New Advances on the Dispersive and Polar Surface Properties of Poly(styrene-co-butadiene) Using Inverse Gas Chromatography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solvents and Materials
- -
- The Lewis’s acid solvents such as: chloroform (DN = 0, AN = 22.54) and dichloromethane (DN = 5.02, AN = 16.27)
- -
- The amphoteric solvents such as: benzene (DN = 0.42, AN = 0.72), ethanol (DN = 80.35, AN = 43.27), acetonitrile (DN = 59.01, AN = 19.65), and toluene (DN = 16.32, AN = 3.98)
- -
- The Lewis’s base solvents such as: acetone (DN = 71.15, AN = 10.49), ethyl acetate (DN = 71.56, AN = 6.39), diethyl ether (DN = 80.35, AN = 5.91), cyclohexane (DN = 5.89, AN = 0.17), and tetrahydrofuran (THF) (DN = 83.70, AN = 2.29)
2.2. Inverse Gas Chromatography
2.3. Thermodynamic Methods
3. Experimental Results
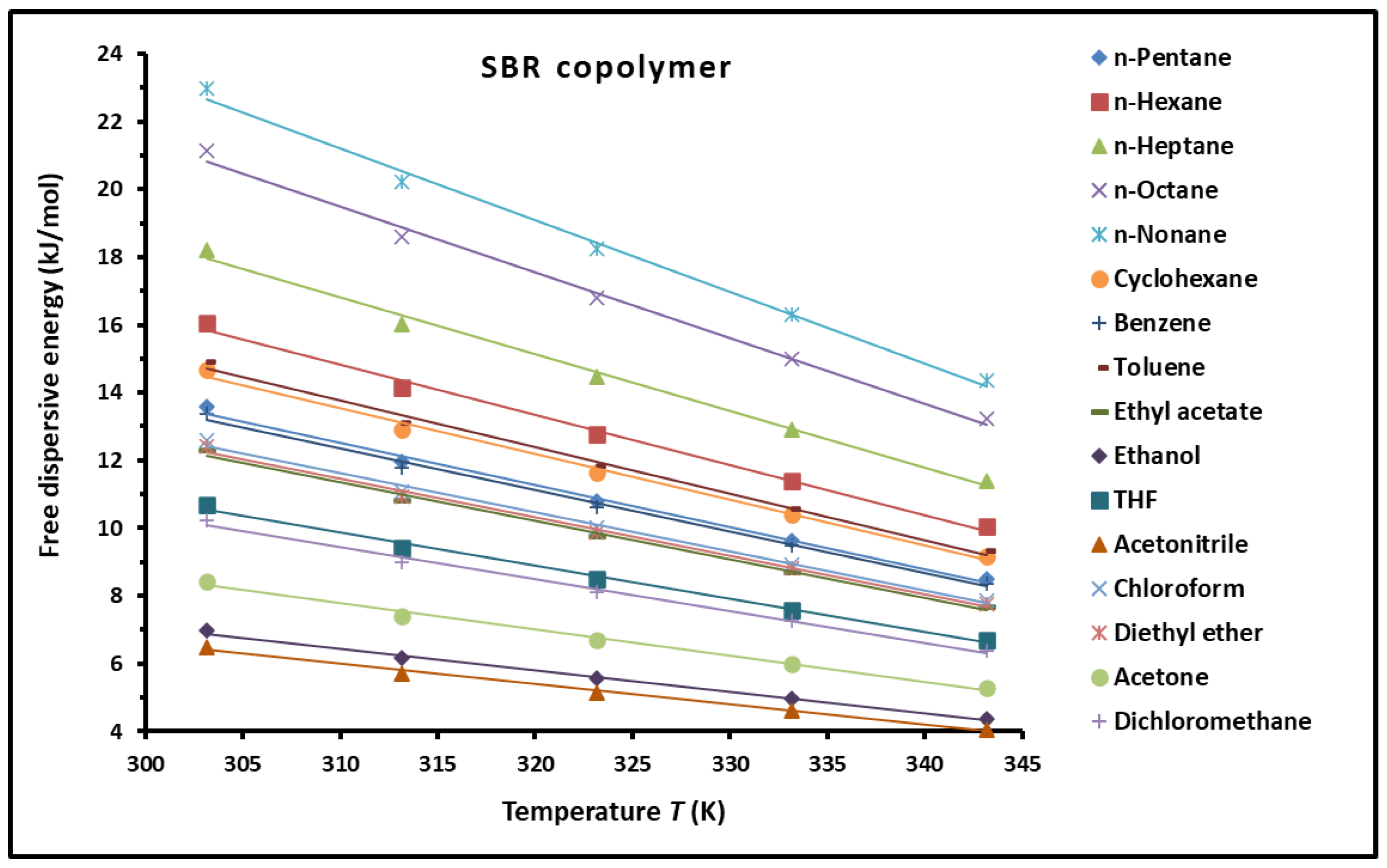
3.1. London Dispersive Surface Energy of the SBR Copolymer
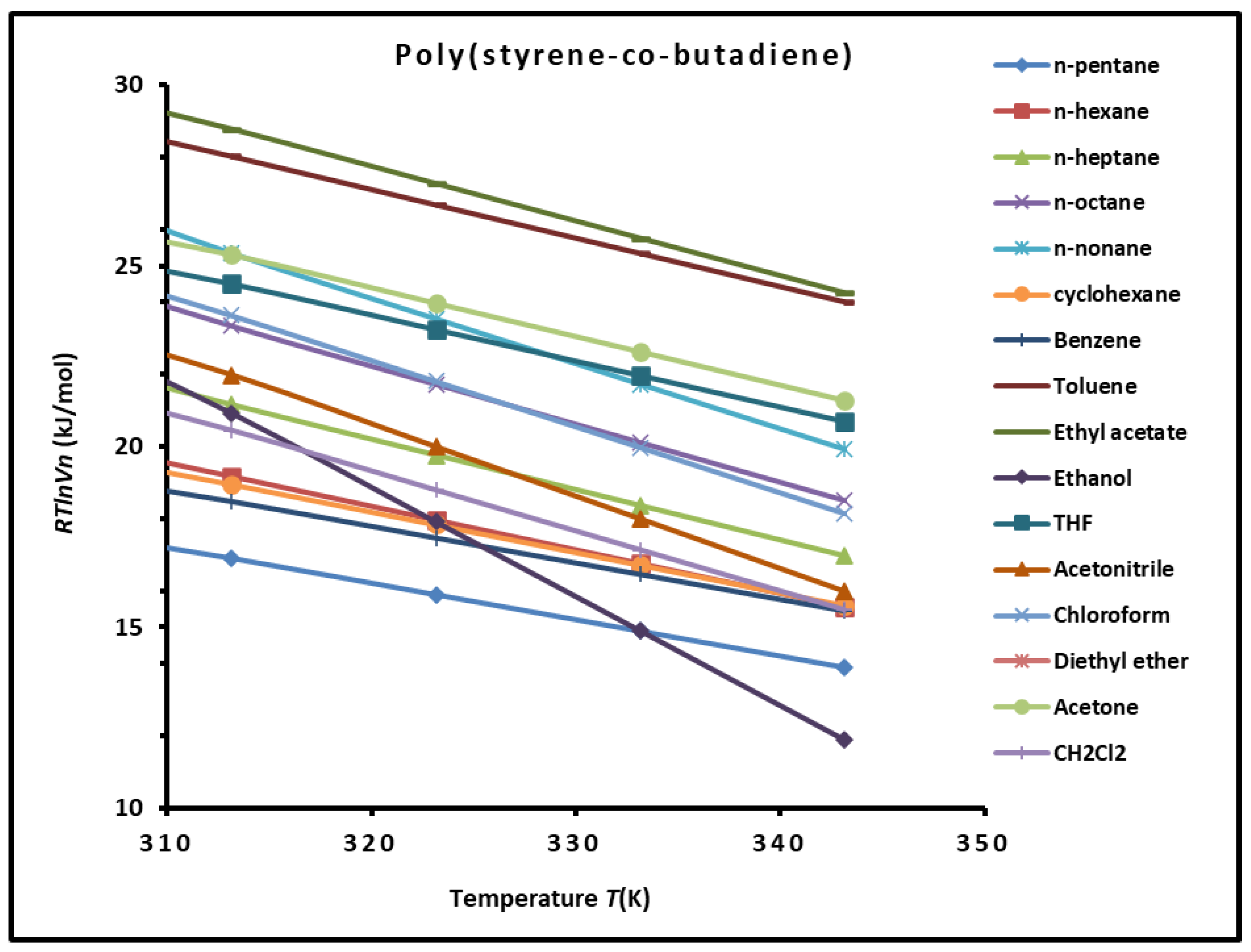
3.2. Variations of Polar Free Surface Energy () of SBR Copolymer
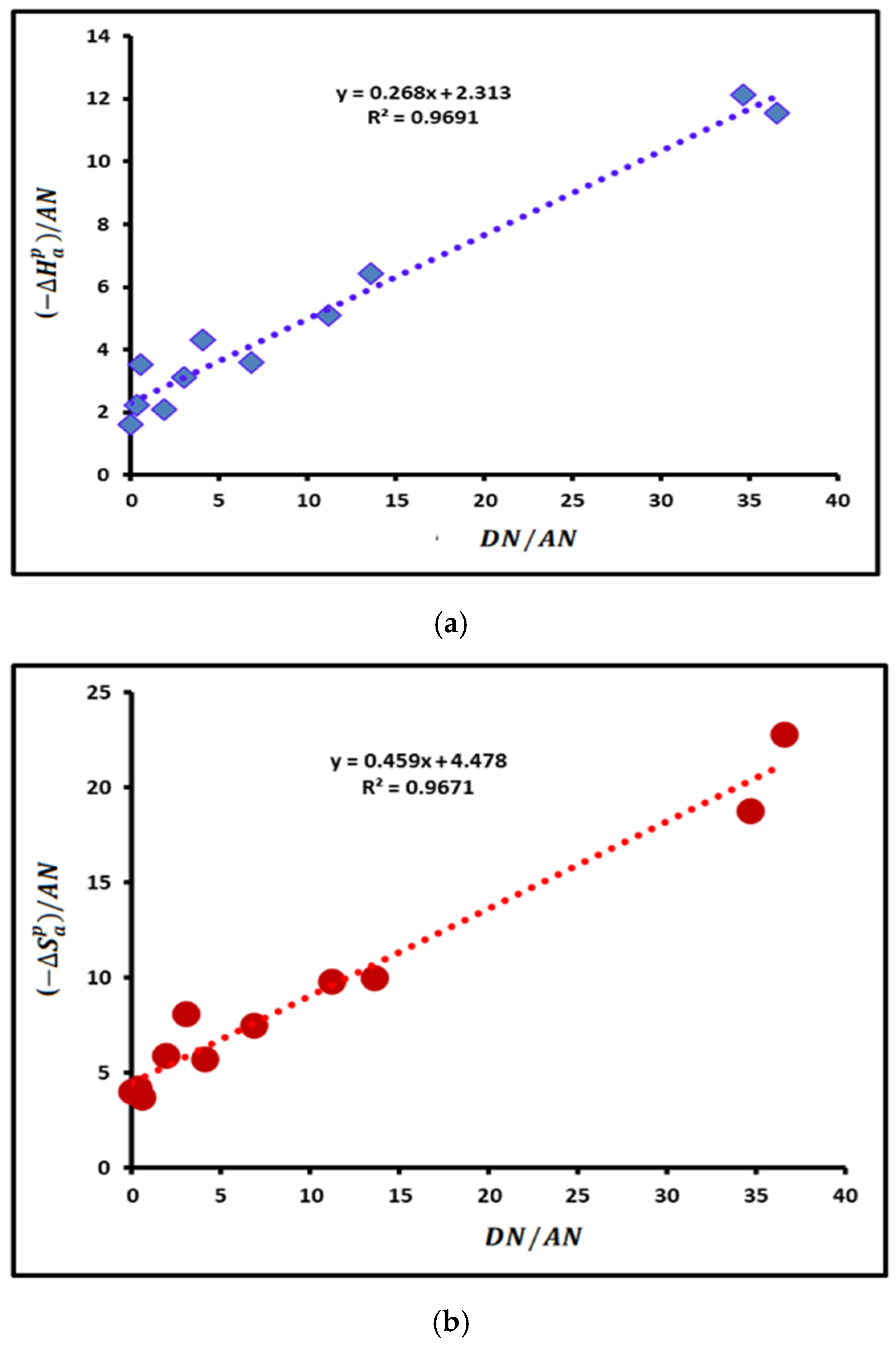
3.3. Lewis Acid–Base Constants of Poly(styrene-co-butadiene)
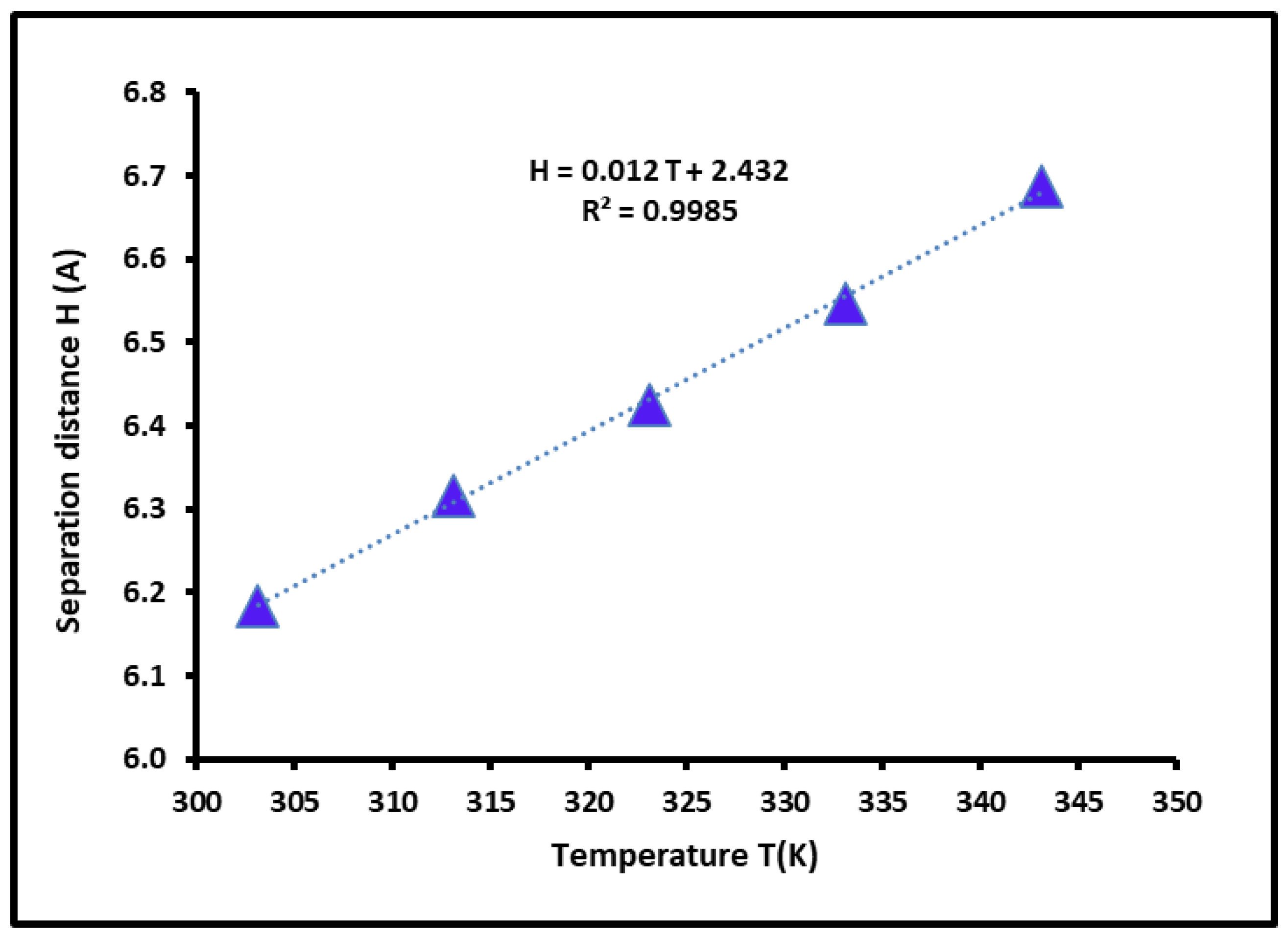
3.4. Dispersive Free Energy and Interaction Distance Between the Solvents and SBR Copolymer
3.5. Determination of Polar Acid–Base Surface Energies and Total Surface Energy of the Copolymer
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ovejero, G.; Romero, M.D.; Díez, E.; Díaz, I. Thermodynamic interactions of three SBS (styrene–butadiene–styrene) triblock copolymers with different solvents, by means of intrinsic viscosity measurements. Eur. Polym. J. 2010, 46, 2261–2268. [Google Scholar] [CrossRef]
- Smith, W.F.; Hashemi, J. Foundations of Materials Science and Technology; McGraw-Hill: Mexico City, Mexico, 2006. [Google Scholar]
- Zuo, B.; Inutsuka, M.; Kawaguchi, D.; Wang, X.; Tanaka, K. Conformational Relaxation of Poly(styrene-co-butadiene) Chains at Substrate Interface in Spin-Coated and Solvent-Cast Films. Macromolecules 2018, 51, 2180–2186. [Google Scholar] [CrossRef]
- Jancar, J.; Douglas, J.; Starr, F.; Kumar, S.; Cassagnau, P.; Lesser, A.; Sternstein, S.; Buehler, M. Current issues in research on structure–property relationships in polymer nanocomposites. Polymer 2010, 51, 3321–3343. [Google Scholar] [CrossRef]
- Song, Y.; Zheng, Q. Concepts and conflicts in nanoparticles reinforcement to polymers beyond hydrodynamics. Prog. Mater. Sci. 2016, 84, 1–58. [Google Scholar] [CrossRef]
- Boonstra, B. Role of particulate fillers in elastomer reinforcement: A review. Polymer 1979, 20, 691–704. [Google Scholar] [CrossRef]
- Edwards, D.C. Polymer-filler interactions in rubber reinforcement. J. Mater. Sci. 1990, 25, 4175–4185. [Google Scholar] [CrossRef]
- Balazs, A.C.; Emrick, T.; Russell, T.P. Nanoparticle Polymer Composites: Where Two Small Worlds Meet. Science 2006, 314, 1107–1110. [Google Scholar] [CrossRef]
- Kumar, S.K.; Benicewicz, B.C.; Vaia, R.A.; Winey, K.I. 50th Anniversary Perspective: Are Polymer Nanocomposites Practical for Applications? Macromolecules 2017, 50, 714–731. [Google Scholar] [CrossRef]
- Radhakrishnan, C.K.; Sujith, A.; Unnikrishnan, G. Thermal behaviour of styrene butadiene rubber/poly(ethylene-co-vinyl acetate) blends. J. Therm. Anal. Calorim. 2007, 90, 191–199. [Google Scholar] [CrossRef]
- McNeill, I.C. Thermal Degradation. In Comprehensive Polymer Science; Allen, G., Ed.; Pergamon Press: New York, NY, USA, 1989; Chapter 15; Volume 6. [Google Scholar]
- Rybiński, P.; Janowska, G.; Antkowicz, W.; Krauze, S. Thermal stability and flammability of butadiene- acrylonitrile rubber cross-linked with iodoform. J. Therm. Anal. Calorim. 2005, 81, 9–13. [Google Scholar] [CrossRef]
- Vo, L.T.; Anastasiadis, S.H.; Giannelis, E.P. Dielectric study of Poly(styrene-co-butadiene) Composites with Carbon Black, Silica, and Nanoclay. Macromolecules 2011, 44, 6162–6171. [Google Scholar] [CrossRef]
- Posadas, P.; Bernal-Ortega, P.; Bernal, M.M.; Nogales, A.; Navarro, R.; Valentín, J.L. From Nanoscale to Macroscale Characterization of Sulfur-Modified Oxidized Carbon Nanotubes in Styrene Butadiene Rubber Compounds. ACS Omega 2024, 9, 31669–31683. [Google Scholar] [CrossRef] [PubMed]
- Clément, F.; Lapra, A.; Bokobza, L.; Monnerie, L.; Ménez, P. Atomic force microscopy investigation of filled elastomers and comparison with transmission electron microscopy—Application to silica-filled silicone elastomers. Polymer 2001, 42, 6259–6270. [Google Scholar] [CrossRef]
- Špírková, M.; Pavličević, J.; Strachota, A.; Poreba, R.; Bera, O.; Kaprálková, L.; Baldrian, J.; Šlouf, M.; Lazić, N.; Budinski-Simendić, J. Novel polycarbonate-based polyurethane elastomers: Composition–property relationship. Eur. Polym. J. 2011, 47, 959–972. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, Y.; Liang, J.; Yu, S. New Fabrication and Mechanical Properties of Styrene-Butadiene Rubber/Carbon Nanotubes Nanocomposite. J. Mater. Sci. Technol. 2010, 26, 1127–1132. [Google Scholar] [CrossRef]
- Bertora, A.; Castellano, M.; Marsano, E.; Alessi, M.; Conzatti, L.; Stagnaro, P.; Colucci, G.; Priola, A.; Turturro, A. A New Modifier for Silica in Reinforcing SBR Elastomers for the Tyre Industry. Macromol. Mater. Eng. 2011, 296, 455–464. [Google Scholar] [CrossRef]
- Ward, A.A.; Khalf, A.I. Electrical and Mechanical Properties of SBR Filled with Carbon Black-Silica Blends. Kautsch. Gummi Kunstst. 2007, 60, 623–630. [Google Scholar]
- Sae-Oui, P.; Suchiva, K.; Sirisinha, C.; Intiya, W.; Yodjun, P.; Thepsuwan, U. Effects of Blend Ratio and SBR Type on Properties of Carbon Black-Filled and Silica-Filled SBR/BR Tire Tread Compounds. Adv. Mater. Sci. Eng. 2017, 2017, 2476101. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Biswas, S.; Bhowmick, A.K. Permeation characteristics and modeling of barrier properties of multifunctional rubber nanocomposites. Polymer 2011, 52, 1562–1576. [Google Scholar] [CrossRef]
- Stephen, R.; Ranganathaiah, C.; Varghese, S.; Joseph, K.; Thomas, S. Gas transport through nano and micro composites of natural rubber (NR) and their blends with carboxylated styrene butadiene rubber (XSBR) latex membranes. Polymer 2006, 47, 858–870. [Google Scholar] [CrossRef]
- Lu, Y.-L.; Li, Z.; Yu, Z.-Z.; Tian, M.; Zhang, L.-Q.; Mai, Y.-W. Microstructure and properties of highly filled rubber/clay nanocomposites prepared by melt blending. Compos. Sci. Technol. 2007, 67, 2903–2913. [Google Scholar] [CrossRef]
- Peddini, S.; Bosnyak, C.; Henderson, N.; Ellison, C.; Paul, D. Nanocomposites from styrene–butadiene rubber (SBR) and multiwall carbon nanotubes (MWCNT) part 2: Mechanical properties. Polymer 2015, 56, 443–451. [Google Scholar] [CrossRef]
- Bokobza, L.; Bresson, B.; Garnaud, G.; Zhang, J. Mechanical and AFM investigations of elastomers filled with multiwall carbon nanotubes. Compos. Interfaces 2012, 19, 285–295. [Google Scholar] [CrossRef]
- Díez, E.; Ovejero, G.; Romero, M.D.; Díaz, I. Polymer–solvent interaction parameters of SBS rubbers by inverse gas chromatography measurements. Fluid Phase Equilibria 2011, 308, 107–113. [Google Scholar] [CrossRef]
- Benguergoura, H.; Allel, A.; Saeed, W.S.; Aouak, T. Capillary column inverse gas chromatography to determine the thermodynamic parameters of binary solvent poly (styrene-block-butadiene) rubber systems. Arab. J. Chem. 2021, 14, 103040. [Google Scholar] [CrossRef]
- Farshchi, N.; Abbasian, A.; Larijani, K. Is Inverse Gas Chromatography (IGC) a Convenient Method to Determine Compatibility of Rubber Materials? Chromatographia 2019, 82, 1709–1719. [Google Scholar] [CrossRef]
- Sawyer, D.T.; Brookman, D.J. Thermodynamically based gas chromatographic retention index for organic molecules using salt-modified aluminas and porous silica beads. Anal. Chem. 1968, 40, 1847–1850. [Google Scholar] [CrossRef]
- Conder, J.R.; Young, C.L. Physical Measurements by Gas Chromatography; John Wiley & Sons: Hoboken, NJ, USA, 1979; p. 632. [Google Scholar]
- Schultz, J.; Lavielle, L.; Martin, C. The Role of the Interface in Carbon Fibre-Epoxy Composites. J. Adhes. 1987, 23, 45–60. [Google Scholar] [CrossRef]
- Flour, C.S.; Papirer, E. Gas-solid chromatography. A method of measuring surface free energy characteristics of short carbon fibers. 1. Through adsorption isotherms. Ind. Eng. Chem. Prod. Res. Dev. 1982, 21, 337–341. [Google Scholar] [CrossRef]
- Flour, C.S.; Papirer, E. Gas-solid chromatography: Method of measuring surface free energy characteristics of short fibers. 2. Through retention volumes measured near zero surface coverage. Ind. Eng. Chem. Prod. Res. Dev. 1982, 21, 666–669. [Google Scholar] [CrossRef]
- Donnet, J.B.; Park, S.J.; Balard, H. Evaluation of specific interactions of solid surfaces by inverse gas chromatography. Chromatographia 1991, 31, 434–440. [Google Scholar] [CrossRef]
- Brendlé, E.; Papirer, E. A New Topological Index for Molecular Probes Used in Inverse Gas Chromatography for the Surface Nanorugosity Evaluation, 1. Method of Evaluation. J. Colloid Interface Sci. 1997, 194, 207–216. [Google Scholar] [CrossRef]
- Brendlé, E.; Papirer, E. A New Topological Index for Molecular Probes Used in Inverse Gas Chromatography. J. Colloid Interface Sci. 1997, 194, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Hamieh, T.; Schultz, J. New approach to characterise physicochemical properties of solid substrates by inverse gas chromatography at infinite dilution: I. Some new methods to determine the surface areas of some molecules adsorbed on solid surfaces. J. Chromatogr. A 2002, 969, 17–47. [Google Scholar] [CrossRef] [PubMed]
- Voelkel, A. Inverse Gas Chromatography: Characterization of Polymers, Fibers, Modified Silicas, and Surfactants. Crit. Rev. Anal. Chem. 1991, 22, 411–439. [Google Scholar] [CrossRef]
- Papadopoulou, S.K.; Panayiotou, C. Assessment of the thermodynamic properties of poly(2,2,2-trifluoroethyl methacrylate) by inverse gas chromatography. J. Chromatogr. A 2014, 1324, 207–214. [Google Scholar] [CrossRef]
- Hamieh, T. Study of the temperature effect on the surface area of model organic molecules, the dispersive surface energy and the surface properties of solids by inverse gas chromatography. J. Chromatogr. A 2020, 1627, 461372. [Google Scholar] [CrossRef]
- Hamieh, T. New Methodology to Study the Dispersive Component of the Surface Energy and Acid–Base Properties of Silica Particles by Inverse Gas Chromatography at Infinite Dilution. J. Chromatogr. Sci. 2021, 60, 126–142. [Google Scholar] [CrossRef]
- Hamieh, T.; Ahmad, A.A.; Roques-Carmes, T.; Toufaily, J. New approach to determine the surface and interface thermodynamic properties of H-β-zeolite/rhodium catalysts by inverse gas chromatography at infinite dilution. Sci. Rep. 2020, 10, 20894. [Google Scholar] [CrossRef]
- Hamieh, T. Some Irregularities in the Evaluation of Surface Parameters of Solid Materials by Inverse Gas Chromatography. Langmuir 2023, 39, 17059–17070. [Google Scholar] [CrossRef]
- Fowkes, F.M. Surface and Interfacial Aspects of Biomedical Polymers; Andrade, J.D., Ed.; Plenum Press: New York, NY, USA, 1985; Volume I, pp. 337–372. [Google Scholar]
- Hamieh, T. New physicochemical methodology for the determination of the surface thermodynamic properties of solid particles. Appl. Chem. 2023, 3, 229–255. [Google Scholar] [CrossRef]
- Hamieh, T. New Progress on London Dispersive Energy, Polar Surface Interactions, and Lewis’s Acid–Base Properties of Solid Surfaces. Molecules 2024, 29, 949. [Google Scholar] [CrossRef] [PubMed]
- Hamieh, T. London Dispersive and Lewis Acid-Base Surface Energy of 2D Single-Crystalline and Polycrystalline Covalent Organic Frameworks. Crystals 2024, 14, 148. [Google Scholar] [CrossRef]
- Hamieh, T. Inverse Gas Chromatography to Characterize the Surface Properties of Solid Materials. Chem. Mater. 2024, 36, 2231–2244. [Google Scholar] [CrossRef]
- Hamieh, T. Effect of Tacticity on London Dispersive Surface Energy, Polar Free Energy and Lewis Acid-Base Surface Energies of Poly Methyl Methacrylate by Inverse Gas Chromatography. Macromol 2024, 4, 356–375. [Google Scholar] [CrossRef]
- Hamieh, T. The Effect of Temperature on the London Dispersive and Lewis Acid-Base Surface Energies of Polymethyl Methacrylate Adsorbed on Silica by Inverse Gas Chromatography. Thermo 2024, 4, 202–221. [Google Scholar] [CrossRef]
- Hamieh, T. Temperature Dependence of the Polar and Lewis Acid–Base Properties of Poly Methyl Methacrylate Adsorbed on Silica via Inverse Gas Chromatography. Molecules 2024, 29, 1688. [Google Scholar] [CrossRef]
- Gutmann, V. The Donor-Acceptor Approach to Molecular Interactions; Plenum: New York, NY, USA, 1978. [Google Scholar]
- Riddle, F.L.; Fowkes, F.M. Spectral shifts in acid-base chemistry. Van der Waals contributions to acceptor numbers, Spectral shifts in acid-base chemistry. 1. van der Waals contributions to acceptor numbers. J. Am. Chem. Soc. 1990, 112, 3259–3264. [Google Scholar] [CrossRef]
- Dorris, G.M.; Gray, D.G. Adsorption of n-alkanes at zero surface coverage on cellulose paper and wood fibers. J. Colloid Interface Sci. 1980, 77, 353–362. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Good, R.J.; Chaudhury, M.K. Additive and nonadditive surface tension components and the interpretation of contact angles. Langmuir 1988, 4, 884–891. [Google Scholar] [CrossRef]
- Hamieh, T. Exploring the Application of Advanced Chromatographic Methods to Characterize the Surface Physicochemical Properties and Transition Phenomena of Polystyrene-b-poly(4-vinylpyridine). Molecules 2024, 29, 4812. [Google Scholar] [CrossRef]


| Molecular Model | (mJ/m2) | (mJ m−2 K−1) | (mJ/m2) | (K) | R2 |
|---|---|---|---|---|---|
| Kiselev | = −0.43 T + 158.83 | −0.43 | 158.83 | 367.66 | 0.9809 |
| Cylindrical | = −0.40 T + 148.22 | −0.40 | 148.22 | 368.61 | 0.9815 |
| VDW | = −0.45 T + 165.22 | −0.45 | 165.22 | 367.40 | 0.9808 |
| Geometric | = −0.29 T + 108.25 | −0.29 | 108.25 | 370.09 | 0.9821 |
| Redlich-Kwong | = −0.74 T + 271.53 | −0.74 | 271.53 | 368.28 | 0.9811 |
| Spherical | = −1.32 T + 483.45 | −1.32 | 483.45 | 365.59 | 0.9795 |
| Dorris–Gray | = −0.45 T + 165.93 | −0.45 | 165.93 | 371.13 | 0.9817 |
| Gray-Hamieh | = −0.60 T + 219.89 | −0.60 | 219.89 | 366.91 | 0.9799 |
| Hamieh | = −0.59 T + 215.36 | −0.59 | 215.36 | 365.45 | 0.9793 |
| Global average | = −0.59 T + 215.19 | −0.59 | 215.19 | 367.5 | 0.9824 |
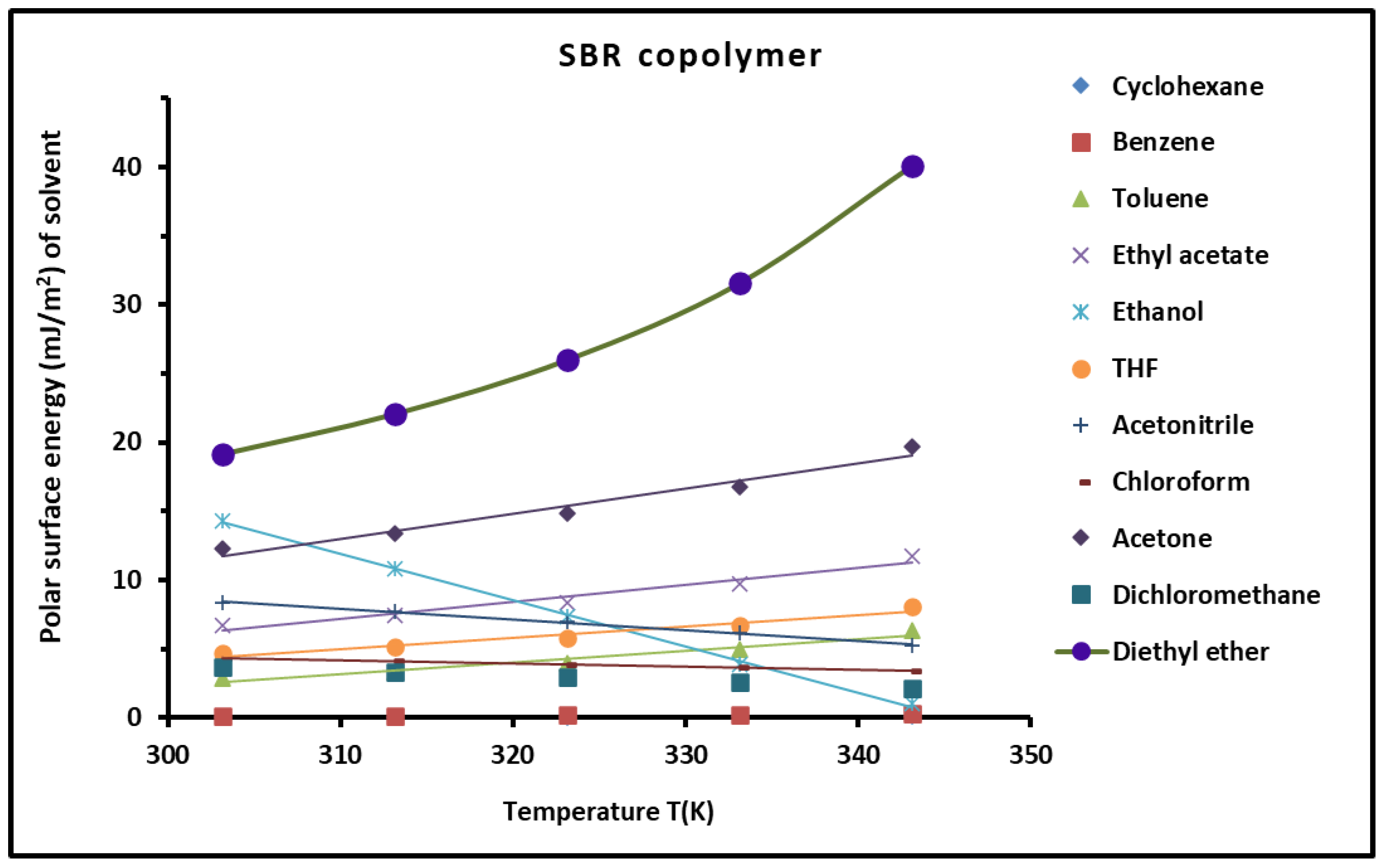
| Probes | Equation of ( (kJ/mol)) |
|---|---|
| Cyclohexane | () = −0.0032T + 2.060 |
| Benzene | () = −0.0027T + 2.542 |
| Toluene | () = −0.023T + 17.108 |
| Ethyl acetate | ) = −0.0627T + 32.598 |
| Ethanol | () = 0.2574T +90.377 |
| THF | () = −0.0522T + 26.434 |
| Acetonitrile | () = −0.1103T + 91.466 |
| Chloroform | () = 0.0918T + 76.300 |
| Diethyl ether | () = −0.0593T + 37.928 |
| Acetone | () = −0.0791T + 57.669 |
| Dichloromethane | () = −0.0689T + 36.204 |
| Probes | (J/k.mol) | (kJ/mol) |
|---|---|---|
| Cyclohexane | 3.2 | 2.060 |
| Benzene | 2.7 | 2.542 |
| Toluene | 23.0 | 17.108 |
| Ethyl acetate | 62.7 | 32.598 |
| Ethanol | 257.4 | 90.377 |
| THF | 52.2 | 26.434 |
| Acetonitrile | 160.3 | 61.466 |
| Chloroform | 91.8 | 36.300 |
| Diethyl ether | 59.3 | 37.928 |
| Acetone | 79.1 | 37.669 |
| Dichloromethane | 68.9 | 36.204 |
| Lewis’s Acid–Base Parameter | Values | R2 |
|---|---|---|
| 0.268 | 0.9691 | |
| 2.313 | 0.9691 | |
| / | 8.631 | 0.9691 |
| 2.581 | 0.9691 | |
| 0.459 | 0.9671 | |
| 4.478 | 0.9671 | |
| 9.756 | 0.9671 | |
| 4.937 | 0.9671 |
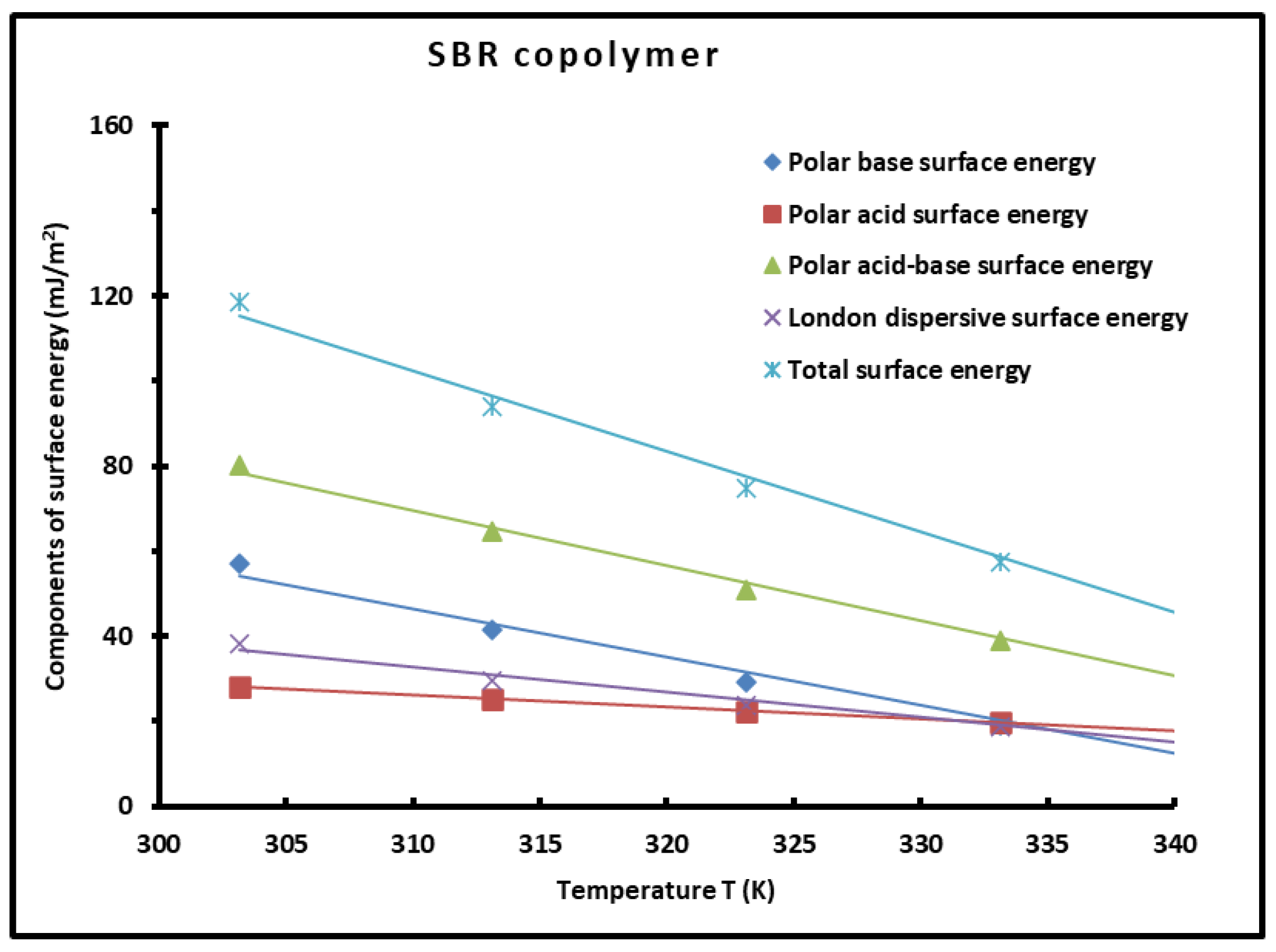
| T(K) | 303.15 | 313.15 | 323.15 | 333.15 | 343.15 | |
|---|---|---|---|---|---|---|
| 56.95 | 41.59 | 29.09 | 19.17 | 11.57 | = −1.132T + 397.42 | |
| 28.17 | 25.1 | 22.26 | 19.65 | 17.25 | = −0.273T + 110.70 | |
| 80.11 | 64.62 | 50.9 | 38.82 | 28.25 | = −1.295T + 471.08 | |
| 38.36 | 29.47 | 23.75 | 18.69 | 14.28 | = −0.590T + 215.36 | |
| 118.47 | 94.08 | 74.65 | 57.5 | 42.53 | = −1.885T + 686.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamieh, T. New Advances on the Dispersive and Polar Surface Properties of Poly(styrene-co-butadiene) Using Inverse Gas Chromatography. Polymers 2024, 16, 3233. https://doi.org/10.3390/polym16233233
Hamieh T. New Advances on the Dispersive and Polar Surface Properties of Poly(styrene-co-butadiene) Using Inverse Gas Chromatography. Polymers. 2024; 16(23):3233. https://doi.org/10.3390/polym16233233
Chicago/Turabian StyleHamieh, Tayssir. 2024. "New Advances on the Dispersive and Polar Surface Properties of Poly(styrene-co-butadiene) Using Inverse Gas Chromatography" Polymers 16, no. 23: 3233. https://doi.org/10.3390/polym16233233
APA StyleHamieh, T. (2024). New Advances on the Dispersive and Polar Surface Properties of Poly(styrene-co-butadiene) Using Inverse Gas Chromatography. Polymers, 16(23), 3233. https://doi.org/10.3390/polym16233233






