Material Aspects of Thin-Film Composite Membranes for CO2/N2 Separation: Metal–Organic Frameworks vs. Graphene Oxides vs. Ionic Liquids
Abstract
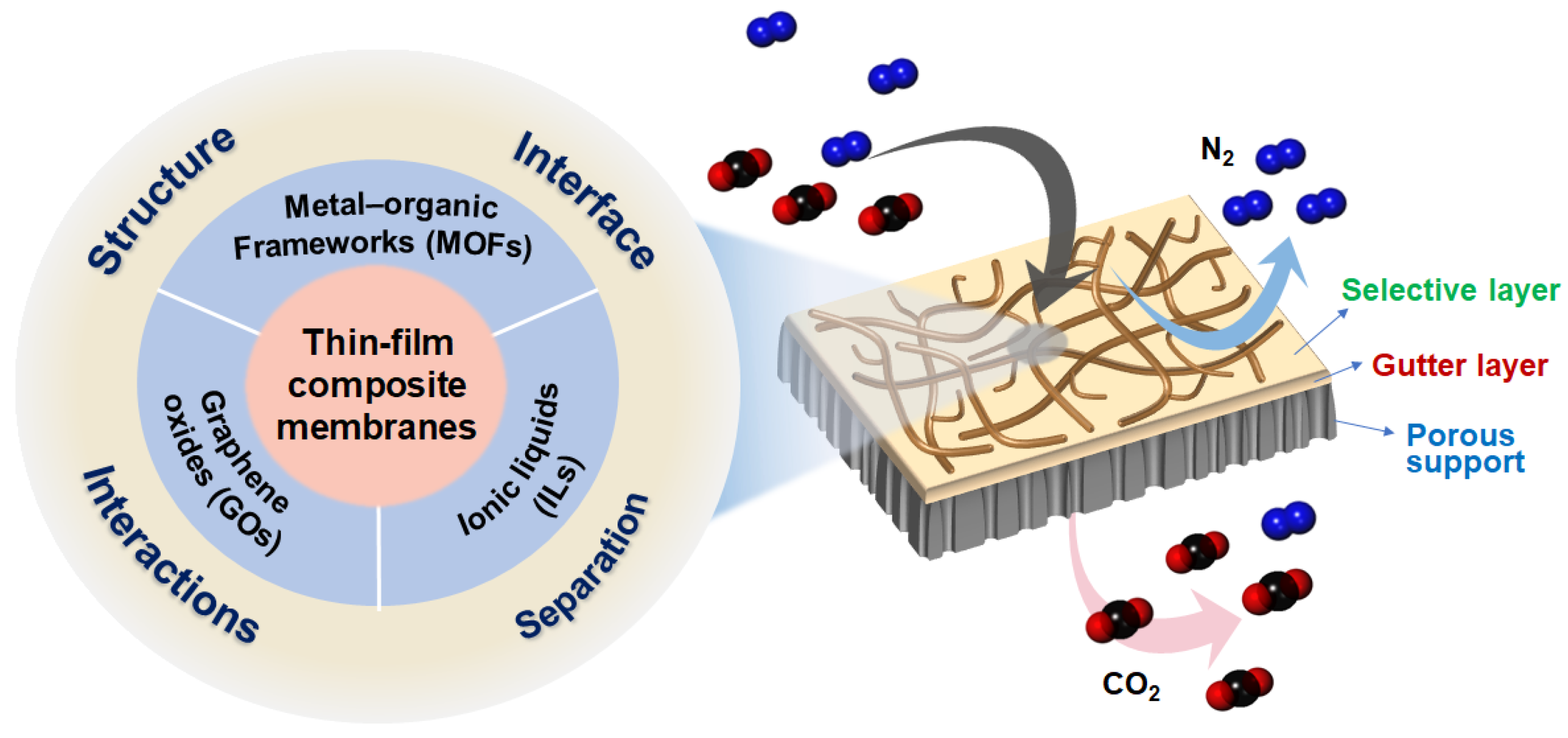
:1. Introduction
2. MOF-Containing TFC Membranes
3. GO-Containing TFC Membranes
4. IL-Containing TFC Membranes
5. Conclusions and Future Perspective
- (1)
- The majority of TFC-MMMs documented in the literature utilize flat sheets instead of hollow fibers, primarily because producing defect-free submicron-thick selective layers within hollow fibers presents challenges. Some studies have explored the fabrication of TFC hollow fiber membranes using external surface coatings instead of internal surface coatings, but the latter can pose difficulties in the development of large-scale hollow fiber membrane modules.
- (2)
- High-performance TFC-MMMs meeting the commercial criteria for CO2/N2 separation from flue gas were mainly developed using PTMSP or PDMS gutter layers, with a minority incorporating MOFs or GOs gutter layers. This highlights the crucial role of the gutter layer in preventing excessive infiltration of the selective layer into the pores of the support. PTMSP has high permeance but undergoes physical aging over time. In contrast, PDMS has lower permeance but offers long-term stability. Being more hydrophobic than PTMSP, PDMS presents challenges when trying to apply a hydrophilic selective layer directly. As a result, surface modification of PDMS is often necessary, such as oxygen plasma.
- (3)
- Membranes containing porous fillers like MOFs and GOs aimed to reduce non-selective interfacial defects between the fillers and polymer matrix, prevent pore blockage of the fillers by the polymer, and minimize the decrease in polymer chain mobility caused by the fillers. In contrast, membranes with ILs exhibit better interfacial contact, no pore blockage, and enhanced chain mobility due to the plasticizing effects of the ILs.
- (4)
- In general, TFC-MMMs containing MOFs showed the best performance, followed by the membranes with GOs. The membranes with ILs showed relatively low separation performance among the three types of additives. This indicates the use of porous fillers is more effective in improving the separation performance. Additionally, careful matching between the additive and the polymer matrix is more important than the intrinsic properties of the additives.
- (5)
- For MOF and GOs, the improved performances primarily stem from heightened diffusivity through the pores. Overall, MOF-based membranes exhibited greater CO2 permeance (in GPU), while GO-based membranes displayed higher CO2/N2 selectivity. Conversely, for ILs, the enhanced performance arises from increased solubility facilitated by specific interactions between ILs and CO2.
- (6)
- PEO-based membranes such as Pebax are widely recognized for their extensive usage, demonstrating promising performance and suitability for commercial applications. These membranes provide several advantages: (1) ether oxygen enhances CO2 solubility via Lewis acid-base interactions, (2) their high solubility in mild solvents like alcohol and water allows for easy coating on porous supports without causing damage, (3) their flexible rubbery properties promote intimate contact with rigid fillers, and (4) their excellent mechanical strength facilitates easier membrane preparation using roll-to-roll processes.
- (7)
- There are only a few studies on TFC membranes incorporating PAF or COF porous fillers as alternatives to MOFs for CO2 separation. Although the reported separation performance of TFC membranes containing these materials still falls short of that achieved by MOF-based MMMs, there is significant potential for growth in this research area.
- (8)
- The combination of binary mixtures such as MOF/IL, GO/IL, and MOF/GO has often demonstrated a synergistic effect in enhancing separation performance. However, as of yet, there has been no exploration of ternary mixtures (MOF/GO/IL) in both bulk and TFC membranes for gas separation. The porous structures of MOF and GO significantly enhance gas diffusivity and size-sieving mechanisms. Conversely, IL serves as an enhancer for CO2 solubility and acts as a compatibilizer, improving the interfacial properties between the rigid MOF (or GO) and the soft polymer matrix. This leads to a uniform and defect-free coating of the TFC layer, further optimizing membrane performance.
- (9)
- Surface modification and functionalization of MOFs and GO with organic groups are commonly employed to improve their interfacial properties with a polymer matrix, enhancing gas separation performance. The interactions between fillers and the matrix are primarily driven by secondary bonds like hydrogen bonding and dipole–dipole interactions. Some fillers form strong covalent bonds with the polymer matrix, resulting in more intimate contact at the interface. A major challenge, though, is the tendency of MOF and GO particles to aggregate, particularly at smaller particle sizes and higher loadings (e.g., >30%). This issue contrasts with the behavior of ILs, which do not exhibit such a tendency.
- (10)
- The controlling factors for additives include dispersion, loading, pore size and structure, particle size, CO₂ adsorption capacity, and interfacial compatibility with the polymer. In general, the cost of MOFs, GO, and ILs is higher than that of polymer matrices due to their complex synthesis, expensive raw materials, and limited scalability. For TFC membranes containing these additives to be commercially viable, they must be cost-competitive with other CO₂ separation technologies, such as amine absorption or cryogenic distillation, to achieve large-scale adoption.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zickfeld, K.; MacIsaac, A.J.; Canadell, J.G.; Fuss, S.; Jackson, R.B.; Jones, C.D.; Lohila, A.; Matthews, H.D.; Peters, G.P.; Rogelj, J.; et al. Net-zero approaches must consider Earth system impacts to achieve climate goals. Nat. Clim. Chang. 2023, 13, 1298–1305. [Google Scholar] [CrossRef]
- Huo, J.; Wang, Z.; Oberschelp, C.; Guillén-Gosálbez, G.; Hellweg, S. Net-zero transition of the global chemical industry with CO2-feedstock by 2050: Feasible yet challenging. Green Chem. 2023, 25, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Nath, F.; Mahmood, M.N.; Yousuf, N. Recent advances in CCUS: A critical review on technologies, regulatory aspects and economics. Geoenergy Sci. Eng. 2024, 238, 212726. [Google Scholar] [CrossRef]
- Mon, M.T.; Tansuchat, R.; Yamaka, W. CCUS Technology and Carbon Emissions: Evidence from the United States. Energies 2024, 17, 1748. [Google Scholar] [CrossRef]
- Yao, J.; Han, H.; Yang, Y.; Song, Y.; Li, G. A Review of Recent Progress of Carbon Capture, Utilization, and Storage (CCUS) in China. Appl. Sci. 2023, 13, 1169. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, G.; Liu, J.; Li, G.; Zhao, Y.; Wang, Y.; Wu, C.; Zhang, Y.; Xu, Y. Investigation of CO2 adsorption performance of amine impregnated adsorbents using amine-support matching strategies. Sep. Purif. Technol. 2023, 310, 123178. [Google Scholar] [CrossRef]
- Xiang, J.; Wei, D.; Mao, W.; Liu, T.; Luo, Q.; Huang, Y.; Liang, Z.; Luo, X. Comprehensive kinetic study of carbon dioxide absorption in blended tertiary/secondary amine solutions: Experiments and simulations. Sep. Purif. Technol. 2024, 330, 125310. [Google Scholar] [CrossRef]
- Li, X.; Li, R.; Peng, K.; Zhao, K.; Bai, M.; Li, H.; Gao, W.; Gong, Z. Amine-impregnated porous carbon–silica sheets derived from vermiculite with superior adsorption capability and cyclic stability for CO2 capture. Chem. Eng. J. 2023, 464, 142662. [Google Scholar] [CrossRef]
- Moradi, M.R.; Torkashvand, A.; Ramezanipour Penchah, H.; Ghaemi, A. Amine functionalized benzene based hypercrosslinked polymer as an adsorbent for CO2/N2 adsorption. Sci. Rep. 2023, 13, 9214. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, G.; Xu, Y.; Zhang, Q.; Liu, J.; Li, G.; Zhao, Y.; Wang, Y.; Zhang, Y. High CO2 adsorption on amine-functionalized improved macro-/mesoporous multimodal pore silica. Fuel 2022, 315, 123195. [Google Scholar] [CrossRef]
- Liu, H.; Lu, X.; Liu, L.; Wang, J.; Wang, P.; Gao, P.; Ren, T.; Tian, G.; Wang, D. Application of amine-loaded activated carbon fiber in CO2 capture and separation. Korean J. Chem. Eng. 2022, 39, 2513–2522. [Google Scholar] [CrossRef]
- Zhang, R.; Li, Y.; He, X.; Niu, Y.; Li, C.e.; Amer, M.W.; Barzagli, F. Investigation of the improvement of the CO2 capture performance of aqueous amine sorbents by switching from dual-amine to trio-amine systems. Sep. Purif. Technol. 2023, 316, 123810. [Google Scholar] [CrossRef]
- Pasichnyk, M.; Stanovsky, P.; Polezhaev, P.; Zach, B.; Šyc, M.; Bobák, M.; Jansen, J.C.; Přibyl, M.; Bara, J.E.; Friess, K.; et al. Membrane technology for challenging separations: Removal of CO2, SO2 and NOx from flue and waste gases. Sep. Purif. Technol. 2023, 323, 124436. [Google Scholar] [CrossRef]
- Olabi, A.G.; Alami, A.H.; Ayoub, M.; Aljaghoub, H.; Alasad, S.; Inayat, A.; Abdelkareem, M.A.; Chae, K.-J.; Sayed, E.T. Membrane-based carbon capture: Recent progress, challenges, and their role in achieving the sustainable development goals. Chemosphere 2023, 320, 137996. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Chen, D.; Liang, Z.; Yang, F. Insight and Comparison of Energy-efficient Membrane Processes for CO2 Capture from Flue Gases in Power Plant and Energy-intensive Industry. Carbon Capture Sci. Technol. 2022, 2, 100020. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.-K. Sub-ambient membrane process for CO2 removal in the industrial sector: Iron and steel, cement, and refinery. J. Membr. Sci. 2023, 686, 122018. [Google Scholar] [CrossRef]
- Chen, D.; Wang, K.; Yuan, Z.; Lin, Z.; Zhang, M.; Li, Y.; Tang, J.; Liang, Z.; Li, Y.; Chen, L.; et al. Boosting membranes for CO2 capture toward industrial decarbonization. Carbon Capture Sci. Technol. 2023, 7, 100117. [Google Scholar] [CrossRef]
- Guiver, M.D. Field Grand Challenge for Membrane Science and Technology. Front. Membr. Sci. Technol. 2022, 1, 878879. [Google Scholar] [CrossRef]
- Freeman, B.D. Basis of Permeability/Selectivity Tradeoff Relations in Polymeric Gas Separation Membranes. Macromolecules 1999, 32, 375–380. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Singh, R.; Prasad, B.; Ahn, Y.-H. Recent developments in gas separation membranes enhancing the performance of oxygen and nitrogen separation: A comprehensive review. Gas Sci. Eng. 2024, 123, 205256. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, eaab0530. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.B.; Beal, J.L.; Tamaddondar, M.; Luque-Alled, J.M.; Robertson, B.; Mathias, M.; Gorgojo, P.; Budd, P.M. Importance of small loops within PIM-1 topology on gas separation selectivity in thin film composite membranes. J. Mater. Chem. A 2021, 9, 21807–21823. [Google Scholar] [CrossRef]
- Kim, J.H. Grand Challenges in Membrane Applications—Gas and Vapor. Front. Membr. Sci. Technol. 2022, 1, 853402. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.; Zhang, J.; Li, X. Boosting CO2 transport in mixed matrix membranes by chitosan-MOF networks. J. Membr. Sci. 2024, 697, 122569. [Google Scholar] [CrossRef]
- Yang, L.; Park, S.; Han, W.Z.; Razak, M.A.; Zhen, L.Y.; Faires, M.; Omar, R.; Choong, T.S.Y.; Rohani, R.; Hamid, M.R.A. Systematic Narrowing of ZIF-8 Apertures via Controlled 2-Ethylimidazole Doping for Hydrogen/Carbon Dioxide Separation. Korean J. Chem. Eng. 2024, 41, 2277–2285. [Google Scholar] [CrossRef]
- Pirouzfar, V.; Roustaie, N.; Su, C.-H. Gas transport characteristics of mixed matrix membrane containing MIL-100 (Fe) metal-organic frameworks and PEBAX precursors. Korean J. Chem. Eng. 2023, 40, 2138–2148. [Google Scholar] [CrossRef]
- Su, P.; Chen, S.; Chen, L.; Li, W. Constructing polymer/metal-organic framework nanohybrids to design compatible polymer-filler-polymer membranes for CO2 separation. J. Membr. Sci. 2024, 691, 122246. [Google Scholar] [CrossRef]
- Zhao, Q.; Sun, Y.; Zhang, J.; Fan, F.; Li, T.; He, G.; Ma, C. Mixed matrix membranes incorporating amino-functionalized ZIF-8-NH2 in a carboxylic polyimide for molecularly selective gas separation. J. Membr. Sci. 2024, 693, 122326. [Google Scholar] [CrossRef]
- Chen, K.; Ni, L.; Guo, X.; Xiao, C.; Yang, Y.; Zhou, Y.; Zhu, Z.; Qi, J.; Li, J. Introducing pyrazole-based MOF to polymer of intrinsic microporosity for mixed matrix membranes with enhanced CO2/CH4 separation performance. J. Membr. Sci. 2023, 688, 122110. [Google Scholar] [CrossRef]
- Yahia, M.; Lozano, L.A.; Zamaro, J.M.; Téllez, C.; Coronas, J. Microwave-assisted synthesis of metal–organic frameworks UiO-66 and MOF-808 for enhanced CO2/CH4 separation in PIM-1 mixed matrix membranes. Sep. Purif. Technol. 2024, 330, 125558. [Google Scholar] [CrossRef]
- Husna, A.; Hossain, I.; Jeong, I.; Kim, T.-H. Mixed Matrix Membranes for Efficient CO2 Separation Using an Engineered UiO-66 MOF in a Pebax Polymer. Polymers 2022, 14, 655. [Google Scholar] [CrossRef]
- Fajrina, N.; Yusof, N.; Ismail, A.F.; Jaafar, J.; Aziz, F.; Salleh, W.N.W. Metal organic framework (MOF)-based composite filler incorporated thin film nanocomposite of hollow fiber membrane for carbon dioxide permeance. Mater. Today Proc. 2022, 65, 3060–3065. [Google Scholar] [CrossRef]
- Shin, H.; Chi, W.S.; Bae, S.; Kim, J.H.; Kim, J. High-performance thin PVC-POEM/ZIF-8 mixed matrix membranes on alumina supports for CO2/CH4 separation. J. Ind. Eng. Chem. 2017, 53, 127–133. [Google Scholar] [CrossRef]
- Mohsenpour, S.; Ameen, A.W.; Leaper, S.; Skuse, C.; Almansour, F.; Budd, P.M.; Gorgojo, P. PIM-1 membranes containing POSS-Graphene oxide for CO2 separation. Sep. Purif. Technol. 2022, 298, 121447. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, P.; Wu, H.; Sun, S.; You, X.; Yuan, B.; Hou, J.; Duan, C.; Jiang, Z. Incorporating amino acids functionalized graphene oxide nanosheets into Pebax membranes for CO2 separation. Sep. Purif. Technol. 2022, 288, 120682. [Google Scholar] [CrossRef]
- Pakizeh, M.; Karami, M.; Kooshki, S.; Rahimnia, R. Advanced toluene/n-heptane separation by pervaporation: Investigating the potential of graphene oxide (GO)/PVA mixed matrix membrane. J. Taiwan Inst. Chem. Eng. 2023, 150, 105025. [Google Scholar] [CrossRef]
- Chen, D.; Li, L.; Semiat, R.; He, X. Process Parametric Investigation of Graphene-Oxide-Embedded Composite Membranes for Boosting CO2/N2 Separation. Energy Fuels 2023, 37, 11187–11196. [Google Scholar] [CrossRef]
- Kheirtalab, M.; Abedini, R.; Ghorbani, M. Pebax/poly(vinyl alcohol) mixed matrix membrane incorporated by amine-functionalized graphene oxide for CO2 separation. J. Polym. Sci. 2024, 62, 517–535. [Google Scholar] [CrossRef]
- Niu, Z.; Wang, Y.; Dai, Y.; Zhong, S.; Li, J.; Mu, P.; Li, J. Enhanced CO2 Separation by Functionalized g-C3N4 Nanosheets as Composite Filler to Fabricate Mixed Matrix Membranes. Macromolecules 2023, 56, 6461–6469. [Google Scholar] [CrossRef]
- Yang, F.; Jin, Y.; Liu, J.; Zhu, H.; Xu, R.; Xiangli, F.; Liu, G.; Jin, W. Regulation of interlayer channels of graphene oxide nanosheets in ultra-thin Pebax mixed-matrix membranes for CO2 capture. Chin. J. Chem. Eng. 2024, 67, 257–267. [Google Scholar] [CrossRef]
- Xu, W.; Lindbråthen, A.; Janakiram, S.; Ansaloni, L.; Deng, L. Enhanced CO2/H2 separation by GO and PVA-GO embedded PVAm nanocomposite membranes. J. Membr. Sci. 2023, 671, 121397. [Google Scholar] [CrossRef]
- Vroulias, D.; Staurianou, E.; Ioannides, T.; Deimede, V. Poly(ethylene oxide)-Based Copolymer-IL Composite Membranes for CO2 Separation. Membranes 2023, 13, 26. [Google Scholar] [CrossRef]
- Mulk, W.U.; Ali, S.A.; Shah, S.N.; Shah, M.U.H.; Zhang, Q.-J.; Younas, M.; Fatehizadeh, A.; Sheikh, M.; Rezakazemi, M. Breaking boundaries in CO2 capture: Ionic liquid-based membrane separation for post-combustion applications. J. CO2 Util. 2023, 75, 102555. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, L.; Yuan, Z.; Semiat, R.; He, X. CO2-Philic Imidazolium-Based Poly(Ionic Liquid) Composite Membranes for Enhanced CO2/N2 Separation. Ind. Eng. Chem. Res. 2023, 62, 8902–8910. [Google Scholar] [CrossRef]
- Ravula, S.; Wise, K.W.; Shinde, P.S.; Bara, J.E. Design and Performance of Di- and Tricationic Poly(ionic liquid) + Ionic Liquid Composite Membranes for CO2 Separation. Macromolecules 2023, 56, 6126–6141. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, L.; Wang, K.; Lin, R.; Xiao, Z.; Semiat, R.; He, X. Molecular Engineering of Copoly(ionic liquids)-Based Membranes for CO2 Separation. ACS Appl. Polym. Mater. 2024, 6, 1853–1863. [Google Scholar] [CrossRef]
- Zhang, M.; Semiat, R.; He, X. Highly CO2-selective composite membranes from amino-functionalized imidazolium-based Poly(ionic liquids). Sep. Purif. Technol. 2024, 345, 127281. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, X.; Zhang, C.; Chen, J.; Lin, W.; Meng, J. A tough double-network ion gel membrane based on poly (ionic liquid) for efficient carbon capture. Sep. Purif. Technol. 2024, 331, 125591. [Google Scholar] [CrossRef]
- Patil, T.; Dharaskar, S.; Sinha, M.k.; Pandya, J.; Shinde, S.; kumar Jampa, S.S.; Sillanpaa, M.; Yoo, C. Efficient CO2/CH4 separation using [Bmim][Ac]/Pebax-1657 supported ionic liquid membranes and its prediction by density functional theory. Int. J. Greenh. Gas Control 2023, 124, 103856. [Google Scholar] [CrossRef]
- He, S.; Kamio, E.; Zhang, J.; Matsuoka, A.; Nakagawa, K.; Yoshioka, T.; Matsuyama, H. Development of an ion gel membrane containing a CO2-philic ionic liquid in interpenetrating semi-crystalline and crosslinkable polymer networks. J. Membr. Sci. 2023, 685, 121912. [Google Scholar] [CrossRef]
- Lee, C.S.; Song, E.; Park, J.T.; Kim, J.H. Ultrathin, Highly Permeable Graphene Oxide/Zeolitic Imidazole Framework Polymeric Mixed-Matrix Composite Membranes: Engineering the CO2-Philic Pathway. ACS Sustain. Chem. Eng. 2021, 9, 11903–11915. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Q.; Su, J.; Ma, B.; Wan, Y.; Zhong, R.; Zou, R. Graphene-Oxide-Modified Metal–Organic Frameworks Embedded in Mixed-Matrix Membranes for Highly Efficient CO2/N2 Separation. Nanomaterials 2024, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Zunita, M.; Natola, O.W.; David, M.; Lugito, G. Integrated metal organic framework/ionic liquid-based composite membrane for CO2 separation. Chem. Eng. J. Adv. 2022, 11, 100320. [Google Scholar] [CrossRef]
- Habib, N.; Durak, Ö.; Uzun, A.; Keskin, S. Incorporation of a pyrrolidinium-based ionic liquid/MIL-101(Cr) composite into Pebax sets a new benchmark for CO2/N2 selectivity. Sep. Purif. Technol. 2023, 312, 123346. [Google Scholar] [CrossRef]
- Iqbal, Z.; Shamair, Z.; Usman, M.; Gilani, M.A.; Yasin, M.; Saqib, S.; Khan, A.L. One pot synthesis of UiO-66@IL composite for fabrication of CO2 selective mixed matrix membranes. Chemosphere 2022, 303, 135122. [Google Scholar] [CrossRef] [PubMed]
- Ghadiri, M.; Aroujalian, A.; Pazani, F.; Salimi, P. Tailoring filler/gas vs. filler/polymer interactions via optimizing Co/Zn ratio in bimetallic ZIFs and decorating on GO nanosheets for enhanced CO2 separation. Sep. Purif. Technol. 2024, 330, 125315. [Google Scholar] [CrossRef]
- Habib, N.; Durak, O.; Zeeshan, M.; Uzun, A.; Keskin, S. A novel IL/MOF/polymer mixed matrix membrane having superior CO2/N2 selectivity. J. Membr. Sci. 2022, 658, 120712. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, Y.; Geng, C.; Zhang, Z.; Qiao, Z.; Zhong, C. Improved CO2/N2 separation performance by relatively continuous and defect-free distribution of IL-encapsulated ZIF-67 in ion gel membranes. J. Membr. Sci. 2023, 683, 121818. [Google Scholar] [CrossRef]
- Lai, W.-H.; Wang, D.K.; Wey, M.-Y.; Tseng, H.-H. ZIF-8/styrene-IL polymerization hollow fiber membrane for improved CO2/N2 separation. J. Clean Prod. 2022, 372, 133785. [Google Scholar] [CrossRef]
- Sun, Y.; Qin, Z.; Geng, C.; Zhang, Z.; Qiao, Z.; Chen, A. Enhancing CO2/N2 separation performances by turning membrane affinity for CO2. Sep. Purif. Technol. 2024, 337, 126377. [Google Scholar] [CrossRef]
- Gebremariam, S.K.; Mathai Varghese, A.; Reddy, K.S.K.; Fowad AlWahedi, Y.; Dumée, L.F.; Karanikolos, G.N. Polymer-aided microstructuring of moisture-stable GO-hybridized MOFs for carbon dioxide capture. Chem. Eng. J. 2023, 473, 145286. [Google Scholar] [CrossRef]
- Sanders, D.F.; Smith, Z.P.; Guo, R.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-efficient polymeric gas separation membranes for a sustainable future: A review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef]
- Baker, R.W.; Wijmans, J.G.; Huang, Y. Permeability, permeance and selectivity: A preferred way of reporting pervaporation performance data. J. Membr. Sci. 2010, 348, 346–352. [Google Scholar] [CrossRef]
- Du, N.; Park, H.B.; Dal-Cin, M.M.; Guiver, M.D. Advances in high permeability polymeric membrane materials for CO2 separations. Energy Environ. Sci. 2012, 5, 7306–7322. [Google Scholar] [CrossRef]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane Gas Separation: A Review/State of the Art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Fu, Q.; Halim, A.; Kim, J.; Scofield, J.M.P.; Gurr, P.A.; Kentish, S.E.; Qiao, G.G. Highly permeable membrane materials for CO2 capture. J. Mater. Chem. A 2013, 1, 13769–13778. [Google Scholar] [CrossRef]
- Mokarinezhad, N.; Hosseini, S.S.; Nxumalo, E.N. Development of polyamide/polyacrylonitrile thin film composite RO membranes by interfacial polymerization assisted with an aromatic/aliphatic organic solvent mixture. J. Appl. Polym. Sci. 2023, 140, e53811. [Google Scholar] [CrossRef]
- Almansour, F.; Foster, A.B.; Ameen, A.W.; Mohsenpour, S.; Budd, P.M.; Gorgojo, P. High gas permeance in CO2-selective thin film composite membranes from bis(phenyl)fluorene-containing blends with PIM-1. J. Membr. Sci. 2024, 699, 122652. [Google Scholar] [CrossRef]
- Liu, M.; Nothling, M.D.; Zhang, S.; Fu, Q.; Qiao, G.G. Thin film composite membranes for postcombustion carbon capture: Polymers and beyond. Prog. Polym. Sci. 2022, 126, 101504. [Google Scholar] [CrossRef]
- Zhao, H.; Wei, M.; Ding, X.; Song, T.; Wang, Y.; Dong, J.; Sun, S.; Hou, J.; Ma, Q.; Tan, X. Polymerization-induced self-assembly amino acid ionic liquid/poly(ethylene oxide) thin-film composite membranes for CO2 separation. J. Membr. Sci. 2024, 707, 123033. [Google Scholar] [CrossRef]
- Fan, S.-T.; Wang, J.-X.; Liao, L.-G.; Feng, J.-F.; Li, B.-J.; Zhang, S. Enhanced selectivity in thin film composite membrane for CO2 capture through improvement to support layer. Chem. Eng. J. 2023, 468, 143645. [Google Scholar] [CrossRef]
- Han, H.; Scofield, J.M.P.; Gurr, P.A.; Webley, P.A.; Qiao, G.G. Breaking barriers: Unleashing CO2 selectivity with ultrathin poly(1,3-dioxolane) composite membranes produced by continuous assembly of polymers. J. Membr. Sci. 2024, 692, 122272. [Google Scholar] [CrossRef]
- Yu, C.; Cen, X.; Ao, D.; Qiao, Z.; Zhong, C. Preparation of thin-film composite membranes with ultrahigh MOFs loading through polymer-template MOFs induction secondary interfacial polymerization. Appl. Surf. Sci. 2023, 614, 156186. [Google Scholar] [CrossRef]
- Roh, D.K.; Kim, S.J.; Jeon, H.; Kim, J.H. Nanocomposites with graft copolymer-templated mesoporous MgTiO3 perovskite for CO2 capture applications. ACS Appl. Mater. Interfaces 2013, 5, 6615–6621. [Google Scholar] [CrossRef]
- Kim, N.U.; Park, B.J.; Lee, J.H.; Kim, J.H. High-performance ultrathin mixed-matrix membranes based on an adhesive PGMA-co-POEM comb-like copolymer for CO2 capture. J. Mater. Chem. A 2019, 7, 14723–14731. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, Z.; Dai, Y.; Zhong, S.; Li, J. Efficient CO2 separation by ionic liquid nanoconfined in ultra-thin TCOH@Pebax-1657 MMM. Sep. Purif. Technol. 2023, 325, 124667. [Google Scholar] [CrossRef]
- Song, S.; Zhao, M.; Guo, Z.; Ren, Y.; Wang, J.; Liang, X.; Pu, Y.; Wang, S.; Ma, H.; Wang, X.; et al. Mixed matrix composite membranes with MOF-protruding structure for efficient CO2 separation. J. Membr. Sci. 2023, 669, 121340. [Google Scholar] [CrossRef]
- Pazani, F.; Shariatifar, M.; Salehi Maleh, M.; Alebrahim, T.; Lin, H. Challenge and promise of mixed matrix hollow fiber composite membranes for CO2 separations. Sep. Purif. Technol. 2023, 308, 122876. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, N.; Tang, S.; He, X.; Chu, J.; Zeng, L.; Zhang, P.; Tang, K. PEI@MOFs thin film nanocomposite (TFN) membrane for efficient CO2 separation. Appl. Surf. Sci. 2023, 640, 158414. [Google Scholar] [CrossRef]
- Fakoori, M.; Azdarpour, A.; Abedini, R.; Honarvar, B. Effect of Cu-MOFs incorporation on gas separation of Pebax thin film nanocomposite (TFN) membrane. Korean J. Chem. Eng. 2021, 38, 121–128. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, X.; Wang, Y.; Hu, F.; Lu, X.; Li, J. Mixed matrix composite membranes based on Pebax and nano-amorphous MIP-202 for CO2 separation. J. Membr. Sci. 2024, 692, 122290. [Google Scholar] [CrossRef]
- Zeng, H.; He, S.; Hosseini, S.S.; Zhu, B.; Shao, L. Emerging nanomaterial incorporated membranes for gas separation and pervaporation towards energetic-efficient applications. Adv. Membr. 2022, 2, 100015. [Google Scholar] [CrossRef]
- Li, W.-L.; Fu, P.; Lin, W.-T.; Zhang, Z.-L.; Luo, X.-W.; Yu, Y.-H.; Xu, Z.-K.; Wan, L.-S. High-performance thin-film composite (TFC) membranes with 2D nanomaterial interlayers: An overview. Results Eng. 2024, 21, 101932. [Google Scholar] [CrossRef]
- Xie, K.; Fu, Q.; Xu, C.; Lu, H.; Zhao, Q.; Curtain, R.; Gu, D.; Webley, P.A.; Qiao, G.G. Continuous assembly of a polymer on a metal–organic framework (CAP on MOF): A 30 nm thick polymeric gas separation membrane. Energy Environ. Sci. 2018, 11, 544–550. [Google Scholar] [CrossRef]
- Martínez-Izquierdo, L.; García-Comas, C.; Dai, S.; Navarro, M.; Tissot, A.; Serre, C.; Téllez, C.; Coronas, J. Ultrasmall Functionalized UiO-66 Nanoparticle/Polymer Pebax 1657 Thin-Film Nanocomposite Membranes for Optimal CO2 Separation. ACS Appl. Mater. Interfaces 2024, 16, 4024–4034. [Google Scholar] [CrossRef]
- Wong, K.C.; Goh, P.S.; Ismail, A.F. Gas separation performance of thin film nanocomposite membranes incorporated with polymethyl methacrylate grafted multi-walled carbon nanotubes. Int. Biodeterior. Biodegrad. 2015, 102, 339–345. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Zhang, Y.; Ding, X.; Liu, J. Thin film composite membranes functionalized with montmorillonite and hydrotalcite nanosheets for CO2/N2 separation. Sep. Purif. Technol. 2017, 189, 128–137. [Google Scholar] [CrossRef]
- Zhu, J.; Hou, J.; Uliana, A.; Zhang, Y.; Tian, M.; Van der Bruggen, B. The rapid emergence of two-dimensional nanomaterials for high-performance separation membranes. J. Mater. Chem. A 2018, 6, 3773–3792. [Google Scholar] [CrossRef]
- Wu, H.; Li, Q.; Guo, B.; Sheng, M.; Wang, D.; Mao, S.; Ye, N.; Qiao, Z.; Kang, G.; Cao, Y.; et al. Industrial-scale spiral-wound facilitated transport membrane modules for post-combustion CO2 capture: Development, investigation and optimization. J. Membr. Sci. 2023, 670, 121368. [Google Scholar] [CrossRef]
- Bui, V.; Satti, V.R.; Haddad, E.; Hu, L.; Deng, E.; Zhu, L.; Lee, W.-I.; Yin, Y.; Kisslinger, K.; Zhang, Y.; et al. Ultrathin polyorganosilica membranes synthesized by oxygen-plasma treatment of polysiloxanes for H2/CO2 separation. J. Membr. Sci. 2023, 688, 122099. [Google Scholar] [CrossRef]
- Lie, J.A.; Vassbotn, T.; Hägg, M.-B.; Grainger, D.; Kim, T.-J.; Mejdell, T. Optimization of a membrane process for CO2 capture in the steelmaking industry. Int. J. Greenh. Gas Control 2007, 1, 309–317. [Google Scholar] [CrossRef]
- Qin, Z.; Feng, X.; Yin, D.; Xin, B.; Jin, Z.; Deng, Y.; Yang, L.; Yao, L.; Jiang, W.; Liu, C.; et al. Impact of Humidity on the CO2/N2 Separation Performance of Pebax-MOF Mixed Matrix Membranes. Ind. Eng. Chem. Res. 2023, 62, 14034–14046. [Google Scholar] [CrossRef]
- Lin, R.-B.; Xiang, S.; Zhou, W.; Chen, B. Microporous Metal-Organic Framework Materials for Gas Separation. Chem 2020, 6, 337–363. [Google Scholar] [CrossRef]
- Demir, H.; Aksu, G.O.; Gulbalkan, H.C.; Keskin, S. MOF Membranes for CO2 Capture: Past, Present and Future. Carbon Capture Sci. Technol. 2022, 2, 100026. [Google Scholar] [CrossRef]
- Yan, J.; Sun, Y.; Ji, T.; Zhang, C.; Liu, L.; Liu, Y. Room-temperature synthesis of defect-engineered Zirconium-MOF membrane enabling superior CO2/N2 selectivity with zirconium-oxo cluster source. J. Membr. Sci. 2022, 653, 120496. [Google Scholar] [CrossRef]
- Qian, Q.; Asinger, P.A.; Lee, M.J.; Han, G.; Mizrahi Rodriguez, K.; Lin, S.; Benedetti, F.M.; Wu, A.X.; Chi, W.S.; Smith, Z.P. MOF-Based Membranes for Gas Separations. Chem. Rev. 2020, 120, 8161–8266. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, W. 2D Metal-Organic Framework Materials for Membrane-Based Separation. Adv. Mater. Interfaces 2020, 7, 1901514. [Google Scholar] [CrossRef]
- Lee, H.; Park, S.C.; Roh, J.S.; Moon, G.H.; Shin, J.E.; Kang, Y.S.; Park, H.B. Metal–organic frameworks grown on a porous planar template with an exceptionally high surface area: Promising nanofiller platforms for CO2 separation. J. Mater. Chem. A 2017, 5, 22500–22505. [Google Scholar] [CrossRef]
- Yin, L.; Li, D.; Guo, H.; Wang, S.; Zhang, T.; Liu, Y.; Gai, F.; Zhao, X. High-performance carbonized ZIF-8-doped hybrid carbon molecular sieve membrane for CO2/N2 separation. J. Membr. Sci. 2022, 655, 120610. [Google Scholar] [CrossRef]
- Ge, C.; Sheng, M.; Yuan, Y.; Shi, F.; Yang, Y.; Zhao, S.; Wang, J.; Wang, Z. Pore-optimized MOF-808 made through a facile method using for fabrication of high-performance mixed matrix composite CO2 capture membranes. Carbon Capture Sci. Technol. 2024, 10, 100156. [Google Scholar] [CrossRef]
- Qu, K.; Huang, K.; Xu, J.; Dai, L.; Wang, Y.; Cao, H.; Xia, Y.; Wu, Y.; Xu, W.; Yao, Z.; et al. High-Efficiency CO2/N2 Separation Enabled by Rotation of Electrostatically Anchored Flexible Ligands in Metal–Organic Framework. Angew. Chem. Int. Edit. 2022, 61, e202213333. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xu, J.; Wang, J.; Zhao, S.; Liu, X.; Wang, Z. High-performance membrane with angstrom-scale manipulation of gas transport channels via polymeric decorated MOF cavities. J. Membr. Sci. 2021, 625, 119175. [Google Scholar] [CrossRef]
- Basu, S.; Cano-Odena, A.; Vankelecom, I.F.J. MOF-containing mixed-matrix membranes for CO2/CH4 and CO2/N2 binary gas mixture separations. Sep. Purif. Technol. 2011, 81, 31–40. [Google Scholar] [CrossRef]
- Asadi, E.; Ghadimi, A.; Hosseini, S.S.; Sadatnia, B.; Rostamizadeh, M.; Nadeali, A. Surfactant-mediated and wet-impregnation approaches for modification of ZIF-8 nanocrystals: Mixed matrix membranes for CO2/CH4 separation. Microporous Mesoporous Mater. 2022, 329, 111539. [Google Scholar] [CrossRef]
- Hou, J.; Sutrisna, P.D.; Zhang, Y.; Chen, V. Formation of Ultrathin, Continuous Metal-Organic Framework Membranes on Flexible Polymer Substrates. Angew. Chem.-Int. Edit. 2016, 55, 3947–3951. [Google Scholar] [CrossRef] [PubMed]
- Culp, J.T.; Sui, L.; Goodman, A.; Luebke, D. Carbon dioxide (CO2) absorption behavior of mixed matrix polymer composites containing a flexible coordination polymer. J. Colloid Interface Sci. 2013, 393, 278–285. [Google Scholar] [CrossRef]
- Kusuma, V.A.; Venna, S.R.; Wickramanayake, S.; Dahe, G.J.; Myers, C.R.; O’Connor, J.; Resnik, K.P.; Anthony, J.H.; Hopkinson, D. An automated lab-scale flue gas permeation membrane testing system at the National Carbon Capture Center. J. Membr. Sci. 2017, 533, 28–37. [Google Scholar] [CrossRef]
- Liu, M.; Xie, K.; Nothling, M.D.; Gurr, P.A.; Tan, S.S.L.; Fu, Q.; Webley, P.A.; Qiao, G.G. Ultrathin Metal-Organic Framework Nanosheets as a Gutter Layer for Flexible Composite Gas Separation Membranes. ACS Nano 2018, 12, 11591–11599. [Google Scholar] [CrossRef]
- Liu, M.; Xie, K.; Nothling, M.D.; Zu, L.; Zhao, S.; Harvie, D.J.E.; Fu, Q.; Webley, P.A.; Qiao, G.G. Ultrapermeable Composite Membranes Enhanced Via Doping with Amorphous MOF Nanosheets. ACS Cent. Sci. 2021, 7, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Elsaidi, S.K.; Ostwal, M.; Zhu, L.; Sekizkardes, A.; Mohamed, M.H.; Gipple, M.; McCutcheon, J.R.; Hopkinson, D. 3D printed MOF-based mixed matrix thin-film composite membranes. RSC Adv. 2021, 11, 25658–25663. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Nothling, M.D.; Webley, P.A.; Jin, J.; Fu, Q.; Qiao, G.G. High-throughput CO2 capture using PIM-1@MOF based thin film composite membranes. Chem. Eng. J. 2020, 396, 125328. [Google Scholar] [CrossRef]
- Bakhtin, D.S.; Kulikov, L.A.; Bondarenko, G.N.; Vasilevskii, V.P.; Maksimov, A.L.; Volkov, A.V. Stabilization of Gas Transport Properties of Composite Membranes with a Thin PTMSP Selective Layer by Adding Porous Aromatic Framework Nanoparticles and Simultaneous Polymer Crosslinking. Pet. Chem. 2018, 58, 790–796. [Google Scholar] [CrossRef]
- Bakhtin, D.S.; Kulikov, L.A.; Legkov, S.A.; Khotimskiy, V.S.; Levin, I.S.; Borisov, I.L.; Maksimov, A.L.; Volkov, V.V.; Karakhanov, E.A.; Volkov, A.V. Aging of thin-film composite membranes based on PTMSP loaded with porous aromatic frameworks. J. Membr. Sci. 2018, 554, 211–220. [Google Scholar] [CrossRef]
- Lee, C.S.; Kang, M.; Kim, K.C.; Kim, J.H. In-situ formation of asymmetric thin-film, mixed-matrix membranes with ZIF-8 in dual-functional imidazole-based comb copolymer for high-performance CO2 capture. J. Membr. Sci. 2022, 642, 119913. [Google Scholar] [CrossRef]
- Kang, M.; Kim, T.-H.; Han, H.H.; Min, H.J.; Bae, Y.-S.; Kim, J.H. Submicron-thick, mixed-matrix membranes with metal-organic frameworks for CO2 separation: MIL-140C vs. UiO-67. J. Membr. Sci. 2022, 659, 120788. [Google Scholar] [CrossRef]
- Min, H.J.; Kang, M.; Bae, Y.-S.; Blom, R.; Grande, C.A.; Kim, J.H. Thin-film composite mixed-matrix membrane with irregular micron-sized UTSA-16 for outstanding gas separation performance. J. Membr. Sci. 2023, 669, 121295. [Google Scholar] [CrossRef]
- Min, H.J.; Kim, M.-B.; Bae, Y.-S.; Thallapally, P.K.; Lee, J.H.; Kim, J.H. Polymer-Infiltrated Metal–Organic Frameworks for Thin-Film Composite Mixed-Matrix Membranes with High Gas Separation Properties. Membranes 2023, 13, 287. [Google Scholar] [CrossRef]
- Aliyev, E.; Warfsmann, J.; Tokay, B.; Shishatskiy, S.; Lee, Y.J.; Lillepaerg, J.; Champness, N.R.; Filiz, V. Gas Transport Properties of the Metal-Organic Framework (MOF)-Assisted Polymer of Intrinsic Microporosity (PIM-1) Thin-Film Composite Membranes. ACS Sustain. Chem. Eng. 2021, 9, 684–694. [Google Scholar] [CrossRef]
- Bakhtin, D.S.; Malakhov, A.O.; Volkov, A.V.; Kulikov, L.A.; Petrova, I.V.; Borisov, I.L.; Bazhenov, S.D. Mitigating of Thin-Film Composite PTMSP Membrane Aging by Introduction of Porous Rigid and Soft Branched Polymeric Additives. Membranes 2023, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Feng, W.; Sheng, M.; Yuan, Y.; Wang, B.; Wang, J.; Wang, Z. Covalent organic frameworks-incorporated thin film composite membranes prepared by interfacial polymerization for efficient CO2 separation. Chin. J. Chem. Eng. 2022, 43, 152–160. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, Y.; Shi, F.; Li, Q.; Zhao, S.; Wang, J.; Sheng, M.; Wang, Z. Enhancing dispersibility of nanofiller via polymer-modification for preparation of mixed matrix membrane with high CO2 separation performance. J. Membr. Sci. 2023, 683, 121791. [Google Scholar] [CrossRef]
- Liu, G.; Jin, W.; Xu, N. Graphene-based membranes. Chem. Soc. Rev. 2015, 44, 5016–5030. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Liu, G.; Huang, K.; Jin, W.; Lee, K.-R.; Xu, N. Membranes with Fast and Selective Gas-Transport Channels of Laminar Graphene Oxide for Efficient CO2 Capture. Angew. Chem.-Int. Edit. 2015, 54, 578–582. [Google Scholar] [CrossRef]
- Kim, H.W.; Yoon, H.W.; Yoon, S.-M.; Yoo, B.M.; Ahn, B.K.; Cho, Y.H.; Shin, H.J.; Yang, H.; Paik, U.; Kwon, S.; et al. Selective Gas Transport Through Few-Layered Graphene and Graphene Oxide Membranes. Science 2013, 342, 91–95. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Y.; Zhang, H.; Wang, S.; Jiang, Z.; Guo, R.; Wu, H. Efficient CO2 Capture by Functionalized Graphene Oxide Nanosheets as Fillers To Fabricate Multi-Permselective Mixed Matrix Membranes. ACS Appl. Mater. Interfaces 2015, 7, 5528–5537. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, M.; Liu, G.; Guan, K.; Jin, W. Size effects of graphene oxide on mixed matrix membranes for CO2 separation. AlChE J. 2016, 62, 2843–2852. [Google Scholar] [CrossRef]
- Karunakaran, M.; Shevate, R.; Kumar, M.; Peinemann, K.V. CO2-selective PEO–PBT (PolyActive™)/graphene oxide composite membranes. Chem. Commun. 2015, 51, 14187–14190. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, K.; Yang, Z.; Bauer, R.A.; Hong, N.; Verweij, H. Nanometer-Thick Supported Graphene Oxide Membrane for CO2 Capture. ACS Appl. Nano Mater. 2020, 3, 6654–6663. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Zhang, X.; Li, J.; Liu, C.; Li, N.; Xie, Z. Polyvinylamine/graphene oxide/PANI@CNTs mixed matrix composite membranes with enhanced CO2/N2 separation performance. J. Membr. Sci. 2019, 589, 117246. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Lee, J.; Song, J.; Bae, T.-H. Carbon Molecular Sieve Membranes Comprising Graphene Oxides and Porous Carbon for CO2/N2 Separation. Membranes 2021, 11, 284. [Google Scholar] [CrossRef] [PubMed]
- Mehdinia Lichaei, M.; Pazani, F.; Aroujalian, A.; Rodrigue, D. Two-step surface functionalization/alignment strategy to improve CO2/N2 separation from mixed matrix membranes based on PEBAX and graphene oxide. Process Saf. Environ. Protect. 2022, 163, 36–47. [Google Scholar] [CrossRef]
- Wang, S.; Wu, Y.; Zhang, N.; He, G.; Xin, Q.; Wu, X.; Wu, H.; Cao, X.; Guiver, M.D.; Jiang, Z. A highly permeable graphene oxide membrane with fast and selective transport nanochannels for efficient carbon capture. Energy Environ. Sci. 2016, 9, 3107–3112. [Google Scholar] [CrossRef]
- Ying, Y.; Liu, D.; Ma, J.; Tong, M.; Zhang, W.; Huang, H.; Yang, Q.; Zhong, C. A GO-assisted method for the preparation of ultrathin covalent organic framework membranes for gas separation. J. Mater. Chem. A 2016, 4, 13444–13449. [Google Scholar] [CrossRef]
- Janakiram, S.; Martín Espejo, J.L.; Yu, X.; Ansaloni, L.; Deng, L. Facilitated transport membranes containing graphene oxide-based nanoplatelets for CO2 separation: Effect of 2D filler properties. J. Membr. Sci. 2020, 616, 118626. [Google Scholar] [CrossRef]
- Choi, O.; Hossain, I.; Jeong, I.; Park, C.H.; Kim, Y.; Kim, T.H. Modified graphene oxide-incorporated thin-film composite hollow fiber membranes through interface polymerization on hydrophilic substrate for CO2 separation. Membranes 2021, 11, 650. [Google Scholar] [CrossRef]
- Li, H.; Ding, X.; Zhang, Y.; Liu, J. Porous graphene nanosheets functionalized thin film nanocomposite membrane prepared by interfacial polymerization for CO2/N2 separation. J. Membr. Sci. 2017, 543, 58–68. [Google Scholar] [CrossRef]
- Wong, K.C.; Goh, P.S.; Ismail, A.F. Highly permeable and selective graphene oxide-enabled thin film nanocomposite for carbon dioxide separation. Int. J. Greenh. Gas Control 2017, 64, 257–266. [Google Scholar] [CrossRef]
- Olivieri, L.; Meneguzzo, S.; Ligi, S.; Saccani, A.; Giorgini, L.; Orsini, A.; Pettinau, A.; De Angelis, M.G. Reducing ageing of thin PTMSP films by incorporating graphene and graphene oxide: Effect of thickness, gas type and temperature. J. Membr. Sci. 2018, 555, 258–267. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Q.; Hou, J.; Sutrisna, P.D.; Chen, V. Shear-aligned graphene oxide laminate/Pebax ultrathin composite hollow fiber membranes using a facile dip-coating approach. J. Mater. Chem. A 2017, 5, 7732–7737. [Google Scholar] [CrossRef]
- Lee, C.S.; Moon, J.; Park, J.T.; Kim, J.H. Engineering CO2-philic pathway via grafting poly (ethylene glycol) on graphene oxide for mixed matrix membranes with high CO2 permeance. Chem. Eng. J. 2023, 453, 139818. [Google Scholar] [CrossRef]
- Dai, Z.; Noble, R.D.; Gin, D.L.; Zhang, X.; Deng, L. Combination of ionic liquids with membrane technology: A new approach for CO2 separation. J. Membr. Sci. 2016, 497, 1–20. [Google Scholar] [CrossRef]
- Zeng, S.; Zhang, X.; Bai, L.; Zhang, X.; Wang, H.; Wang, J.; Bao, D.; Li, M.; Liu, X.; Zhang, S. Ionic-Liquid-Based CO2 Capture Systems: Structure, Interaction and Process. Chem. Rev. 2017, 117, 9625–9673. [Google Scholar] [CrossRef]
- Uk Hong, S.; Park, D.; Ko, Y.; Baek, I. Polymer-ionic liquid gels for enhanced gas transport. Chem. Commun. 2009, 2009, 7227–7229. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Z.; Li, P.; Chung, T.-S. PVDF/ionic liquid polymer blends with superior separation performance for removing CO2 from hydrogen and flue gas. Int. J. Hydrogen Energy 2012, 37, 11796–11804. [Google Scholar] [CrossRef]
- Pishva, S.; Hassanajili, S. Investigation on effect of ionic liquid on CO2 separation performance and properties of novel co-casted dual-layer PEBAX-ionic liquid/PES composite membrane. J. Ind. Eng. Chem. 2022, 107, 180–196. [Google Scholar] [CrossRef]
- Aghajohari, M.; Fazeli-Khosh, H.; Adibi, M.; Sharif, A. Thin Film Composite Membrane Comprising Ionic Liquid/Graphene Oxide in the Selective Layer for Enhanced CO2 Separation. ACS Appl. Polym. Mater. 2024, 6, 2576–2585. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Z.; Yang, C.; Liu, J.; Shen, H.; Yang, K.; Wang, Z. PIM-based mixed-matrix membranes containing MOF-801/ionic liquid nanocomposites for enhanced CO2 separation performance. J. Membr. Sci. 2021, 636, 119581. [Google Scholar] [CrossRef]
- Habib, N.; Tarhanlı, I.; Senses, E.; Keskin, S.; Uzun, A. IL-modified MOF-177 filler boosts the CO2/N2 selectivity of Pebax membrane. J. Membr. Sci. 2024, 710, 123143. [Google Scholar] [CrossRef]
- Pandya, I.; El Seoud, O.A.; Assiri, M.A.; Kumar Kailasa, S.; Malek, N.I. Ionic liquid/ metal organic framework composites as a new class of materials for CO2 capture: Present scenario and future perspective. J. Mol. Liq. 2024, 395, 123907. [Google Scholar] [CrossRef]
- Klepić, M.; Fuoco, A.; Monteleone, M.; Esposito, E.; Friess, K.; Izák, P.; Jansen, J.C. Effect of the CO2-philic ionic liquid [BMIM][Tf2N] on the single and mixed gas transport in PolyActive™ membranes. Sep. Purif. Technol. 2021, 256, 117813. [Google Scholar] [CrossRef]
- Ghasemi Estahbanati, E.; Omidkhah, M.; Ebadi Amooghin, A. Preparation and characterization of novel Ionic liquid/Pebax membranes for efficient CO2/light gases separation. J. Ind. Eng. Chem. 2017, 51, 77–89. [Google Scholar] [CrossRef]
- Lim, J.Y.; Lee, J.H.; Park, M.S.; Kim, J.H.; Kim, J.H. Hybrid membranes based on ionic-liquid-functionalized poly(vinyl benzene chloride) beads for CO2 capture. J. Membr. Sci. 2019, 572, 365–373. [Google Scholar] [CrossRef]
- Fam, W.; Mansouri, J.; Li, H.; Hou, J.; Chen, V. Effect of Inorganic Salt Blending on the CO2 Separation Performance and Morphology of Pebax1657/Ionic Liquid Gel Membranes. Ind. Eng. Chem. Res. 2019, 58, 3304–3313. [Google Scholar] [CrossRef]
- Chi, W.S.; Hong, S.U.; Jung, B.; Kang, S.W.; Kang, Y.S.; Kim, J.H. Synthesis, structure and gas permeation of polymerized ionic liquid graft copolymer membranes. J. Membr. Sci. 2013, 443, 54–61. [Google Scholar] [CrossRef]
- Jomekian, A.; Bazooyar, B.; Behbahani, R.M. Experimental and modeling study of CO2 separation by modified Pebax 1657 TFC membranes. Korean J. Chem. Eng. 2020, 37, 2020–2040. [Google Scholar] [CrossRef]
- Fam, W.; Mansouri, J.; Li, H.; Chen, V. Improving CO2 separation performance of thin film composite hollow fiber with Pebax®1657/ionic liquid gel membranes. J. Membr. Sci. 2017, 537, 54–68. [Google Scholar] [CrossRef]
- Huang, G.; Isfahani, A.P.; Muchtar, A.; Sakurai, K.; Shrestha, B.B.; Qin, D.; Yamaguchi, D.; Sivaniah, E.; Ghalei, B. Pebax/ionic liquid modified graphene oxide mixed matrix membranes for enhanced CO2 capture. J. Membr. Sci. 2018, 565, 370–379. [Google Scholar] [CrossRef]
- Nikolaeva, D.; Azcune, I.; Sheridan, E.; Sandru, M.; Genua, A.; Tanczyk, M.; Jaschik, M.; Warmuzinski, K.; Jansen, J.C.; Vankelecom, I.F.J. Poly(vinylbenzyl chloride)-based poly(ionic liquids) as membranes for CO2 capture from flue gas. J. Mater. Chem. A 2017, 5, 19808–19818. [Google Scholar] [CrossRef]
- Nikolaeva, D.; Verachtert, K.; Azcune, I.; Jansen, J.C.; Vankelecom, I.F.J. Influence of ionic liquid-like cationic pendants composition in cellulose based polyelectrolytes on membrane-based CO2 separation. Carbohydr. Polym. 2021, 255, 117375. [Google Scholar] [CrossRef] [PubMed]
- Fam, W.; Mansouri, J.; Li, H.; Hou, J.; Chen, V. Gelled Graphene Oxide-Ionic Liquid Composite Membranes with Enriched Ionic Liquid Surfaces for Improved CO2 Separation. ACS Appl. Mater. Interfaces 2018, 10, 7389–7400. [Google Scholar] [CrossRef] [PubMed]
- Nikolaeva, D.; Loïs, S.; Dahl, P.I.; Sandru, M.; Jaschik, J.; Tanczyk, M.; Fuoco, A.; Jansen, J.C.; Vankelecom, I.F.J. Water Vapour promotes CO2 transport in poly(ionic liquid)/ionic liquid-based thin-film composite membranes containing zinc salt for flue gas treatment. Appl. Sci. 2020, 10, 3859. [Google Scholar] [CrossRef]
- Kang, M.; Min, H.J.; Kim, S.-J.; Kim, J.H. Thin-film composite mixed-matrix membrane based on polymerizable ionic liquid comb copolymer for CO2 separation. J. Membr. Sci. 2024, 698, 122611. [Google Scholar] [CrossRef]
- Nikolaeva, D.; Azcune, I.; Tanczyk, M.; Warmuzinski, K.; Jaschik, M.; Sandru, M.; Dahl, P.I.; Genua, A.; Loïs, S.; Sheridan, E.; et al. The performance of affordable and stable cellulose-based poly-ionic membranes in CO2/N2 and CO2/CH4 gas separation. J. Membr. Sci. 2018, 564, 552–561. [Google Scholar] [CrossRef]
- Zhang, J.; Kamio, E.; Matsuoka, A.; Nakagawa, K.; Yoshioka, T.; Matsuyama, H. Fundamental investigation on the development of composite membrane with a thin ion gel layer for CO2 separation. J. Membr. Sci. 2022, 663, 121032. [Google Scholar] [CrossRef]
- Jiang, X.; Goh, K.; Wang, R. Air plasma assisted spray coating of Pebax-1657 thin-film composite membranes for post-combustion CO2 capture. J. Membr. Sci. 2022, 658, 120741. [Google Scholar] [CrossRef]
- Wang, Z.-X.; Sun, W.-S.; Zhang, W.-H.; Li, S.; Yin, M.-J.; An, Q.-F. Construction of high-performance thin-film composite membrane for CO2 separation via interface engineering. Sep. Purif. Technol. 2023, 322, 124348. [Google Scholar] [CrossRef]

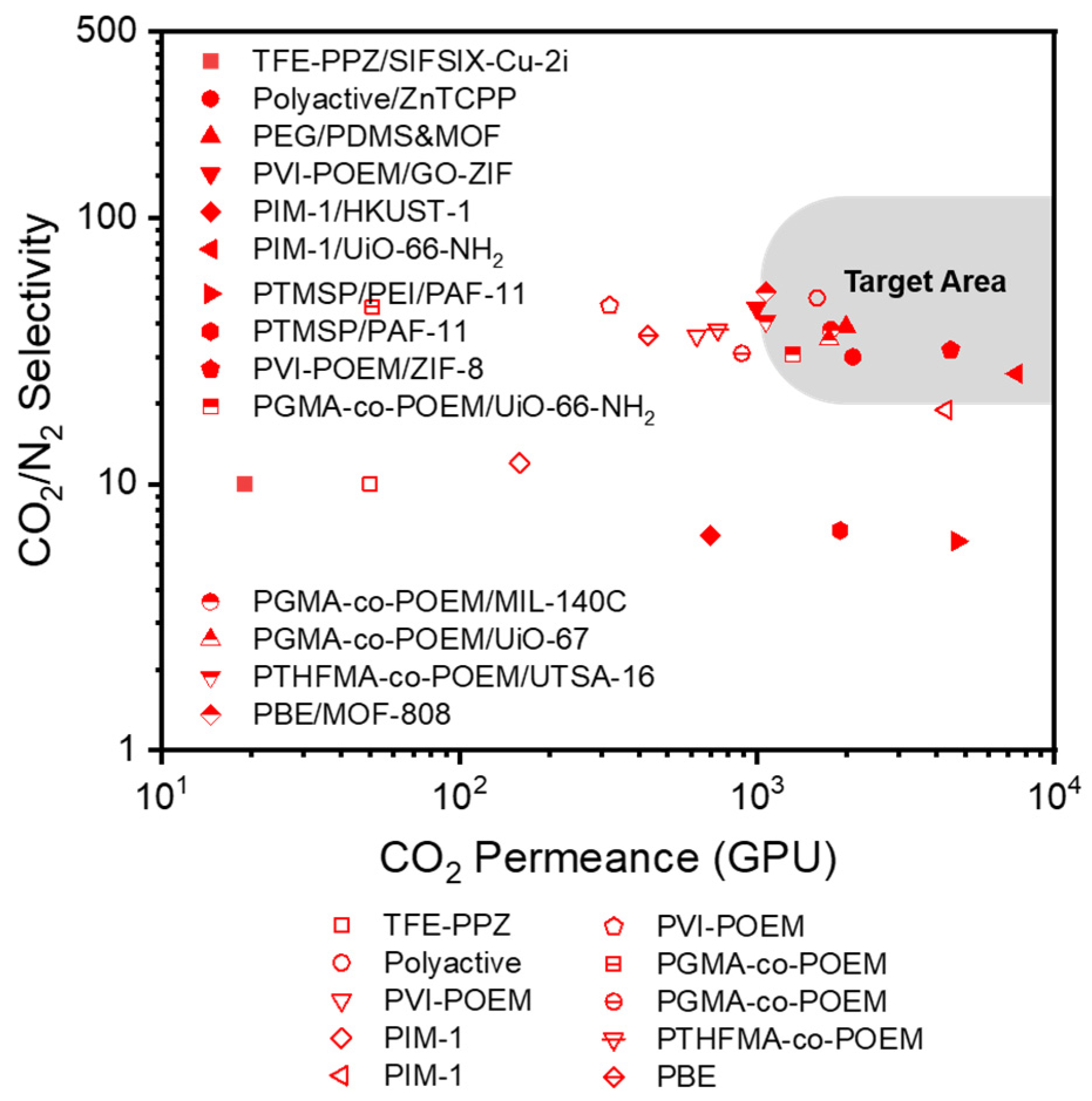

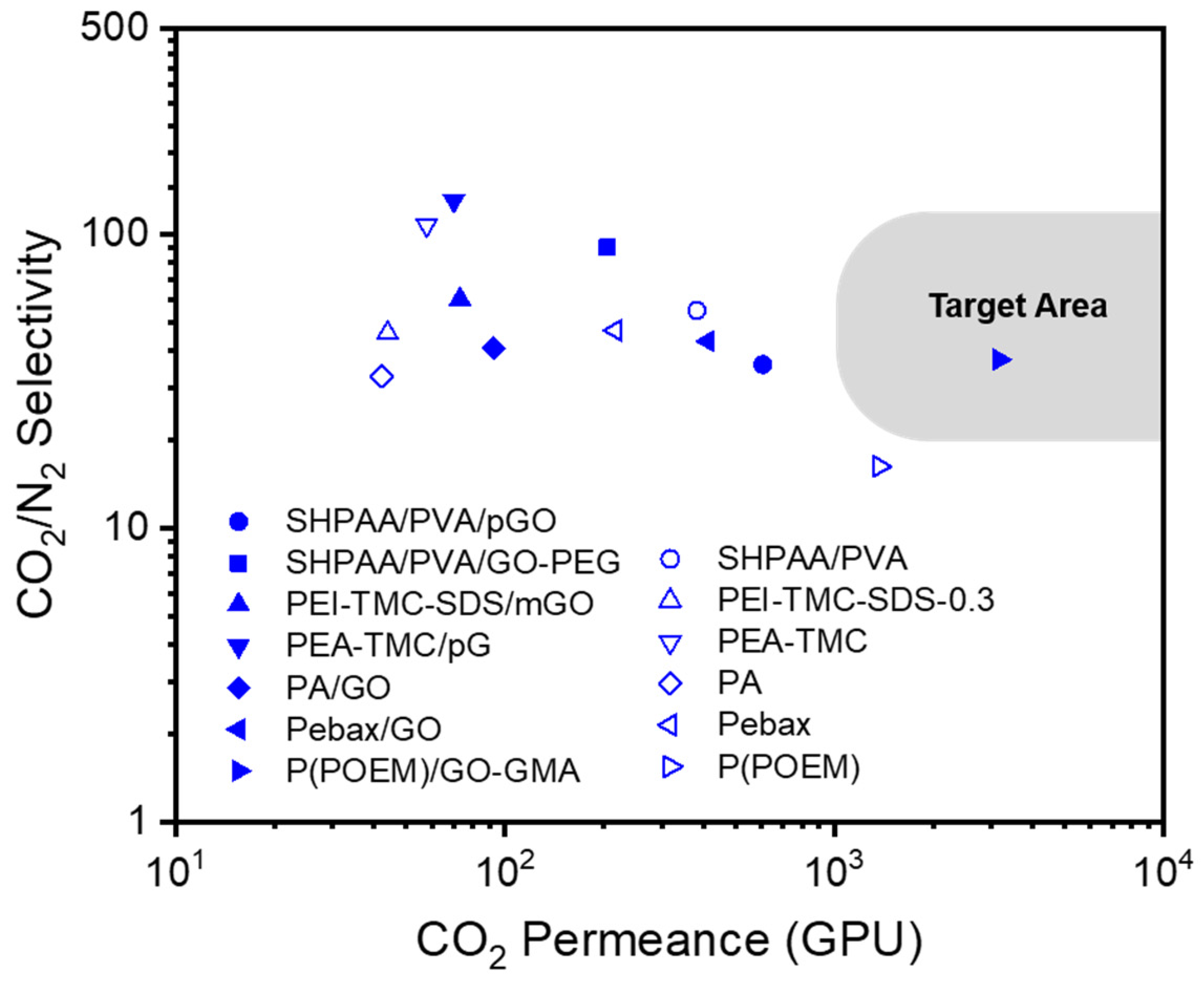
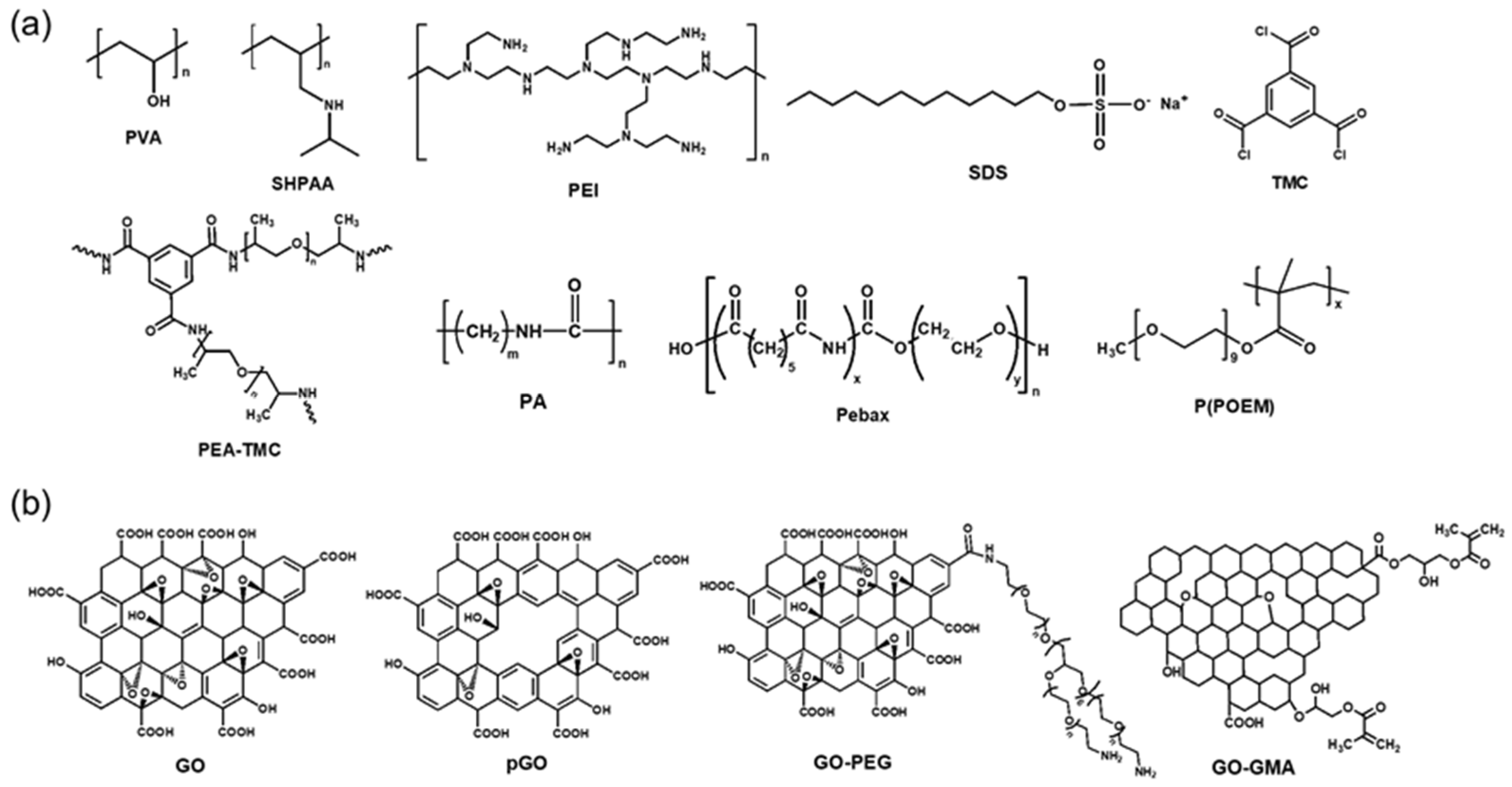
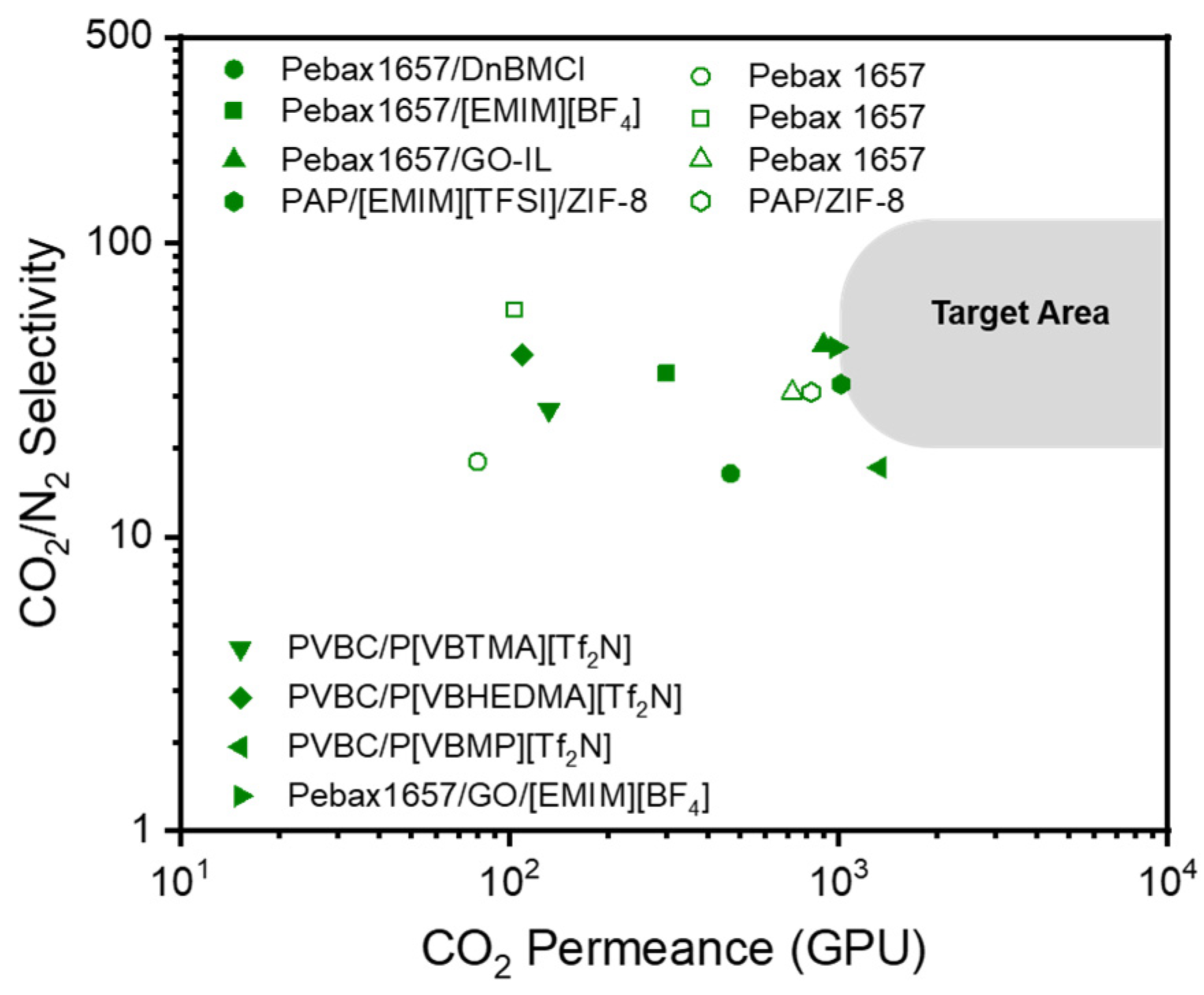
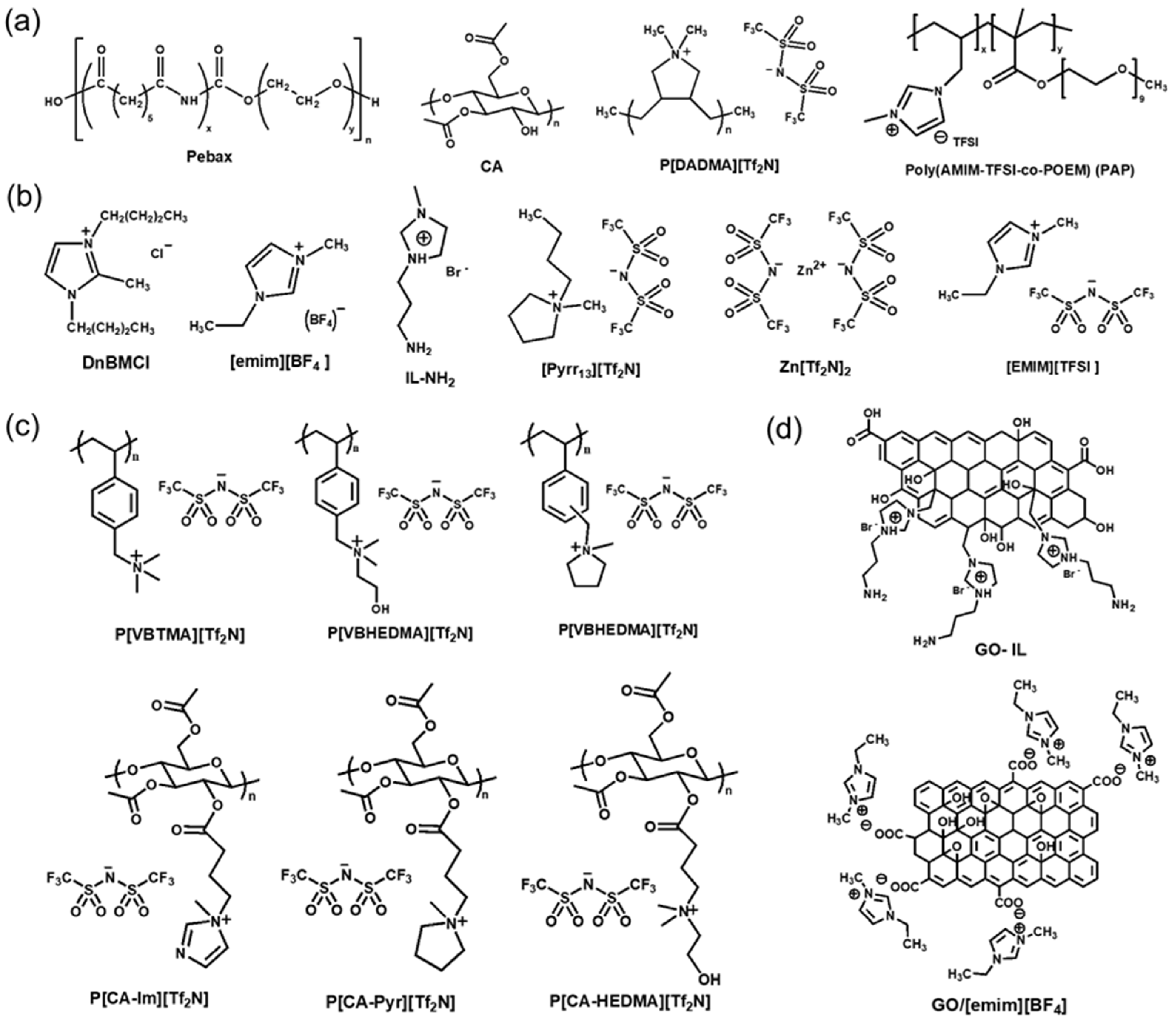


| Support Material | Selective Polymer | Gutter Layer | MOF | Membrane Type | Measurement Conditions | Feed | CO2 Permeance (GPU) | CO2/N2 Selectivity | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Torlon fiber | TFE-PPZ | N/A | SIFSIX-Cu-2i | Hollow fiber | 1.3 bar, 40 °C | Flue gas | 19 | 10 | [109] |
| PAN | Polyactive | ZnTCPP | ZnTCPP | Flat sheet | 1–5 bar, 35 °C | Pure gas | 2100 | 32 | [110] |
| PAN | PEG | PDMS/amorphous MOF nanosheet | N/A | Flat sheet | 1 bar, 35 °C | Pure gas | 1990 | 39 | [111] |
| PSf | PVI-POEM copolymer | PTMSP | GO-ZIF | Flat sheet | 1 bar, 25 °C | Pure gas | 995.1 | 45.9 | [53] |
| PAN | PIM-1 | N/A | HKUST-1 | Flat sheet | 1 bar, 22 °C | Pure gas | 696 | 6.4 | [112] |
| PAN | PIM-1 | PDMS/MOF | UiO-66-NH2 | Flat sheet | 1 bar, 35 °C | Pure gas | 4660–7460 | 26–33 | [113] |
| PAN | PTMSP/PEI | PTMSP | PAF-11 | Flat sheet | 2 bar, 25 °C | Pure gas | 4715 | 6.1 | [114] |
| PAN | PTMSP | PTMSP | PAF-11 | Flat sheet | 2 bar, 25 °C | Pure gas | 1900 | 6.7 | [115] |
| PSf | PVI-POEM copolymer | PTMSP | ZIF-8 | Flat sheet | 1 bar, 30 °C | Pure gas | 4474 | 32 | [116] |
| PSf | PGMA-co-POEM | PTMSP | UiO-66-NH2 | Flat sheet | 1 bar, 25 °C | Pure gas | 1320 | 30.8 | [77] |
| PSf | PGMA-co-POEM | PTMSP | MIL-140C | Flat sheet | 1 bar, 25 °C | Pure gas | 1768 | 38 | [117] |
| UiO-67 | 1745 | 35 | |||||||
| PSf | PTHFMA-co-POEM | PTMSP | UTSA-16 | Flat sheet | 1 bar, 30 °C | Pure gas | 1070 | 41 | [118] |
| PSf | PBE copolymer | PTMSP | MOF-808 | Flat sheet | 1 bar, 30 °C | Pure gas | 1069 | 52.7 | [119] |
| Support Material | Selective Polymer | Gutter Layer | GO | Membrane Type | Measurement Conditions | Feed | CO2 Permeance (GPU) | CO2/N2 Selectivity | Ref |
|---|---|---|---|---|---|---|---|---|---|
| PVDF | SHPAA/PVA | FC-72 | pGO | Flat sheet | 1.7–2 bar, 35 °C | Mixed gas (10/90 v/v of CO2/N2) | 607 | 36 | [136] |
| GO-PEG | 205 | 90 | |||||||
| PES | PEI-TMC-SDS | N/A | mGO | Hollow fiber | 0.25 bar, 25 °C | Pure gas | 73 | 60 | [137] |
| PSf | PEA-TMC | N/A | pG | Flat sheet | 1 bar, 30 °C | Pure gas | 70 | 130 | [138] |
| PSf | PA | N/A | GO | Flat sheet | 2 bar, 25 °C | Pure gas | 92.4 | 41 | [139] |
| PP | PTMSP | N/A | GO | Flat sheet | 1.3 bar, 30 °C | Pure gas | N/A | N/A | [140] |
| PVDF | Pebax | PTMSP | GO | Hollow fiber | 2 bar, 25 °C | Pure gas | 413.3 | 43.2 | [141] |
| PSf | P(POEM) | PTMSP | GO-GMA | Flat sheet | 1 bar, 25 °C | Pure gas | 3169 | 37.4 | [142] |
| Support Material | Selective Polymer | Gutter Layer | IL | Membrane Type | Measurement Conditions | Feed | CO2 Permeance (GPU) | CO2/N2 Selectivity | Ref |
|---|---|---|---|---|---|---|---|---|---|
| PC | Pebax 1657 | N/A | DnBMCl | Flat sheet | 4 bar, 20 °C | Pure gas | 470 | 16.4 | [157] |
| PVDF | Pebax 1657 | PTMSP | [emim][BF4] | Hollow fiber | 3 bar, 35 °C | Pure gas | 300 | 36 | [158] |
| PVDF | Pebax 1657 | PTMSP | GO-IL | Flat sheet | 4 bar, 25 °C | Pure gas | 900 | 45 | [159] |
| Matrimid | PVBC | PDMS protective layer | P[VBTMA] [Tf2N] | Flat sheet | 5 bar, 26 °C | Mixed gas (15/85 v/v of CO2/N2) | 132 | 27.0 | [160] |
| P[VBHEDMA] [Tf2N] | 109 | 41.6 | |||||||
| P[VBMP] [Tf2N] | 1334 | 17.2 | |||||||
| Matrimid | CA | PDMS protective layer | [Im][Tf2N] | Flat sheet | 5 bar, 26 °C | Mixed gas (15/85 v/v of CO2/N2) | N/A | N/A | [161] |
| [Pyr][Tf2N] | |||||||||
| [HEDMA][Tf2N] | |||||||||
| PVDF | Pebax 1657/GO | PTMSP | [emim][BF4] | Hollow fiber | 3 bar, 35 °C | Mixed gas (20/80 v/v of CO2/N2) | 981 | 44 | [162] |
| Matrimid | P[DADMA] [Tf2N] | PDMS protective layer | [Pyrr14] [Tf2N] | Flat sheet | 1.2 bar, 26 °C | Mixed gas (15/85 v/v of CO2/N2) | N/A | N/A | [163] |
| Zn[Tf2N]2 | |||||||||
| PSf | PAP copolymer | PTMSP | [EMIM][TFSI], ZIF-8 | Flat sheet | 1 bar, 25 °C | Pure gas | 1017 | 33 | [164] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, N.Y.; Lee, S.Y.; Lee, J.; Min, H.J.; Hosseini, S.S.; Patel, R.; Kim, J.H. Material Aspects of Thin-Film Composite Membranes for CO2/N2 Separation: Metal–Organic Frameworks vs. Graphene Oxides vs. Ionic Liquids. Polymers 2024, 16, 2998. https://doi.org/10.3390/polym16212998
Oh NY, Lee SY, Lee J, Min HJ, Hosseini SS, Patel R, Kim JH. Material Aspects of Thin-Film Composite Membranes for CO2/N2 Separation: Metal–Organic Frameworks vs. Graphene Oxides vs. Ionic Liquids. Polymers. 2024; 16(21):2998. https://doi.org/10.3390/polym16212998
Chicago/Turabian StyleOh, Na Yeong, So Youn Lee, Jiwon Lee, Hyo Jun Min, Seyed Saeid Hosseini, Rajkumar Patel, and Jong Hak Kim. 2024. "Material Aspects of Thin-Film Composite Membranes for CO2/N2 Separation: Metal–Organic Frameworks vs. Graphene Oxides vs. Ionic Liquids" Polymers 16, no. 21: 2998. https://doi.org/10.3390/polym16212998
APA StyleOh, N. Y., Lee, S. Y., Lee, J., Min, H. J., Hosseini, S. S., Patel, R., & Kim, J. H. (2024). Material Aspects of Thin-Film Composite Membranes for CO2/N2 Separation: Metal–Organic Frameworks vs. Graphene Oxides vs. Ionic Liquids. Polymers, 16(21), 2998. https://doi.org/10.3390/polym16212998









