A UV-Protective Textile Coating Based on Recycled Poly(vinyl butyral) (PVB): A New Life for a Waste Polymer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of re-PVB Formulations for Coating Application
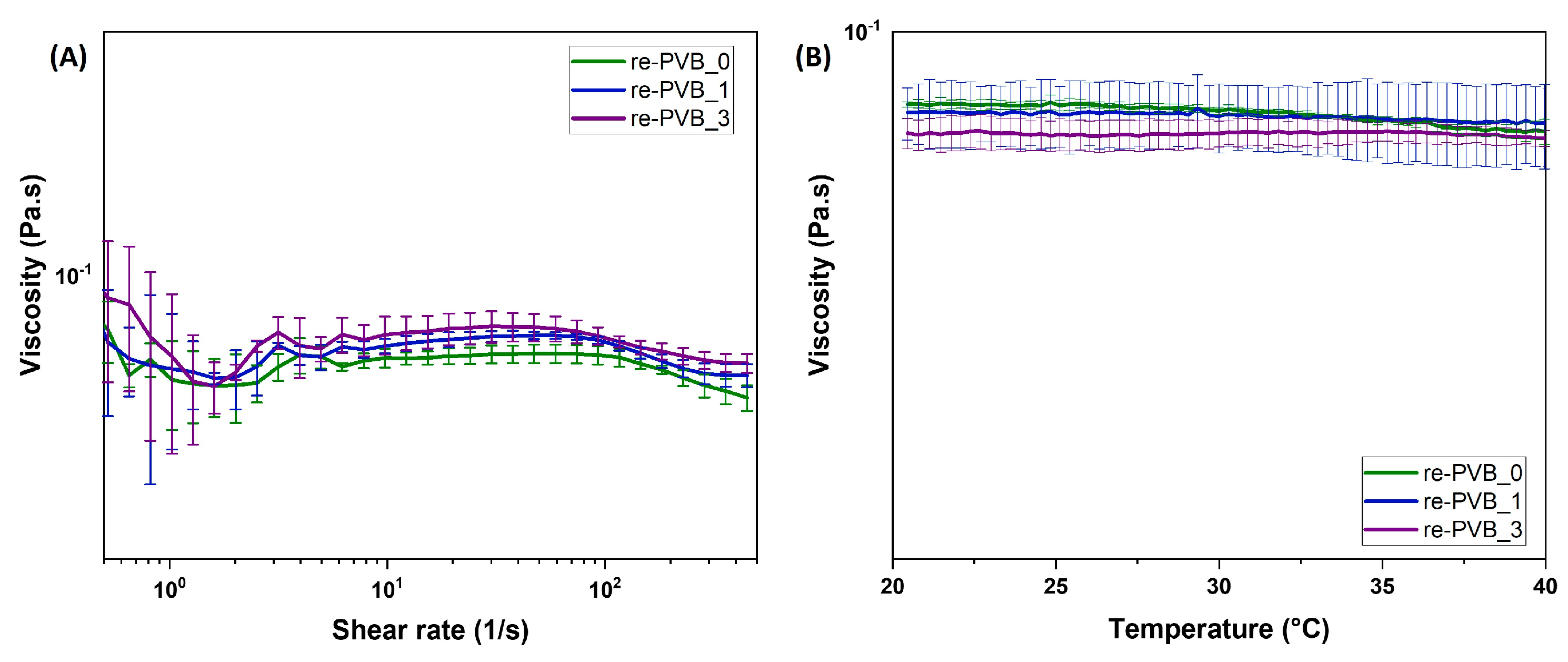
2.2. Rheology of re-PVB Formulations
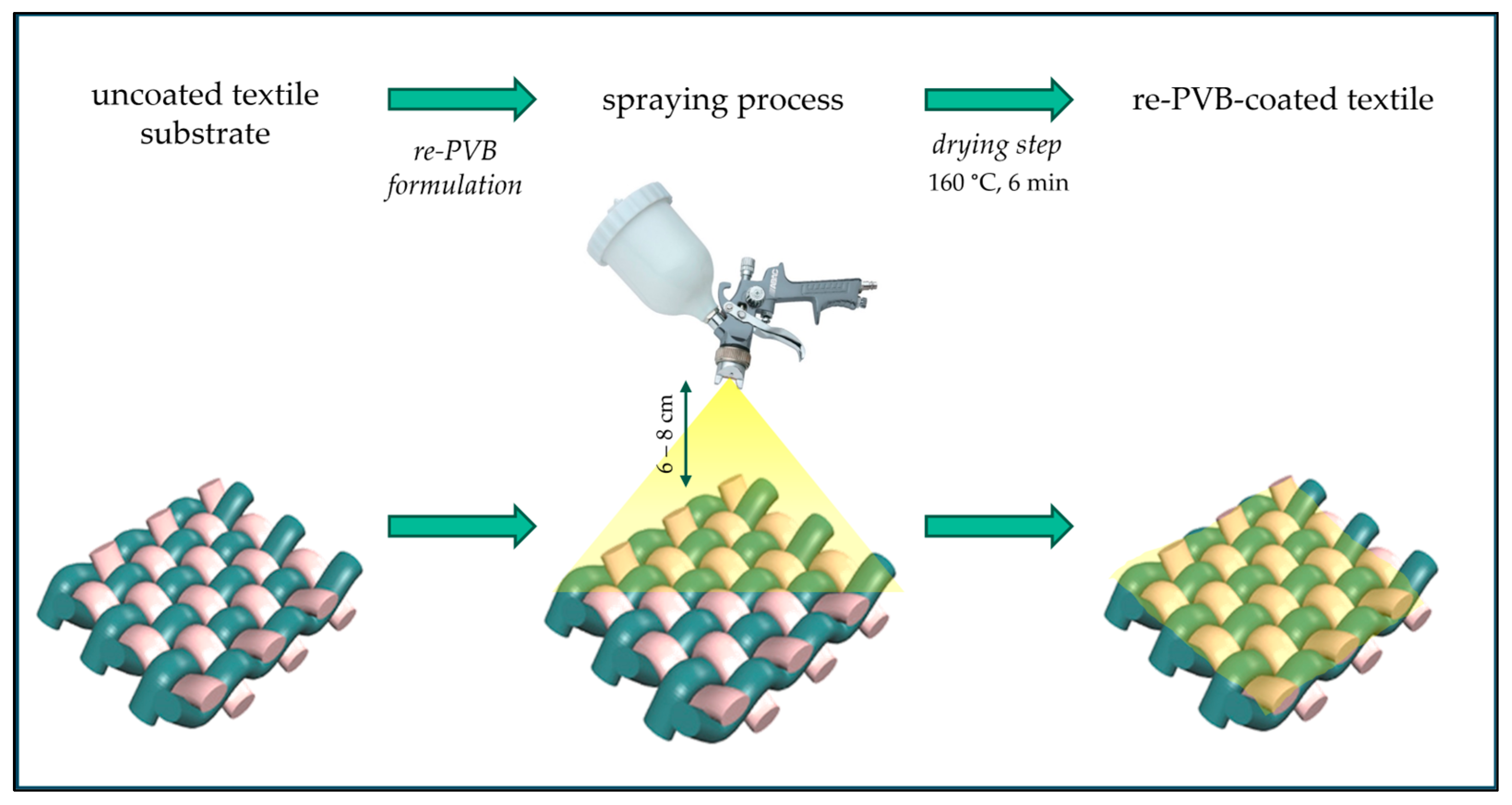
2.3. Coating Application Process
2.4. Evaluation of Coated Textile Properties
Fourier Transform–Infrared (FT-IR) Spectroscopy
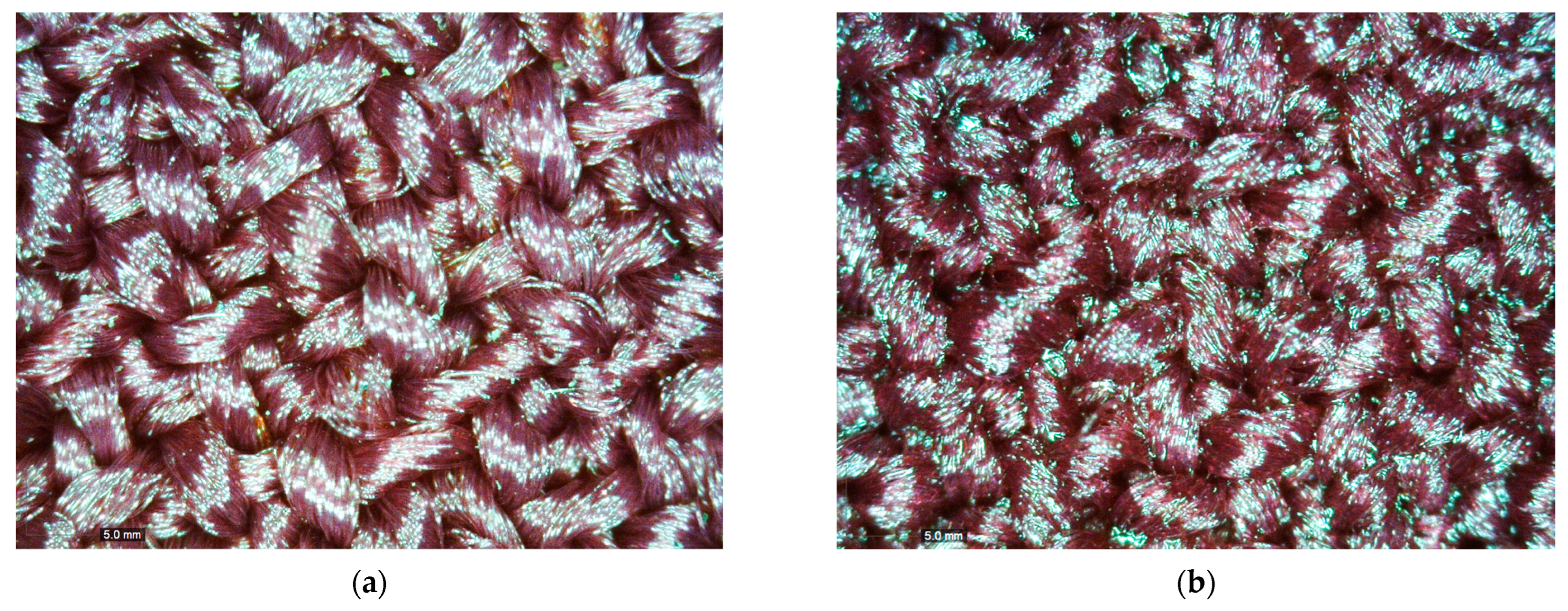
2.5. Microscopic Analysis
2.6. Nuclear Magnetic Resonance (NMR)
2.7. Washing Test
2.8. Mechanical Test
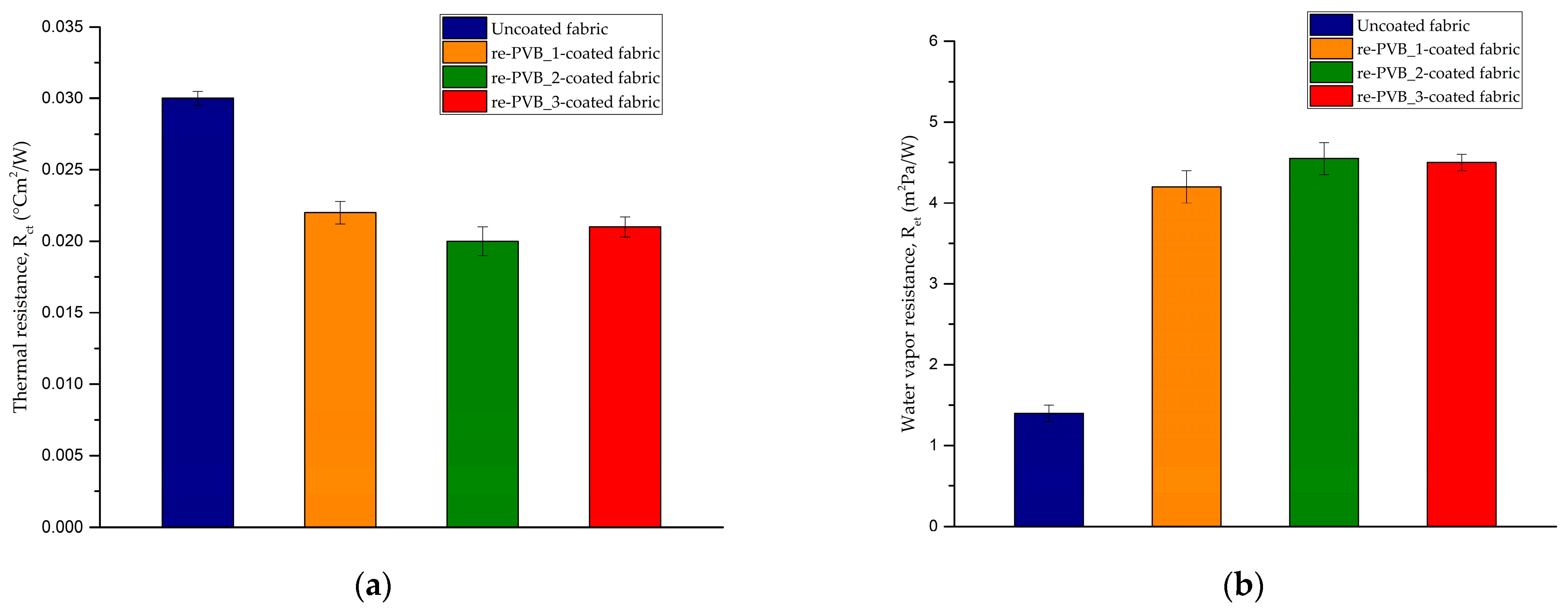
2.9. Thermal and Water Vapor Resistance Test
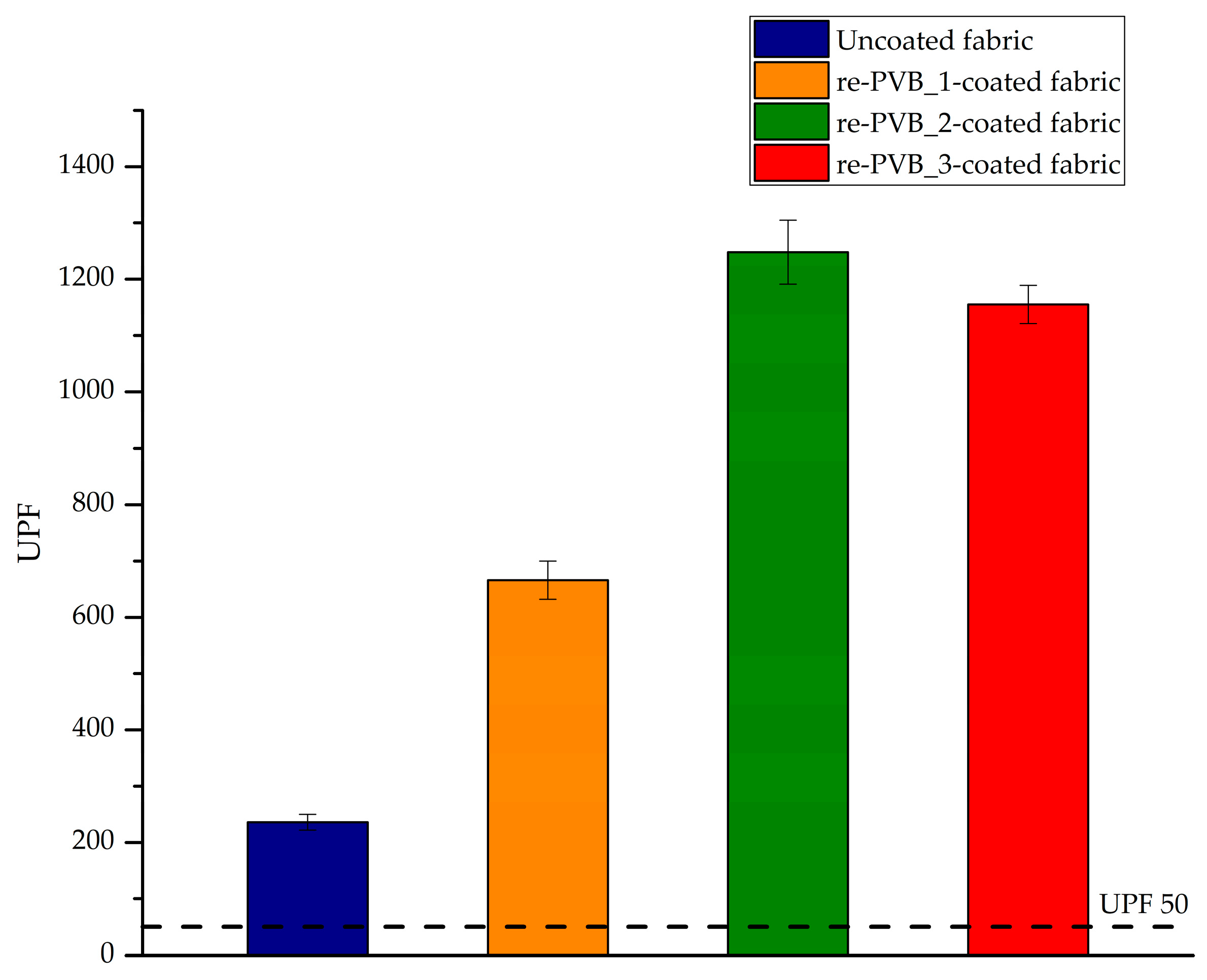
2.10. Ultraviolet Protection Factor (UPF) Analysis
3. Results and Discussion
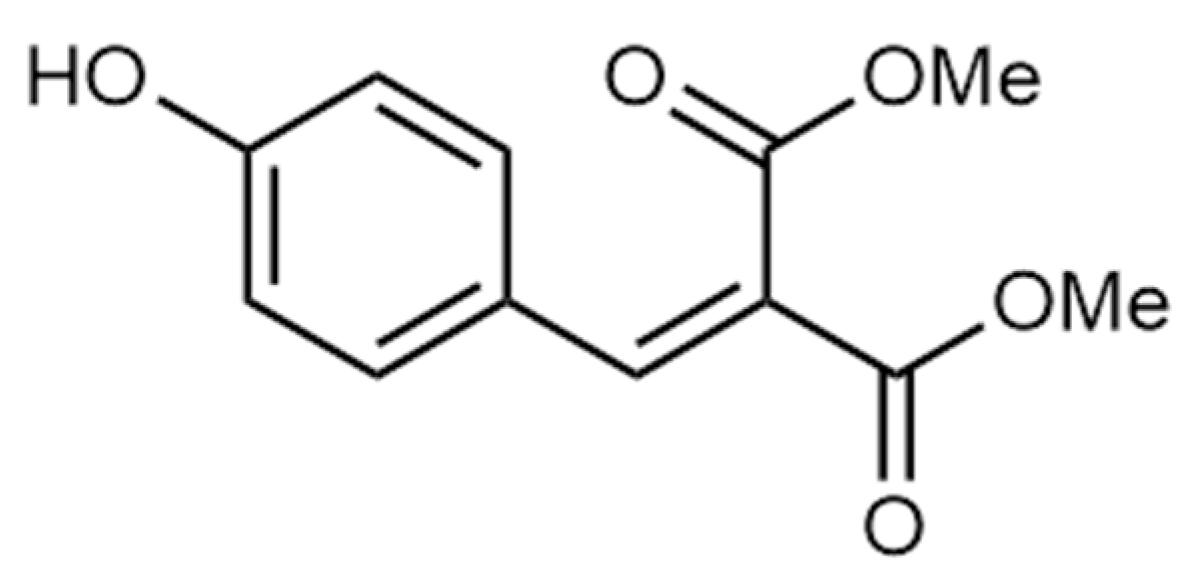
3.1. The re-PVB Formulations
3.2. The re-PVB Crosslinked Films
3.3. The re-PVB-Coated Fabrics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EURATEX. Facts & Key Figures 2024 of the European Textile and Clothing Industry; EURATEX: Brussels, Belgium, 2024; pp. 1–30. [Google Scholar]
- European Commission. EU Strategy for Sustainable and Circular Textiles; European Commission: Brussels, Belgium, 2022. [Google Scholar]
- Helmcke, S.; Hundertmark, T.; Musso, C.; Ong, W.J.; Oxgaard, J.; Wallach, J. Climate Impact of Plastics; McKinsey & Company: New York, NY, USA, 2022; pp. 1–30. [Google Scholar]
- European Commission. A New Circular Economy Action Plan for a Cleaner and More Competitive Europe; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Billah, S.M.R. Textile Coatings. In Functional Polymers; Polymers and Polymeric Composites: A Reference, Series; Jafar Mazumder, M.A., Sheardown, H., Al-Ahmed, A., Eds.; Springer International Publishing: Edinburgh, UK, 2019; Volume XXII, p. 1156. ISBN 978-3-319-95986-3. [Google Scholar]
- Paul, R. Functional finishes for textiles: An overview. In Functional Finishes for Textiles; Woodhead Publishing Series in Textiles; Woodhead Publishing: Delhi, India, 2015; pp. 1–14. ISBN 978-0-85709-839-9. [Google Scholar]
- Kovacevic, S.; Ujevic, D.; Brnada, S. Coated Textile Materials. In Woven Fabric Engineering; Sciyo: Rijeka, Croatia, 2010; pp. 241–254. ISBN 978-953-307-194-7. [Google Scholar]
- Brendgen, R.; Graßmann, C.; Grethe, T.; Mahltig, B.; Schwarz-Pfeiffer, A. Coatings with Recycled Polyvinyl Butyral on Polyester and Polyamide Mono- and Multifilament Yarns. J. Coat. Technol. Res. 2021, 18, 819–829. [Google Scholar] [CrossRef]
- Bidoki, S.M.; Wittlinger, R. Environmental and Economical Acceptance of Polyvinyl Chloride (PVC) Coating Agents. J. Clean. Prod. 2010, 18, 219–225. [Google Scholar] [CrossRef]
- Plakantonaki, S.; Kiskira, K.; Zacharopoulos, N.; Chronis, I.; Coelho, F.; Togiani, A.; Kalkanis, K.; Priniotakis, G. A Review of Sustainability Standards and Ecolabeling in the Textile Industry. Sustainability 2023, 15, 11589. [Google Scholar] [CrossRef]
- Mariotti, N.; Viada, G.; Galliano, S.; Menozzi, A.; Tammaro, F.; Giannelli, W.; Bonomo, M.; Barolo, C. Increasing Circular and Bio-Based Content of a Thermosetting Polyurethane for Encapsulation of Optoelectronic Devices: A Multivariate Investigation. J. Clean. Prod. 2023, 408, 137161. [Google Scholar] [CrossRef]
- Nikitakos, V.; Porfyris, A.D.; Beltsios, K.; Papaspyrides, C.; Bordignon, S.; Chierotti, M.R.; Nejrotti, S.; Bonomo, M.; Barolo, C.; Piovano, A.; et al. An Integrated Characterization Strategy on Board for Recycling of Poly(Vinyl Butyral) (PVB) from Laminated Glass Wastes. Polymers 2024, 16, 10. [Google Scholar] [CrossRef]
- Grethe, T.; Schwarz-Pfeiffer, A.; Grassmann, C.; Engelhardt, E.; Feld, S.; Guo, F.; De Vrieze, M.; Mahltig, B. Polyvinylbutyral (PVB) Coatings for Optical Modification of Textile Substrates. In Polymer Research: Communicating Current Advances, Contributions, Applications and Educational Aspects; Formatex Research Center S.L.: Extremadura, Spain, 2018; pp. 36–45. ISBN 978-84-947512-4-0. [Google Scholar]
- Carrot, C.; Bendaoud, A.; Pillon, C. Polyvinyl Butyral. In Handbook of Thermoplastics; Plastics Engineering; CRC Press: Boca Raton, FL, USA, 2015; p. 1012. ISBN 978-1-4665-7723-7. [Google Scholar]
- Corroyer, E.; Brochier-Salon, M.-C.; Chaussy, D.; Wery, S.; Belgacem, M.N. Characterization of Commercial Polyvinylbutyrals. Int. J. Polym. Anal. Charact. 2013, 18, 346–357. [Google Scholar] [CrossRef]
- Behúnová, A.; Knapčíková, L.; Behún, M.; Mandičák, T.; Mésároš, P. Intelligent Designing and Increasing the Variability of Healthy Residential Buildings by Customizing Recycled Polyvinyl Butyral. Sustainability 2021, 13, 9073. [Google Scholar] [CrossRef]
- Luan, W.; Sun, L.; Zeng, Z.; Xue, W. Optimization of a Polyvinyl Butyral Synthesis Process Based on Response Surface Methodology and Artificial Neural Network. RSC Adv. 2023, 13, 7682–7693. [Google Scholar] [CrossRef] [PubMed]
- Oehlmann, J.; Schulte-Oehlmann, U.; Kloas, W.; Jagnytsch, O.; Lutz, I.; Kusk, K.O.; Wollenberger, L.; Santos, E.M.; Paull, G.C.; Van Look, K.J.W.; et al. A Critical Analysis of the Biological Impacts of Plasticizers on Wildlife. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2047–2062. [Google Scholar] [CrossRef]
- Heudorf, U.; Mersch-Sundermann, V.; Angerer, J. Phthalates: Toxicology and Exposure. Int. J. Hyg. Environ. Health 2007, 210, 623–634. [Google Scholar] [CrossRef]
- Global Polyvinyl Butyral (PVB) Market; Chemicals and Materials; Mordor Intelligence: Telangana, India, 2022; pp. 1–46.
- Šooš, Ľ.; Matúš, M.; Pokusová, M.; Čačko, V.; Bábics, J. The Recycling of Waste Laminated Glass through Decomposition Technologies. Recycling 2021, 6, 26. [Google Scholar] [CrossRef]
- Guner, B.; Bulbul, Y.E.; Dilsiz, N. Recycling of Polyvinyl Butyral from Waste Automotive Windshield and Fabrication of Their Electrospun Fibrous Materials. J. Taiwan Inst. Chem. Eng. 2022, 132, 104136. [Google Scholar] [CrossRef]
- Roshni, S.B.; Jayakrishnan, M.P.; Mohanan, P.; Surendran, K.P. Design and Fabrication of an E-Shaped Wearable Textile Antenna on PVB-Coated Hydrophobic Polyester Fabric. Smart Mater. Struct. 2017, 26, 105011. [Google Scholar] [CrossRef]
- Obradović, V.; Sejkot, P.; Zabloudil, A.; Machalická, K.V.; Vokáč, M. Degradation Effect of Moisture on Mechanical Properties of Kevlar/PVB Composites with TiO2 Nanoparticles. Buildings 2024, 14, 409. [Google Scholar] [CrossRef]
- Yalcinkaya, F.; Komarek, M. Polyvinyl Butyral (PVB) Nanofiber/Nanoparticle-Covered Yarns for Antibacterial Textile Surfaces. Int. J. Mol. Sci. 2019, 20, 4317. [Google Scholar] [CrossRef]
- Ray, A.; Singha, K.; Pandit, P.; Maity, S. Advanced Ultraviolet Protective Agents for Textiles And Clothing. In Advances in Functional and Protective Textiles; Woodhead Publishing: Delhi, India, 2020; pp. 243–260. ISBN 978-0-12-820257-9. [Google Scholar]
- Louris, E.; Sfiroera, E.; Priniotakis, G.; Makris, R.; Siemos, H.; Efthymiou, C.; Assimakopoulos, M.N. Evaluating the Ultraviolet Protection Factor (UPF) of Various Knit Fabric Structures. IOP Conf. Ser. Mater. Sci. Eng. 2018, 459, 012051. [Google Scholar] [CrossRef]
- Mahltig, B.; Böttcher, H.; Rauch, K.; Dieckmann, U.; Nitsche, R.; Fritz, T. Optimized UV Protecting Coatings by Combination of Organic and Inorganic UV Absorbers. Thin Solid Films 2005, 485, 108–114. [Google Scholar] [CrossRef]
- Bashari, A.; Shakeri, M.; Shirvan, A.R. UV-Protective Textiles. In The Impact and Prospects of Green Chemistry for Textile Technology; Woodhead Publishing: Delhi, India, 2019; pp. 327–365. ISBN 978-0-08-102491-1. [Google Scholar]
- Khan, A.; Nazir, A.; Rehman, A.; Naveed, M.; Ashraf, M.; Iqbal, K.; Basit, A.; Maqsood, H.S. A Review of UV Radiation Protection on Humans by Textiles and Clothing. Int. J. Cloth. Sci. Technol. 2020, 32, 869–890. [Google Scholar] [CrossRef]
- Arfa, U.; Alshareef, M.; Nadeem, N.; Javid, A.; Nawab, Y.; Alshammari, K.F.; Zubair, U. Sunlight-Driven Photocatalytic Active Fabrics through Immobilization of Functionalized Doped Titania Nanoparticles. Polymers 2023, 15, 2775. [Google Scholar] [CrossRef]
- Textile Exchange. Materials Market Report; Textile Exchange: Lamesa, TX, USA, 2023; pp. 1–75. [Google Scholar]
- Grant Agreement No 958243 (SUNRISE Project Horizon 2020, Call: H2020-LOW-CARBON-CIRCULAR-INDUSTRIES-2020). Available online: https://sunrise-project.eu/ (accessed on 3 December 2024).
- Broniarz-Press, L.; Sosnowski, T.R.; Matuszak, M.; Ochowiak, M.; Jabłczyńska, K. The Effect of Shear and Extensional Viscosities on Atomization of Newtonian and Non-Newtonian Fluids in Ultrasonic Inhaler. Int. J. Pharm. 2015, 485, 41–49. [Google Scholar] [CrossRef]
- Carvalho, F.K.; Antuniassi, U.R.; Chechetto, R.G.; Mota, A.A.B.; De Jesus, M.G.; De Carvalho, L.R. Viscosity, Surface Tension and Droplet Size of Sprays of Different Formulations of Insecticides and Fungicides. Crop Prot. 2017, 101, 19–23. [Google Scholar] [CrossRef]
- Zheng, S.X.; Chen, H.S. Correlations of Rheological Methods to Coatings’ Performance. Prog. Org. Coat. 2023, 177, 107403. [Google Scholar] [CrossRef]
- Nasar, A.S.; Libni, G. Forward and Reverse Reactions of N-Methylaniline-Blocked Polyisocyanates: A Clear Step into Double Arrhenius Plots and Equilibrium Temperature of Thermally Reversible Reactions. RSC Adv. 2017, 7, 34149–34159. [Google Scholar] [CrossRef]
- Mohammadian-Kohol, M.; Asgari, M.; Shakur, H.R. A Detailed Investigation of the Gamma-Ray Radiation Effects on the Optical Properties of Polyvinyl Butyral Film. Optik 2016, 127, 7459–7468. [Google Scholar] [CrossRef]
- Gaina, C.; Ursache, O.; Gaina, V.; Ionita, D. Study on the Chemical Modification of Poly(Vinyl Alcohol) with 4-Maleimidophenyl Isocyanate. Polym.-Plast. Technol. Mater. 2012, 51, 65–70. [Google Scholar] [CrossRef]
- Lee, J.; Lee, U.; Jeong, K.; Seo, Y.; Park, S.; Kim, H. Preparation and Characterization of Poly(Vinyl Alcohol) Nanofiber Mats Crosslinked with Blocked Isocyanate Prepolymer. Polym. Int. 2010, 59, 1683–1689. [Google Scholar] [CrossRef]
- Krumova, M.; López, D.; Benavente, R.; Mijangos, C.; Pereña, J.M. Effect of Crosslinking on the Mechanical and Thermal Properties of Poly(Vinyl Alcohol). Polymer 2000, 41, 9265–9272. [Google Scholar] [CrossRef]
- Porporato, S.; Darjazi, H.; Gastaldi, M.; Piovano, A.; Perez, A.; Yécora, B.; Fina, A.; Meligrana, G.; Elia, G.A.; Gerbaldi, C. On the Use of Recycled PVB to Develop Sustainable Separators for Greener Li-Ion Batteries. Adv. Sustain. Syst. 2024, 2400569. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P. FTIR Spectroscopy Characterization of Poly (Vinyl Alcohol) Hydrogel with Different Hydrolysis Degree and Chemically Crosslinked with Glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Cura, K.; Rintala, N.; Kamppuri, T.; Saarimäki, E.; Heikkilä, P. Textile Recognition and Sorting for Recycling at an Automated Line Using Near Infrared Spectroscopy. Recycling 2021, 6, 11. [Google Scholar] [CrossRef]
- Phan, K.; Ügdüler, S.; Harinck, L.; Denolf, R.; Roosen, M.; O’Rourke, G.; De Vos, D.; Van Speybroeck, V.; De Clerck, K.; De Meester, S. Analysing the Potential of the Selective Dissolution of Elastane from Mixed Fiber Textile Waste. Resour. Conserv. Recycl. 2023, 191, 106903. [Google Scholar] [CrossRef]
- Zavec Pavlinic, D.; Geršak, J. Investigations of the Relation Between Fabric Mechanical Properties and Behaviour. Int. J. Cloth. Sci. Technol. 2003, 15, 231–240. [Google Scholar] [CrossRef]
- Kazmi, K.; Javed, Z.; Salman, M.; Iftikhar, F.; Ahmed, N.; Naeem, J.; Jabbar, A.; Karahan, M.; Naeem, M.S. Optimization of Knitted Fabrics for Better Thermo-Physiological Comfort by Using Taguchi-Based Principal Component Analysis. Tekstilec 2023, 66, 18–30. [Google Scholar] [CrossRef]
- Skrzetuska, E.; Puszkarz, A.K.; Nosal, J. Assessment of the Impact of the Surface Modification Processes of Cotton and Polyester Fabrics with Various Techniques on Their Structural, Biophysical, Sensory, and Mechanical Properties. Polymers 2022, 14, 796. [Google Scholar] [CrossRef]
- Morton, W.E.; Hearle, J.W.S. Thermal Properties. In Physical Properties of Textile Fibres; Woodhead Publishing: Delhi, India, 2008; pp. 168–177. ISBN 978-1-84569-220-9. [Google Scholar]
- Sankaran, A.; Kamboj, A.; Samant, L.; Jose, S. Synthetic and Natural UV Protective Agents for Textile Finishing. In Innovative and Emerging Technologies for Textile Dyeing and Finishing; Rather, L.J., Haji, A., Shabbir, M., Eds.; Scrivener Publishing LLC: Beverly, MA, USA, 2021; pp. 301–324. ISBN 978-1-119-71028-8. [Google Scholar]

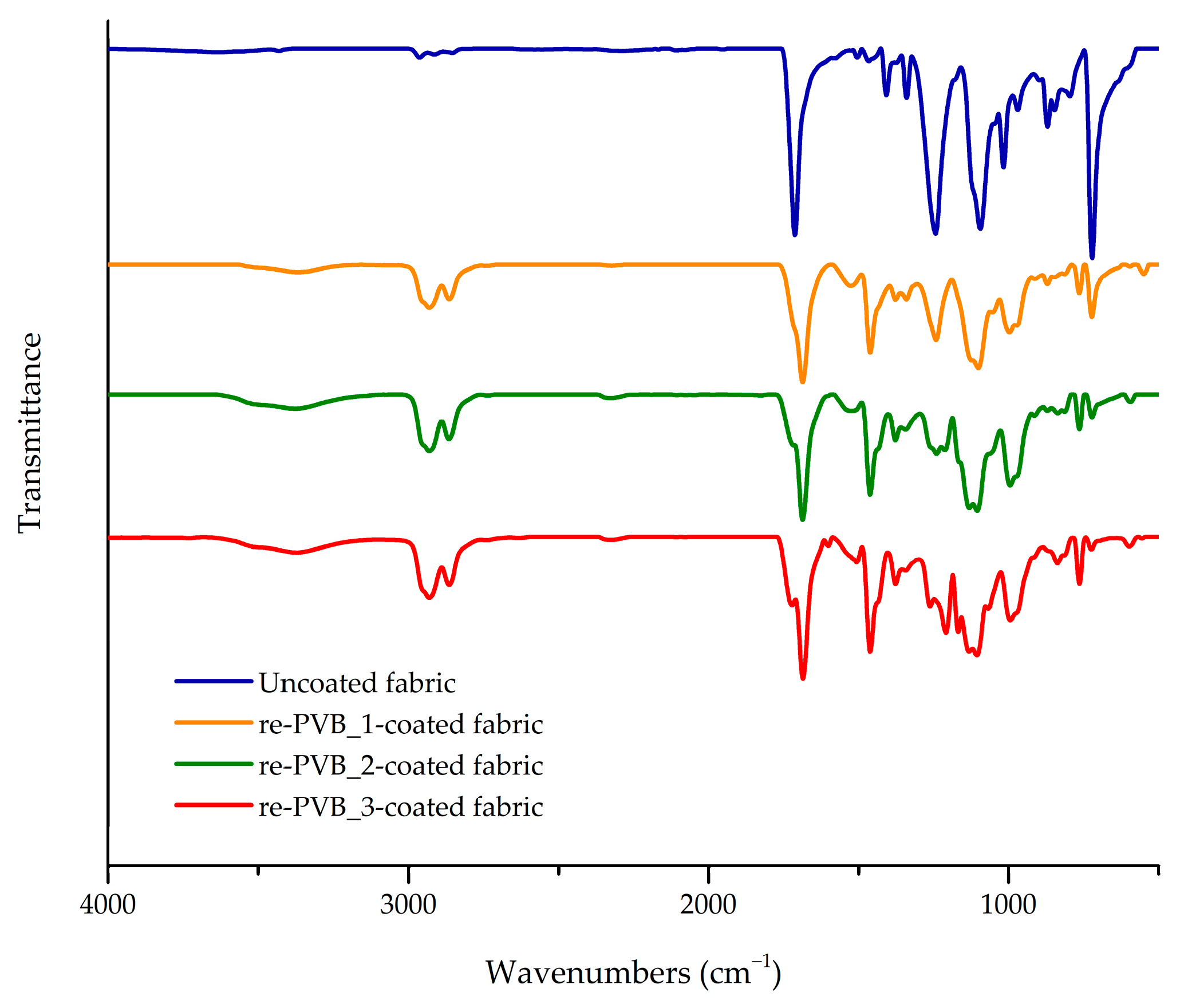

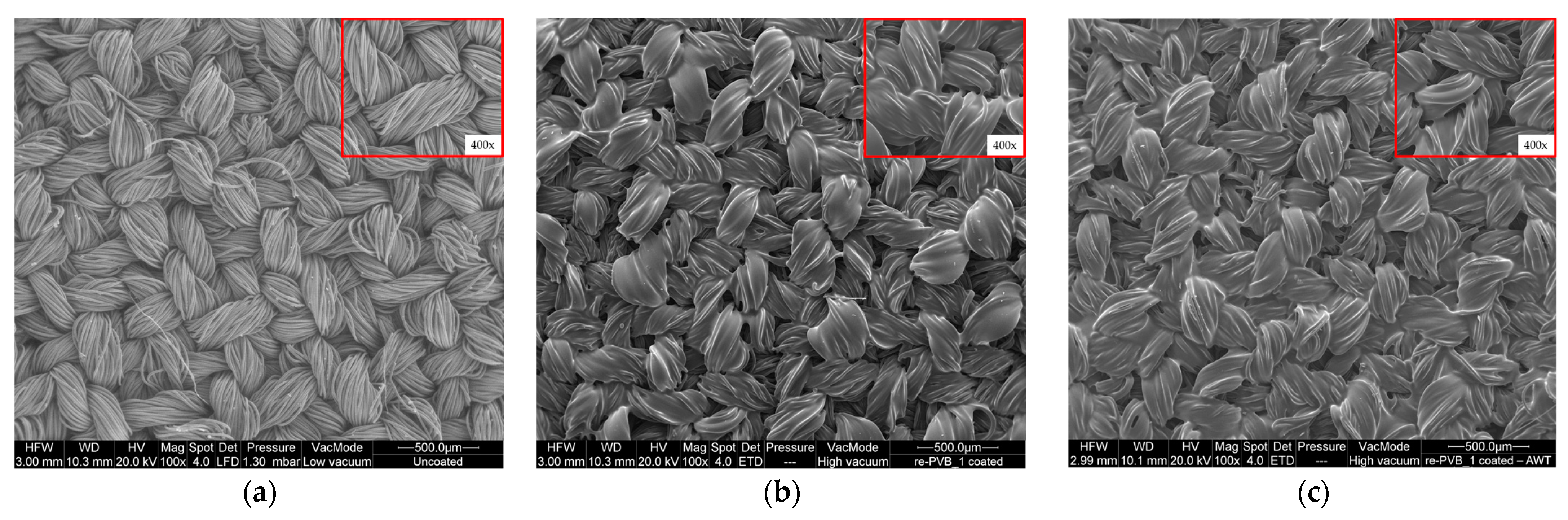
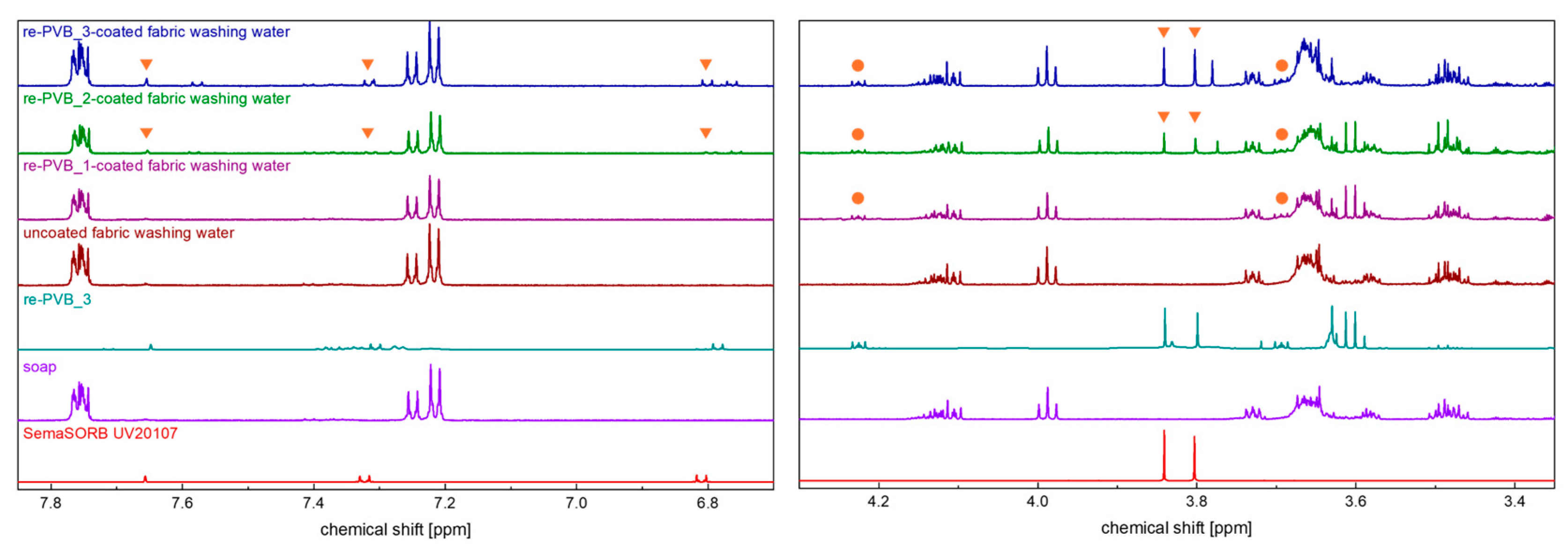
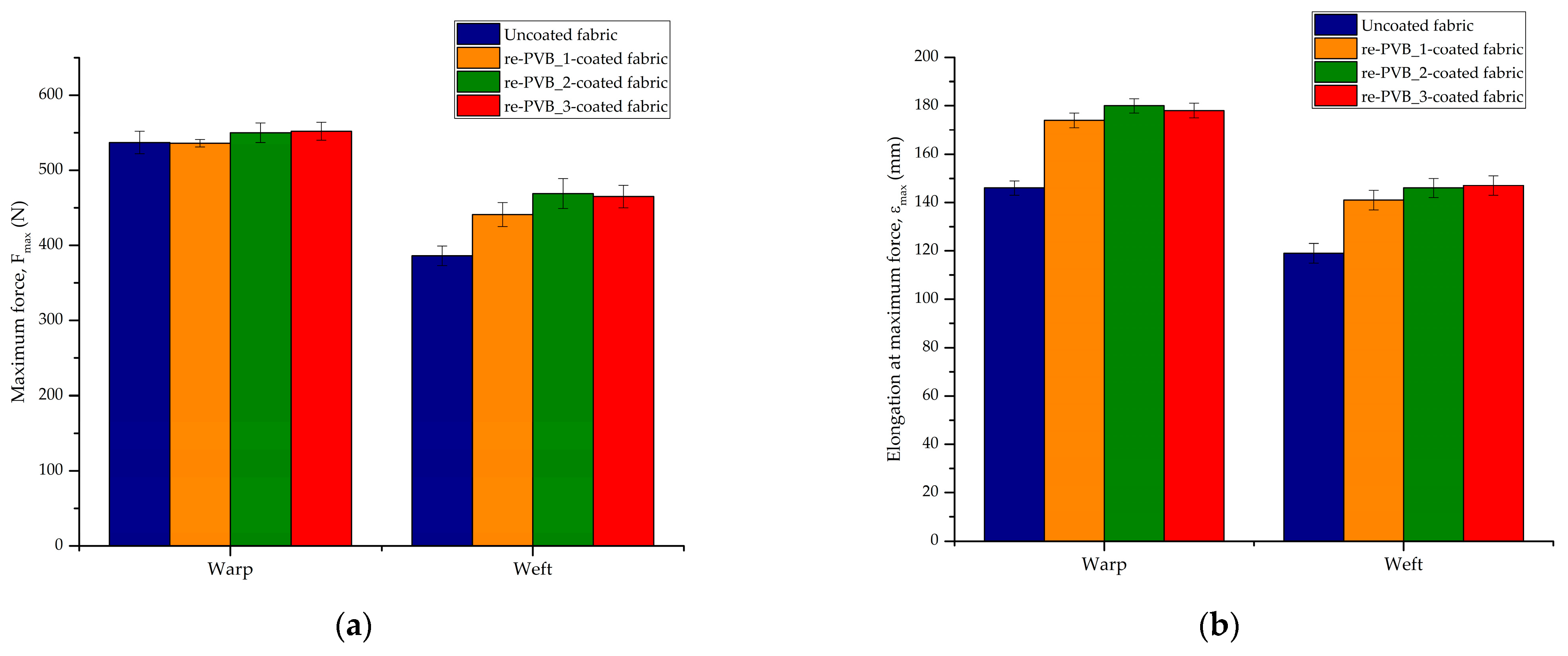

| Material | Wavenumber (cm−1) | Description | Ref. |
|---|---|---|---|
| re-PVB | 3600–3200 | O–H stretching (VA unit) | [12,14,15,38] |
| re-PVB_1 film | 3600–3200 | O–H and N–H stretching (VA unit + urethane group) | [12,14,15,38,39] |
| re-PVB | 2960–2850 | C–H stretching (alkyl groups) | [12,14,15,38] |
| re-PVB_1 film | 2960–2850 | C–H stretching (alkyl groups) | |
| re-PVB | 1735 | C=O stretching (plasticizers) | [12] |
| re-PVB_1 film | 1735–1690 | C=O stretching (plasticizers + urethane group) | [12,39,40] |
| Re-PVB_1 film | 1530 | CO–NH bending (urethane group) | [39,40,41,42] |
| re-PVB | 1460–1350 | CH2 and CH3 bending (alkyl groups) | [38,43] |
| re-PVB_1 film | 1460–1350 | CH2 and CH3 bending (alkyl groups) | |
| re-PVB | 1140–1050 | C–O–C stretching (VB unit + plasticizers) | [12,14,15,38] |
| re-PVB_1 film | 1140–1050 | C–O–C stretching (VB unit + plasticizers) | |
| re-PVB | 1000 | C–O stretching (VB unit) | [12,15,38] |
| re-PVB_1 film | 1000 | C–O stretching (VB unit) |
| Sample | UPF | UVA Transmittance (%) | UVB Transmittance (%) |
|---|---|---|---|
| Uncoated fabric | 236 ± 14 | 2.0 ± 0.1 | 0.20 ± 0.02 |
| re-PVB_1-coated fabric | 666 ± 34 | 0.90 ± 0.04 | 0.10 ± 0.01 |
| re-PVB_2-coated fabric | 1248 ± 57 | 0.5 ± 0.1 | 0.10 ± 0.01 |
| re-PVB_3-coated fabric | 1155 ± 34 | 0.60 ± 0.02 | 0.10 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cei, N.; Canesi, I.; Nejrotti, S.; Montalbano, G.; Darjazi, H.; Piovano, A.; Bonomo, M.; Fina, A.; Yecora, B.; Perez, A.; et al. A UV-Protective Textile Coating Based on Recycled Poly(vinyl butyral) (PVB): A New Life for a Waste Polymer. Polymers 2024, 16, 3439. https://doi.org/10.3390/polym16233439
Cei N, Canesi I, Nejrotti S, Montalbano G, Darjazi H, Piovano A, Bonomo M, Fina A, Yecora B, Perez A, et al. A UV-Protective Textile Coating Based on Recycled Poly(vinyl butyral) (PVB): A New Life for a Waste Polymer. Polymers. 2024; 16(23):3439. https://doi.org/10.3390/polym16233439
Chicago/Turabian StyleCei, Noemi, Ilaria Canesi, Stefano Nejrotti, Giorgia Montalbano, Hamideh Darjazi, Alessandro Piovano, Matteo Bonomo, Alberto Fina, Beatriz Yecora, Angelica Perez, and et al. 2024. "A UV-Protective Textile Coating Based on Recycled Poly(vinyl butyral) (PVB): A New Life for a Waste Polymer" Polymers 16, no. 23: 3439. https://doi.org/10.3390/polym16233439
APA StyleCei, N., Canesi, I., Nejrotti, S., Montalbano, G., Darjazi, H., Piovano, A., Bonomo, M., Fina, A., Yecora, B., Perez, A., Barolo, C., Gerbaldi, C., & Spinelli, D. (2024). A UV-Protective Textile Coating Based on Recycled Poly(vinyl butyral) (PVB): A New Life for a Waste Polymer. Polymers, 16(23), 3439. https://doi.org/10.3390/polym16233439












