Development of Innovative Thermoplastic Foam Materials Using Two Additive Manufacturing Technologies for Application in Evaporative Cooling Systems
Abstract
1. Introduction and State of the Art
2. Material and Methods
2.1. Selection of Materials and Manufacturing of Samples
2.2. Characterization of Foam Materials
2.2.1. Capillarity
2.2.2. Water Absorption
2.2.3. Surface Analysis
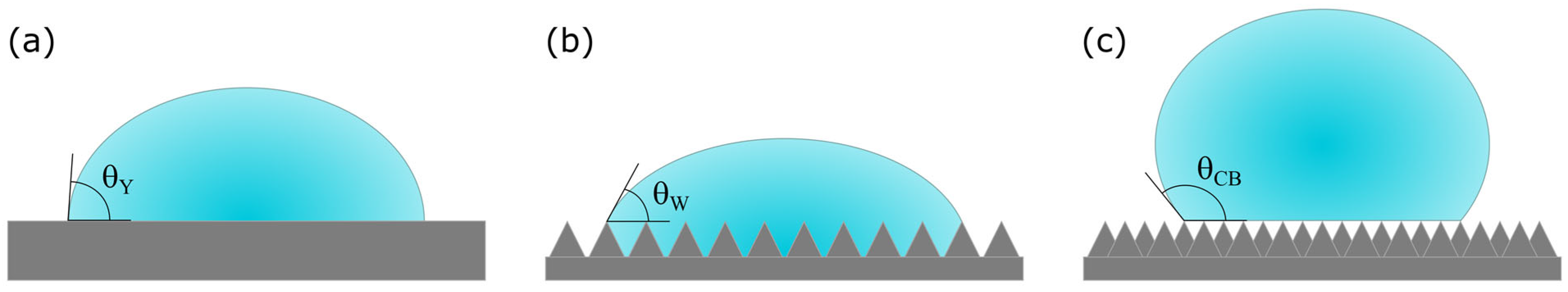
2.2.4. Wettability
3. Results and Discussion
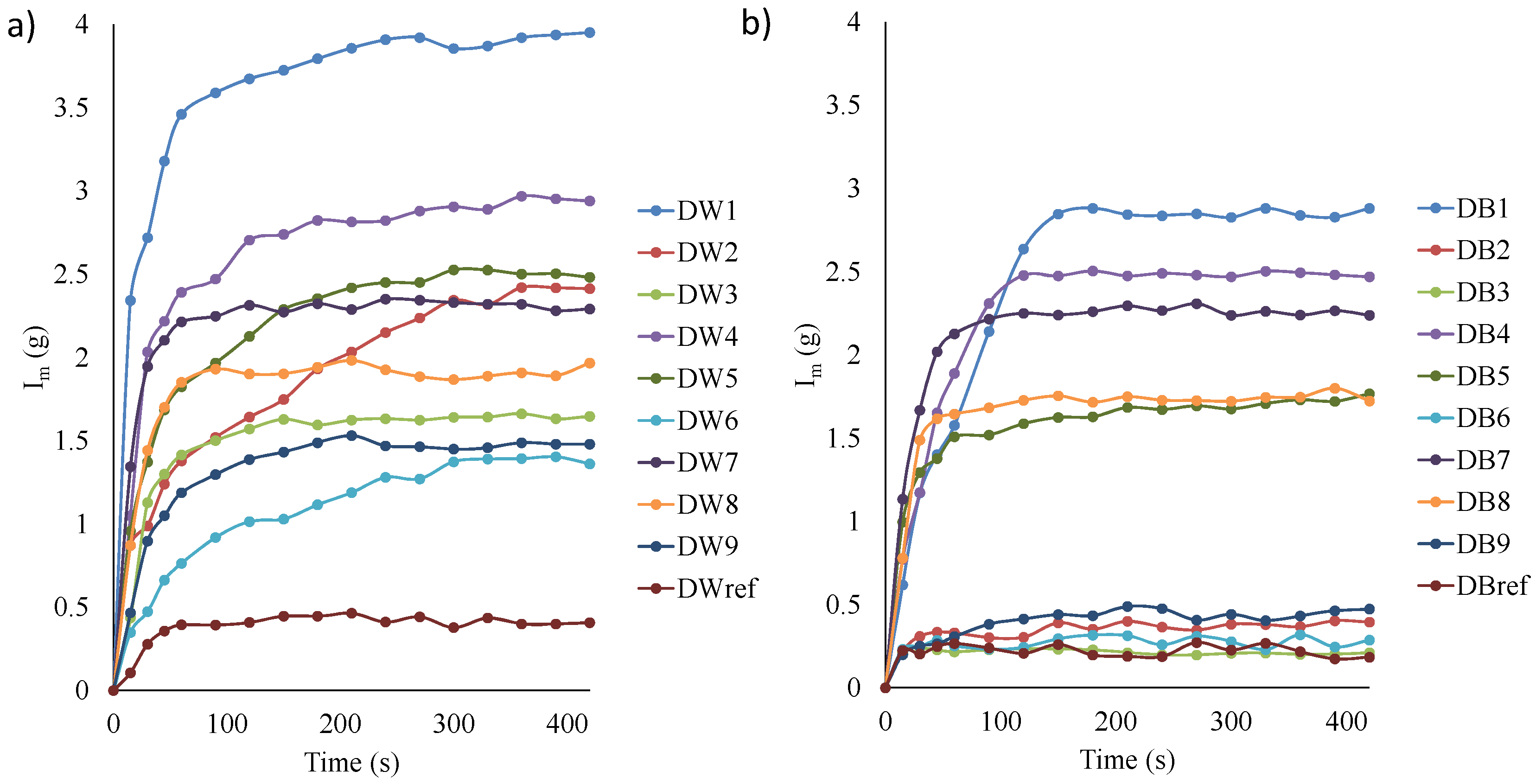
3.1. Analysis of Water Absorption by Capillarity and Immersion
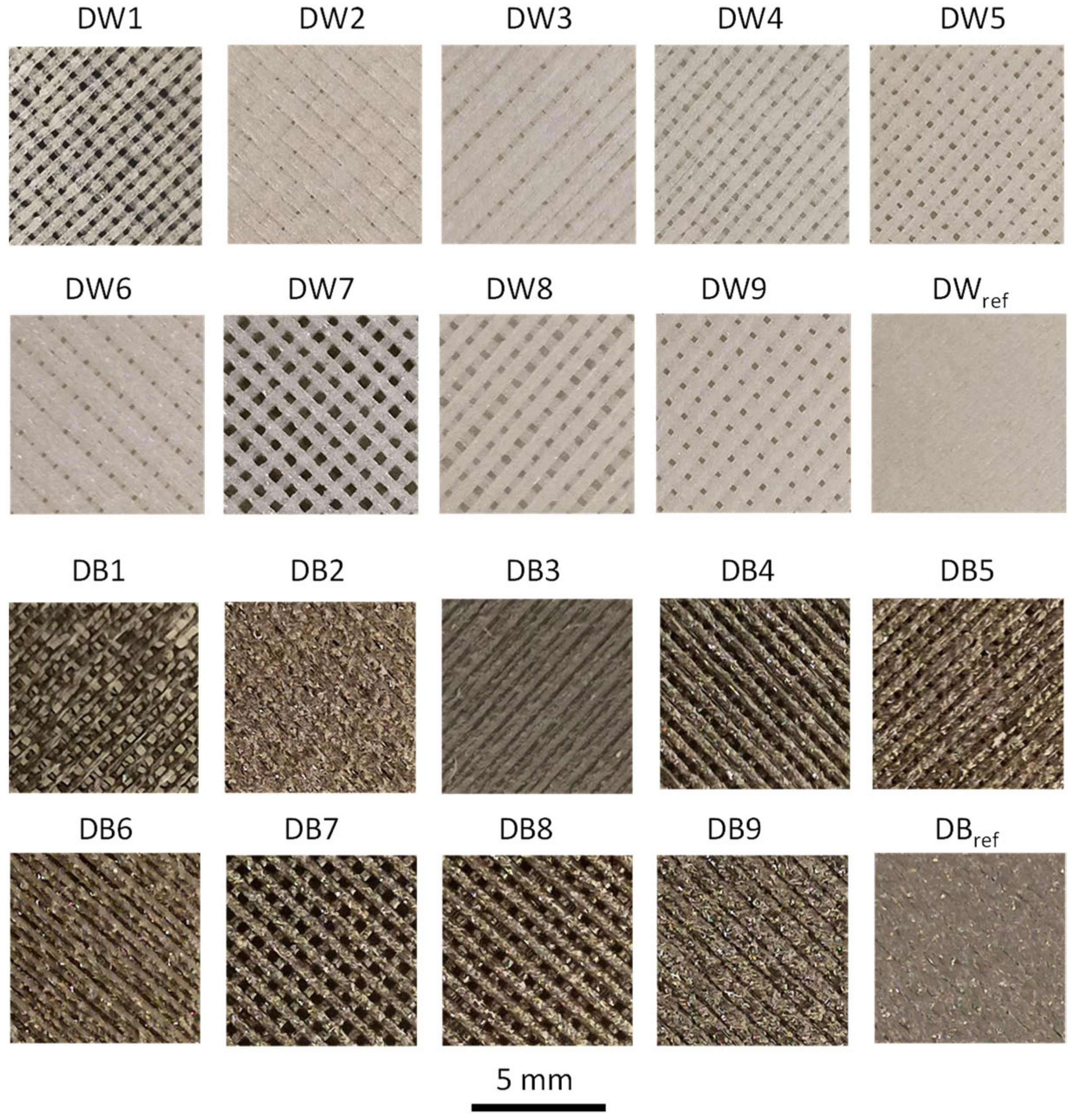
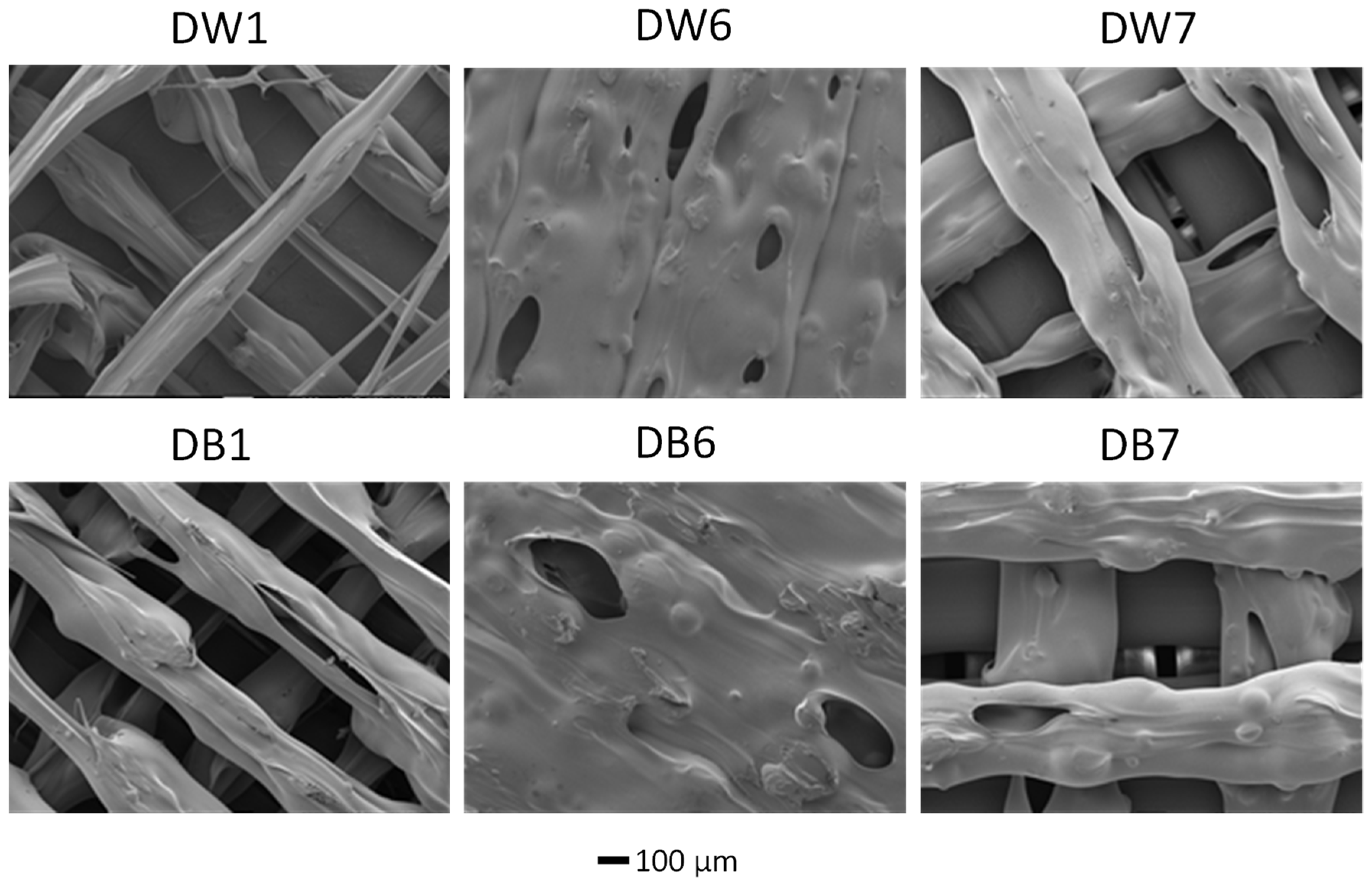
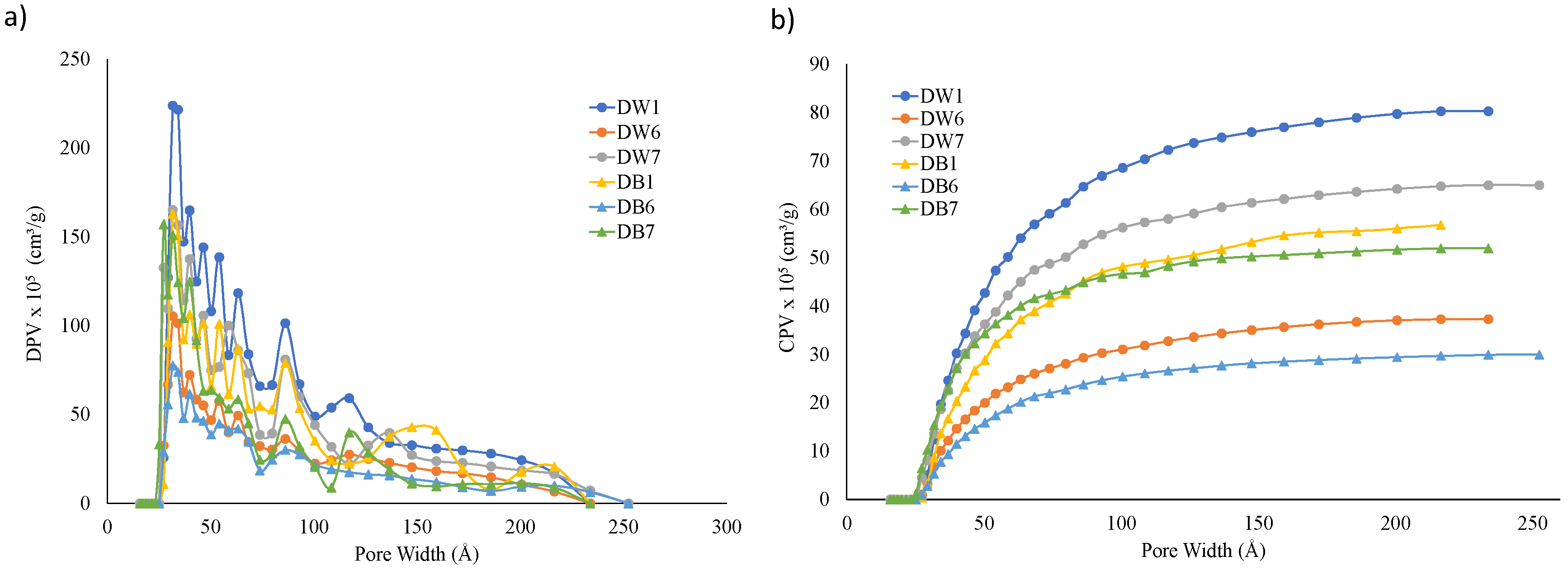
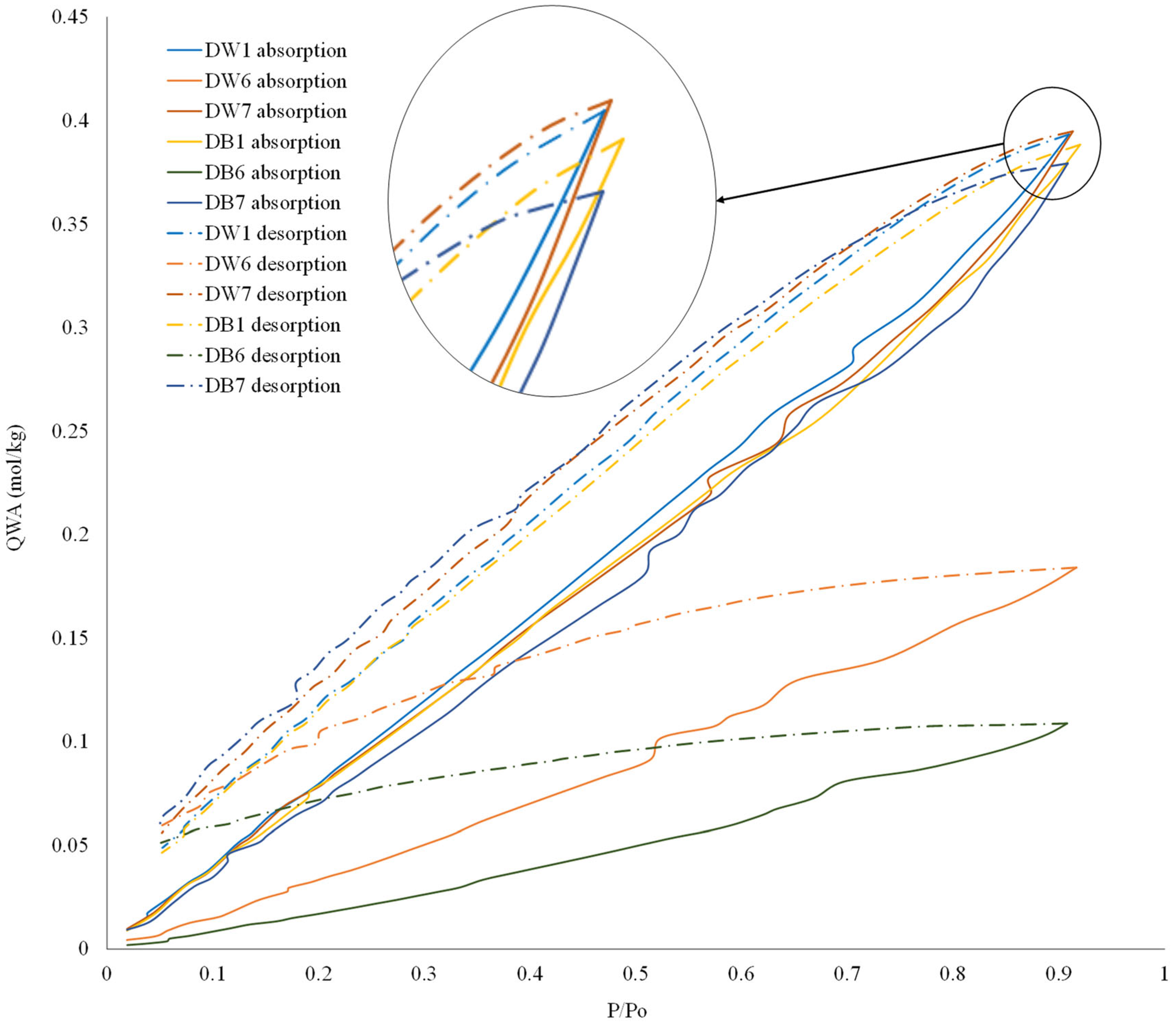
3.2. Surface Analysis of the Foam Materials
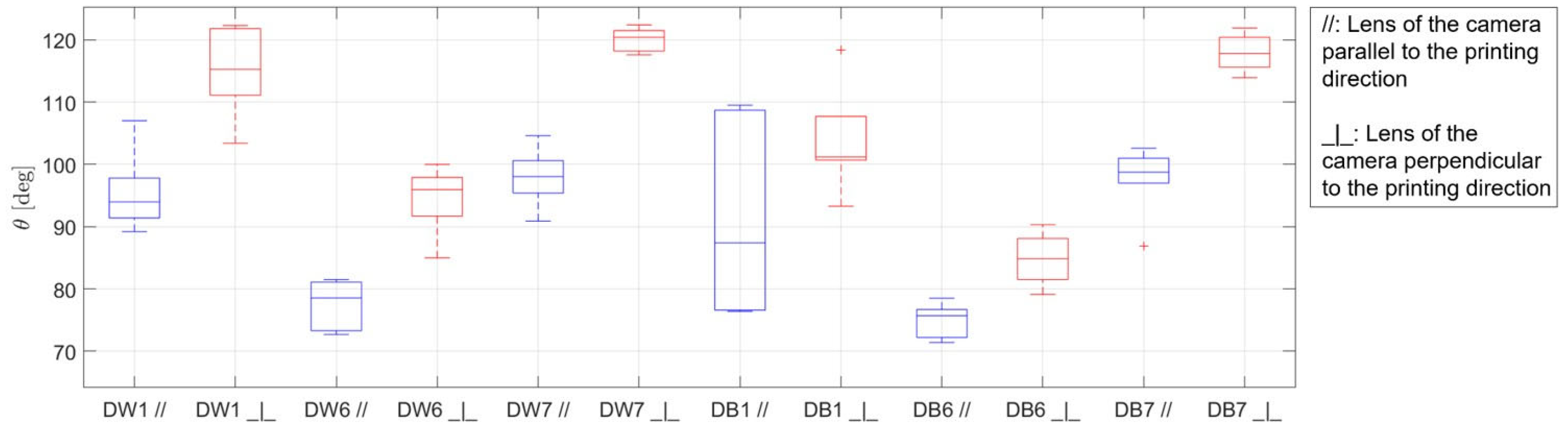
3.3. Wettability
4. Conclusions
- Regarding water absorption and capillarity height, the flow rate was identified as the most influential process parameter, followed by line width, while the effects of the layer height and the speed were negligible. The optimal process parameters for maximizing the capillarity height and water absorption for both foam materials, DW and DB, were achieved with the following specific set of parameters: 50% flow, 0.4 mm line width, 40 mm/s speed, and 0.2 mm layer height.
- In terms of accumulated micropores and mesopores, the trends observed across different configurations were similar to those in the capillarity and water absorption tests. Samples with the D1 configuration presented greater surface area (21% more) and porosity (40% more), which resulted in a taller capillarity height and increased water absorption. These outcomes aligned with the results from the water adsorption and capillarity tests; that is, the greater the porosity, the increased the water absorption and capillarity effect.
- The results of the wettability tests were consistent with those of the capillary rise and macropore analyses. They demonstrated that the surface wetting behavior of the samples, characterized by roughness and micro-grooves due to the manufacturing technique, could be attributed to a Cassie–Baxter wetting state. Considering an intermediate contact angle as being advantageous for improving the evaporative cooling effect, the D1 configuration presented the best results, which aligned with the findings regarding capillarity height, water absorption, and porosity. Additionally, the wettability tests in both the along-grooves and cross-grooves directions demonstrated that the contact angle in the direction parallel to the printing orientation was always lower than that in the perpendicular orientation, as expected. Thus, the orientation of the material within evaporative cooling systems could influence their performance.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Irshad, K.; Habib, K.; Algarni, S.; Saha, B.B.; Jamil, B. Sizing and life-cycle assessment of building integrated thermoelectric air cooling and photovoltaic wall system. Appl. Therm. Eng. 2019, 154, 302–314. [Google Scholar] [CrossRef]
- Duan, Z.; Wang, M.; Dong, X.; Liu, J.; Zhao, X. Experimental and numerical investigation of wicking and evaporation performance of fibrous materials for evaporative cooling. Energy Build. 2022, 255, 111675. [Google Scholar] [CrossRef]
- Amer, O.; Boukhanouf, R.; Ibrahim, H.G. A Review of Evaporative Cooling Technologies. Int. J. Environ. Sci. Dev. 2014, 6, 111–117. [Google Scholar] [CrossRef]
- Castillo-González, J.; Comino, F.; Navas-Martos, F.J.; de Adana, M.R. Manufacturing and experimental analysis of a dew-point indirect evaporative cooler using fused deposition modelling 3D printing and polymeric materials. Appl. Therm. Eng. 2023, 230, 120683. [Google Scholar] [CrossRef]
- Caruana, R.; De Antonellis, S.; Marocco, L.; Guilizzoni, M. Modeling of Indirect Evaporative Cooling Systems: A Review. Fluids 2023, 8, 303. [Google Scholar] [CrossRef]
- Chen, Q.; Burhan, M.; Ja, M.K.; Shahzad, M.W.; Ybyraiymkul, D.; Zheng, H.; Cui, X.; Ng, K.C. Hybrid Indirect Evaporative Cooling-Mechanical Vapor Compression System: A Mini-Review. Energies 2022, 15, 7810. [Google Scholar] [CrossRef]
- Lu, Z.; Leroy, A.; Zhang, L.; Patil, J.J.; Wang, E.N.; Grossman, J.C. Significantly enhanced sub-ambient passive cooling enabled by evaporation, radiation, and insulation. Cell Rep. Phys. Sci. 2022, 3, 101068. [Google Scholar] [CrossRef]
- Castillo-González, J.; Comino, F.; Navas-Martos, F.J.; De Adana, M.R. Life cycle assessment of an experimental solar HVAC system and a conventional HVAC system. Energy Build. 2022, 256, 111697. [Google Scholar] [CrossRef]
- Harrouz, J.P.; Ghali, K.; Ghaddar, N. Integrated solar–Windcatcher with dew–point indirect evaporative cooler for classrooms. Appl. Therm. Eng. 2021, 188, 116654. [Google Scholar] [CrossRef]
- Pacak, A.; Worek, W. Review of Dew Point Evaporative Cooling Technology for Air Conditioning Applications. Appl. Sci. 2021, 11, 934. [Google Scholar] [CrossRef]
- Yang, H.; Shi, W.; Chen, Y.; Min, Y. Research development of indirect evaporative cooling technology: An updated review. Renew. Sustain. Energy Rev. 2021, 145, 111082. [Google Scholar] [CrossRef]
- Lv, J.; Xu, H.; Zhu, M.; Dai, Y.; Liu, H.; Li, Z. The performance and model of porous materials in the indirect evaporative cooling system: A review. J. Build. Eng. 2021, 41, 102741. [Google Scholar] [CrossRef]
- Duan, Z.; Zhan, C.; Zhang, X.; Mustafa, M.; Zhao, X.; Alimohammadisagvand, B.; Hasan, A. Indirect evaporative cooling: Past, present and future potentials. Renew. Sustain. Energy Rev. 2012, 16, 6823–6850. [Google Scholar] [CrossRef]
- Pineault, H. Wetting Transition on 3D-printed Featured Surface. Univ. Akron 2021. [Google Scholar]
- Cao, Z.; Stevens, M.J.; Carrillo, J.-M.Y.; Dobrynin, A.V. Adhesion and Wetting of Soft Nanoparticles on Textured Surfaces: Transition between Wenzel and Cassie-Baxter States. Langmuir 2015, 31, 1693–1703. [Google Scholar] [CrossRef]
- Daiki, M.; Hiroshi, J.; Atsushi, T. Wetting Transition from the Cassie−Baxter State to the Wenzel State on Textured Polymer Sur-faces. Langmuir 2014, 30, 2061–2067. [Google Scholar] [CrossRef]
- Caruana, R.; De Antonellis, S.; Marocco, L.; Liberati, P.; Guilizzoni, M. Experimental Characterization of the Wettability of Coated and Uncoated Plates for Indirect Evaporative Cooling Systems. Fluids. 2023, 8, 122. [Google Scholar] [CrossRef]
- Wang, X.; Fu, C.; Zhang, C.; Qiu, Z.; Wang, B. A Comprehensive Review of Wetting Transition Mechanism on the Surfaces of Microstructures from Theory and Testing Methods. Materials 2022, 15, 4747. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, Z.; Zhang, Y.; Meng, Q. Impact of climatic factors on evaporative cooling of porous building materials. Energy Build. 2018, 173, 601–612. [Google Scholar] [CrossRef]
- Wang, J.; Meng, Q.; Zhang, L.; Zhang, Y.; He, B.J.; Zheng, S.; Santamouris, M. Impacts of the water absorption capability on the evaporative cooling effect of pervious paving materials. Build. Environ. 2019, 151, 187–197. [Google Scholar] [CrossRef]
- Abada, D.; Maalouf, C.; Sotehi, O.; Rouag-Saffidine, D.; Polidori, G.; Boudjabi, A.F.; Derghout, Z. Performance evaluation of fabrics for evaporative cooling applications. Energy Build 2022, 266, 112120. [Google Scholar] [CrossRef]
- Xu, P.; Ma, X.; Zhao, X.; Fancey, K.S. Experimental investigation on performance of fabrics for indirect evaporative cooling applications. Build. Environ. 2016, 110, 104–114. [Google Scholar] [CrossRef]
- Khalid, O.; Butt, Z.; Tanveer, W.; Rao, H.I. Design and experimental analysis of counter-flow heat and mass exchanger incorpo-rating (M-cycle) for evaporative cooling. Heat Mass Transf. 2016, 53, 1391–1403. [Google Scholar] [CrossRef]
- Felber, R.A.; Nellis, G.; Rudolph, N. Design and modeling of 3D-printed air-cooled heat exchangers. In Proceedings of the 27th Annual International Solid Freeform Fabrication Symposium—An Additive Manufacturing Conference, Austin, TX, USA, 8–10 August 2016; 1763. Available online: http://docs.lib.purdue.edu/iracc/1763 (accessed on 11 July 2016).
- Arie, M.A.; Shooshtari, A.H.; Tiwari, R.; Dessiatoun, S.V.; Ohadi, M.M.; Pearce, J.M. Experimental characterization of heat transfer in an additively manufactured polymer heat exchanger. Appl. Therm. Eng. 2017, 113, 575–584. [Google Scholar] [CrossRef]
- Tarancón, A.; Esposito, V. 3D Printing For Energy Applications; John Wiley Sons, Inc.: Hoboken, NJ, USA, 2012; Available online: https://eur-lex.europa.eu/legal-content/PT/TXT/PDF/?uri=CELEX:32016R0679&from=PT (accessed on 1 June 2021).
- Wang, W.; Lai, H.; Wang, F.; Wang, D.; Li, Y.; Li, S.; Luo, D.; Yamaguchi, T. The feasibility of resistance seam welding as an additive manufacturing technology for Al 1060. J. Mater. Res. Technol. 2024, 30, 6609–6618. [Google Scholar] [CrossRef]
- Rodrigues, S.; Miri, S.; Cole, R.G.; Postigo, A.A.; Saleh, M.A.; Dondish, A.; Melenka, G.W.; Fayazbakhsh, K. Towards optimization of polymer filament tensile test for material extrusion additive manufacturing process. J. Mater. Res. Technol. 2023, 24, 8458–8472. [Google Scholar] [CrossRef]
- Ateeq, M.; Shafique, M.; Azam, A.; Rafiq, M. A review of 3D printing of the recycled carbon fiber reinforced polymer composites: Processing, potential, and perspectives. J. Mater. Res. Technol. 2023, 26, 2291–2309. [Google Scholar] [CrossRef]
- Al Rashid, A.; Khan, S.A.; Al-Ghamdi, S.G.; Koc, M. Additive manufacturing of polymer nanocomposites: Needs and challenges in materials, processes, and applications. J. Mater. Res. Technol. 2021, 14, 910–941. [Google Scholar] [CrossRef]
- Afgan, S.; Ullah, N.; Sulaiman, M.; Ali, I.; Iqbal, T.; Younas, M.; Rezakazemi, M. High strength insulating polymeric composite based on recycled/virgin polyethylene terephthalate (PET) reinforced with hydrous magnesium silicate (talc). J. Mater. Res. Technol. 2022, 21, 3579–3593. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, S.; Riffat, S. Comparative study of heat and mass exchanging materials for indirect evaporative cooling systems. Build. Environ. 2008, 43, 1902–1911. [Google Scholar] [CrossRef]
- Choi, W.J.; Hwang, K.S.; Kwon, H.J.; Lee, C.; Kim, C.H.; Kim, T.H.; Heo, S.W.; Kim, J.-H.; Lee, J.-Y. Rapid development of dual porous poly(lactic acid) foam using fused deposition modeling (FDM) 3D printing for medical scaffold application. Mater. Sci. Eng. C. 2020, 110, 110693. [Google Scholar] [CrossRef] [PubMed]
- Marascio, M.G.M.; Antons, J.; Pioletti, D.P.; Bourban, P.-E. 3D Printing of Polymers with Hierarchical Continuous Porosity. Adv. Mater. Technol. 2017, 2, 1700145. [Google Scholar] [CrossRef]
- Feng, J.; Fu, J.; Shang, C.; Lin, Z.; Niu, X.; Li, B. Efficient generation strategy for hierarchical porous scaffolds with freeform external geometries. Addit. Manuf. 2020, 31, 100943. [Google Scholar] [CrossRef]
- Ishutov, S.; Hasiuk, F.J.; Harding, C.; Gray, J.N. 3D printing sandstone porosity models, Interpret. 3D Vis. 2015, 3, SX49–SX61. [Google Scholar] [CrossRef]
- Mackiewicz, E.; Wejrzanowski, T.; Adamczyk-Cieślak, B.; Oliver, G.J. Polymer–Nickel Composite Filaments for 3D Printing of Open. Materials 2022, 15, 1360. [Google Scholar] [CrossRef]
- S, B.H.; Bonthu, D.; Prabhakar, P.; Doddamani, M. Three-dimensional printed lightweight composite foams. ACS Omega 2020, 5, 22536–22550. [Google Scholar] [CrossRef]
- Babel, B.M.; Massar, C.J.; Day, J. Material Processing of a HIPS/AC FDM Filament for Water Filtration. Ph.D. Thesis, Worcester Polytechnic Institute, Worcester, MA, USA, 2018. [Google Scholar]
- Kalsoom, U.; Hasan, C.K.; Tedone, L.; Desire, C.T.; Li, F.; Breadmore, M.C.; Nesterenko, P.N.; Paull, B. Low-Cost Passive Sampling Device with Integrated Porous Membrane Produced Using Multimaterial 3D Printing. Anal. Chem. 2018, 90, 12081–12089. [Google Scholar] [CrossRef]
- Nofar, M.; Utz, J.; Geis, N.; Altstädt, V.; Ruckdäschel, H. Foam 3D Printing of Thermoplastics: A Symbiosis of Additive Manufacturing and Foaming Technology. Adv. Sci. 2022, 9, 5701. [Google Scholar] [CrossRef]
- Gunasekaran, H.B.; Ponnan, S.; Thirunavukkarasu, N.; Laroui, A.; Wu, L.; Wang, J. Rapid Carbon Dioxide Foaming of 3D Printed Thermoplastic Polyurethane Elastomers. Appl. Polym. Mater. 2022, 4, 1497–1511. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, K.; Wang, K.; Pei, X.; Dong, Z.; Hong, Y.; Zhang, X. Combination of fused deposition modeling and gas foaming technique to fabricated hierarchical macro/microporous polymer scaffolds. Mater. Des. 2016, 109, 415–424. [Google Scholar] [CrossRef]
- Zhang, J.; Li, D.; Zhu, W.; Li, Y.; Zhu, W. In situ 3D printing of poly-ether-ether-ketone/poly-ether-imide hierarchical cellular foams containing electromagnetic absorbent. Addit. Manuf. 2022, 59, 103181. [Google Scholar] [CrossRef]
- Tammaro, D.; Gatta, R.D.; Villone, M.M.; Maffettone, P.L. Continuous 3D Printing of Hierarchically Structured Microfoamed Objects. Adv. Eng. Mater. 2021, 24, 1226. [Google Scholar] [CrossRef]
- Li, M.; Jiang, J.; Hu, B.; Zhai, W. Fused deposition modeling of hierarchical porous polyetherimide assisted by an in-situ CO2 foaming technology. Compos. Sci. Technol. 2020, 200, 108454. [Google Scholar] [CrossRef]
- Gama, N.; Ferreira, A.; Barros-Timmons, A. 3D printed cork/polyurethane composite foams. Mater. Des. 2019, 179, 107905. [Google Scholar] [CrossRef]
- Tammaro, D.; Villone, M.M.; Maffettone, P.L. Microfoamed Strands by 3D Foam Printing. Polymers 2022, 14, 3214. [Google Scholar] [CrossRef] [PubMed]
- Johnston, W.; Sharma, B. Additive manufacturing of fibrous sound absorbers. Addit. Manuf. 2021, 41, 101984. [Google Scholar] [CrossRef]
- Shigueoka, M.O.; Volpato, N. Expanding manufacturing strategies to advance in porous media planning with material extrusion additive manufacturing. Addit. Manuf. 2021, 38, 101760. [Google Scholar] [CrossRef]
- Ayrilmis, N.; Kariz, M.; Kwon, J.H.; Kuzman, M.K. Effect of printing layer thickness on water absorption and mechanical properties of 3D-printed wood/PLA composite materials. Int. J. Adv. Manuf. Technol. 2019, 102, 2195–2200. [Google Scholar] [CrossRef]
- Duigou, A.L.; Castro, M.; Bevan, R.; Martin, N. 3D printing of wood fibre biocomposites: From mechanical to actuation functionality. Mater. Des. 2016, 96, 106–114. [Google Scholar] [CrossRef]
- Rodríguez, J.; Restrepo, D. Elaboración y Caracterización de Componentes Plásticos Porosos, Mediante Impresión 3D Para Aplicaciones de Regeneración Ósea. Ph.D. Thesis, Universidad Santo Tomás, Bogotá, Colombia, 2015. [Google Scholar] [CrossRef]
- Zeng, Z.; Song, P.; Gui, X.; Zhang, B.; Zhao, L.; Feng, P.; Deng, Z.; Wang, L.; Wei, W.; Fan, C.; et al. Song, 3D printed Nanohydroxyapatite/Polyamide 66 scaffolds with balanced mechanical property and osteogenic ability for bone repair. Mater. Des. 2024, 241, 112896. [Google Scholar] [CrossRef]
- Kharaghani, A. Drying and Wetting of Capillary Porous Materials: Insights from Imaging and Pysics-Based Modeling. Ph.D. Thesis, Otto-von-Guericke-Universität Magdeburg, Magdeburg, Germany, 2020. [Google Scholar]
- Berg, J.C. An Introduction to Interfaces and Colloids, Bridg to Nanosci. World Sci. 2009, 39–155. [Google Scholar] [CrossRef]
- UNE-EN ISO 62:2008; Plastic—Water Absorption Determination. ISO: Geneva, Switzerland, 2008.
- Caruana, R.; Marocco, L.; Liberati, P.; Guilizzoni, M. Experimental Analysis of the Effect of Limescale on the Wettability of Indirect Evaporative Cooling System Plates. Fluids 2024, 9, 76. [Google Scholar] [CrossRef]
- Santini, M.; Guilizzoni, M.; Fest-Santini, S.; Lorenzi, M. A novel technique for investigation of complete and partial anisotropic wetting on structured surface by X-ray microtomography. Rev. Sci. Instrum. 2015, 86, 1–10. [Google Scholar] [CrossRef]
- Guilizzoni, M. Drop shape visualization and contact angle measurement on curved surfaces. J. Colloid Interface Sci. 2011, 364, 230–236. [Google Scholar] [CrossRef]
- Guilizzoni, M.; Sapienza, J. Axisymmetric Drop Shape Analysis using a low-cost home-made setup. J. Phys. Conf. Ser. 2021, 1977, 012003. [Google Scholar] [CrossRef]
- Santini, M.; Guilizzoni, M. 3D X-ray Micro Computed Tomography on Multiphase Drop Interfaces: From Biomimetic to Functional Applications. Colloids Interface Sci. Commun. 2014, 1, 14–17. [Google Scholar] [CrossRef][Green Version]
- Guilizzoni, M.; Santini, M.; Lorenzi, M.; Knisel, V.; Fest-Santini, S. Micro computed tomography and CFD simulation of drop deposition on gas diffusion layers. J. Phys. Conf. Ser. 2014, 547, 012028. [Google Scholar] [CrossRef]
- Galindo, S.; Ureña-Núñez, F. Enhanced surface hydrophobicity of poly(lactic acid) by Co60 gamma ray irradiation. Rev. Mex. Fis. 2018, 64, 1–7. [Google Scholar] [CrossRef]
- Kingman, J.; Dymond, M.K. Fused filament fabrication and water contact angle anisotropy: The effect of layer height and raster width on the wettability of 3D printed polylactic acid parts. Chem. Data Collect. 2022, 40, 100884. [Google Scholar] [CrossRef]
- Erbil, H.Y. Evaporation of pure liquid sessile and spherical suspended drops: A review. Adv. Colloid Interface Sci. 2012, 170, 67–86. [Google Scholar] [CrossRef]
- Teng, L.; Wang, W.; Huang, X.; Luo, X.; Li, W.; Li, J.; Yin, P.; Luo, Y.; Jiang, L. Evaporation of sessile droplet on surfaces with various wettability. Chem. Eng. Sci. 2023, 268, 118413. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Li, L. Investigation of the falling water flow with evaporation for the passive containment cooling system and its scaling-down criteria. Heat Mass Transf. 2018, 54, 473–482. [Google Scholar] [CrossRef]

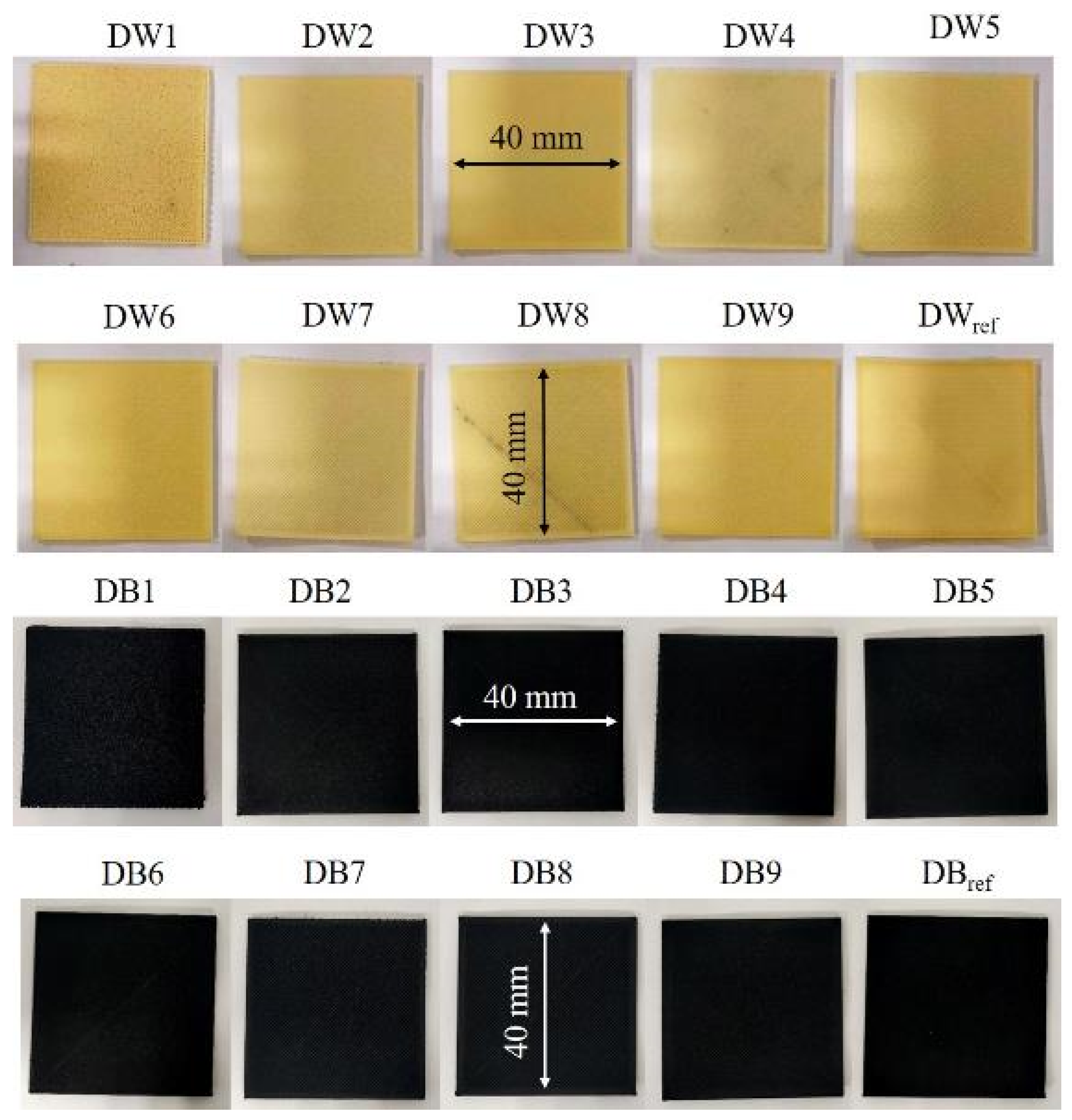
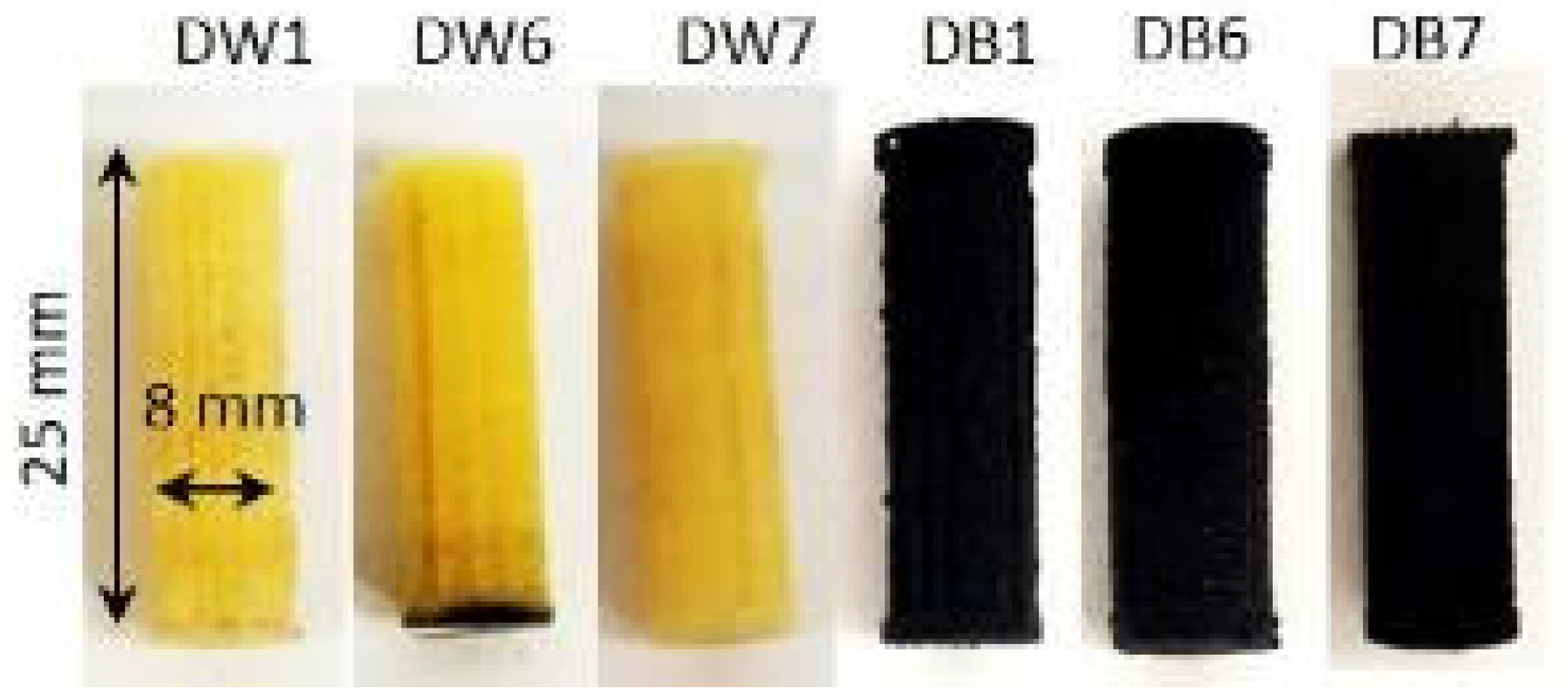



| Sample | Dref | D1 | D2 | D3 | D4 | D5 | D6 | D7 | D8 | D9 |
|---|---|---|---|---|---|---|---|---|---|---|
| Sample reference | DWref DBref | DW1 DB1 | DW2 DB2 | DW3 DB3 | DW4 DB4 | DW5 DB5 | DW6 DB6 | DW7 DB7 | DW8 DB8 | DW9 DB9 |
| LW (mm) | 0.4 | 0.4 | 0.4 | 0.4 | 0.6 | 0.6 | 0.6 | 0.8 | 0.8 | 0.8 |
| F (%) | 100 | 50 | 70 | 90 | 50 | 70 | 90 | 50 | 70 | 90 |
| S (mm/s) | 40 | 40 | 60 | 80 | 60 | 80 | 40 | 80 | 60 | 40 |
| LH (mm) | 0.2 | 0.2 | 0.3 | 0.4 | 0.4 | 0.2 | 0.3 | 0.3 | 0.4 | 0.2 |
| T (°C) | 210 | 250 | 250 | 250 | 250 | 250 | 250 | 250 | 250 | 250 |
| CH-DW | Im-DW | CH-DB | Im-DB | |
|---|---|---|---|---|
| LW (mm) | 0.006 | 0.033 | 0.582 | 0.542 |
| F (%) | 0.005 | 0.003 | 0.047 | 0.008 |
| S (mm/s) | 0.378 | 0.375 | 0.567 | 0.729 |
| LH (mm) | 0.017 | 0.154 | 0.963 | 0.736 |
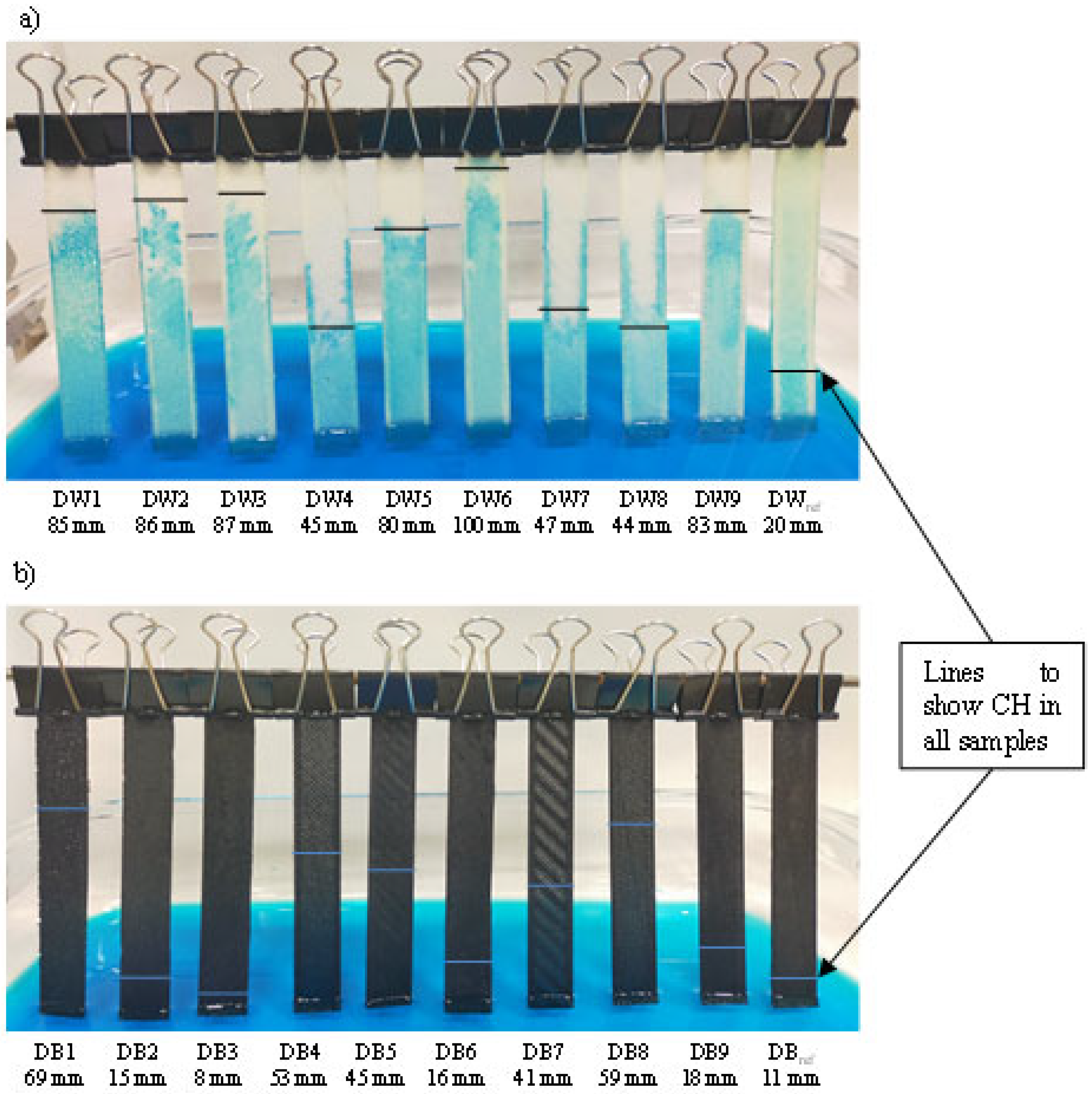
| Specimen | CH [mm] | = 60°) [μm] | Macropores at Visual Observation? |
|---|---|---|---|
| DW1 | 85 | 86 | yes |
| DW2 | 86 | 85 | yes |
| DW3 | 87 | 84 | yes |
| DW4 | 45 | 163 | yes |
| DW5 | 80 | 92 | yes |
| DW6 | 100 | 73 | yes |
| DW7 | 47 | 156 | yes |
| DW8 | 44 | 167 | yes |
| DW9 | 83 | 88 | yes |
| DWref | 20 | 367 | no |
| DB1 | 69 | 106 | yes |
| DB2 | 15 | 489 | no |
| DB3 | 8 | 917 | no |
| DB4 | 53 | 138 | yes |
| DB5 | 45 | 163 | yes |
| DB6 | 16 | 459 | no |
| DB7 | 41 | 179 | yes |
| DB8 | 59 | 124 | yes |
| DB9 | 18 | 407 | no |
| DBref | 11 | 667 | no |
| Parameter | DW1 | DW6 | DW7 | DB1 | DB6 | DB7 |
|---|---|---|---|---|---|---|
| SA (m2/g) | 1.17 | 0.54 | 0.86 | 0.81 | 0.42 | 0.80 |
| PVA (cm3/g) | 0.00087 | 0.00041 | 0.00071 | 0.00061 | 0.00032 | 0.00057 |
| PVD × 105 (cm3/g) | 22 | 12 | 15 | 14 | 6 | 13 |
| APWA (Å) | 29.73 | 30.05 | 32.71 | 31.54 | 30.79 | 28.16 |
| APWD (Å) | 7.55 | 8.41 | 6.75 | 6.93 | 5.74 | 6.45 |
| Specimen | θCB [°] | [-] |
|---|---|---|
| DW1 | 105.2 | 0.492 |
| DW6 | 83.2 | 0.746 |
| DW7 | 111.1 | 0.427 |
| DB1 | 100.8 | 0.542 |
| DB6 | 78.8 | 0.796 |
| DB7 | 108.2 | 0.458 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo-González, J.; Comino, F.; Caruana, R.; Guilizzoni, M.; Conrat, P.; Ruiz de Adana, M.; Navas-Martos, F.J. Development of Innovative Thermoplastic Foam Materials Using Two Additive Manufacturing Technologies for Application in Evaporative Cooling Systems. Polymers 2024, 16, 3190. https://doi.org/10.3390/polym16223190
Castillo-González J, Comino F, Caruana R, Guilizzoni M, Conrat P, Ruiz de Adana M, Navas-Martos FJ. Development of Innovative Thermoplastic Foam Materials Using Two Additive Manufacturing Technologies for Application in Evaporative Cooling Systems. Polymers. 2024; 16(22):3190. https://doi.org/10.3390/polym16223190
Chicago/Turabian StyleCastillo-González, Jesús, Francisco Comino, Roberta Caruana, Manfredo Guilizzoni, Paula Conrat, Manuel Ruiz de Adana, and Francisco J. Navas-Martos. 2024. "Development of Innovative Thermoplastic Foam Materials Using Two Additive Manufacturing Technologies for Application in Evaporative Cooling Systems" Polymers 16, no. 22: 3190. https://doi.org/10.3390/polym16223190
APA StyleCastillo-González, J., Comino, F., Caruana, R., Guilizzoni, M., Conrat, P., Ruiz de Adana, M., & Navas-Martos, F. J. (2024). Development of Innovative Thermoplastic Foam Materials Using Two Additive Manufacturing Technologies for Application in Evaporative Cooling Systems. Polymers, 16(22), 3190. https://doi.org/10.3390/polym16223190









