Green Chemically Synthesized Iron Oxide Nanoparticles–Chitosan Coatings for Enhancing Strawberry Shelf-Life
Abstract
1. Introduction
2. Material and Methods
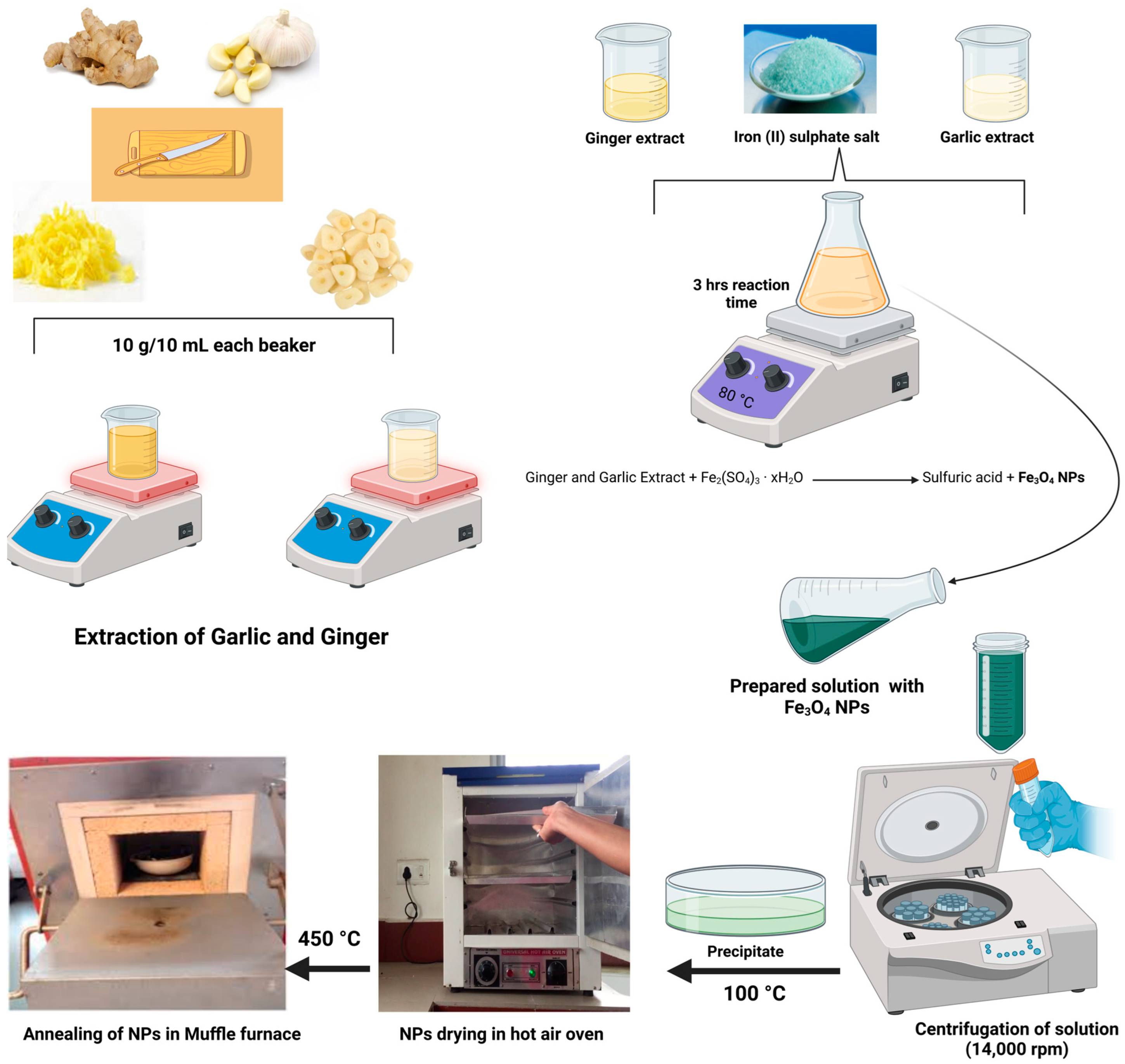
2.1. Lemon Juice and Ginger and Garlic Extraction
2.1.1. Filtration of Lemon Juice
2.1.2. Extraction of Ginger and Garlic Extract
2.2. Synthesis of Nanoparticles
Characterization Techniques of NPs
2.3. Preparation of Coating Solution
Coating Solution Characterization Parameters
2.4. Characterization Parameters of the Coating Solution
2.4.1. Determination of Weight Loss and Firmness
2.4.2. Determination of pH, Total Soluble Solids, and Titratable Acidity
2.4.3. Determination of Antioxidant Activity and Ascorbic Acid
2.4.4. Determination of Total Reducing Sugar, Total Phenolic Compounds, and % Infection
3. Results and Discussion
3.1. Fe3O4 NPs Characterization
3.1.1. XRD
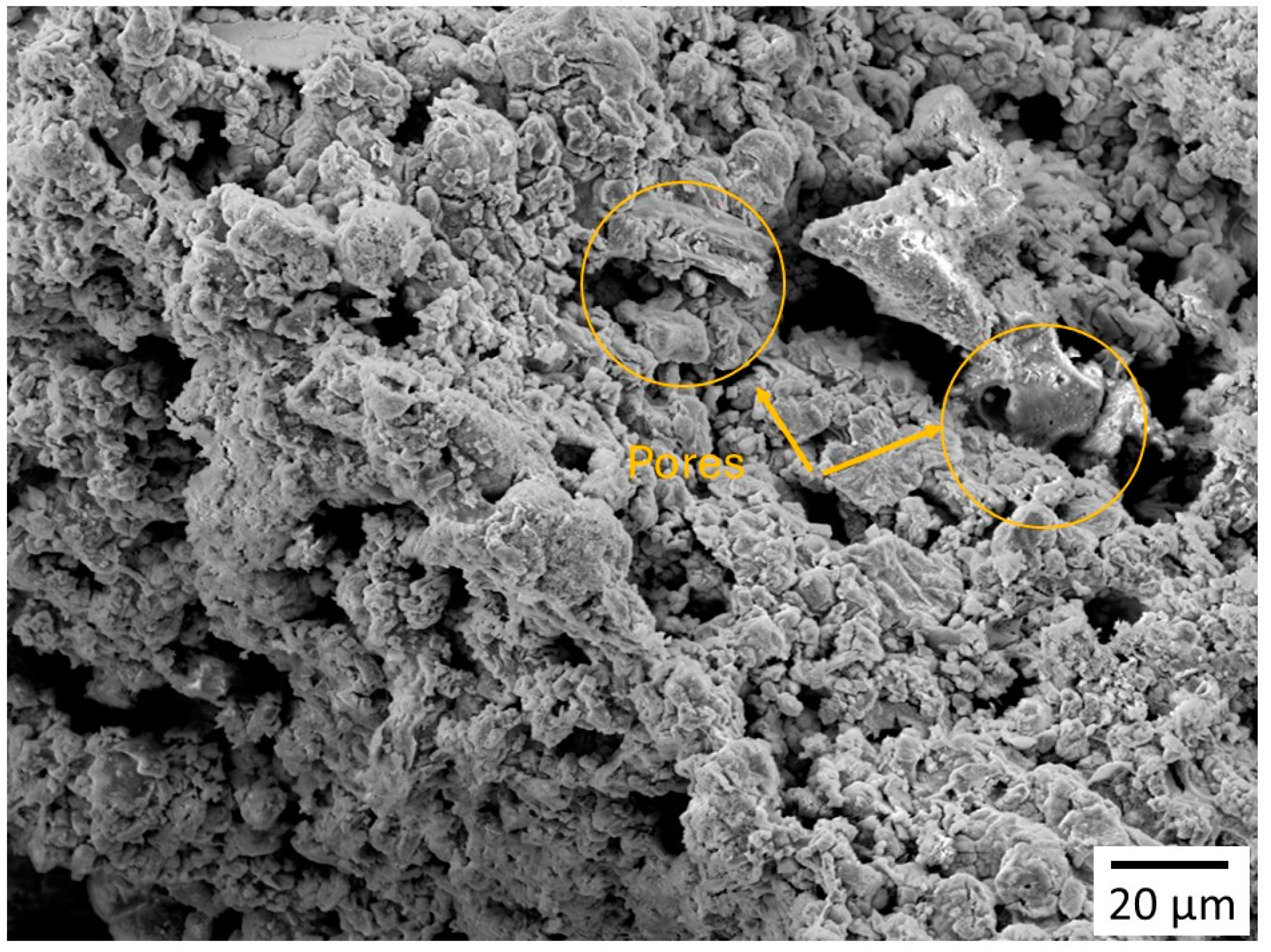
3.1.2. Scanning Electron Microscopy
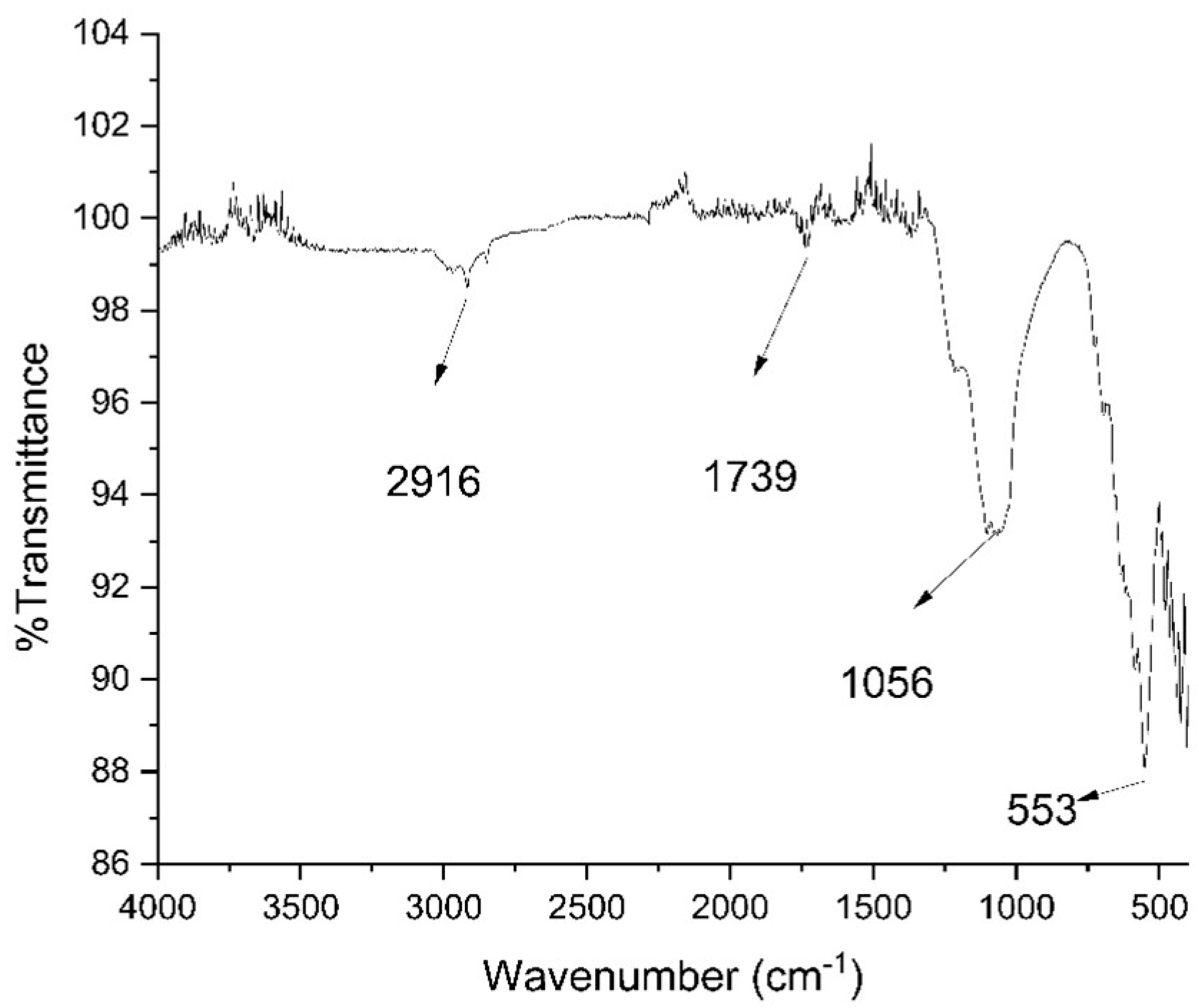
3.1.3. Fourier Transform Infrared Spectroscopy
3.2. Coating Solution and Fruit Characterizations
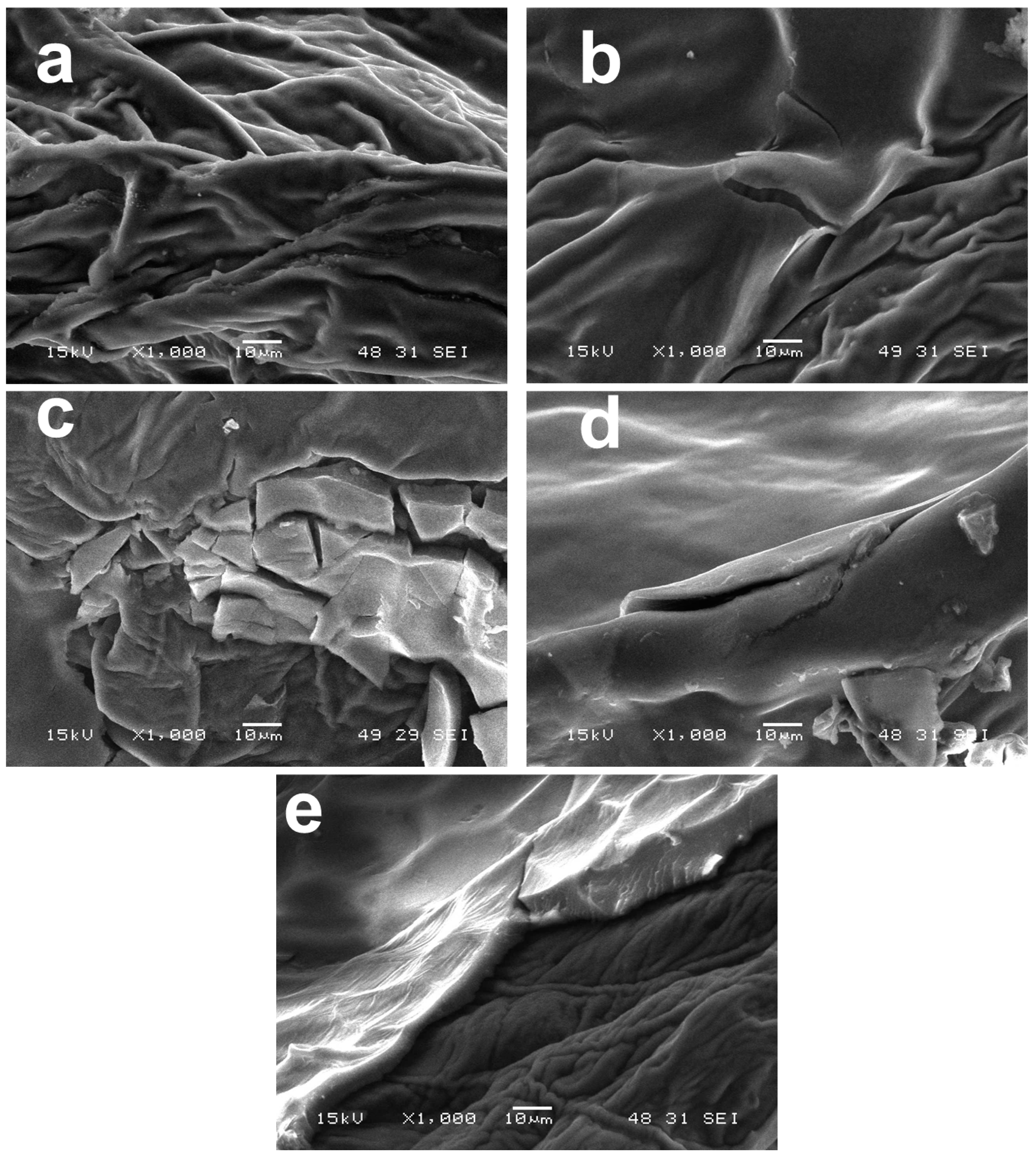
3.2.1. Scanning Electron Microscopy of Fruit
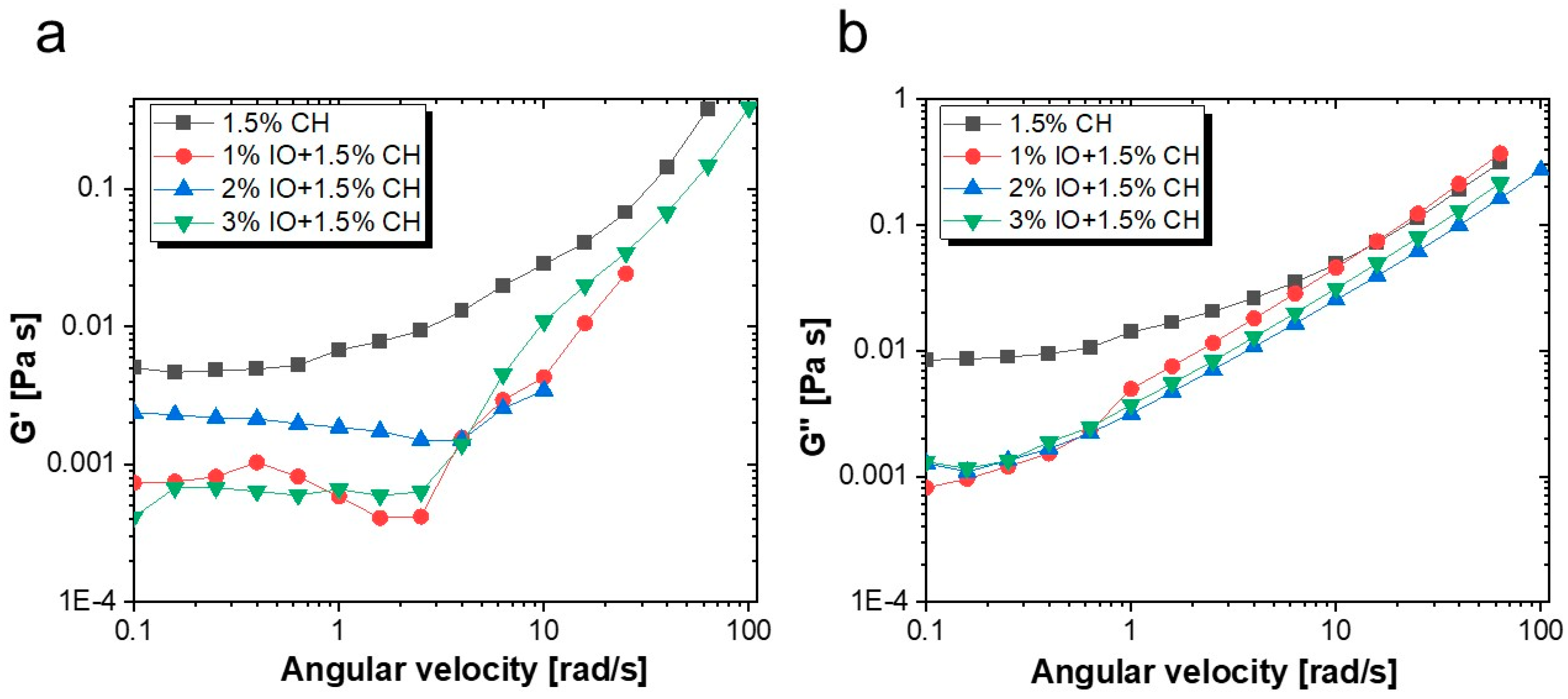
3.2.2. Rheological Properties
3.3. Quality Parameters of Food Coatings
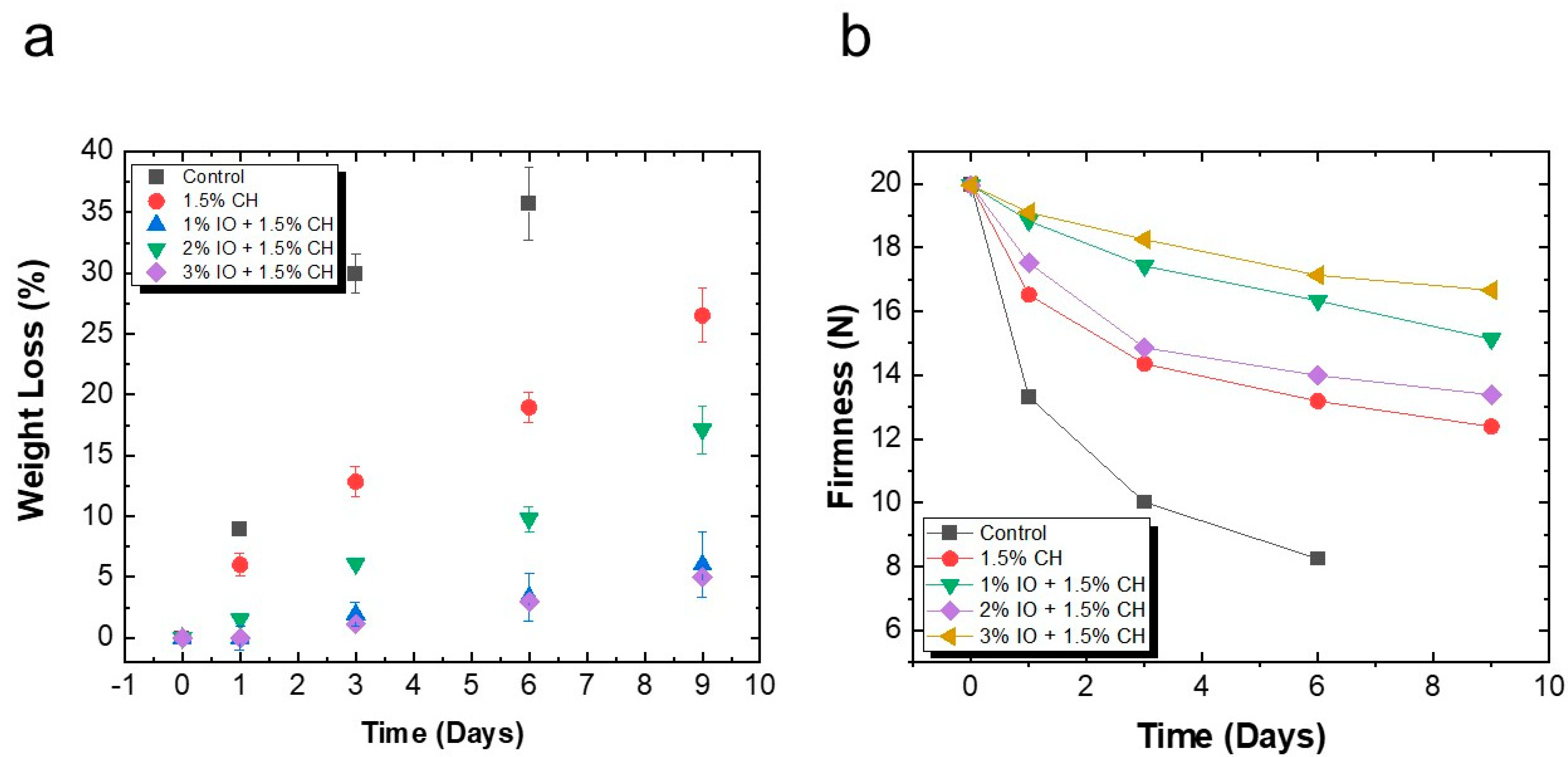
3.3.1. Determination of Weight Loss and Firmness
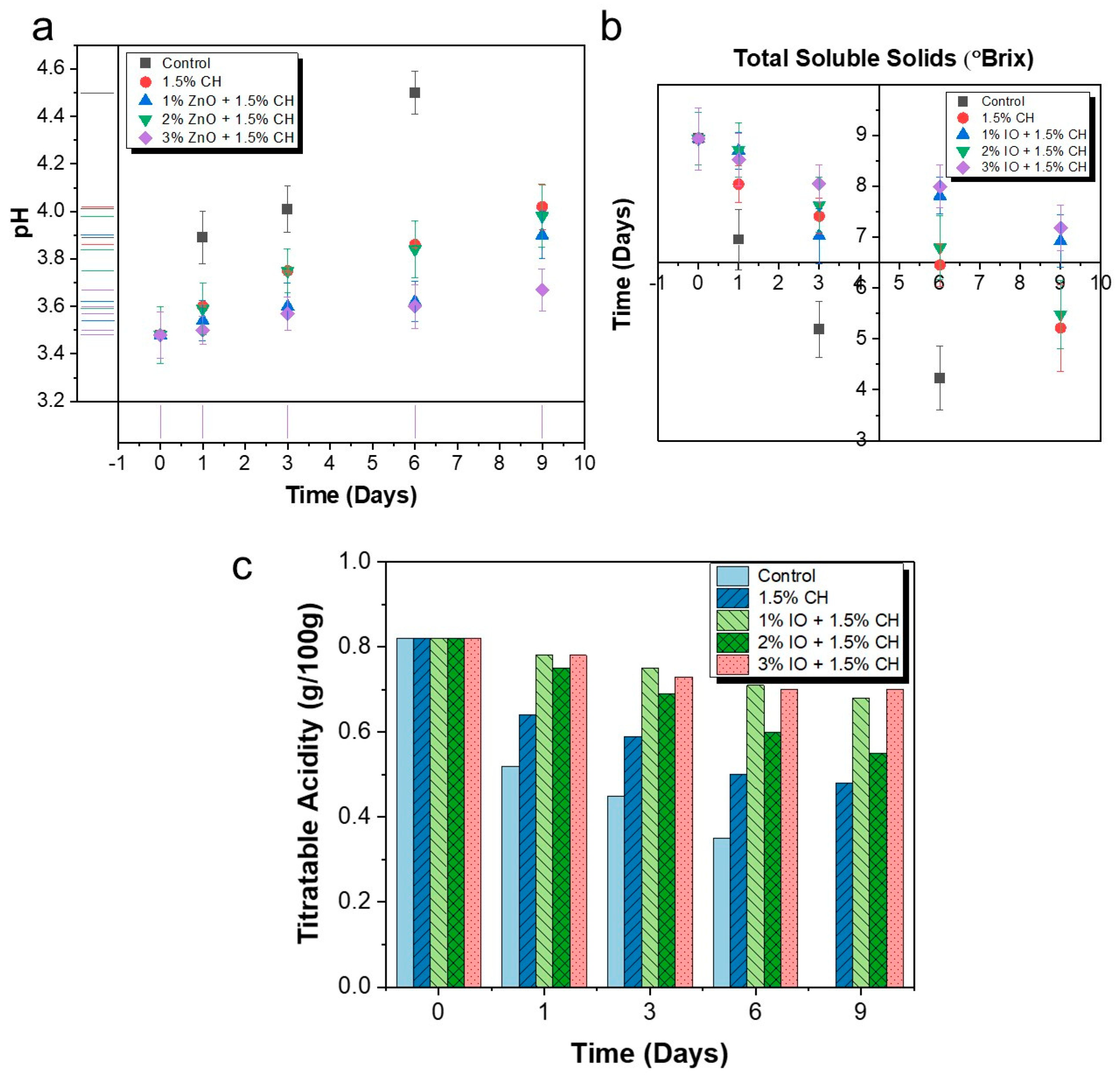
3.3.2. Determination of pH, Total Soluble Solid and Titratable Acidity
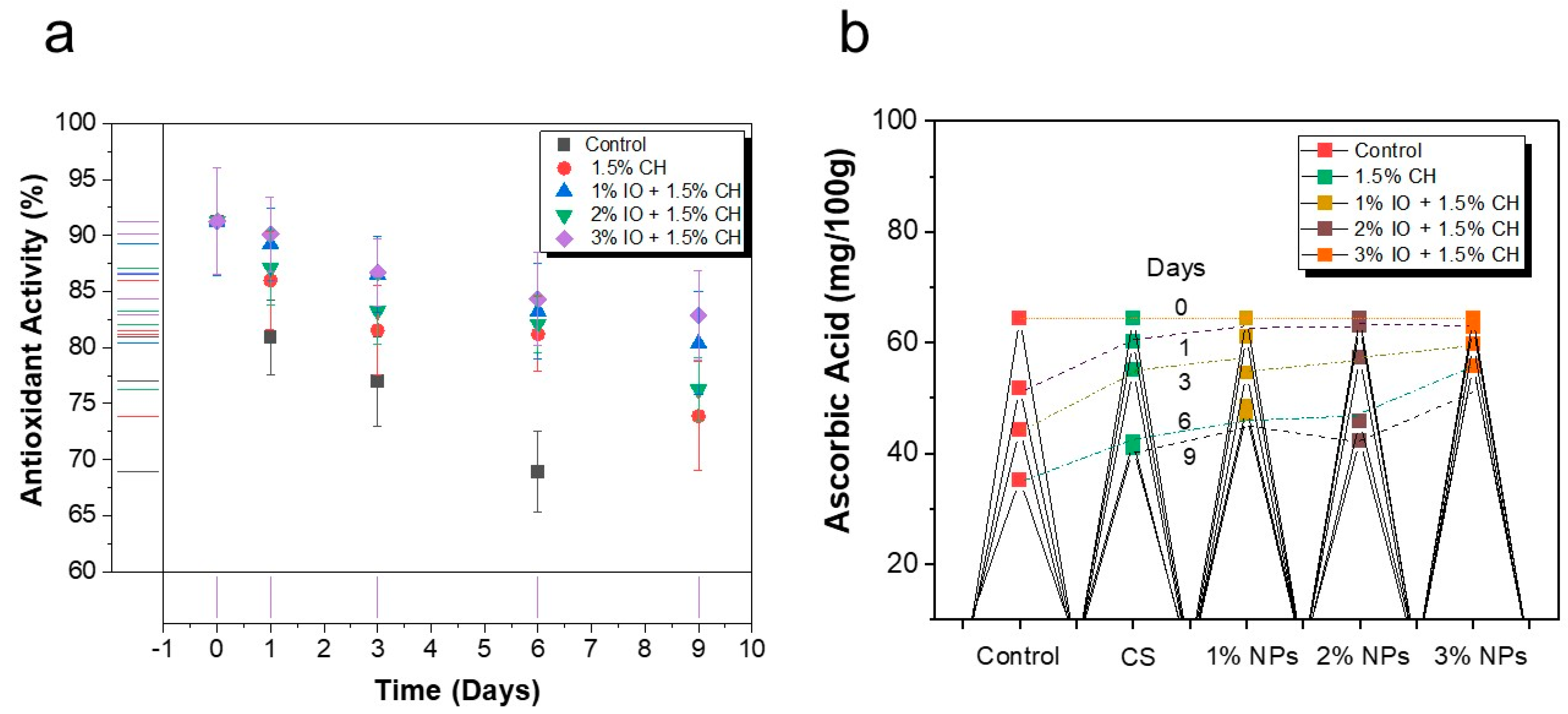
3.3.3. Determination of Antioxidant Activity and Ascorbic Acid
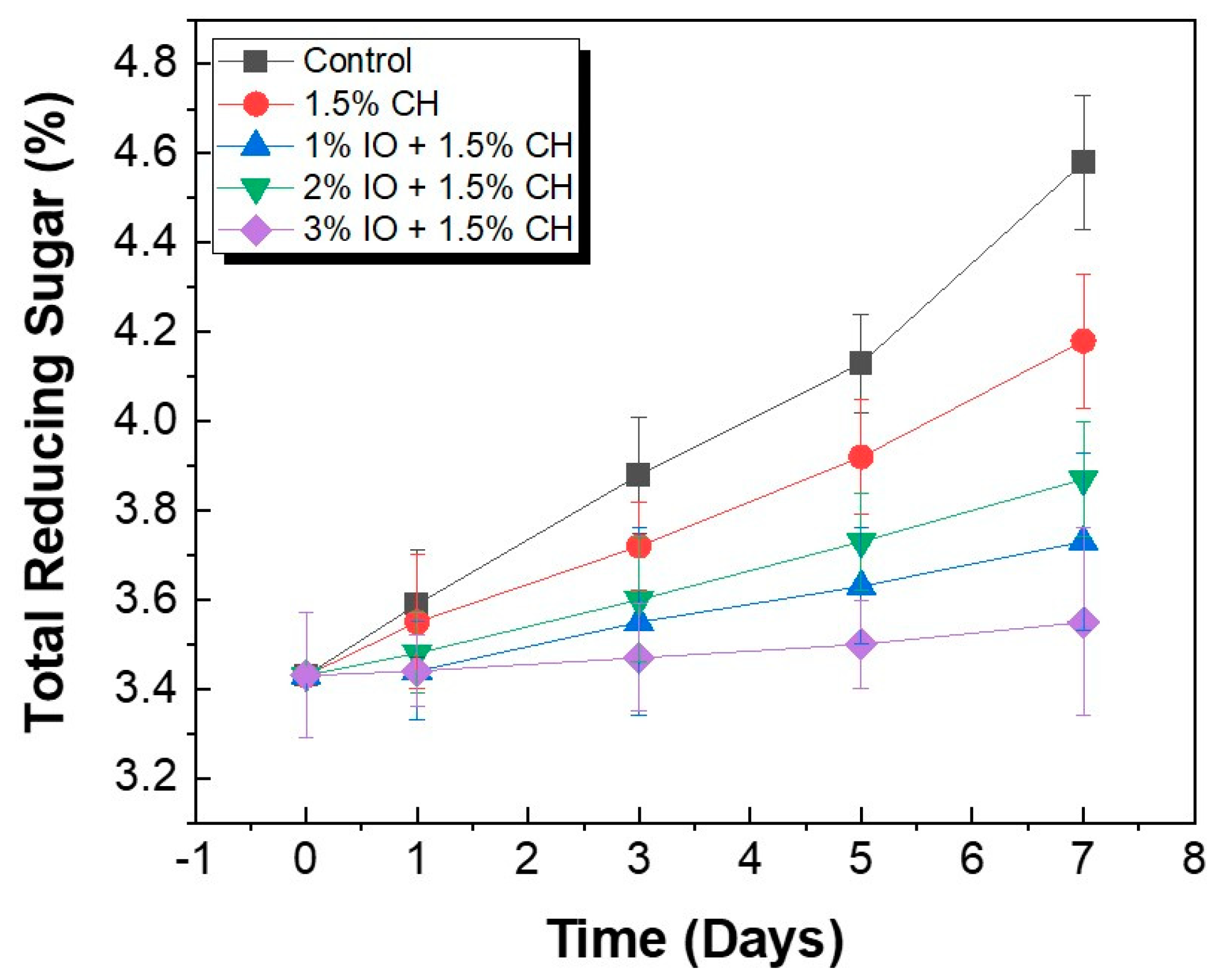
3.3.4. Determination of Total Reducing Sugar
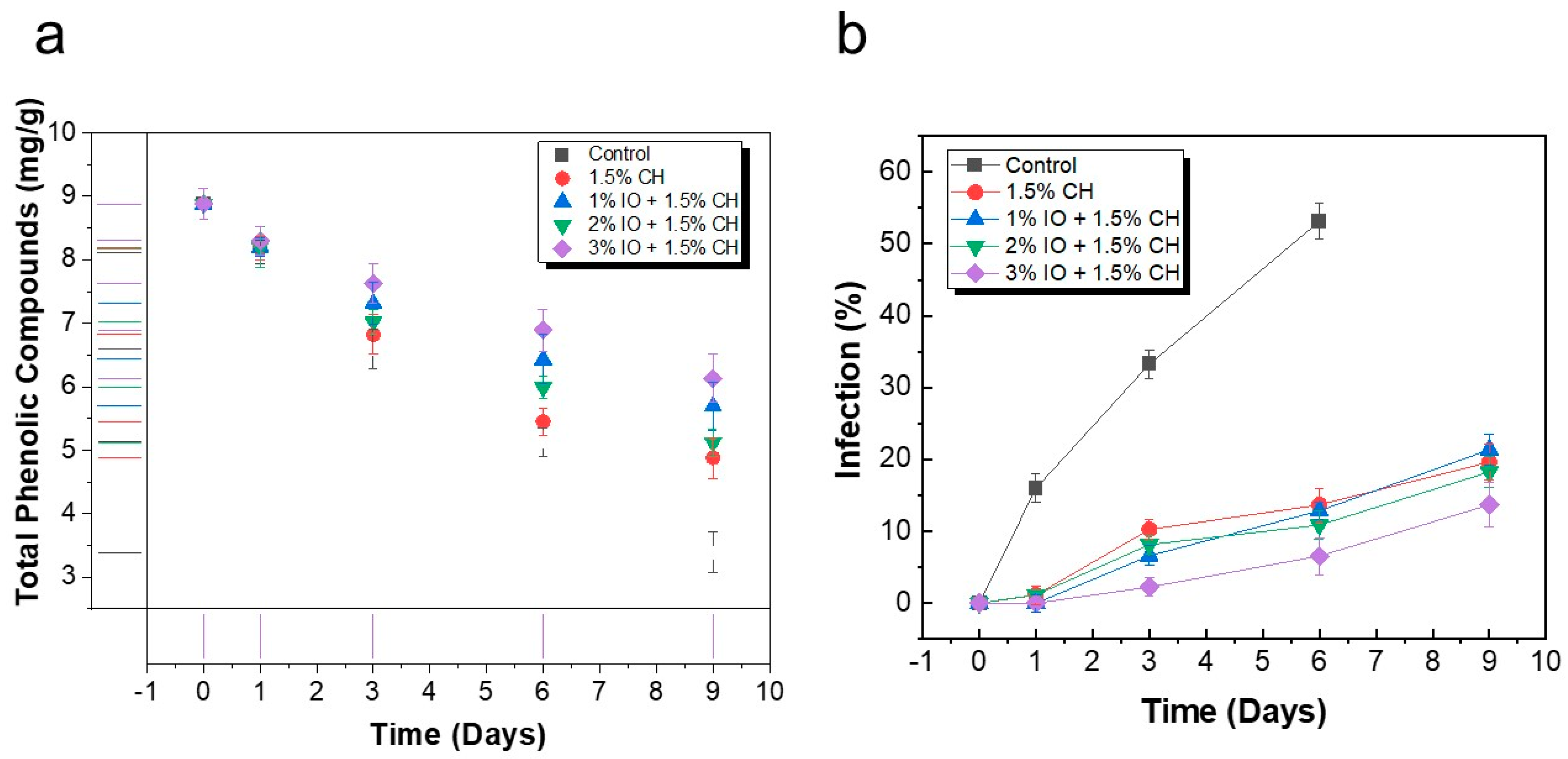
3.3.5. Determination Total Phenolic Compounds, and % Infection
4. Conclusions
5. Future Directions
6. Project Details
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karanth, S.; Feng, S.; Patra, D.; Pradhan, A.K. Linking microbial contamination to food spoilage and food waste: The role of smart packaging, spoilage risk assessments, and date labeling. Front. Microbiol. 2023, 14, 1198124. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, J.; Khan, R.A. Characterization of natural antimicrobials in food system. Adv. Microbiol. 2018, 8, 894. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhou, W.; Pang, C.; Deng, W.; Xu, C.; Wang, X. Multifunctional chitosan-based coating with liposomes containing laurel essential oils and nanosilver for pork preservation. Food Chem. 2019, 295, 16–25. [Google Scholar] [CrossRef]
- Jadhav, R.; Pawar, P.; Choudhari, V.; Topare, N.; Raut-Jadhav, S.; Bokil, S.; Khan, A. An overview of antimicrobial nanoparticles for food preservation. Mater. Today Proc. 2023, 72, 204–216. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Hussein, D.E.; Algammal, A.M.; George, T.T.; Jeandet, P.; Al-Snafi, A.E.; Tiwari, A.; Pagnossa, J.P.; Lima, C.M.; Thorat, N.D. Application of natural antimicrobials in food preservation: Recent views. Food Control 2021, 126, 108066. [Google Scholar] [CrossRef]
- de Almeida Roger, J.; Magro, M.; Spagnolo, S.; Bonaiuto, E.; Baratella, D.; Fasolato, L.; Vianello, F. Antimicrobial and magnetically removable tannic acid nanocarrier: A processing aid for Listeria monocytogenes treatment for food industry applications. Food Chem. 2018, 267, 430–436. [Google Scholar] [CrossRef]
- Vasile, C.; Baican, M. Progresses in Food Packaging, Food Quality, and Safety-Controlled-Release Antioxidant and/or Antimicrobial Packaging. Molecules 2021, 26, 1263. [Google Scholar] [CrossRef]
- Khandeparkar, A.S.; Paul, R.; Sridhar, A.; Lakshmaiah, V.V.; Nagella, P. Eco-friendly innovations in food packaging: A sustainable revolution. Sustain. Chem. Pharm. 2024, 39, 101579. [Google Scholar] [CrossRef]
- Almeida, T.; Karamysheva, A.; Valente, B.F.A.; Silva, J.M.; Braz, M.; Almeida, A.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Biobased ternary films of thermoplastic starch, bacterial nanocellulose and gallic acid for active food packaging. Food Hydrocoll. 2023, 144, 108934. [Google Scholar] [CrossRef]
- Ghosh, T.; Priyadarshi, R.; Krebs de Souza, C.; Angioletti, B.L.; Rhim, J.-W. Advances in pullulan utilization for sustainable applications in food packaging and preservation: A mini-review. Trends Food Sci. Technol. 2022, 125, 43–53. [Google Scholar] [CrossRef]
- Bastante, C.C.; Silva, N.H.C.S.; Cardoso, L.C.; Serrano, C.M.; Martínez de la Ossa, E.J.; Freire, C.S.R.; Vilela, C. Biobased films of nanocellulose and mango leaf extract for active food packaging: Supercritical impregnation versus solvent casting. Food Hydrocoll. 2021, 117, 106709. [Google Scholar] [CrossRef]
- Abdullah; Cai, J.; Hafeez, M.A.; Wang, Q.; Farooq, S.; Huang, Q.; Tian, W.; Xiao, J. Biopolymer-based functional films for packaging applications: A review. Front. Nutr. 2022, 9, 1000116. [Google Scholar] [CrossRef] [PubMed]
- García-Guzmán, L.; Cabrera-Barjas, G.; Soria-Hernández, C.G.; Castaño, J.; Guadarrama-Lezama, A.Y.; Rodríguez Llamazares, S. Progress in starch-based materials for food packaging applications. Polysaccharides 2022, 3, 136–177. [Google Scholar] [CrossRef]
- Saedi, S.; Garcia, C.V.; Kim, J.T.; Shin, G.H. Physical and chemical modifications of cellulose fibers for food packaging applications. Cellulose 2021, 28, 8877–8897. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, F.; Ma, R.; Tian, Y. Advances in gum Arabic utilization for sustainable applications as food packaging: Reinforcement strategies and applications in food preservation. Trends Food Sci. Technol. 2023, 142, 104215. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S. 6—The use of chitin and chitosan for food packaging applications. In Environmentally Compatible Food Packaging; Chiellini, E., Ed.; Woodhead Publishing: Cambridge, UK, 2008; pp. 137–158. [Google Scholar]
- Flórez, M.; Guerra-Rodríguez, E.; Cazón, P.; Vázquez, M. Chitosan for food packaging: Recent advances in active and intelligent films. Food Hydrocoll. 2022, 124, 107328. [Google Scholar] [CrossRef]
- Kontominas, M.G. Use of Alginates as Food Packaging Materials. Foods 2020, 9, 1440. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Villagrán-de la Mora, Z.; Rodríguez-Barajas, N.; Ruvalcaba-Gómez, J.M.; Iñiguez-Muñoz, L.E.; Maytorena-Verdugo, C.I.; Montalvo-González, E.; Pérez-Larios, A. Polysaccharide-based packaging functionalized with inorganic nanoparticles for food preservation. Polysaccharides 2021, 2, 400–428. [Google Scholar] [CrossRef]
- Nešić, A.; Cabrera-Barjas, G.; Dimitrijević-Branković, S.; Davidović, S.; Radovanović, N.; Delattre, C. Prospect of polysaccharide-based materials as advanced food packaging. Molecules 2019, 25, 135. [Google Scholar] [CrossRef]
- Shivangi, S.; Dorairaj, D.; Negi, P.S.; Shetty, N.P. Development and characterisation of a pectin-based edible film that contains mulberry leaf extract and its bio-active components. Food Hydrocoll. 2021, 121, 107046. [Google Scholar] [CrossRef]
- Santhosh, R.; Nath, D.; Sarkar, P. Novel food packaging materials including plant-based byproducts: A review. Trends Food Sci. Technol. 2021, 118, 471–489. [Google Scholar] [CrossRef]
- Jiang, A.; Patel, R.; Padhan, B.; Palimkar, S.; Galgali, P.; Adhikari, A.; Varga, I.; Patel, M. Chitosan Based Biodegradable Composite for Antibacterial Food Packaging Application. Polymers 2023, 15, 2235. [Google Scholar] [CrossRef] [PubMed]
- Samrot, A.V.; Sahithya, C.S.; Selvarani, J.; Purayil, S.K.; Ponnaiah, P. A review on synthesis, characterization and potential biological applications of superparamagnetic iron oxide nanoparticles. Curr. Res. Green Sustain. Chem. 2021, 4, 100042. [Google Scholar] [CrossRef]
- Jain, S.; Nehra, M.; Dilbaghi, N.; Singhal, N.K.; Marrazza, G.; Kim, K.-H.; Kumar, S. Insight into the antifungal effect of chitosan-conjugated metal oxide nanoparticles decorated on cellulosic foam filter for water filtration. Int. J. Food Microbiol. 2022, 372, 109677. [Google Scholar] [CrossRef]
- Konwar, A.; Kalita, S.; Kotoky, J.; Chowdhury, D. Chitosan–iron oxide coated graphene oxide nanocomposite hydrogel: A robust and soft antimicrobial biofilm. ACS Appl. Mater. Interfaces 2016, 8, 20625–20634. [Google Scholar] [CrossRef]
- Baneshi, M.; Aryee, A.N.A.; English, M.; Mkandawire, M. Designing Plant-Based Smart Food Packaging Solutions for Prolonging Consumable Life of Perishable Foods. Food Chem. Adv. 2024, 5, 100769. [Google Scholar] [CrossRef]
- Satgurunathan, T.; Bhavan, P.S.; Kalpana, R.; Jayakumar, T.; Sheu, J.R.; Manjunath, M. Influence of Garlic (Allium sativum) Clove-Based Selenium Nanoparticles on Status of Nutritional, Biochemical, Enzymological, and Gene Expressions in the Freshwater Prawn Macrobrachium rosenbergii (De Man, 1879). Biol. Trace Elem. Res. 2023, 201, 2036–2057. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Shang, A.; Cao, S.-Y.; Xu, X.-Y.; Gan, R.-Y.; Tang, G.-Y.; Corke, H.; Mavumengwana, V.; Li, H.-B. Bioactive compounds and biological functions of garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef]
- Miraghajani, M.; Rafie, N.; Hajianfar, H.; Larijani, B.; Azadbakht, L. Aged garlic and cancer: A systematic review. Int. J. Prev. Med. 2018, 9, 84. [Google Scholar] [PubMed]
- Lee, J.; Zhao, N.; Fu, Z.; Choi, J.; Lee, H.J.; Chung, M. Effects of garlic intake on cancer: A systematic review of randomized clinical trials and cohort studies. Nutr. Res. Pract. 2021, 15, 773–788. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, T.; Vestine, A.; Kim, K.D.; Kwon, S.J.; Sivanesan, I.; Chun, S.C. Antibacterial Activity of Nanoparticles of Garlic (Allium sativum) Extract against Different Bacteria Such as Streptococcus mutans and Poryphormonas gingivalis. Appl. Sci. 2022, 12, 3491. [Google Scholar] [CrossRef]
- Han, Y.A.; Song, C.W.; Koh, W.S.; Yon, G.H.; Kim, Y.S.; Ryu, S.Y.; Kwon, H.J.; Lee, K.H. Anti-inflammatory effects of the Zingiber officinale roscoe constituent 12-dehydrogingerdione in lipopolysaccharide-stimulated Raw 264.7 cells. Phytother. Res. 2013, 27, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Hitomi, S.; Ono, K.; Terawaki, K.; Matsumoto, C.; Mizuno, K.; Yamaguchi, K.; Imai, R.; Omiya, Y.; Hattori, T.; Kase, Y.; et al. [6]-gingerol and [6]-shogaol, active ingredients of the traditional Japanese medicine hangeshashinto, relief oral ulcerative mucositis-induced pain via action on Na+ channels. Pharmacol. Res. 2017, 117, 288–302. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, S.M.; Ezzat, M.I.; Okba, M.M.; Menze, E.T.; Abdel-Naim, A.B. The hidden mechanism beyond ginger (Zingiber officinale Rosc.) potent in vivo and in vitro anti-inflammatory activity. J. Ethnopharmacol. 2018, 214, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.M.S.; Atta, A.M.; Al-Lohedan, H.A.; Alkhathlan, H.Z.; Khan, M.; Ezzat, A.O. Synthesis of Green Recyclable Magnetic Iron Oxide Nanomaterials Coated by Hydrophobic Plant Extracts for Efficient Collection of Oil Spills. Nanomaterials 2019, 9, 1505. [Google Scholar] [CrossRef]
- Antunes, J.C.; Domingues, J.M.; Miranda, C.S.; Silva, A.F.G.; Homem, N.C.; Amorim, M.T.P.; Felgueiras, H.P. Bioactivity of Chitosan-Based Particles Loaded with Plant-Derived Extracts for Biomedical Applications: Emphasis on Antimicrobial Fiber-Based Systems. Mar. Drugs 2021, 19, 359. [Google Scholar] [CrossRef]
- Luo, M.-X.; Hua, S.; Shang, Q.-Y. Application of nanotechnology in drug delivery systems for respiratory diseases. Mol. Med. Rep. 2021, 23, 325. [Google Scholar] [CrossRef]
- Du, B.; Yu, M.; Zheng, J. Transport and interactions of nanoparticles in the kidneys. Nat. Rev. Mater. 2018, 3, 358–374. [Google Scholar] [CrossRef]
- Vijayaram, S.; Razafindralambo, H.; Sun, Y.Z.; Vasantharaj, S.; Ghafarifarsani, H.; Hoseinifar, S.H.; Raeeszadeh, M. Applications of Green Synthesized Metal Nanoparticles—A Review. Biol. Trace Elem. Res. 2024, 202, 360–386. [Google Scholar] [CrossRef] [PubMed]
- Babaei-Ghazvini, A.; Acharya, B.; Korber, D.R. Antimicrobial Biodegradable Food Packaging Based on Chitosan and Metal/Metal-Oxide Bio-Nanocomposites: A Review. Polymers 2021, 13, 2790. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, M.V.; Vasiljevic, Z.Z.; Auger, S.; Vidic, J. Metal oxide nanoparticles for safe active and intelligent food packaging. Trends Food Sci. Technol. 2021, 116, 655–668. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Alabbosh, K.F.; Manan, S.; Khan, S.; Ahmad, F.; Ullah, M.W. Chitosan-based nanostructured biomaterials: Synthesis, properties, and biomedical applications. Adv. Ind. Eng. Polym. Res. 2024, 7, 79–99. [Google Scholar] [CrossRef]
- Shabib Akhtar, M.; Chandrasekaran, K.; Saminathan, S.; Rajalingam, S.R.; Mohsin, N.; Awad Alkarem Ahmed, K.A.; Alhazmi, Y.; Walbi, I.A.; Abdel-Wahab, B.A.; Gholap, A.D.; et al. Nanoengineered chitosan functionalized titanium dioxide biohybrids for bacterial infections and cancer therapy. Sci. Rep. 2024, 14, 3705. [Google Scholar] [CrossRef]
- Miceli, M.; Frontera, P.; Macario, A.; Malara, A. Recovery/Reuse of Heterogeneous Supported Spent Catalysts. Catalysts 2021, 11, 591. [Google Scholar] [CrossRef]
- Garvasis, J.; Prasad, A.R.; Shamsheera, K.O.; Nidheesh Roy, T.A.; Joseph, A. A facile one-pot synthesis of phyto-conjugate superparamagnetic magnetite nanoparticles for the rapid removal of hexavalent chromium from water bodies. Mater. Res. Bull. 2023, 160, 112130. [Google Scholar] [CrossRef]
- Ismail, A.M.; Tiama, T.M.; Farghaly, A.; Elhaes, H.; Ibrahim, M.A. Assessment of the Functionalization of Chitosan/Iron Oxide Nanoparticles. Biointerface Res. Appl. Chem. 2023, 13, 582. [Google Scholar]
- Laniado, M.; Chachuat, A. The endorem tolerance profile. Radiologe 1995, 35 (Suppl. 2), S266–S270. [Google Scholar]
- Markides, H.; Rotherham, M.; El Haj, A.J. Biocompatibility and Toxicity of Magnetic Nanoparticles in Regenerative Medicine. J. Nanomater. 2012, 2012, 614094. [Google Scholar] [CrossRef]
- Lin, J.; Li, Y.; Li, Y.; Wu, H.; Yu, F.; Zhou, S.; Xie, L.; Luo, F.; Lin, C.; Hou, Z. Drug/dye-loaded, multifunctional PEG–chitosan–iron oxide nanocomposites for methotraxate synergistically self-targeted cancer therapy and dual model imaging. ACS Appl. Mater. Interfaces 2015, 7, 11908–11920. [Google Scholar] [CrossRef] [PubMed]
- Haruehansapong, S.; Pulngern, T.; Chucheepsakul, S. Effect of Nanosilica Particle Size on the Water Permeability, Abrasion Resistance, Drying Shrinkage, and Repair Work Properties of Cement Mortar Containing Nano-SiO2. Adv. Mater. Sci. Eng. 2017, 2017, 4213690. [Google Scholar] [CrossRef]
- Hassan, D.; Khalil, A.T.; Saleem, J.; Diallo, A.; Khamlich, S.; Shinwari, Z.K.; Maaza, M. Biosynthesis of pure hematite phase magnetic iron oxide nanoparticles using floral extracts of Callistemon viminalis (bottlebrush): Their physical properties and novel biological applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 693–707. [Google Scholar] [CrossRef] [PubMed]
- Hassan, D.; Khalil, A.T.; Solangi, A.R.; El-Mallul, A.; Shinwari, Z.K.; Maaza, M. Physiochemical properties and novel biological applications of Callistemon viminalis-mediated α-Cr2O3 nanoparticles. Appl. Organomet. Chem. 2019, 33, e5041. [Google Scholar] [CrossRef]
- El-Gioushy, S.F.; Abdelkader, M.F.M.; Mahmoud, M.H.; Abou El Ghit, H.M.; Fikry, M.; Bahloul, A.M.E.; Morsy, A.R.; A., L.A.; Abdelaziz, A.M.R.A.; Alhaithloul, H.A.S.; et al. The Effects of a Gum Arabic-Based Edible Coating on Guava Fruit Characteristics during Storage. Coatings 2022, 12, 90. [Google Scholar] [CrossRef]
- Wantat, A.; Rojsitthisak, P.; Seraypheap, K. Inhibitory effects of high molecular weight chitosan coating on ‘Hom Thong’ banana fruit softening. Food Packag. Shelf Life 2021, 29, 100731. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, L.; Chen, Y.; Liao, L.; Li, R.; Wang, H.; Mo, Y.; Lin, L.; Liu, K. The combined effect of ascorbic acid and chitosan coating on postharvest quality and cell wall metabolism of papaya fruits. LWT 2022, 171, 114134. [Google Scholar] [CrossRef]
- Robles-Sánchez, R.M.; Rojas-Graü, M.A.; Odriozola-Serrano, I.; González-Aguilar, G.; Martin-Belloso, O. Influence of alginate-based edible coating as carrier of antibrowning agents on bioactive compounds and antioxidant activity in fresh-cut Kent mangoes. LWT - Food Sci. Technol. 2013, 50, 240–246. [Google Scholar] [CrossRef]
- Dixit, S.; Rana, S. Investigation of Immunomodulation Activity in the Leaves of Dalbergia dissoo. Glob. J. Pharm. Pharm. Sci. 2018, 5, 23–28. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Y.; Li, X.; Jia, J.; Chen, Y.; Hua, Z. Antioxidant capacities and main reducing substance contents in 110 fruits and vegetables eaten in China. Food Nutr. Sci. 2014, 2014, 42650. [Google Scholar] [CrossRef]
- Kim, A.-N.; Lee, K.-Y.; Jeong, E.J.; Cha, S.W.; Kim, B.G.; Kerr, W.L.; Choi, S.-G. Effect of vacuum–grinding on the stability of anthocyanins, ascorbic acid, and oxidative enzyme activity of strawberry. LWT 2021, 136, 110304. [Google Scholar] [CrossRef]
- Krivorotova, T.; Sereikaite, J. Determination of fructan exohydrolase activity in the crude extracts of plants. Electron. J. Biotechnol. 2014, 17, 329–333. [Google Scholar] [CrossRef]
- Anjum, M.A.; Akram, H.; Zaidi, M.; Ali, S. Effect of gum arabic and Aloe vera gel based edible coatings in combination with plant extracts on postharvest quality and storability of ‘Gola’ guava fruits. Sci. Hortic. 2020, 271, 109506. [Google Scholar] [CrossRef]
- Qayoom, M.; Shah, K.A.; Pandit, A.H.; Firdous, A.; Dar, G.N. Dielectric and electrical studies on iron oxide (α-Fe2O3) nanoparticles synthesized by modified solution combustion reaction for microwave applications. J. Electroceram. 2020, 45, 7–14. [Google Scholar] [CrossRef]
- Baroudi, A.; García-Payo, C.; Khayet, M. Chitosan-Based Composite Membranes with Different Biocompatible Metal Oxide Nanoparticles: Physicochemical Properties and Drug-Release Study. Polymers 2023, 15, 2804. [Google Scholar] [CrossRef]
- Jiang, R.; Zhu, H.-Y.; Zang, X.; Fu, Y.-Q.; Jiang, S.-T.; Li, J.-B.; Wang, Q. A review on chitosan/metal oxide nanocomposites for applications in environmental remediation. Int. J. Biol. Macromol. 2024, 254, 127887. [Google Scholar] [CrossRef]
- Silva, A.O.; Cunha, R.S.; Hotza, D.; Machado, R.A.F. Chitosan as a matrix of nanocomposites: A review on nanostructures, processes, properties, and applications. Carbohydr. Polym. 2021, 272, 118472. [Google Scholar] [CrossRef]
- Kwon, S.H.; Jung, H.S.; Choi, H.J.; Strecker, Z.; Roupec, J. Effect of octahedral typed iron oxide particles on magnetorheological behavior of carbonyl iron dispersion. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 685–690. [Google Scholar] [CrossRef]
- Leong, S.A.N.; Samin, P.M.; Idris, A.; Mazlan, S.A.; Rahman, A.H.A. Synthesis, characterization and magnetorheological properties of carbonyl iron suspension with superparamagnetic nanoparticles as an additive. Smart Mater. Struct. 2016, 25, 025025. [Google Scholar] [CrossRef]
- Javanbakht, T.; Laurent, S.; Stanicki, D.; David, E. Related physicochemical, rheological, and dielectric properties of nanocomposites of superparamagnetic iron oxide nanoparticles with polyethyleneglycol. J. Appl. Polym. Sci. 2020, 137, 48280. [Google Scholar] [CrossRef]
- Alam, M.S.; Ahmed, N.; Salam, M.A. Study on rheology and filtration properties of field used mud using iron (III) oxide nanoparticles. Upstream Oil Gas Technol. 2021, 7, 100038. [Google Scholar] [CrossRef]
- Zhu, W.; Dong, X.; Huang, H.; Qi, M. Iron nanoparticles-based magnetorheological fluids: A balance between MR effect and sedimentation stability. J. Magn. Magn. Mater. 2019, 491, 165556. [Google Scholar] [CrossRef]
- Firdous, N.; Khan, M.R.; Butt, M.S.; Shahid, M. Application of Aloevera Gel Based Edible Coating to Maintain Postharvest Quality of Tomatoes. Pak. J. Agric. Sci. 2020, 57, 245–249. [Google Scholar]
- Abhirami, P.; Modupalli, N.; Natarajan, V. Novel postharvest intervention using rice bran wax edible coating for shelf-life enhancement of Solanum lycopersicum fruit. J. Food Process. Preserv. 2020, 44, e14989. [Google Scholar] [CrossRef]
- Patel, H.; Taghavi, T.; Samtani, J.B. Fruit Quality of Several Strawberry Cultivars during the Harvest Season under High Tunnel and Open Field Environments. Horticulturae 2023, 9, 1084. [Google Scholar] [CrossRef]
- Hussain, S.B.; Shi, C.-Y.; Guo, L.-X.; Kamran, H.; Sadka, A.; Liu, Y. Recent Advances in the Regulation of Citric Acid Metabolism in Citrus Fruit. Crit. Rev. Plant Sci. 2017, 36, 241–256. [Google Scholar] [CrossRef]
- Shehata, S.A.; Abdeldaym, E.A.; Ali, M.R.; Mohamed, R.M.; Bob, R.I.; Abdelgawad, K.F. Effect of Some Citrus Essential Oils on Post-Harvest Shelf Life and Physicochemical Quality of Strawberries during Cold Storage. Agronomy 2020, 10, 1466. [Google Scholar] [CrossRef]
- Riaz, A.; Aadil, R.M.; Amoussa, A.M.O.; Bashari, M.; Abid, M.; Hashim, M.M. Application of chitosan-based apple peel polyphenols edible coating on the preservation of strawberry (Fragaria ananassa cv Hongyan) fruit. J. Food Process. Preserv. 2021, 45, e15018. [Google Scholar] [CrossRef]
- Kibar, H.F.; Sabir, F.K. Chitosan coating for extending postharvest quality of tomatoes (Lycopersicon esculentum Mill.) maintained at different storage temperatures. AIMS Agric. Food 2018, 3, 97–108. [Google Scholar]
- Wang, S.Y.; Lewers, K.S. Antioxidant Capacity and Flavonoid Content in Wild Strawberries. J. Am. Soc. Hortic. Sci. 2007, 132, 629–637. [Google Scholar] [CrossRef]
- Muthukumaran, S.; Tranchant, C.; Shi, J.; Ye, X.; Xue, S.J. Ellagic acid in strawberry (Fragaria spp.): Biological, technological, stability, and human health aspects. Food Qual. Saf. 2017, 1, 227–252. [Google Scholar] [CrossRef]
- Gao, F.; Shao, T.; Yu, Y.; Xiong, Y.; Yang, L. Surface-bound reactive oxygen species generating nanozymes for selective antibacterial action. Nat. Commun. 2021, 12, 745. [Google Scholar] [CrossRef] [PubMed]


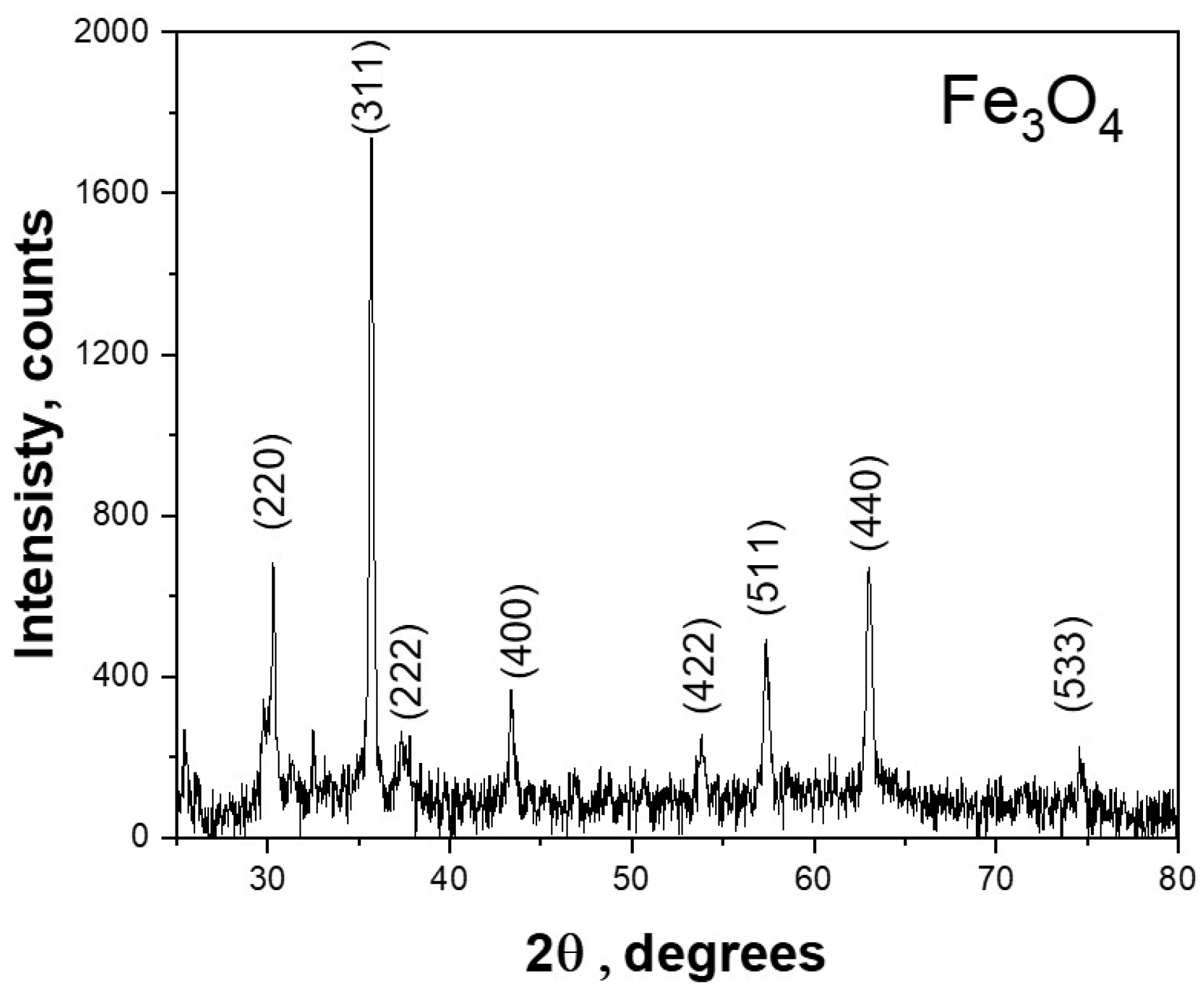

| Peak | Size, nm |
|---|---|
| 220 | 75.56 |
| 311 | 32.27 |
| 222 | 78.82 |
| 400 | 42.92 |
| 422 | 30.81 |
| 511 | 40.33 |
| 440 | 42.90 |
| 533 | 41.50 |
| Average Crystalline Size | 48.138 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sani, A.; Hassan, D.; Chanihoon, G.Q.; Melo Máximo, D.V.; Sánchez-Rodríguez, E.P. Green Chemically Synthesized Iron Oxide Nanoparticles–Chitosan Coatings for Enhancing Strawberry Shelf-Life. Polymers 2024, 16, 3239. https://doi.org/10.3390/polym16233239
Sani A, Hassan D, Chanihoon GQ, Melo Máximo DV, Sánchez-Rodríguez EP. Green Chemically Synthesized Iron Oxide Nanoparticles–Chitosan Coatings for Enhancing Strawberry Shelf-Life. Polymers. 2024; 16(23):3239. https://doi.org/10.3390/polym16233239
Chicago/Turabian StyleSani, Ayesha, Dilawar Hassan, Ghulam Qadir Chanihoon, Dulce Viridiana Melo Máximo, and Elvia Patricia Sánchez-Rodríguez. 2024. "Green Chemically Synthesized Iron Oxide Nanoparticles–Chitosan Coatings for Enhancing Strawberry Shelf-Life" Polymers 16, no. 23: 3239. https://doi.org/10.3390/polym16233239
APA StyleSani, A., Hassan, D., Chanihoon, G. Q., Melo Máximo, D. V., & Sánchez-Rodríguez, E. P. (2024). Green Chemically Synthesized Iron Oxide Nanoparticles–Chitosan Coatings for Enhancing Strawberry Shelf-Life. Polymers, 16(23), 3239. https://doi.org/10.3390/polym16233239







