Prevention and Control of Biofouling Coatings in Limnoperna fortunei: A Review of Research Progress and Strategies
Abstract
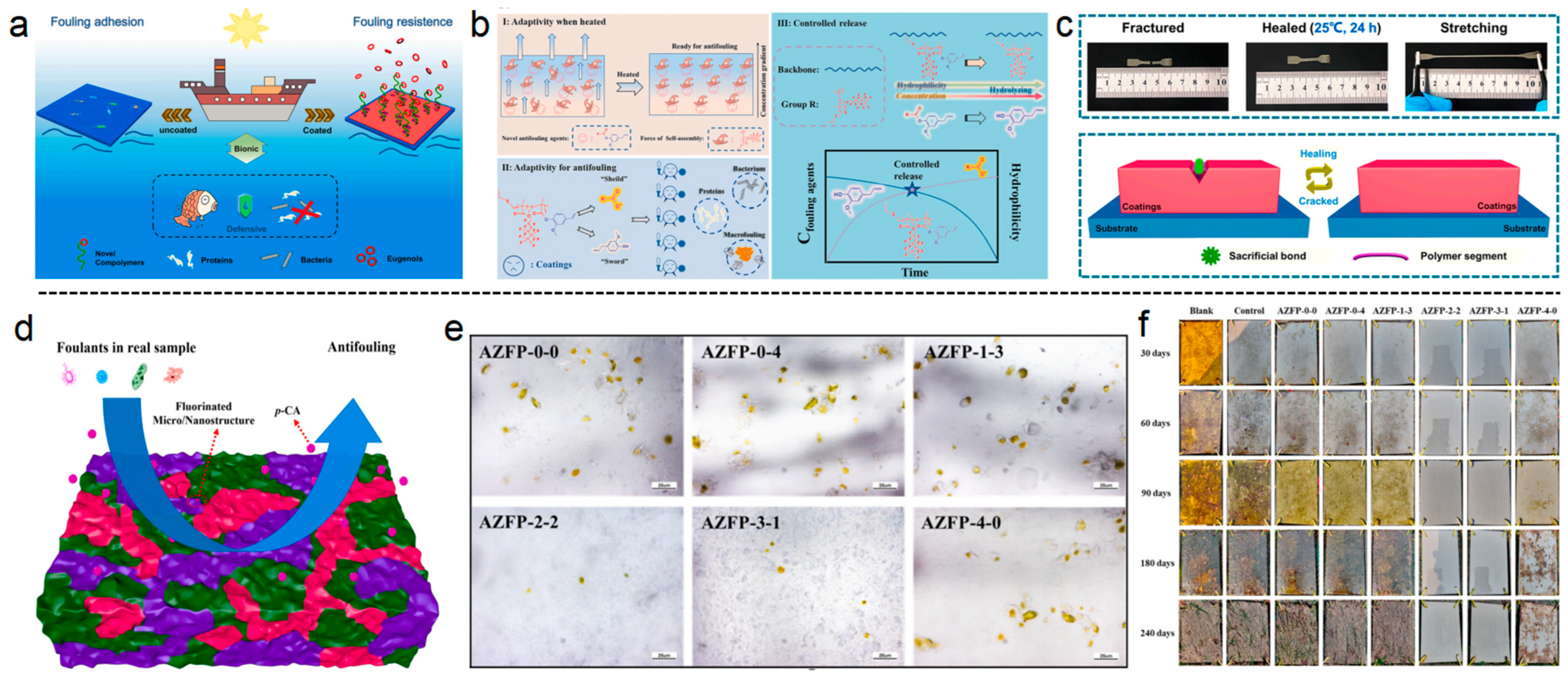
1. Introduction
2. Biological Characteristics of Limnoperna fortunei
2.1. Limnoperna fortunei
2.2. Biological Characteristics
- (1)
- Larval Stage
- (2)
- Juvenile Stage
- (3)
- Mature Stage
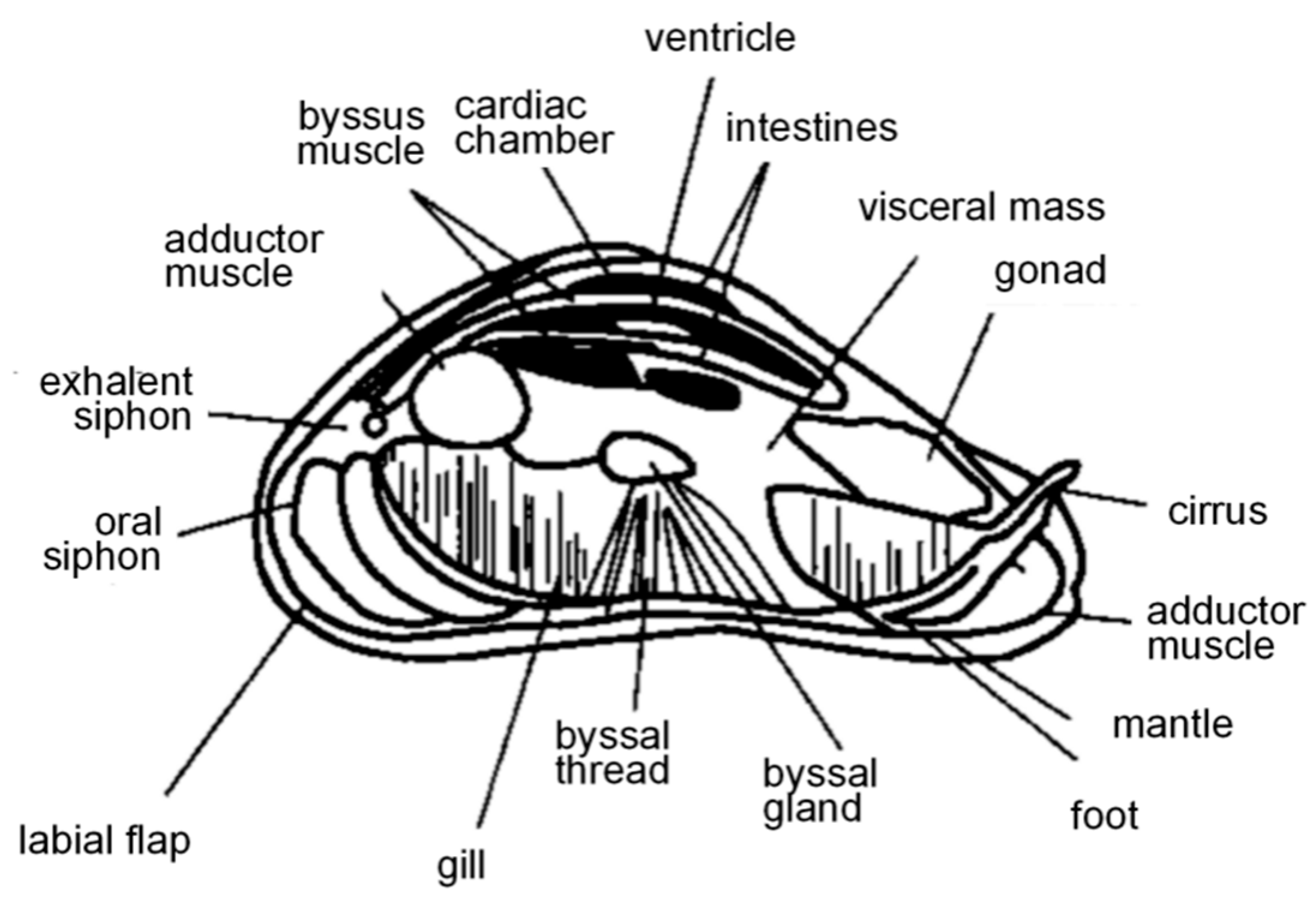
2.3. Growth and Attachment Mechanisms
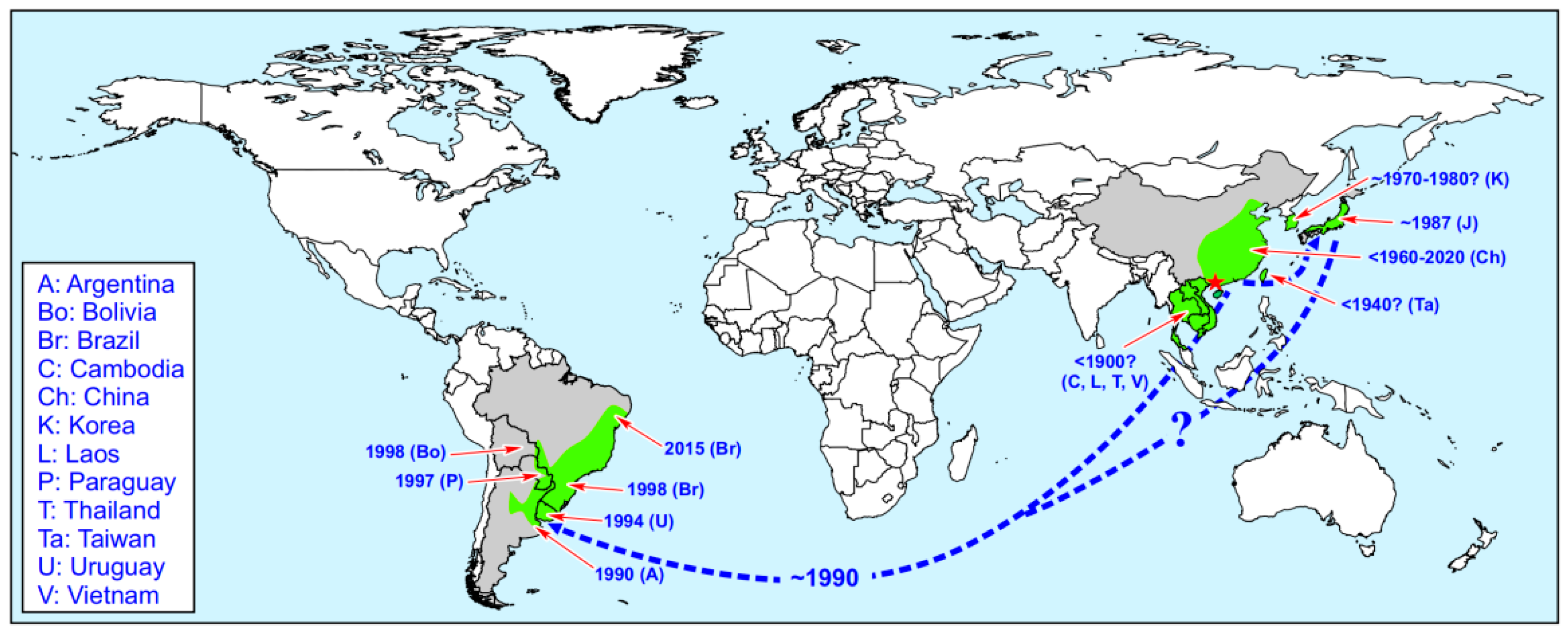
2.4. Habitat and Distribution of Limnoperna fortunei
3. Antifouling Mechanisms of Coatings
3.1. Conventional Antifouling Coatings
3.1.1. Environmental Impact and Limitations of Conventional Antifouling Coatings
- (1)
- Biotoxicity: Conventional coatings contain heavy metals and organotin substances that are highly toxic to aquatic life [68]. These toxic components gradually disperse into the aquatic environment, affecting the reproduction and growth of aquatic organisms and potentially leading to the long-term degradation of some species.
- (2)
- Bioaccumulation: Toxic substances released into the environment can accumulate in the food chain, eventually affecting higher-level consumers, including humans.
- (3)
- Ecological Disruption: The presence of harmful components in conventional coatings may disperse through water currents, potentially harming the entire aquatic ecosystem and threatening the survival and reproduction of other organisms.
3.1.2. Regulatory and Policy Environment
3.2. Antifouling Mechanisms of Novel Coatings
3.2.1. Eco-Friendly Coatings
- (1)
- Natural Antifouling Compounds
- (2)
- Low Surface Energy Materials
- (3)
- Enzyme Inhibitors
3.2.2. Biodegradable Coatings
- (1)
- Biodegradability
- (2)
- Controlled Release Technology
3.2.3. Nano-Antifouling Coatings
- (1)
- Nano-filler Effect
- (2)
- Photocatalytic Effects
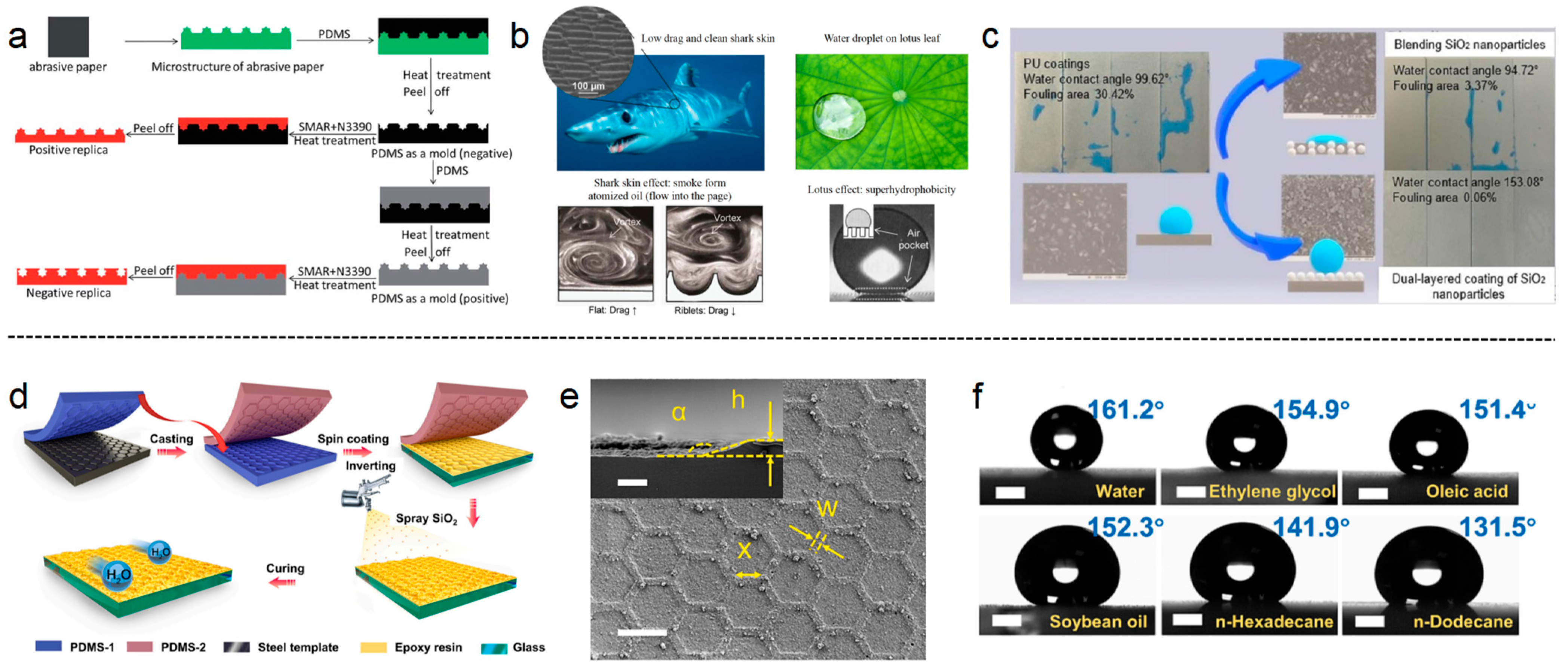
3.2.4. Structured Surface Coatings
- (1)
- Microtextural Effect
- (2)
- Superhydrophobic Properties
4. Conclusions
4.1. Challenges
4.2. Current Technological Limitations
4.3. Balancing Sustainable Development and Ecological Safety
4.4. Future Research Directions
- (1)
- Development of eco-friendly coating: Future studies should focus on innovating eco-friendly antifouling coatings derived from natural products, in order to minimize environmental and ecological impacts [109,135,136]. At the same time, we are investigating the long-term stability and durability of these materials to ensure that antifouling coatings can maintain their performance for a long time in practical applications. Furthermore, when developing new environmentally friendly coatings, a comprehensive ecological safety assessment is necessary. This includes assessing the environmental impact of these coatings throughout their entire lifecycle, from preparation to disposal, as well as their potential hazards to aquatic organisms and ecosystems. Future research should focus on longitudinal studies to observe the long-term effects of biodegradable coatings on mussel populations in different ecological regions. These studies will help validate theoretical predictions with empirical data, ensuring the ecological safety and effectiveness of these coatings.
- (2)
- Advancement through nanotechnology: The utilization of nanotechnology has the potential to significantly enhance the antifouling performance of coatings. Research should be directed towards developing intelligent coatings with self-repairing properties [108,137,138,139] and multifunctional coatings [140,141]. The polydimethylsiloxane with low surface energy was combined with polyurethane, which forms the novel type of waterborne polyurethane coating with good antifouling and self-healing abilities through incorporating polydimethylsiloxane and disulfide bonds [139]. These advanced coatings should adapt their chemical properties in response to environmental conditions, thereby improving durability and antifouling efficacy. For instance, by designing coatings with a multi-layered structure, the synergistic effect of multiple functions can be achieved, enhancing the overall performance of the coating. Future research should aim to elucidate the response mechanisms of these intelligent systems, enhance their performance characteristics, and explore their potential applications in complex aquatic environments.
- (3)
- Improving formulation and process: The focus should extend to improving the anti-adhesion properties, durability, and environmental performance of antifouling coatings through formulation and process innovations. Future research should concentrate on examining the interaction mechanisms between coatings and diverse substrates, developing universal coatings that are suitable for multiple substrates, and providing customized coating solutions for specific substrates.
- (4)
- Interdisciplinary integration: There is a compelling need to integrate knowledge and technologies from materials science, environmental science, biology, and chemistry into one integrated approach. This interdisciplinary approach will accelerate the development of sophisticated antifouling strategies tailored to manage biofouling by Limnoperna fortunei and potentially other invasive species.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hong, H.T.; Deng, A.J.; Tang, Y.; Liu, Z.X. How to identify biofouling species in marine and freshwater. Biofouling 2024, 40, 130–152. [Google Scholar] [CrossRef] [PubMed]
- Gizer, G.; Onal, U.; Ram, M.; Sahiner, N. Biofouling and mitigation methods: A review. Biointerface Res. Appl. Chem. 2023, 13, 185. [Google Scholar] [CrossRef]
- Hong, H.T.; Lv, J.W.; Deng, A.J.; Tang, Y.; Liu, Z.X. A review of experimental assessment processes of material resistance to marine and freshwater biofouling. J. Environ. Manag. 2024, 357, 120766. [Google Scholar] [CrossRef] [PubMed]
- Walter, T.; Langbein, M.; Blenk, P.; Tesler, A.B.; Prado, L.H.; Bornstein, D.; Virtanen, S.; Castiglione, K.; Vogel, N. Performance of environmentally friendly, liquid-infused coatings against biofouling: Evaluation of macrofouling and microbially induced corrosion in freshwater environments. J. Mater. Chem. A 2024, 12, 15278–15289. [Google Scholar] [CrossRef]
- Nakano, D.; Strayer, D.L. Biofouling animals in fresh water: Biology, impacts, and ecosystem engineering. Front. Ecol. Environ. 2014, 12, 167–175. [Google Scholar] [CrossRef]
- Zhang, R.H.; Zhang, Y.H.; Fei, X.L.; Hou, Y.N.; Shi, J.; Li, E.C.; Chu, W.H. Limnoperna fortunei as an invasive biofouling bivalve species in freshwater: A review of its occurrence, biological traits, risks, and control strategies. AQUA 2022, 71, 1364–1383. [Google Scholar] [CrossRef]
- Boltovskoy, D.; Paolucci, E.; MacIsaac, H.J.; Zhan, A.B.; Xia, Z.Q.; Correa, N. What we know and don’t know about the invasive golden mussel. Hydrobiologia 2022, 1–48. [Google Scholar] [CrossRef]
- Liu, Y.H.; He, X.Y.; Yang, Y.; Bai, X.Q.; Yuan, C.Q. Distribution, tolerance, growth, behaviour and control methods of (Dunker, 1857) (Bivalvia: Mytilidae): A review. Aquat. Conserv. 2024, 34, e4217. [Google Scholar] [CrossRef]
- Paolucci, E.M.; Burlakova, L.E.; Yarza, N.; Correa, N.; Boltovskoy, D.; Karatayev, A.Y. Planktonic larvae of the invasive bivalves dreissena spp. and Limnoperna fortunei: Review of their effects on freshwater communities. Hydrobiologia 2024. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, M.Z.; Chen, X.Y.; Zhang, J.H.; Wang, S.L.; Zhu, J.Y.; Fu, X.D. Establishment risk of invasive golden mussel in a water diversion project: An assessment framework. Environ. Sci. Ecotech. 2024, 17, 100305. [Google Scholar] [CrossRef]
- Boltovskoy, D. Limnoperna fortunei: The Ecology, Distribution and Control of a Swiftly Spreading Invasive Fouling Musse; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Wang, Y.P.; Pitet, L.M.; Finlay, J.A.; Brewer, L.H.; Cone, G.; Betts, D.E.; Callow, M.E.; Callow, J.A.; Wendt, D.E.; Hillmyer, M.A.; et al. Investigation of the role of hydrophilic chain length in amphiphilic perfluoropolyether/poly(ethylene glycol) networks: Towards high-performance antifouling coatings. Biofouling 2011, 27, 1139–1150. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, H.S.; Cho, Y.J.; Dimitriou, M.D.; Weinman, C.J.; Finlay, J.A.; Cone, G.; Callow, M.E.; Callow, J.A.; Kramer, E.J.; Ober, C.K. Fluorine-free mixed amphiphilic polymers based on PDMS and PEG side chains for fouling release applications. Biofouling 2011, 27, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Yang, C.G.; Xu, D.K.; Sun, D.; Nan, L.; Sun, Z.Q.; Li, Q.; Gu, T.Y.; Yang, K. Laboratory investigation of the microbiologically influenced corrosion (MIC) resistance of a novel Cu-bearing 2205 duplex stainless steel in the presence of an aerobic marine biofilm. Biofouling 2015, 31, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Ashassi-Sorkhabi, H.; Moradi-Haghighi, M.; Zarrini, G.; Javaherdashti, R. Corrosion behavior of carbon steel in the presence of two novel iron-oxidizing bacteria isolated from sewage treatment plants. Biodegradation 2012, 23, 69–79. [Google Scholar] [CrossRef]
- de Paula, R.S.; Reis, M.D.; de Oliveira, R.B.; Andrade, G.R.; de Carvalho, M.D.; Cardoso, A.N.V.; Jorge, E.C. Genetic and functional repertoires of (Dunker, 1857) (Mollusca, Mytilidae): A review on the use of molecular techniques for the detection and control of the golden mussel. Hydrobiologia 2020, 847, 2193–2202. [Google Scholar] [CrossRef]
- Fujita, D.S.; Takeda, A.M.; Coutinho, R.; Fernandes, F.C. Influence of antifouling paint on freshwater invertebrates (Mytilidae, Chironomidae and Naididae): Density, richness and composition. Braz. J. Biol. 2015, 75, S70–S78. [Google Scholar] [CrossRef]
- Li, S.G.; Li, X.; Cheng, J.W.; Zhan, A.B. Effectiveness and mechanisms of recoverable magnetic nanoparticles on mitigating golden mussel biofouling. Environ. Sci. Technol. 2021, 55, 2500–2510. [Google Scholar] [CrossRef]
- Elizárraga, V.H.H.; Ballantyne, S.; O’Brien, L.G.; Americo, J.A.; Suhr, S.T.; Senut, M.C.; Minerich, B.; Merkes, C.M.; Edwards, T.M.; Klymus, K.; et al. Toward invasive mussel genetic biocontrol: Approaches, challenges, and perspectives. Iscience 2023, 26, 108027. [Google Scholar] [CrossRef]
- Guan, F.; Zhang, X. Research on the oxygen consumption and ammonia excretion of invasive mussel Limnoperna fortunei in raw water transport pipeline. Water Wastewater Eng. 2005, 31, 23–26. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, A.; Wang, S.; Xu, M. Impact and control measures for Limnoperna fortunei (golden mussel) biofouling in water diversion proiects. J. Hydroecol. 2020, 41, 110–116. [Google Scholar] [CrossRef]
- Molina, F.R.; Paggi, J.C.; Devercelli, M. Zooplanktophagy in the natural diet and selectivity of the invasive mollusk Limnoperna fortunei. Biol. Invasions 2010, 12, 1647–1659. [Google Scholar] [CrossRef]
- Barbosa, N.P.U.; Ferreira, J.A.; Nascimento, C.A.R.; Silva, F.A.; Carvalho, V.A.; Xavier, E.R.S.; Ramon, L.; Almeida, A.C.; Carvalho, M.D.; Cardoso, A.V. Prediction of future risk of invasion by Limnoperna fortunei (Dunker, 1857) (Mollusca, Bivalvia, Mytilidae) in Brazil with cellular automata. Ecol. Indic. 2018, 92, 30–39. [Google Scholar] [CrossRef]
- Nakano, D.; Kobayashi, T.; Sakaguchi, I. Differences in larval dynamics of golden mussel between dam reservoirs with and without an aeration system. Landsc. Ecol. Eng. 2010, 6, 53–60. [Google Scholar] [CrossRef]
- Li, D. Study on the influence of Limnoperna fortunei on the water transport capacity of water transport buildings. Water Wastewater Eng. 2009, 45, 94–96. [Google Scholar] [CrossRef]
- Li, M.; Su, X. Analysis of the causes of shellfish growth in long-distance water pipes and culverts and prevention and control measures. Pearl River 2007, 3, 29–30+34. [Google Scholar]
- Xia, Z.Q.; Barker, J.R.; Zhan, A.B.; Haffner, G.D.; MacIsaac, H.J. Golden mussel (Limnoperna fortunei) survival during winter at the northern invasion front implies a potential high-latitude distribution. Divers. Distrib. 2021, 27, 1422–1434. [Google Scholar] [CrossRef]
- Nakano, D.; Kobayashi, T.; Sakaguchi, I. Reproduction and larval developmental stages of the freshwater mussel at three sites in a temperate reservoir. Invertebr. Reprod. Dev. 2017, 61, 128–135. [Google Scholar] [CrossRef]
- Perepelizin, P.V.; Boltovskoy, D. Effects of 254 nm UV irradiation on the mobility and survival of larvae of the invasive fouling mussel. Biofouling 2014, 30, 197–202. [Google Scholar] [CrossRef]
- Oliveira, M.D.; Hamilton, S.K.; Calheiros, D.F.; Jacobi, C.M. Oxygen Depletion Events Control the Invasive Golden Mussel (Limnoperna fortunei) in a Tropical Floodplain. Wetlands 2010, 30, 705–716. [Google Scholar] [CrossRef]
- Oliveira, M.D.; Calheiros, D.F.; Jacobi, C.M.; Hamilton, S.K. Abiotic factors controlling the establishment and abundance of the invasive golden mussel Limnoperna fortunei. Biol. Invasions 2011, 13, 717–729. [Google Scholar] [CrossRef]
- Tan, X.; Liu, D.; Zhang, J.; You, R.; Wei, X.; Gong, D. Behavioral and survival characteristics of fouling organisms Limnoperna fortunei in different eutrophic status. Resour. Environ. Yangtze Basin 2017, 26, 2120–2128. [Google Scholar]
- Boltovskoy, D.; Correa, N.; Bordet, F.; Leites, V.; Cataldo, D. Toxic microcystis (cyanobacteria) inhibit recruitment of the bloom-enhancing invasive bivalve Limnoperna fortunei. Freshwater Biol. 2013, 58, 1968–1981. [Google Scholar] [CrossRef]
- Ayroza, D.M.M.D.; do Carmo, C.F.; Camargo, A.F.M.; de Oliveira, M.D.; Petesse, M.L. Net cages enhance golden mussel (Limnoperna fortunei) larval density and condition factor. Freshwater Biol. 2019, 64, 1593–1602. [Google Scholar] [CrossRef]
- Sylvester, F.; Cataldo, D.H.; Notaro, C.; Boltovskoy, D. Fluctuating salinity improves survival of the invasive freshwater golden mussel at high salinity: Implications for the introduction of aquatic species through estuarine ports. Biol. Invasions 2013, 15, 1355–1366. [Google Scholar] [CrossRef]
- Pessotto, M.A.; Nogueira, M.G. More than two decades after the introduction of (Dunker 1857) in La Plata Basin. Braz. J. Biol. 2018, 78, 773–784. [Google Scholar] [CrossRef]
- Wei, X.; Yang, Z.; Liu, D.; Ma, J.; Tan, X. Behavioral characteristics of fouling organisms Limnoperna fortunei in hydraulic engineering in different environmental conditions. J. Hydroelec. Eng. 2016, 35, 73–80. [Google Scholar] [CrossRef]
- Li, S.G.; Xia, Z.Q.; Chen, Y.Y.; Gao, Y.C.; Zhan, A.B. Byssus structure and protein composition in the highly invasive fouling mussel. Front. Physiol. 2018, 9, 418. [Google Scholar] [CrossRef]
- Lee, B.P.; Messersmith, P.B.; Israelachvili, J.N.; Waite, J.H. Mussel-inspired adhesives and coatings. Annu. Rev. Mater. Res. 2011, 41, 99–132. [Google Scholar] [CrossRef]
- Waite, J.H.; Andersen, N.H.; Jewhurst, S.; Sun, C. Mussel adhesion: Finding the tricks worth mimicking. J. Adhes. 2005, 81, 297–317. [Google Scholar] [CrossRef]
- Zhao, H.; Waite, J.H. Proteins in load-bearing junctions: The histidine-rich metal-binding protein of mussel byssus. Biochemistry 2006, 45, 14223–14231. [Google Scholar] [CrossRef]
- Deacon, M.P.; Davis, S.S.; Waite, J.H.; Harding, S.E. Structure and mucoadhesion of mussel glue protein in dilute solution. Biochemistry 1998, 37, 14108–14112. [Google Scholar] [CrossRef] [PubMed]
- Bandara, N.; Zeng, H.B.; Wu, J.P. Marine mussel adhesion: Biochemistry, mechanisms, and biomimetics. J. Adhes. Sci. Technol. 2013, 27, 2139–2162. [Google Scholar] [CrossRef]
- Papov, V.V.; Diamond, T.V.; Biemann, K.; Waite, J.H. Hydroxyarginine-containing polyphenolic proteins in the adhesive plaques of the marine mussel Mytilus edulis. J. Biol. Chem. 1995, 270, 20183–20192. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Waite, J.H. Linking adhesive and structural proteins in the attachment plaque of Mytilus californianus. J. Biol. Chem. 2006, 281, 26150–26158. [Google Scholar] [CrossRef]
- Waite, J.H.; Qin, X. Polyphosphoprotein from the adhesive pads of Mytilus edulis. Biochemistry 2001, 40, 2887–2893. [Google Scholar] [CrossRef]
- Cha, H.J.; Hwang, D.S.; Lim, S. Development of bioadhesives from marine mussels. Biotechnol. J. 2008, 3, 631–638. [Google Scholar] [CrossRef]
- Waite, J.H.; Qin, X.X.; Coyne, K.J. The peculiar collagens of mussel byssus. Matrix Biol. 1998, 17, 93–106. [Google Scholar] [CrossRef]
- Coyne, K.J.; Qin, X.X.; Waite, J.H. Extensible collagen in mussel byssus: A natural block copolymer. Science 1997, 277, 1830–1832. [Google Scholar] [CrossRef]
- Qin, X.; Waite, J. A potential mediator of collagenous block copolymer gradients in mussel byssal threads. Proc. Natl. Acad. Sci. USA 1998, 95, 10517–10522. [Google Scholar] [CrossRef]
- Cmiel, A.M.; Struzynski, A.; Wyrebek, M.; Lipinska, A.M.; Zajac, K.; Zajac, T. Response of freshwater mussel recruitment to hydrological changes in a eutrophic floodplain lake. Sci. Total Environ. 2020, 703, 135467. [Google Scholar] [CrossRef]
- Chatelain, M.; Guizien, K. Modelling coupled turbulence—Dissolved oxygen dynamics near the sediment-water interface under wind waves and sea swell. Water Res. 2010, 44, 1361–1372. [Google Scholar] [CrossRef] [PubMed]
- de Castro, M.C.T.; Fileman, T.W.; Hall-Spencer, J.M. Invasive species in the Northeastern and Southwestern Atlantic Ocean: A review. Mar. Pollut. Bull. 2017, 116, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Kumar, R.; Anand, J.; Gupta, R.; Gupta, A.; Pant, K.; Dohare, S.; Tiwari, P.; Kesari, K.K.; Krishnan, S.; et al. Nanotechnology-based solutions for antibiofouling applications: An overview. ACS Appl. Nano Mater. 2023, 6, 12828–12848. [Google Scholar] [CrossRef]
- Silva, I.; Naya, D.; de Mello, F.T.; D’Anatro, A.; Tesitore, G.; Clavijo, C.; González-Bergonzoni, I. Fish vs. Aliens: Predatory fish regulate populations of mitigating impacts on native macroinvertebrate communities. Hydrobiologia 2021, 848, 2303. [Google Scholar] [CrossRef]
- Zhou, N.; Zhang, R.J.; Liu, B.D.; Cui, B.; Du, Z.L.; Chen, P.F.; Zhu, B.F.; Lin, C.; Dong, H.T.; Zhou, W.Y.; et al. Effects of ultrasound on invasive golden mussel mortality and tissue lesions. Sci. Total Environ. 2021, 761, 144134. [Google Scholar] [CrossRef]
- Carteau, D.; Vallée-Réhel, K.; Linossier, I.; Quiniou, F.; Davy, R.; Compère, C.; Delbury, M.; Faÿ, F. Development of environmentally friendly antifouling paints using biodegradable polymer and lower toxic substances. Prog. Org. Coat. 2014, 77, 485–493. [Google Scholar] [CrossRef]
- Castritsi-Catharios, J.; Neofitou, N.; Vorloou, A.A. Comparison of heavy metal concentrations in fish samples from three fish farms (Eastern Mediterranean) utilizing antifouling paints. Toxicol. Environ. Chem. 2015, 97, 116–123. [Google Scholar] [CrossRef]
- Nurioglu, A.G.; Esteves, A.C.C.; de With, G. Non-toxic, non-biocide-release antifouling coatings based on molecular structure design for marine applications. J. Mater. Chem. B 2015, 3, 6547–6570. [Google Scholar] [CrossRef]
- Silver, S.; Phung, L.T.; Silver, G. Silver as biocides in burn and wound dressings and bacterial resistance to silver compounds. J. Ind. Microbiol. Biotechnol. 2006, 33, 627–634. [Google Scholar] [CrossRef]
- Gibbs, P.E.; Bryan, G.W.; Pascoe, P.L.; Burt, G.R. The use of the dog-whelk, Nucella lapillus, as an indicator of tributyltin (TBT) contamination. J. Mar. Biol. Assoc. UK 1987, 67, 507–523. [Google Scholar] [CrossRef]
- Lee, S.H.; Chen, Y.S.; Chen, C.F.; Albarico, F.P.J.B.; Lim, Y.C.; Wang, M.H.; Chen, C.W.; Dong, C.D. Butyltin contamination in fishing port sediments after the ban of tributyltin antifouling paint: A case of Qianzhen Fishing Port in Taiwan. Water-Sui 2022, 14, 813. [Google Scholar] [CrossRef]
- Law, R.J.; Bolam, T.; James, D.; Barry, J.; Deaville, R.; Reid, R.J.; Penrose, R.; Jepson, P.D. Butyltin compounds in liver of harbour porpoises (Phocoena phocoena) from the UK prior to and following the ban on the use of tributyltin in antifouling paints (1992–2005&2009). Mar. Pollut. Bull. 2012, 64, 2576–2580. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.R.L.; Ross, P.M. Recovery of the New Zealand muricid dogwhelk Haustrum scobina from TBT-induced imposex. Mar. Pollut. Bull. 2018, 126, 396–401. [Google Scholar] [CrossRef] [PubMed]
- MacCallum, N.; Howell, C.; Kim, P.; Sun, D.; Friedlander, R.; Ranisau, J.; Ahanotu, O.; Lin, J.J.; Vena, A.; Hatton, B.; et al. Liquid-infused silicone as a biofouling-free medical material. ACS Biomater. Sci. Eng. 2015, 1, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Amini, S.; Kolle, S.; Petrone, L.; Ahanotu, O.; Sunny, S.; Sutanto, C.N.; Hoon, S.; Cohen, L.; Weaver, J.C.; Aizenberg, J.; et al. Preventing mussel adhesion using lubricant-infused materials. Science 2017, 357, 668–673. [Google Scholar] [CrossRef]
- Ware, C.S.; Smith-Palmer, T.; Peppou-Chapman, S.; Scarratt, L.R.J.; Humphries, E.M.; Balzer, D.; Neto, C. Marine antifouling behavior of lubricant-infused nanowrinkled polymeric surfaces. ACS Appl. Mater. Interfaces 2018, 10, 4173–4182. [Google Scholar] [CrossRef]
- Li, Z.C.; Liu, P.; Chen, S.W.; Liu, X.T.; Yu, Y.W.; Li, T.W.; Wan, Y.; Tang, N.; Liu, Y.X.; Gu, Y.X. Bioinspired marine antifouling coatings: Antifouling mechanisms, design strategies and application feasibility studies. Eur. Polym. J. 2023, 190, 111997. [Google Scholar] [CrossRef]
- IMO. International Convention for the Prevention of Pollution from Ships; International Maritime Organization (IMO): London, UK, 2021. [Google Scholar]
- Chemical Watch Group. US EPA Proposes Ban on Irgarol in Antifouling Paints; Chemical Watch: Brussels, Belgium, 2020. [Google Scholar]
- Blossom, N.; Szafranski, F.; Yacht, A.; Lotz, A. Use of Copper-Based Antifouling Paint: A US Regulatory Update. Coatingstech 2018, 15, 63–68. [Google Scholar]
- GB 38469-2019; Limit of Harmful Substances of Marine Coatings. State Administration for Market Regulation, Standardization Administration of the People’s Republic of China: Beijing, China, 2020.
- Pradhan, S.; Kumar, S.; Mohanty, S.; Nayak, S.K. Environmentally Benign Fouling-Resistant Marine Coatings: A Review. Polym-Plast. Technol. Mater. 2019, 58, 498–518. [Google Scholar] [CrossRef]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling Coatings: Recent Developments in the Design of Surfaces That Prevent Fouling by Proteins, Bacteria, and Marine Organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef]
- Braga, E.F.; Ayroza, D.M.M.D.; Silva, M.C.D.; Nascimento, T.S.; Sanches, E.G.; Carmo, C.F.D.; Pereira, L.P.F.; Albert, A.L.M.; Batista, W.R.; Lopes, R.S.C.; et al. Synthesis of lysoglycerophosphocholines from crude soybean lecithins as sustainable and non-toxic antifouling agents against the golden mussel Limnoperna fortunei. ACS Omega 2022, 7, 45197–45207. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hower, J.; Chen, S.F.; Bernards, M.T.; Chang, Y.; Jiang, S.Y. Molecular simulation studies of protein interactions with zwitterionic phosphorylcholine self-assembled monolayers in the presence of water. Langmuir 2008, 24, 10358–10364. [Google Scholar] [CrossRef] [PubMed]
- Batista, W.R.; Fernandes, F.C.; Neves, M.H.C.B.; Nascimento, T.S.; Lopes, R.S.C.; Lopes, C.C.; Ziegler, G.P.; Soler-Figueroa, B.M.; Sparks, D.; Fontaine, D.N.; et al. Synthetic lipids as a biocide candidate for disinfection of ballast water. Mar. Pollut. Bull. 2018, 137, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Batista, W.R.; Neves, M.H.C.B.; Coutinho, R.; Lopes, C.C.; Lopes, R.S.C. The synthesis of glycerophospholipids for use as a booster biocide in antifouling coatings. Quim. Nova 2015, 38, 917–923. [Google Scholar] [CrossRef]
- Prieto, I.M.; Paola, A.; Pérez, M.; García, M.; Blustein, G.; Schejter, L.; Palermo, J.A. Antifouling diterpenoids from the Sponge Dendrilla antarctica. Chem. Biodivers. 2022, 19, e202100618. [Google Scholar] [CrossRef] [PubMed]
- Selvin, J.; Lipton, A.P. Biopotentials of secondary metabolites isolated from marine sponges. Hydrobiologia 2004, 513, 231–238. [Google Scholar] [CrossRef]
- Kjelleberg, S.; Steinberg, P. Surface warfare in the sea. Microbiol. Today 2001, 28, 134–135. [Google Scholar]
- Nys, R.D.; Steinberg, P.D.; Willemsen, P.; Dworjanyn, S.A.; Gabelish, C.L.; King, R.J. Broad spectrum effects of secondary metabolites from the red alga delisea pulchra in antifouling assays. Biofouling 1995, 8, 259–271. [Google Scholar] [CrossRef]
- Hao, X.P.; Chen, S.G.; Qin, D.; Zhang, M.T.; Li, W.; Fan, J.C.; Wang, C.; Dong, M.Y.; Zhang, J.X.; Cheng, F.; et al. Antifouling and antibacterial behaviors of capsaicin-based pH responsive smart coatings in marine environments. Mater. Sci. Eng. C 2020, 108, 110361. [Google Scholar] [CrossRef]
- Lu, Z.W.; Chen, Z.; Guo, Y.; Ju, Y.Y.; Liu, Y.; Feng, R.; Xiong, C.X.; Ober, C.K.; Dong, L.J. Flexible hydrophobic antifouling coating with oriented nanotopography and nonleaking capsaicin. ACS Appl. Mater. Interfaces 2018, 10, 9718–9726. [Google Scholar] [CrossRef]
- Vitali, A.; Stringaro, A.; Colone, M.; Muntiu, A.; Angiolella, L. Antifungal carvacrol loaded chitosan nanoparticles. Antibiotics 2022, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- He, G.L.; Liu, Y.H.; Liu, W.Y.; Dong, L.; Yu, L.M.; Wang, L. A highly antifouling and eco-friendly hydrogel coating based on capsaicin derivative-functionalized polymer. J. Clean. Prod. 2023, 429, 139538. [Google Scholar] [CrossRef]
- Beyazkilic, Z.; Faccini, M.; Escobar, A.M.; Bautista, L. Eco-friendly capsaicin-containing water-based antifouling coatings for marine aquaculture. Coatings 2023, 13, 1616. [Google Scholar] [CrossRef]
- Qiao, Z.X.; Yang, H.; Liu, Y.; Chen, X.Y.; Feng, X.H.; Liu, X.M.; Zhang, B.T.; Huang, J.; Dan, Y.X.; Boshkov, N.; et al. A comparative study on anti-corrosion and antifouling performance of marine high density polyethylene-capsaicin composite coatings with different biocide content. J. Therm. Spray Technol. 2024, 33, 88–100. [Google Scholar] [CrossRef]
- Liu, Y.; Ru, R.T.; Ge, C.J.; Zhang, L.L.; Sun, H.F. Construction of highly hydrated and mechanically strong anti-fouling coatings for deep-sea probes based on poly(vinyl alcohol) tannic acid zwitterion coatings. New J. Chem. 2024, 48, 11729–11740. [Google Scholar] [CrossRef]
- Xu, G.; Neoh, K.G.; Kang, E.T.; Teo, S.L.M. Switchable antimicrobial and antifouling coatings from tannic acid-scaffolded binary polymer brushes. ACS Sustain. Chem. Eng. 2020, 8, 2586–2595. [Google Scholar] [CrossRef]
- Han, Z.L.; Chen, S.Y.; Deng, L.L.; Liang, Q.Q.; Qu, X.Y.; Li, J.; Wang, B.X.; Wang, H.P. Anti-fouling, adhesive polyzwitterionic hydrogel electrodes toughened using a tannic acid nanoflower. ACS Appl. Mater. Interfaces 2022, 14, 45954–45965. [Google Scholar] [CrossRef]
- Wisespongpand, P.; Kuniyoshi, M. Bioactive phloroglucinols from the brown alga Zonaria diesingiana. J. Appl. Phycol. 2003, 15, 225–228. [Google Scholar] [CrossRef]
- Guo, H.Y.; Song, L.N.; Hu, J.K.; Lin, T.F.; Li, X.H.; Yu, H.J.; Cheng, D.G.; Hou, Y.; Zhan, X.L.; Zhang, Q.H. Enhanced antifouling strategy with a strong synergistic effect of fluorescent antifouling and contact bacteriostasis using 7-amino-4-methylcoumarin. Chem. Eng. J. 2021, 420, 127676. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Gao, H.P.; Zhang, G.L.; Sun, Z.Y.; Zhang, J.W.; Wang, L.; Lin, C.G. Design and synthesis of a new bioactive compound for marine antifouling inspired by natural products. Nat. Prod. Res. 2024, 38, 1624–1628. [Google Scholar] [CrossRef]
- Salta, M.; Wharton, J.A.; Stoodley, P.; Dennington, S.P.; Goodes, L.R.; Werwinski, S.; Mart, U.; Wood, R.J.K.; Stokes, K.R. Designing biomimetic antifouling surfaces. Philos. Trans. R. Soc. A 2010, 368, 4729–4754. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.C.; Tian, L.M.; Bing, W.; Zhao, J.; Ren, L.Q. Toward the application of graphene for combating marine biofouling. Adv. Sustain. Syst. 2021, 5, 2000076. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, F.; Sun, X.; Jin, L.; Shi, L.; Hang, J.H. Preparation and properties of amphiphilic fluorinated silicone modified acrylic resin coating containing PEG for anti-fouling application. Acta Polym. Sin. 2016, 8, 1112–1120. [Google Scholar] [CrossRef]
- Jin, H.C.; Tian, L.M.; Bing, W.; Zhao, J.; Ren, L.Q. Bioinspired marine antifouling coatings: Status, prospects, and future. Prog. Mater. Sci. 2022, 124, 100889. [Google Scholar] [CrossRef]
- Wang, W.; Luo, Q.; Li, J.Y.; Li, L.Q.; Li, Y.H.; Huo, X.B.; Du, X.W.; Li, Z.S.; Wang, N. Photothermal-amplified single atom nanozyme for biofouling control in seawater. Adv. Funct. Mater. 2022, 32, 2205461. [Google Scholar] [CrossRef]
- Fang, G.; Kang, R.N.; Cai, S.W.; Ge, C.C. Insight into nanozymes for their environmental applications as antimicrobial and antifouling agents: Progress, challenges and prospects. Nano Today 2023, 48, 101755. [Google Scholar] [CrossRef]
- Shimeta, J.; Wilding-McBride, G.; Bott, N.J.; Piola, R.; Santander, R.; Leary, M.; Scardino, A.J. Growth of marine biofilms and macrofouling organisms on biocide-infused, 3D-printed thermoplastics. Front. Mar. Sci. 2023, 10, 1172942. [Google Scholar] [CrossRef]
- Ma, J.L.; Ma, C.F.; Yang, Y.; Xu, W.T.; Zhang, G.Z. Biodegradable polyurethane carrying antifoulants for inhibition of marine biofouling. Ind. Eng. Chem. Res. 2014, 53, 12753–12759. [Google Scholar] [CrossRef]
- Yang, J.X.; Li, L.W.; Ma, C.F.; Ye, X.D. Degradable polyurethane with poly(2-ethyl-2-oxazoline) brushes for protein resistance. RSC Adv. 2016, 6, 69930–69938. [Google Scholar] [CrossRef]
- Ma, J.L.; Ma, C.F.; Zhang, G.Z. Degradable polymer with protein resistance in a marine environment. Langmuir 2015, 31, 6471–6478. [Google Scholar] [CrossRef]
- Chen, S.S.; Ma, C.F.; Zhang, G.Z. Biodegradable polymer as controlled release system of organic antifoulant to prevent marine biofouling. Prog. Org. Coat. 2017, 104, 58–63. [Google Scholar] [CrossRef]
- Chen, S.S.; Ma, C.F.; Zhang, G.Z. Biodegradable polymers for marine antibiofouling: Poly(ε-caprolactone)/poly(butylene succinate) blend as controlled release system of organic antifoulant. Polymer 2016, 90, 215–221. [Google Scholar] [CrossRef]
- Sun, Z.Y.; Gu, L.H.; Zheng, J.Y.; Zhang, J.W.; Wang, L.; Xu, F.L.; Lin, C.G. A controlled release strategy of antifouling agent in coating based on intercalated layered double hydroxides. Mater. Lett. 2016, 172, 105–108. [Google Scholar] [CrossRef]
- Liu, J.H.; Li, Q.; Gui, T.J.; Guo, H.Y.; Zhang, K.; Meng, F.D.; Zhan, X.L.; Liu, Q.; Zhang, Q.H. Carp-inspired self-regulating marine antifouling coating: Featuring robust controlled release of eugenol and high-efficiency self-healing performance. Chem. Eng. J. 2024, 486, 149929. [Google Scholar] [CrossRef]
- Ma, C.F.; Zhang, W.P.; Zhang, G.Z.; Qian, P.Y. Environmentally friendly antifouling coatings based on biodegradable polymer and natural antifoulant. ACS Sustain. Chem. Eng. 2017, 5, 6304–6309. [Google Scholar] [CrossRef]
- Yu, Z.Q.; Sun, W.; Wang, L.D.; Yang, Z.Q.; Zhang, H.; Liu, G.C.; Zhang, Q. Excellent synergistic antifouling polymers based on controlled release of cinnamic acid and hydrolysis-induced fluorinated micro/nanostructure. Mater. Chem. Phys. 2022, 282, 125913. [Google Scholar] [CrossRef]
- Selim, M.S.; El-Safty, S.A.; Shenashen, M.A.; Higazy, S.A.; Elmarakbi, A. Progress in biomimetic leverages for marine antifouling using nanocomposite coatings. J. Mater. Chem. B 2020, 8, 3701–3732. [Google Scholar] [CrossRef]
- Selim, M.S.; Yang, H.; Wang, F.Q.; Li, X.; Huang, Y.; Fatthallah, N.A. Silicone/Ag@SiO2 core-shell nanocomposite as a self-cleaning antifouling coating material. RSC Adv. 2018, 8, 9910–9921. [Google Scholar] [CrossRef]
- Guo, X.M.; Pan, G.T.; Fang, L.N.; Liu, Y.; Rui, Z.B. Z-Scheme CuOx/Ag/TiO2 heterojunction as promising photoinduced anticorrosion and antifouling integrated coating in seawater. Molecules 2023, 28, 456. [Google Scholar] [CrossRef]
- Deng, Y.J.; Song, G.L.; Zheng, D.J.; Zhang, Y.M. Fabrication and synergistic antibacterial and antifouling effect of an organic/inorganic hybrid coating embedded with nanocomposite Ag@TA-SiO2 particles. Colloids Surf. A 2021, 613, 126085. [Google Scholar] [CrossRef]
- Wang, K.; Xu, K.W.; Tian, J.J.; Li, Z.Z.; Shao, G.S. Tailoring the micro-galvanic dissolution behavior and antifouling performance through laminated-structured Cu-X composite coating. J. Therm. Spray Technol. 2021, 30, 1566–1581. [Google Scholar] [CrossRef]
- Zhang, J.W.; Wang, Y.X.; Zhou, S.G.; Wang, Y.C.; Wang, C.T.; Guo, W.M.; Lu, X.J.; Wang, L.P. Tailoring self-lubricating, wear-resistance, anticorrosion and antifouling properties of Ti/(Cu, MoS2)-DLC coating in marine environment by controlling the content of Cu dopant. Tribol. Int. 2020, 143, 106029. [Google Scholar] [CrossRef]
- Sui, X.D.; Xu, R.N.; Liu, J.; Zhang, S.T.; Wu, Y.; Yang, J.; Hao, J.Y. Tailoring the tribocorrosion and antifouling performance of (Cr, Cu)-GLC coatings for marine application. ACS Appl. Mater. Interfaces 2018, 10, 36531–36539. [Google Scholar] [CrossRef] [PubMed]
- Selim, M.S.; El-Safty, S.A.; Fatthallah, N.A.; Shenashen, M.A. Silicone/graphene oxide sheet-alumina nanorod ternary composite for superhydrophobic antifouling coating. Prog. Org. Coat. 2018, 121, 160–172. [Google Scholar] [CrossRef]
- Amiri, S.; Vatanpour, V.; He, T. Antifouling thin-film nanocomposite NF membrane with polyvinyl alcohol-sodium alginate-graphene oxide nanocomposite hydrogel coated layer for As(III) removal. Chemosphere 2023, 322, 138159. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Mohanty, S.; Nayak, S.K. Preparation of hydrophobic epoxy-polydimethylsiloxane-graphene oxide nanocomposite coatings for antifouling application. Soft Matter 2020, 16, 1211–1226. [Google Scholar] [CrossRef] [PubMed]
- Selim, M.S.; Azzam, A.M.; Higazy, S.A.; El-Safty, S.A.; Shenashen, M.A. Novel graphene-based ternary nanocomposite coatings as ecofriendly antifouling brush surfaces. Prog. Org. Coat. 2022, 167, 106803. [Google Scholar] [CrossRef]
- Kyei, S.K.; Darko, G.; Akaranta, O. Chemistry and application of emerging ecofriendly antifouling paints: A review. J. Coat. Technol. Res. 2020, 17, 315–332. [Google Scholar] [CrossRef]
- Selim, M.S.; Yang, H.; Wang, F.Q.; Fatthallah, N.A.; Huang, Y.; Kuga, S. Silicone/ZnO nanorod composite coating as a marine antifouling surface. Appl. Surf. Sci. 2019, 466, 40–50. [Google Scholar] [CrossRef]
- Kumar, S.; Ye, F.; Mazinani, B.; Dobretsov, S.; Dutta, J. Chitosan nanocomposite coatings containing chemically resistant ZnO-SnOx core-shell nanoparticles for photocatalytic antifouling. Int. J. Mol. Sci. 2021, 22, 4513. [Google Scholar] [CrossRef] [PubMed]
- Al-Belushi, M.A.; Myint, M.T.Z.; Kyaw, H.H.; Al-Naamani, L.; Al-Mamari, R.; Al-Abri, M.; Dobretsov, S. ZnO nanorod-chitosan composite coatings with enhanced antifouling properties. Int. J. Biol. Macromol. 2020, 162, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Waterhouse, G.I.N.; Jia, M.Y.; Jiang, X.H.; Zhang, Z.M.; Yu, L.M. The feasibility of polyaniline-TiO2 coatings for photocathodic antifouling: Antibacterial effect. Synth. Met. 2019, 257, 116175. [Google Scholar] [CrossRef]
- Yoshioka, K.; Okuda, T.; Fujii, H.; Kamimoto, Y.; Kamiya, H. Effect of TiO2 nanoparticles aggregation in silicate thin coating films on photocatalytic behavior for antifouling materials. Adv. Powder Technol. 2013, 24, 886–890. [Google Scholar] [CrossRef]
- Chen, Z.F.; Zhao, W.J.; Mo, M.T.; Zhou, C.X.; Liu, G.; Zeng, Z.X.; Wu, X.D.; Xue, Q.J. Architecture of modified silica resin coatings with various micro/nano patterns for fouling resistance: Microstructure and antifouling performance. RSC Adv. 2015, 5, 97862–97873. [Google Scholar] [CrossRef]
- Xu, J.H.; Zhao, W.J.; Peng, S.S.; Zeng, Z.X.; Zhang, X.; Wu, X.D.; Xue, Q.J. Investigation of the biofouling properties of several algae on different textured chemical modified silicone surfaces. Appl. Surf. Sci. 2014, 311, 703–708. [Google Scholar] [CrossRef]
- He, X.Y.; Liu, Y.; Gong, Y.F.; Zhou, C.X.; Li, H. Autoclaving-induced in-situ grown alumina on arc-sprayed aluminum coatings: Multiscaled topography facilitates antifouling performances. Surf. Coat. Technol. 2017, 309, 295–300. [Google Scholar] [CrossRef]
- Bixler, G.D.; Bhushan, B. Rice and butterfly wing effect inspired low drag and antifouling surfaces: A review. Crit. Rev. Solid State Mater. Sci. 2015, 40, 1–37. [Google Scholar] [CrossRef]
- Pechook, S.; Pokroy, B. Self-assembling, bioinspired wax crystalline surfaces with time-dependent wettability. Adv. Funct. Mater. 2012, 22, 745–750. [Google Scholar] [CrossRef]
- Li, H.; Tu, S.H.; Tu, H.Y.; Chen, M.; Zhou, S.X.; Wu, L.M. Construction of transparent, robust and haze-selectable superhydrophobic coatings with honeycomb structure. Chem. Eng. J. 2024, 483, 149319. [Google Scholar] [CrossRef]
- Kanthasamy, R.; Algarni, M.; Peng, L.C.; Zakaria, N.A.; Zwawi, M. The effects of solvent on superhydrophobic polyurethane coating incorporated with hydrophilic SiO2 nanoparticles as antifouling paint. Polymers 2023, 15, 1328. [Google Scholar] [CrossRef]
- Al Solami, L.; Satheesh, S. Testing the efficacy of apple cider vinegar as an environmentally friendly natural product antifoulant for marine applications. Int. J. Environ. Sci. Technol. 2024, 21, 4587–4598. [Google Scholar] [CrossRef]
- Siless, G.E.; García, M.; Pérez, M.; Blustein, G.; Palermo, J.A. Large-scale purification of pachydictyol A from the brown alga obtained from algal wash and evaluation of its antifouling activity against the freshwater mollusk Limnoperna fortunei. J. Appl. Phycol. 2018, 30, 629–636. [Google Scholar] [CrossRef]
- Pan, S.Y.; Chen, M.; Wu, L.M. Smart superhydrophobic surface with restorable microstructure and self-healable surface chemistry. ACS Appl. Mater. Interfaces 2020, 12, 5157–5165. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.B.; Li, B.C.; Zhao, X.; Tian, N.; Zhang, J.P. Totally waterborne, nonfluorinated, mechanically robust, and self-healing superhydrophobic coatings for actual anti-icing. ACS Appl. Mater. Interfaces 2018, 10, 39391–39399. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.R.; Liu, H.H.; Li, M.; Fu, Z.N.; Liu, L.Q.; Zhang, H.J.; Fan, C.J.; Xu, J.; Wang, J. Multifunctional polyurethane coatings with excellent antifouling and self-healing properties. J. Coat. Technol. Res. 2024, 21, 1333–1342. [Google Scholar] [CrossRef]
- Jin, H.C.; Bing, W.; Jin, E.; Tian, L.M.; Jiang, Y.G. Bioinspired PDMS-phosphor-silicone rubber sandwich-structure coatings for combating biofouling. Adv. Mater. Interfaces 2020, 7, 1901577. [Google Scholar] [CrossRef]
- Peres, R.S.; Zmozinski, A.V.; Brust, F.R.; Macedo, A.J.; Armelin, E.; Alemán, C.; Ferreira, C.A. Multifunctional coatings based on silicone matrix and propolis extract. Prog. Org. Coat. 2018, 123, 223–231. [Google Scholar] [CrossRef]



| Protein | Molecular Weight /kDa | Functions | References |
|---|---|---|---|
| Mefp-1 | ~110 | Forms a protective coating over the byssus threads, shielding them from aqueous dissolution and microbial degradation. Exhibits limited adhesion capabilities. | [42] |
| Mefp-2 | ~40/45 | A primary structural protein of the attachment base, potentially involved in cross-linking with byssal pad adhesion proteins. Contains 5 mol% of DOTA. | [43] |
| Mefp-3 | ~5–7 | Localized at the interface between the attachment base and the substrate, acting as the principal adhesive protein in bond formation. | [44] |
| Mefp-4 | ~70–80 | An adhesion protein in the byssal pad, responsible for linking collagen-like proteins (such as preCoD) within the byssus threads to the adhesion proteins of the byssal pad. | [45] |
| Mefp-5 | ~9.5 | Predominantly found at the junction between the attachment base and external materials, considered the main adhesive protein facilitating the bond between mussel byssal pads and solid external surfaces. | [46] |
| Mefp-6 | ~11.6 | An adhesion protein present in the byssal pad. | [47] |
| PreCol-D | ~80 | Distal Collagen Prepolymers: These confer significant toughness and superior extensibility to the terminal regions of byssal fibers, enhancing their mechanical properties under stress. | [48] |
| PreCol-P | ~95 | Proximal Collagen Prepolymers: These polymers imbue the byssal fibers with both resilience and elasticity, crucial for maintaining fiber integrity during dynamic environmental interactions. | [49] |
| PreCol-NG | ~76 | Non-Gradient Collagen Prepolymers: Serve as connectors between PreCol-D and PreCol-P, forming the core structural framework of the byssal fibers. | [50] |
| Types | Mechanism | Sources | References |
|---|---|---|---|
| Terpenes | Inhibition of mycelial growth or destruction of cell membrane structure | Secondary metabolites from sponge dendrilla antarctica | [79,80] |
| Halogenated furanone | Inhibiting the attachment of fouling organisms | Red alga | [81,82] |
| Capsaicin | Inhibit adhesion, aggregation and accumulation of chlorella vulgaris | Solanaceae plants | [86,87,88] |
| Carvacrol | Destruction of cell wall and membrane integrity | Phenolic essential oils from plants in the family Lamiaceae | [85] |
| Tannic acid | Deactivate enzymes and proteins | The rhizomes, bark, and seeds of various plants | [89,90,91] |
| Triphenyl compounds | Inhibit the growth of diatoms | Brown algae | [92] |
| Coumarin | Fluorescent antifouling and contact bacteriostasis mechanisms | Leguminous plants | [93] |
| Paeonol | Bacteriostatic activity | Ranunculaceae plants and Asclepiadaceae plants | [94] |
| Type | Antifouling Effectiveness | Environmental Impact | Cost Control |
|---|---|---|---|
| Conventional Antifouling Coatings | Significantly reduce biofouling in the short term but require frequent recoating. | Utilize toxic antifoulants, leading to considerable environmental pollution. | Initial investment is relatively low, but long-term maintenance costs are high. |
| Eco-Friendly Coatings | Provide long-lasting fouling resistance and reduce environmental pollution. | Use natural or low-toxicity components, environmentally friendly. | Research and development costs are high, but overall cost-effectiveness is favorable. |
| Biodegradable Coatings | Coatings naturally degrade, minimizing long-term pollution; controlled release technology provides sustained antifouling effects. | Good biodegradability, low environmental impact over their lifecycle. | High research and development costs, but reduce subsequent coating treatment and removal expenses. |
| Nano-Antifouling Coatings | Highly effective fouling resistance due to significant nanoscale effects. | Safety of nanomaterials requires further validation | High cost of nanomaterials and complex preparation processes increase the overall coating costs. |
| Structured Surface Coatings | Prevents biofouling through physical barriers and superhydrophobic properties. | Minimal impact on aquatic ecosystems, but susceptibility to wear could lead to failure. | High manufacturing and processing costs and relatively low maintenance costs. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Ding, Q.; Zhang, Y.; Lu, G.; Liu, Y.; Tong, Y. Prevention and Control of Biofouling Coatings in Limnoperna fortunei: A Review of Research Progress and Strategies. Polymers 2024, 16, 3070. https://doi.org/10.3390/polym16213070
Zhang H, Ding Q, Zhang Y, Lu G, Liu Y, Tong Y. Prevention and Control of Biofouling Coatings in Limnoperna fortunei: A Review of Research Progress and Strategies. Polymers. 2024; 16(21):3070. https://doi.org/10.3390/polym16213070
Chicago/Turabian StyleZhang, Hailong, Qingjie Ding, Yonghui Zhang, Guangyi Lu, Yangyu Liu, and Yuping Tong. 2024. "Prevention and Control of Biofouling Coatings in Limnoperna fortunei: A Review of Research Progress and Strategies" Polymers 16, no. 21: 3070. https://doi.org/10.3390/polym16213070
APA StyleZhang, H., Ding, Q., Zhang, Y., Lu, G., Liu, Y., & Tong, Y. (2024). Prevention and Control of Biofouling Coatings in Limnoperna fortunei: A Review of Research Progress and Strategies. Polymers, 16(21), 3070. https://doi.org/10.3390/polym16213070







