Synthesis Characterization and Physicochemical Properties of Rigid Alicyclic Polyimide Films Based on Bicyclo[2.2.2]oct-7-ene-2,3,5,6-tetracarboxylic Dianhydride
Abstract
:1. Introduction
2. Experimental Materials
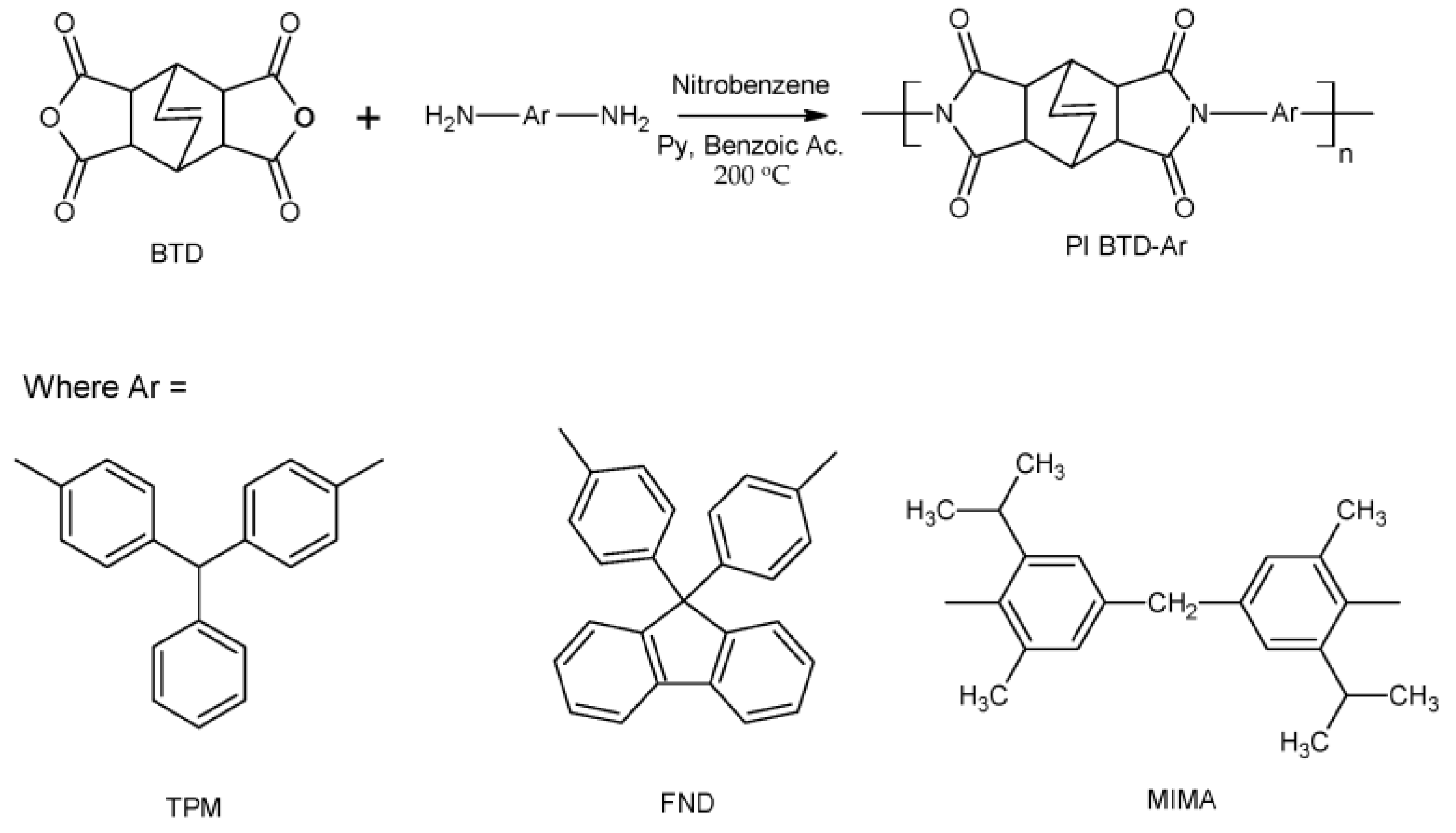
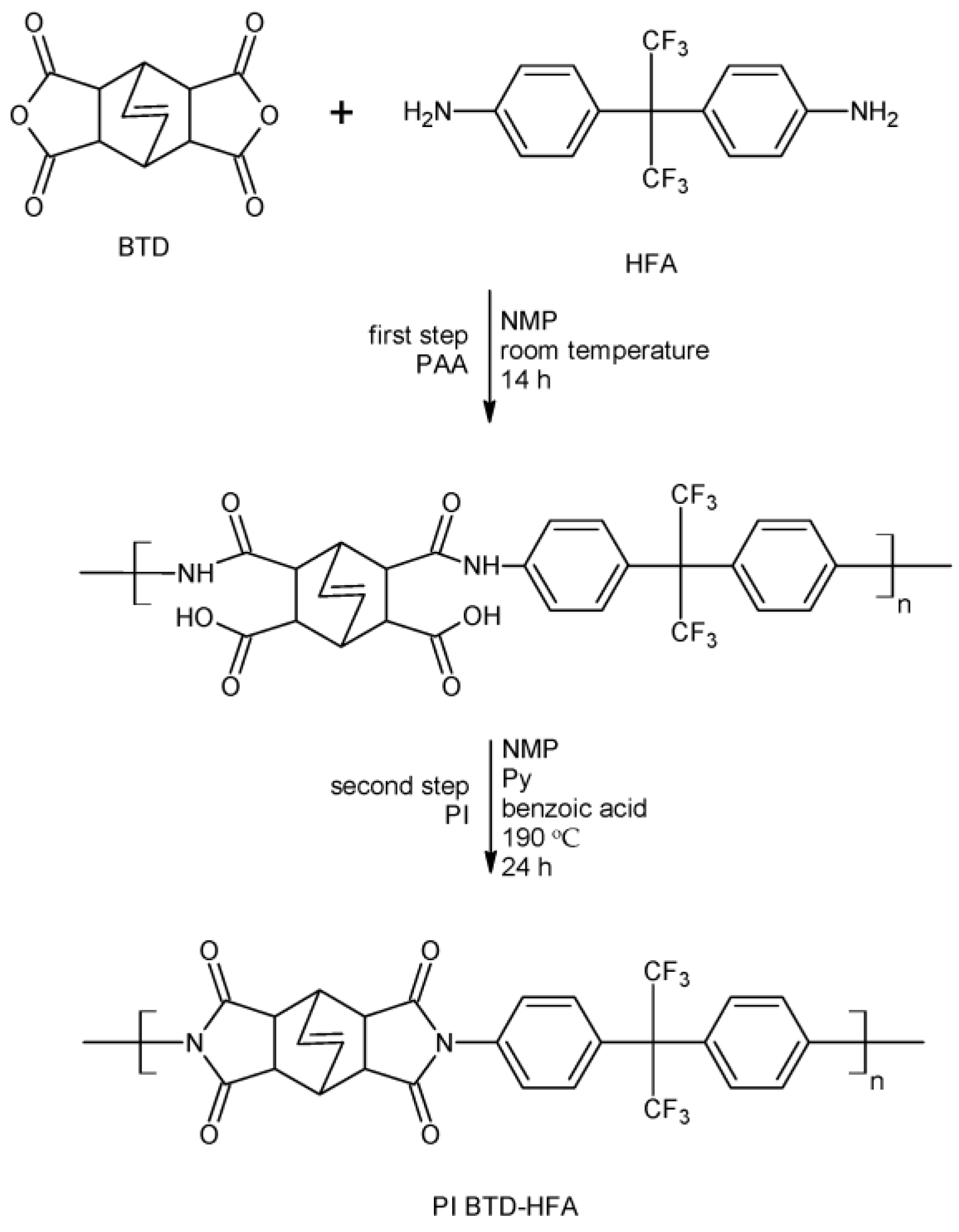
2.1. Polyimide Synthesis
2.2. Membrane Preparation
2.3. Characterization
2.4. Gas Transport Properties
3. Results and Discussion
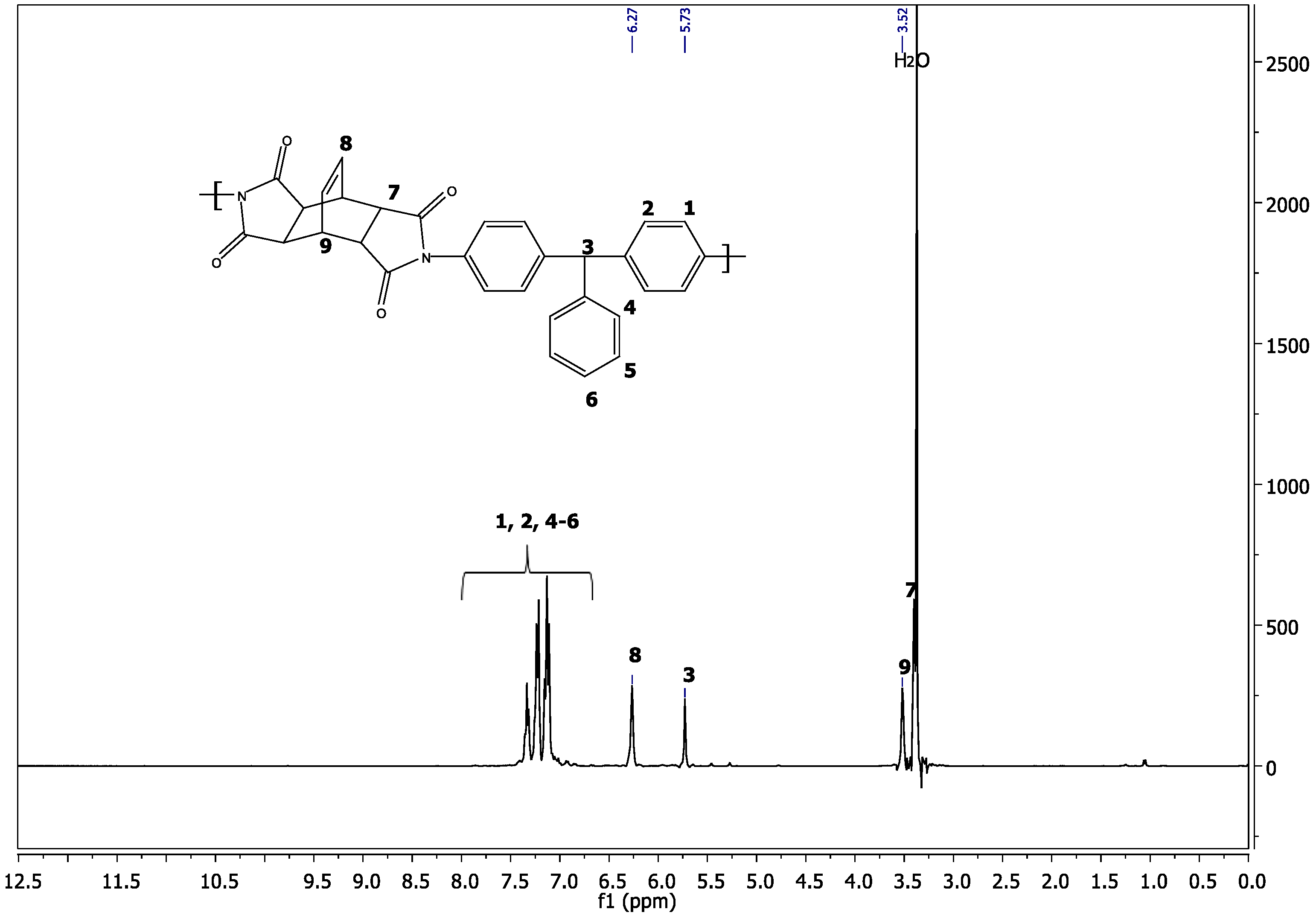
3.1. Polyimide Synthesis and Characterization
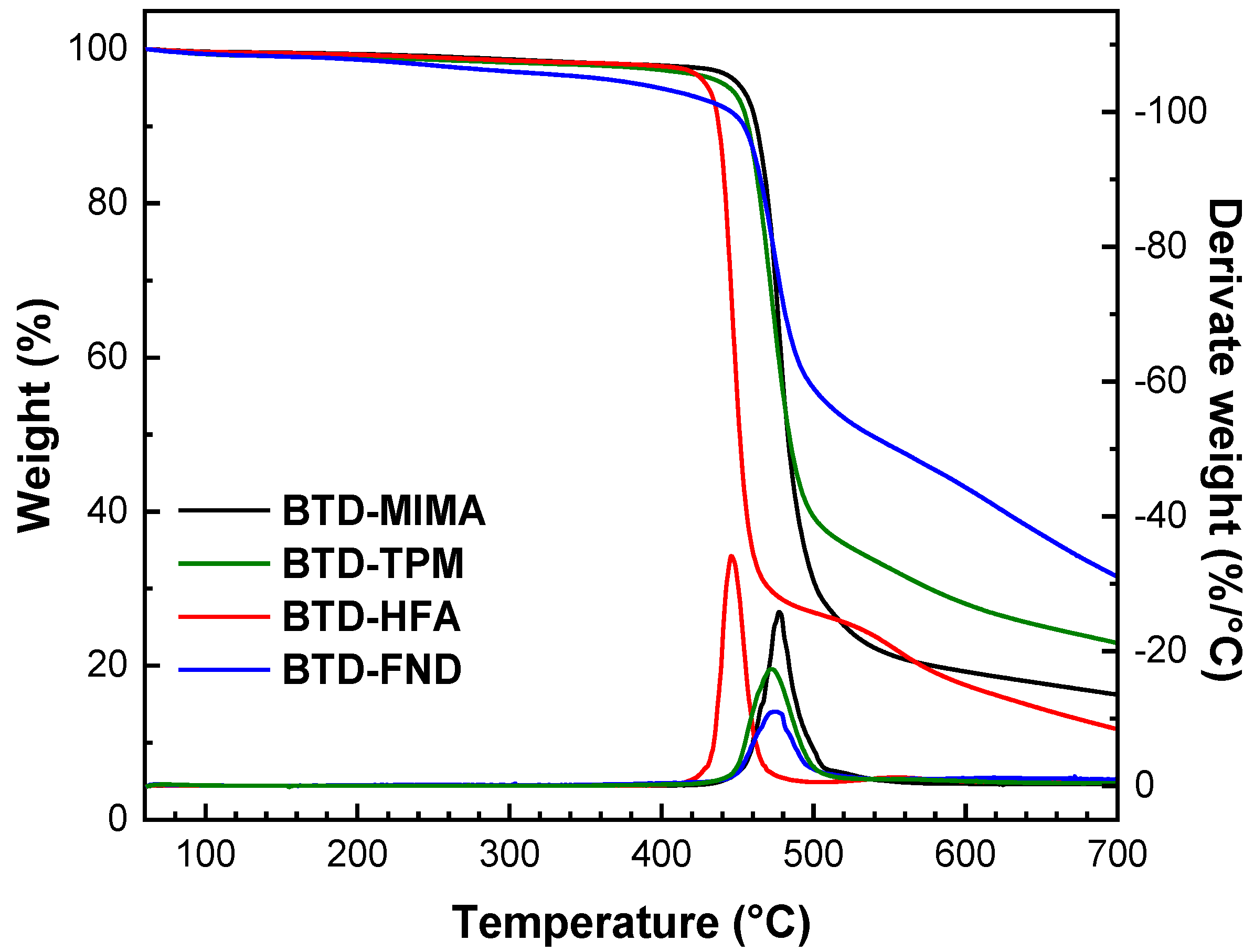
3.2. Thermal Analysis
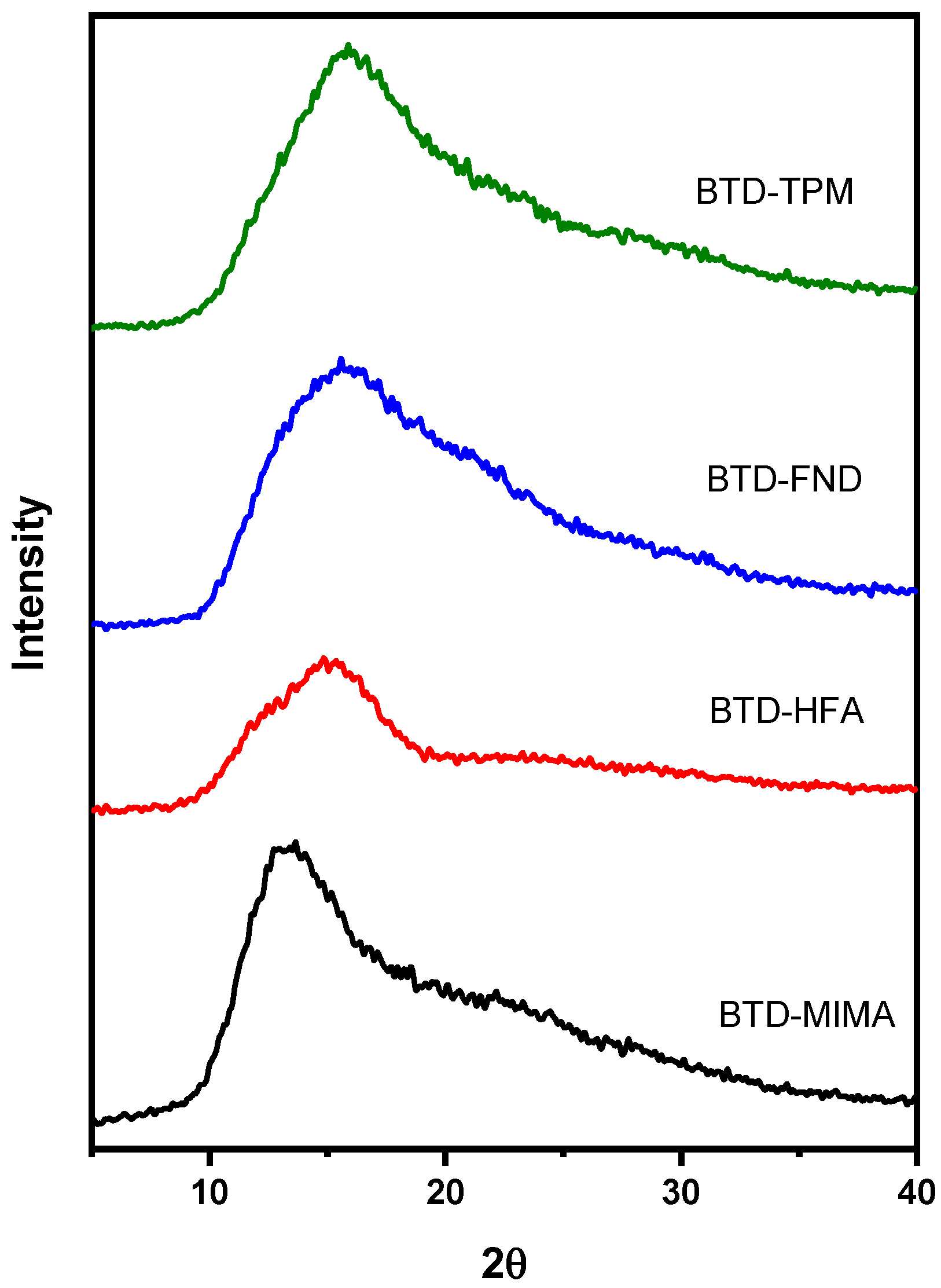
3.3. X-Ray Diffraction
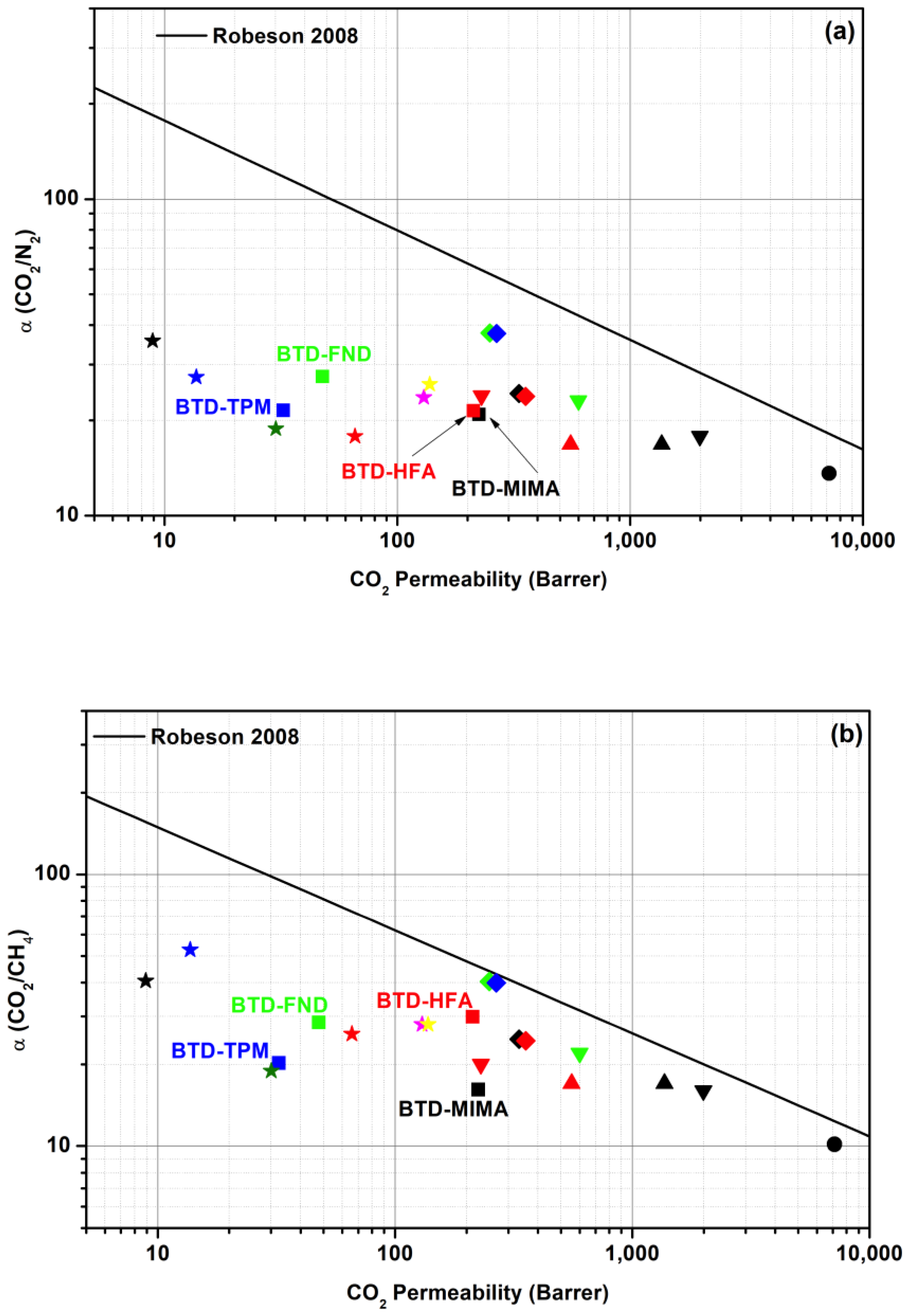
3.4. Gas Transport Through Rigid BTD-Based Polyimide Membranes
| Polyimide | Permeability (Barrer) | Selectivity | |||||
|---|---|---|---|---|---|---|---|
| He | O2 | N2 | CH4 | CO2 | αCO2/CH4 | αCO2/N2 | |
| BTD-MIMA | 165.9 | 42.8 | 10.7 | 13.9 | 224.6 | 16.1 | 20.8 |
| BTD-HFA | 274.6 | 41.9 | 9.9 | 7.1 | 212.9 | 29.9 | 21.4 |
| BTD-FND | 61.6 | 8.6 | 1.7 | 1.7 | 47.7 | 28.5 | 27.4 |
| BTD-TPM | 40.5 | 5.9 | 1.5 | 1.6 | 32.3 | 20.2 | 21.5 |
| PIM-EA-TB [38] | 2150 | 525 | 699 | 7140 | 10.2 | 13.6 | |
| Ac-CoPI-TB1 [10] | 919 | 331 | 81 | 33 | 1366 | 17 | 16.8 |
| Ac-CoPI-TB2 [10] | 540 | 166 | 33 | 33 | 555 | 17 | 16.8 |
| BTA-CANAL2 [12] | 392 | 112 | 125 | 1995 | 16 | 17.8 | |
| BTA-CANAL4 [12] | 115 | 26 | 30 | 600 | 20 | 23.1 | |
| BTA-CANAL3 [12] | 40 | 9.6 | 11 | 230 | 22 | 23.9 | |
| POBI-1 [39] | 57 | 13.7 | 13.5 | 334 | 24.7 | 24.3 | |
| POBI-2 [39] | 61 | 14.9 | 14.6 | 356 | 24.4 | 23.8 | |
| POBI-3 [39] | 39 | 6.6 | 6.2 | 250 | 40.3 | 37.8 | |
| POBI-4 [39] | 41 | 7.1 | 6.7 | 267 | 39.9 | 37.6 | |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Álvarez, C.; Lozano, A.E.; de la Campa, J.G. High-productivity gas separation membranes derived from pyromellitic dianhydride and nonlinear diamines. J. Membr. Sci. 2016, 501, 191–198. [Google Scholar] [CrossRef]
- Qiu, Z.; Chen, G.; Zhang, Q.; Zhang, S. Synthesis and gas transport property of polyimide from 2,2′-disubstituted biphenyltetracarboxylic dianhydrides (BPDA). Eur. Polym. J. 2007, 43, 194–204. [Google Scholar] [CrossRef]
- Toshihiko, M.; Daisuke, M.; Takahiro, H.; Motoaki, K.; Risa, T.; Shuichi, K. Alicyclic polyimides—A colorless and thermally stable polymer for opto-electronic devices. J. Phys. Conf. Ser. 2009, 187, 012005. [Google Scholar] [CrossRef]
- Sulub-Sulub, R.; Loría-Bastarrachea, M.I.; Vázquez-Torres, H.; Santiago-García, J.L.; Aguilar-Vega, M. Highly permeable polyimide membranes with a structural pyrene containing tert-butyl groups: Synthesis, characterization and gas transport. J. Membr. Sci. 2018, 563, 134–141. [Google Scholar] [CrossRef]
- Suzuki, H.; Kondo, Y.; Endo, K.; Narita, M.; Hamada, F. Synthesis of; Bis (4′-Oxa-3′; Dioxotricyclo-[4.3.0.12, 5] Decane-8′-Yloxy) Ethane Involving a Flexible Bridging Moiety by Combination of Heteropolyacids as Solid Acid Catalysts and 1, 4-Dioxane as a Bridging Reagent. Int. J. Soc. Mater. Eng. Resour. 2001, 9, 14–16. [Google Scholar] [CrossRef]
- Guan, Y.; Wang, D.; Song, G.; Dang, G.; Chen, C.; Zhou, H.; Zhao, X. Novel soluble polyimides derived from 2,2′-bis [4-(5-amino-2-pyridinoxy)phenyl]hexafluoropropane: Preparation, characterization, and optical, dielectric properties. Polymer 2014, 55, 3634–3641. [Google Scholar] [CrossRef]
- Chun, B.-W. Preparation and characterization of organic-soluble optically transparent polyimides from alicyclic dianhydride, bicyclo[2.2.2]-oct-7-ene-2,3,5,6-tetracarboxylic dianhydride. Polymer 1994, 35, 4203–4208. [Google Scholar] [CrossRef]
- Matsumoto, T.; Feger, C. Optical Properties of Polyalicyclic Polyimides. J. Photopolym. Sci. Technol. 1998, 11, 231–236. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kurosaki, T. Soluble Polyimides with Polyalicyclic Structure. 4. Colorless Polyimides from Bicyclo[2.2.1]heptane-2-endo,3-endo,5-exo,6-exo-tetracarboxylic 2,3:5,6-Dianhydride. Macromolecules 1995, 28, 5684–5685. [Google Scholar] [CrossRef]
- Zhang, Y.; Lee, W.H.; Seong, J.G.; Bae, J.Y.; Zhuang, Y.; Feng, S.; Wan, Y.; Lee, Y.M. Alicyclic segments upgrade hydrogen separation performance of intrinsically microporous polyimide membranes. J. Membr. Sci. 2020, 611, 118363. [Google Scholar] [CrossRef]
- Abdulhamid, M.A.; Ma, X.; Ghanem, B.S.; Pinnau, I. Synthesis and Characterization of Organo-Soluble Polyimides Derived from Alicyclic Dianhydrides and a Dihydroxyl-Functionalized Spirobisindane Diamine. ACS Appl. Polym. Mater. 2019, 1, 63–69. [Google Scholar] [CrossRef]
- Yuan, P.; Zhang, M.; Pang, Y.; Chen, A.; Wang, Z.; Yan, J. Intrinsically Microporous Polyimides from Norbornyl Bis-benzocyclobutene-Containing Diamines and Rigid Dianhydrides for Membrane-Based Gas Separation. ACS Appl. Polym. Mater. 2023, 5, 1420–1429. [Google Scholar] [CrossRef]
- Likhatchev, D.; Alexandrova, L.; Tlenkopatchev, M.; Vilar, R.; Vera-Graziano, R. Soluble aromatic polyimides and polyamides based on 4,4′-diaminotriphenylmethane. J. Appl. Polym. Sci. 1995, 57, 37–44. [Google Scholar] [CrossRef]
- Sulub-Sulub, R.; Loría-Bastarrachea, M.I.; Santiago-García, J.L.; Aguilar-Vega, M. Synthesis and characterization of new polyimides from diphenylpyrene dianhydride and ortho methyl substituted diamines. RSC Adv. 2018, 8, 31881–31888. [Google Scholar] [CrossRef]
- Carrera-Figueiras, C.; Aguilar-Vega, M. Gas permeability and selectivity of hexafluoroisopropylidene aromatic isophthalic copolyamides. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 2625–2638. [Google Scholar] [CrossRef]
- Ghosh, M.K.; Mittal, K.L. (Eds.) Polyimides: Fundamentals and Applications; Marcel Dekker, Inc.: New York, NY, USA, 1996. [Google Scholar]
- Likhatchev, B.D.; Vera-Graziano, R. Polyimide in High Performance Films. In Polymeric Materials Encyclopedia, Twelve Volume Set; Salamone, J.C., Ed.; CRC Press: Boca Raton, FL, USA, 1996; pp. 6275–6285. [Google Scholar] [CrossRef]
- Kuznetsov, A.A. One-Pot Polyimide Synthesis in Carboxylic Acid Medium. High Perform. Polym. 2000, 12, 445–460. [Google Scholar] [CrossRef]
- Korshak, V.V.; Vinogradova, S.V.; Vygodskii, Y.S.; Pavlova, S.A.; Boiko, L.V. Thermally stable soluble polyimides, Bulletin of the Academy of Sciences of the USSR. Div. Chem. Sci. 1967, 16, 2172–2178. [Google Scholar]
- An, H.-Y.; Zhan, M.-S.; Wang, K. Synthesis and properties of fluorene-based polyimide adhesives. Polym. Eng. Sci. 2011, 51, 1533–1540. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kurosaki, T. Soluble and Colorless Polyimides from Bicyclo[2.2.2]octane-2,3,5,6-tetracarboxylic 2,3:5,6-Dianhydrides. Macromolecules 1997, 30, 993–1000. [Google Scholar] [CrossRef]
- Huang, X.; Li, H.; Liu, C.; Wei, C. Design and synthesis of high heat-resistant, soluble, and hydrophobic fluorinated polyimides containing pyridine and trifluoromethylthiophenyl units. High Perform. Polym. 2019, 31, 107–115. [Google Scholar] [CrossRef]
- Santiago-García, J.L.; Pérez-Francisco, J.M.; Loría-Bastarrachea, M.I.; Aguilar-Vega, M. Synthesis and characterization of novel polyamides containing dibenzobarrelene pendant groups. Des. Monomers Polym. 2015, 18, 350–359. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Ren, X.; Wang, Z.; He, Z.; Yang, C.; Qi, Y.; Han, S.; Chen, S.; Yu, H.; Liu, J. Synthesis and Characterization of Organo-Soluble Polyimides Based on Polycondensation Chemistry of Fluorene-Containing Dianhydride and Amide-Bridged Diamines with Good Optical Transparency and Glass Transition Temperatures over 400 °C. Polymers 2023, 15, 3549. [Google Scholar] [CrossRef] [PubMed]
- Loría-Bastarrachea, M.I.; Aguilar-Vega, M. Synthesis of hexafluoroisopropylidene isophthalic polyesters and copolyesters and the relationship between their structure and gas transport properties. J. Appl. Polym. Sci. 2007, 103, 2207–2216. [Google Scholar] [CrossRef]
- López-Nava, R.; Vázquez-Moreno, F.S.; Palí-Casanova, R.; Aguilar-Vega, M. Gas permeability coefficients of isomeric aromatic Polyamides obtained from 4,4′-(9-fluorenylidene) diamine and aromatic diacid chlorides. Polym. Bull. 2002, 49, 165–172. [Google Scholar] [CrossRef]
- Pérez-Francisco, J.M.; Santiago-García, J.L.; Loría-Bastarrachea, M.I.; Aguilar-Vega, M. Evaluation of Gas Transport Properties of Highly Rigid Aromatic PI DPPD-IMM/PBI Blends. Ind. Eng. Chem. Res. 2017, 56, 9355–9366. [Google Scholar] [CrossRef]
- Santiago-García, J.L.; Álvarez, C.; Sánchez, F.; de la Campa, J.G. Gas transport properties of new aromatic polyimides based on 3,8-diphenylpyrene-1,2,6,7-tetracarboxylic dianhydride. J. Membr. Sci. 2015, 476, 442–448. [Google Scholar] [CrossRef]
- Aguilar-Lugo, C.; Santiago-García, J.L.; Loría-Bastarrachea, M.I.; Guzmán-Lucero, D.; Alexandrova, L.; Aguilar-Vega, M. Synthesis; characterization, and structure-property relationships of aromatic polyimides containing 4,4′-diaminotriphenylmethane. J. Polym. Res. 2016, 23, 49. [Google Scholar] [CrossRef]
- Sathiskumar, P.S.; Madras, G. Synthesis; characterization, degradation of biodegradable castor oil based polyesters. Polym. Degrad. Stab. 2011, 96, 1695–1704. [Google Scholar] [CrossRef]
- Fan, F.; Sun, Y.; Zhao, Q.; Zhang, J.; Guan, J.; He, G.; Ma, C. Fluorinated-cardo-based Co-polyimide membranes with enhanced selectivity for CO2 separation. Sep. Purif. Technol. 2023, 324, 124511. [Google Scholar] [CrossRef]
- Yong, W.F.; Li, F.Y.; Xiao, Y.C.; Chung, T.S.; Tong, Y.W. High performance PIM-1/Matrimid hollow fiber membranes for CO2/CH4, O2/N2 and CO2/N2 separation. J. Membr. Sci. 2013, 443, 156–169. [Google Scholar] [CrossRef]
- Ma, X.; Salinas, O.; Litwiller, E.; Pinnau, I. Novel Spirobifluorene- and Dibromospirobifluorene-Based Polyimides of Intrinsic Microporosity for Gas Separation Applications. Macromolecules 2013, 46, 9618–9624. [Google Scholar] [CrossRef]
- Budd, P.M.; McKeown, N.B.; Fritsch, D. Free volume and intrinsic microporosity in polymers. J. Mater. Chem. 2005, 15, 1977–1986. [Google Scholar] [CrossRef]
- Cetina-Mancilla, E.; Camacho-Zuñiga, C.; González-Díaz, M.O.; Alondra, C.T.; Ruiz-Treviño, A.F.; Vivaldo-Lima, E.; Vera-Graziano, R.; Zolotukhin, M.G.; Sulub-Sulub, R.; Aguilar-Vega, M. Room temperature synthesis, characterization and enhanced gas transport properties of novel poly(oxindolylidene arylene)s with dibenzothiophene, dibenzothiophene-S-oxide and dibenzothiophene-S,S-dioxide fragments in the main chain. Sep. Purif. Technol. 2024, 341, 126853. [Google Scholar] [CrossRef]
- Espeso, J.; Lozano, A.E.; de la Campa, J.G.; de Abajo, J. Effect of substituents on the permeation properties of polyamide membranes. J. Membr. Sci. 2006, 280, 659–665. [Google Scholar] [CrossRef]
- Carta, M.; Malpass-Evans, R.; Croad, M.; Rogan, Y.; Jansen, J.C.; Bernardo, P.; Bazzarelli, F.; McKeown, N.B. An Efficient Polymer Molecular Sieve for Membrane Gas Separations. Science 2013, 339, 303–307. [Google Scholar] [CrossRef]
- Chen, H.; Dai, F.; Wang, M.; Ke, Z.; Yan, K.; Li, D.; Chen, C.; Qian, G.; Yu, Y. Synthesis, characterization and properties of polyimides with spirobisbenzoxazole scaffold structure. Polymer 2022, 254, 125091. [Google Scholar] [CrossRef]
- Zhang, C.; Li, P. Preparation and Gas Separation Properties of Spirobichroman-Based Polyimides. Macromol. Chem. Phys. 2018, 219, 1800157. [Google Scholar] [CrossRef]
- Yong, W.F.; Li, F.Y.; Xiao, Y.C.; Li, P.; Pramoda, K.P.; Tong, Y.W.; Chung, T.S. Molecular engineering of PIM-1/Matrimid blend membranes for gas separation. J. Membr. Sci. 2012, 407–408, 47–57. [Google Scholar] [CrossRef]
- Shao, L.; Liu, L.; Cheng, S.-X.; Huang, Y.-D.; Ma, J. Comparison of diamino cross-linking in different polyimide solutions and membranes by precipitation observation and gas transport. J. Membr. Sci. 2008, 312, 174–185. [Google Scholar] [CrossRef]
- Zhang, C. Synthesis and characterization of bis(phenyl)fluorene-based cardo polyimide membranes for H2/CH4 separation. J. Mater. Sci. 2019, 54, 10560–10569. [Google Scholar] [CrossRef]
- Canto-Acosta, R.J.; Loría-Bastarrachea, M.I.; Carrillo-Escalante, H.J.; Hernández-Núñez, E.; Aguilar-Vega, M.; Santiago-García, J.L. Synthesis and characterization of poly(amide-imide)s derived from a new ortho-functional unsymmetrical dicarboxylic acid. RSC Adv. 2018, 8, 284–290. [Google Scholar] [CrossRef]
| Polyimide | Mw (Dalton) | Mn (Dalton) | PDI | η a (dL/g) |
|---|---|---|---|---|
| BTD-MIMA b | 146,326 | 69,517 | 2.10 | 1.37 |
| BTD-HFA c | 62,927 | 39,412 | 1.59 | 0.43 |
| BTD-FND b | 68,052 | 43,419 | 1.56 | 0.48 |
| BTD-TPM b | 99,611 | 48,303 | 2.06 | 0.63 |
| Polyimide | NMP | DMF | DMAc | CHCl3 | DCE | TCE | THF | DMSO |
|---|---|---|---|---|---|---|---|---|
| BTD-MIMA | + | + | + | + | − | − | − | + |
| BTD-HFA | − | ± | − | − | − | − | − | + |
| BTD-FND | + | + | + | − | − | + | − | + |
| BTD-TPM | + | + | + | − | − | + | − | + |
| DDE1 | + | + | + | − | + | |||
| DDM1 | + | + | + | − | NR | NR | NR | + |
| BAB1 | + | + | + | + | NR | NR | NR | + |
| PI-DP2 | ± | − | ± | − | NR | NR | − | − |
| PI-6F2 | + | + | + | + | NR | NR | + | + |
| Polyimide | Td(°C) | Tg(°C) | d-Spacing (Å) | Density (g/cm3) | Vw (cm3/mol) | FFV |
|---|---|---|---|---|---|---|
| BTD-MIMA | 460 | 345 | 7.32 | 1.177 | 299.51 | 0.1232 |
| BTD-HFA | 437 | 355 | 6.56 | 1.427 | 256.57 | 0.1289 |
| BTD-FND | 450 | - | 6.23 | 1.289 | 296.01 | 0.1150 |
| BTD-TPM | 454 | 272 | 6.17 | 1.279 | 261.16 | 0.1070 |
| Polyimide | Diffusion Coefficient (10−8 cm2/s) | Diffusion Selectivity | ||||
|---|---|---|---|---|---|---|
| O2 | N2 | CH4 | CO2 | αCO2/CH4 | αCO2/N2 | |
| BTD-MIMA | 31.4 | 7.3 | 2.9 | 9.5 | 3.3 | 1.3 |
| BTD-HFA | 32.0 | 6.6 | 2.4 | 10.7 | 4.4 | 1.6 |
| BTD-FND | 6.9 | 1.7 | 1.0 | 3.7 | 3.7 | 2.1 |
| BTD-TPM | 4.8 | 1.5 | 0.9 | 2.9 | 3.2 | 1.9 |
| Polyimide | Solubility Coefficient (10−2 cm3(STP)/cm3cm Hg) | Solubility Selectivity | ||||
|---|---|---|---|---|---|---|
| O2 | N2 | CH4 | CO2 | αCO2/CH4 | αCO2/N2 | |
| BTD-MIMA | 1.36 | 1.47 | 4.72 | 23.47 | 4.97 | 15.96 |
| BTD-HFA | 1.31 | 1.49 | 2.96 | 19.90 | 6.72 | 13.35 |
| BTD-FND | 1.25 | 1.00 | 1.68 | 12.73 | 7.57 | 12.73 |
| BTD-TPM | 1.22 | 1.02 | 1.79 | 11.01 | 6.15 | 10.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Francisco, J.M.; Aguilar-Lugo, C.; Alexandrova, L.; Gonzalez-Diaz, M.O.; Sulub-Sulub, R.; Loría-Bastarrachea, M.I.; Aguilar-Vega, M. Synthesis Characterization and Physicochemical Properties of Rigid Alicyclic Polyimide Films Based on Bicyclo[2.2.2]oct-7-ene-2,3,5,6-tetracarboxylic Dianhydride. Polymers 2024, 16, 3188. https://doi.org/10.3390/polym16223188
Pérez-Francisco JM, Aguilar-Lugo C, Alexandrova L, Gonzalez-Diaz MO, Sulub-Sulub R, Loría-Bastarrachea MI, Aguilar-Vega M. Synthesis Characterization and Physicochemical Properties of Rigid Alicyclic Polyimide Films Based on Bicyclo[2.2.2]oct-7-ene-2,3,5,6-tetracarboxylic Dianhydride. Polymers. 2024; 16(22):3188. https://doi.org/10.3390/polym16223188
Chicago/Turabian StylePérez-Francisco, José Manuel, Carla Aguilar-Lugo, Larissa Alexandrova, María O. Gonzalez-Diaz, Rita Sulub-Sulub, María Isabel Loría-Bastarrachea, and Manuel Aguilar-Vega. 2024. "Synthesis Characterization and Physicochemical Properties of Rigid Alicyclic Polyimide Films Based on Bicyclo[2.2.2]oct-7-ene-2,3,5,6-tetracarboxylic Dianhydride" Polymers 16, no. 22: 3188. https://doi.org/10.3390/polym16223188
APA StylePérez-Francisco, J. M., Aguilar-Lugo, C., Alexandrova, L., Gonzalez-Diaz, M. O., Sulub-Sulub, R., Loría-Bastarrachea, M. I., & Aguilar-Vega, M. (2024). Synthesis Characterization and Physicochemical Properties of Rigid Alicyclic Polyimide Films Based on Bicyclo[2.2.2]oct-7-ene-2,3,5,6-tetracarboxylic Dianhydride. Polymers, 16(22), 3188. https://doi.org/10.3390/polym16223188








