A Novel Polymer Film to Develop Heart Valve Prostheses
Abstract
:1. Introduction
- (1)
- Polyhedral oligomeric silsesquioxane poly(carbonate–urea) urethane (POSS-PCU);
- (2)
- Siloxane poly(urethane–urea) (SiPUU, LifePolymer);
- (3)
- Poly(styrene-b-isobutylene-b-styrene) (SIBS) and poly(styrene-b-4-vinylbenzocyclobuteneb-isobutylene-b-styrene-b-4-vinylbenzocylcobutene) (xSIBS);
- (4)
2. Materials and Methods
2.1. Materials
Bovine Pericardium Treatment
2.2. Non-Implanted Sample Studies
2.2.1. Water Contact Angle Measurement
2.2.2. Mechanical Testing
2.2.3. Hemocompatibility Study
Platelet Aggregation Test
Platelet Adhesion Test
2.2.4. Atomic Force Microscopy (AFM)
2.2.5. Cytocompatibility Evaluation
Materials for Cell Cultivation
Evaluation of Cell Adhesion, Proliferation, and Viability
2.3. Implanted-Sample Studies
2.3.1. Subcutaneous Implantation in Rats
2.3.2. Histological Studies
2.4. Scanning Electron Microscopy (SEM) and Energy Dispersive Spectrometry (EDS) Analysis
2.5. Statistical Analysis
3. Results
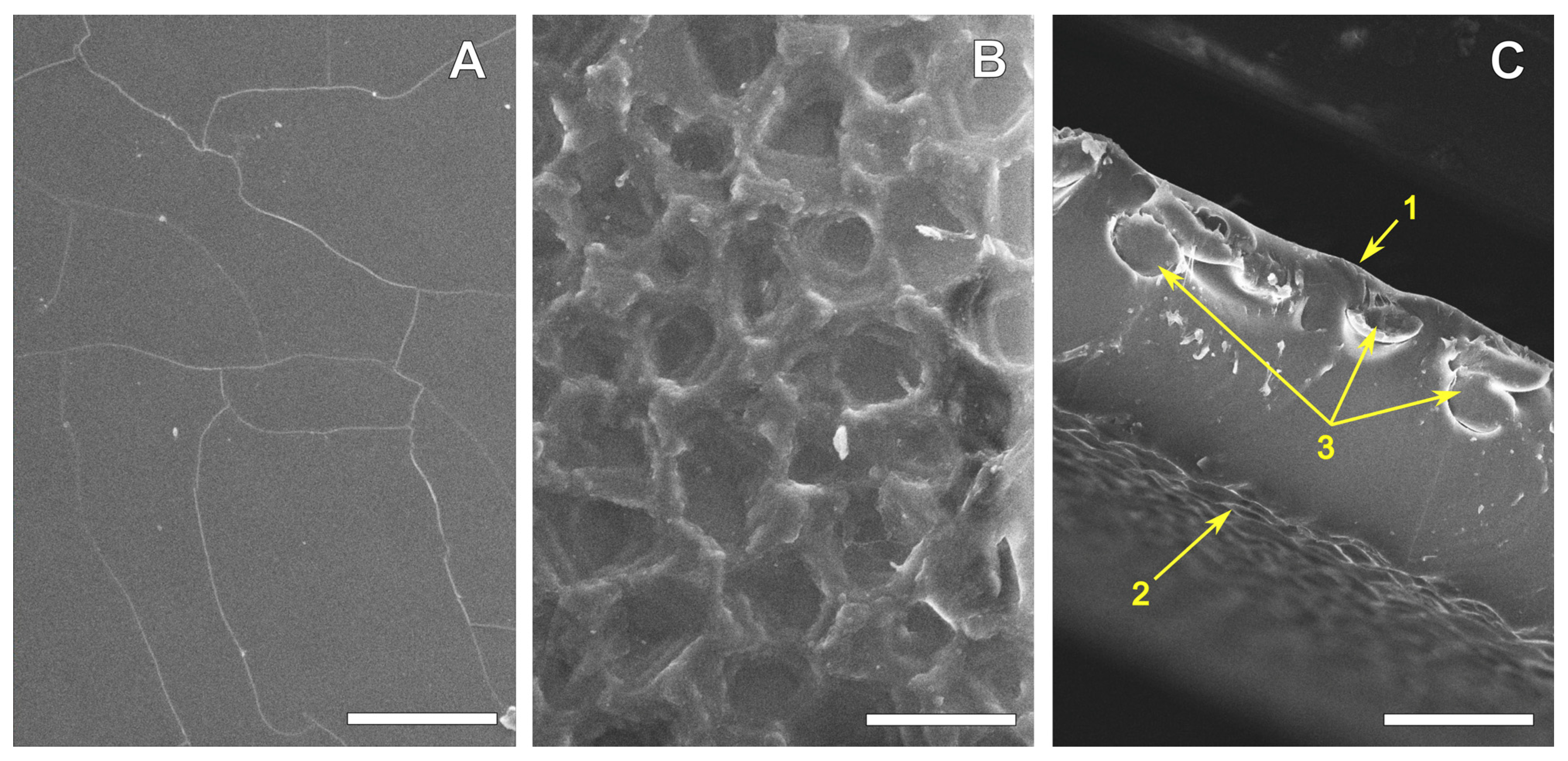
3.1. REPEREN® Film Structure and Hydrophilicity
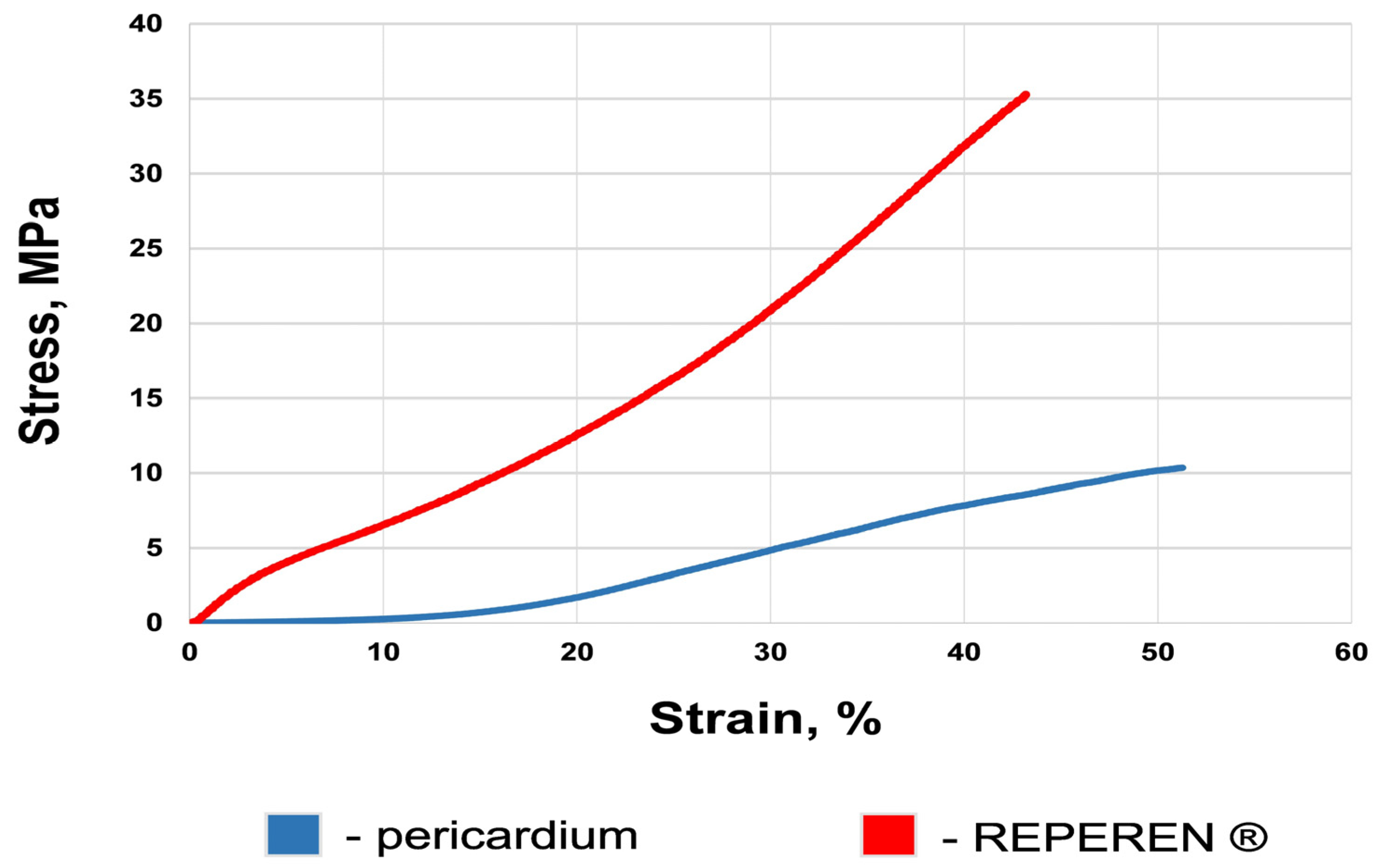
3.2. Mechanical Features of the Materials
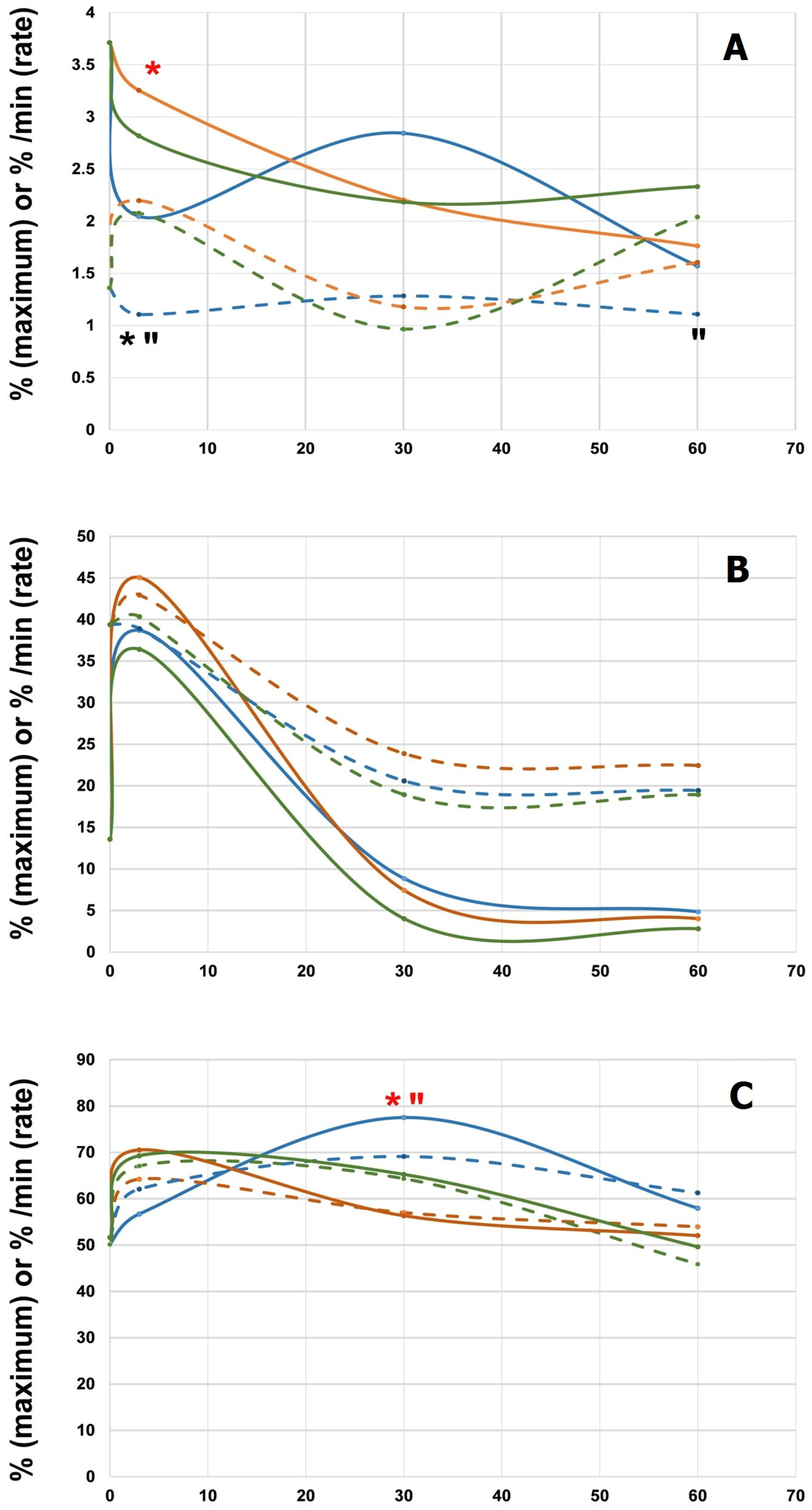
3.3. Hemocompatibility
3.4. Cytocompatibility (In Vitro Test)
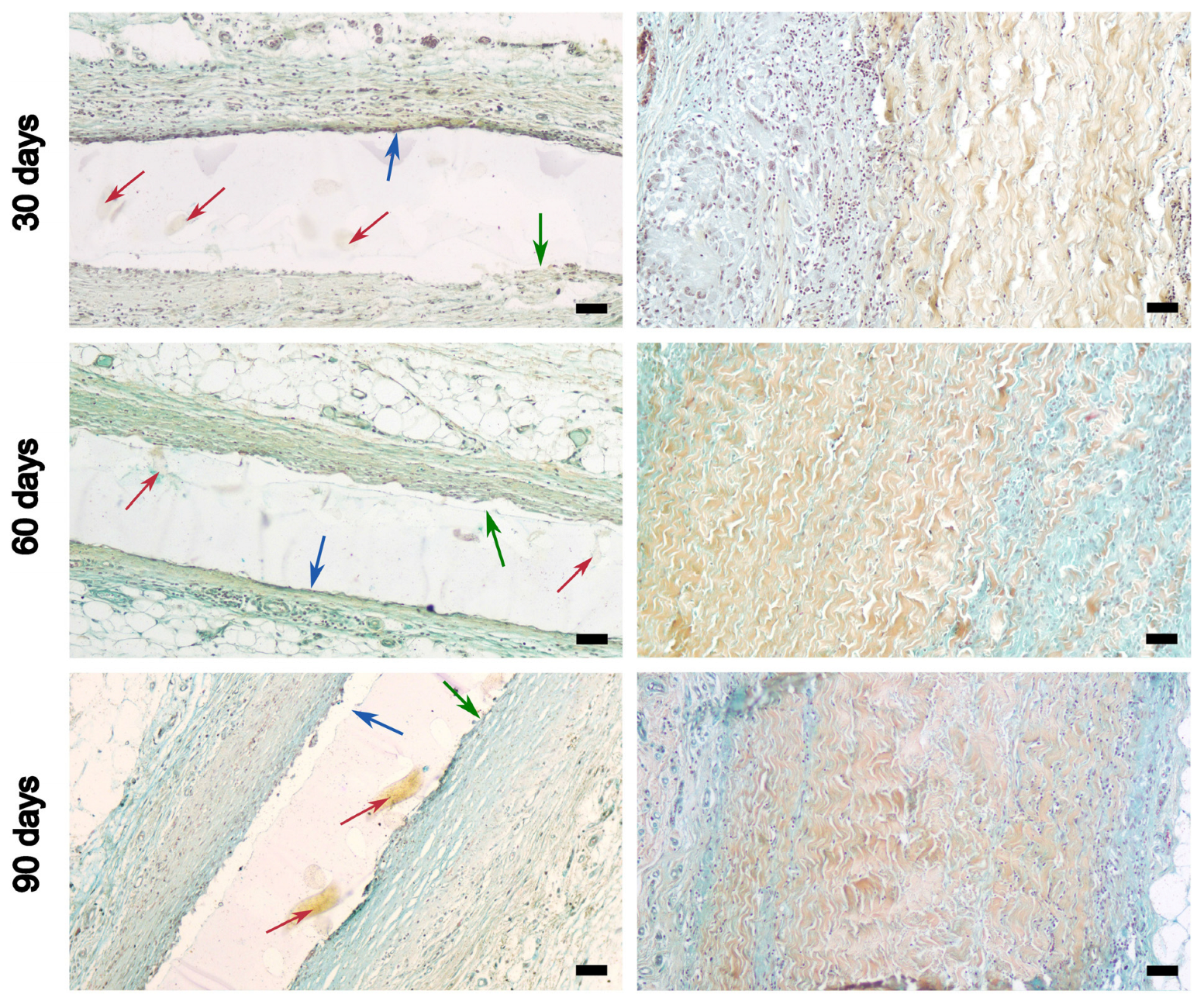
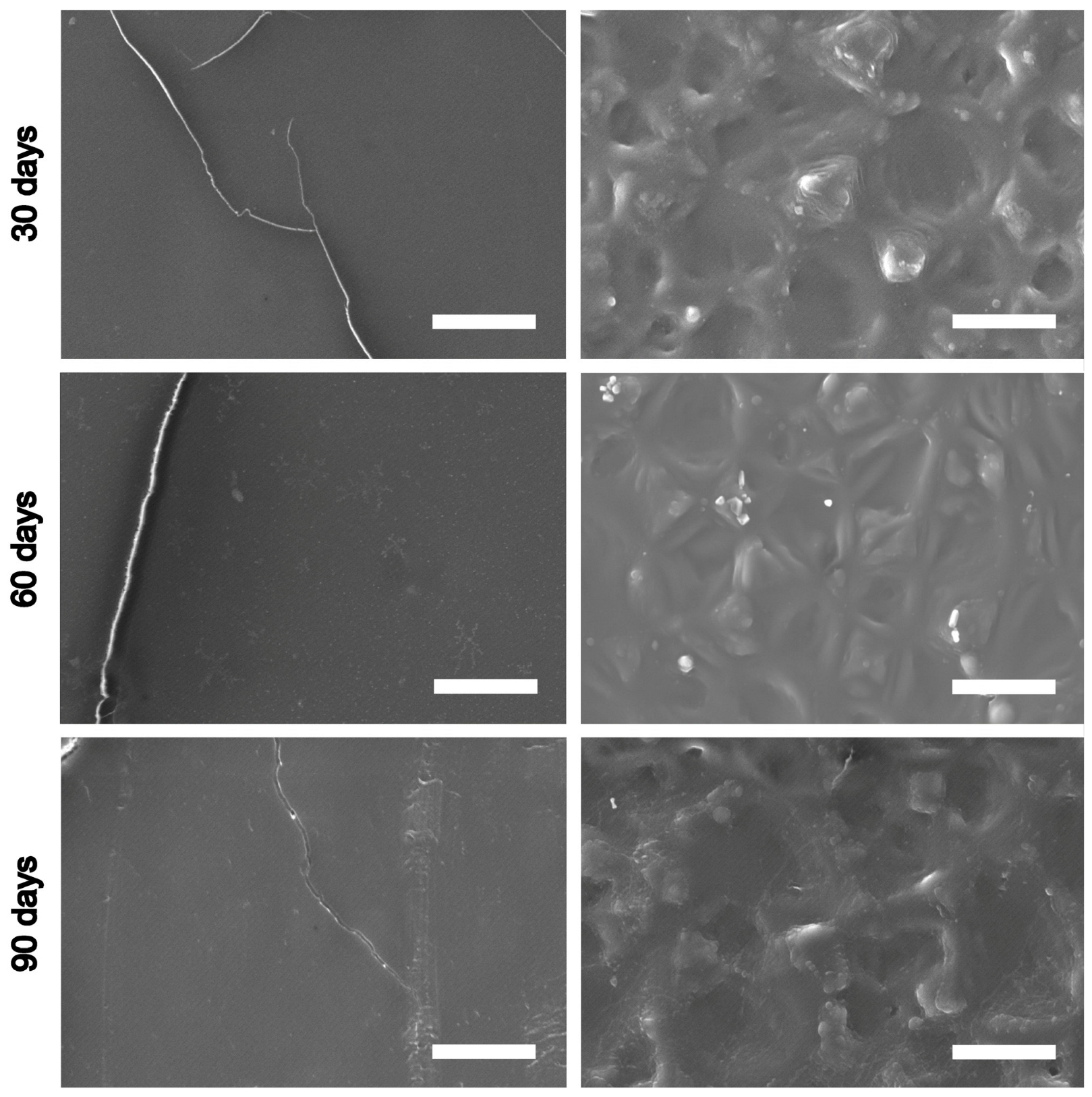
3.5. Results of Subcutaneous Implantation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Carroll, J.D.; Mack, M.J.; Vemulapalli, S.; Herrmann, H.C.; Gleason, T.G.; Hanzel, G.; Deeb, G.M.; Thourani, V.H.; Cohen, D.J.; Desai, N.; et al. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. Ann. Thorac. Surg. 2021, 111, 701–722. [Google Scholar] [CrossRef] [PubMed]
- Donmazov, S.; Piskin, S.; Gölcez, T.; Kul, D.; Arnaz, A.; Pekkan, K. Mechanical characterization and torsional buckling of pediatric cardiovascular materials. Biomech. Model. Mechanobiol. 2024, 23, 845–860. [Google Scholar] [CrossRef] [PubMed]
- Noble, C.; Hooke, A.; Rajotia, A.; Morse, D.; Dragomir-Daescu, D.; Salisbury, J.; Young, M.D.; Lerman, A. Effect of mechanical fatigue on commercial bioprosthetic TAVR valve mechanical and microstructural properties. J. Mech. Behav. Biomed. Mater. 2024, 154, 106441. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Zhao, L.; Wu, Z.; Li, B.; Liu, R.; He, H.; Wang, L.; Wang, W. Comparison on the properties of bovine pericardium and porcine pericardium used as leaflet materials of transcatheter heart valve. Artif. Organs 2022, 46, 427–438. [Google Scholar] [CrossRef]
- Liang, X.; Zheng, C.; Ding, K.; Huang, X.; Zhang, S.; Lei, Y.; Yu, K.; Wang, Y. Arginine-grafted porcine pericardium by copolymerization to improve the cytocompatibility, hemocompatibility and anti-calcification properties of bioprosthetic heart valve materials. J. Mater. Chem. B 2022, 10, 5571–5581. [Google Scholar] [CrossRef]
- Wu, B.; Zheng, C.; Ding, K.; Huang, X.; Li, M.; Zhang, S.; Lei, Y.; Guo, Y.; Wang, Y. Cross-Linking Porcine Pericardium by 3,4-Dihydroxybenzaldehyde: A Novel Method to Improve the Biocompatibility of Bioprosthetic Valve. Biomacromolecules 2021, 22, 823–836. [Google Scholar] [CrossRef]
- Sakaue, T.; Koyama, T.; Nakamura, Y.; Okamoto, K.; Kawashima, T.; Umeno, T.; Nakayama, Y.; Miyamoto, S.; Shikata, F.; Hamaguchi, M.; et al. Bioprosthetic Valve Deterioration: Accumulation of Circulating Proteins and Macrophages in the Valve Interstitium. JACC Basic Transl. Sci. 2023, 8, 862–880. [Google Scholar] [CrossRef]
- Patel, S.P.; Garcia, S.; Sathananthan, J.; Tang, G.H.L.; Albaghdadi, M.S.; Pibarot, P.; Cubeddu, R.J. Structural Valve Deterioration in Transcatheter Aortic Bioprostheses: Diagnosis, Pathogenesis, and Treatment. Struct. Heart 2023, 7, 100155. [Google Scholar] [CrossRef]
- Xue, Y.; Kossar, A.P.; Abramov, A.; Frasca, A.; Sun, M.; Zyablitskaya, M.; Paik, D.; Kalfa, D.; Della Barbera, M.; Thiene, G.; et al. Age-related enhanced degeneration of bioprosthetic valves due to leaflet calcification, tissue crosslinking, and structural changes. Cardiovasc. Res. 2023, 119, 302–315. [Google Scholar] [CrossRef]
- Schoen, F.J.; Levy, R.J. Bioprosthetic Heart Valve Calcification: Clinicopathologic Correlations, Mechanisms, and Prevention. In Cardiovascular Calcification and Bone Mineralization; Aikawa, E., Hutcheson, J.D., Eds.; Humana Press: Totowa, NJ, USA; Springer Nature Switzerland AG: Cham, Switzerland, 2020; pp. 183–218. [Google Scholar]
- Balmforth, D.; Dimagli, A.; Benedetto, U.; Uppal, R. Fifty years of the pericardial valve: Long-term results in the aortic position. J. Card. Surg. 2021, 36, 2865–2875. [Google Scholar] [CrossRef]
- Sharifulin, R.; Bogachev-Prokophiev, A.; Demin, I.; Afanasyev, A.; Ovcharov, M.; Pivkin, A.; Sapegin, A.; Zhuravleva, I.; Karaskov, A. Allografts and xenografts for right ventricular outflow tract reconstruction in Ross patients. Eur. J. Cardiothorac. Surg. 2021, 59, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Nichay, N.R.; Zhuravleva, I.Y.; Kulyabin, Y.Y.; Zubritskiy, A.V.; Voitov, A.V.; Soynov, I.A.; Gorbatykh, A.V.; Bogachev-Prokophiev, A.V.; Karaskov, A.M. Diepoxy-Versus Glutaraldehyde-Treated Xenografts: Outcomes of Right Ventricular Outflow Tract Reconstruction in Children. World J. Pediatr. Congenit. Heart Sur. 2020, 11, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Nichay, N.R.; Dokuchaeva, A.A.; Kulyabin, Y.Y.; Boyarkin, E.V.; Kuznetsova, E.V.; Rusakova, Y.L.; Murashov, I.S.; Vaver, A.A.; Bogachev-Prokophiev, A.V.; Zhuravleva, I.Y. Epoxy- versus Glutaraldehyde-Treated Bovine Jugular Vein Conduit for Pulmonary Valve Replacement: A Comparison of Morphological Changes in a Pig Model. Biomedicines 2023, 11, 3101. [Google Scholar] [CrossRef] [PubMed]
- Zhuravleva, I.Y.; Karpova, E.V.; Dokuchaeva, A.A.; Titov, A.T.; Timchenko, T.P.; Vasilieva, M.B. Calcification of Various Bioprosthetic Materials in Rats: Is It Really Different? Int. J. Mol. Sci. 2023, 24, 7274. [Google Scholar] [CrossRef]
- Bezuidenhout, D.; Williams, D.F.; Zilla, P. Polymeric heart valves for surgical implantation, catheter-based technologies and heart assist devices. Biomaterials 2015, 36, 6–25. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, Y.; Wang, Q.; Kong, D.; Wang, Z.; Liu, J. Recent advancements in polymeric heart valves: From basic research to clinical trials. Mater. Today Bio 2024, 28, 101194. [Google Scholar] [CrossRef]
- Rezvova, M.A.; Klyshnikov, K.Y.; Gritskevich, A.A.; Ovcharenko, E.A. Polymeric Heart Valves Will Displace Mechanical and Tissue Heart Valves: A New Era for the Medical Devices. Int. J. Mol. Sci. 2023, 24, 3963. [Google Scholar] [CrossRef]
- Choi, K.Y.; Sung, S.C.; Kim, H.; Lee, H.D.; Kim, G.; Ko, H.; Byun, J.-H.; Kim, W.-H.; Kim, E.R.; Park, H.K.; et al. Simplified Tricuspid Polytetrafluoroethylene Valved Conduit: Midterm Results of Multicenter Study. Ann. Thorac. Surg. 2019, 108, 1228–1233. [Google Scholar] [CrossRef]
- Chang, T.-I.; Hsu, K.-H.; Li, S.-J.; Chuang, M.-K.; Luo, C.-W.; Chen, Y.-J.; Chang, C.-I. Evolution of pulmonary valve reconstruction with focused review of expanded polytetrafluoroethylene handmade valves. Interact. Cardiovasc. Thorac. Surg. 2021, 32, 585–592. [Google Scholar] [CrossRef]
- Ghanbari, H.; Kidane, A.G.; Burriesci, G.; Ramesh, B.; Darbyshire, A.; Seifalian, A.M. The anti-calcification potential of a silsesquioxane nanocomposite polymer under in vitro conditions: Potential material for synthetic leaflet heart valve. Acta Biomater. 2010, 6, 4249–4260. [Google Scholar] [CrossRef]
- Ghanbari, H.; de Mel, A.; Seifalian, A.M. Cardiovascular application of polyhedral oligomeric silsesquioxane nanomaterials: A glimpse into prospective horizons. Intern. J. Nanomed. 2011, 6, 775–786. [Google Scholar] [CrossRef]
- Rahmani, B.; Tzamtzis, S.; Ghanbari, H.; Burriesci, G.; Seifalian, A.M. Manufacturing and hydrodynamic assessment of a novel aortic valve made of a new nanocomposite polymer. J. Biomech. 2012, 45, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Tschorn, P.; Schröter, F.; Hartrumpf, M.; Kühnel, R.-U.; Ostovar, R.; Albes, J.M. Engineering a New Polymeric Heart Valve Using 3D Printing—TRISKELION. Medicina 2022, 58, 1695. [Google Scholar] [CrossRef] [PubMed]
- Schröter, F.; Kühnel, R.-U.; Hartrumpf, M.; Ostovar, R.; Albes, J.M. Progress on a Novel, 3D-Printable Heart Valve Prosthesis. Polymers 2023, 15, 4413. [Google Scholar] [CrossRef]
- Ge, J.; Zhou, D.; Zhang, X.; Hou, S.; Chen, S.; Jin, Q.; Pan, W.; Li, W.; Pan, C.; Qian, J. Preliminary Implantation of a Novel TAVR Device with Polymeric Leaflets for Symptomatic Calcific Aortic Disease. JACC Case Rep. 2023, 25, 101901. [Google Scholar] [CrossRef]
- Dandeniyage, L.S.; Gunatillake, P.A.; Adhikari, R.; Bown, M.; Shanks, R.; Adhikari, B. Development of high strength siloxane poly(urethane-urea) elastomers based on linked macrodiols for heart valve application. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 1712–1720. [Google Scholar] [CrossRef]
- Dandeniyage, L.S.; Adhikari, R.; Bown, M.; Shanks, R.; Adhikari, B.; Easton, C.D.; Gengenbach, T.R.; Cookson, D.; Gunatillake, P.A. Morphology and surface properties of high strength siloxane poly(urethane-urea)s developed for heart valve application. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 112–121. [Google Scholar] [CrossRef]
- Dandeniyage, L.S.; Knower, W.; Adhikari, R.; Bown, M.; Shanks, R.; Adhikari, B.; Gunatillake, P.A. In vitro oxidative stability of high strength siloxane poly(urethane-urea) elastomers based on linked-macrodiol. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 2557–2565. [Google Scholar] [CrossRef]
- Kereiakes, D.J.; Answini, G.A.; Yakubov, S.J.; Rai, B.; Smith, J.M.; Duff, S.; Shannon, F.L.; Sakwa, M.; Beith, J.; Heimansohn, D. Preliminary evaluation of a novel polymeric valve following surgical implantation for symptomatic aortic valve disease. JACC Cardiovasc. Interv. 2021, 14, 2754–2756. [Google Scholar] [CrossRef]
- TRIA™ Polymer Surgical Valves Surpass 200 Patient Life Years. Available online: https://www.pcronline.com/News/Press-releases/2024/TRIA-polymer-surgical-valves-surpass-200-patient-life-years (accessed on 29 October 2024).
- Singh, S.K.; Kachel, M.; Castillero, E.; Xue, Y.; Kalfa, D.; Ferrari, G.; George, I. Polymeric prosthetic heart valves: A review of current technologies and future directions. Front. Cardiovasc. Med. 2023, 10, 1137827. [Google Scholar] [CrossRef]
- Ovcharenko, E.A.; Seifalian, A.; Rezvova, M.A.; Klyshnikov, K.Y.; Glushkova, T.V.; Akenteva, T.N.; Antonova, L.V.; Velikanova, E.A.; Chernonosova, V.S.; Shevelev, G.Y.; et al. A new nanocomposite copolymer Based on functionalised Graphene oxide for Development of Heart Valves. Sci. Rep. 2020, 10, 5271. [Google Scholar] [CrossRef] [PubMed]
- Slepičková Kasálková, N.; Rimpelová, S.; Vacek, C.; Fajstavr, D.; Švorčík, V.; Sajdl, P.; Slepička, P. Surface activation of Hastalex by vacuum argon plasma for cytocompatibility enhancement. Heliyon 2024, 10, e27816. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.; Yadav, V.; Ishaq, A.; Sanipini, S.; Ekhator, C.; Khleif, R.; Beheshtaein, A.; Jhajj, L.K.; Khan, A.W.; Al Khalifa, A.; et al. Trends and Challenges in the Development of 3D-Printed Heart Valves and Other Cardiac Implants: A Review of Current Advances. Cureus 2023, 15, e43204. [Google Scholar] [CrossRef] [PubMed]
- Zhuravleva, I.Y. Biocidal Composition for Aseptic Storage of Preserved Prosthetic Material from Tissues of Animal Origin. Patent RU 2580621 C1, 10 April 2016. Available online: https://patents.google.com/patent/RU2580621C1/en (accessed on 29 October 2024).
- Zhuravleva, I.Y.; Karpova, E.V.; Oparina, L.A.; Poveschenko, O.V.; Surovtseva, M.A.; Titov, A.T.; Ksenofontov, A.L.; Vasilieva, M.B.; Kuznetsova, E.V.; Bogachev-Prokophiev, A.V.; et al. Cross-linking method using pentaepoxide for improving bovine and porcine bioprosthetic pericardia: A multiparametric assessment study. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111473. [Google Scholar] [CrossRef]
- Gauvin, R.; Marinov, G.; Mehri, Y.; Klein, J.; Li, B.; Larouche, D.; Guzman, R.; Zhang, Z.; Germain, L.; Guidoin, R. A comparative study of bovine and porcine pericardium to highlight their potential advantages to manufacture percutaneous cardiovascular implants. J. Biomater. Appl. 2013, 28, 552–565. [Google Scholar] [CrossRef]
- Marmur, A. The Lotus effect: Superhydrophobicity and metastability. Langmuir 2004, 20, 3517–3519. [Google Scholar] [CrossRef]
- Dokuchaeva, A.A.; Timchenko, T.P.; Karpova, E.V.; Vladimirov, S.V.; Soynov, I.A.; Zhuravleva, I.Y. Effects of Electrospinning Parameter Adjustment on the Mechanical Behavior of Poly-ε-caprolactone Vascular Scaffolds. Polymers 2022, 14, 349. [Google Scholar] [CrossRef]
- Liu, H.; Wu, F.-Y.; Zhong, G.-J.; Li, Z.-M. Predicting the complex stress-strain curves of polymeric solids by classification-embedded dual neural network. Mater. Des. 2023, 227, 111773. [Google Scholar] [CrossRef]
- Available online: https://iconlab.ru/ (accessed on 29 October 2024).
- Zhuravleva, I.Y.; Surovtseva, M.A.; Vaver, A.A.; Suprun, E.A.; Kim, I.I.; Bondarenko, N.A.; Kuzmin, O.S.; Mayorov, A.P.; Poveshchenko, O.V. Effect of the Nanorough Surface of TiO2 Thin Films on the Compatibility with Endothelial Cells. Int. J. Mol. Sci. 2023, 24, 6699. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, Y.; Cui, B. New perspectives on the roles of nanoscale surface topography in modulating intracellular signaling. Curr. Opin. Solid State Mater. Sci. 2021, 25, 100873. [Google Scholar] [CrossRef]
- Horev, M.B.; Zabary, Y.; Zarka, R.; Sorrentino, S.; Medalia, O.; Zaritsky, A.; Geiger, B. Differential dynamics of early stages of platelet adhesion and spreading on collagen IV- and fibrinogen-coated surfaces. F1000Res 2020, 9, 449. [Google Scholar] [CrossRef]
- Shin, E.K.; Park, H.; Noh, J.Y.; Lim, K.M.; Chung, J.H. Platelet Shape Changes and Cytoskeleton Dynamics as Novel Therapeutic Targets for Anti-Thrombotic Drugs. Biomol. Ther. 2017, 25, 223–230. [Google Scholar] [CrossRef]
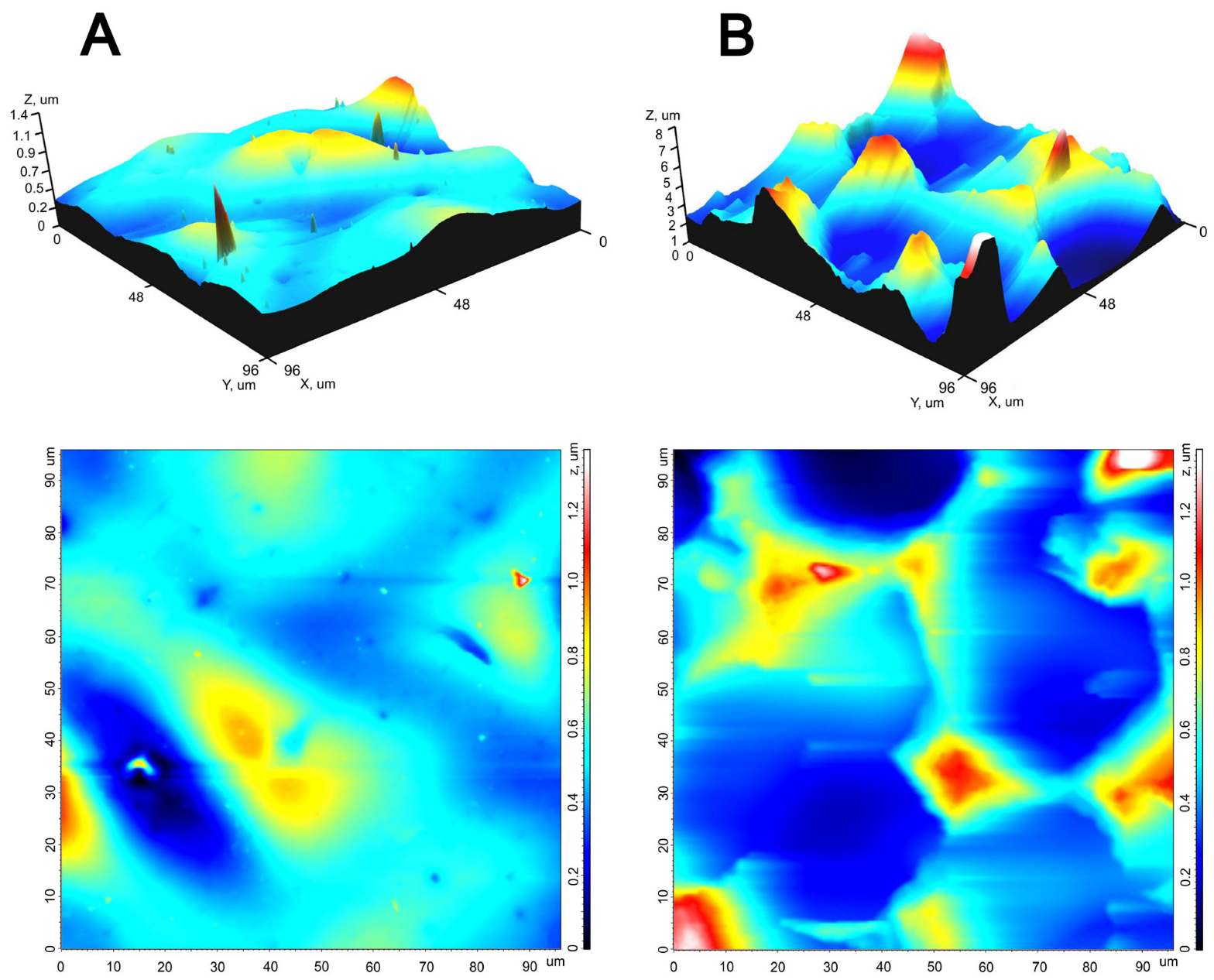


| Parameter | Smooth Surface | Rough Surface | p |
|---|---|---|---|
| Sq, μm | 0.20 (0.15; 0.24) | 1.40 (1.31; 1.63) | 0.0001 |
| Sp, μm | 1.32 (1.21; 1.41) | 5.92 (5.62; 7.56) | 0.0026 |
| Sv, μm | 0.76 (0.65; 0.87) | 2.78 (2.39; 3.30) | 0.003 |
| Sz, μm | 2.08 (1.86; 2.28) | 9.02 (8.01; 10.85) | 0.00001 |
| Sds, 1/μm2 | 0.04 (0.03; 0.07) | 0.01 (0.01; 0.02) | 0.0015 |
| Ssc, 1/μm | 0.15 (0.12; 0.15) | 0.64 (0.41; 0.85) | 0.001 |
| Surface | Contact Angle, ° | Drop Image | |
|---|---|---|---|
| Ellipse-Fitting | The Young-Laplace Algorithm | ||
| Smooth, 0 s | 82 ± 3 | 82 ± 4 |  |
| rough, 0–38 s | 89 ± 6 | 91 ± 5 |  |
| rough, 60 s | 77.2 ± 0.3 | 76.3 ± 0.6 |  |
| Parameter | DE-Treated BP | REPEREN® Film |
|---|---|---|
| Ultimate tensile stress, MPa | 10.3 (9.0; 12.5) | 35.7 (31.4; 40.7) |
| Deformability, % | 49.9 (44.7; 56.2) | 40.0 (32.0; 59.7) |
| Elastic modulus | 17.5 (14.6; 21.6) | 81.8 (65.6; 95.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuravleva, I.Y.; Dokuchaeva, A.A.; Vaver, A.A.; Kreiker, L.V.; Mochalova, A.B.; Chepeleva, E.V.; Surovtseva, M.A.; Kolodin, A.N.; Kuznetsova, E.V.; Grek, R.I. A Novel Polymer Film to Develop Heart Valve Prostheses. Polymers 2024, 16, 3373. https://doi.org/10.3390/polym16233373
Zhuravleva IY, Dokuchaeva AA, Vaver AA, Kreiker LV, Mochalova AB, Chepeleva EV, Surovtseva MA, Kolodin AN, Kuznetsova EV, Grek RI. A Novel Polymer Film to Develop Heart Valve Prostheses. Polymers. 2024; 16(23):3373. https://doi.org/10.3390/polym16233373
Chicago/Turabian StyleZhuravleva, Irina Yu., Anna A. Dokuchaeva, Andrey A. Vaver, Ludmila V. Kreiker, Alexandra B. Mochalova, Elena V. Chepeleva, Maria A. Surovtseva, Aleksei N. Kolodin, Elena V. Kuznetsova, and Rostislav I. Grek. 2024. "A Novel Polymer Film to Develop Heart Valve Prostheses" Polymers 16, no. 23: 3373. https://doi.org/10.3390/polym16233373
APA StyleZhuravleva, I. Y., Dokuchaeva, A. A., Vaver, A. A., Kreiker, L. V., Mochalova, A. B., Chepeleva, E. V., Surovtseva, M. A., Kolodin, A. N., Kuznetsova, E. V., & Grek, R. I. (2024). A Novel Polymer Film to Develop Heart Valve Prostheses. Polymers, 16(23), 3373. https://doi.org/10.3390/polym16233373










