Fabrication of Functionalized Graphene Oxide–Aluminum Hypophosphite Nanohybrids for Enhanced Fire Safety Performance in Polystyrene
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
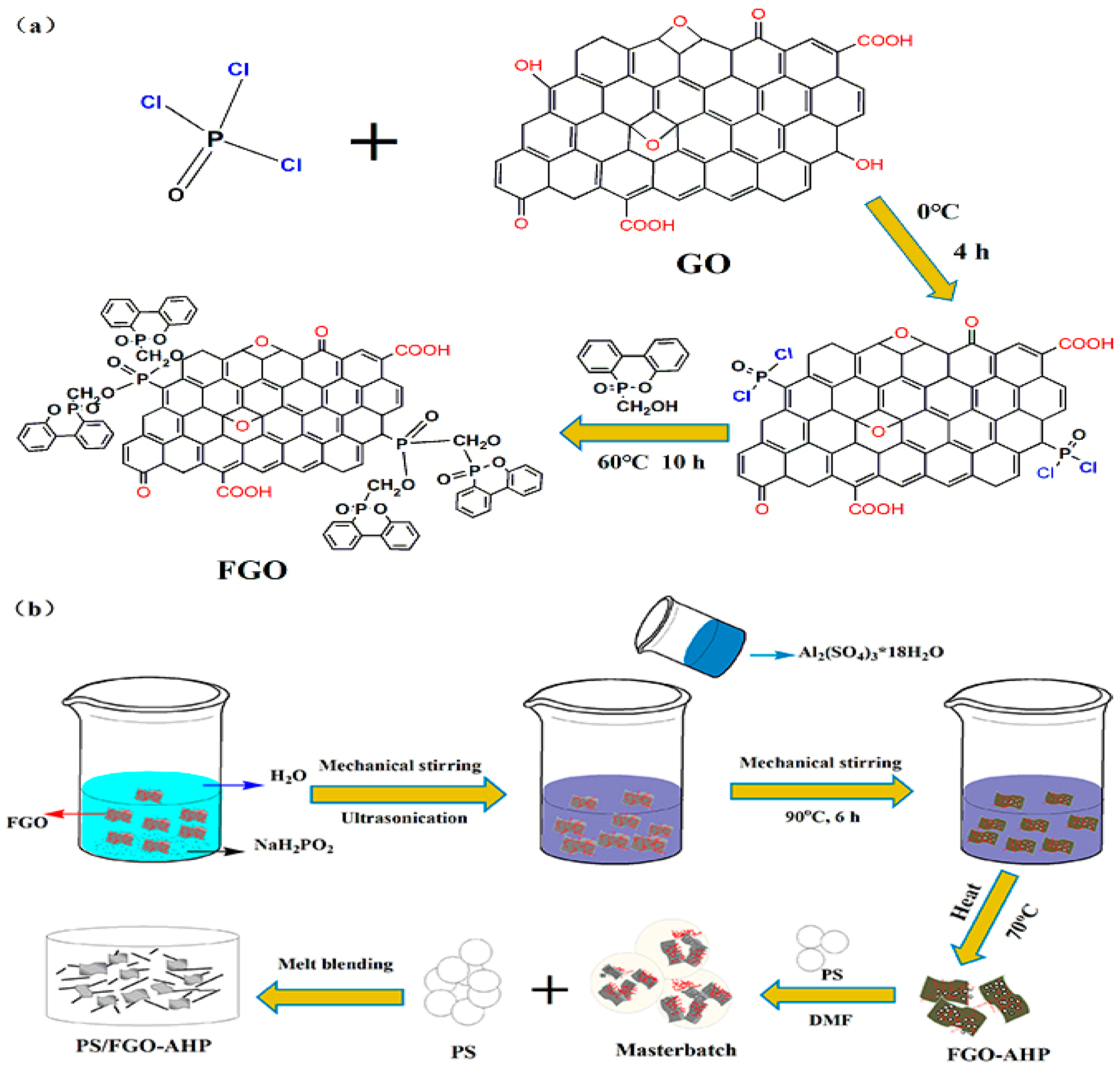
2.2. Fabrication of FGO and FGO–AHP
2.3. Preparation of PS and PS Nanocomposites
2.4. Characterization
3. Result and Discussion
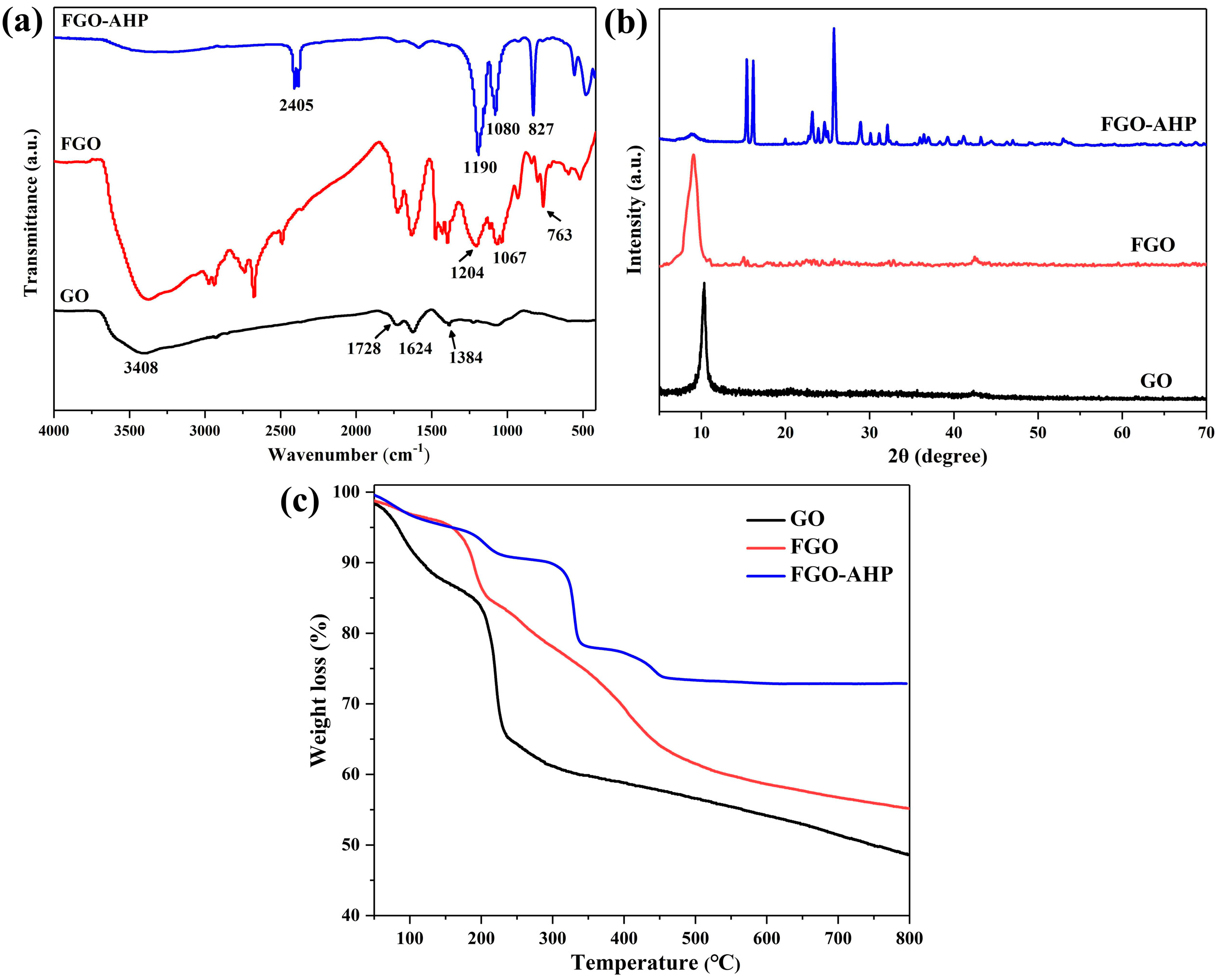
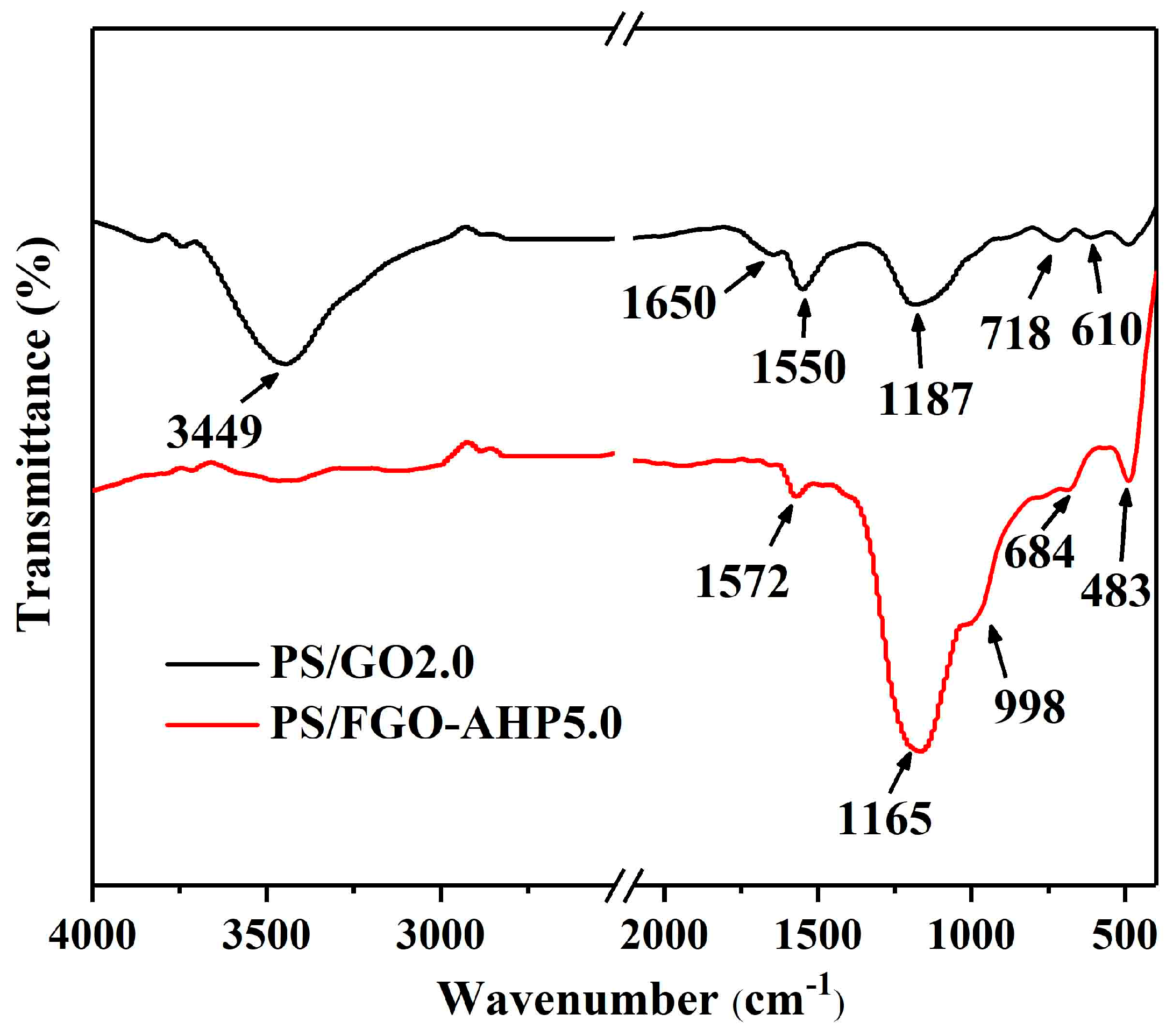
3.1. Characterization of GO, FGO, and FGO–AHP
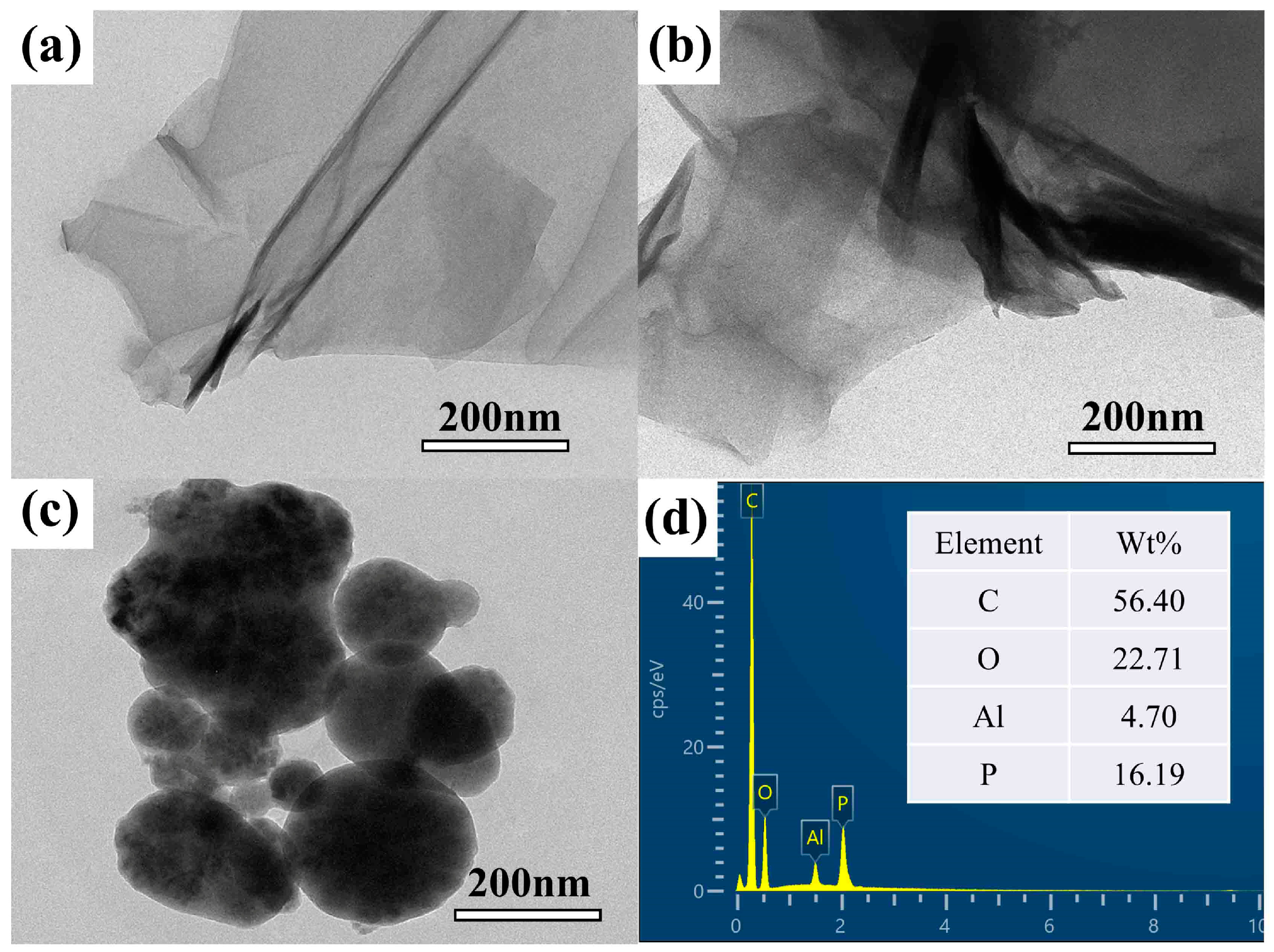
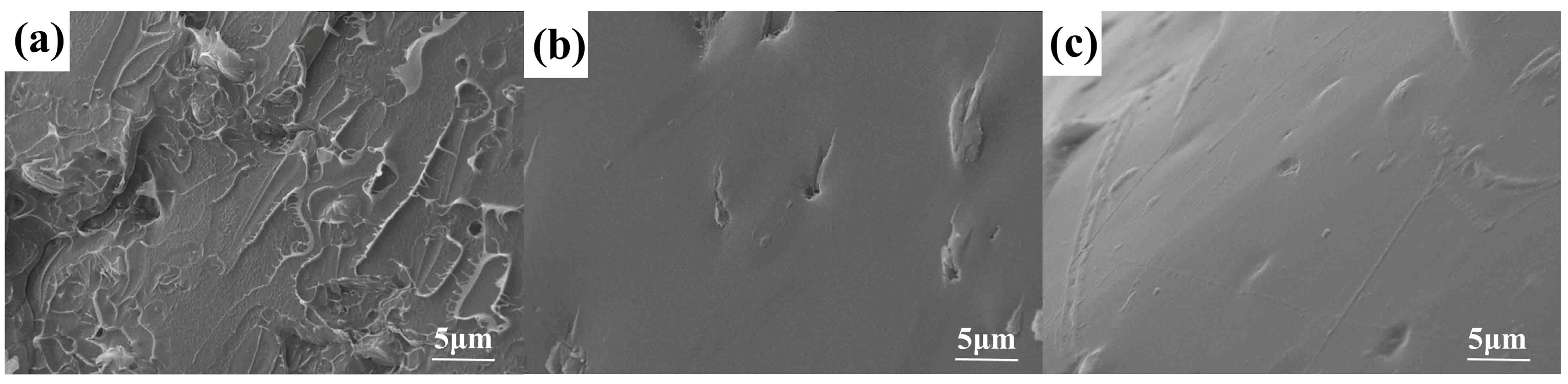
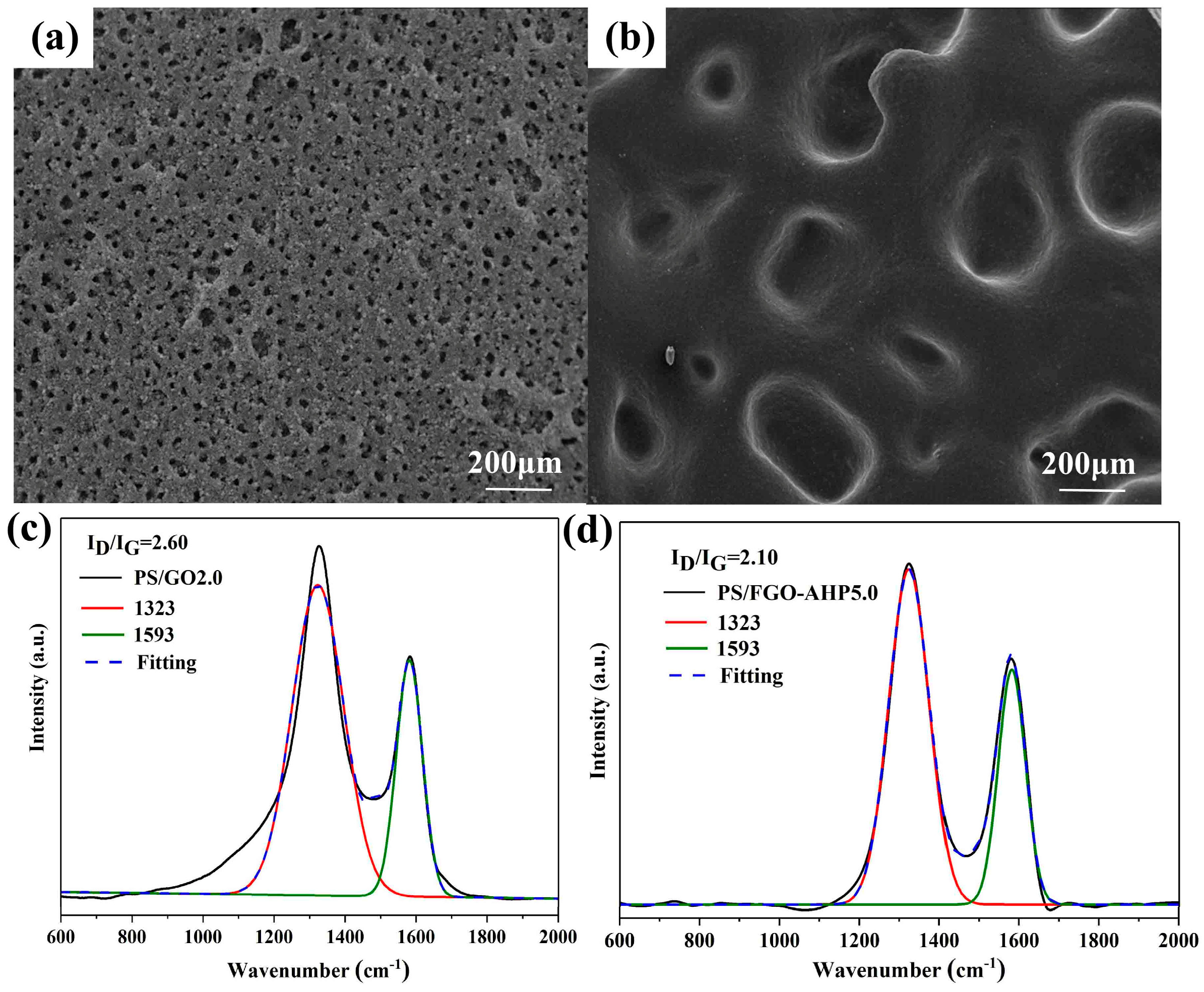
3.2. Morphological Analysis
3.3. Thermal Stability
3.4. Flammability of PS Nanocomposites
3.5. Toxic Smoke and Gaseous Volatiles Analysis
3.6. Char Residue Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, F.; Yang, K.; Liu, G.; Chen, Y.; Wang, M.; Li, S.; Li, R. Recent advances on graphene: Synthesis, properties and applications. Compos. Part A Appl. Sci. Manuf. 2022, 160, 107051. [Google Scholar] [CrossRef]
- Govindaraj, P.; Sokolova, A.; Salim, N.; Juodkazis, S.; Fuss, F.K.; Fox, B.; Hameed, N. Distribution states of graphene in polymer nanocomposites: A review. Compos. Part B Eng. 2021, 226, 109353. [Google Scholar] [CrossRef]
- Han, Y.; Wang, T.; Gao, X.; Li, T.; Zhang, Q. Preparation of thermally reduced graphene oxide and the influence of its reduction temperature on the thermal, mechanical, flame retardant performances of PS nanocomposites. Compos. Part A Appl. Sci. Manuf. 2016, 84, 336–343. [Google Scholar] [CrossRef]
- Kalali, E.N.; Guo, W.; Wang, X.; Xing, W.; Song, L.; Hu, Y. Effect of metal-based nanoparticles decorated graphene hybrids on flammability of epoxy nanocomposites. Compos. Part A Appl. Sci. Manuf. 2020, 129, 105694. [Google Scholar] [CrossRef]
- Gnanasekar, P.; Chen, H.; Tratnik, N.; Feng, M.; Yan, N. Enhancing performance of phosphorus containing vanillin-based epoxy resins by P–N non-covalently functionalized graphene oxide nanofillers. Compos. Part B Eng. 2021, 207, 108585. [Google Scholar] [CrossRef]
- Yu, W.; Sisi, L.; Haiyan, Y.; Jie, L. Progress in the functional modification of graphene/graphene oxide: A review. RSC Adv. 2020, 10, 15328–15345. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, L.; Guan, Q.; Liang, G.; Gu, A. Synergistically building flame retarding thermosetting composites with high toughness and thermal stability through unique phosphorus and silicone hybridized graphene oxide. Compos. Part A Appl. Sci. Manuf. 2017, 98, 174–183. [Google Scholar] [CrossRef]
- Sang, B.; Li, Z.-W.; Li, X.-H.; Yu, L.-G.; Zhang, Z.-J. Graphene-based flame retardants: A review. J. Mater. Sci. 2016, 51, 8271–8295. [Google Scholar] [CrossRef]
- Liao, S.-H.; Liu, P.-L.; Hsiao, M.-C.; Teng, C.-C.; Wang, C.-A.; Ger, M.-D.; Chiang, C.-L. One-step reduction and functionalization of graphene oxide with phosphorus-based compound to produce flame-retardant epoxy nanocomposite. Ind. Eng. Chem. Res. 2012, 51, 4573–4581. [Google Scholar] [CrossRef]
- Hou, Y.; Hu, W.; Liu, L.; Gui, Z.; Hu, Y. In-situ synthesized CNTs/Bi2Se3 nanocomposites by a facile wet chemical method and its application for enhancing fire safety of epoxy resin. Compos. Sci. Technol. 2018, 157, 185–194. [Google Scholar] [CrossRef]
- Feng, Y.; Li, X.; Zhao, X.; Ye, Y.; Zhou, X.; Liu, H.; Liu, C.; Xie, X. Synergetic improvement in thermal conductivity and flame retardancy of epoxy/silver nanowires composites by incorporating “branch-like” flame-retardant functionalized graphene. ACS Appl. Mater. Interfaces 2018, 10, 21628–21641. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yun, Y.; Fan, A.; Yuan, B.; Shang, S.; He, S. The assembly nanohybrid of graphene with lamellar zirconium phenylphosphonate for improving flame retardancy and mechanical properties of polypropylene. Polym. Compos. 2019, 40, E1757–E1765. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, L.; Wang, Z. Iron-phosphorus-nitrogen functionalized reduced graphene oxide for epoxy resin with reduced fire hazards and improved impact toughness. Compos. Part B Eng. 2020, 199, 108283. [Google Scholar] [CrossRef]
- Hu, W.; Yu, B.; Jiang, S.-D.; Song, L.; Hu, Y.; Wang, B. Hyper-branched polymer grafting graphene oxide as an effective flame retardant and smoke suppressant for polystyrene. J. Hazard. Mater. 2015, 300, 58–66. [Google Scholar] [CrossRef]
- Hou, Y.; Liu, L.; Qiu, S.; Zhou, X.; Gui, Z.; Hu, Y. DOPO-modified two-dimensional Co-based metal–organic framework: Preparation and application for enhancing fire safety of poly (lactic acid). ACS Appl. Mater. Interfaces 2018, 10, 8274–8286. [Google Scholar] [CrossRef]
- Shi, X.; Peng, X.; Zhu, J.; Lin, G.; Kuang, T. Synthesis of DOPO-HQ-functionalized graphene oxide as a novel and efficient flame retardant and its application on polylactic acid: Thermal property, flame retardancy, and mechanical performance. J. Colloid Interface Sci. 2018, 524, 267–278. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, B.; Zheng, Y.; Yang, J.; Duan, Z.; Hu, Y. Design of reduced graphene oxide decorated with DOPO-phosphanomidate for enhanced fire safety of epoxy resin. J. Colloid Interface Sci. 2018, 521, 160–171. [Google Scholar] [CrossRef]
- Mihis, A.G.; Cotet, L.C.; Cadar, C.; Pop, L.C.; Todea, M.; Rusu, M.M.; Vulpoi, A.; Székely, I.; Sălăgean, C.A.; Magyari, K. Structural and flame retardancy properties of GO-DOPO-HAK composite. J. Mater. Sci. 2023, 58, 7025–7047. [Google Scholar] [CrossRef]
- Liu, M.; Xie, Z.; Ye, H.; Li, W.; Shi, W.; Liu, Y.; Zhang, Y. Waste polystyrene foam–Chitosan composite materials as high-efficient scavenger for the anionic dyes. Colloids Surf. A 2021, 627, 127155. [Google Scholar] [CrossRef]
- Lu, J.; Wang, D.; Jiang, P.; Zhang, S.; Chen, Z.; Bourbigot, S.; Fontaine, G.; Wei, M. Design of fire resistant, sound-absorbing and thermal-insulated expandable polystyrene based lightweight particleboard composites. Constr. Build. Mater. 2021, 305, 124773. [Google Scholar] [CrossRef]
- Benchouia, H.E.; Boussehel, H.; Guerira, B.; Sedira, L.; Tedeschi, C.; Becha, H.E.; Cucchi, M. An experimental evaluation of a hybrid bio-composite based on date palm petiole fibers, expanded polystyrene waste, and gypsum plaster as a sustainable insulating building material. Constr. Build. Mater. 2024, 422, 135735. [Google Scholar] [CrossRef]
- Zhu, Y.; Shi, Y.; Huang, Z.-Q.; Duan, L.; Tai, Q.; Hu, Y. Novel graphite-like carbon nitride/organic aluminum diethylhypophosphites nanohybrid: Preparation and enhancement on thermal stability and flame retardancy of polystyrene. Compos. Part A Appl. Sci. Manuf. 2017, 99, 149–156. [Google Scholar] [CrossRef]
- Hou, B.; Song, X.; Song, K.; Geng, Z.; Pan, Y.-T.; Song, P.; Yang, R. Synchronous preparation and modification of LDH hollow polyhedra by polydopamine: Synthesis and application. J. Colloid Interface Sci. 2024, 654, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.-P.; Liao, S.-F.; Zhang, Y.; Chen, M.-J.; Xiao, Y.; Liu, X.-Y.; Liu, Z.-G.; Wang, D.-Y. Cu (0) and Cu (II) decorated graphene hybrid on improving fireproof efficiency of intumescent flame-retardant epoxy resins. Compos. Part B Eng. 2019, 175, 107189. [Google Scholar] [CrossRef]
- Xu, B.; Liu, Y.; Wei, S.; Zhao, S.; Qian, L.; Chen, Y.; Shan, H.; Zhang, Q. A phosphorous-based bi-functional flame retardant based on phosphaphenanthrene and aluminum hypophosphite for an epoxy thermoset. Int. J. Mol. Sci. 2022, 23, 11256. [Google Scholar] [CrossRef]
- Tawiah, B.; Yu, B.; Fei, B. Advances in flame retardant poly (lactic acid). Polymers 2018, 10, 876. [Google Scholar] [CrossRef]
- Li, J.; Qin, Z.; Zhai, C.; Yang, R. Study on the synergistic flame-retardancy of phenyl/vinyl siliconesquioxane and aluminum diethyl phosphinate on polyethylene terephthalate. Polym. Degrad. Stab. 2024, 220, 110660. [Google Scholar] [CrossRef]
- Lou, Y.; Ma, H.; Jia, Y.; Sun, S.; Wang, M.; Xu, J. One-pot synthesis of aluminum hypophosphite encapsulation in expanded halloysite lumen for reducing fire hazard and environmental pollution. Polym. Degrad. Stab. 2024, 219, 110599. [Google Scholar] [CrossRef]
- Ge, H.; Tang, G.; Hu, W.-Z.; Wang, B.-B.; Pan, Y.; Song, L.; Hu, Y. Aluminum hypophosphite microencapsulated to improve its safety and application to flame retardant polyamide 6. J. Hazard. Mater. 2015, 294, 186–194. [Google Scholar] [CrossRef]
- Bansal, S.; Prakash, K.; Sharma, K.; Sardana, N.; Kumar, S.; Gupta, N.; Singh, A.K. A highly efficient bilayer graphene/ZnO/silicon nanowire based heterojunction photodetector with broadband spectral response. Nanotechnology 2020, 31, 405205. [Google Scholar] [CrossRef]
- Bansal, S.; Das, A.; Prakash, K.; Sharma, K.; Khanal, G.M.; Sardana, N.; Kumar, S.; Gupta, N.; Singh, A.K. Bilayer graphene/HgCdTe heterojunction based novel GBn infrared detectors. Micro Nanostruct. 2022, 169, 207345. [Google Scholar] [CrossRef]
- Dai, K.; Sun, S.; Xu, W.; Song, Y.; Deng, Z.; Qian, X. Covalently-functionalized graphene oxide via introduction of bifunctional phosphorus-containing molecules as an effective flame retardant for polystyrene. RSC Adv. 2018, 8, 24993–25000. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Hu, J.; Xue, Y.; He, C.; Zhou, X.; Xie, X.; Ye, Y.; Mai, Y.-W. Simultaneous improvement in the flame resistance and thermal conductivity of epoxy/Al2O3 composites by incorporating polymeric flame retardant-functionalized graphene. J. Mater. Chem. A 2017, 5, 13544–13556. [Google Scholar] [CrossRef]
- Ji, P.; Cui, Y.; Liu, D.; Zhang, T.; Lv, J. The Bi-DOPO derivative functionalized graphene oxide: Preparation and its flame-retardation on epoxy resin. Polym. Adv. Technol. 2021, 32, 2843–2855. [Google Scholar] [CrossRef]
- Lin, Y.; Jiang, S.; Hu, Y.; Chen, G.; Shi, X.; Peng, X. Hybrids of aluminum hypophosphite and ammonium polyphosphate: Highly effective flame retardant system for unsaturated polyester resin. Polym. Compos. 2018, 39, 1763–1770. [Google Scholar] [CrossRef]
- Xu, W.; Wang, X.; Wu, Y.; Li, W.; Chen, C. Functionalized graphene with Co-ZIF adsorbed borate ions as an effective flame retardant and smoke suppression agent for epoxy resin. J. Hazard. Mater. 2019, 363, 138–151. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Z.; Li, X.; Yu, L.; Zhang, Z.; Wu, Z. Zinc ferrite nanoparticle decorated boron nitride nanosheet: Preparation, magnetic field arrangement, and flame retardancy. Chem. Eng. J. 2019, 356, 680–692. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Shi, Y.; Fu, L.; Liu, M.; Feng, Y.; Yu, B.; Gao, J.; Yang, F. Interface assembly of hypophosphite/ultrathin MXene nanosheets towards fire safe polylactic acid composites. Compos. Commun. 2022, 34, 101270. [Google Scholar] [CrossRef]
- Peyravi, A.; Ahmadijokani, F.; Arjmand, M.; Hashisho, Z. Graphene oxide enhances thermal stability and microwave absorption/regeneration of a porous polymer. J. Hazard. Mater. 2022, 433, 128792. [Google Scholar] [CrossRef]
- Imran, M.; Raza, M.; Noor, H.; Faraz, S.M.; Raza, A.; Farooq, U.; Khan, M.E.; Ali, S.K.; Bakather, O.Y.; Ali, W. Insight into mechanism of excellent visible-light photocatalytic activity of CuO/MgO/ZnO nanocomposite for advanced solution of environmental remediation. Chemosphere 2024, 359, 142224. [Google Scholar] [CrossRef]
- Wang, J.; Jin, X.; Li, C.; Wang, W.; Wu, H.; Guo, S. Graphene and graphene derivatives toughening polymers: Toward high toughness and strength. Chem. Eng. J. 2019, 370, 831–854. [Google Scholar] [CrossRef]
- He, W.; Song, P.; Yu, B.; Fang, Z.; Wang, H. Flame retardant polymeric nanocomposites through the combination of nanomaterials and conventional flame retardants. Prog. Mater. Sci. 2020, 114, 100687. [Google Scholar] [CrossRef]
- Noor, H.; Zafar, A.; Raza, A.; Baqi, A.; Farooq, U.; Khan, M.E.; Ali, W.; Ali, S.K.; Bashiri, A.H.; Zakri, W. Advancement in optical and dielectric properties of unsaturated polyester resin/zinc oxide nanocomposite: Synthesis to application in electronics. J. Mater. Sci. Mater. Electron. 2024, 35, 1598. [Google Scholar] [CrossRef]
- Wiacek, M.; Wesolek, D.; Rojewski, S.; Bujnowicz, K.; Schab-Balcerzak, E. Halogeno-modified polystyrene: Monomer reactivity ratios, thermal behaviour and flammability. Polym. Int. 2014, 63, 1982–1990. [Google Scholar] [CrossRef]
- Hou, Y.; Hu, W.; Hu, Y. Preparation of layered organic-inorganic aluminum phosphonate for enhancing fire safety of polystyrene. Mater. Chem. Phys. 2017, 196, 109–117. [Google Scholar] [CrossRef]
- Zhu, Z.-M.; Rao, W.-H.; Kang, A.-H.; Liao, W.; Wang, Y.-Z. Highly effective flame retarded polystyrene by synergistic effects between expandable graphite and aluminum hypophosphite. Polym. Degrad. Stab. 2018, 154, 1–9. [Google Scholar] [CrossRef]
- Wang, G.; Bai, S. Synergistic effect of expandable graphite and melamine phosphate on flame-retardant polystyrene. J. Appl. Polym. Sci. 2017, 134, 45474. [Google Scholar] [CrossRef]
- Amirabadi, S.; Kheradmandkeysomi, M.; Zandieh, A.; Serles, P.; Tanguy, N.; Filleter, T.; Sain, M.; Park, C.B. Highly tough and flame retardant polystyrene composites by elastomeric nanofibers and hexagonal boron nitride. J. Mater. Sci. Technol. 2024, 198, 208–220. [Google Scholar] [CrossRef]
- Huang, R.; Gao, C.; Shi, Y.; Fu, L.; Feng, Y.; Shui, W. Synergistic function between phosphorus-containing flame retardant and multi-walled carbon nanotubes towards fire safe polystyrene composites with enhanced electromagnetic interference shielding. Int. J. Mol. Sci. 2022, 23, 13434. [Google Scholar] [CrossRef]
- Zhou, K.; Gui, Z.; Hu, Y. The influence of graphene based smoke suppression agents on reduced fire hazards of polystyrene composites. Compos. Part A Appl. Sci. Manuf. 2016, 80, 217–227. [Google Scholar] [CrossRef]
- Hu, G.; Zhang, X.; Bu, M.; Lei, C. Toughening and strengthening epoxy resins with a new bi-DOPO biphenyl reactive flame retardant. Eur. Polym. J. 2022, 178, 111488. [Google Scholar] [CrossRef]
- Liu, M.; Gong, Z.; Wang, G.; Liu, X.; Hou, Y.; Tang, G. Melamine resin coordinated cobalt@piperazine pyrophosphate microcapsule: An innovative strategy for imparting long-lasting fire safety to rigid polyurethane foams. Polym. Degrad. Stab. 2024, 219, 110605. [Google Scholar] [CrossRef]
- Xia, Z.; Kiratitanavit, W.; Facendola, P.; Thota, S.; Yu, S.; Kumar, J.; Mosurkal, R.; Nagarajan, R. Fire resistant polyphenols based on chemical modification of bio-derived tannic acid. Polym. Degrad. Stab. 2018, 153, 227–243. [Google Scholar] [CrossRef]
- Yuan, B.; Hu, Y.; Chen, X.; Shi, Y.; Niu, Y.; Zhang, Y.; He, S.; Dai, H. Dual modification of graphene by polymeric flame retardant and Ni(OH)2 nanosheets for improving flame retardancy of polypropylene. Compos. Part A Appl. Sci. Manuf. 2017, 100, 106–117. [Google Scholar] [CrossRef]
- Wang, H.; Li, S.; Zhu, Z.; Yin, X.; Wang, L.; Weng, Y.; Wang, X. A novel DOPO-based flame retardant containing benzimidazolone structure with high charring ability towards low flammability and smoke epoxy resins. Polym. Degrad. Stab. 2021, 183, 109426. [Google Scholar] [CrossRef]
- Tang, G.; Wang, X.; Xing, W.; Zhang, P.; Wang, B.; Hong, N.; Yang, W.; Hu, Y.; Song, L. Thermal degradation and flame retardance of biobased polylactide composites based on aluminum hypophosphite. Ind. Eng. Chem. Res. 2012, 51, 12009–12016. [Google Scholar] [CrossRef]

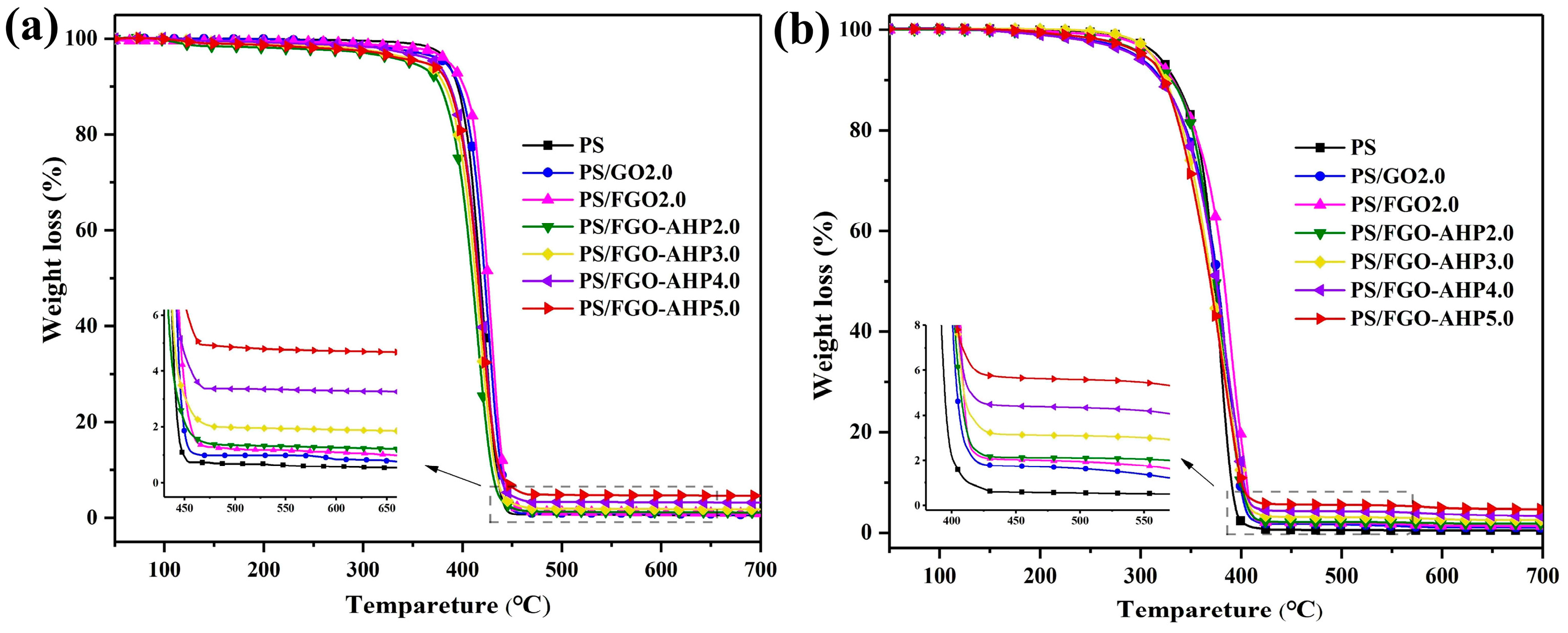
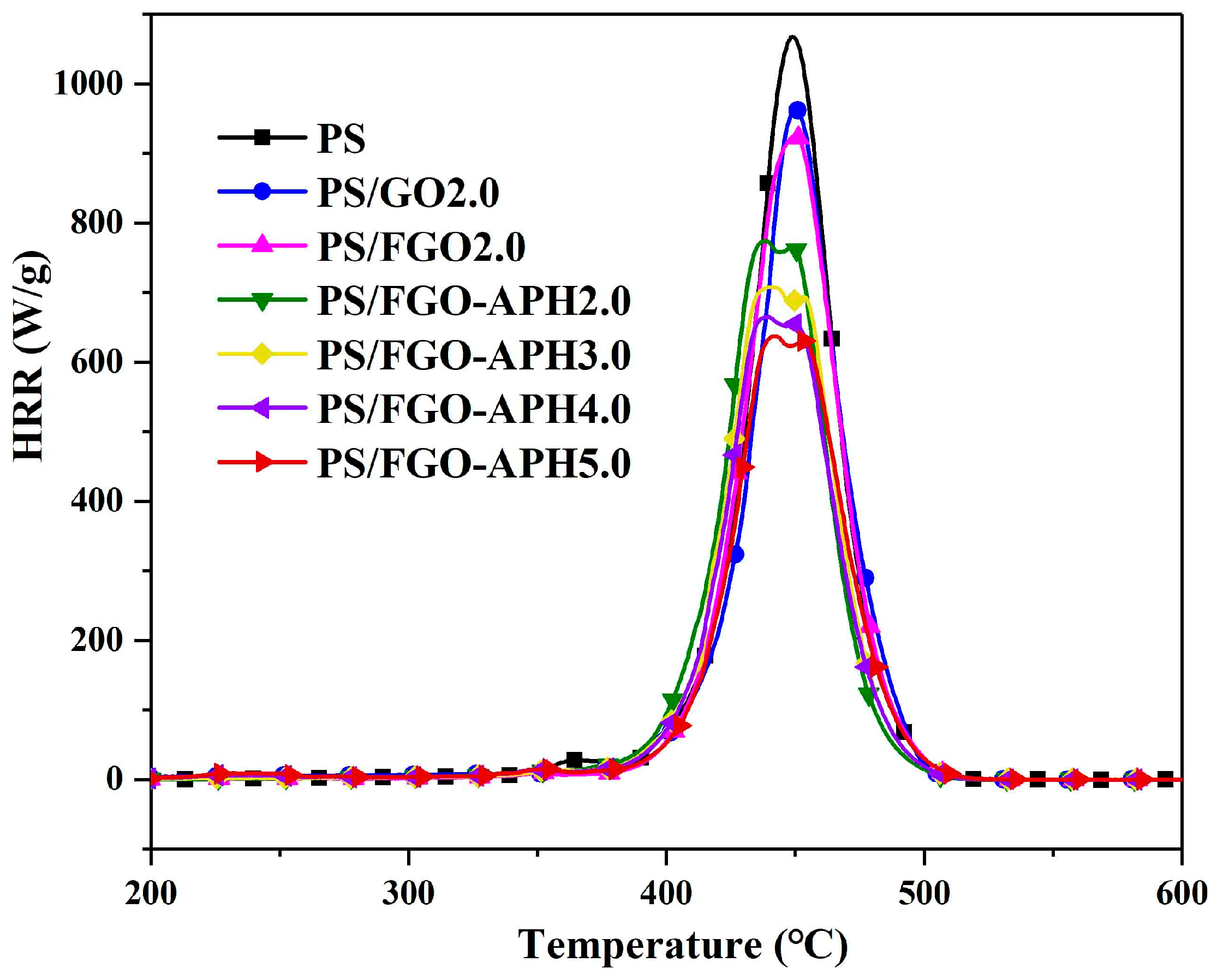
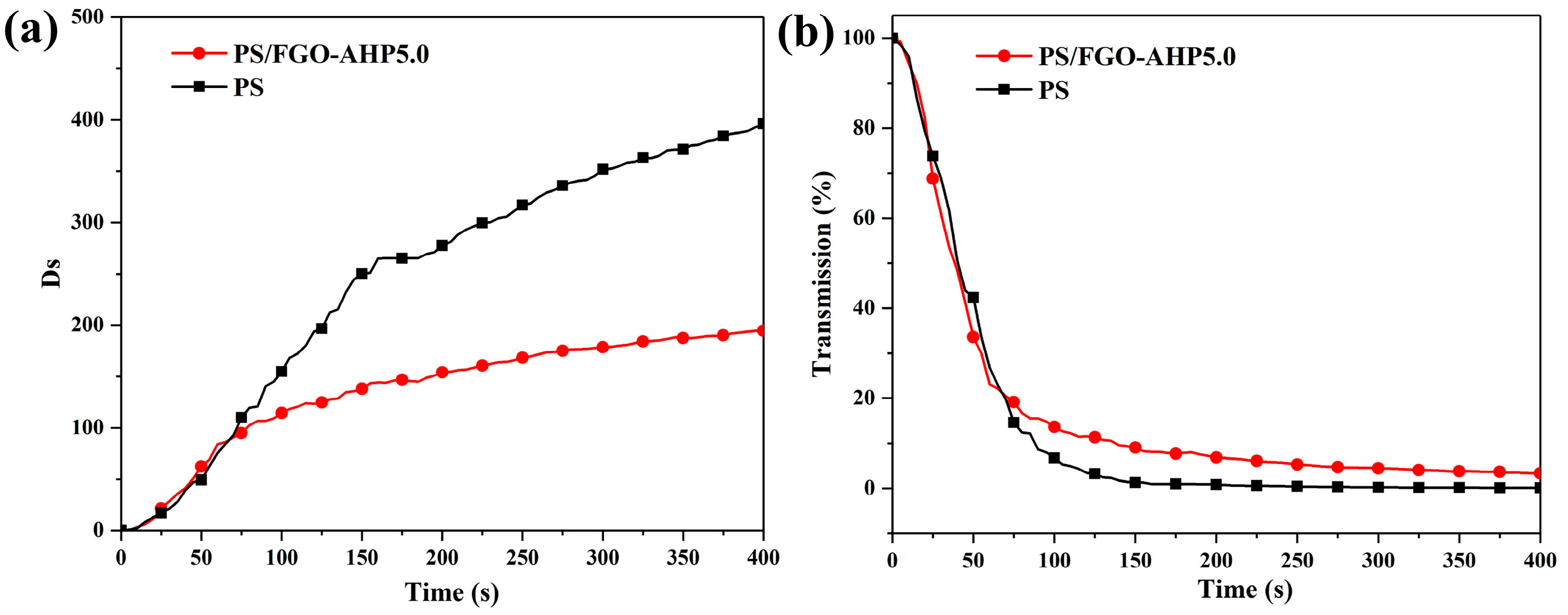
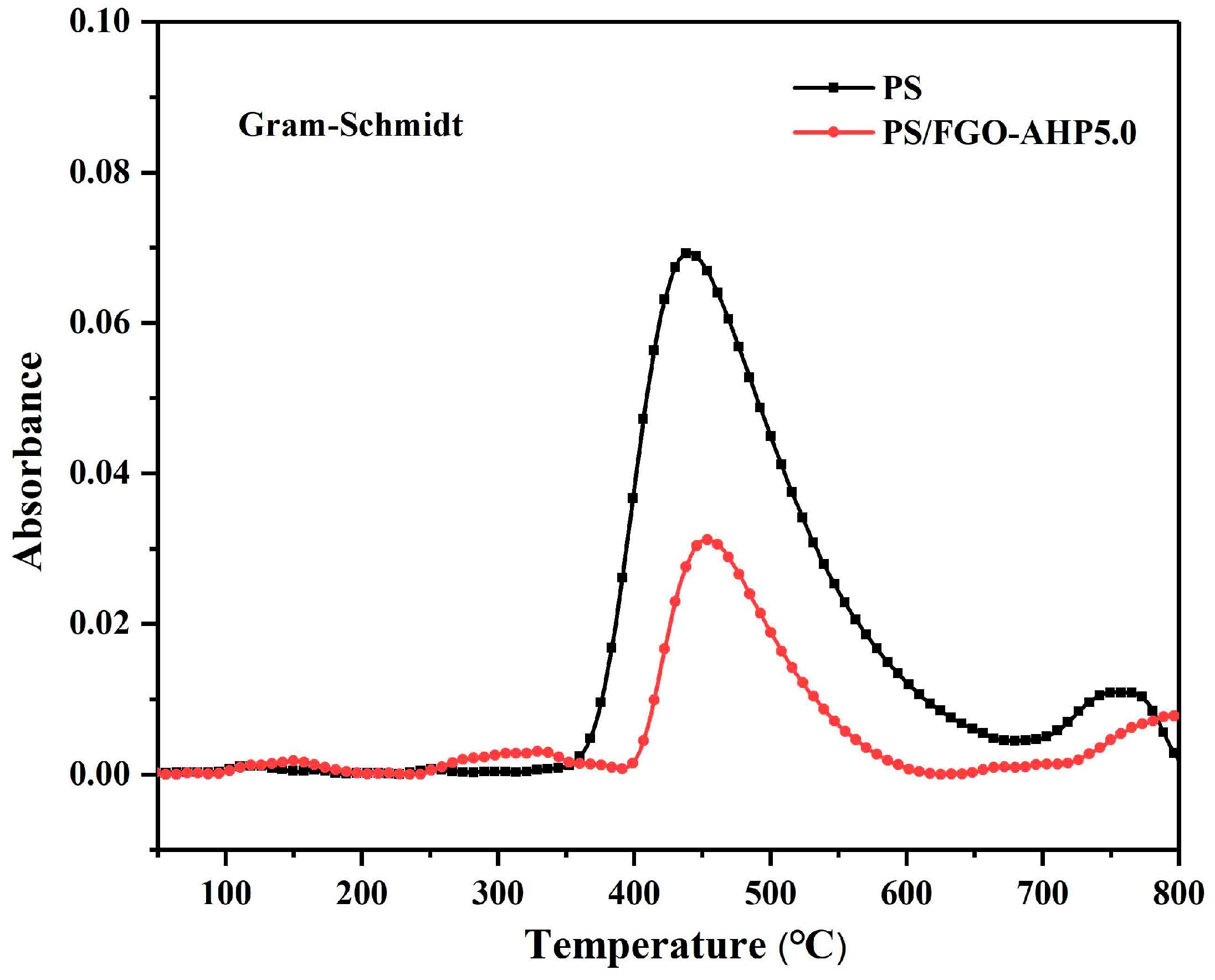

| Sample | T0.1 (°C) | T0.2 (°C) | Char Residue (800 °C, wt%) |
|---|---|---|---|
| GO | 117.4 | 211.7 | 48.2 |
| FGO | 189.3 | 274.5 | 55.1 |
| FGO–AHP | 295.6 | 334.3 | 73.6 |
| Samples | T0.1 (°C) (N2/Air) | Tmax (°C) (N2/Air) | MMLR (%/°C) (N2/Air) | Residues at 700 °C (wt%) (N2/Air) |
|---|---|---|---|---|
| Error | ±1 | ±1 | ±0.01 | ±0.01 |
| PS | 395/335 | 420/378 | 2.76/2.75 | 0.53/0.46 |
| PS/GO2.0 | 397/322 | 420/384 | 2.60/2.00 | 0.61/1.04 |
| PS/FGO2.0 | 401/332 | 416/390 | 2.49/1.98 | 0.89/1.34 |
| PS/FGO–AHP2.0 | 378/330 | 420/379 | 2.45/1.82 | 1.19/1.80 |
| PS/FGO–AHP3.0 | 382/325 | 420/384 | 2.42/1.46 | 1.83/2.49 |
| PS/FGO–AHP4.0 | 386/323 | 419/381 | 2.40/1.38 | 3.24/3.32 |
| PS/FGO–AHP5.0 | 388/321 | 418/383 | 2.34/1.29 | 4.66/4.73 |
| Samples | PHRR (W/g) | HRC (J/g·K) | THR (kJ/g) |
|---|---|---|---|
| Error | ±1 | ±1 | ±0.1 |
| PS | 1063 | 1051 | 45.6 |
| PS/GO2.0 | 964 | 963 | 42.5 |
| PS/FGO2.0 | 925 | 921 | 41.4 |
| PS/FGO–AHP2.0 | 774 | 764 | 39.6 |
| PS/FGO–AHP3.0 | 708 | 702 | 37.9 |
| PS/FGO–AHP4.0 | 664 | 656 | 35.8 |
| PS/FGO–AHP5. 0 | 639 | 627 | 34.9 |
| Matrix | Filler (wt%) | Reduction in PHRR (%) | Reduction in THR (%) | Reference |
|---|---|---|---|---|
| PS | TGO (5%) | 23.9% | 20.0% | [3] |
| PS | MP-EG (20%) | 35.3% | 21.8% | [47] |
| PS | hBN-SBC (20%) | 29.0% | 14.1% | [48] |
| PS | SiAPP/aMWCNT (20%) | 29.6% | 25.2% | [49] |
| PS | Fc-GNS (2%) | 27.0% | 12.0% | [50] |
| PS | FGO–AHP (2%) | 27.2% | 13.2% | Present work |
| PS | FGO–AHP (3%) | 33.4% | 16.9% | Present work |
| PS | FGO–AHP (4%) | 37.5% | 21.5% | Present work |
| PS | FGO–AHP (5%) | 39.9% | 23.5% | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Z.; Tang, T.; Huo, J.; He, H.; Dai, K. Fabrication of Functionalized Graphene Oxide–Aluminum Hypophosphite Nanohybrids for Enhanced Fire Safety Performance in Polystyrene. Polymers 2024, 16, 3083. https://doi.org/10.3390/polym16213083
Deng Z, Tang T, Huo J, He H, Dai K. Fabrication of Functionalized Graphene Oxide–Aluminum Hypophosphite Nanohybrids for Enhanced Fire Safety Performance in Polystyrene. Polymers. 2024; 16(21):3083. https://doi.org/10.3390/polym16213083
Chicago/Turabian StyleDeng, Zhenzhen, Tao Tang, Junjie Huo, Hui He, and Kang Dai. 2024. "Fabrication of Functionalized Graphene Oxide–Aluminum Hypophosphite Nanohybrids for Enhanced Fire Safety Performance in Polystyrene" Polymers 16, no. 21: 3083. https://doi.org/10.3390/polym16213083
APA StyleDeng, Z., Tang, T., Huo, J., He, H., & Dai, K. (2024). Fabrication of Functionalized Graphene Oxide–Aluminum Hypophosphite Nanohybrids for Enhanced Fire Safety Performance in Polystyrene. Polymers, 16(21), 3083. https://doi.org/10.3390/polym16213083







