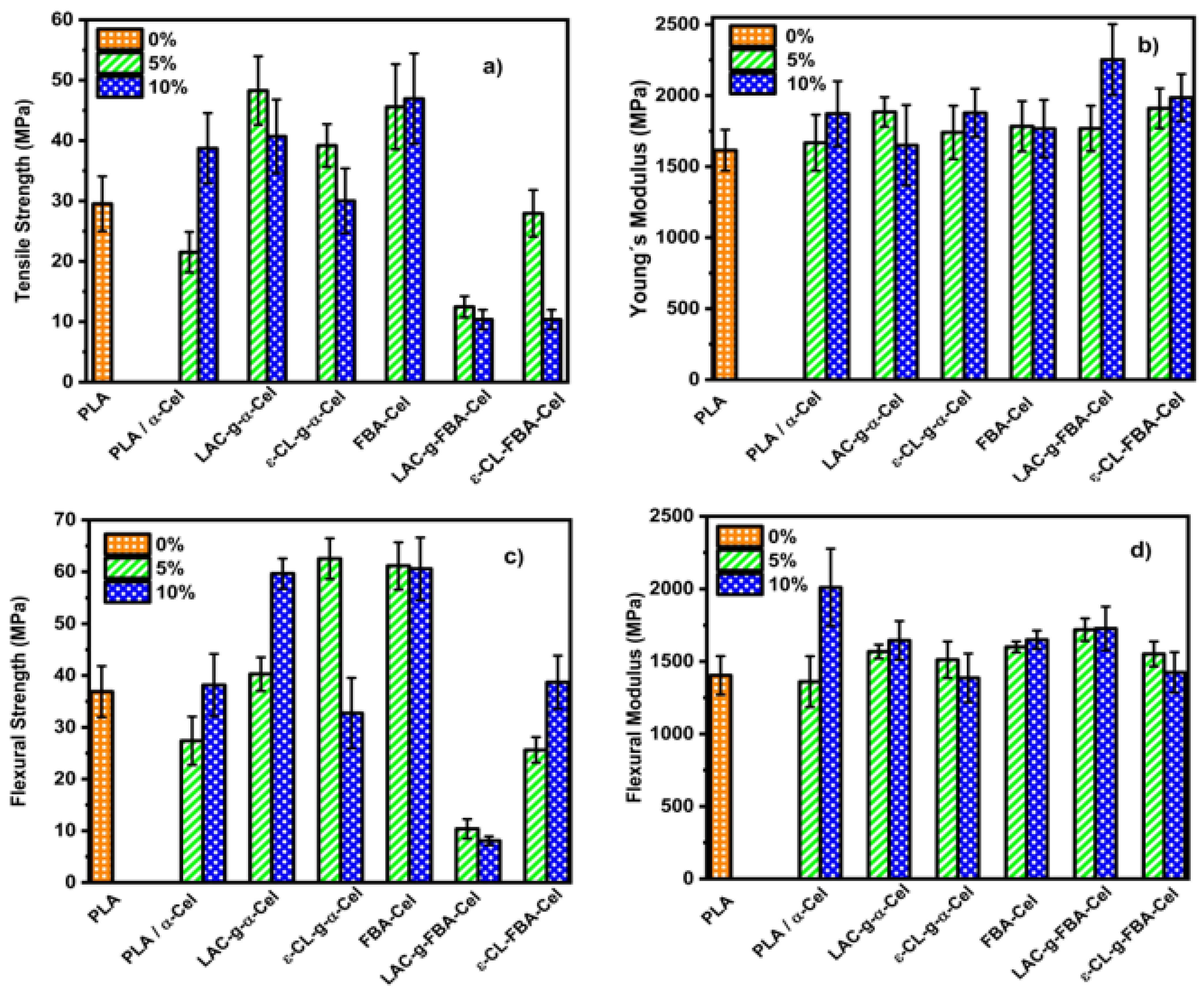
3.4. PLA Composites with α-Cel and FBA-Cel
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Auras, R.; Lim, L.T.; Selke, S.E.M.; Tsuji, H. Poly (Lactic Acid): Synthesis, Structures, Properties, Processing, and Applications, 1st ed.; Wiley: Hoboken, NJ, USA, 2011; p. 3. [Google Scholar]
- Zhou, C.; Shi, Q.; Guo, W.; Terrel, L.; Qureshi, A.; Hayes, D.; Wu, Q. Electrospun bio-nanocomposite scaffolds for bone tissue engineering by cellulose nanocrystals reinforcing maleic anhydride grafted PLA. ACS Appl. Mater. Interfaces 2013, 59, 3847–3854. [Google Scholar] [CrossRef]
- Chen, L.; Qiu, X.; Deng, M.; Hong, Z.; Luo, R.; Chen, X.; Jing, X. The starch grafted poly (L-lactide) and the physical properties of its blending composites. Polymer 2005, 46, 5723–5729. [Google Scholar] [CrossRef]
- Zhou, L.; Ke, K.; Yang, M.B.; Yang, W. Recent progress on chemical modification of cellulose for high mechanical-performance Poly (lactic acid)/Cellulose composite: A review. Compos. Commun. 2021, 23, 100548. [Google Scholar] [CrossRef]
- Teramoto, Y.; Yoshioka, M.; Shiraishi, N.; Nishio, Y. Plasticization of cellulose diacetate by graft copolymerization of ε-caprolactone and lactic acid. J. Appl. Polym. Sci. 2002, 84, 2621–2628. [Google Scholar] [CrossRef]
- Lönnberg, H.; Zhou, Q.; Brumer, H.; Teeri, T.T.; Malmström, E.; Hult, A. Grafting of cellulose fibers with poly (ε-caprolactone) and poly (l-lactic acid) via ring-opening polymerization. Biomacromolecules 2006, 7, 2178–2185. [Google Scholar] [CrossRef]
- Lönnberg, H.; Fogelström, L.; Zhou, Q.; Hult, A.; Berglund, L.; Malmström, E. Investigation of the graft length impact on the interfacial toughness in a cellulose/poly (ε-caprolactone) bilayer laminate. Compos. Sci. Technol. 2011, 71, 9–12. [Google Scholar] [CrossRef]
- Muiruri, J.K.; Liu, S.; Teo, W.S.; Kong, J.; He, C. Highly biodegradable and harsh polylactic acid–cellulose nanocrystal composite. ACS Sustain. Chem. Eng. 2017, 5, 3929–3937. [Google Scholar] [CrossRef]
- Gupta, A.; Katiyar, V. Cellulose functionalized high molecular weight stereocomplex polylactic acid biocomposite films with improved gas barrier, thermomechanical properties. ACS Sustain. Chem. Engin. 2017, 5, 6835–6844. [Google Scholar] [CrossRef]
- Yu, Y.; Gao, X.; Jiang, Z.; Zhang, W.; Ma, J.; Liu, X.; Zhang, L. Homogeneous grafting of cellulose with polycaprolactone using quaternary ammonium salt systems and its application for ultraviolet-shielding composite films. RSC Adv. 2018, 8, 10865–10872. [Google Scholar] [CrossRef]
- Canché-Escamilla, G.; los Santos-Hernández, D.; Andrade-Canto, S.; Gómez-Cruz, R. Obtención de celulosa a partir de los desechos agrícolas del banano. Inf. Tecnológica 2005, 16, 83–88. [Google Scholar] [CrossRef]
- Chen, L.; Xie, Z.; Zhuang, X.; Chen, X.; Jing, X. Controlled release of urea encapsulated by starch-g-poly (L-lactide). Carbohydr. Polym. 2008, 72, 342–348. [Google Scholar] [CrossRef]
- Kowalski, A.; Duda, A.; Penczek, S. Kinetics and mechanism of cyclic esters polymerization initiated with tin (II) octoate. 3. Polymerization of L, L-dilactide. Macromolecules 2000, 33, 7359–7370. [Google Scholar] [CrossRef]
- Carlsson, L. Surface Modification of Cellulose by Covalent Grafting and Physical Adsorption. Doctoral Dissertation, KTH Royal Institute of Technology, Stockholm, Sweden, 2014. [Google Scholar]
- ASTM D638; Standard Test Method for Tensile Properties of Plastics. American Society for Testing and Materials: West Conshohocken, PA, USA, 2022.
- ASTM D790; Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. ASTM International: Conshohocken, PA, USA, 2017.
- Wang, G.; Zhang, D.; Li, B.; Wan, G.; Zhao, G.; Zhang, A. Strong and thermal-resistance glass fiber-reinforced polylactic acid (PLA) composites enabled by heat treatment. Int. J. Biol. Macromol. 2019, 129, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Benahmed, A.; Azzaoui, K.; El Idrissi, A.; Belkheir, H.; Said Hassane, S.O.; Touzani, R.; Rhazi, L. Cellulose acetate-g-polycaprolactone copolymerization using diisocyanate intermediates and the effect of polymer chain length on surface, thermal, and antibacterial properties. Molecules 2022, 27, 1408. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.K.; Thakur, M.K.; Gupta, R.K. Development of functionalized cellulosic biopolymers by graft copolymerization. Int. J. Biol. Macromol. 2013, 62, 44–51. [Google Scholar] [CrossRef]
- Jacob, J.; Linson, N.; Mayelil-Sam, R.; Maria, H.J.; Pothan, L.A.; Thomas, S.; Kabdrakhmanova, S.; Laroze, D. Poly (lactic acid)/nanocellulose biocomposites for sustainable food packing. Cellulose 2024, 31, 5997–6042. [Google Scholar] [CrossRef]
- Hosen, M.S.; Rahaman, M.H.; Gafur, M.A.; Ahme, A.N. Development of Thermal Properties and Surface Morphology of Poly (lactic acid)/Chitosan blend with Microcrystalline Cellulose obtained from natural Jute Fiber. Int. Res. J. Pure Appl. Chem. 2017, 15, 1–8. [Google Scholar] [CrossRef]
- Hospodarova, V.; Singovszka, E.; Stevulova, N. Characterization of cellulosic fibers by FTIR spectroscopy for their further implementation to building materials. Am. J. Anal. Chem. 2018, 9, 303–310. [Google Scholar] [CrossRef]
- Lafia-Araga, R.A.; Sabo, R.; Nabinejad, O.; Matuana, L.; Stark, N. Influence of lactic acid surface modification of cellulose nanofibrils on the properties of cellulose nanofibril films and cellulose nanofibril–poly (Lactic acid) composites. Biomolecules 2021, 11, 1346. [Google Scholar] [CrossRef]
- VP, S.; Mohanty, S.; Nayak, S.K. Effect of poly (lactic acid)-graft-glycidyl methacrylate as a compatibilizer on properties of poly (lactic acid)/banana fiber biocomposites. Polym. Adv. Technol. 2016, 27, 515–524. [Google Scholar] [CrossRef]
- Abdelrazek, E.M.; Hezma, A.M.; El-Khodary, A.; Elzayat, A.M. Spectroscopic studies and thermal properties of PCL/PMMA biopolymer blend. Egypt. J. Basic Appl. Sci. 2016, 3, 10–15. [Google Scholar] [CrossRef]
- Huda, M.S.; Mohanty, A.K.; Drzal, L.T.; Schut, E.; Misra, M. “Green” composites from recycled cellulose and poly (lactic acid): Physico-mechanical and morphological properties evaluation. J. Mater. Sci. 2005, 40, 4221–4229. [Google Scholar] [CrossRef]
- Khoo, R.Z.; Chow, W.S. Mechanical and thermal properties of poly (lactic acid)/sugarcane bagasse fiber green composites. J. Thermoplast. Compos. Mater. 2017, 30, 1091–1102. [Google Scholar] [CrossRef]
- Hou, A.L.; Qu, J.P. Super-Toughened Poly(Lactic Acid) with Poly(ε-caprolactone) and Ethylene-Methyl Acrylate-Glycidyl Methacrylate by Reactive Melt Blending. Polymers 2019, 11, 771. [Google Scholar] [CrossRef]
- Awal, A.; Rana, M.; Sain, M. Thermorheological and mechanical properties of cellulose reinforced PLA bio-composites. Mech. Mater. 2015, 80, 87–95. [Google Scholar] [CrossRef]
- Negaresh, M.; Javadi, A.; Garmabi, H. Poly (lactic acid)/poly (ε-caprolactone) blends the effect of nanocalcium carbonate and glycidyl methacrylate on interfacial characteristics. Front. Mater. 2024, 11, 1377340. [Google Scholar] [CrossRef]
- Zhang, C.; Salick, M.R.; Cordie, T.M.; Ellingham, T.; Dan, Y.; Turng, L.S. Incorporation of poly (ethylene glycol) grafted cellulose nanocrystals in poly (lactic acid) electrospun nanocomposite fibers as potential scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2015, 49, 463–471. [Google Scholar] [CrossRef]
- Goffin, A.L.; Raquez, J.M.; Duquesne, E.; Siqueira, G.; Habibi, Y.; Dufresne, A.; Dubois, P. From interfacial ring-opening polymerization to melt processing of cellulose nanowhisker-filled polylactide-based nanocomposites. Biomacromolecules 2011, 12, 2456–2465. [Google Scholar] [CrossRef]








| Sample | Tg (°C) | Tc (°C) | ΔHc (W/g) | Tm (°C) | ΔHm (W/g) | Xc (%) |
|---|---|---|---|---|---|---|
| PLA | 63.28 | 94.65 | 21.19 | 170.43 | 44.95 | 25.54 |
| 95 PLA/5 α-Cel | 62.14 | 93.40 | 28.43 | 169.4 | 40.25 | 13.37 |
| 90 PLA/10 α-Cel | 59.32 | 91.97 | 20.02 | 169.63 | 32.05 | 14.37 |
| 95 PLA/ 5 LAC-g-α-Cel | 59.19 | 100.45 | 32.49 | 167.87 | 41.50 | 10.19 |
| 90 PLA/ 10 LAC-g-α-Cel | 57.64 | 97.43 | 15.19 | 166.31 | 35.50 | 24.26 |
| 95 PLA/ 5 ε-CL-g-α-Cel | 58.82 | 98.71 | 38.78 | 164.44 | 45.71 | 7.84 |
| 90 PLA/ 10 ε-CL-g-α-Cel | 57.55 | 91.13 | 29.72 | 167.20 | 39.28 | 11.42 |
| 95 PLA/5 FBA-Cel | 57.77 | 117.81 | 19.21 | 149.33 | 29.61 | 11.77 |
| 90 PLA/10 FBA-Cel | 61.03 | 114.99 | 16.72 | 148.84 | 31.67 | 17.88 |
| 95 PLA/ 5 LAC-g-FBA-Cel | 57.36 | 101.18 | 10.59 | 164.43 | 46.33 | 40.45 |
| 90 PLA/ 10 LAC-g-FBA-Cel | 57.72 | 91.79 | 2.41 | 164.97 | 45.41 | 51.37 |
| 95 PLA/ 5 ε-CL-g-FBA-Cel | 59.07 | 89.52 | 25.72 | 167.19 | 36.08 | 11.72 |
| 90 PLA/ 10 ε-CL-FBA-Cel | 57.60 | 86.01 | 23.41 | 166.04 | 33.62 | 12.19 |
| Sample | Binding Energy (eV) | Atomic Percentage (%) | Rate O/C |
|---|---|---|---|
| C1S | 285.05 | 36.30 | |
| 287.30 | 15.97 | ||
| 289.37 | 10.83 | ||
| O1S | 532.12 | 20.37 | |
| 533.69 | 16.53 | 0.58 | |
| C1S | 285.09 | 31.07 | |
| 286.55 | 20.47 | ||
| 289.03 | 10.26 | ||
| O1S | 532.02 | 21.45 | |
| 533.03 | 16.75 | 0.62 | |
| C1S | 285.0 | 22.63 | |
| 286.28 | 18.04 | ||
| 288.89 | 14.15 | ||
| O1S | 532.19 | 39.18 | |
| 533.88 | 6.00 | 0.82 | |
| C1S | 284.88 | 26.54 | |
| 286.90 | 22.47 | ||
| 289..17 | 10.51 | ||
| O1S | 531.89 | 11.75 | |
| 533.43 | 28.73 | 0.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valle Reyes, O.S.; Orozco-Guareño, E.; Hernández-Montelongo, R.; Alvarado Mendoza, A.G.; Martínez Chávez, L.; González Núñez, R.; Aguilar Martínez, J.; Moscoso Sánchez, F.J. Grafting of Lactic Acid and ε-Caprolactone onto Alpha-Cellulose and Sugarcane Bagasse Cellulose: Evaluation of Mechanical Properties in Polylactic Acid Composites. Polymers 2024, 16, 2964. https://doi.org/10.3390/polym16212964
Valle Reyes OS, Orozco-Guareño E, Hernández-Montelongo R, Alvarado Mendoza AG, Martínez Chávez L, González Núñez R, Aguilar Martínez J, Moscoso Sánchez FJ. Grafting of Lactic Acid and ε-Caprolactone onto Alpha-Cellulose and Sugarcane Bagasse Cellulose: Evaluation of Mechanical Properties in Polylactic Acid Composites. Polymers. 2024; 16(21):2964. https://doi.org/10.3390/polym16212964
Chicago/Turabian StyleValle Reyes, Oscar Salvador, Eulogio Orozco-Guareño, Rosaura Hernández-Montelongo, Abraham Gabriel Alvarado Mendoza, Liliana Martínez Chávez, Rubén González Núñez, Jacobo Aguilar Martínez, and Francisco Javier Moscoso Sánchez. 2024. "Grafting of Lactic Acid and ε-Caprolactone onto Alpha-Cellulose and Sugarcane Bagasse Cellulose: Evaluation of Mechanical Properties in Polylactic Acid Composites" Polymers 16, no. 21: 2964. https://doi.org/10.3390/polym16212964
APA StyleValle Reyes, O. S., Orozco-Guareño, E., Hernández-Montelongo, R., Alvarado Mendoza, A. G., Martínez Chávez, L., González Núñez, R., Aguilar Martínez, J., & Moscoso Sánchez, F. J. (2024). Grafting of Lactic Acid and ε-Caprolactone onto Alpha-Cellulose and Sugarcane Bagasse Cellulose: Evaluation of Mechanical Properties in Polylactic Acid Composites. Polymers, 16(21), 2964. https://doi.org/10.3390/polym16212964








