Repurposing ABS to Produce Polyamide 6 (PA6)-Based Blends: Reactive Compatibilization with SAN-g-MA of a High Degree of Functionalization
Abstract
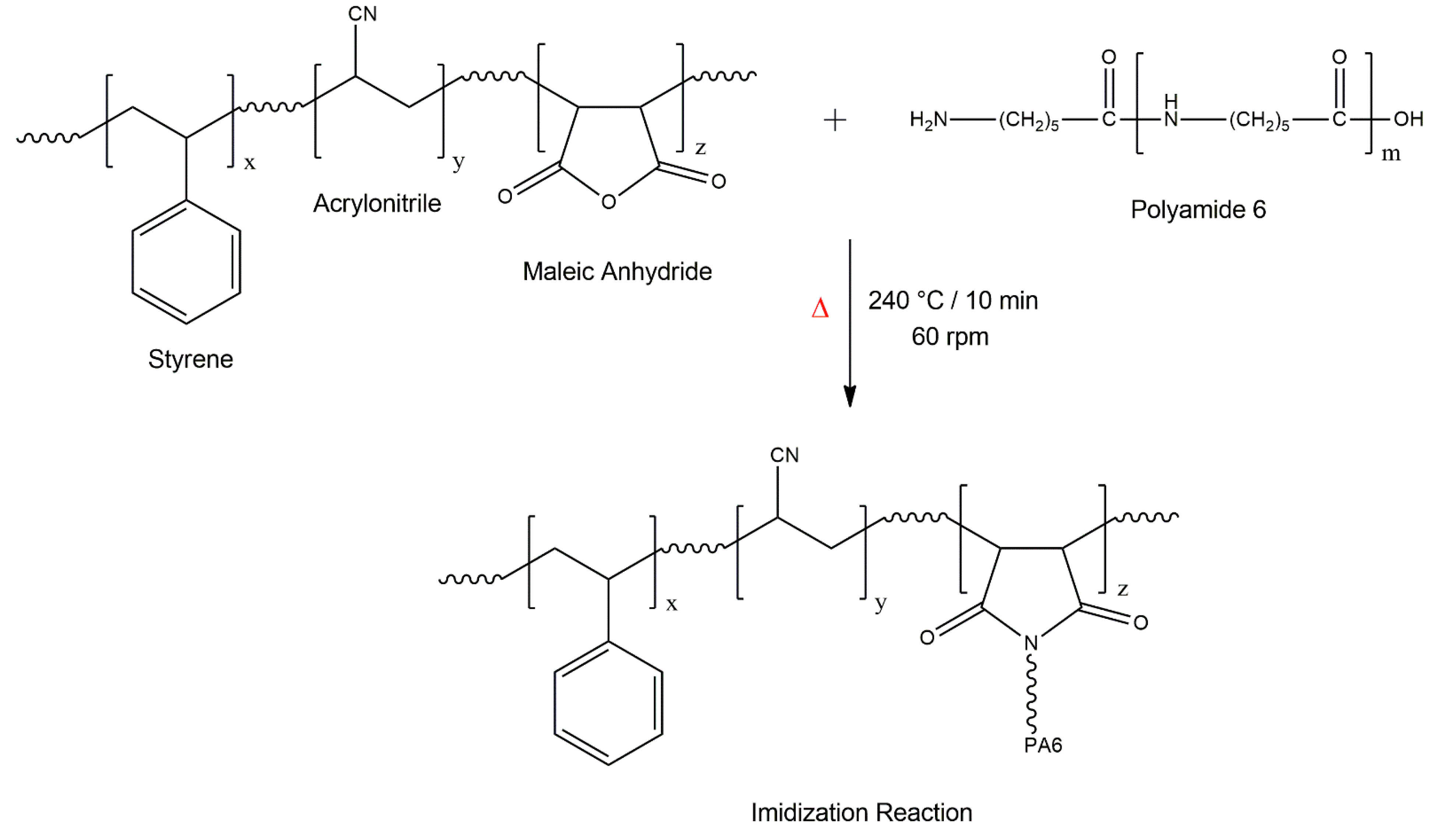
:1. Introduction
2. Materials and Methods
2.1. Materials
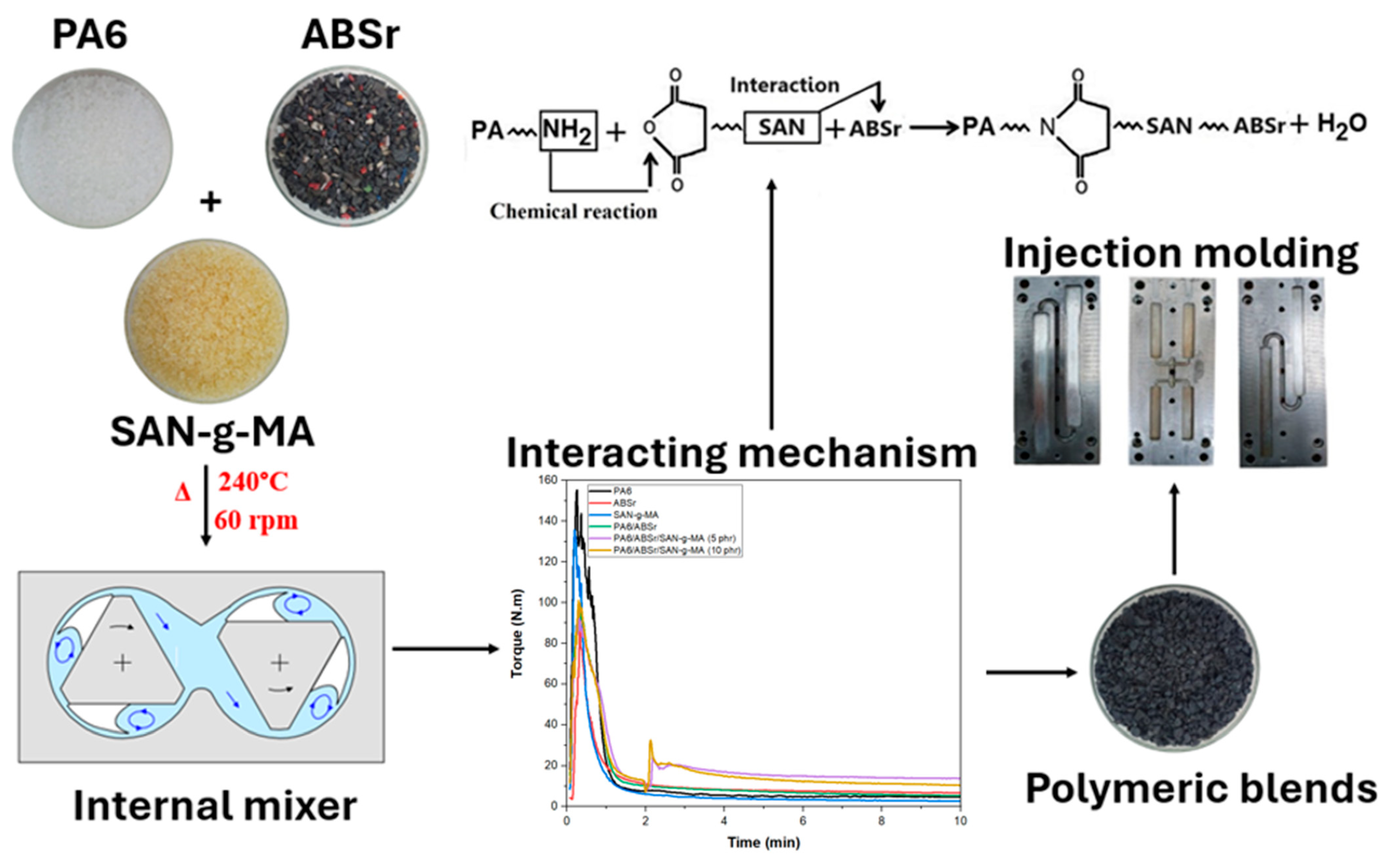
2.2. Preparation and Molding of Polymer Blends
2.3. Characterization of Polymer Blends
3. Results and Discussion
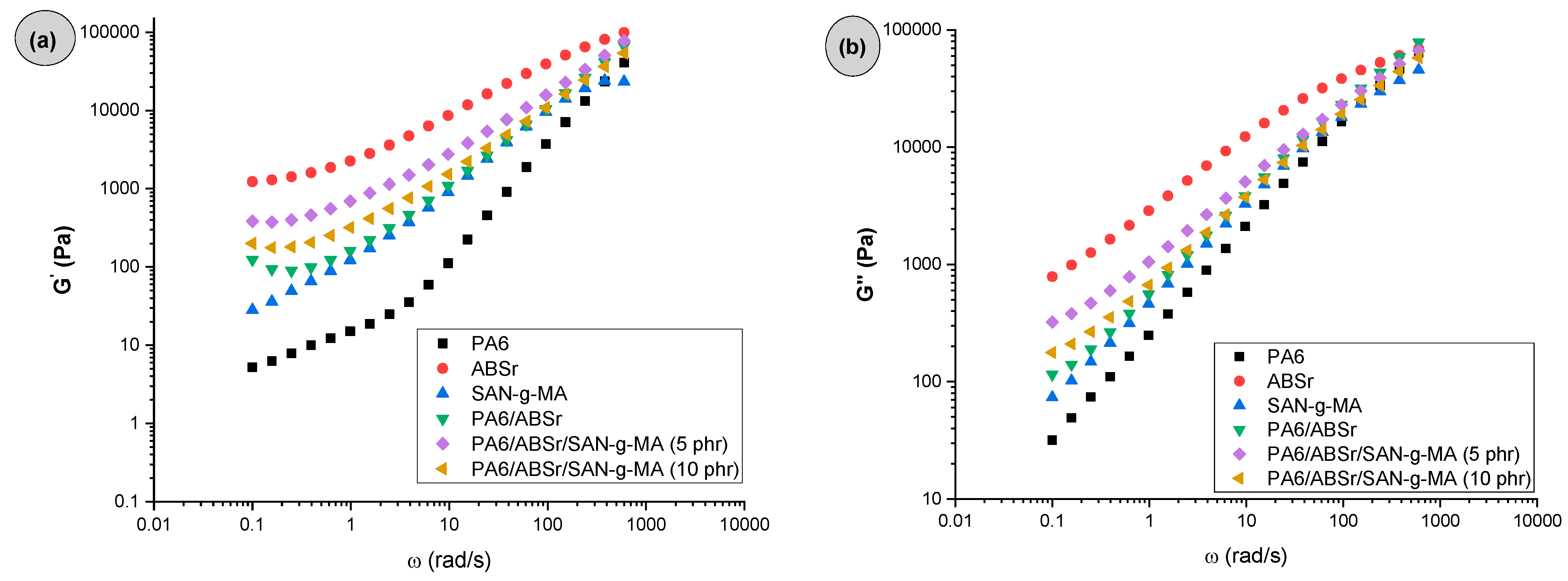
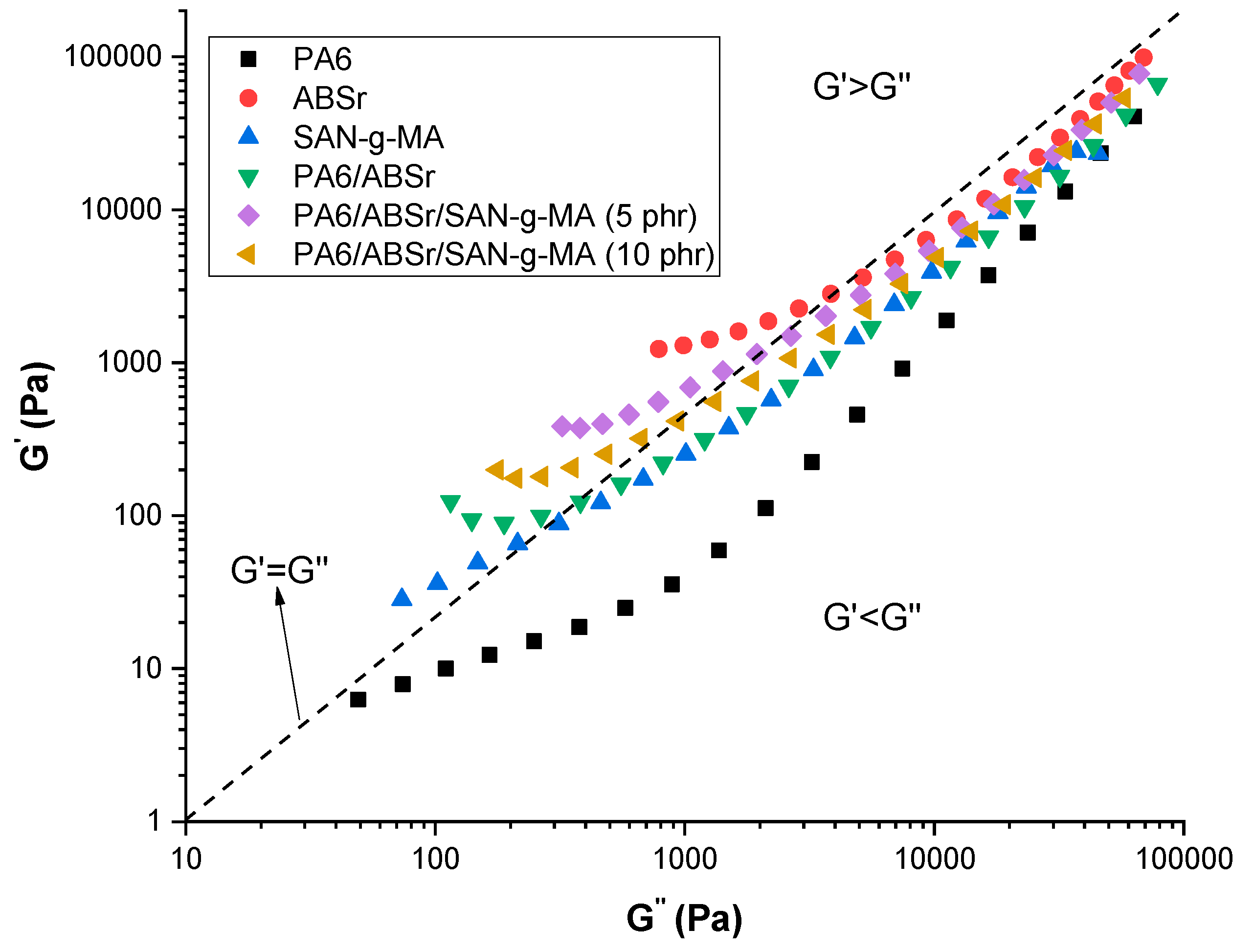
3.1. Dynamic-Oscillatory Rheology
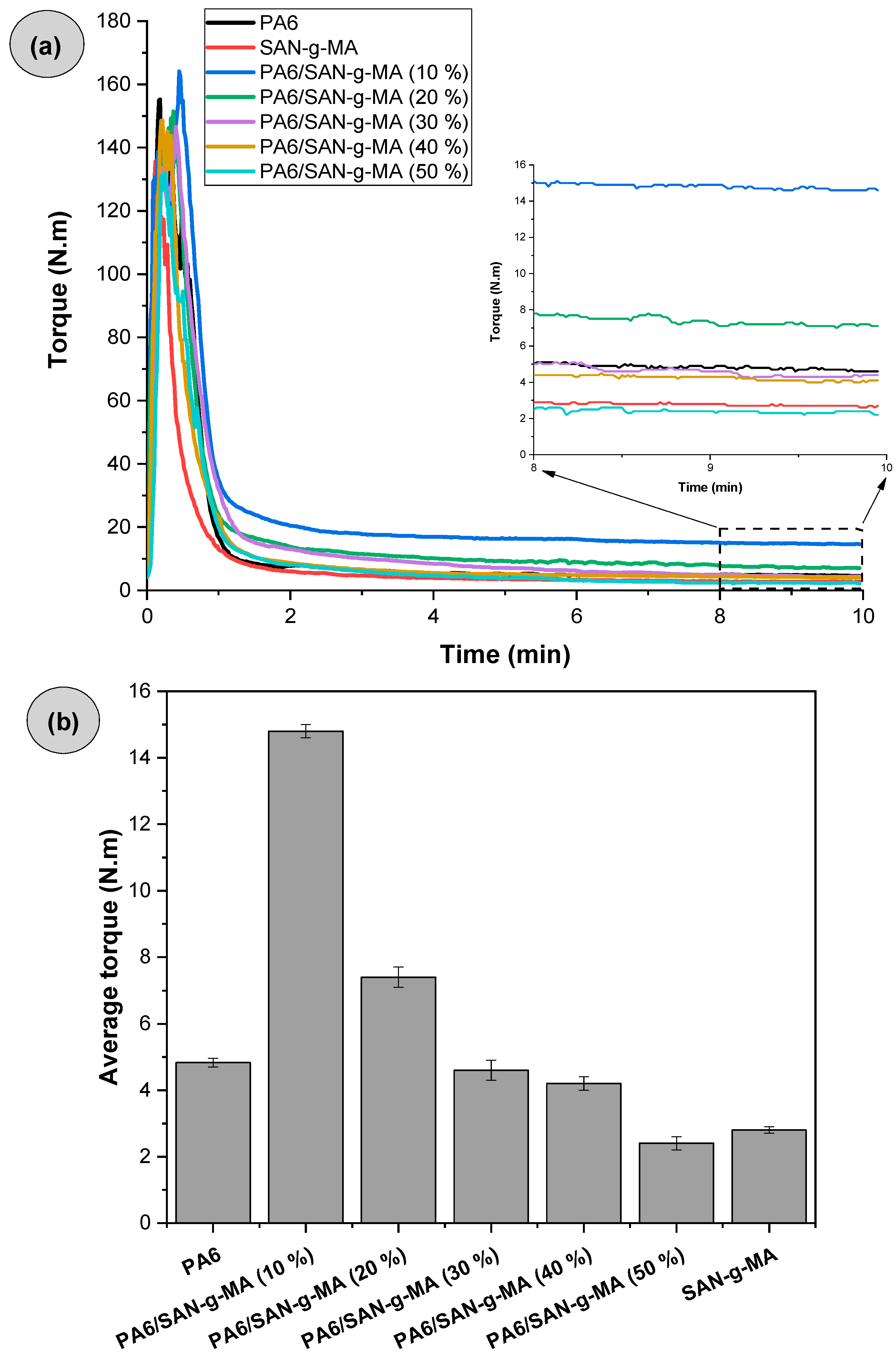
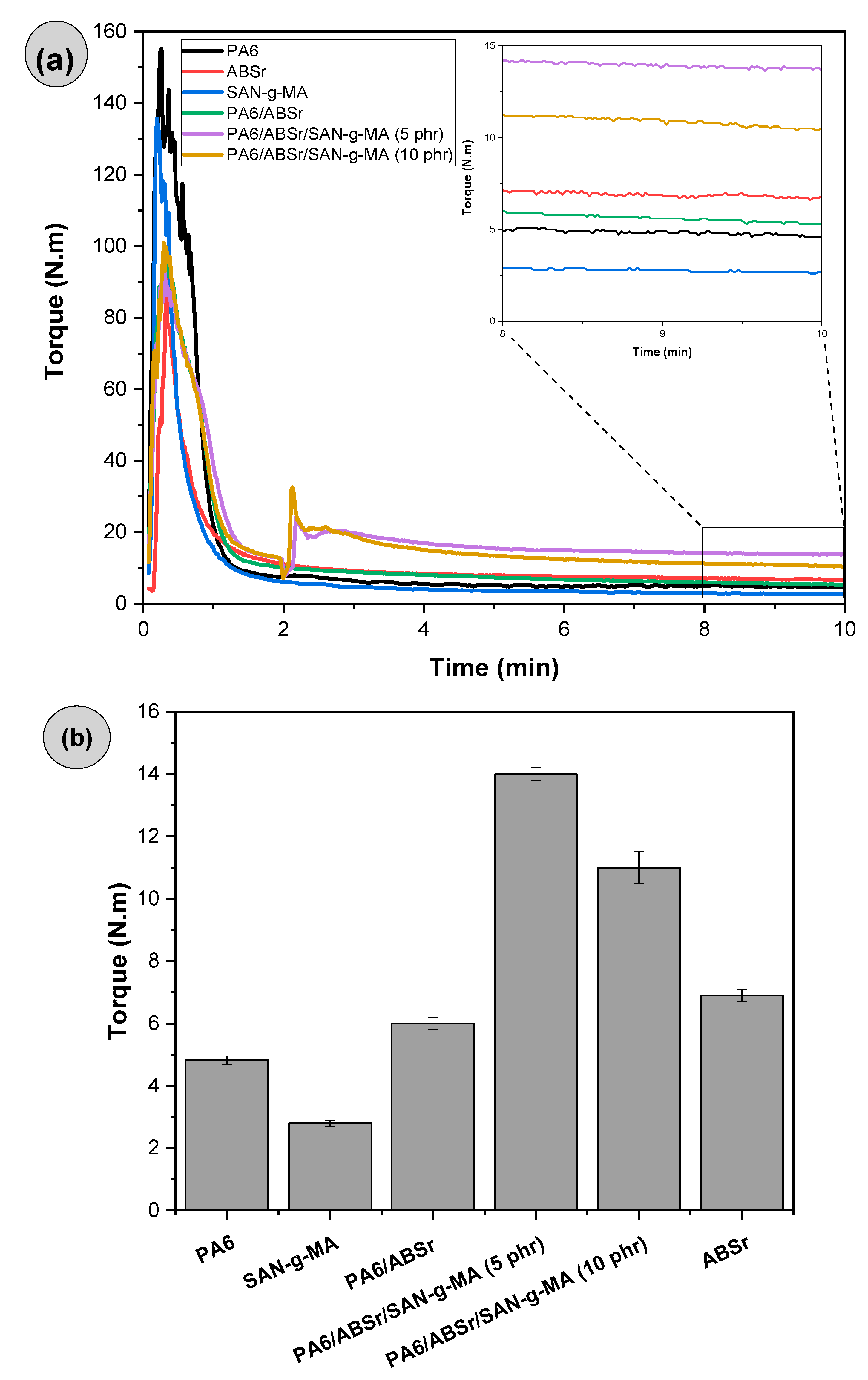
3.2. Torque Rheometry
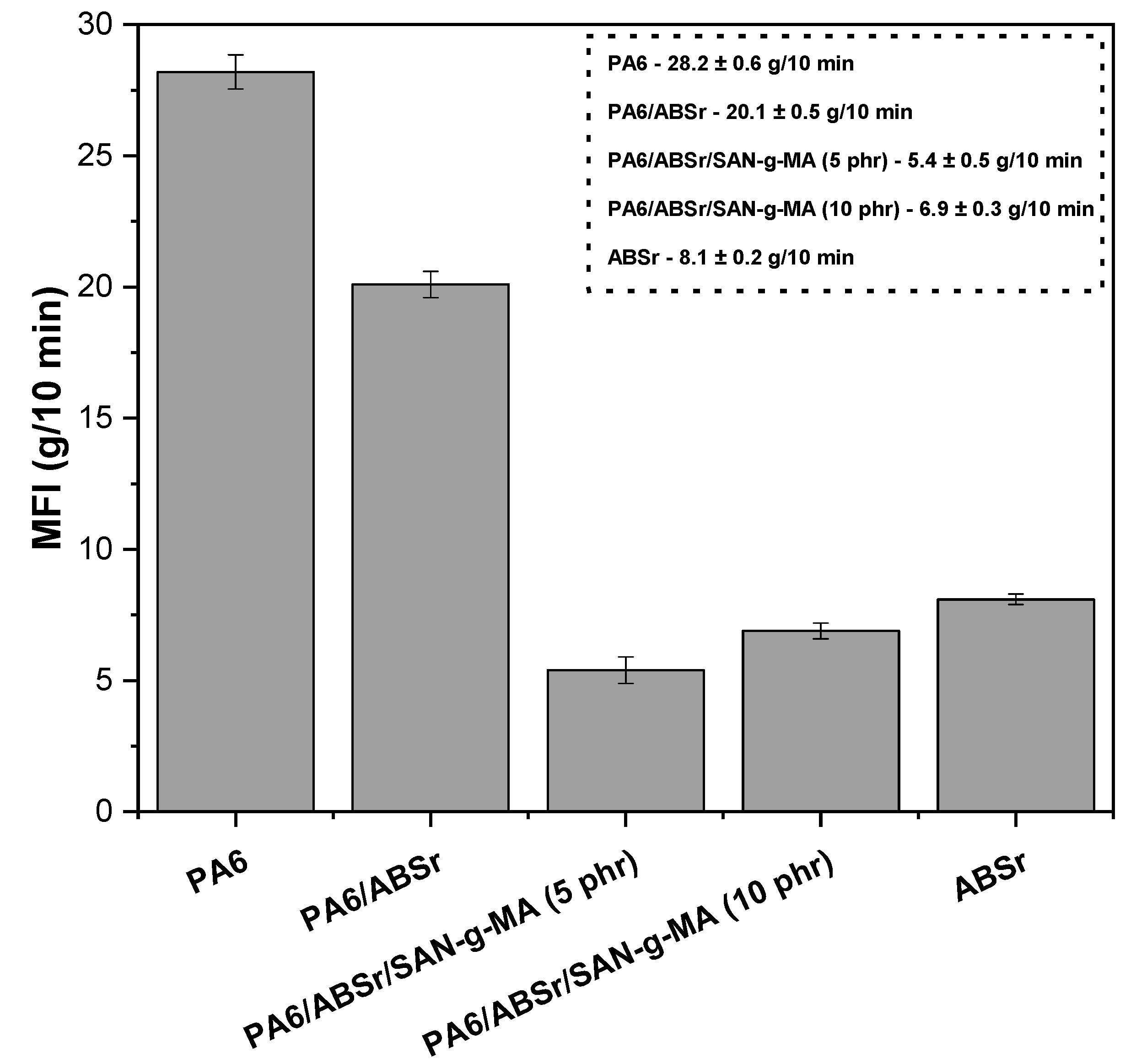
3.3. Melt Flow Index (MFI)
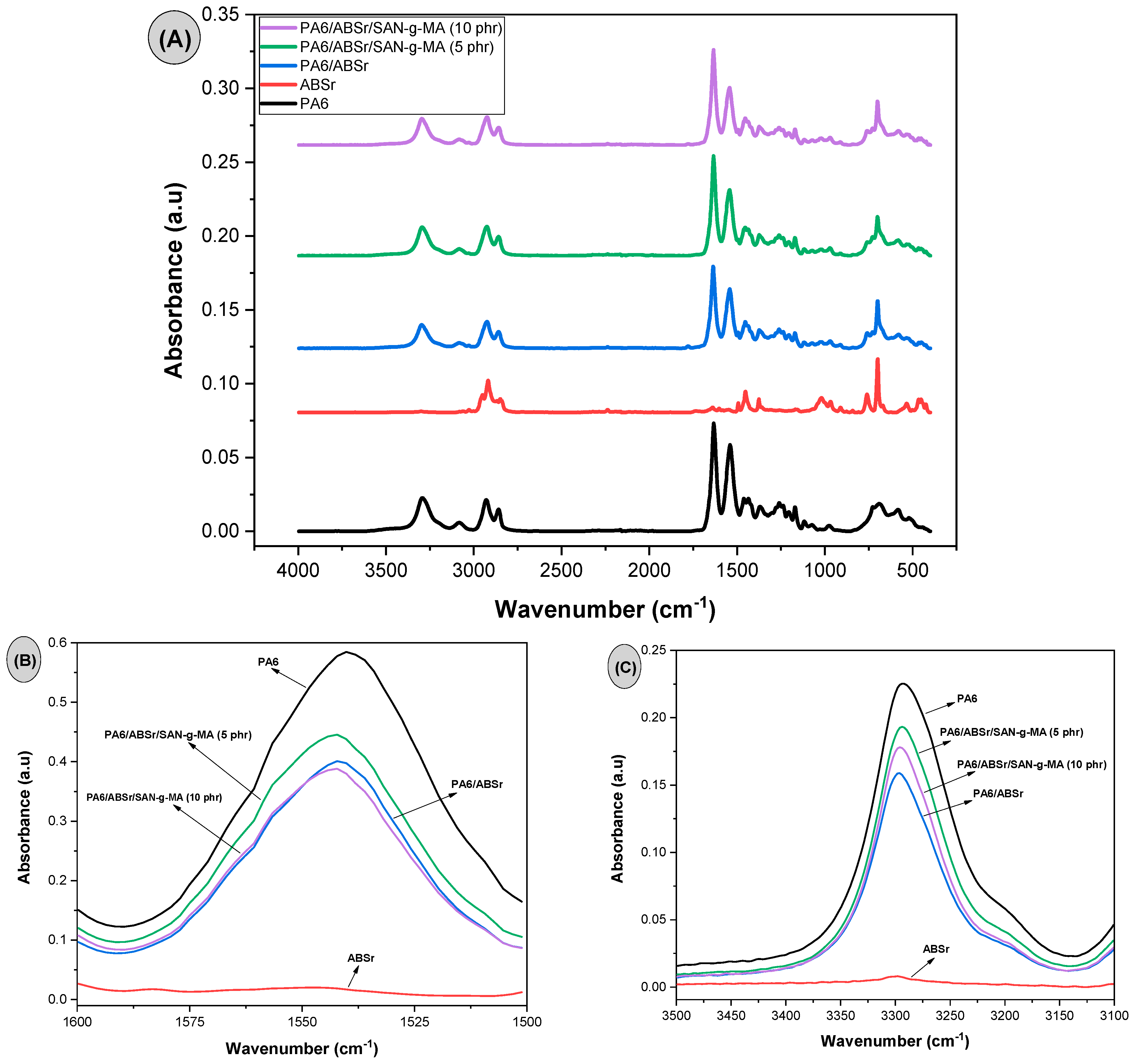
3.4. Fourier Transform Infrared Spectroscopy (FTIR)
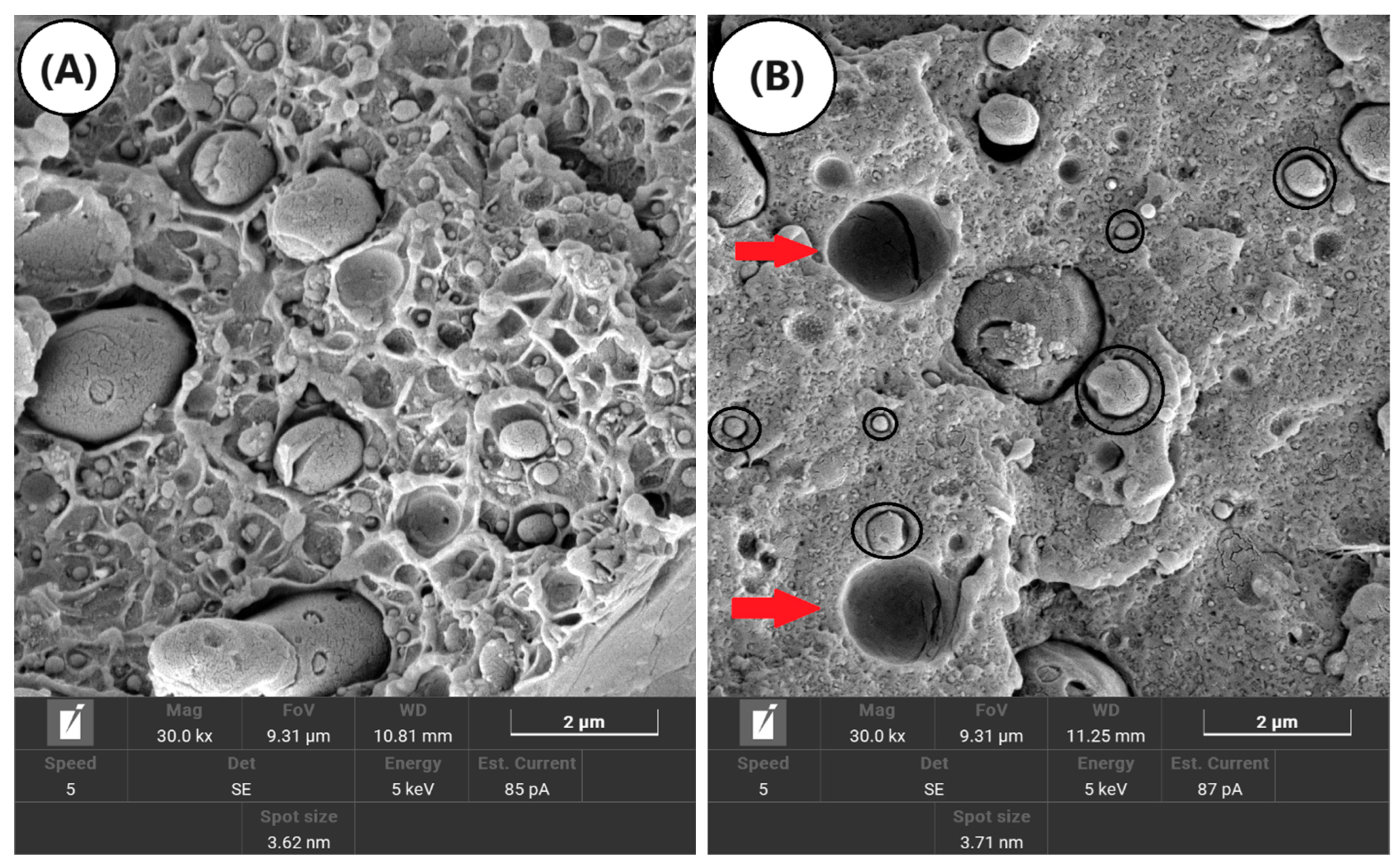
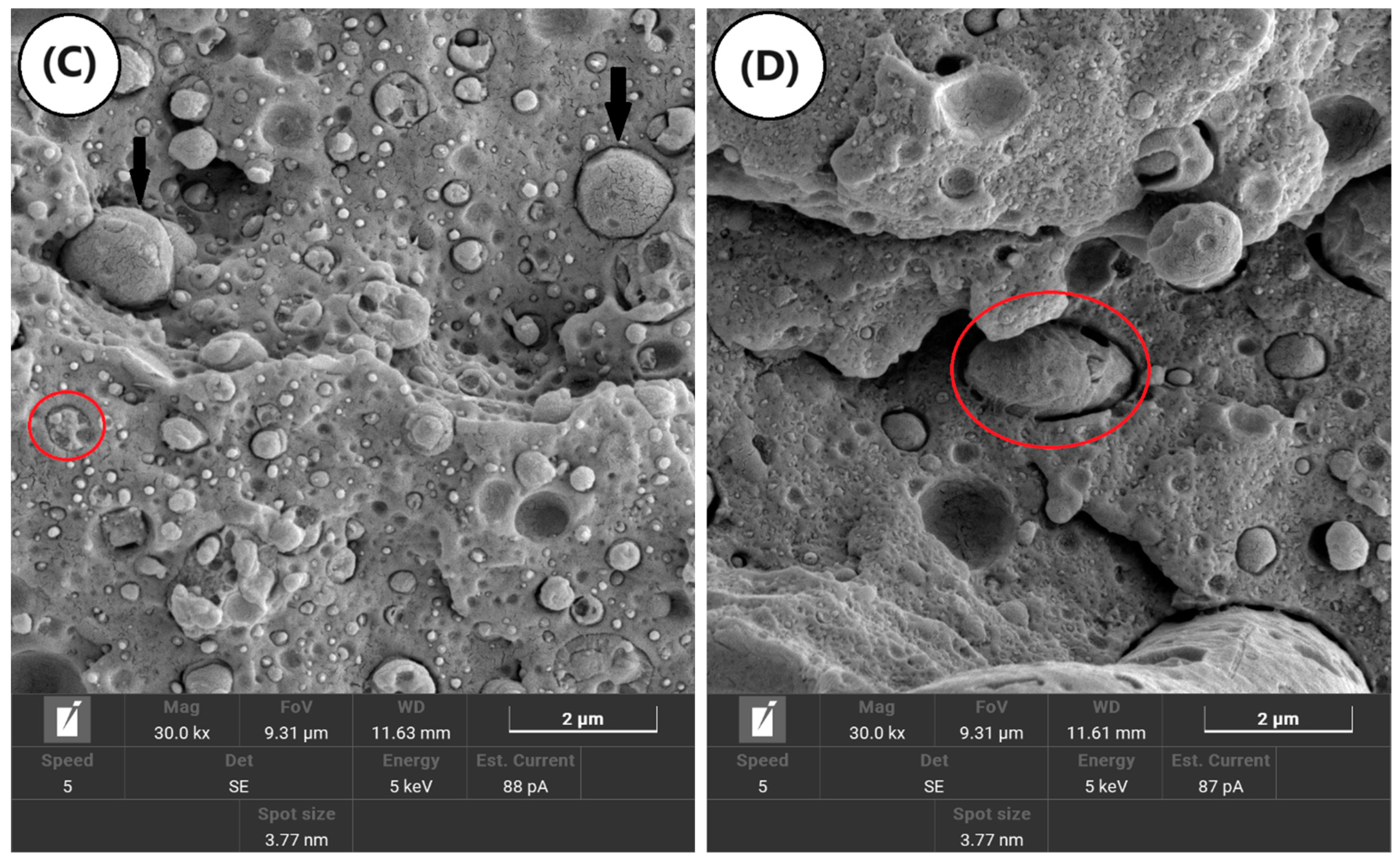
3.5. Scanning Electron Microscopy (SEM)
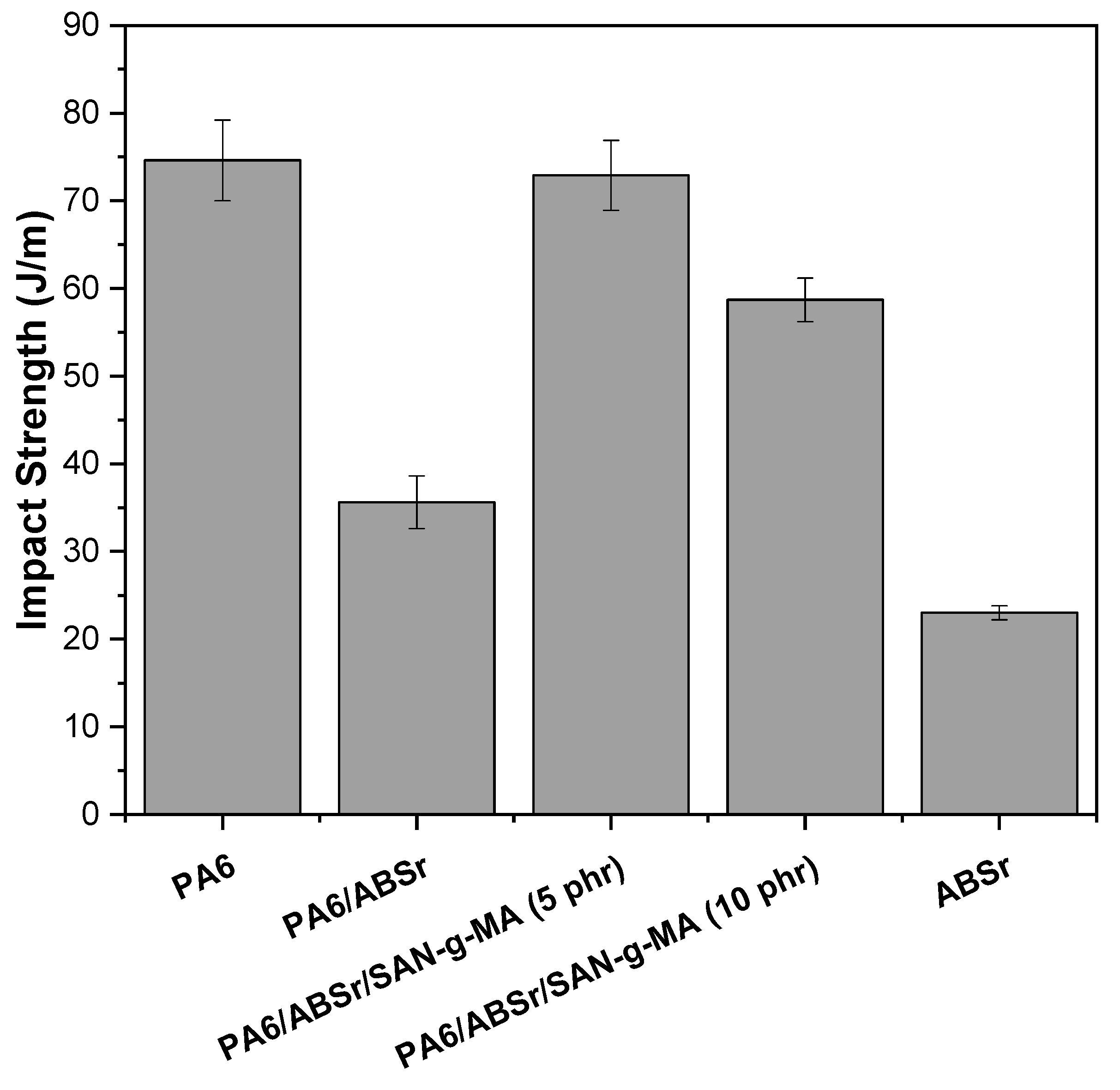
3.6. Impact Strength
3.7. Tensile Strength
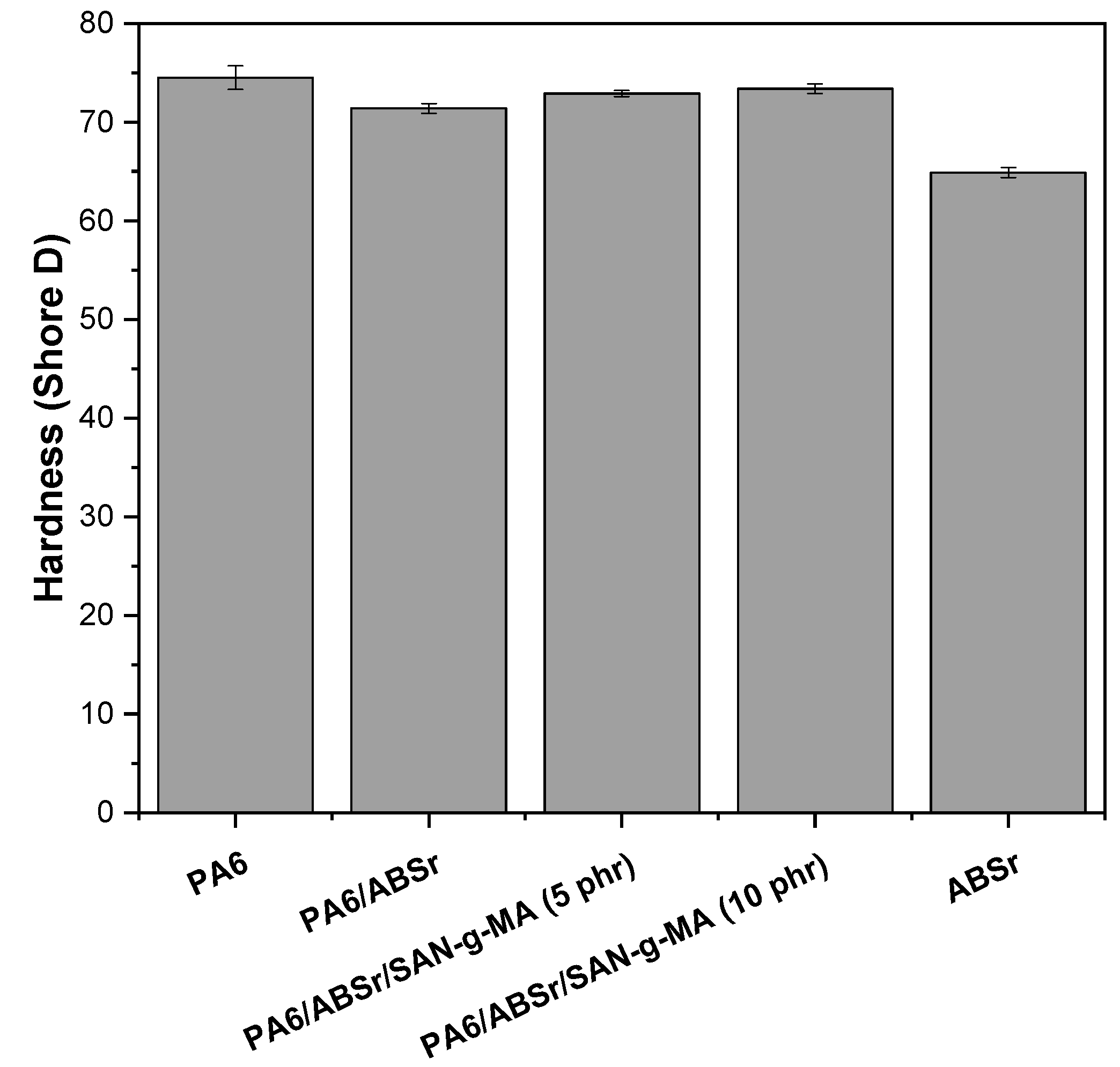
3.8. Shore D Hardness
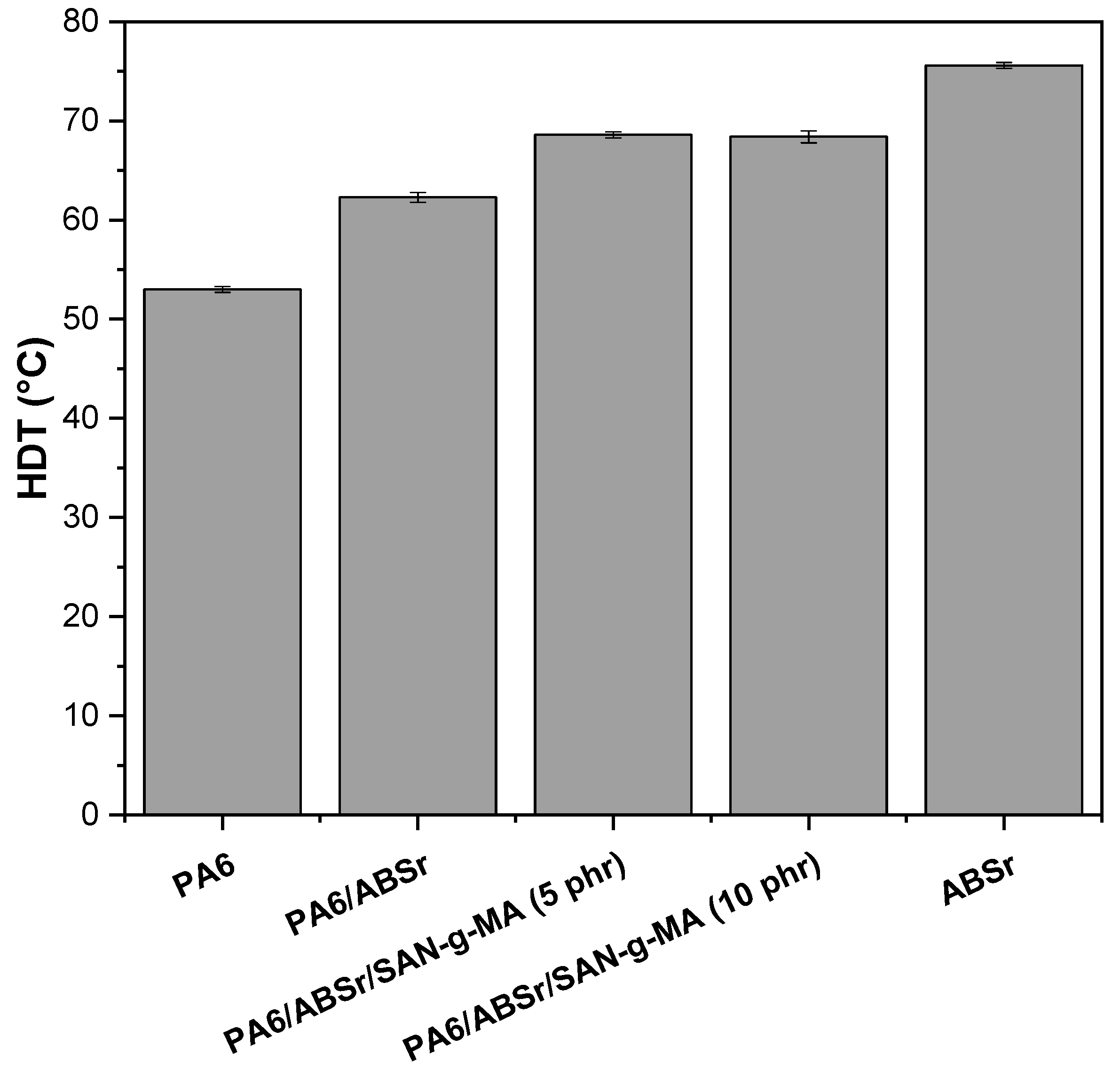
3.9. Heat Deflection Temperature (HDT)
3.10. Differential Scanning Calorimetry (DSC)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salahuddin, U.; Sun, J.; Zhu, C.; Wu, M.; Zhao, B.; Gao, P. Plastic Recycling: A Review on Life Cycle, Methods, Misconceptions, and Techno-Economic Analysis. Adv. Sustain. Syst. 2023, 7, 2200471. [Google Scholar] [CrossRef]
- Kouhi, F.; Vahidifar, A.; Naderi, G.; Esmizadeh, E. Tire-derived reclaimed rubber as a secondary raw material for rubber foams: In the framework of circular economy strategy. J. Polym. Res. 2023, 30, 97. [Google Scholar] [CrossRef]
- Mangold, H.; von Vacano, B. The Frontier of Plastics Recycling: Rethinking Waste as a Resource for High-Value Applications. Macromol. Chem. Phys. 2022, 223, 2100488. [Google Scholar] [CrossRef]
- Seixas, A.A.A.; Figueiredo, L.R.F.; Santos, A.S.F.; Medeiros, E.S. Influence of the addition of glycerol-derived polymers on the properties of post-consumer recycled PET. J. Polym. Res. 2023, 30, 372. [Google Scholar] [CrossRef]
- Muthukumar, J.; Kandukuri, V.A.; Chidambaram, R. A critical review on various treatment, conversion, and disposal approaches of commonly used polystyrene. Polym. Bull. 2024, 81, 2819–2845. [Google Scholar] [CrossRef]
- Rosales, C.; Hocine, N.A.; Bernal, C.; Pettarin, V. Toughness improvement of LLDPE/PP blend by incorporation of GTR waste. Polym. Bull. 2024, 81, 6743–6760. [Google Scholar] [CrossRef]
- Kolluru, S.; Thakur, A.; Tamakuwala, D.; Kumar, V.V.; Ramakrishna, S.; Chandran, S. Sustainable recycling of polymers: A comprehensive review. Polym. Bull. 2024, 81, 9569–9610. [Google Scholar] [CrossRef]
- Ror, C.K.; Negi, S.; Mishra, V. Development and characterization of sustainable 3D printing filaments using post-consumer recycled PET: Processing and characterization. J. Polym. Res. 2023, 30, 350. [Google Scholar] [CrossRef]
- Ekinci, A.; Öksüz, M.; Ates, M.; Aydin, I. Thermal and Mechanical Properties of Polypropylene/Post-consumer Poly (ethylene terephthalate) Blends: Bottle-to-Bottle recycling. J. Polym. Res. 2022, 29, 433. [Google Scholar] [CrossRef]
- Adhikari, S.; Kelkar, V.; Kumar, R.; Halden, R.U. Methods and challenges in the detection of microplastics and nanoplastics: A mini-review. Polym. Int. 2022, 71, 543–551. [Google Scholar] [CrossRef]
- Chen, J.; Wu, J.; Sherrell, P.C.; Chen, J.; Wang, H.; Zhang, W.; Yang, J. How to Build a Microplastics-Free Environment: Strategies for Microplastics Degradation and Plastics Recycling. Adv. Sci. 2022, 9, 2103764. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, D.; Nunnenkamp, L.A.; Braga, F.H.G.; Saron, C. Enhanced mechanical properties of recycled blends acrylonitrile–butadiene–styrene/high–impact polystyrene from waste electrical and electronic equipment using compatibilizers and virgin polymers. J. Appl. Polym. Sci. 2022, 139, 51873. [Google Scholar] [CrossRef]
- Nogueira, J.A.d.S.; Luna, C.B.B.; Ferreira, E.d.S.B.; Filho, E.A.S.; Costa, A.R.M.; Henrique, M.A.; Araújo, E.M. Selective dispersion of SEBS copolymer in the compatibilization of PS/PP recycled copolymer blend: Towards the circular economy. J. Polym. Res. 2023, 30, 362. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, J.; Luo, Z.; Li, J.; Xue, B.; Chen, X.; Li, X.; Yang, L.; Linghu, C.; Tao, Y. Structures of the co-branching reactive products of isotactic polypropylene with high-density polyethylene and the effect on the in situ compatibilization of mixed recycled materials. J. Appl. Polym. Sci. 2022, 139, e53170. [Google Scholar] [CrossRef]
- Kumar, R.; Sadeghi, K.; Jang, J.; Seo, J. Mechanical, chemical, and bio-recycling of biodegradable plastic: A review. Sci. Total Environ. 2023, 882, 163446. [Google Scholar] [CrossRef]
- Jung, H.; Shin, G.; Kwak, H.; Hao, L.T.; Jegal, J.; Kim, H.J.; Jeon, H.; Park, J.; Oh, D.X. Review of polymer technologies for improving the recycling and upcycling efficiency of plastic waste. Chemosphere 2023, 320, 138089. [Google Scholar] [CrossRef]
- Lamba, P.; Kaur, D.P.; Raj, S.; Sorout, J. Recycling/reuse of plastic waste as construction material for sustainable development: A review. Environ. Sci. Pollut. Res. 2022, 29, 86156–86179. [Google Scholar] [CrossRef]
- Junior, A.J.A.; Saron, C. Mechanical recycling of expanded polystyrene and tire rubber waste as compatibilized and toughened blends. J. Appl. Polym. Sci. 2023, 140, e54267. [Google Scholar] [CrossRef]
- Liu, S.; Wu, H.; Li, Y.; Yin, F.; Liang, D. Research on the co-pyrolysis kinetic and synergistic effect of waste PP and LDPE mixed plastics. J. Polym. Res. 2024, 31, 132. [Google Scholar] [CrossRef]
- Heller, B.; Simon-Stőger, L.; Makó, E.; Varga, C. A practical manner to GTR recycling in waste-HDPE/ABS. J. Polym. Res. 2022, 29, 329. [Google Scholar] [CrossRef]
- Son, Y.; Lee, S. Compatibility between aliphatic polyketone terpolymer and Nylon6 polymer blend. J. Polym. Res. 2023, 30, 33. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y. Compatibility evaluation and mechanical properties of isotactic polypropylene/high density polyethylene (iPP/HDPE) blends. J. Polym. Res. 2024, 31, 161. [Google Scholar] [CrossRef]
- Zeng, S.; Zhang, H.; Li, Z.; Hu, C.; Wang, Z. Effect of mechanical recycling on crystallization, mechanical, and rheological properties of recycled high-density polyethylene and reinforcement based on virgin high-density polyethylene. J. Appl. Polym. Sci. 2024, 141, e55097. [Google Scholar] [CrossRef]
- Traxler, I.; Marschik, C.; Farthofer, M.; Laske, S.; Fischer, J. Application of Mixing Rules for Adjusting the Flowability of Virgin and Post-Consumer Polypropylene as an Approach for Design from Recycling. Polymers 2022, 14, 2699. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Huang, Z.; Ma, K.; Dong, L.; Li, J.; Wang, Q.-C.; Xu, Z.; Chen, W.-Q. Life cycle assessment of recycling high impact polystyrene and acrylonitrile butadiene styrene plastic from waste refrigerators. J. Clean. Prod. 2024, 435, 140294. [Google Scholar] [CrossRef]
- Teixeira, F.d.S.M.; Peres, A.C.C.; Pacheco, E.B.A.V. Mechanical recycling of acrylonitrile-butadiene-styrene copolymer and high impact polystyrene from waste electrical and electronic equipment to comply with the circular economy. Front. Sustain. 2023, 4, 1203457. [Google Scholar] [CrossRef]
- Filho, V.A.S.; Filho, M.A.A.; Lira, M.C.A.; Pedrosa, T.C.; da Fonseca, L.S.; Araújo, S.S.; Henrique, M.A.; Ferreira, E.S.B.; Araújo, E.M.; Luna, C.B.B. Efficiency assessment of the SEBS, SEP, and SBS copolymers in the compatibilization of the PS/ABS blend. J. Polym. Res. 2023, 30, 425. [Google Scholar] [CrossRef]
- Sanchez, E.M.S.; Ferreira, M.M.C.; Felisberti, M.I. Thermal degradation and Photo-oxidation of the ABS used for automotive applications. Polímeros Ciência Tecnol. 1999, 9, 116–122. [Google Scholar] [CrossRef]
- Mishra, V.; Ror, C.K.; Negi, S.; Kar, S.; Borah, L.N. 3D printing with recycled ABS resin: Effect of blending and printing temperature. Mater. Chem. Phys. 2023, 309, 128317. [Google Scholar] [CrossRef]
- Verma, N.; Awasthi, P.; Pandey, P.M.; Banerjee, S.S. Development of material extrusion 3D printing compatible tailorable thermoplastic elastomeric materials from acrylonitrile butadiene styrene and styrene-(ethylene-butylene)-styrene block copolymer blends. J. Appl. Polym. Sci. 2022, 139, e53039. [Google Scholar] [CrossRef]
- Hassan, M.M. Mechanical, thermal, and morphological behavior of the polyamide 6/acrylonitrile–butadiene–styrene blends irradiated with gamma rays. Polym. Eng. Sci. 2008, 48, 373–380. [Google Scholar] [CrossRef]
- Arsad, A.; Rahmat, A.R.; Hassan, A.; Mokhtar, M.; Dali, S.N.M. Flow Characteristics and Dynamic Behavior of Polyamide 6/Acrylonitile Butadiene Styrene (PA6/ABS) Blends. Int. J. Polym. Mater. Polym. Biomater. 2013, 62, 209–214. [Google Scholar] [CrossRef]
- Howe, D.V.; Wolkowicz, M.D. Structure-property relationships in polyamide/acrylonitrile-butadiene-styrene (ABS) blends. Polym. Eng. Sci. 1987, 27, 1582–1590. [Google Scholar] [CrossRef]
- Handge, U.A.; Galeski, A.; Kim, S.C.; Dijkstra, D.J.; Götz, C.; Fischer, F.; Lim, G.T.; Altstädt, V.; Gabriel, C.; Weber, M.; et al. Melt processing, mechanical, and fatigue crack propagation properties of reactively compatibilized blends of polyamide 6 and acrylonitrile–butadiene–styrene copolymer. J. Appl. Polym. Sci. 2021, 124, 740–754. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, Y.; Han, Y.; Chen, M.; Sun, S.; Cao, C.; Zhou, C.; Zhang, H. Toughening of Polyamide-6 with a Maleic Anhydride Functionalized Acrylonitrile-Styrene-Butyl Acrylate Copolymer. Ind. Eng. Chem. Res. 2012, 51, 9235–9240. [Google Scholar] [CrossRef]
- Aparna, S.; Purnima, D.; Adusumalli, R.B. Review on Various Compatibilizers and its Effect on Mechanical Properties of Compatibilized Nylon Blends. Polym.-Plast. Technol. Eng. 2016, 56, 617–634. [Google Scholar] [CrossRef]
- Correa, C.A.; Yamakawa, R.S.; Razzino, C.A.; Junior, E.H. Fracture Toughness of PA 6/Abs Evaluated by the EWF Method (Essential Work of Fracture)—Part A: On the Effect of the Compatibilizer. Polímeros Ciência Tecnol. 2007, 17, 36–45. [Google Scholar] [CrossRef]
- Ozkoc, G.; Bayram, G.; Bayramli, E. Impact essential work of fracture toughness of ABS/polyamide-6 blends compatibilized with olefin based copolymers. J. Mater. Sci. 2008, 43, 2642–2652. [Google Scholar] [CrossRef]
- Kudva, R.; Keskkula, H.; Paul, D. Properties of compatibilized nylon 6/ABS blends: Part II. Effects of compatibilizer type and processing history. Polymer 2000, 41, 239–258. [Google Scholar] [CrossRef]
- de Oliveira, A.D.; Larocca, N.M.; Pessan, L.A. Effect from the Blending Sequence on the Properties of PA6/ABS Blends Compatibilized with SMA Copolymer. Polímeros 2011, 21, 27–33. [Google Scholar] [CrossRef]
- Ren, J.; Wang, H.; Jian, L.; Zhang, J.; Yang, S. Morphological, Thermal and Mechanical Properties of Compatibilized Nylon 6/ABS Blends. J. Macromol. Sci. Part B 2008, 47, 712–722. [Google Scholar] [CrossRef]
- Castro, L.; Oliveira, A.; Kersch, M.; Altstädt, V.; Pessan, L. Effect of organoclay incorporation and blending protocol on performance of PA6/ABS nanocomposites compatibilized with SANMA. Polym. Eng. Sci. 2017, 57, 1147–1154. [Google Scholar] [CrossRef]
- Kusmono; Ishak, Z.M.; Chow, W.; Takeichi, T. Rochmadi Influence of SEBS-g-MA on morphology, mechanical, and thermal properties of PA6/PP/organoclay nanocomposites. Eur. Polym. J. 2008, 44, 1023–1039. [Google Scholar] [CrossRef]
- Luna, C.B.B.; Ferreira, E.d.S.B.; Costa, A.R.d.M.; de Almeida, Y.M.B.; Melo, J.B.d.C.A.d.; Araújo, E.M. Toward Reactive Processing of Polyamide 6 Based Blends with Polyethylene Grafted with Maleic Anhydride and Acrylic Acid: Effect of Functionalization Degree. Macromol. React. Eng. 2023, 17, 2300031. [Google Scholar] [CrossRef]
- Roeder, J.; Oliveira, R.; Gonçalves, M.; Soldi, V.; Pires, A. Polypropylene/polyamide-6 blends: Influence of compatibilizing agent on interface domains. Polym. Test. 2002, 21, 815–821. [Google Scholar] [CrossRef]
- Silva, A.L.N.; Cipriano, T.F.; Silva, A.H.M.F.T.d.; Sousa, A.M.F.; Silva, G.M. Thermal, Rheological and Morphological Properties of Poly (Lactic Acid) (PLA) and Talc Composites. Polímeros 2014, 24, 276–282. [Google Scholar] [CrossRef]
- Lima, J.C.C.; Araújo, J.P.; Agrawal, P.; Mélo, T.J.A. Efeito do teor do copolímero SEBS no comportamento reológico da blenda PLA/SEBS. Rev. Eletrônica Mater. E Process. 2016, 11, 10–17. [Google Scholar]
- Feng, L.; Bian, X.; Chen, Z.; Li, G.; Chen, X. Mechanical, aging, optical and rheological properties of toughening polylactide by melt blending with poly(ethylene glycol) based copolymers. Polym. Degrad. Stab. 2013, 98, 1591–1600. [Google Scholar] [CrossRef]
- Salmani, F.; Shemshadi, R.; Moghaddampour, I.M.; Ahmadi, S.; Naderi, G.; Sahraeian, R.; Paran, S.M.R. Mechanical, Rheological, and Heat Seal Properties of 1-Octene Grafted Low Density Polyethylene Films. Macromol. React. Eng. 2023, 18, 2300060. [Google Scholar] [CrossRef]
- Cruz, S.A.; Farah, M.; Zanin, M.; Bretas, R.E.S. Evaluation of Rheological Properties of Virgin HDPE/Recycled HDPE Blends. Polímeros 2008, 18, 144–151. [Google Scholar] [CrossRef]
- Cho, K.; Seo, K.H.; Ahn, T.O. Morphology and Rheological Behavior of Amorphous Polyamide/(Styrene-Acrylonitrile/Styrene Maleic Anhydride) Reactive Blends. Polym. J. 1997, 29, 987–991. [Google Scholar] [CrossRef]
- Sousa, F.M.; Costa, A.R.M.; Reul, L.T.A.; Cavalcanti, F.B.; Carvalho, L.H.; Almeida, T.G.; Canedo, E.L. Rheological and thermal characterization of PCL/PBAT blends. Polym. Bull. 2019, 76, 1573–1593. [Google Scholar] [CrossRef]
- Andrade, D.S.C.; Canedo, E.L.; de Carvalho, L.H.; Barbosa, R.; Alves, T.S. Characterization of Poly(Ethylene Terephthalate) by Torque Rheometry. Mater. Res. 2021, 24, e20200238. [Google Scholar] [CrossRef]
- Jiang, C.; Filippi, S.; Magagnini, P. Reactive compatibilizer precursors for LDPE/PA6 blends. II: Maleic anhydride grafted polyethylenes. Polymer 2003, 44, 2411–2422. [Google Scholar] [CrossRef]
- de Oliveira, A.D.; de Castro, L.D.C.; Beatrice, C.A.G.; Lucas, A.d.A.; Pessan, L.A. Effect of Maleic Anhydride Content in Properties of PA6/AES Blends Compatibilized with MMA-MA. Mater. Res. 2017, 20, 1630–1637. [Google Scholar] [CrossRef]
- Komalan, C.; George, K.; Jacob, S.; Thomas, S. Reactive compatibilization of nylon copolymer/EPDM blends: Experimental aspects and their comparison with theory. Polym. Adv. Technol. 2008, 19, 351–360. [Google Scholar] [CrossRef]
- Xu, M.; Qiu, W.; Qiu, G. Ethylene-propylene elastomer grafted maleic anhydride toughened polyamide-6 morphology and properties. J. Macromol. Sci. Part B 2013, 52, 155–166. [Google Scholar] [CrossRef]
- Lee, J.-M.; Busquets, R.; Choi, I.-C.; Lee, S.-H.; Kim, J.-K.; Campos, L.C. Photocatalytic Degradation of Polyamide 66; Evaluating the Feasibility of Photocatalysis as a Microfibre-Targeting Technology. Water 2020, 12, 3551. [Google Scholar] [CrossRef]
- Liu, K.; Li, Y.; Tao, L.; Xiao, R. Preparation and characterization of polyamide 6 fibre based on a phosphorus-containing flame retardant. RSC Adv. 2018, 8, 9261–9271. [Google Scholar] [CrossRef]
- Krylova, V.; Dukštienė, N. The structure of PA-Se-S-Cd composite materials probed with FTIR spectroscopy. Appl. Surf. Sci. 2019, 470, 462–471. [Google Scholar] [CrossRef]
- Matos, M.; Favis, B.D.; Lomellini, P. Interfacial modification of polymer blends—The emulsification curve: 1. Influence of molecular weight and chemical composition of the interfacial modifier. Polymer 1995, 36, 3899–3907. [Google Scholar] [CrossRef]
- Fortelný, I.; Jůza, J. The Effects of Copolymer Compatibilizers on the Phase Structure Evolution in Polymer Blends—A Review. Materials 2021, 14, 7786. [Google Scholar] [CrossRef] [PubMed]
- Okada, O.; Keskkula, H.; Paul, D. Fracture toughness of nylon 6 blends with maleated ethylene/propylene rubbers. Polymer 2000, 41, 8061–8074. [Google Scholar] [CrossRef]
- Rabello, M.S. Additivation of Polymers—Impact Modifiers; Artliber Editora: São Paulo, Brazil, 2000; pp. 211–228. [Google Scholar]
- Agrawal, P.; Araújo, E.M.; Mélo, T.J. ATorque Rheometry, Mechanical Properties and Morphology of Compatibilized PA6/HDPE Blends. Polímeros Ciência Tecnol. 2008, 18, 152–157. [Google Scholar] [CrossRef]
- Ferreira, L.A.S.; Pessan, L.A.; Júnior, E.H. Mechanical and thermo-mechanical behavior of PBT/ABS polymer blends. Polímeros 1997, 7, 67–72. [Google Scholar] [CrossRef]
- Luna, C.B.B.; Siqueira, D.D.; Araújo, E.M.; do Nascimento, E.P.; da Costa Agra de Melo, J.B. Evaluation of the SEBS copolymer in the compatibility of PP/ABS blends through mechanical, thermal, thermomechanical properties, and morphology. Polym. Adv. Technol. 2022, 33, 111–124. [Google Scholar] [CrossRef]
- Sui, X.; Xie, X.-M. Creating super-tough and strong PA6/ABS blends using multi-phase compatibilizers. Chin. Chem. Lett. 2019, 30, 149–152. [Google Scholar] [CrossRef]
- Essabir, H.; El Mechtali, F.Z.; Nekhlaoui, S.; Raji, M.; Bensalah, M.O.; Rodrigue, D.; Bouhfid, R.; Qaiss, A. Compatibilization of PA6/ABS blend by SEBS-g-MA: Morphological, mechanical, thermal, and rheological properties. Int. J. Adv. Manuf. Technol. 2020, 110, 1095–1111. [Google Scholar] [CrossRef]
- Kudva, R.; Keskkula, H.; Paul, D. Properties of compatibilized nylon 6/ABS blends: Part I. Effect of ABS type. Polymer 2000, 41, 225–237. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Castro, L.; Mareau, V.; Pessan, L.; Gonon, L. New insights on the compatibilization of PA6/ABS blends: A co-localized AFM-Raman study. Polymer 2018, 146, 151–160. [Google Scholar] [CrossRef]
- Daghigh, V.; Lacy, T.E.; Daghigh, H.; Gu, G.; Baghaei, K.T.; Horstemeyer, M.F.; Pittman, C.U. Heat deflection temperatures of bio-nano-composites using experiments and machine learning predictions. Mater. Today Commun. 2020, 22, 100789. [Google Scholar] [CrossRef]
- Wong, A.-Y. Heat deflection characteristics of polypropylene and polypropylene/polyethylene binary systems. Compos. Part B Eng. 2003, 34, 199–208. [Google Scholar] [CrossRef]
- Castro, L.; Oliveira, A.; Kersch, M.; Altstädt, V.; Pessan, L. Effects of mixing protocol on morphology and properties of PA6/ABS blends compatibilized with MMA-MA. J. Appl. Polym. Sci. 2016, 133, 43612. [Google Scholar] [CrossRef]
- Spadetti, C.; Filho, E.A.d.S.; de Sena, G.L.; de Melo, C.V.P. Thermal and mechanical properties of post-consumer polypropylene composites reinforced with cellulose fibers. Polímeros 2017, 27, 84–90. [Google Scholar] [CrossRef]
- Vieira, K.P.; Reichert, A.A.; Cholant, G.M.; Marin, D.; Beatrice, C.A.G.; de Oliveira, A.D. Sustainable composites of eco-friendly polyethylene reinforced with eggshells and bio-calcium carbonate. Polímeros 2023, 33, e20230026. [Google Scholar] [CrossRef]
- Yordanov, C.; Minkova, L. Fractionated crystallization of compatibilized LDPE/PA6 blends. Eur. Polym. J. 2005, 41, 527–534. [Google Scholar] [CrossRef]
- Coelho, P.H.d.S.L.; Morales, A.R. The Effect of Organophilic Montmorillonite on Compatibilization, Morphology and Mechanical and Thermal Properties of PA6/ LDPE Blends. Polímeros 2013, 23, 410–416. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.; Zhang, D.; Wang, Y.; Wang, L.; Qiu, Y.; Wang, L.; Chen, T.; Zhao, L. Crystallization and Performance of Polyamide Blends Comprising Polyamide 4, Polyamide 6, and Their Copolymers. Polymers 2023, 15, 3399. [Google Scholar] [CrossRef]



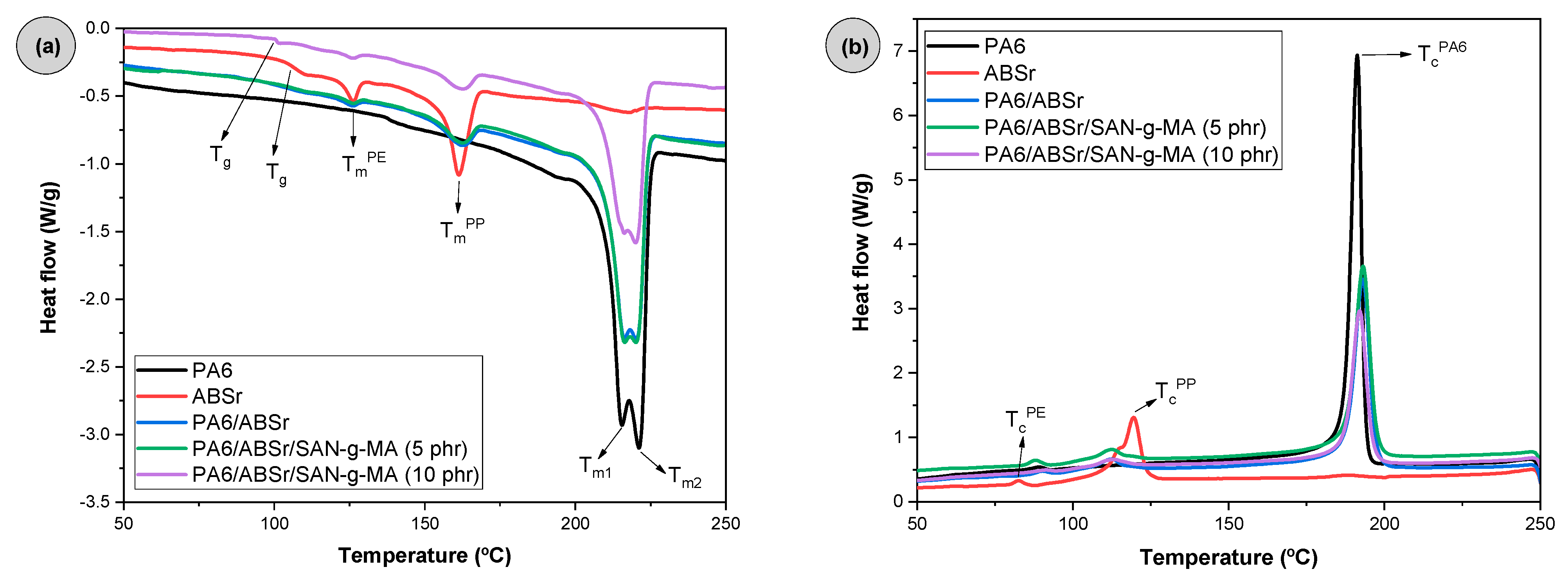
| Samples | Tg (°C) | TmPA6 (°C) | TcPA6 (°C) | XcPA6 (%) | |
|---|---|---|---|---|---|
| Tm1 (°C) | Tm2 (°C) | ||||
| PA6 | - | 215.6 | 221.2 | 191.3 | 28.5 |
| ABSr | 107.0 | - | - | - | - |
| PA6/ABSr | 104.7 | 216.3 | 220.3 | 193.1 | 26.8 |
| PA6/ABSr/SAN-g-MA (5 phr) | 101.2 | 216.3 | 220.2 | 193.2 | 28.0 |
| PA6/ABSr/SAN-g-MA (10 phr) | 101.8 | 216.1 | 220.1 | 192.1 | 23.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torquato, J.V.M.; Luna, C.B.B.; dos Santos Filho, E.A.; do Nascimento, E.P.; de Mélo, T.J.A.; Wellen, R.M.R.; Araújo, E.M.; Morais, D.D.d.S. Repurposing ABS to Produce Polyamide 6 (PA6)-Based Blends: Reactive Compatibilization with SAN-g-MA of a High Degree of Functionalization. Polymers 2024, 16, 3103. https://doi.org/10.3390/polym16223103
Torquato JVM, Luna CBB, dos Santos Filho EA, do Nascimento EP, de Mélo TJA, Wellen RMR, Araújo EM, Morais DDdS. Repurposing ABS to Produce Polyamide 6 (PA6)-Based Blends: Reactive Compatibilization with SAN-g-MA of a High Degree of Functionalization. Polymers. 2024; 16(22):3103. https://doi.org/10.3390/polym16223103
Chicago/Turabian StyleTorquato, Jonathan Vinícius Moreira, Carlos Bruno Barreto Luna, Edson Antonio dos Santos Filho, Emanuel Pereira do Nascimento, Tomás Jeferson Alves de Mélo, Renate Maria Ramos Wellen, Edcleide Maria Araújo, and Dayanne Diniz de Souza Morais. 2024. "Repurposing ABS to Produce Polyamide 6 (PA6)-Based Blends: Reactive Compatibilization with SAN-g-MA of a High Degree of Functionalization" Polymers 16, no. 22: 3103. https://doi.org/10.3390/polym16223103
APA StyleTorquato, J. V. M., Luna, C. B. B., dos Santos Filho, E. A., do Nascimento, E. P., de Mélo, T. J. A., Wellen, R. M. R., Araújo, E. M., & Morais, D. D. d. S. (2024). Repurposing ABS to Produce Polyamide 6 (PA6)-Based Blends: Reactive Compatibilization with SAN-g-MA of a High Degree of Functionalization. Polymers, 16(22), 3103. https://doi.org/10.3390/polym16223103







