Poly(HEMA-co-MMA) Hydrogel Scaffold for Tissue Engineering with Controllable Morphology and Mechanical Properties Through Self-Assembly
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
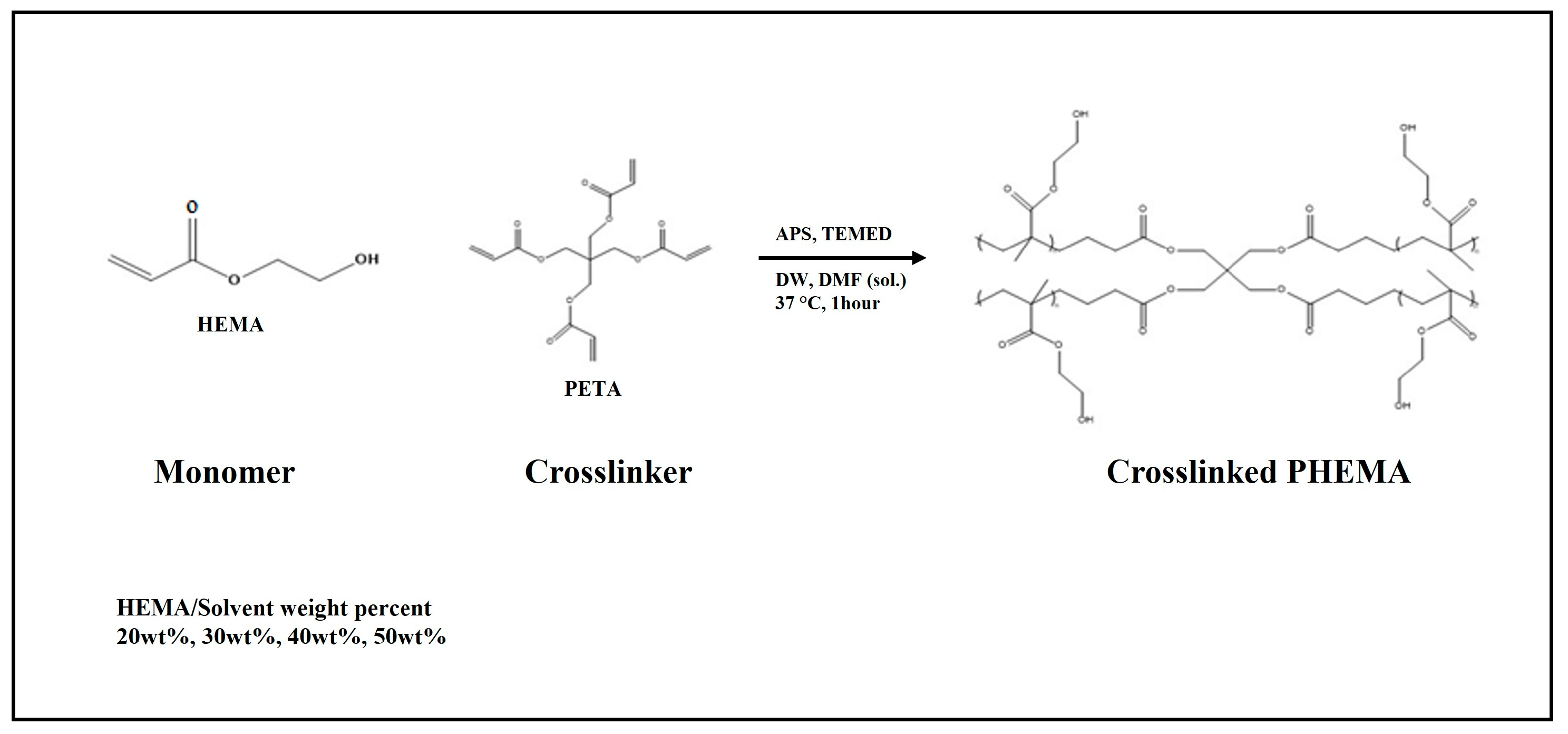
2.2. Hydrogel Scaffold Preparation
2.2.1. PHEMA Hydrogel Scaffold Preparation
2.2.2. Poly(HEMA-co-MMA) Hydrogel Scaffold Preparation
2.3. Analytical Methods
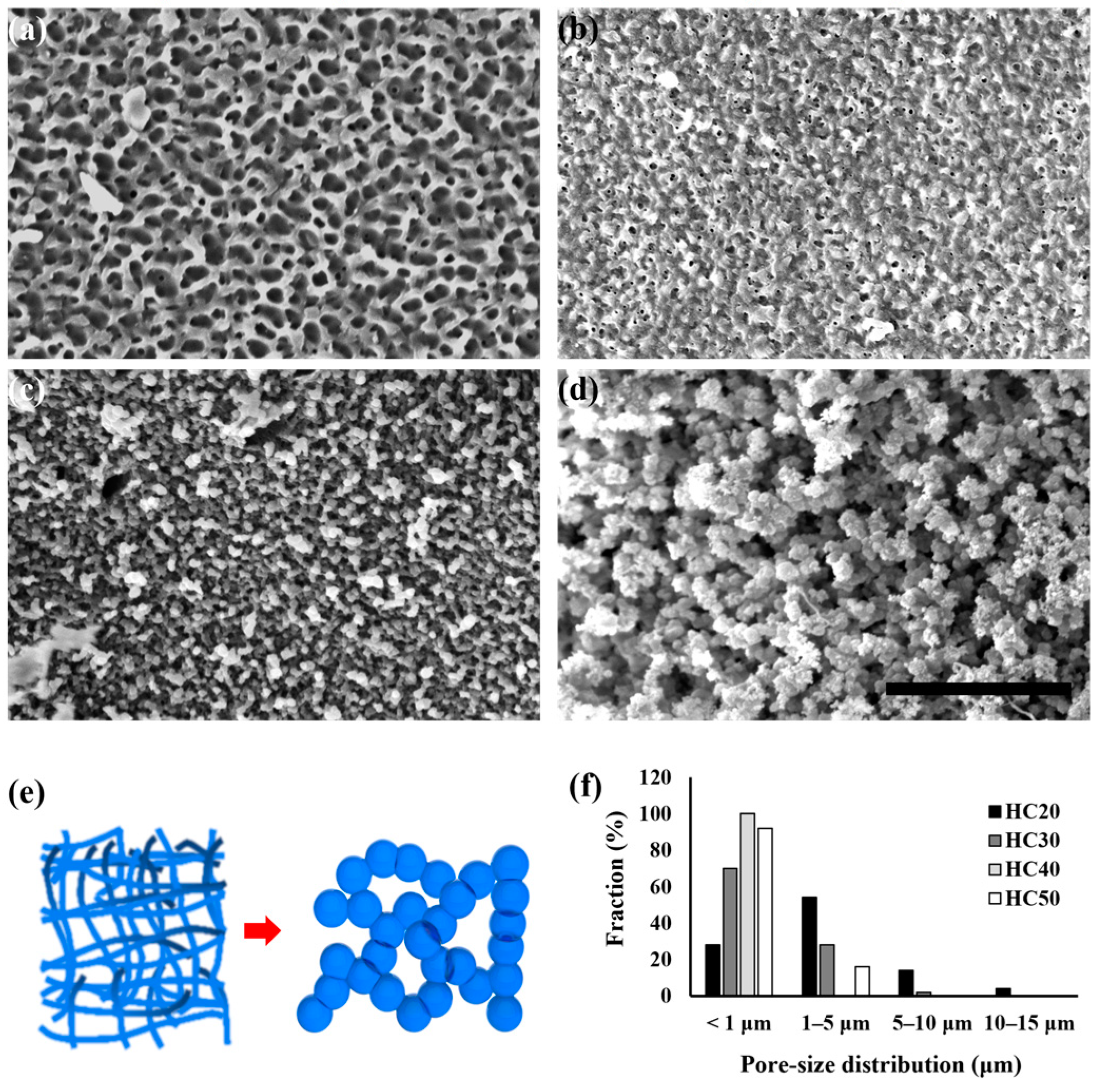
2.3.1. Scanning Electron Microscope (SEM) and Pore Size Distribution
2.3.2. Fourier-Transform Infrared (FT-IR) Spectroscopy
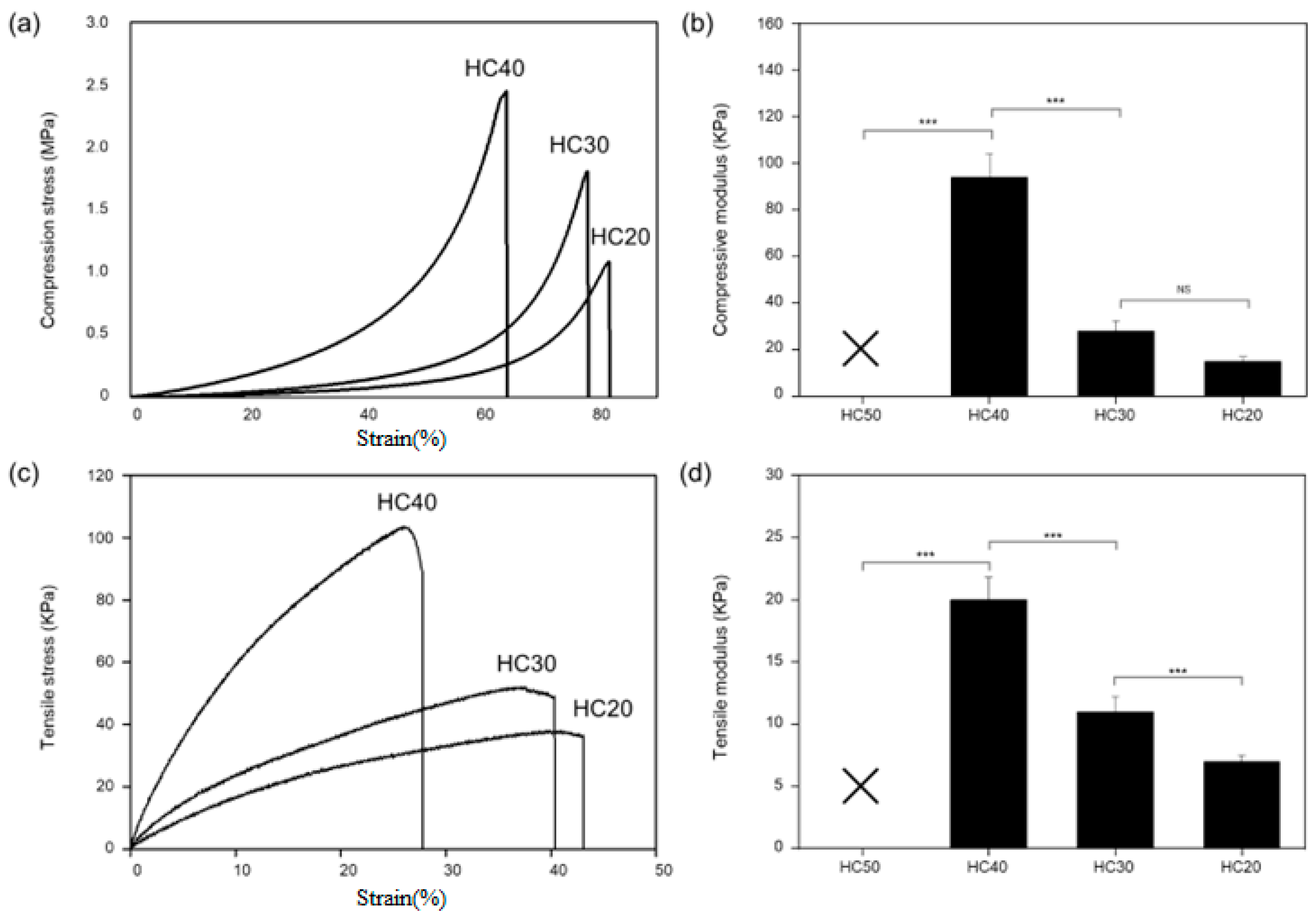
2.3.3. Mechanical Test
2.3.4. Wettability
2.3.5. Cell Culture and Cell Proliferation Assay
2.3.6. Cell Adhesion Observation Via SEM
2.3.7. Statistical Analysis
3. Results and Discussion
3.1. Comparison of PHEMA Hydrogel Scaffold Morphology According to HEMA Concentration
3.2. Evaluation of PHEMA Hydrogel Scaffold Characteristics According to HEMA Concentration
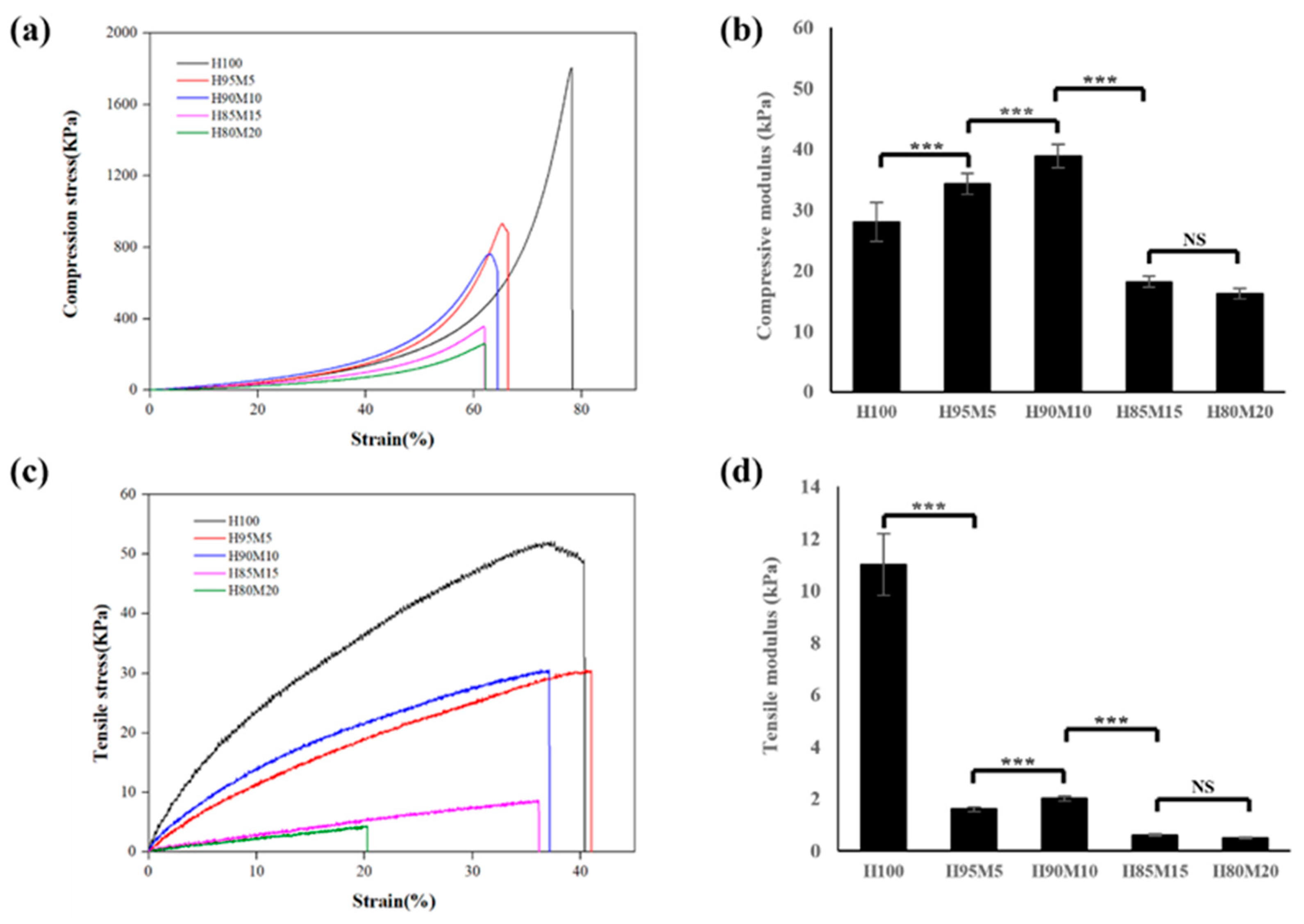
3.3. Assessment of Poly(HEMA-co-MMA) Hydrogel Scaffold Characteristics According to MMA Concentration
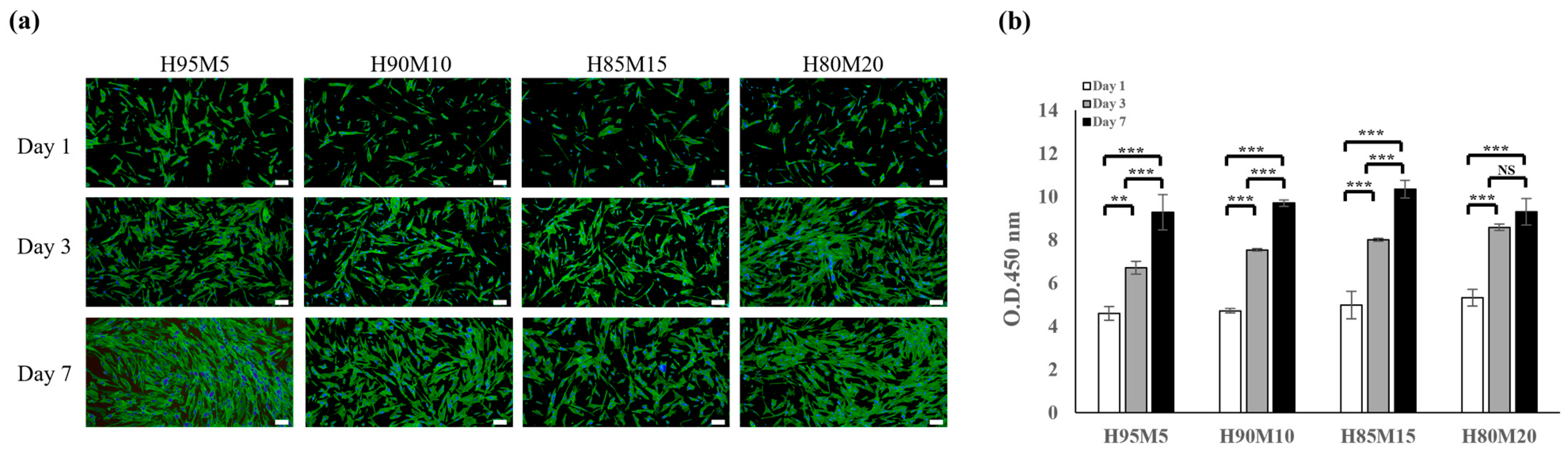
3.4. Biocompatibility of Poly(HEMA-co-MMA) Hydrogel Scaffolds with Various Concentrations of HEMA and MMA
3.5. Cell Adhesion and Morphology of Poly(HEMA-co-MMA) Hydrogel Scaffolds with Various Concentrations of HEMA and MMA
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chan, B.P.; Leong, K.W. Scaffolding in Tissue Engineering: General Approaches and Tissue-Specific Considerations. Eur. Spine J. 2008, 17 (Suppl. S4), 467–479. [Google Scholar] [CrossRef] [PubMed]
- Willerth, S.M.; Sakiyama-Elbert, S.E. Combining Stem Cells and Biomaterial Scaffolds for Constructing Tissues and Cell Delivery. In Stembook [Internet]; Harvard Stem Cell Institute: Cambridge, MA, USA, 2008; pp. 1–18. [Google Scholar] [CrossRef]
- Asadi, N.; Del Bakhshayesh, A.R.; Davaran, S.; Akbarzadeh, A. Common Biocompatible Polymeric Materials for Tissue Engineering and Regenerative Medicine. Mater. Chem. Phys. 2020, 242, 122528. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Petrie Aronin, C.E.; Sadik, K.W.; Lay, A.L.; Rion, D.B.; Tholpady, S.S.; Ogle, R.C.; Botchwey, E.A. Comparative Effects of Scaffold Pore Size, Pore Volume, and Total Void Volume on Cranial Bone Healing Patterns Using Microsphere-Based Scaffolds. J. Biomed. Mater. Res. A 2009, 89, 632–641. [Google Scholar] [CrossRef]
- Rufaihah, A.J.; Cheyyatraivendran, S.; Mazlan, M.D.M.; Lim, K.; Chong, M.S.K.; Mattar, C.N.Z.; Chan, J.K.Y.; Kofidis, T.; Seliktar, D. The Effect of Scaffold Modulus on the Morphology and Remodeling of Fetal Mesenchymal Stem Cells. Front. Physiol. 2018, 9, 1555. [Google Scholar] [CrossRef]
- Nisal, A.; Sayyad, R.; Dhavale, P.; Khude, B.; Deshpande, R.; Mapare, V.; Shukla, S.; Venugopalan, P. Silk Fibroin Micro-Particle Scaffolds with Superior Compression Modulus and Slow Bioresorption for Effective Bone Regeneration. Sci. Rep. 2018, 8, 7235. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, C.; Pandey, H.; Pandey, A.C.; Patil, S.; Ramteke, P.W.; Laux, P.; Luch, A.; Singh, A.V. In Vivo Biocompatibility of Electrospun Biodegradable Dual Carrier (Antibiotic + Growth Factor) in a Mouse Model-Implications for Rapid Wound Healing. Pharmaceutics 2019, 11, 180. [Google Scholar] [CrossRef]
- Ranella, A.; Barberoglou, M.; Bakogianni, S.; Fotakis, C.; Stratakis, E. Tuning Cell Adhesion by Controlling the Roughness and Wettability of 3D Micro/Nano Silicon Structures. Acta Biomater. 2010, 6, 2711–2720. [Google Scholar] [CrossRef]
- Zamani, F.; Amani-Tehran, M.; Latifi, M.; Shokrgozar, M.A. The Influence of Surface Nanoroughness of Electrospun PLGA Nanofibrous Scaffold on Nerve Cell Adhesion and Proliferation. J. Mater. Sci. Mater. Med. 2013, 24, 1551–1560. [Google Scholar] [CrossRef]
- Vaquette, C.; Mitchell, J.; Fernandez-Medina, T.; Kumar, S.; Ivanovski, S. Resorbable Additively Manufactured Scaffold Imparts Dimensional Stability to Extraskeletally Regenerated Bone. Biomaterials 2021, 269, 120671. [Google Scholar] [CrossRef]
- Calore, A.R.; Srinivas, V.; Anand, S.; Albillos-Sanchez, A.; Looijmans, S.F.S.P.; van Breemen, L.C.A.; Mota, C.; Bernaerts, K.; Harings, J.A.W.; Moroni, L. Shaping and Properties of Thermoplastic Scaffolds in Tissue Regeneration: The Effect of Thermal History on Polymer Crystallization, Surface Characteristics and Cell Fate. J. Mater. Res. 2021, 36, 3914–3935. [Google Scholar] [CrossRef]
- Cun, X.; Hosta-Rigau, L. Topography: A Biophysical Approach to Direct the Fate of Mesenchymal Stem Cells in Tissue Engineering Applications. Nanomaterials 2020, 10, 2070. [Google Scholar] [CrossRef] [PubMed]
- Grover, C.N.; Gwynne, J.H.; Pugh, N.; Hamaia, S.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Crosslinking and Composition Influence the Surface Properties, Mechanical Stiffness and Cell Reactivity of Collagen-Based Films. Acta Biomater. 2012, 8, 3080–3090. [Google Scholar] [CrossRef] [PubMed]
- Vyas, C.; Ates, G.; Aslan, E.; Hart, J.; Huang, B.; Bartolo, P. Three-Dimensional Printing and Electrospinning Dual-Scale Polycaprolactone Scaffolds with Low-Density and Oriented Fibers to Promote Cell Alignment. 3D Print Addit Manuf 2020, 7, 105–113. [Google Scholar] [CrossRef]
- Weisgrab, G.; Guillaume, O.; Guo, Z.; Heimel, P.; Slezak, P.; Poot, A.; Grijpma, D.; Ovsianikov, A. 3D Printing of Large-Scale and Highly Porous Biodegradable Tissue Engineering Scaffolds from Poly(Trimethylene-Carbonate) Using Two-Photon-Polymerization. Biofabrication 2020, 12, 045036. [Google Scholar] [CrossRef]
- Cho, Y.S.; Choi, S.; Lee, S.H.; Kim, K.K.; Cho, Y.S. Assessments of Polycaprolactone/Hydroxyapatite Composite Scaffold with Enhanced Biomimetic Mineralization by Exposure to Hydroxyapatite via a 3D-Printing System and Alkaline Erosion. Eur. Polym. J. 2019, 113, 340–348. [Google Scholar] [CrossRef]
- Cho, Y.S.; Quan, M.; Lee, S.H.; Hong, M.W.; Kim, Y.Y.; Cho, Y.S. Assessment of Osteogenesis for 3D-Printed Polycaprolactone/Hydroxyapatite Composite Scaffold with Enhanced Exposure of Hydroxyapatite Using Rat Calvarial Defect Model. Compos. Sci. Technol. 2019, 184, 107844. [Google Scholar] [CrossRef]
- Xiang, T.; Hou, J.; Xie, H.; Liu, X.; Gong, T.; Zhou, S. Biomimetic Micro/Nano Structures for Biomedical Applications. Nano Today 2020, 35, 100980. [Google Scholar] [CrossRef]
- Norzain, N.A.; Yu, Z.W.; Lin, W.C.; Su, H.H. Micropatterned Fibrous Scaffold Produced by Using Template-Assisted Electrospinning Technique for Wound Healing Application. Polymers 2021, 13, 2821. [Google Scholar] [CrossRef]
- Yang, W.; Yu, H.; Li, G.; Wang, Y.; Liu, L. Facile Modulation of Cell Adhesion to a Poly(Ethylene Glycol) Diacrylate Film with Incorporation of Polystyrene Nano-Spheres. Biomed. Microdevices 2016, 18, 107. [Google Scholar] [CrossRef]
- Jeon, H.; Lee, H.; Kim, G. A Surface-Modified Poly(ε-Caprolactone) Scaffold Comprising Variable Nanosized Surface-Roughness Using a Plasma Treatment. Tissue Eng. Part C Methods 2014, 20, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Saez-Martinez, V.; Mann, A.; Lydon, F.; Molock, F.; Layton, S.A.; Toolan, D.T.W.; Howse, J.R.; Topham, P.D.; Tighe, B.J. The Influence of Structure and Morphology on Ion Permeation in Commercial Silicone Hydrogel Contact Lenses. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Jirásková, N.; Rozsival, P.; Burova, M.; Kalfertova, M. AlphaCor Artificial Cornea: Clinical Outcome. Eye 2011, 25, 1138. [Google Scholar] [CrossRef] [PubMed]
- Yadav, I.; Purohit, S.D.; Singh, H.; Bhushan, S.; Yadav, M.K.; Velpandian, T.; Chawla, R.; Hazra, S.; Mishra, N.C. Vitreous Substitutes: An Overview of the Properties, Importance, and Development. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1156–1176. [Google Scholar] [CrossRef] [PubMed]
- Negussie, A.H.; de Ruiter, Q.M.B.; Britton, H.; Donahue, D.R.; Boffi, Q.; Kim, Y.S.; Pritchard, W.F.; Moonen, C.; Storm, G.; Lewis, A.L.; et al. Synthesis, Characterization, and Imaging of Radiopaque Bismuth Beads for Image-Guided Transarterial Embolization. Sci. Rep. 2021, 11, 533. [Google Scholar] [CrossRef]
- Tsou, T.L.; Tang, S.T.; Huang, Y.C.; Wu, J.R.; Young, J.J.; Wang, H.J. Poly(2-Hydroxyethyl Methacrylate) Wound Dressing Containing Ciprofloxacin and Its Drug Release Studies. J. Mater. Sci. Mater. Med. 2005, 16, 95–100. [Google Scholar] [CrossRef]
- Xiang, T.; Lu, T.; Zhao, W.F.; Zhao, C.S. Ionic-Strength Responsive Zwitterionic Copolymer Hydrogels with Tunable Swelling and Adsorption Behaviors. Langmuir 2019, 35, 1146–1155. [Google Scholar] [CrossRef]
- McKee, C.T.; Last, J.A.; Russell, P.; Murphy, C.J. Indentation Versus Tensile Measurements of Young’s Modulus for Soft Biological Tissues. Tissue Eng. Part B Rev. 2011, 17, 155. [Google Scholar] [CrossRef]
- Boazak, E.M.; Greene, V.K.; Auguste, D.T. The Effect of Heterobifunctional Crosslinkers on HEMA Hydrogel Modulus and Toughness. PLoS ONE 2019, 14, e0215895. [Google Scholar] [CrossRef]
- Gomez, C.G.; Alvarez Igarzabal, C.I.; Strumia, M.C. Effect of the Crosslinking Agent on Porous Networks Formation of Hema-Based Copolymers. Polymer 2004, 45, 6189–6194. [Google Scholar] [CrossRef]
- Wang, X.; Lin, X.; Xie, Z.; Giesy, J.P. Preparation and Evaluation of a Neutral Methacrylate-Based Monolithic Column for Hydrophilic Interaction Stationary Phase by Pressurized Capillary Electrochromatography. J. Chromatogr. A 2009, 1216, 4611–4617. [Google Scholar] [CrossRef] [PubMed]
- Barbera, L.; Madrid-Wolff, J.; Emma, R.; Masania, K.; Boniface, A.; Loterie, D.; Delrot, P.; Moser, C.; Studart, A.R. Multimaterial Volumetric Printing of Silica-Based Glasses. Adv. Mater. Technol. 2024, 9, 2202117. [Google Scholar] [CrossRef]
- Frieß, F.V.; Gallei, M. An Anchor for Help: A Cross-Linking Moiety for Block Copolymer Membrane Stabilization for Ultrafiltration Applications in Water. Chem. Commun. 2024, 60, 8462–8465. [Google Scholar] [CrossRef]
- Zaszczyńska, A.; Kołbuk, D.; Gradys, A.; Sajkiewicz, P. Development of Poly(Methyl Methacrylate)/Nano-Hydroxyapatite (PMMA/NHA) Nanofibers for Tissue Engineering Regeneration Using an Electrospinning Technique. Polymers 2024, 16, 531. [Google Scholar] [CrossRef]
- Chung, J.J.; Yoo, J.; Sum, B.S.T.; Li, S.; Lee, S.; Kim, T.H.; Li, Z.; Stevens, M.M.; Georgiou, T.K.; Jung, Y.; et al. 3D Printed Porous Methacrylate/Silica Hybrid Scaffold for Bone Substitution. Adv. Healthc. Mater. 2021, 10, 2100117. [Google Scholar] [CrossRef]
- Álvarez-Carrasco, F.; Varela, P.; Sarabia-Vallejos, M.A.; García-Herrera, C.; Saavedra, M.; Zapata, P.A.; Zárate-Triviño, D.; Martínez, J.J.; Canales, D.A. Development of Bioactive Hybrid Poly(Lactic Acid)/Poly(Methyl Methacrylate) (PLA/PMMA) Electrospun Fibers Functionalized with Bioglass Nanoparticles for Bone Tissue Engineering Applications. Int. J. Mol. Sci. 2024, 25, 6843. [Google Scholar] [CrossRef]
- Hisatome, T.; Yasunaga, Y.; Ikuta, Y.; Fujimoto, Y. Effects on Articular Cartilage of Subchondral Replacement with Polymethylmethacrylate and Calcium Phosphate Cement. J. Biomed. Mater. Res. 2002, 59, 490–498. [Google Scholar] [CrossRef]
- Radev, B.R.; Kase, J.A.; Askew, M.J.; Weiner, S.D. Potential for Thermal Damage to Articular Cartilage by PMMA Reconstruction of a Bone Cavity Following Tumor Excision: A Finite Element Study. J. Biomech. 2009, 42, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Frazer, R.Q.; Byron, R.T.; Osborne, P.B.; West, K.P. PMMA: An Essential Material in Medicine and Dentistry. J. Long-Term Eff. Med. Implants 2005, 15, 629–639. [Google Scholar] [CrossRef]
- Gautam, R.; Singh, R.D.; Sharma, V.P.; Siddhartha, R.; Chand, P.; Kumar, R. Biocompatibility of Polymethylmethacrylate Resins Used in Dentistry. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100B, 1444–1450. [Google Scholar] [CrossRef]
- Arcos, D.; Ragel, C.V.; Vallet-Regí, M. Bioactivity in Glass/PMMA Composites Used as Drug Delivery System. Biomaterials 2001, 22, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Ayre, W.N.; Birchall, J.C.; Evans, S.L.; Denyer, S.P. A Novel Liposomal Drug Delivery System for PMMA Bone Cements. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1510–1524. [Google Scholar] [CrossRef] [PubMed]
- Musgrave, C.S.A.; Fang, F. Contact Lens Materials: A Materials Science Perspective. Materials 2019, 12, 261. [Google Scholar] [CrossRef] [PubMed]
- Holland, G.; Pandit, A.; Sánchez-Abella, L.; Haiek, A.; Loinaz, I.; Dupin, D.; Gonzalez, M.; Larra, E.; Bidaguren, A.; Lagali, N.; et al. Artificial Cornea: Past, Current, and Future Directions. Front. Med. 2021, 8, 770780. [Google Scholar] [CrossRef]
- Ronan, S.J.; Eaton, L.; Lehman, A.; Pilcher, B.; Erickson, C.P. Histologic Characterization of Polymethylmethacrylate Dermal Filler Biostimulatory Properties in Human Skin. Dermatol. Surg. 2019, 45, 1580–1584. [Google Scholar] [CrossRef]
- Zahari, N.K.; Idrus, R.B.H.; Chowdhury, S.R. Laminin-Coated Poly(Methyl Methacrylate) (PMMA) Nanofiber Scaffold Facilitates the Enrichment of Skeletal Muscle Myoblast Population. Int. J. Mol. Sci. 2017, 18, 2242. [Google Scholar] [CrossRef]
- Hu, J.; Kai, D.; Ye, H.; Tian, L.; Ding, X.; Ramakrishna, S.; Loh, X.J. Electrospinning of Poly(Glycerol Sebacate)-Based Nanofibers for Nerve Tissue Engineering. Mater. Sci. Eng. C 2017, 70, 1089–1094. [Google Scholar] [CrossRef]
- Patel, S.; Thakar, R.G.; Wong, J.; McLeod, S.D.; Li, S. Control of Cell Adhesion on Poly(Methyl Methacrylate). Biomaterials 2006, 27, 2890–2897. [Google Scholar] [CrossRef] [PubMed]
- Lampin, M.; Warocquier-Clérout, R.; Legris, C.; Degrange, M.; Sigot-Luizard, M.F. Correlation between Substratum Roughness and Wettability, Cell Adhesion, and Cell Migration. J. Biomed. Mater. Res. 1997, 36, 99–108. [Google Scholar] [CrossRef]
- Ahn, J.; Son, S.J.; Min, J. The Control of Cell Adhesion on a PMMA Polymer Surface Consisting of Nanopillar Arrays. J. Biotechnol. 2013, 164, 543–548. [Google Scholar] [CrossRef]
- Yan, T.; Sun, R.; Li, C.; Tan, B.; Mao, X.; Ao, N. Immobilization of Type-I Collagen and Basic Fibroblast Growth Factor (BFGF) onto Poly (HEMA-Co-MMA) Hydrogel Surface and Its Cytotoxicity Study. J. Mater. Sci. Mater. Med. 2010, 21, 2425–2433. [Google Scholar] [CrossRef] [PubMed]
- Dalton, P.D.; Flynn, L.; Shoichet, M.S. Manufacture of Poly(2-Hydroxyethyl Methacrylate-Co-Methyl Methacrylate) Hydrogel Tubes for Use as Nerve Guidance Channels. Biomaterials 2002, 23, 3843–3851. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Hwang, Y.; Kashif, M.; Jeong, D.; Kim, G. Evaluation of Bone Regeneration on Polyhydroxyethyl-Polymethyl Methacrylate Membrane in a Rabbit Calvarial Defect Model. In Vivo 2016, 30, 587. [Google Scholar]
- Çetin, D.; Kahraman, A.S.; Gümüsderelioglu, M. Novel Scaffolds Based on Poly(2-Hydroxyethyl Methacrylate) Superporous Hydrogels for Bone Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2011, 22, 1157–1178. [Google Scholar] [CrossRef]
- Sreeja, S.; Parameshwar, R.; Varma, P.R.H.; Sailaja, G.S. Hierarchically Porous Osteoinductive Poly(Hydroxyethyl Methacrylate- Co-Methyl Methacrylate) Scaffold with Sustained Doxorubicin Delivery for Consolidated Osteosarcoma Treatment and Bone Defect Repair. ACS Biomater. Sci. Eng. 2021, 7, 701–717. [Google Scholar] [CrossRef]
- Zare, M.; Bigham, A.; Zare, M.; Luo, H.; Rezvani Ghomi, E.; Ramakrishna, S. Phema: An Overview for Biomedical Applications. Int. J. Mol. Sci. 2021, 22, 6376. [Google Scholar] [CrossRef]
- Zumbro, E.; Alexander-Katz, A. Polymer Stiffness Regulates Multivalent Binding and Liquid-Liquid Phase Separation. Biophys. J. 2020, 119, 1849–1864. [Google Scholar] [CrossRef]
- Rusakov, D.; Menner, A.; Bismarck, A. High-Performance Polymer Foams by Thermally Induced Phase Separation. Macromol. Rapid Commun. 2020, 41, 2000110. [Google Scholar] [CrossRef] [PubMed]
- Staub, M.C.; Kim, S.; Yu, S.; Li, C.Y. Porous Crystalsomes via Emulsion Crystallization and Polymer Phase Separation. ACS Macro Lett. 2022, 11, 1022–1027. [Google Scholar] [CrossRef]
- Tipduangta, P.; Belton, P.; McAuley, W.J.; Qi, S. The Use of Polymer Blends to Improve Stability and Performance of Electrospun Solid Dispersions: The Role of Miscibility and Phase Separation. Int. J. Pharm. 2021, 602, 120637. [Google Scholar] [CrossRef]
- Hsu, S.-H.; Huang, S.; Wang, Y.-C.; Kuo, Y.-C. Novel Nanostructured Biodegradable Polymer Matrices Fabricated by Phase Separation Techniques for Tissue Regeneration. Acta Biomater. 2013, 9, 6915–6927. [Google Scholar] [CrossRef] [PubMed]
- Bahners, T.; Gutmann, J.S.; Müssig, J. Application-Related Optimization of Adhesion of Polymers Using Photochemical Surface Modification. In Polymer Surface Modification to Enhance Adhesion; Scrivener Publishing LLC: Beverly, MA, USA, 2024; pp. 155–198. ISBN 9781394231034. [Google Scholar]
- Chen, C.; Garber, L.; Smoak, M.; Fargason, C.; Scherr, T.; Blackburn, C.; Bacchus, S.; Lopez, M.J.; Pojman, J.A.; Del Piero, F.; et al. In Vitro and in Vivo Characterization of Pentaerythritol Triacrylate-Co-Trimethylolpropane Nanocomposite Scaffolds as Potential Bone Augments and Grafts. Tissue Eng. Part A 2015, 21, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Paterson, S.M.; Shadforth, A.M.A.; Shaw, J.A.; Brown, D.H.; Chirila, T.V.; Baker, M.V. Improving the Cellular Invasion into PHEMA Sponges by Incorporation of the RGD Peptide Ligand: The Use of Copolymerization as a Means to Functionalize PHEMA Sponges. Mater. Sci. Eng. C 2013, 33, 4917–4922. [Google Scholar] [CrossRef] [PubMed]


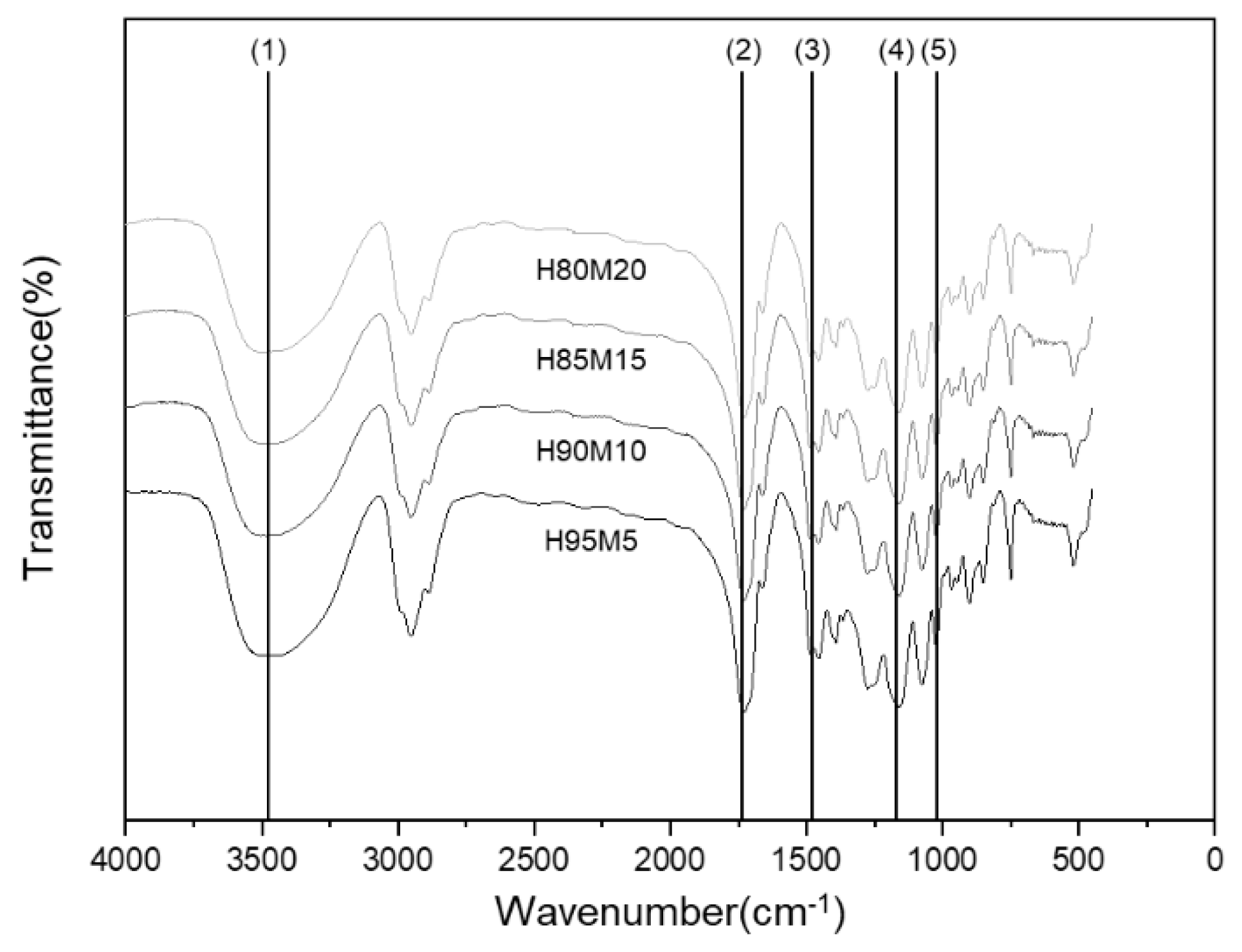


| Peak No. | Wavelength (cm−1) | Functional Group |
|---|---|---|
| 1 | 3463 | −OH |
| 2 | 2944 | SP3 Carbon |
| 3 | 1734 | C = O |
| 4 | 1475 | −COOsym |
| 5 | 1166 | −COOasym |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-R.; Cho, Y.S.; Park, J.-H.; Kim, T.-H. Poly(HEMA-co-MMA) Hydrogel Scaffold for Tissue Engineering with Controllable Morphology and Mechanical Properties Through Self-Assembly. Polymers 2024, 16, 3014. https://doi.org/10.3390/polym16213014
Kim J-R, Cho YS, Park J-H, Kim T-H. Poly(HEMA-co-MMA) Hydrogel Scaffold for Tissue Engineering with Controllable Morphology and Mechanical Properties Through Self-Assembly. Polymers. 2024; 16(21):3014. https://doi.org/10.3390/polym16213014
Chicago/Turabian StyleKim, Ja-Rok, Yong Sang Cho, Jae-Hong Park, and Tae-Hyun Kim. 2024. "Poly(HEMA-co-MMA) Hydrogel Scaffold for Tissue Engineering with Controllable Morphology and Mechanical Properties Through Self-Assembly" Polymers 16, no. 21: 3014. https://doi.org/10.3390/polym16213014
APA StyleKim, J.-R., Cho, Y. S., Park, J.-H., & Kim, T.-H. (2024). Poly(HEMA-co-MMA) Hydrogel Scaffold for Tissue Engineering with Controllable Morphology and Mechanical Properties Through Self-Assembly. Polymers, 16(21), 3014. https://doi.org/10.3390/polym16213014







