Mechanical and Thermal Characteristics of Films from Glycerol Mixed Emulsified Carnauba Wax/Polyvinyl Alcohol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Preparation
2.3. Tensile Properties
2.4. Functional Groups
2.5. Surface Morphology
2.6. Film Solubility
2.7. Contact Angle
2.8. Thermal Gravimetric Analysis (TGA)
2.9. Differential Scanning Calorimetry
2.10. Data Analysis
3. Results and Discussion
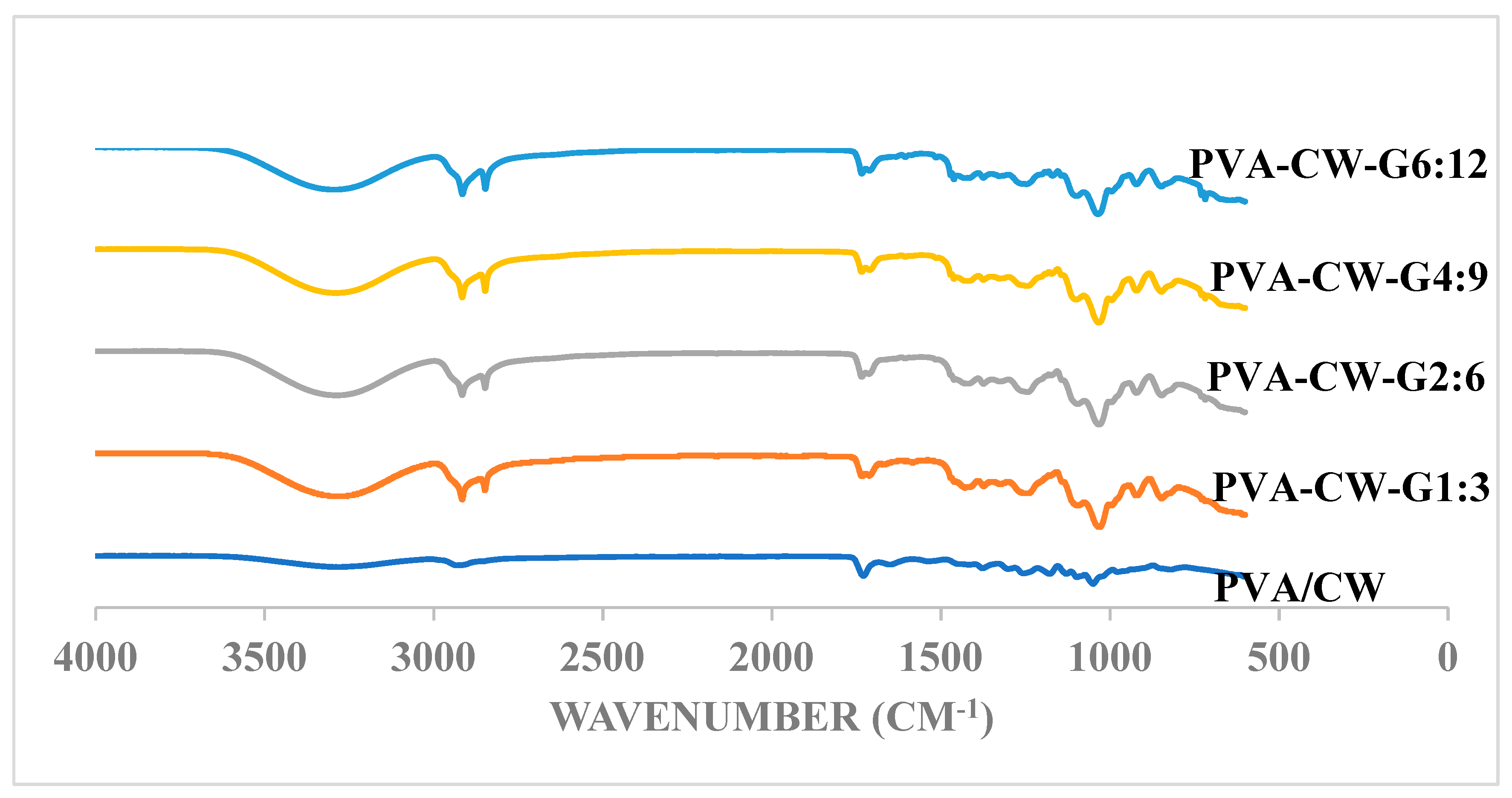
3.1. Functional Group Study
3.2. Tensile Strength of PVA Films
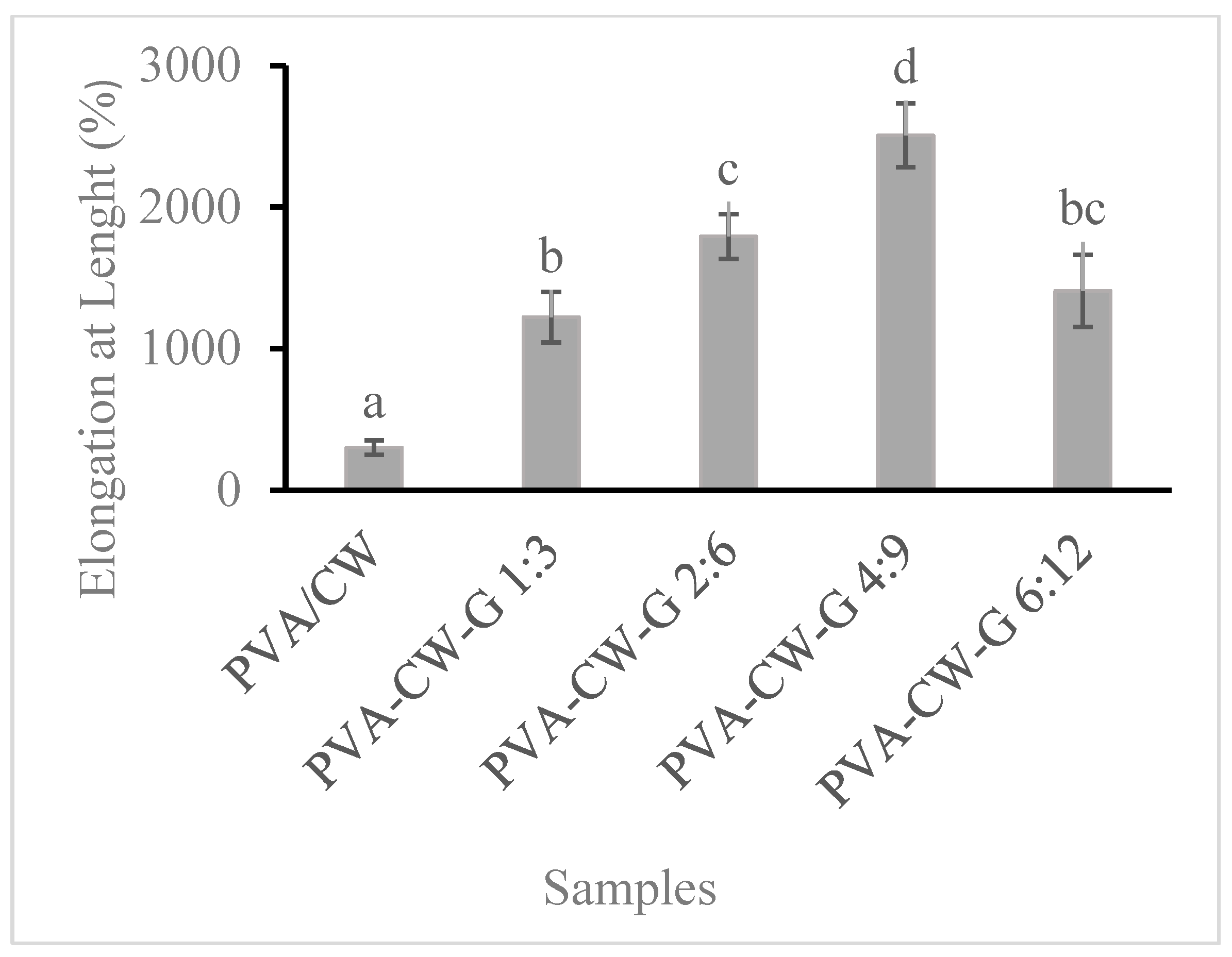
3.3. Film Elongation
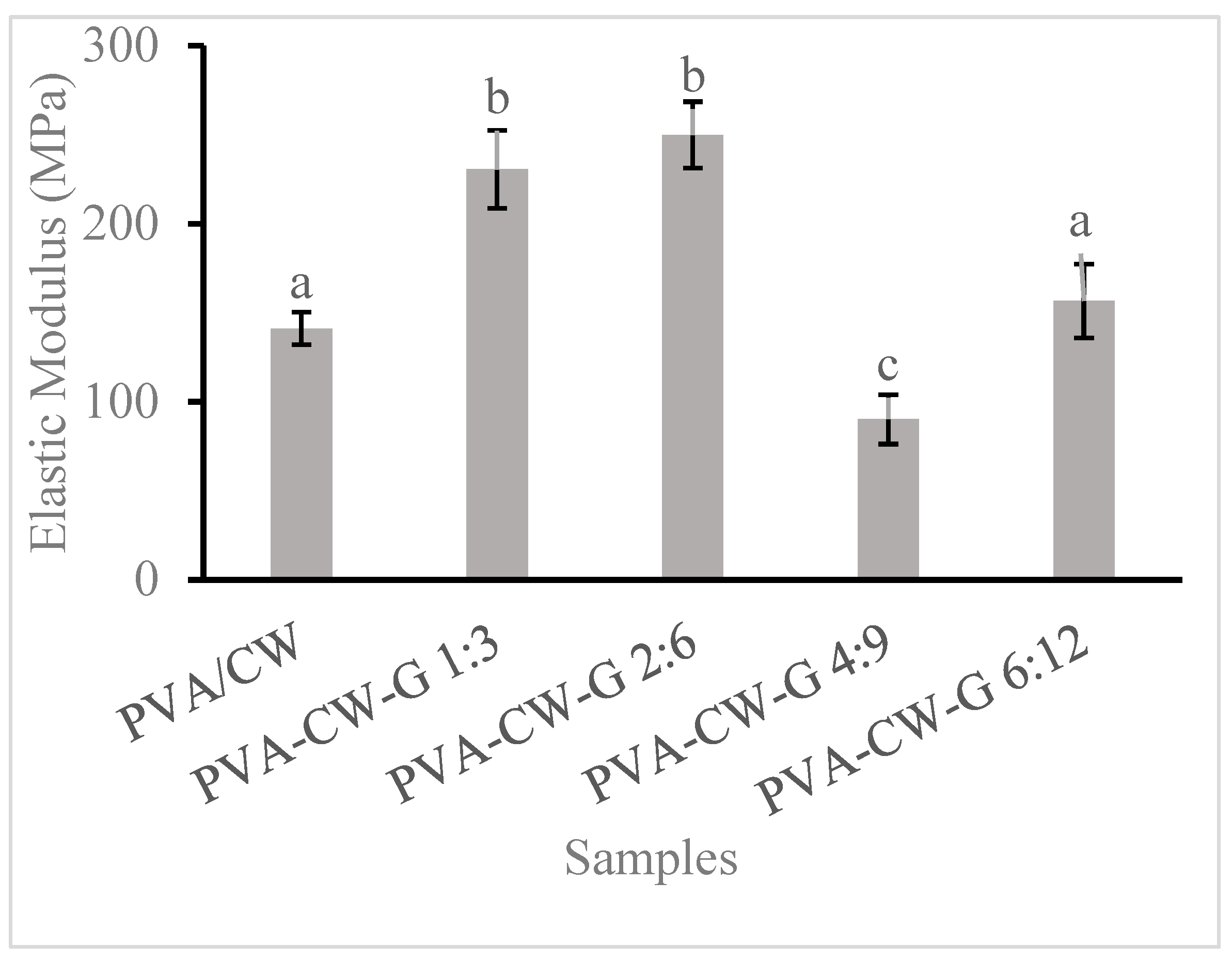
3.4. Film Young’s Modulus
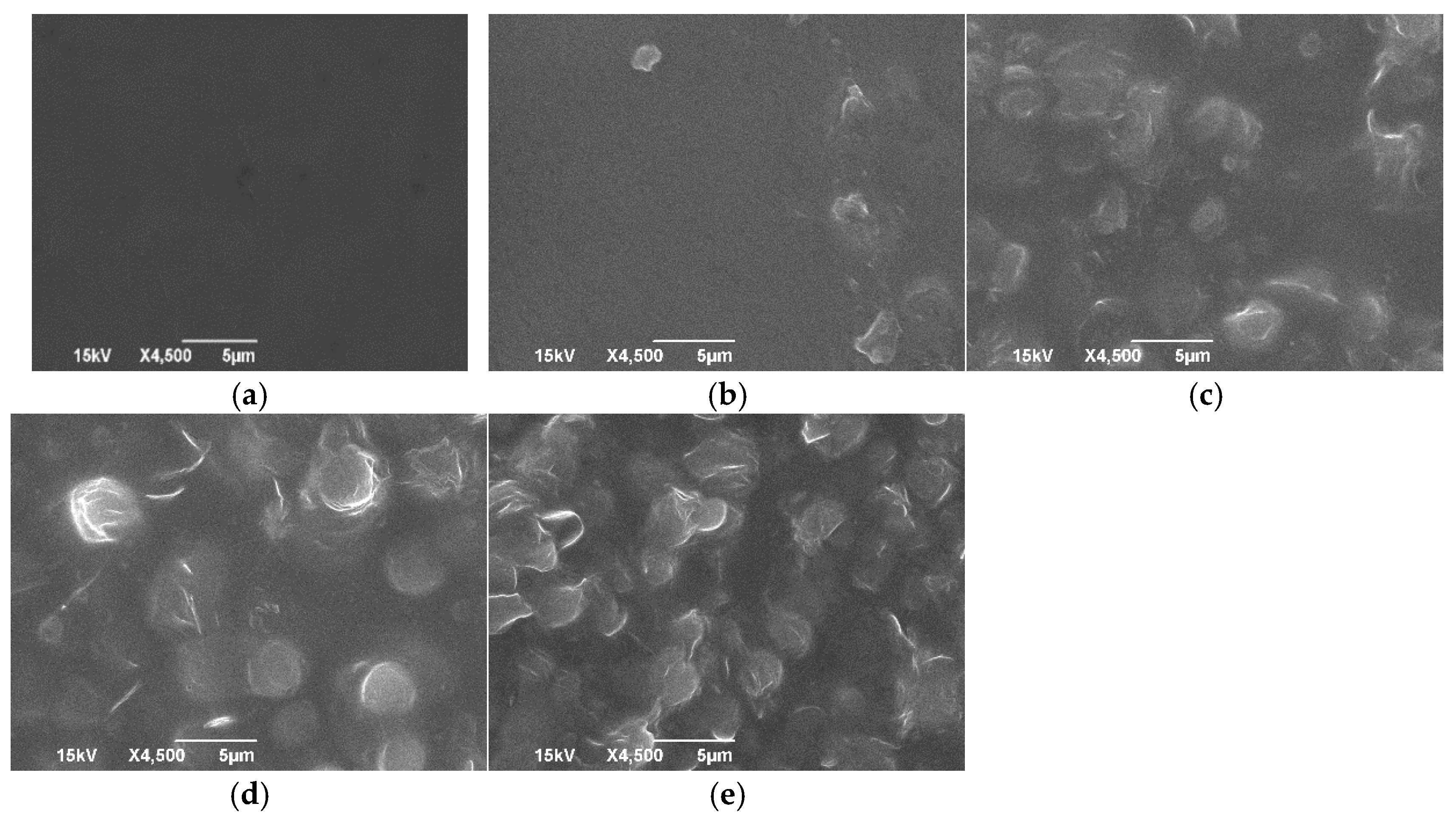
3.5. Scanning Electron Microscope
3.6. Water Resistance Analysis of Films
3.6.1. Water Contact Angle Test
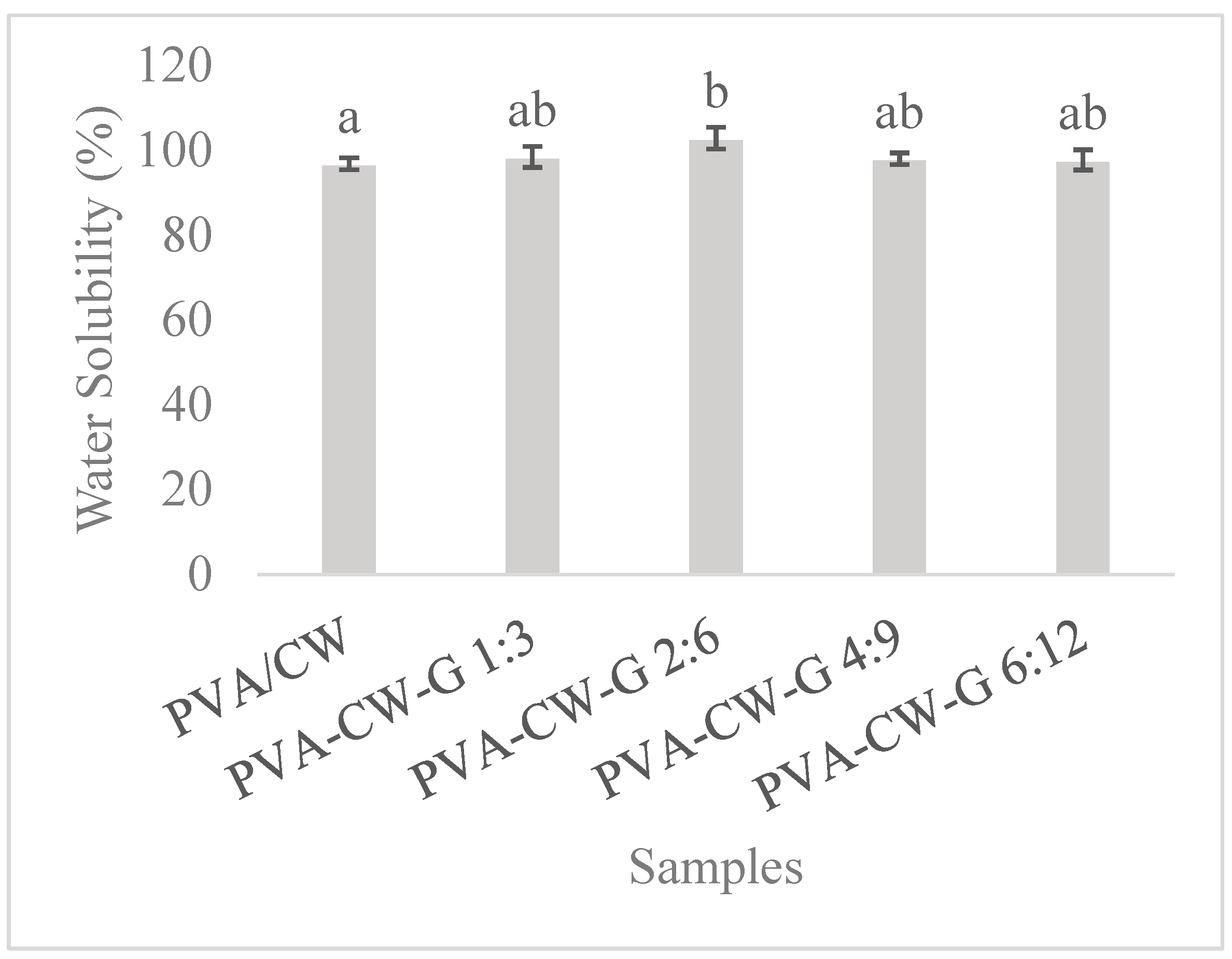
3.6.2. Solubility
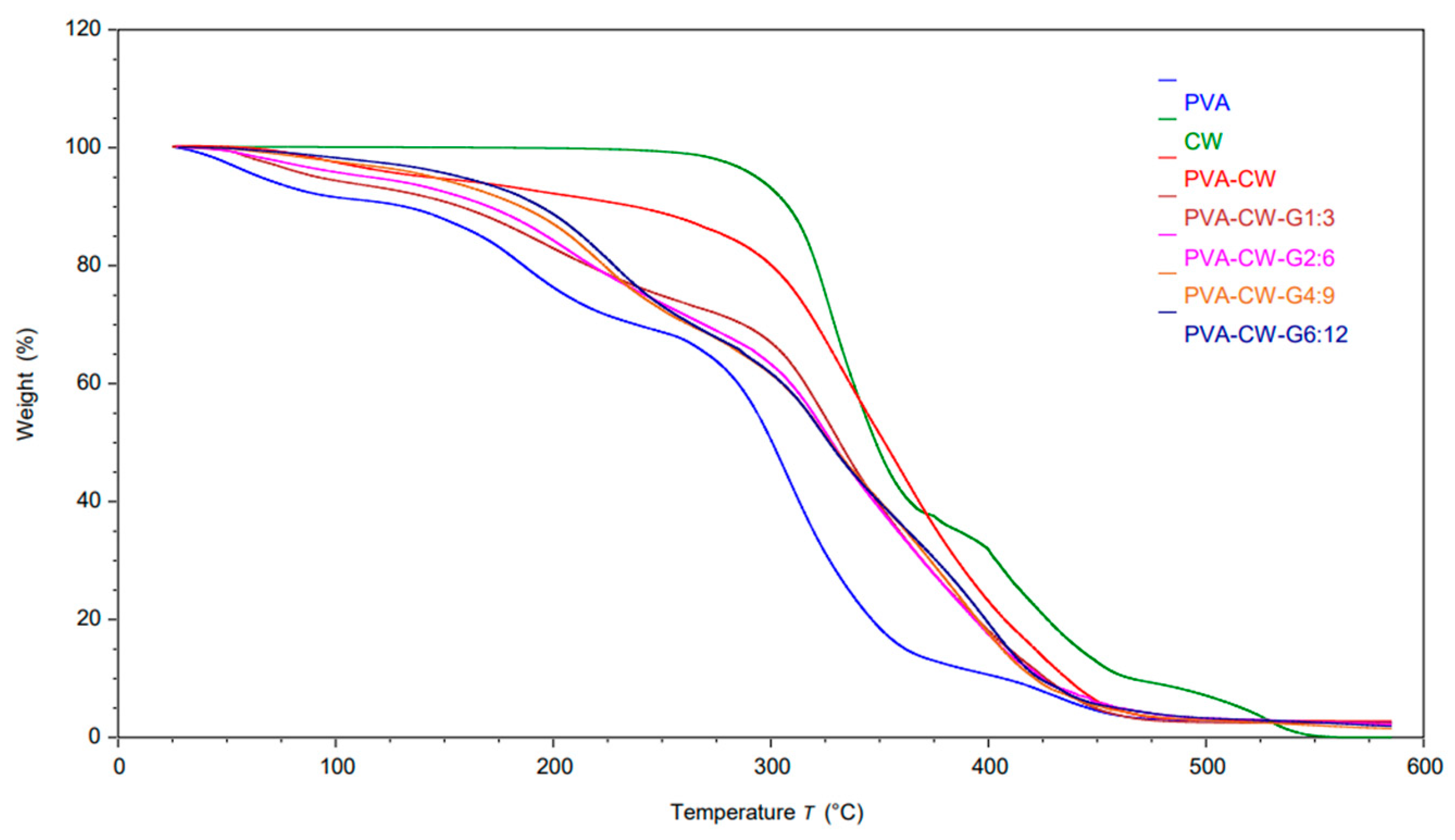
3.7. Thermal Analysis
3.8. Differential Scanning Calorimetric Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gobi, R.; Ravichandiran, P.; Babu, R.S.; Yoo, D.J. Biopolymer and Synthetic Polymer-Based Nanocomposites in Wound Dressing Applications: A Review. Polymers 2021, 13, 1962. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.D.A.D.; Matos, L.C.; Mendonça, M.C.; Lago, R.C.D.; Muguet, M.C.D.S.; Damásio, R.A.P.; Ponzecchi, A.; Soares, J.R.; Sanadi, A.R.; Tonoli, G.H.D. Evaluation of paper coated with cationic starch and carnauba wax mixtures regarding barrier properties. Ind. Crops Prod. 2023, 203, 117177. [Google Scholar] [CrossRef]
- Musa, B.H.; Hameed, N.J. Study of the mechanical properties of polyvinyl alcohol/starch blends. Mater. Today Proc. 2020, 20, 439–442. [Google Scholar] [CrossRef]
- Andrade, J.; González-Martínez, C.; Chiralt, A. The Incorporation of Carvacrol into Poly (vinyl alcohol) Films Encapsulated in Lecithin Liposomes. Polymers 2020, 12, 497. [Google Scholar] [CrossRef]
- Dos Santos, F.K.G.; Silva, K.N.d.O.; Xavier, T.D.N.; Leite, R.H.d.L.; Aroucha, E.M.M. Effect of the Addition of Carnauba Wax on Physicochemical Properties of Chitosan Films. Mater. Res. 2017, 20, 479–484. [Google Scholar] [CrossRef]
- Rullier-Birat, B.; Cazalbou, S.; Nassar, M.A.; Sandrine, C.; Tourrette, A. New backing layer for transdermal drug delivery systems: Coatings based on fatty acid and beeswax on chitosan films. J. Adhes. Sci. Technol. 2015, 29, 245–255. [Google Scholar] [CrossRef]
- Tijani, A.T.; Ayodele, T.; Liadi, M.; Clementson, C.; Hammed, A. Review of bioactive wax emulsified films. J. Appl. Polym. Sci. 2024, 141, e55718. [Google Scholar] [CrossRef]
- Nurul Syahida, S.; Ismail-Fitry, M.R.; Ainun, Z.M.A.; Nur Hanani, Z.A. Effects of palm wax on the physical, mechanical and water barrier properties of fish gelatin films for food packaging application. Food Packag. Shelf Life 2020, 23, 100437. [Google Scholar] [CrossRef]
- Hazrol, M.D.; Sapuan, S.M.; Zainudin, E.S.; Zuhri, M.Y.M.; Abdul Wahab, N.I. Corn Starch (Zea mays) Biopolymer Plastic Reaction in Combination with Sorbitol and Glycerol. Polymers 2021, 13, 242. [Google Scholar] [CrossRef]
- Muscat, D.; Tobin, M.J.; Guo, Q.; Adhikari, B. Understanding the distribution of natural wax in starch–wax films using synchrotron-based FTIR (S-FTIR). Carbohydr. Polym. 2014, 102, 125–135. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Souza, B.W.S.; Teixeira, J.A.; Vicente, A.A. Effect of glycerol and corn oil on physicochemical properties of polysaccharide films—A comparative study. Food Hydrocoll. 2012, 27, 175–184. [Google Scholar] [CrossRef]
- Matet, M.; Heuzey, M.-C.; Pollet, E.; Ajji, A.; Avérous, L. Innovative thermoplastic chitosan obtained by thermo-mechanical mixing with polyol plasticizers. Carbohydr. Polym. 2013, 95, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Mukaila, T.; Adeniyi, A.; Bello, I.; Sarker, N.C.; Monono, E.; Hammed, A. Synthesis and characterization of polylactic acid-lecithin-starch bioplastic film. J. Appl. Polym. Sci. 2024, 141, e55345. [Google Scholar] [CrossRef]
- Shafik, S.S.; Majeed, K.J.; Kamil, M.I. Preparation of PVA/corn starch blend films and studying the influence of gamma irradiation on mechanical properties. Int. J. Mater. Sci. Appl. 2014, 3, 25–28. [Google Scholar]
- Ibrahim, M.I.J.; Sapuan, S.M.; Zainudin, E.S.; Zuhri, M.Y.M. Physical, thermal, morphological, and tensile properties of cornstarch-based films as affected by different plasticizers. Int. J. Food Prop. 2019, 22, 925–941. [Google Scholar] [CrossRef]
- Santos, T.M.; Pinto, A.M.B.; De Oliveira, A.V.; Ribeiro, H.L.; Caceres, C.A.; Ito, E.N.; Azeredo, H.M.C. Physical properties of cassava starch–carnauba wax emulsion films as affected by component proportions. Int. J. Food Sci. Technol. 2014, 49, 2045–2051. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhai, X.; Wu, Y.; Li, C.; Zhang, R.; Sun, C.; Wang, W.; Hou, H. Effects of natural wax types on the physicochemical properties of starch/gelatin edible films fabricated by extrusion blowing. Food Chem. 2023, 401, 134081. [Google Scholar] [CrossRef]
- Muscat, D.; Adhikari, R.; McKnight, S.; Guo, Q.; Adhikari, B. The physicochemical characteristics and hydrophobicity of high amylose starch–glycerol films in the presence of three natural waxes. J. Food Eng. 2013, 119, 205–219. [Google Scholar] [CrossRef]
- Hiremani, V.D.; Gasti, T.; Sataraddi, S.; Vanjeri, V.N.; Goudar, N.; Masti, S.P.; Chougale, R.B. Characterization of Mechanical and Thermal Properties of Glycerol Mixed Oxidized Maize Starch/Polyvinyl alcohol Blend Films. Chem. Data Collect. 2020, 28, 100416. [Google Scholar] [CrossRef]
- Palma-Rodríguez, H.M.; Aguirre-Álvarez, G.; Chavarría-Hernández, N.; Rodríguez-Hernández, A.I.; Bello-Pérez, L.A.; Vargas-Torres, A. Oxidized banana starch–polyvinyl alcohol film: Partial characterization. Starch-Stärke 2012, 64, 882–889. [Google Scholar] [CrossRef]
- Rao, M.S.; Kanatt, S.R.; Chawla, S.P.; Sharma, A. Chitosan and guar gum composite films: Preparation, physical, mechanical and antimicrobial properties. Carbohydr. Polym. 2010, 82, 1243–1247. [Google Scholar] [CrossRef]
- Rivero, S.; García, M.A.; Pinotti, A. Crosslinking capacity of tannic acid in plasticized chitosan films. Carbohydr. Polym. 2010, 82, 270–276. [Google Scholar] [CrossRef]
- Nordin, N.; Othman, S.H.; Rashid, S.A.; Basha, R.K. Effects of glycerol and thymol on physical, mechanical, and thermal properties of corn starch films. Food Hydrocoll. 2020, 106, 105884. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of Plasticizer Type and Concentration on Dynamic Mechanical Properties of Sugar Palm Starch–Based Films. Int. J. Polym. Anal. Charact. 2015, 20, 627–636. [Google Scholar] [CrossRef]
- Pervaiz, M.; Oakley, P.; Sain, M. Development of Novel Wax-enabled Thermoplastic Starch Blends and Their Morphological, Thermal and Environmental Properties. Int. J. Compos. Mater. 2014, 4, 204–212. [Google Scholar]
- Auras, R.; Arroyo, B.; Selke, S. Production and Properties of Spin-Coated Cassava-Starch-Glycerol-Beeswax Films. Starch-Stärke 2009, 61, 463–471. [Google Scholar] [CrossRef]
- Tomoda, B.T.; Yassue-Cordeiro, P.H.; Ernesto, J.V.; Lopes, P.S.; Péres, L.O.; Da Silva, C.F.; De Moraes, M.A. Characterization of biopolymer membranes and films: Physicochemical, mechanical, barrier, and biological properties. In Biopolymer Membranes and Films; Elsevier: Amsterdam, The Netherlands, 2020; pp. 67–95. [Google Scholar]
- Gomes, Á.V.R.; Gonçalves, F.C.P.; Silva Júnior, M.Q.D.; Leite, R.H.D.L.; Dos Santos, F.K.G.; Aroucha, E.M.M. Effect of Carnauba Wax and Coconut Fiber Contents on Tensile Properties of Corn Starch-Based Biocomposites. Mater. Res. 2019, 22, e20190053. [Google Scholar] [CrossRef]
- Mohsin, M.; Hossin, A.; Haik, Y. Thermal and mechanical properties of poly(vinyl alcohol) plasticized with glycerol. J. Appl. Polym. Sci. 2011, 122, 3102–3109. [Google Scholar] [CrossRef]
- Yang, J.; Ching, Y.C.; Julai J, S.; Chuah, C.H.; Nguyen, D.H.; Lin, P.-C. Comparative study on the properties of starch-based bioplastics incorporated with palm oil and epoxidized palm oil. Polym. Polym. Compos. 2022, 30, 09673911221087595. [Google Scholar] [CrossRef]
- Liu, S.; Li, L.; Li, B.; Zhu, J.; Li, X. Size effect of carnauba wax nanoparticles on water vapor and oxygen barrier properties of starch-based film. Carbohydr. Polym. 2022, 296, 119935. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, D.; Regenstein, J.M.; Xia, W.; Dong, J. A comprehensive review on natural bioactive films with controlled release characteristics and their applications in foods and pharmaceuticals. Trends Food Sci. Technol. 2021, 112, 690–707. [Google Scholar] [CrossRef]
- Bourtoom, T. Plasticizer effect on the properties of biodegradable blend film from rice starch-chitosan. Songklanakarin J. Sci. Technol. 2008, 30, 149–165. [Google Scholar]
- Liu, X.; Wang, Y.; Yu, L.; Tong, Z.; Chen, L.; Liu, H.; Li, X. Thermal degradation and stability of starch under different processing conditions. Starch-Stärke 2013, 65, 48–60. [Google Scholar] [CrossRef]
- Guinesi, L.S.; da Róz, A.L.; Corradini, E.; Mattoso, L.H.C.; Teixeira, E.d.M.; Curvelo, A.A.d.S. Kinetics of thermal degradation applied to starches from different botanical origins by non-isothermal procedures. Thermochim. Acta 2006, 447, 190–196. [Google Scholar] [CrossRef]
- McHugh, T.H.; Krochta, J.M. Sorbitol- vs Glycerol-Plasticized Whey Protein Edible Films: Integrated Oxygen Permeability and Tensile Property Evaluation. J. Agric. Food Chem. 1994, 42, 841–845. [Google Scholar] [CrossRef]
- Białecka-Florjańczyk, E.; Florjańczyk, Z. Solubility of Plasticizers, Polymers and Environmental Pollution. In Thermodynamics, Solubility and Environmental Issues; Elsevier: Amsterdam, The Netherlands, 2007; pp. 397–408. [Google Scholar]
- Ballesteros-Mártinez, L.; Pérez-Cervera, C.; Andrade-Pizarro, R. Effect of glycerol and sorbitol concentrations on mechanical, optical, and barrier properties of sweet potato starch film. NFS J. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Lim, R.; Kiew, P.L.; Lam, M.K.; Yeoh, W.M.; Ho, M.Y. Corn starch/PVA bioplastics—The properties and biodegradability study using cultivation. Asia-Pac. J. Chem. Eng. 2021, 16, e2622. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of plasticizer type and concentration on physical properties of biodegradable films based on sugar palm (arenga pinnata) starch for food packaging. J. Food Sci. Technol. 2016, 53, 326–336. [Google Scholar] [CrossRef]
- Burgos, N.; Martino, V.P.; Jiménez, A. Characterization and ageing study of poly(lactic acid) films plasticized with oligomeric lactic acid. Polym. Degrad. Stab. 2013, 98, 651–658. [Google Scholar] [CrossRef]


| Blends | PVA | Glycerol | Polysorbate-20 | CW/SA |
|---|---|---|---|---|
| PVA/CW | 2.5 | - | 0.3 | 0.1 |
| PVA-CW-G1:3 | 2.5 | 1.5 | 0.3 | 0.5 |
| PVA-CW-G2:6 | 2.5 | 2.5 | 0.3 | 1.0 |
| PVA-CW-G4:9 | 2.5 | 3.5 | 0.3 | 2.0 |
| PVA-CW-G6:12 | 2.5 | 4.5 | 0.3 | 5.0 |
| Blends | Tg (°C) | Tm (°C) | Td (°C) |
|---|---|---|---|
| PVA/CW | 55.39 ± 1.7 a | 110.04 ± 3.2 d | 146.24 ± 1.5 f |
| PVA-CW-G1:3 | 51.45 ± 0.15 b | 77.65 ± 0.5 e | 80.21 ± 2.7 g |
| PVA-CW-G2:6 | 51.95 ± 0.18 b | 77.99 e ± 0.8 | 80.96 ± 3.5 g |
| PVA-CW-G4:9 | 52.58 ± 1.4 c | 77.39 ± 0.6 e | 81.39 ± 1.9 g |
| PVA-CW-G6:12 | 53.01 ± 0.56 bc | 77.60 ± 0.4 e | 81.45 ± 2.2 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tijani, A.T.; Ayodele, T.; Liadi, M.; Sarker, N.C.; Hammed, A. Mechanical and Thermal Characteristics of Films from Glycerol Mixed Emulsified Carnauba Wax/Polyvinyl Alcohol. Polymers 2024, 16, 3024. https://doi.org/10.3390/polym16213024
Tijani AT, Ayodele T, Liadi M, Sarker NC, Hammed A. Mechanical and Thermal Characteristics of Films from Glycerol Mixed Emulsified Carnauba Wax/Polyvinyl Alcohol. Polymers. 2024; 16(21):3024. https://doi.org/10.3390/polym16213024
Chicago/Turabian StyleTijani, Abodunrin Tirmidhi, Tawakalt Ayodele, Musiliu Liadi, Niloy Chandra Sarker, and Ademola Hammed. 2024. "Mechanical and Thermal Characteristics of Films from Glycerol Mixed Emulsified Carnauba Wax/Polyvinyl Alcohol" Polymers 16, no. 21: 3024. https://doi.org/10.3390/polym16213024
APA StyleTijani, A. T., Ayodele, T., Liadi, M., Sarker, N. C., & Hammed, A. (2024). Mechanical and Thermal Characteristics of Films from Glycerol Mixed Emulsified Carnauba Wax/Polyvinyl Alcohol. Polymers, 16(21), 3024. https://doi.org/10.3390/polym16213024






