Effect of Alkyl Side Chain Length on Electrical Performance of Ion-Gel-Gated OFETs Based on Difluorobenzothiadiazole-Based D-A Copolymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Structural Characterisation
2.3. The Preparation of Ion-Gel Based Capacitor
2.4. The Preparation of Ion-Gel-Gated OFETs
2.5. Electrical Properties’ Characterization
3. Results and Discussion
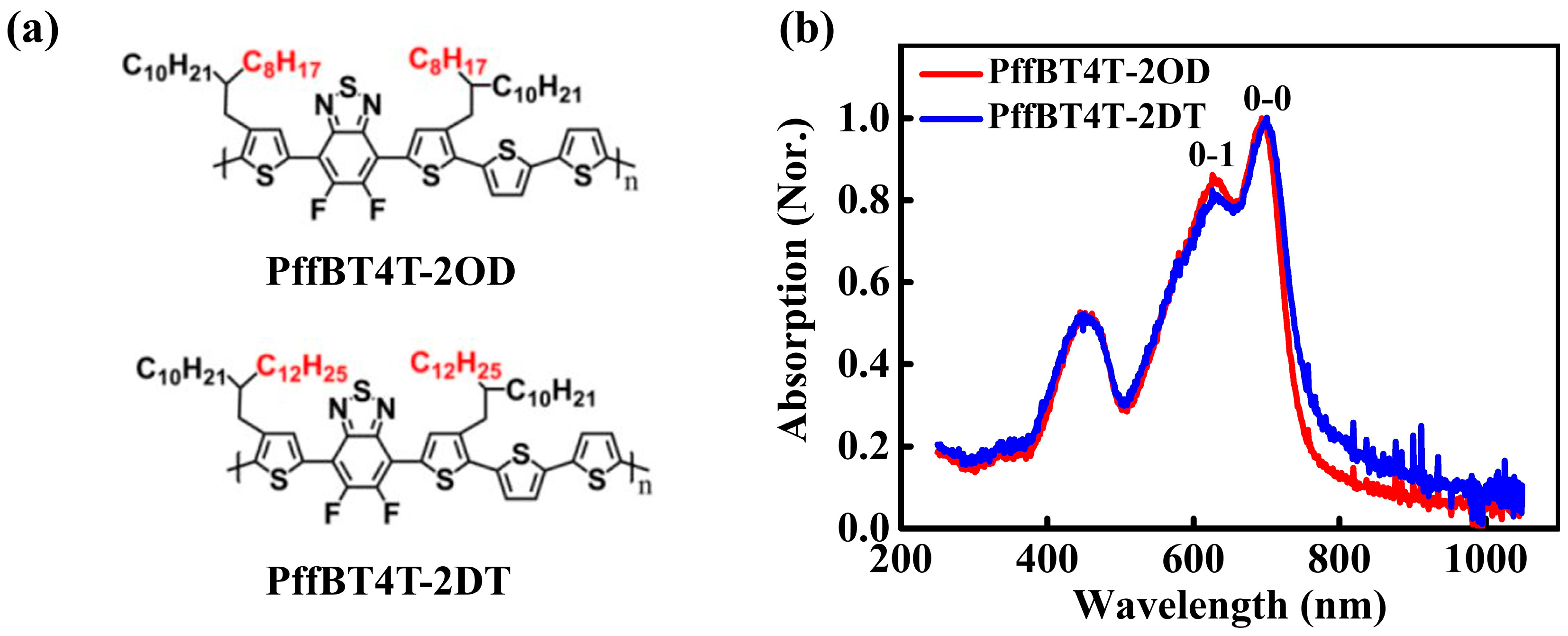
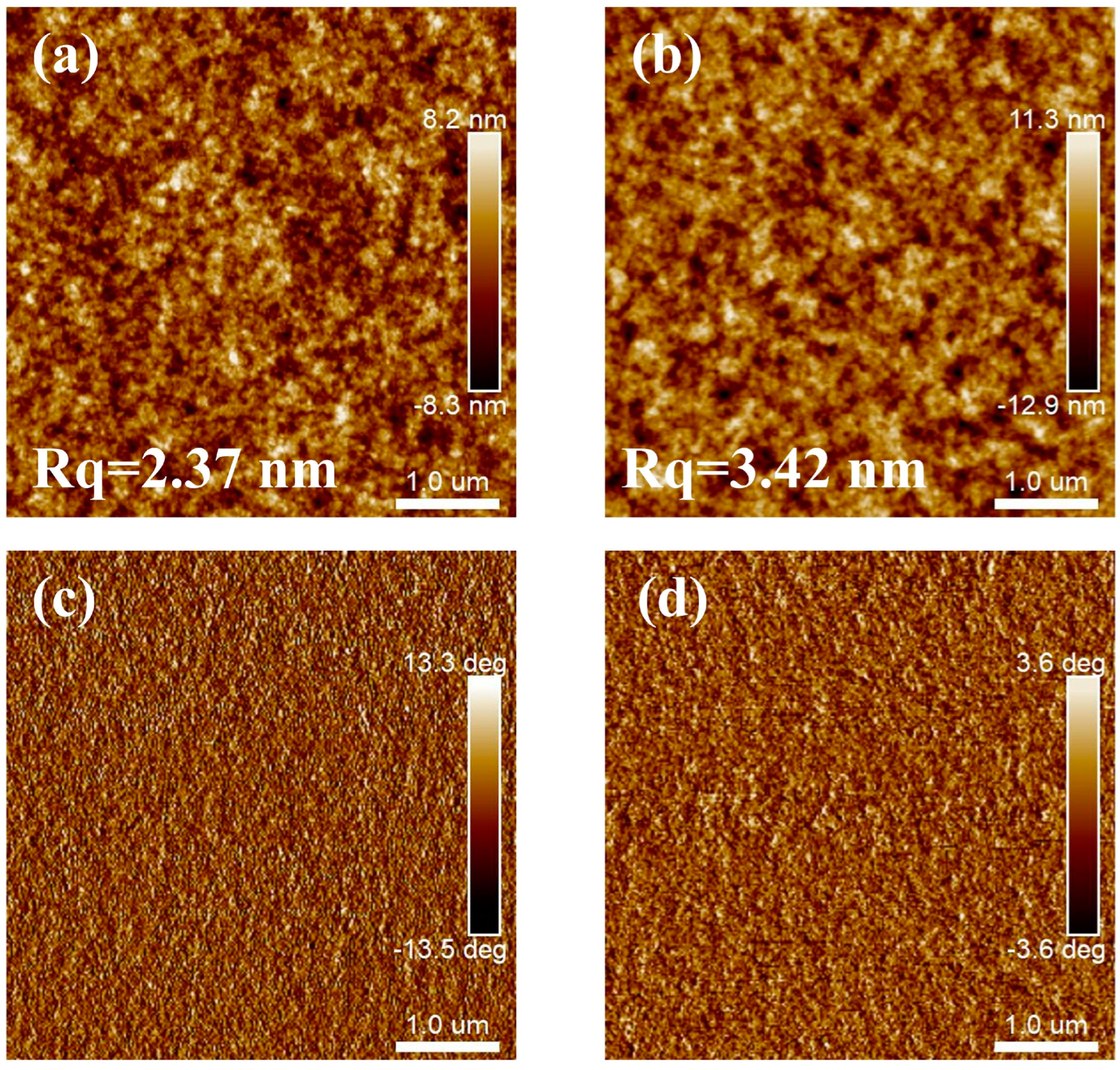
3.1. Microstructures of PffBT4T-2OD and PffBT4T-2DT Films
3.2. Electrical Performance of the SiO2-Gated and Ion-Gel-Gated OFETs
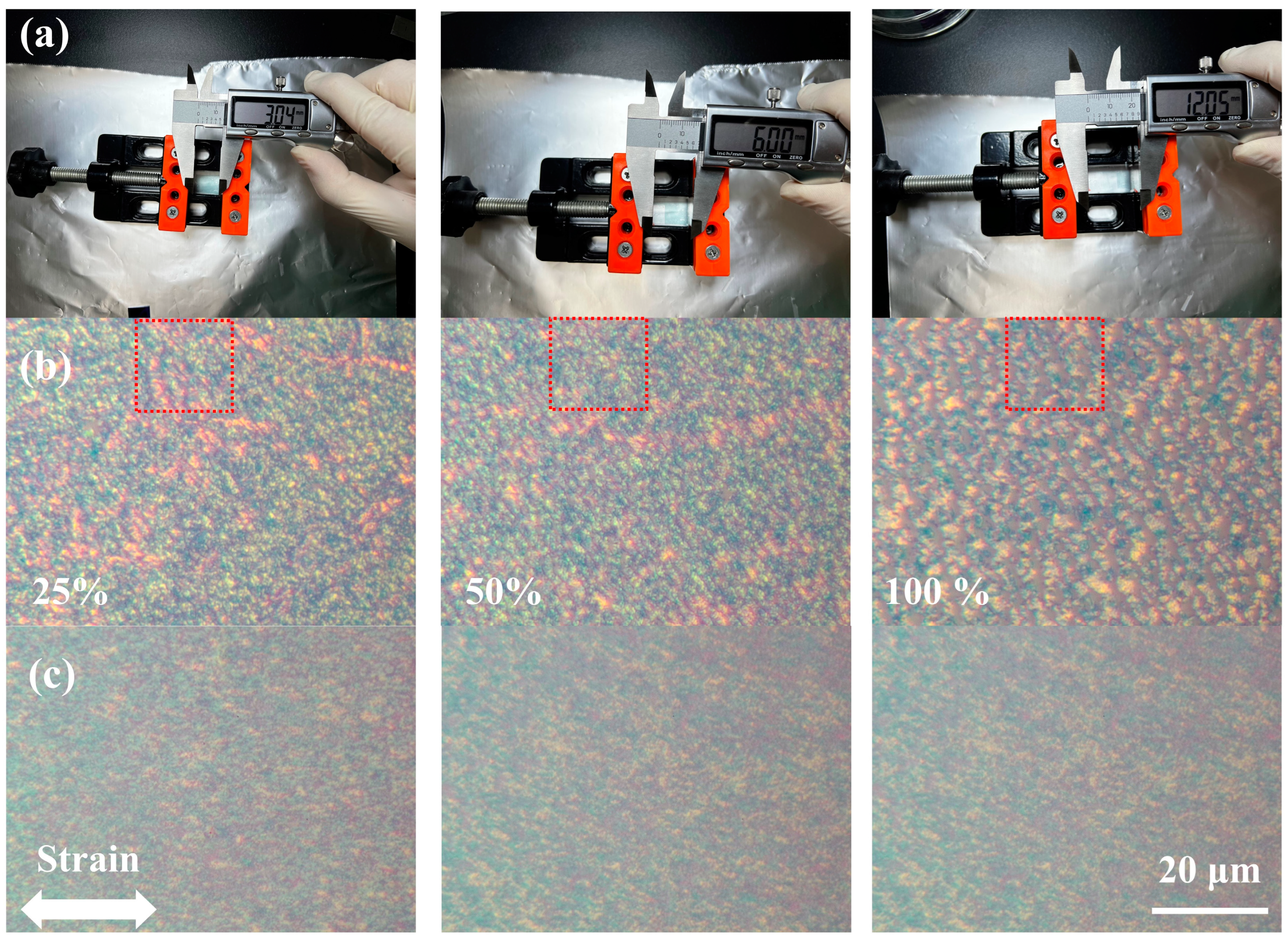
3.3. Electrical Performance of Flexible Ion-Gel-Gated OFETs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Y.; Liu, W.; Huang, Y.; Jin, C.; Zhou, B.; Sun, J.; Yang, J. Recent advances in flexible organic synaptic transistors. Adv. Electron. Mater. 2021, 7, 2100336. [Google Scholar] [CrossRef]
- Hui, Z.; Zhang, L.; Ren, G.; Sun, G.; Yu, H.; Huang, W. Green flexible electronics: Natural materials, fabrication, and applications. Adv. Mater. 2023, 35, 2211202. [Google Scholar] [CrossRef] [PubMed]
- Baruah, R.K.; Yoo, H.; Lee, E.K. Interconnection technologies for flexible electronics: Materials, fabrications, and applications. Micromachines 2023, 14, 1131. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.M.A.; Mahgoub, I.; Du, E.; Leavitt, M.A.; Asghar, W. Advances in healthcare wearable devices. npj Flex. Electron. 2021, 5, 9. [Google Scholar] [CrossRef]
- Haldar, T.; Wollandt, T.; Weis, J.; Zschieschang, U.; Klauk, H.; Weitz, R.T.; Burghartz, J.N.; Geiger, M. High-gain, low-voltage unipolar logic circuits based on nanoscale flexible organic thin-film transistors with small signal delays. Sci. Adv. 2023, 9, d3669. [Google Scholar] [CrossRef]
- Han, S.; Peng, H.; Sun, Q.; Venkatesh, S.; Chung, K.; Lau, S.C.; Zhou, Y.; Roy, V.A.L. An overview of the development of flexible sensors. Adv. Mater. 2017, 29, 1700375. [Google Scholar] [CrossRef]
- Liu, K.; Ouyang, B.; Guo, X.; Guo, Y.; Liu, Y. Advances in flexible organic field-effect transistors and their applications for flexible electronics. Npj Flex. Electron. 2022, 6, 1. [Google Scholar] [CrossRef]
- Wang, X.; Gu, Y.; Xiong, Z.; Cui, Z.; Zhang, T. Electronic skin: Silk-Molded flexible, ultrasensitive, and highly stable electronic skin for monitoring human physiological signals. Adv. Mater. 2014, 26, 1309. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, Y.; Zhong, D.; Zhang, Z.; Choudhury, S.; Lai, J.; Gong, H.; Niu, S.; Yan, X.; Zheng, Y.; et al. Neuromorphic sensorimotor loop embodied by monolithically integrated, low-voltage, soft e-skin. Science 2023, 380, 735–742. [Google Scholar] [CrossRef]
- Ye, H.; Ryu, K.Y.; Kwon, H.; Lee, H.; Wang, R.; Hong, J.; Choi, H.H.; Nam, S.Y.; Lee, J.; Kong, H.; et al. Amorphous fluorinated acrylate polymer dielectrics for flexible transistors and logic gates with high operational stability. ACS Appl. Mater. Inter. 2023, 15, 32610–32620. [Google Scholar] [CrossRef]
- Zhong, D.; Wu, C.; Jiang, Y.; Yuan, Y.; Kim, M.; Nishio, Y.; Shih, C.; Wang, W.; Lai, J.; Ji, X.; et al. High-speed and large-scale intrinsically stretchable integrated circuits. Nature 2024, 627, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Mangalore, D.K.; Zhao, J.; Carpenter, J.H.; Yan, H.; Ade, H.; Yan, H.; Müllen, K.; Blom, P.W.M.; Pisula, W.; et al. Integrated circuits based on conjugated polymer monolayer. Nat. Commun. 2018, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Rondeau-Gagné, S.; Chiu, Y.; Chortos, A.; Lissel, F.; Wang, G.N.; Schroeder, B.C.; Kurosawa, T.; Lopez, J.; Katsumata, T.; et al. Intrinsically stretchable and healable semiconducting polymer for organic transistors. Nature 2016, 539, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Michalek, L.; Liu, Q.; Wu, Y.; Kim, H.; Sayavong, P.; Yu, W.; Zhong, D.; Zhao, C.; Yu, Z.; et al. Environmentally stable and stretchable polymer electronics enabled by surface-tethered nanostructured molecular-level protection. Nat. Nanotechnol. 2023, 18, 1175–1184. [Google Scholar] [CrossRef]
- Zhang, G.; Mcbride, M.; Persson, N.; Lee, S.; Dunn, T.J.; Toney, M.F.; Yuan, Z.; Kwon, Y.; Chu, P.; Risteen, B.; et al. Versatile interpenetrating polymer network approach to robust stretchable electronic devices. Chem. Mater. 2017, 29, 7645–7652. [Google Scholar] [CrossRef]
- Qin, R.; Wu, Y.; Ding, Z.; Zhang, R.; Yu, J.; Huang, W.; Liu, D.; Lu, G.; Liu, S.F.; Zhao, K.; et al. Highly stretchable conjugated Polymer/Elastomer blend films with sandwich structure. Macromol. Rapid Comm. 2024, 45, 2300240. [Google Scholar] [CrossRef]
- Root, S.E.; Savagatrup, S.; Printz, A.D.; Rodriquez, D.; Lipomi, D.J. Mechanical properties of organic semiconductors for stretchable, highly flexible, and mechanically robust electronics. Chem. Rev. 2017, 117, 6467–6499. [Google Scholar] [CrossRef]
- Liu, H.; Liu, D.; Yang, J.; Gao, H.; Wu, Y. Flexible Electronics Based on Organic Semiconductors: From Patterned Assembly to Integrated Applications. Small 2023, 19, 2206938. [Google Scholar] [CrossRef]
- Xu, J.; Wang, S.; Wang, G.N.; Zhu, C.; Luo, S.; Jin, L.; Gu, X.; Chen, S.; Feig, V.R.; To, J.W.F.; et al. Highly stretchable polymer semiconductor films through the nanoconfinement effect. Science 2017, 355, 59–64. [Google Scholar] [CrossRef]
- Yang, J.; Jin, C.; Hu, B.; Liu, G.; Xu, H. Fully stretchable and skin-mountable ionic-gated organic phototransistors based on elastomeric semiconductor and dielectric. Appl. Phys. Lett. 2023, 122, 141104. [Google Scholar] [CrossRef]
- Qian, C.; Sun, J.; Yang, J.; Gao, Y. Flexible organic field-effect transistors on biodegradable cellulose paper with efficient reusable ion gel dielectrics. RSC Adv. 2015, 5, 14567–14574. [Google Scholar] [CrossRef]
- Sekiguchi, A.; Tanaka, F.; Saito, T.; Kuwahara, Y.; Sakurai, S.; Futaba, D.N.; Yamada, T.; Hata, K. Robust and soft elastomeric electronics tolerant to our daily lives. Nano Lett. 2015, 15, 5716–5723. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Sim, K.; Thukral, A.; Yu, C. Rubbery electronics and sensors from intrinsically stretchable elastomeric composites of semiconductors and conductors. Sci. Adv. 2017, 3, e1701114. [Google Scholar] [CrossRef] [PubMed]
- Herlogsson, L.; Crispin, X.; Robinson, N.D.; Sandberg, M.; Hagel, O.J.; Gustafsson, G.; Berggren, M. Low-Voltage Polymer Field-Effect Transistors Gated via a Proton Conductor. Adv. Mater. 2007, 19, 97–101. [Google Scholar] [CrossRef]
- Kim, S.H.; Hong, K.; Xie, W.; Lee, K.H.; Zhang, S.; Lodge, T.P.; Frisbie, C.D. Electrolyte-Gated transistors for organic and printed electronics. Adv. Mater. 2013, 25, 1822–1846. [Google Scholar] [CrossRef]
- Panzer, M.J.; Frisbie, C.D. Polymer Electrolyte-Gated organic Field-Effect transistors: Low-Voltage, High-Current switches for organic electronics and testbeds for probing electrical transport at high charge carrier density. J. Am. Chem. Soc. 2007, 129, 6599–6607. [Google Scholar] [CrossRef]
- Na, Y.; Kim, F.S. Nanodroplet-Embedded semiconducting polymer layers for electrochemically stable and High-Conductance organic Electrolyte-Gated transistors. Chem. Mater. 2019, 31, 4759–4768. [Google Scholar] [CrossRef]
- Nketia-Yawson, B.; Jung, A.; Nguyen, H.D.; Lee, K.; Kim, B.; Noh, Y. Difluorobenzothiadiazole and Selenophene-Based Conjugated Polymer Demonstrating an Effective Hole Mobility Exceeding 5 cm2 V–1 s–1 with Solid-State Electrolyte Dielectric. ACS Appl. Mater. Inter. 2018, 10, 32492–32500. [Google Scholar] [CrossRef]
- Shim, H.; Sim, K.; Ershad, F.; Yang, P.; Thukral, A.; Rao, Z.; Kim, H.; Liu, Y.; Wang, X.; Gu, G.; et al. Stretchable elastic synaptic transistors for neurologically integrated soft engineering systems. Sci. Adv. 2019, 5, x4961. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, G.; Yang, Y.; Mao, J.; Chen, Z.; Chen, S.; Wang, L.; Wu, D. Fabrication of flexible organic field effect transistors with high carrier mobility via sheath gas-assisted direct writing Poly(3-hexylthiophene) solution. Org. Electron. 2023, 119, 106813. [Google Scholar] [CrossRef]
- Campana, A.; Cramer, T.; Simon, D.T.; Berggren, M.; Biscarini, F. Electrocardiographic recording with conformable organic electrochemical transistor fabricated on resorbable bioscaffold. Adv. Mater. 2014, 26, 3874–3878. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.E.; Flagg, L.Q.; Onorato, J.W.; Richter, L.J.; Guo, J.; Luscombe, C.K.; Ginger, D.S. Impact of varying side chain structure on organic electrochemical transistor performance: A series of oligoethylene glycol-substituted polythiophenes. J. Mater. Chem. A 2022, 10, 10738–10749. [Google Scholar] [CrossRef]
- Bischak, C.G.; Flagg, L.Q.; Ginger, D.S. Ion exchange gels allow organic electrochemical transistor operation with hydrophobic polymers in aqueous solution. Adv. Mater. 2020, 32, 2002610. [Google Scholar] [CrossRef] [PubMed]
- Karimi Azari, R.; Gao, Z.; Carrière, A.; Santato, C. Exploring response time and synaptic plasticity in P3HT ion-gated transistors for neuromorphic computing: Impact of P3HT molecular weight and film thickness. RSC Appl. Interfaces 2024, 1, 564–572. [Google Scholar] [CrossRef]
- Liu, G.; Guo, Y.; Liu, Y. Design of ion-gated transistor materials at the molecular level. Matter 2024, 7, 430–455. [Google Scholar] [CrossRef]
- Sirringhaus, H. 25Th anniversary article: Organic Field-Effect transistors: The path beyond amorphous silicon. Adv. Mater. 2014, 26, 1319–1335. [Google Scholar] [CrossRef]
- Rim, Y.S.; Yang, Y.M.; Bae, S.; Chen, H.; Li, C.; Goorsky, M.S.; Yang, Y. Ultrahigh and broad spectral photodetectivity of an organic–inorganic hybrid phototransistor for flexible electronics. Adv. Mater. 2015, 27, 6885–6891. [Google Scholar] [CrossRef]
- Kim, M.; Ryu, S.U.; Park, S.A.; Choi, K.; Kim, T.; Chung, D.; Park, T. Donor–Acceptor-Conjugated polymer for High-Performance organic Field-Effect transistors: A progress report. Adv. Funct. Mater. 2020, 30, 1904545. [Google Scholar] [CrossRef]
- Wang, S.; Ha, M.; Manno, M.; Daniel Frisbie, C.; Leighton, C. Hopping transport and the Hall effect near the insulator–metal transition in electrochemically gated poly(3-hexylthiophene) transistors. Nat. Commun. 2012, 3, 1210. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, S.; Yin, R.; Li, L.; Lou, Z.; Shen, G. Recent advanced applications of ion-gel in ionic-gated transistor. Npj Flex. Electron. 2021, 5, 13. [Google Scholar] [CrossRef]
- Nikolka, M.; Simatos, D.; Foudeh, A.; Pfattner, R.; Mcculloch, I.; Bao, Z. Low-Voltage, Dual-Gate organic transistors with high sensitivity and stability toward electrostatic biosensing. ACS Appl. Mater. Inter. 2020, 12, 40581–40589. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; An, C.; Marszalek, T.; Baumgarten, M.; Yan, H.; Müllen, K.; Pisula, W. Controlling the surface organization of conjugated donor–acceptor polymers by their aggregation in solution. Adv. Mater. 2016, 28, 9430–9438. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Bin, H.; Jiao, X.; Wienk, M.M.; Yan, H.; Janssen, R.A.J. Controlling the microstructure of conjugated polymers in High-Mobility monolayer transistors via the dissolution temperature. Angew. Chem. Int. Ed. 2020, 59, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Bi, Z.; Naveed, H.B.; Mao, Y.; Yan, H.; Ma, W. Importance of nucleation during morphology evolution of the Blade-Cast PffBT4T-2OD-Based organic solar cells. Macromolecules 2018, 51, 6682–6691. [Google Scholar] [CrossRef]
- Chen, Z.; Cai, P.; Chen, J.; Liu, X.; Zhang, L.; Lan, L.; Peng, J.; Ma, Y.; Cao, Y. Low Band-Gap conjugated polymers with strong interchain aggregation and very high hole mobility towards highly efficient Thick-Film polymer solar cells. Adv. Mater. 2014, 26, 2586–2591. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, Q.; Lv, Y.; Deng, K.; Deng, Y.; Li, Q.; Qi, S.; Chen, W.; Zhang, H. Flexible and highly photosensitive Electrolyte-Gated organic transistors with Ion-gel/Silver nanowire membranes. ACS Appl. Mater. Inter. 2017, 9, 18134–18141. [Google Scholar] [CrossRef]
- Choi, J.; Kim, W.; Kim, D.; Kim, S.; Chae, J.; Choi, S.Q.; Kim, F.S.; Kim, T.; Kim, B.J. Importance of critical molecular weight of semicrystalline n-Type polymers for mechanically robust, efficient electroactive thin films. Chem. Mater. 2019, 31, 3163–3173. [Google Scholar] [CrossRef]
- Fu, F.; Liao, K.; Ma, J.; Cheng, Z.; Zheng, D.; Gao, L.; Liu, C.; Li, S.; Li, W. How intermolecular interactions influence electronic absorption spectra: Insights from the molecular packing of uracil in condensed phases. Phys. Chem. Chem. Phys. 2019, 21, 4072–4081. [Google Scholar] [CrossRef]
- Li, X.; Angunawela, I.; Chang, Y.; Zhou, J.; Huang, H.; Zhong, L.; Liebman-Pelaez, A.; Zhu, C.; Meng, L.; Xie, Z.; et al. Effect of the chlorine substitution position of the end-group on intermolecular interactions and photovoltaic performance of small molecule acceptors. Energy Environ. Sci. 2020, 13, 5028–5038. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Li, Z.; Mu, C.; Ma, W.; Hu, H.; Jiang, K.; Lin, H.; Ade, H.; Yan, H. Aggregation and morphology control enables multiple cases of high-efficiency polymer solar cells. Nat. Commun. 2014, 5, 5293. [Google Scholar] [CrossRef]
- Li, M.; Leenaers, P.J.; Wienk, M.M.; Janssen, R.A.J. The effect of alkyl side chain length on the formation of two semi-crystalline phases in low band gap conjugated polymers. J. Mater. Chem. C 2020, 8, 5856–5867. [Google Scholar] [CrossRef]
- Sekhon, S.S.; Lalia, B.S.; Park, J.; Kim, C.; Yamada, K. Physicochemical properties of proton conducting membranes based on ionic liquid impregnated polymer for fuel cells. J. Mater. Chem. 2006, 16, 2256–2265. [Google Scholar] [CrossRef]
- Wang, P.; Zakeeruddin, S.M.; Exnar, I.; Grätzel, M. High efficiency dye-sensitized nanocrystalline solar cells based on ionic liquid polymer gel electrolyte. Chem. Commun. 2002, 24, 2972–2973. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Kang, M.S.; Zhang, S.; Gu, Y.; Lodge, T.P.; Frisbie, C.D. “cut and stick” rubbery ion gels as high capacitance gate dielectrics. Adv. Mater. 2012, 24, 4457–4462. [Google Scholar] [CrossRef] [PubMed]
- Verploegen, E.; Mondal, R.; Bettinger, C.J.; Sok, S.; Toney, M.F.; Bao, Z. Effects of thermal annealing upon the morphology of polymer–fullerene blends. Adv. Funct. Mater. 2010, 20, 3519–3529. [Google Scholar] [CrossRef]
- Son, S.Y.; Park, T.; You, W. Understanding of Face-On crystallites transitioning to Edge-On crystallites in Thiophene-Based conjugated polymers. Chem. Mater. 2021, 33, 4541–4550. [Google Scholar] [CrossRef]
- Giovannitti, A.; Maria, I.P.; Hanifi, D.; Donahue, M.J.; Bryant, D.; Barth, K.J.; Makdah, B.E.; Savva, A.; Moia, D.; Zetek, M.; et al. The role of the side chain on the performance of n-type conjugated polymers in aqueous electrolytes. Chem. Mater. 2018, 30, 2945–2953. [Google Scholar] [CrossRef]
- Flagg, L.Q.; Bischak, C.G.; Onorato, J.W.; Rashid, R.B.; Luscombe, C.K.; Ginger, D.S. Polymer crystallinity controls water uptake in glycol Side-Chain polymer organic electrochemical transistors. J. Am. Chem. Soc. 2019, 141, 4345–4354. [Google Scholar] [CrossRef]
- Xia, D.; Wu, Y.; Wang, Q.; Zhang, A.; Li, C.; Lin, Y.; Colberts, F.J.M.; van Franeker, J.J.; Janssen, R.A.J.; Zhan, X.; et al. Effect of alkyl side chains of conjugated polymer donors on the device performance of Non-Fullerene solar cells. Macromolecules 2016, 49, 6445–6454. [Google Scholar] [CrossRef]
- Huang, W.; Chen, J.; Yao, Y.; Zheng, D.; Ji, X.; Feng, L.; Moore, D.; Glavin, N.R.; Xie, M.; Chen, Y.; et al. Vertical organic electrochemical transistors for complementary circuits. Nature 2023, 613, 496–502. [Google Scholar] [CrossRef]
- Doumbia, A.; Tong, J.; Wilson, R.J.; Turner, M.L. Investigation of the performance of donor–acceptor conjugated polymers in Electrolyte-Gated organic Field-Effect transistors. Adv. Electron. Mater. 2021, 7, 2100071. [Google Scholar] [CrossRef]
- Xu, J.; Wu, H.; Zhu, C.; Ehrlich, A.; Shaw, L.; Nikolka, M.; Wang, S.; Molina-Lopez, F.; Gu, X.; Luo, S.; et al. Multi-scale ordering in highly stretchable polymer semiconducting films. Nat. Mater. 2019, 18, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, Y.; Zhang, L.; Wang, S.; Li, J.; Zuo, J.; Yu, X.; Zhang, Q.; Han, Y. Constructing a desired nanofibril network morphology for stretchable polymer films by weakening the intermolecular interaction of a conjugated polymer in an elastomer matrix and extending the film-forming time. J. Mater. Chem. C 2023, 11, 2302–2315. [Google Scholar] [CrossRef]
- Guo, S.; Tong, Y.; Wang, X.; Zhang, M.; Yu, H.; Ren, H.; Tang, Q.; Lu, G.; Liu, Y. Brittle PCDTPT based elastic hybrid networks for transparent stretchable Skin-Like electronics. Adv. Electron. Mater. 2023, 9, 2200438. [Google Scholar] [CrossRef]
- Lei, Y.; Deng, P.; Zhang, Q.; Xiong, Z.; Li, Q.; Mai, J.; Lu, X.; Zhu, X.; Ong, B.S. Hydrocarbons-Driven crystallization of polymer semiconductors for Low-Temperature fabrication of High-Performance organic Field-Effect transistors. Adv. Funct. Mater. 2018, 28, 1706372. [Google Scholar] [CrossRef]




| π-π Stacking (010) | Lamellar Stacking (100) | |||||||
|---|---|---|---|---|---|---|---|---|
| q (Å−1) | d010 (nm) | FWHM (°) | CCL010 (nm) | q (Å−1) | d100 (nm) | FWHM (°) | CCL100 (nm) | |
| PffBT4T-2OD | 1.763 | 0.356 | 1.02 | 6.59 | 0.282 | 2.23 | 0.21 | 30.17 |
| PffBT4T-2DT | 1.760 | 0.357 | 1.03 | 6.53 | 0.254 | 2.47 | 0.17 | 37.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Cheng, Z.; Pan, G.; Hu, L.; Zhang, F. Effect of Alkyl Side Chain Length on Electrical Performance of Ion-Gel-Gated OFETs Based on Difluorobenzothiadiazole-Based D-A Copolymers. Polymers 2024, 16, 3287. https://doi.org/10.3390/polym16233287
Zhou H, Cheng Z, Pan G, Hu L, Zhang F. Effect of Alkyl Side Chain Length on Electrical Performance of Ion-Gel-Gated OFETs Based on Difluorobenzothiadiazole-Based D-A Copolymers. Polymers. 2024; 16(23):3287. https://doi.org/10.3390/polym16233287
Chicago/Turabian StyleZhou, Han, Zaitian Cheng, Guoxing Pan, Lin Hu, and Fapei Zhang. 2024. "Effect of Alkyl Side Chain Length on Electrical Performance of Ion-Gel-Gated OFETs Based on Difluorobenzothiadiazole-Based D-A Copolymers" Polymers 16, no. 23: 3287. https://doi.org/10.3390/polym16233287
APA StyleZhou, H., Cheng, Z., Pan, G., Hu, L., & Zhang, F. (2024). Effect of Alkyl Side Chain Length on Electrical Performance of Ion-Gel-Gated OFETs Based on Difluorobenzothiadiazole-Based D-A Copolymers. Polymers, 16(23), 3287. https://doi.org/10.3390/polym16233287







