Novel Simple Approach for Production of Elastic Poly(propylene carbonate)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthetic Procedures
2.2.1. Synthesis of the Complex Rac-(salcy)Co(III)OBzF5
2.2.2. Polymer Synthesis
2.3. Instrumentation
3. Results and Discussion
3.1. Polymer Synthesis and Characterization
3.2. Mechanical Properties of PPC
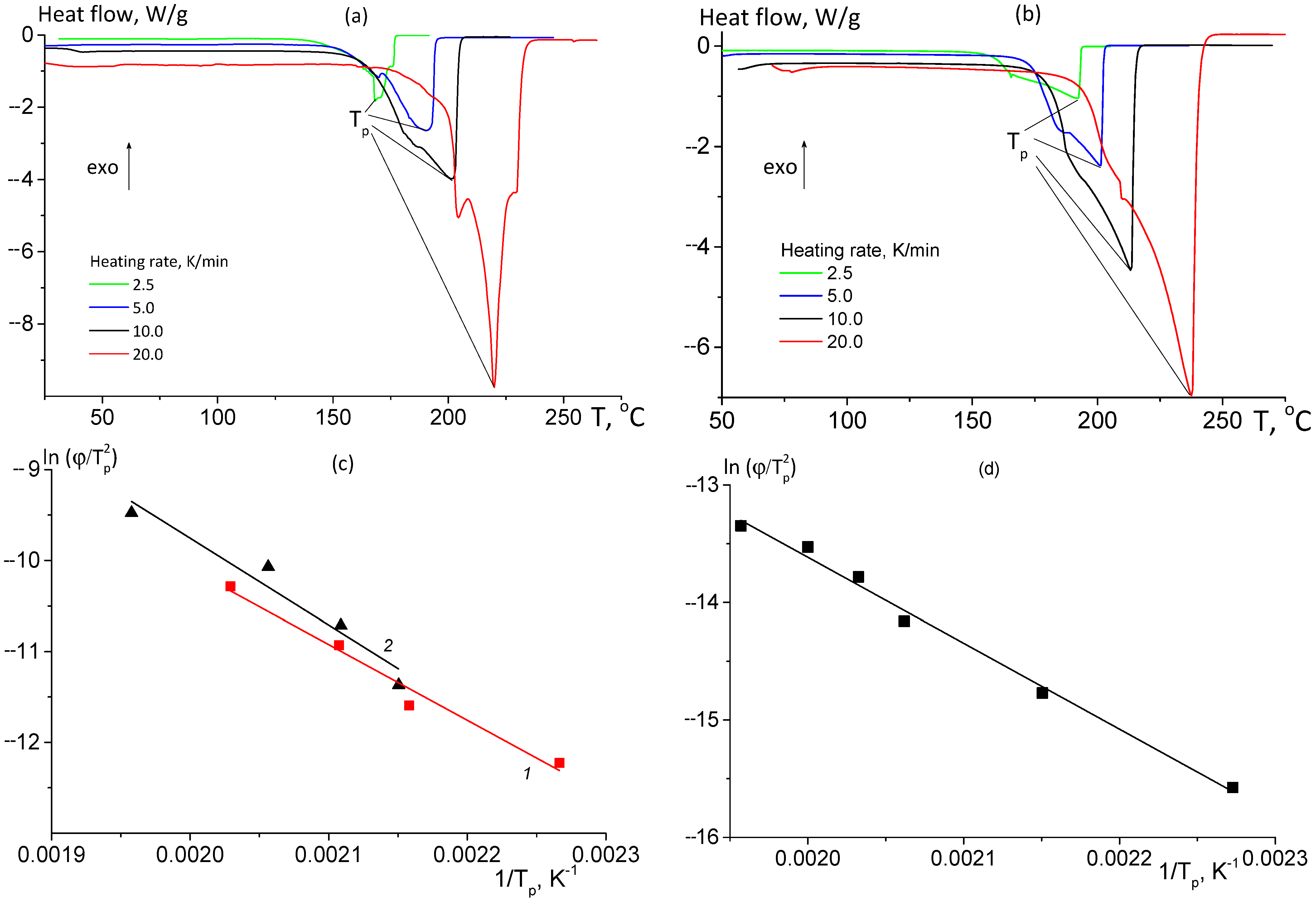
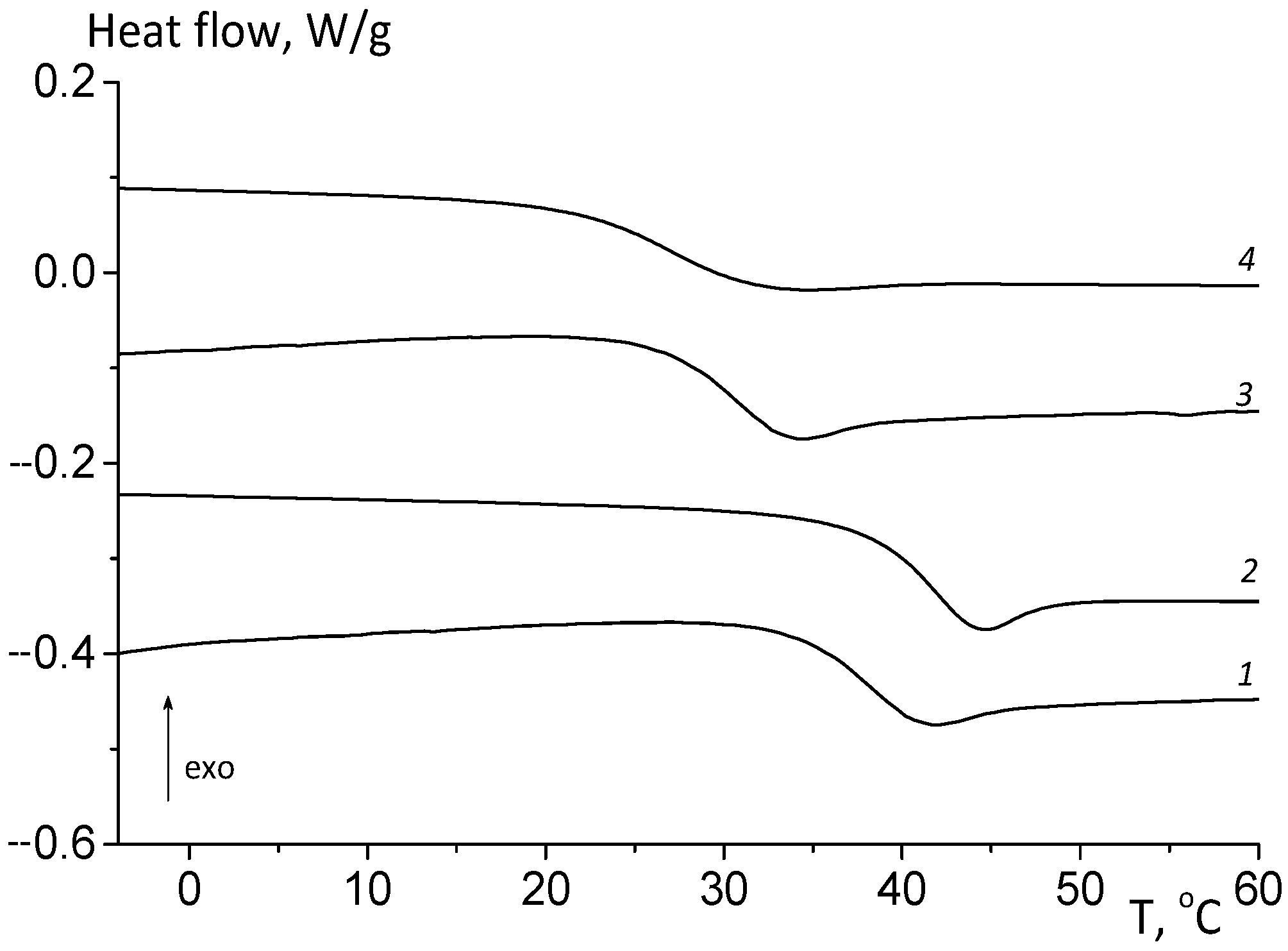
3.2.1. Thermomechanical Analysis
3.2.2. Stress–Strain Test in Uniaxial Stretching Mode
3.2.3. Cyclic Tensile Loading–Unloading Mode (Hysteresis)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Warner, J.C.; Cannon, A.S.; Dye, K.M. Green Chemistry. Environ. Impact Assess. Rev. 2004, 24, 775–799. [Google Scholar] [CrossRef]
- Filiciotto, L.; Rothenberg, G. Biodegradable plastics: Standards, policies, and impact. ChemSusChem 2021, 14, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Luinstra, G.A. Poly(propylene carbonate), old copolymers of propylene oxide and carbon dioxide with new interests: Catalysis and material properties. Polym. Rev. 2008, 48, 192–219. [Google Scholar] [CrossRef]
- Zhou, X.; Zhai, Y.; Ren, K.; Cheng, Z.; Shen, X.; Zhang, T.; Bai, Y.; Jia, Y.; Hong, J. Life cycle assessment of polycarbonate production: Proposed optimization toward sustainability. Resour. Conserv. Recycl. 2023, 189, 106765. [Google Scholar] [CrossRef]
- van der Maas, K.; Weinland, D.H.; van Putten, R.-J.; Wang, B.; Gruter, G.-J.M. Novel elastic rubbers from CO2-based polycarbonates. Green Chem. 2023, 25, 7612–7626. [Google Scholar] [CrossRef]
- Bhat, G.A.; Darensbourg, D.J. Progress in the catalytic reactions of CO2 and epoxides to selectively provide cyclic or polymeric carbonates. Green Chem. 2022, 24, 5007–5034. [Google Scholar] [CrossRef]
- Taherimehr, M.; Al-Amsyar, S.M.; Whiteoak, C.J.; Kleij, A.W.; Pescarmona, P.P. High activity and switchable selectivity in the synthesis of cyclic and polymeric cyclohexene carbonates with iron amino triphenolate catalysts. Green Chem. 2013, 15, 3083–3090. [Google Scholar] [CrossRef]
- Darensbourg, D.J. Comments on the depolymerization of polycarbonates derived from epoxides and carbon dioxide: A mini review. Polym. Degrad. Stab. 2018, 149, 45–51. [Google Scholar] [CrossRef]
- Yang, G.W.; Wang, Y.; Qi, H.; Zhang, Y.Y.; Zhu, X.F.; Lu, C.; Yang, L.; Wu, G.P. Highly Selective Preparation and Depolymerization of Chemically Recyclable Poly(cyclopentene carbonate) Enabled by Organoboron Catalysts. Angew. Chem. Int. Ed. 2022, 61, e202210243. [Google Scholar] [CrossRef]
- Phillips, O.; Schwartz, J.M.; Kohl, P.A. Thermal decomposition of poly(propylene carbonate): End-capping, additives, and solvent effects. Polym. Degrad. Stab. 2016, 125, 129–139. [Google Scholar] [CrossRef]
- Hauenstein, O.; Reiter, M.; Agarwal, S.; Rieger, B.; Greiner, A. Bio-based polycarbonate from limonene oxide and CO2 with high molecular weight, excellent thermal resistance, hardness and transparency. Green Chem. 2016, 18, 760–770. [Google Scholar] [CrossRef]
- Gallin, C.F.; Lee, W.-W.; Byers, J.A. A Simple, Selective, and General Catalyst for Ring Closing Depolymerization of Polyesters and Polycarbonates for Chemical Recycling. Angew. Chem. Int. Ed. 2023, 62, e202303762. [Google Scholar] [CrossRef]
- McGuire, T.M.; Deacy, A.C.; Buchard, A.; Williams, C.K. Solid-State Chemical Recycling of Polycarbonates to Epoxides and Carbon Dioxide Using a Heterodinuclear Mg(II)Co(II) Catalyst. J. Am. Chem. Soc. 2022, 144, 18444–18449. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, H.; Guo, J.Z.; Ren, W.M.; Lu, X.B. Completely Recyclable Monomers and Polycarbonate: Approach to Sustainable Polymers. Angew. Chem. Int. Ed. 2017, 56, 4862–4866. [Google Scholar] [CrossRef]
- Chernikova, E.V.; Beletskaya, I.P. From epoxides and carbon dioxide to polycarbonates: Synthesis, properties and applications. Chem. Rev. 2024, 93, RCR5112. [Google Scholar] [CrossRef]
- Luo, M.; Li, Y.; Zhang, Y.Y.; Zhang, X.H. Using carbon dioxide and its sulfur analogues as monomers in polymer synthesis. Polymer 2016, 82, 406–431. [Google Scholar] [CrossRef]
- Peng, S.; An, Y.; Chen, C.; Fei, B.; Zhuang, Y.; Dong, L. Thermal degradation kinetics of uncapped and end-capped poly(propylene carbonate). Polym. Degrad. Stab. 2003, 80, 141–147. [Google Scholar] [CrossRef]
- Inoue, S.; Koinuma, H.; Tsuruta, T. Copolymerization of Carbon Dioxide and Epoxide with Organometallic Compounds. Die Makromol. Chem. 1969, 130, 210–220. [Google Scholar] [CrossRef]
- Demirel, Y. Sustainability and Economic Analysis of Propylene Carbonate and Polypropylene Carbonate Production Processes Using CO2 and Propylene Oxide. J. Chem. Eng. Process Technol. 2015, 6, 1000236. [Google Scholar] [CrossRef]
- Diment, W.T.; Lindeboom, W.; Fiorentini, F.; Deacy, A.C.; Williams, C.K. Synergic Heterodinuclear Catalysts for the Ring-Opening Copolymerization (ROCOP) of Epoxides, Carbon Dioxide, and Anhydrides. Acc. Chem. Res. 2022, 55, 1997–2010. [Google Scholar] [CrossRef]
- Yang, G.W.; Zhang, Y.Y.; Wu, G.P. Modular Organoboron Catalysts Enable Transformations with Unprecedented Reactivity. Acc.Chem. Res. 2021, 54, 4434–4448. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, L.M.; Ren, B.H.; Lu, X.B. Asymmetric Alternating Copolymerization of CO2 with meso-Epoxides: Ring Size Effects of Epoxides on Reactivity, Enantioselectivity, Crystallization, and Degradation. Macromolecules 2020, 53, 2912–2918. [Google Scholar] [CrossRef]
- Song, Q.W.; Zhou, Z.H.; He, L.N. Efficient, selective and sustainable catalysis of carbon dioxide. Green Chem. 2017, 19, 3707–3728. [Google Scholar] [CrossRef]
- Luinstra, G.A.; Molnar, F. Poly(propylene carbonate), old CO2 coplymer with new attractiveness. Macromol. Symp. 2007, 259, 203. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Wilson, S.J. What’s new with CO2? Recent advances in its copolymerization with oxiranes. Green Chem. 2012, 14, 2665–2671. [Google Scholar] [CrossRef]
- Jiang, G.; Feng, J.; Zhang, M.; Zhang, S.; Huang, H. Structure, thermal and mechanical properties of poly(propylene carbonate) capped with different types of acid anhydride via reactive extrusion. RSC Adv. 2016, 6, 107547–107555. [Google Scholar] [CrossRef]
- Lai, M.F.; Li, J.; Liu, J.J. Thermal and dynamic mechanical properties of poly(propylene carbonate). J. Therm. Analys. Calorim. 2005, 82, 293–298. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Wang, W.-Z.; Wang, L.; Li, L.-L.; Zhang, K.-Y.; Zhao, S.-D. Poly(propylene carbonate) networks with excellent properties: Terpolymerization of carbon dioxide, propylene oxide, and 4,4′-(hexafluoroisopropylidene) diphthalic anhydride. e-Polymers 2021, 21, 511. [Google Scholar] [CrossRef]
- Li, X.; Meng, L.; Zhang, Y.; Qin, Z.; Meng, L.; Li, C.; Liu, M. Research and Application of Polypropylene Carbonate Composite Materials: A Review. Polymers 2022, 14, 2159. [Google Scholar] [CrossRef]
- Cui, S.; Li, L.; Wang, Q. Enhancing Glass Transition Temperature and Mechanical Properties of Poly(propylene carbonate) by Intermacromolecular Complexation with Poly (vinyl alcohol). Compos. Sci. Techn. 2016, 127, 177–184. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Yang, J.; Wu, Q.; Liang, S.; Liu, Z. Poly(Propylene Carbonate)-Based Biodegradable and Environment-Friendly Materials for Biomedical Applications. Int. J. Mol. Sci. 2024, 25, 2938. [Google Scholar] [CrossRef]
- Wu, D.-D.; Li, W.; Zhao, Y.; Deng, Y.-J.; Zhang, H.-L.; Zhang, H.-X.; Dong, L.-S. Thermal, mechanical and rheological properties of biodegradable poly(propylene carbonate) and poly(butylene carbonate) blends. Chin. J. Polym. Sci. 2015, 33, 444–455. [Google Scholar] [CrossRef]
- Chen, L.; Qin, Y.; Wang, X.; Zhao, X.; Wang, F. Plasticizing while toughening and reinforcing poly(propylene carbonate) using low molecular weight urethane: Role of hydrogen-bonding interaction. Polymer 2011, 52, 4873. [Google Scholar] [CrossRef]
- Jiao, J.; Wang, S.J.; Xiao, M.; Xu, Y.; Meng, Y.Z. Processability, Property, and Morphology of Biodegradable Blends of Poly(propylene carbonate) and Poly(ethylene-co-vinyl alcohol). Polym. Eng. Sci. 2007, 47, 174–180. [Google Scholar] [CrossRef]
- Chen, W.; Pang, M.; Xiao, M.; Wang, S.; Wen, L.; Meng, Y. Mechanical, Thermal, and Morphological Properties of Glass Fiber-reinforced Biodegradable Poly(propylene carbonate) Composites. J. Reinf. Plast. Compos. 2010, 29, 1545–1550. [Google Scholar] [CrossRef]
- Li, X.H.; Meng, Y.Z.; Chen, G.Q.; Li, R.K.Y. Thermal Properties and Rheological Behavior of Biodegradable Aliphatic Polycarbonate Derived from Carbon Dioxide and Propylene Oxide. J. Appl. Polym. Sci. 2004, 94, 711–716. [Google Scholar] [CrossRef]
- Zhu, Q.; Meng, Y.Z.; Tjong, S.C.; Zhao, X.S.; Chen, Y.L. Thermally stable and high molecular weight poly(propylene carbonate)s from carbon dioxide and propylene oxide. Polym. Int. 2002, 51, 1079–1085. [Google Scholar] [CrossRef]
- Du, L.; Qu, B.; Meng, Y.; Zhu, Q. Structural characterization and thermal and mechanical properties of poly(propylene carbonate)/MgAl-LDH exfoliation nanocomposite via solution intercalation. Compos. Sci. Techn. 2006, 66, 913–918. [Google Scholar] [CrossRef]
- Liu, H.; Pan, L.; Lin, Q.; Xu, N.; Lu, L.; Pang, S.; Fu, S. Preparation and characterization of poly(propylene carbonate)/polystyrene composite films by melt-extrusion method. e-Polymers 2010, 10, 38. [Google Scholar] [CrossRef]
- Cohen, C.T.; Coates, G.W. Alternating Copolymerization of Propylene Oxide and Carbon Dioxide with Highly Efficient and Selective (Salen)Co(III) Catalysts: Effect of Ligand and Cocatalyst Variation. J. Polym. Sci. Polym. Chem. 2006, 44, 5182–5191. [Google Scholar] [CrossRef]
- Cohen, C.T.; Chu, T.; Coates, G.W. Cobalt Catalysts for the Alternating Copolymerization of Propylene Oxide and Carbon Dioxide: Combining High Activity and Selectivity. J. Am. Chem. Soc. 2005, 127, 10869–10878. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, X.H. Carbon dioxide-based copolymers: Environmental benefits of PPC, an industrially viable catalyst. Biotechnol. J. 2010, 5, 1164–1180. [Google Scholar] [CrossRef]
- Wang, X.L.; Li, R.K.Y.; Cao, Y.X.; Meng, Y.Z. Essential work of fracture analysis of poly(propylene carbonate) with varying molecular weight. Polym. Test. 2005, 24, 699–703. [Google Scholar] [CrossRef]
- Wang, J.T.; Shu, D.; Xiao, M.; Meng, Y.Z. Copolymerization of carbon dioxide and propylene oxide using zinc adipate as catalyst. J. Appl. Polym. Sci. 2006, 99, 200–206. [Google Scholar] [CrossRef]
- Ye, S.; Wang, S.; Lin, L.; Xiao, M.; Meng, Y.Z. CO2 derived biodegradable polycarbonates: Synthesis, modification and applications. Adv. Ind. Eng. Polym. Res. 2019, 2, 143–160. [Google Scholar] [CrossRef]
- Tao, Y.H.; Wang, X.H.; Zhao, X.J.; Li, J.; Wang, F.S. Crosslinkable poly(propylene carbonate): High-yield synthesis and performance improvement. J. Polym. Sci. Polym. Chem. 2006, 44, 5329–5336. [Google Scholar] [CrossRef]
- Lu, L.; Huang, K. Synthesis and characteristics of a novel aliphatic polycarbonate, poly[(propylene oxide)-co-(carbon dioxide)-co-(γ-butyrolactone)]. Polym. Int. 2005, 54, 870–874. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Stafford, N.W.; Katsurao, T. Supercritical carbon dioxide as solvent for the copolymerization of carbon dioxide and propylene oxide using a heterogeneous zinc carboxylate catalyst. J. Molec. Cat. A Chem. 1995, 104, L1–L4. [Google Scholar] [CrossRef]
- Eberhardt, R.; Allmendinger, M.; Zintl, M.; Troll, C.; Luinstra, G.A.; Rieger, B. New Zinc Dicarboxylate Catalysts for the CO2/Propylene Oxide Copolymerization Reaction: Activity Enhancement Through Zn(II)-Ethylsulfinate Initiating Groups. Macromol. Chem. Phys. 2004, 205, 42–47. [Google Scholar] [CrossRef]
- Chisholm, M.H.; Navarro-Llobet, D.; Zhou, Z. Poly(propylene carbonate). 1. More about Poly(propylene carbonate) Formed from the Copolymerization of Propylene Oxide and Carbon Dioxide Employing a Zinc Glutarate Catalyst. Macromolecules 2002, 35, 6494–6504. [Google Scholar] [CrossRef]
- Luinstra, G.A.; Borchardt, E. Material properties of poly(propylene carbonates). Adv. Polym. Sci. 2012, 245, 29–48. [Google Scholar] [CrossRef]
- Nakano, K.; Hashimoto, S.; Nakamura, M.; Kamada, T.; Nozaki, K. Stereocomplex of Poly(propylene carbonate): Synthesis of Stereogradient Poly(propylene carbonate) by Regio- and Enantioselective Copolymerization of Propylene Oxide with Carbon Dioxide. Angew. Chem. Int. Ed. 2011, 50, 4868–4871. [Google Scholar] [CrossRef]
- Dixon, D.D.; Ford, M.E.; Mantell, G.J. Thermal stabilization of poly(alkylene carbonate)s. J. Polym. Sci. Polym. Lett. Ed. 1980, 18, 131–134. [Google Scholar] [CrossRef]
- Lu, X.L.; Zhu, Q.; Meng, Y.Z. Kinetic analysis of thermal decomposition of poly(propylene carbonate). Polym. Degrad. Stab. 2005, 89, 282–288. [Google Scholar] [CrossRef]
- Darensbourg, D.J. Salen Metal Complexes as Catalysts for the Synthesis of Polycarbonates from Cyclic Ethers and Carbon Dioxide. Adv. Polym. Sci. 2012, 245, 1–28. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Wilson, S.J. Synthesis of Poly(indene carbonate) from Indene Oxide and Carbon Dioxide—A Polycarbonate with a Rigid Backbone. J. Am. Chem. Soc. 2011, 133, 18610–18613. [Google Scholar] [CrossRef]
- Li, C.; Sablong, R.J.; Koning, C.E. Chemoselective Alternating Copolymerization of Limonene Dioxide and Carbon Dioxide: A New Highly Functional Aliphatic Epoxy Polycarbonate. Angew. Chem. Int. Ed. 2016, 55, 11572–11576. [Google Scholar] [CrossRef]
- Enriquez, E.; Mohanty, A.K.; Misra, M. Biobased blends of poly(propylene carbonate) and poly(hydroxybutyrate-co-hydroxyvalerate): Fabrication and characterization. J. Appl. Polym. Sci. 2017, 134, 44420. [Google Scholar] [CrossRef]
- Kukushkin, V.Y.; Moiseev, A.I. Convenient synthesis of μ-nitridobis(triphenylphosphonium) chloride ([PPN]Cl) with contribution of PCl5 as chlorinating (PCl5 + PPh3) or deoxygenating (PCl5 + OPPh3) reagent. Inorg. Chim. Acta 1990, 176, 79–81. [Google Scholar] [CrossRef]
- Larrow, J.F.; Jacobsen, E.N. (R,R)-N,N′-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediamino manganese(III) chloride, a highly enantioselective epoxidation catalyst. Org. Synth. Coll. 1998, 75, 1. [Google Scholar] [CrossRef]
- Zhuang, X.; Oyaizu, K.; Niu, Y.; Koshika, K.; Chen, X.; Nishide, H. Synthesis and Electrochemistry of Schiff Base Cobalt(III) Complexes and Their Catalytic Activity for Copolymerization of Epoxide and Carbon Dioxide. Macromol. Chem. Phys. 2010, 211, 669–676. [Google Scholar] [CrossRef]
- Rzhevskiy, S.A.; Shurupova, O.V.; Asachenko, A.F.; Plutalova, A.V.; Chernikova, E.V.; Beletskaya, I.P. The Role of Ligand Exchange in Salen Cobalt Complexes in the Alternating Copolymerization of Propylene Oxide and Carbon Dioxide. Int. J. Mol. Sci. 2024, 25, 10946. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, X.; Wang, X.; Wang, F. Thermal Degradation Kinetics of Poly(propylene carbonate) Obtained from the Copolymerization of Carbon Dioxide and Propylene Oxide. J. Appl. Polym. Sci. 2003, 90, 947–953. [Google Scholar] [CrossRef]
- Lin, S.; Yu, W.; Wang, X.; Zhou, C. Study on the thermal degradation kinetics of biodegradable poly(propylene carbonate) during melt processing by population balance model and rheology. Ind. Eng. Chem. Res. 2014, 53, 18411–18419. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Wei, S.-H. Depolymerization of polycarbonates derived from carbon dioxide and epoxides to provide cyclic carbonates. A kinetic study. Macromolecules 2012, 45, 5916–5922. [Google Scholar] [CrossRef]
- Henke, L.; Zarrinbakhsh, N.; Endres, H.-J.; Misra, M.; Mohanty, A.K. Biodegradable and bio-based green blends from carbon dioxide-derived bioplastic and poly(butylene succinate). J. Polym. Environ. 2016, 25, 499–509. [Google Scholar] [CrossRef]
- Lee, T.-I.; Kim, J.H.; Kim, D.J.; Kim, T.-S. Evaluating Free Thermal Expansion and Glass Transition of Ultrathin Polymer Films on Heated Liquid. ACS Appl. Mater. Interfaces 2024, 16, 30336–30343. [Google Scholar] [CrossRef]
- Eyring, H. Viscosity, plasticity, and diffusion as examples of absolute reaction rates. J. Chem. Phys. 1936, 4, 283–291. [Google Scholar] [CrossRef]
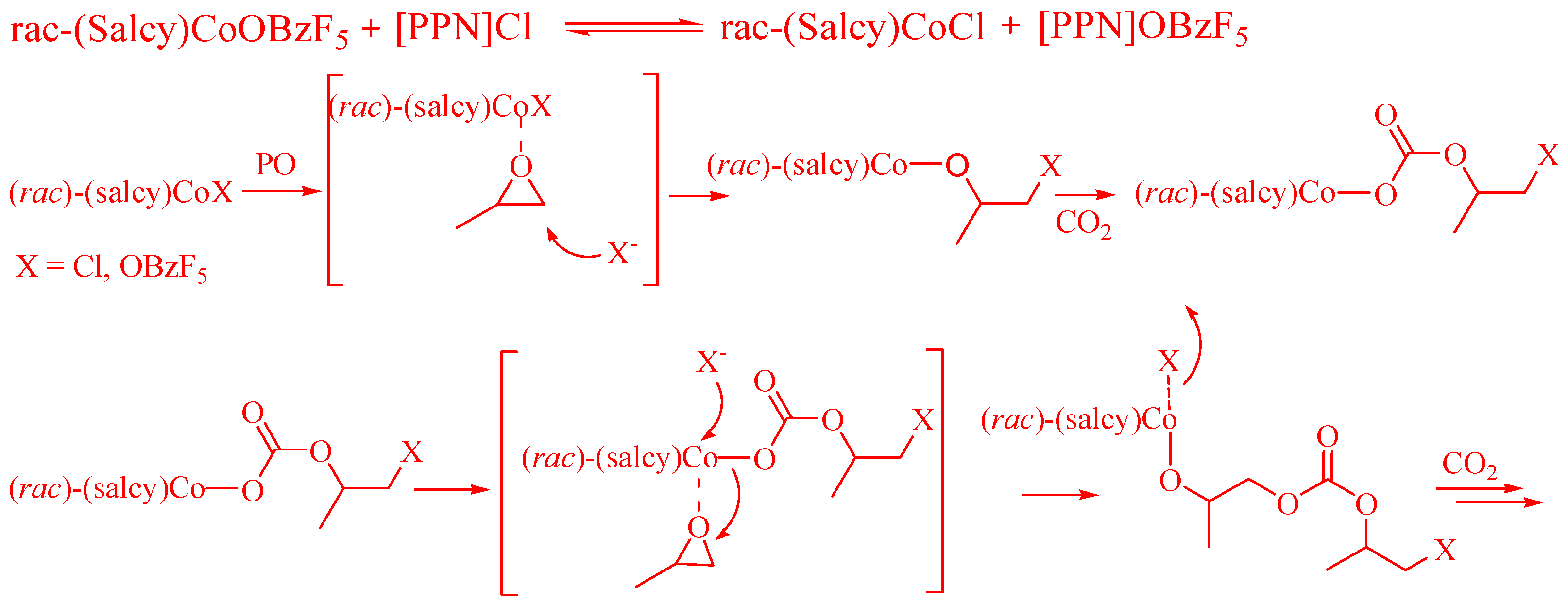


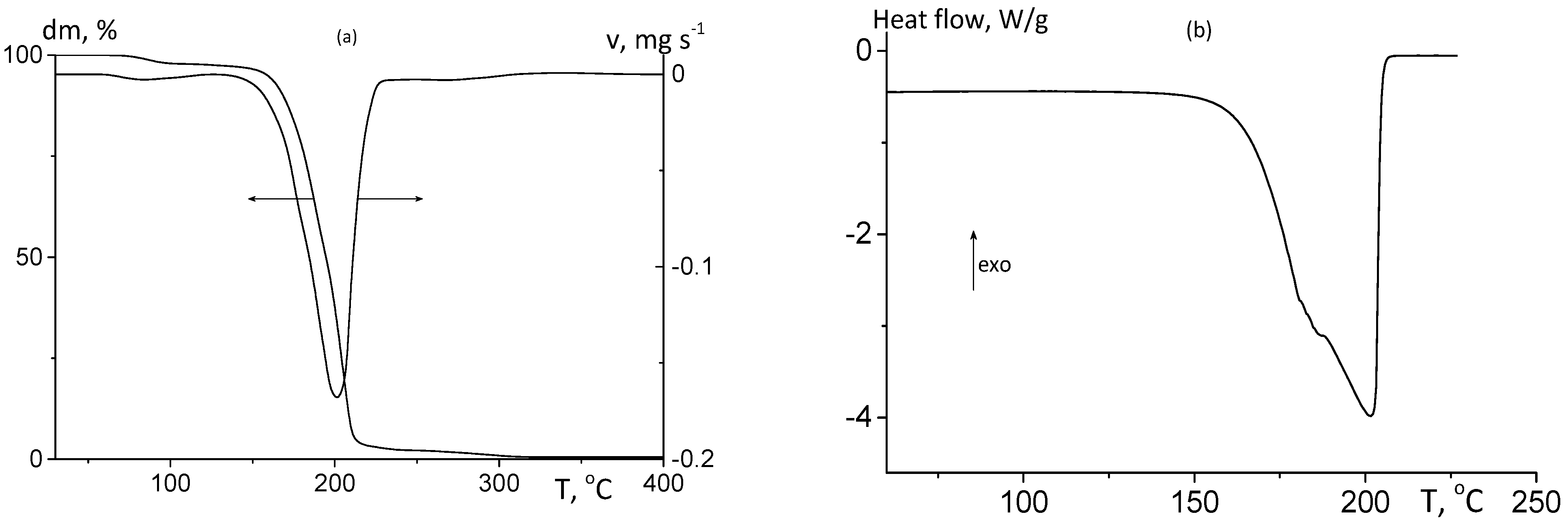
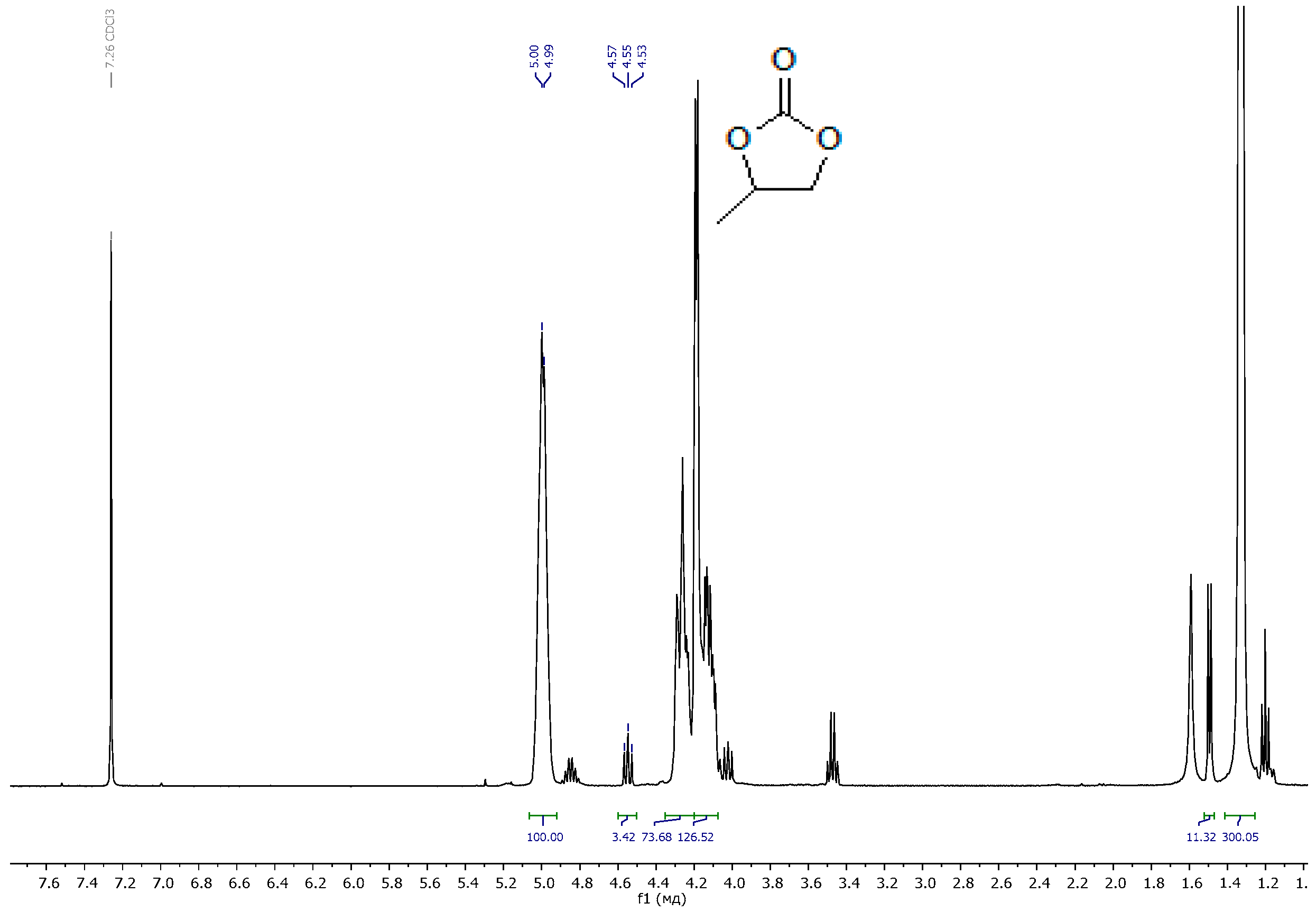
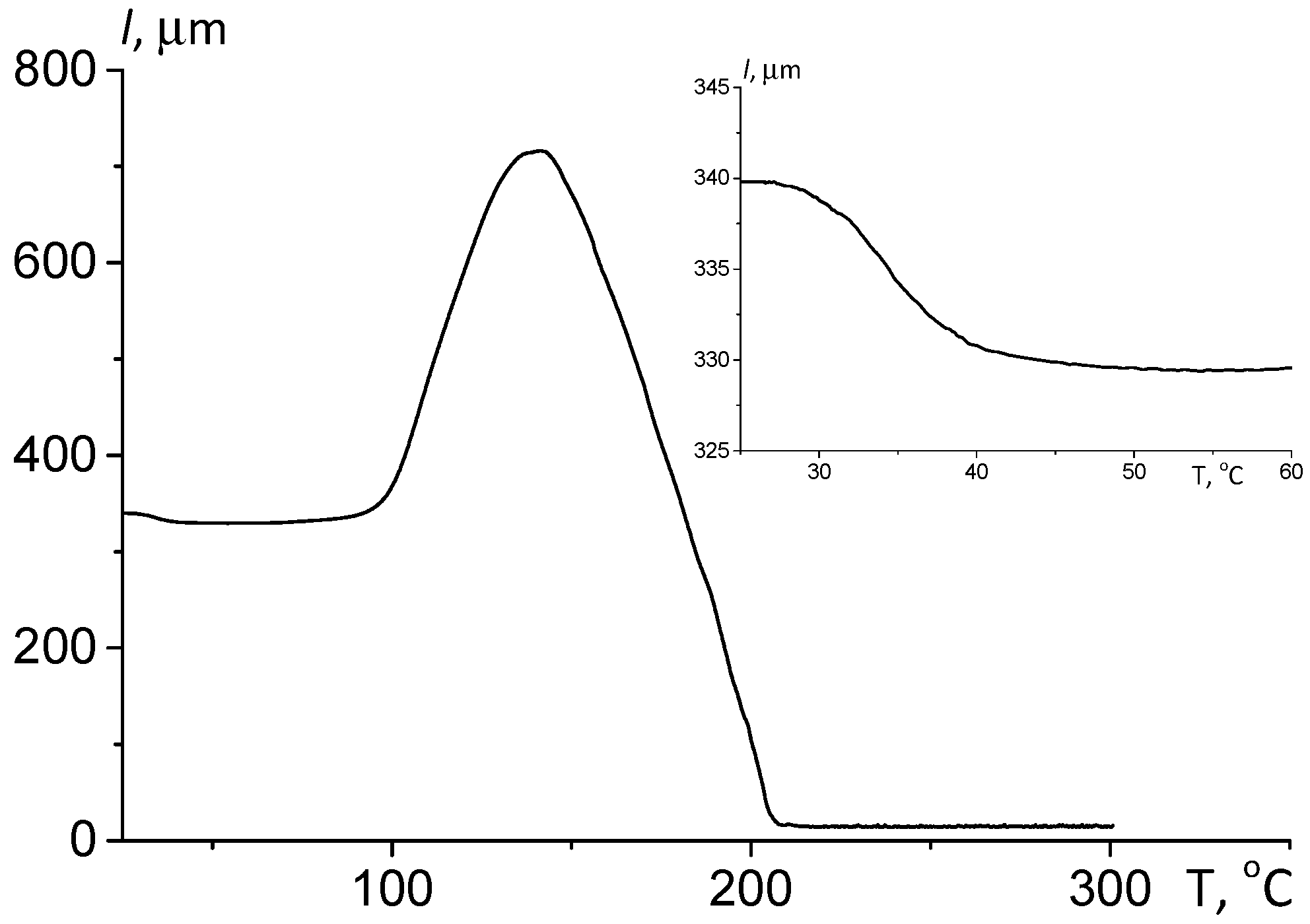
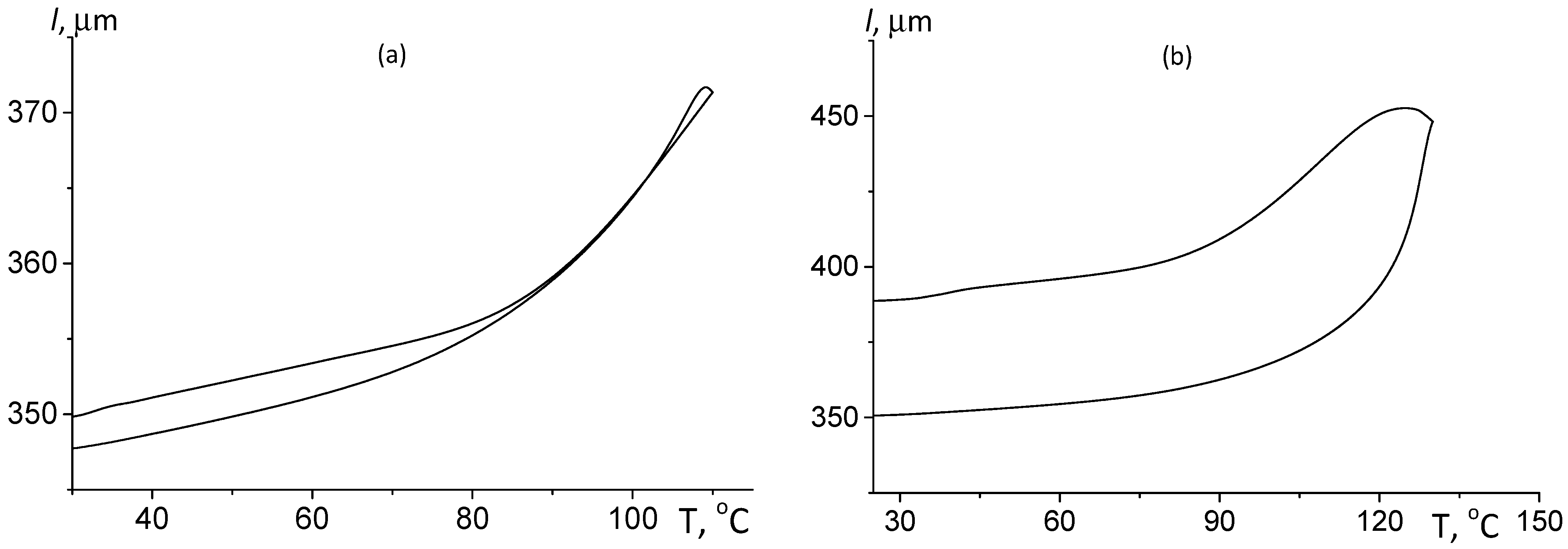
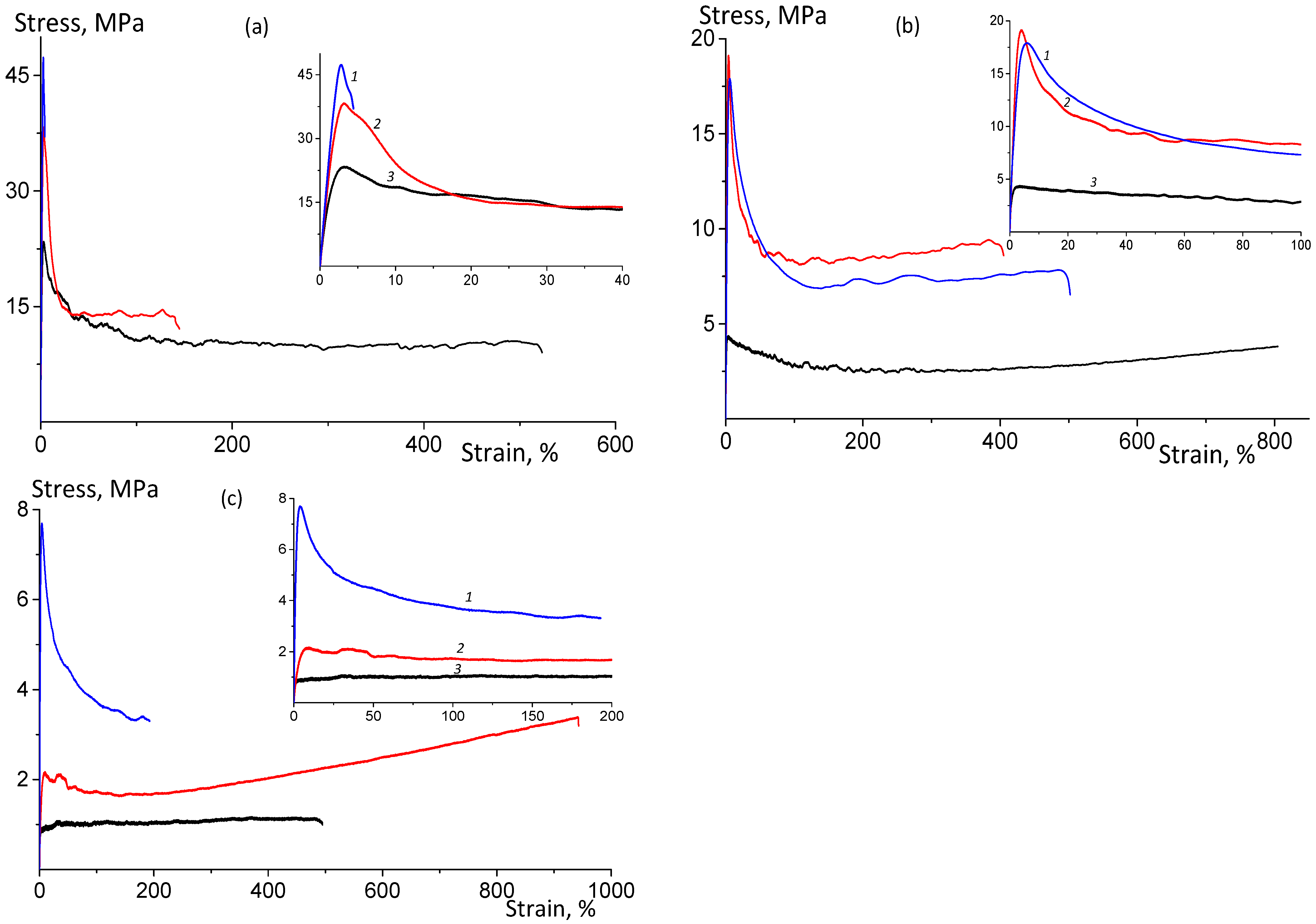
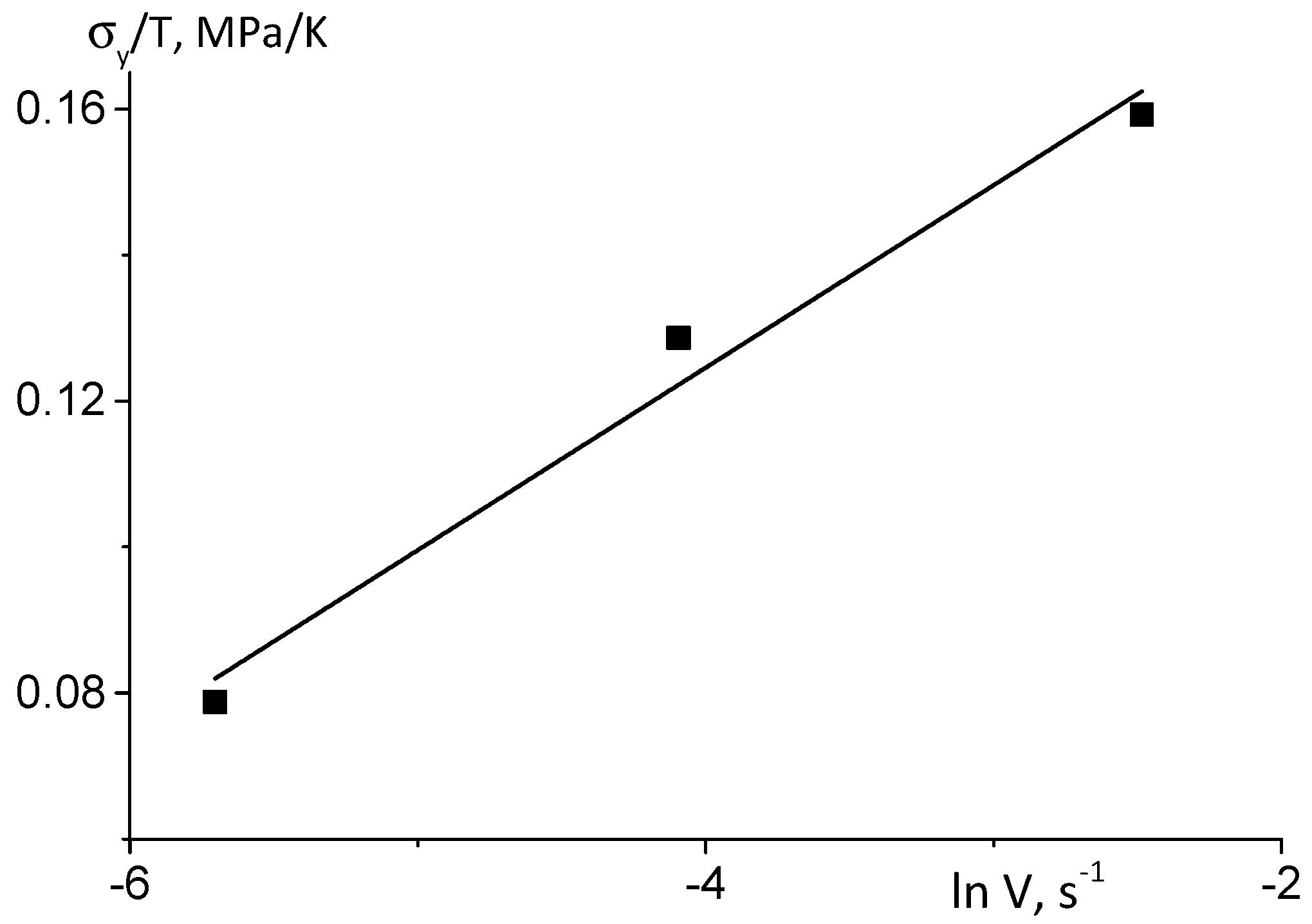
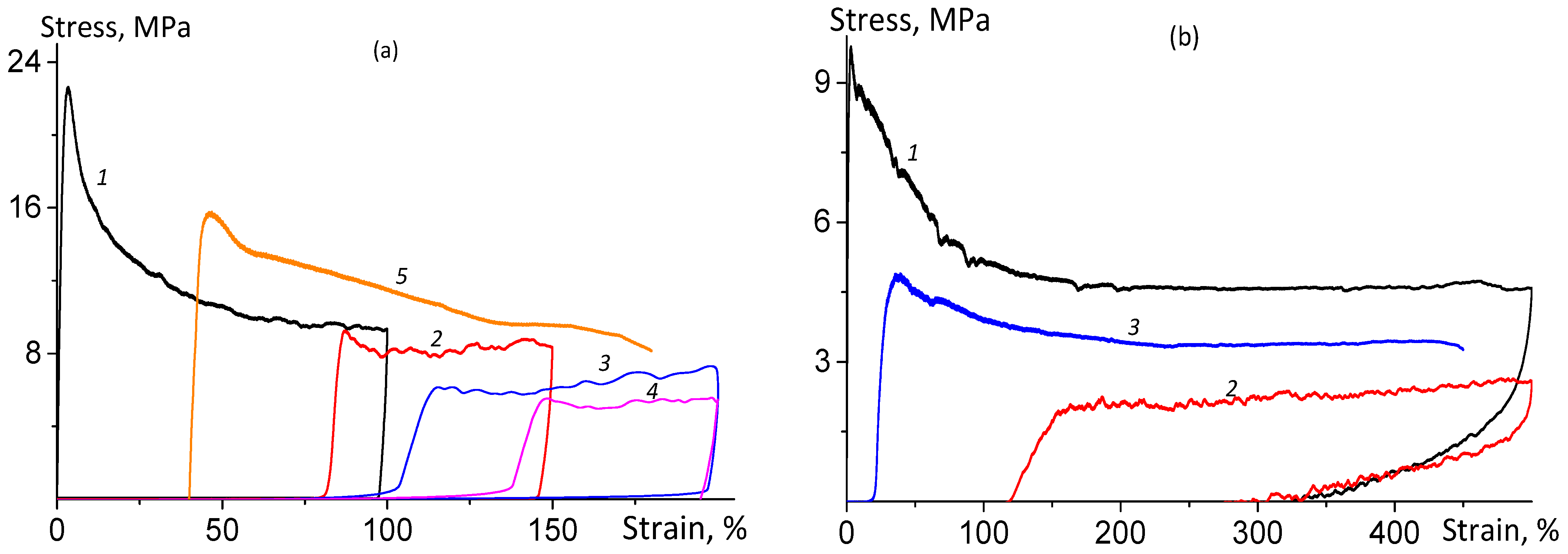
| Sample | Pressing Temperature, °C | Tg, °C | Mn, kDa/ĐM |
|---|---|---|---|
| PPC1 | – | 37.0 | 71.4/1.86 |
| PPC2 | 110 | 39.4 | 65.7/1.78 |
| PPC3 | 130 | 29.4 | 60.3/1.73 |
| PPC4 | 140 | 26.1 | 41.8/2.1 |
| Stretching Rate, mm min−1 | Young’s Modulus, GPa | Yield Point, MPa | Tensile Strength, MPa | Elongation at Break, % |
|---|---|---|---|---|
| Hot pressing at 110 °C | ||||
| 2 | 1.54 ± 0.15 | 23 ± 2 | 10 ± 1 | 510 ± 50 |
| 10 | 1.94 ± 0.20 | 38 ± 2 | 14 ± 1 | 140 ± 30 |
| 50 | 2.12 ± 0.25 | 47 ± 3 | 41 ± 2 | 4 ± 2 |
| Hot pressing at 130 °C | ||||
| 2 | 0.63 ± 0.07 | 3 ± 1 | 2 ± 1 | 810 ± 30 |
| 10 | 0.79 ± 0.08 | 19 ± 2 | 9 ± 2 | 390 ± 30 |
| 50 | 0.89 ± 0.09 | 18 ± 3 | 8 ± 2 | 490 ± 50 |
| Hot pressing at 140 °C | ||||
| 2 | 0.21 ± 0.03 | 1 ± 1 | 1 ± 1 | 480 ± 50 |
| 10 | 0.09 ± 0.05 | 2 ± 1 | 3 ± 1 | 940 ± 50 |
| 50 | 0.51 ± 0.05 | 8 ± 1 | 3 ± 1 | 180 ± 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trofimchuk, E.S.; Chernov, I.V.; Toms, R.V.; Rzhevskiy, S.A.; Asachenko, A.F.; Plutalova, A.V.; Shandryuk, G.A.; Chernikova, E.V.; Beletskaya, I.P. Novel Simple Approach for Production of Elastic Poly(propylene carbonate). Polymers 2024, 16, 3248. https://doi.org/10.3390/polym16233248
Trofimchuk ES, Chernov IV, Toms RV, Rzhevskiy SA, Asachenko AF, Plutalova AV, Shandryuk GA, Chernikova EV, Beletskaya IP. Novel Simple Approach for Production of Elastic Poly(propylene carbonate). Polymers. 2024; 16(23):3248. https://doi.org/10.3390/polym16233248
Chicago/Turabian StyleTrofimchuk, Elena S., Igor V. Chernov, Roman V. Toms, Sergey A. Rzhevskiy, Andrey F. Asachenko, Anna V. Plutalova, George A. Shandryuk, Elena V. Chernikova, and Irina P. Beletskaya. 2024. "Novel Simple Approach for Production of Elastic Poly(propylene carbonate)" Polymers 16, no. 23: 3248. https://doi.org/10.3390/polym16233248
APA StyleTrofimchuk, E. S., Chernov, I. V., Toms, R. V., Rzhevskiy, S. A., Asachenko, A. F., Plutalova, A. V., Shandryuk, G. A., Chernikova, E. V., & Beletskaya, I. P. (2024). Novel Simple Approach for Production of Elastic Poly(propylene carbonate). Polymers, 16(23), 3248. https://doi.org/10.3390/polym16233248









