Mercury Adsorption by Ca-Based Shell-Type Polymers Synthesized by Self-Assembly Mineralization
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Material Preparation
2.2. Morphological Characterization
2.3. The Verification of Adsorbent Uniformity
2.4. Batch Adsorption Testing
2.5. Adsorption Stability Evaluation of Adsorbents
3. Results
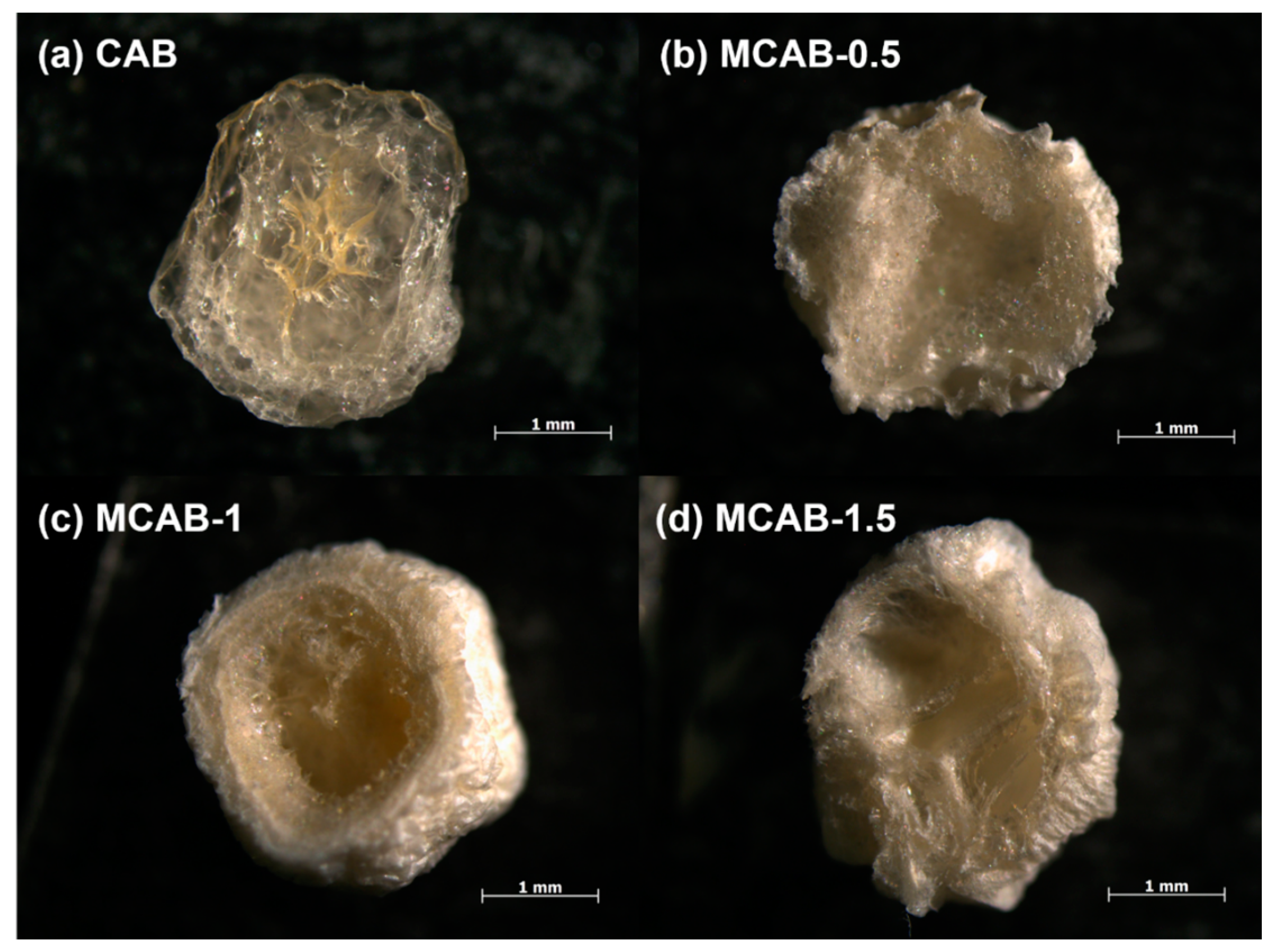
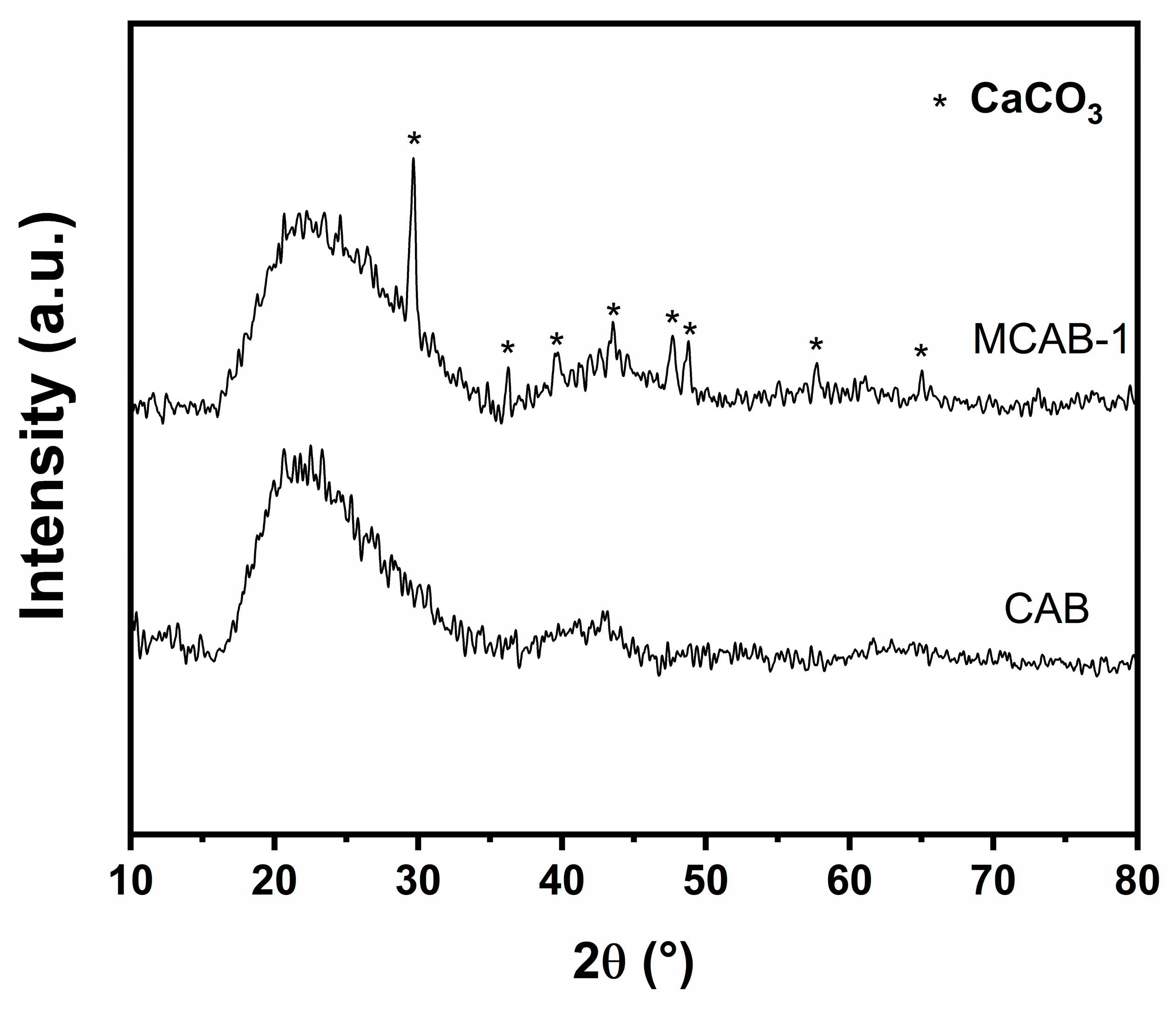
3.1. Sample Characterization
3.2. Effect of Single Factors on the Hg(II) Adsorption Efficiency
3.2.1. Dosage of Adsorbent
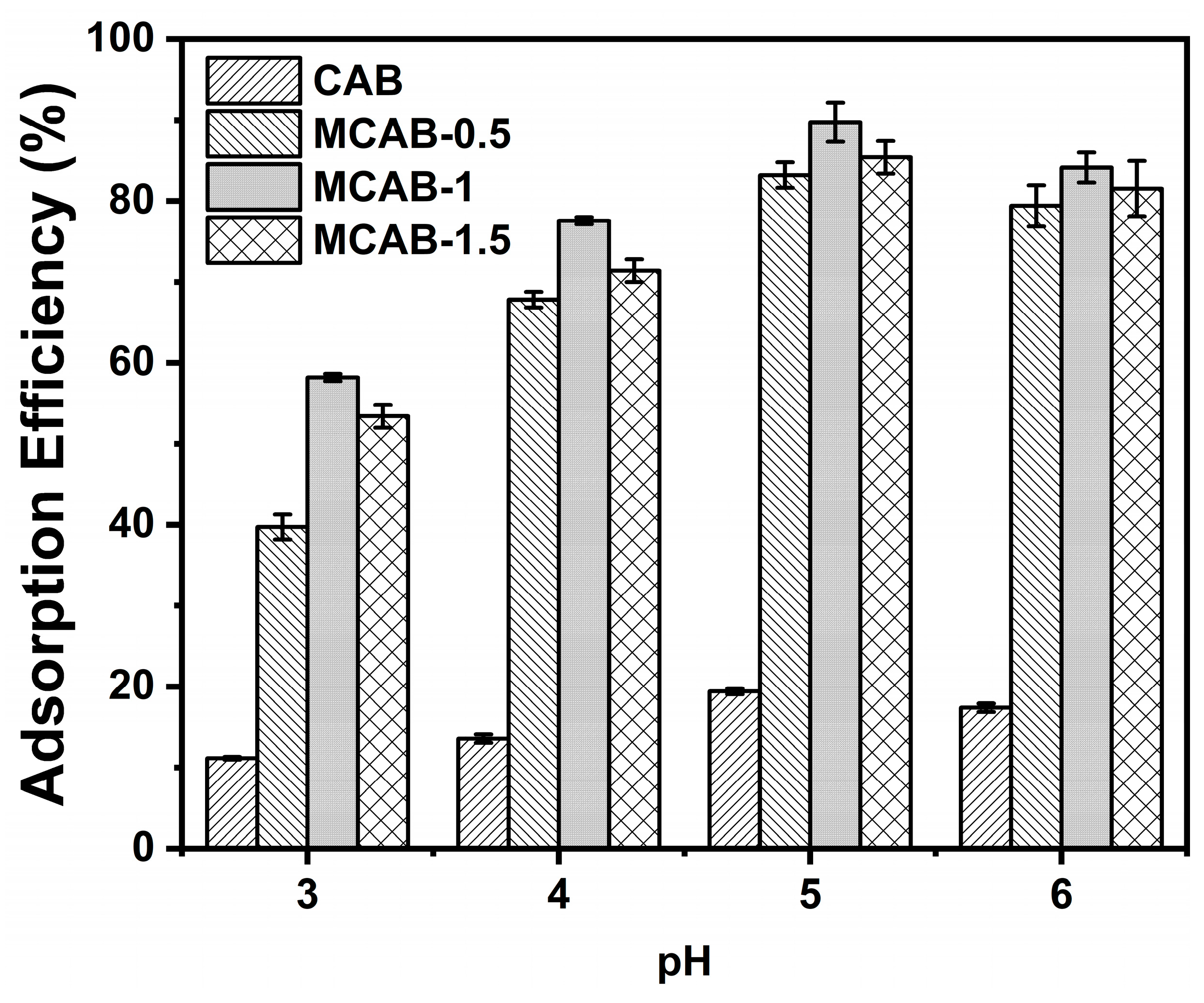
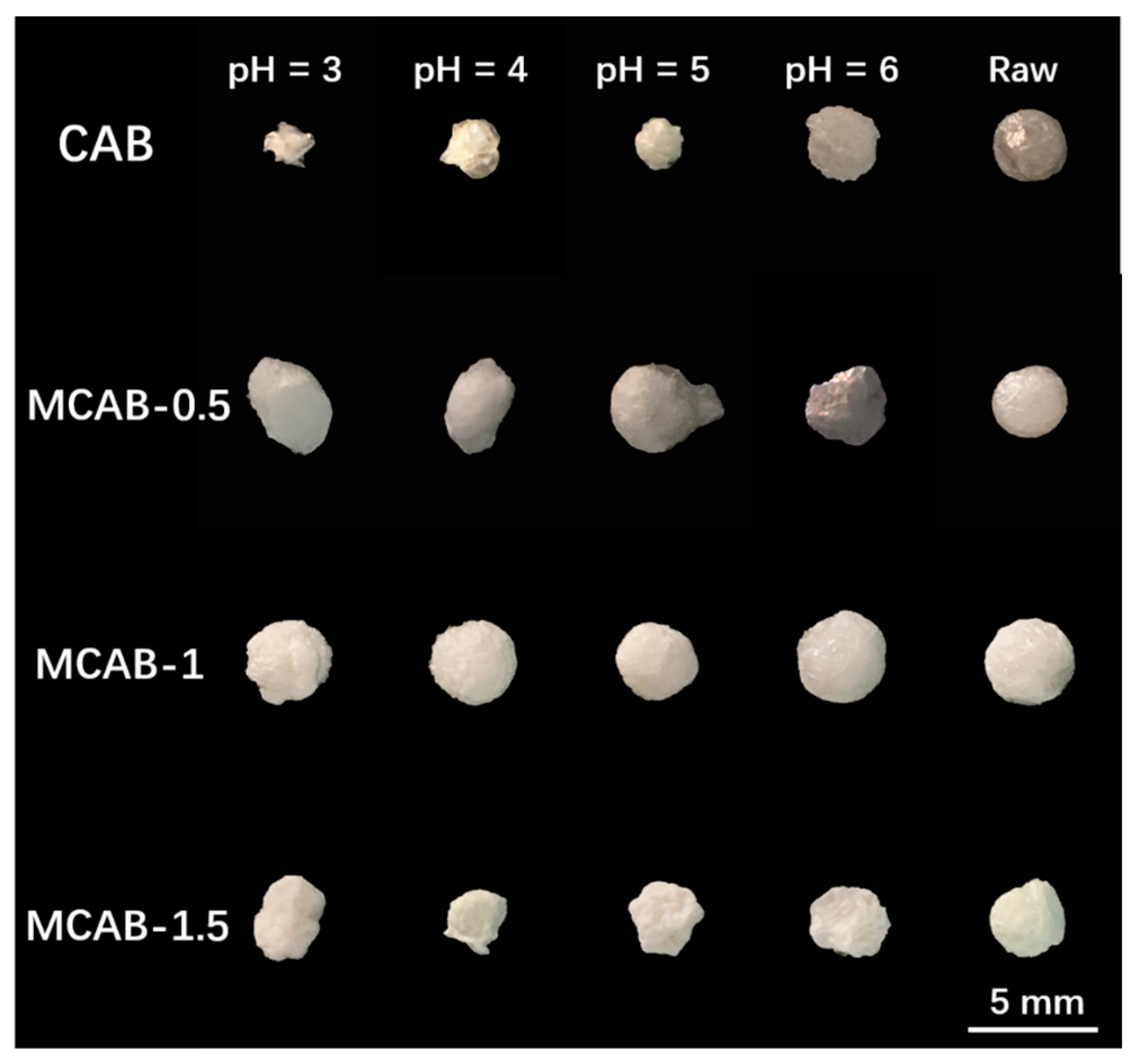
3.2.2. Effect of pH
3.3. The Assessment of Adsorbent Performance in Batch Adsorption
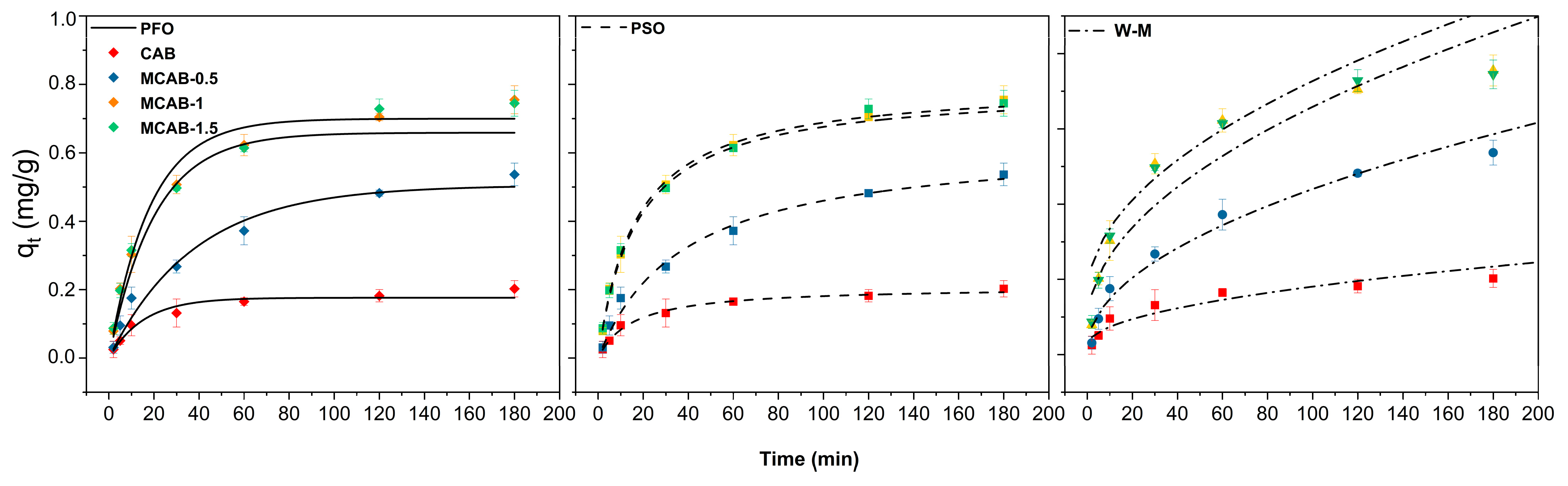
3.3.1. Kinetics Analysis
3.3.2. Adsorption Isotherms
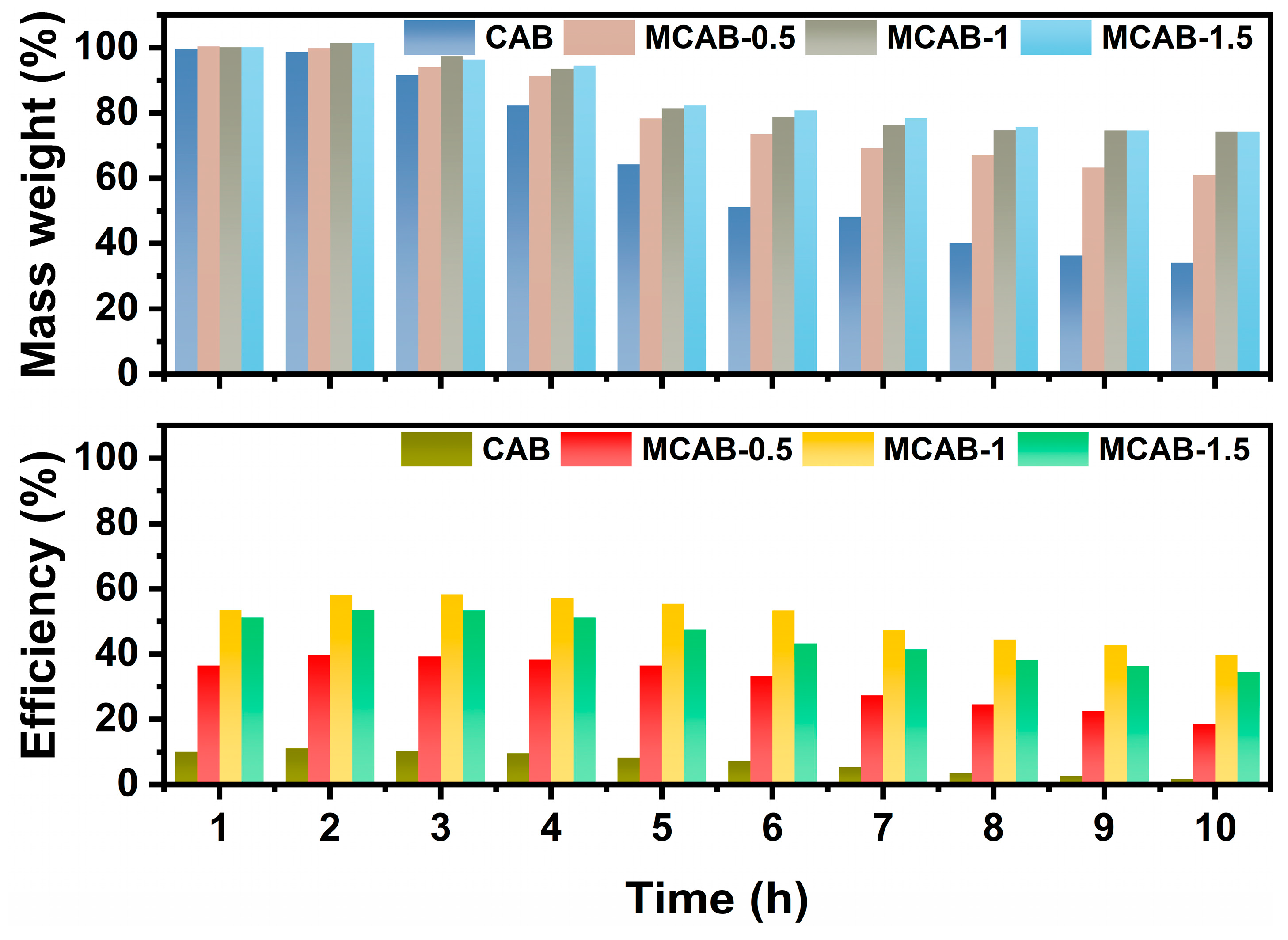
3.4. Long-Term Anti-Wear Test
4. Discussion
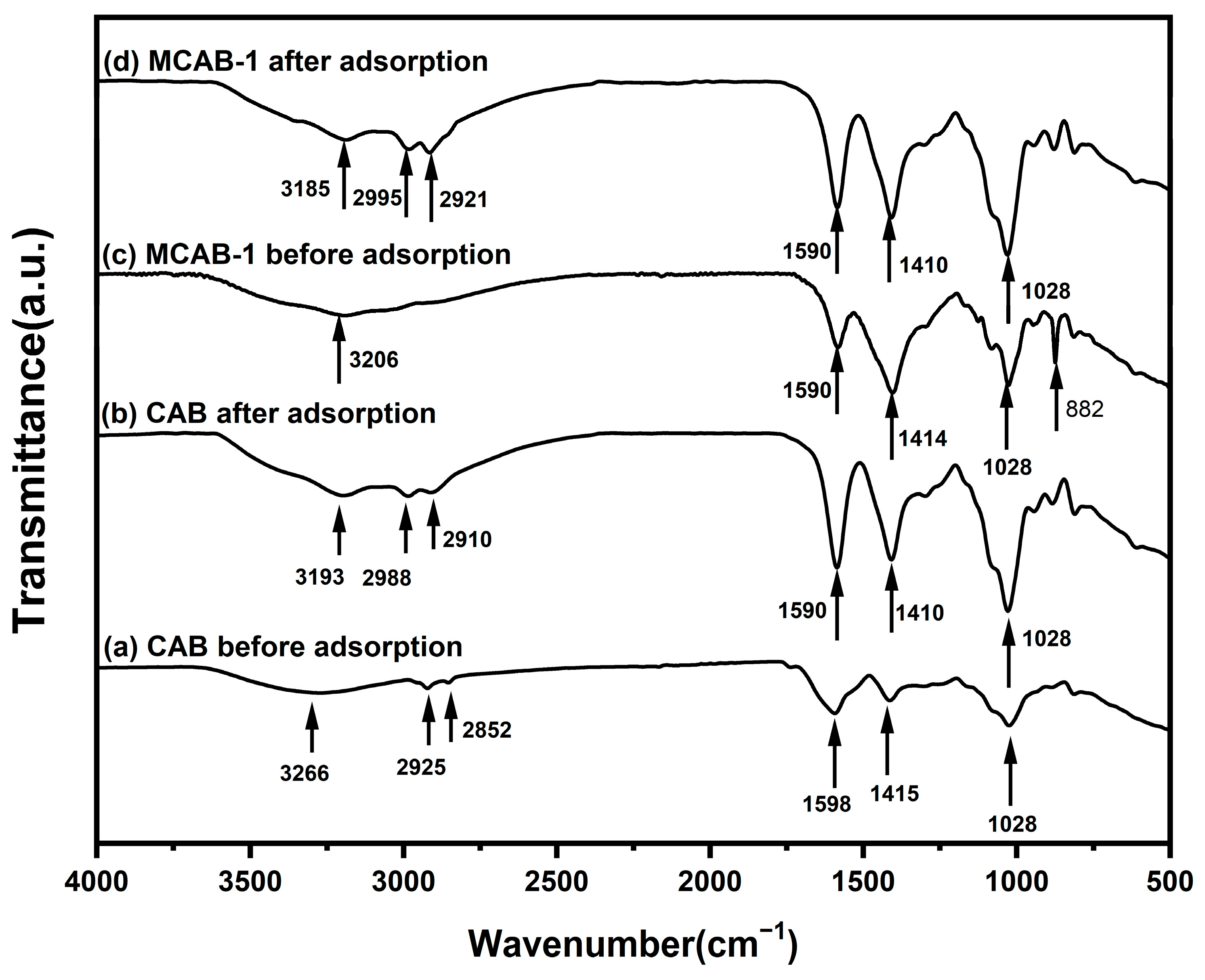
4.1. The Evolution of Functional Groups During Batch Adsorption
4.2. Effect of Mineralization on the Stability of the Adsorption Efficiency and Material Structure
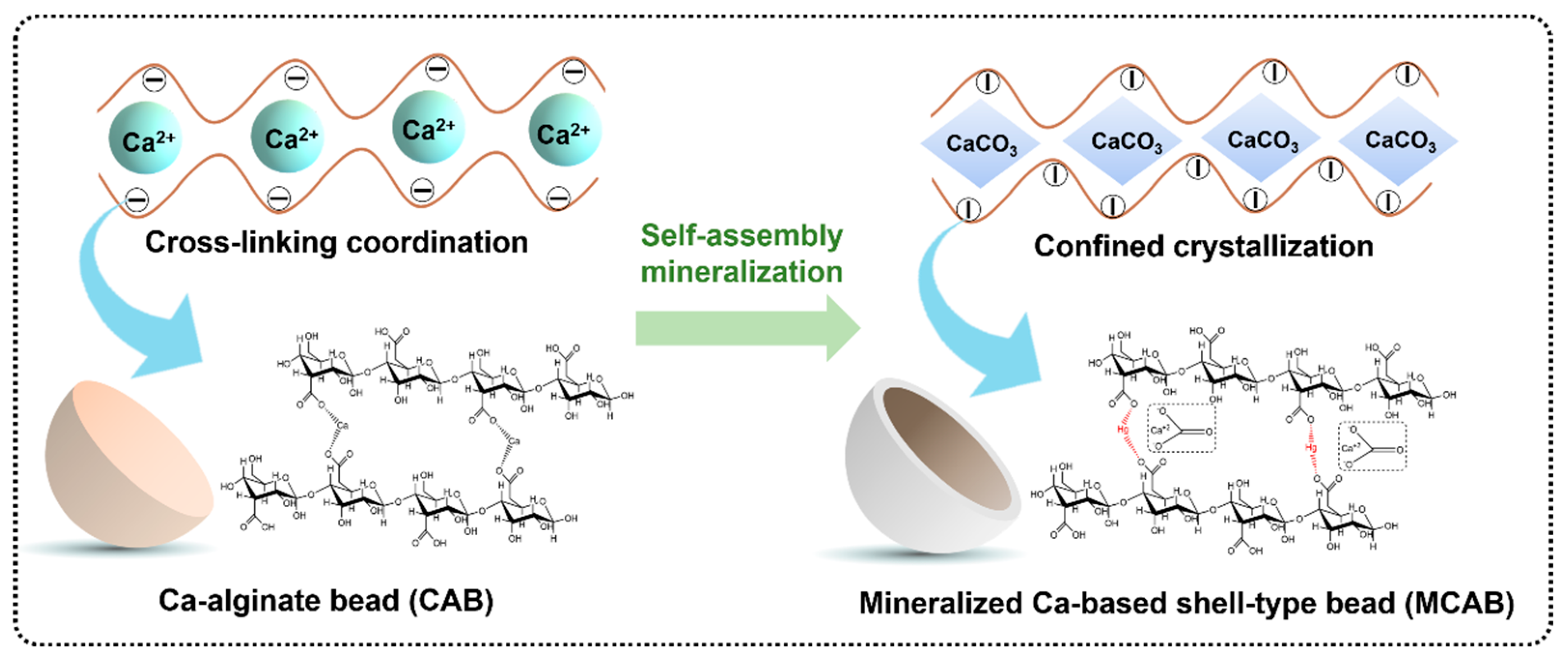
4.3. Mechanism of Mercury Adsorption by Self-Assembly Mineralized Ca-Based Shell-Type Beads
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexandre, J.; Poulain, T.B. Cracking the Mercury Methylation Code. Science 2013, 339, 1280–1281. [Google Scholar]
- Menger-Krug, E.; Niederste-Hollenberg, J.; Hillenbrand, T.; Hiessl, H. Integration of microalgae systems at municipal wastewater treatment plants: Implications for energy and emission balances. Environ. Sci. Technol. 2012, 46, 11505–11514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xu, P.; Qiu, Y.; Yu, Q.; Ma, J.; Wu, H.; Luo, G.; Xu, M.; Yao, H. Increasing oxygen functional groups of activated carbon with non-thermal plasma to enhance mercury removal efficiency for flue gases. Chem. Eng. J. 2015, 263, 1–8. [Google Scholar] [CrossRef]
- Xu, M.; Qiao, Y.; Zheng, C.; Li, L.; Liu, J. Modeling of homogeneous mercury speciation using detailed chemical kinetics. Combust. Flame 2003, 132, 208–218. [Google Scholar] [CrossRef]
- Luo, G.; Yao, H.; Xu, M.; Gupta, R.; Xu, Z. Identifying modes of occurrence of mercury in coal by temperature programmed pyrolysis. Proc. Combust. Inst. 2011, 33, 2763–2769. [Google Scholar] [CrossRef]
- Xu, M.; Yu, D.; Yao, H.; Liu, X.; Qiao, Y. Coal combustion-generated aerosols: Formation and properties. Proc. Combust. Inst. 2011, 33, 1681–1697. [Google Scholar] [CrossRef]
- Yu, D.; Xu, M.; Yao, H.; Sui, J.; Liu, X.; Yu, Y.; Cao, Q. Use of elemental size distributions in identifying particle formation modes. Proc. Combust. Inst. 2007, 31, 1921–1928. [Google Scholar] [CrossRef]
- Zhang, Y.; Kogelnig, D.; Morgenbesser, C.; Stojanovic, A.; Jirsa, F.; Lichtscheidl-Schultz, I.; Krachler, R.; Li, Y.; Keppler, B.K. Preparation and characterization of immobilized [A336][MTBA] in PVA-alginate gel beads as novel solid-phase extractants for an efficient recovery of Hg (II) from aqueous solutions. J. Hazard. Mater. 2011, 196, 201–209. [Google Scholar] [CrossRef]
- Li, F.; Zhang, J.; Jiang, W.; Liu, C.; Zhang, Z.; Zhang, C.; Zeng, G. Spatial health risk assessment and hierarchical risk management for mercury in soils from a typical contaminated site, China. Environ. Geochem. Health 2017, 39, 923. [Google Scholar] [CrossRef]
- Huang, J.; Li, F.; Zeng, G.; Liu, W.; Huang, X.; Xiao, Z.; Wu, H.; Gu, Y.; Li, X.; He, X.; et al. Integrating hierarchical bioavailability and population distribution into potential eco-risk assessment of heavy metals in road dust: A case study in Xiandao District, Changsha city, China. Sci. Total. Environ. 2015, 541, 969. [Google Scholar] [CrossRef]
- Farooq, U.; Kozinski, J.A.; Khan, M.A.; Athar, M. Biosorption of heavy metal ions using wheat based biosorbents--a review of the recent literature. Bioresour. Technol. 2010, 101, 5043–5053. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Wu, T.; Qiao, Y.; Xu, M. In Situ Leaching of Trace Elements in a Coal Ash Dump and Time Dependence Laboratory Evaluation†. Energy Fuels 2010, 24, 84–90. [Google Scholar] [CrossRef]
- Lin, G.; Zeng, B.; Li, J.; Wang, Z.; Wang, S.; Hu, T.; Zhang, L. A systematic review of metal organic frameworks materials for heavy metal removal: Synthesis, applications and mechanism. Chem. Eng. J. 2023, 460, 141710. [Google Scholar] [CrossRef]
- He, J.; Chen, J.P. A comprehensive review on biosorption of heavy metals by algal biomass: Materials, performances, chemistry, and modeling simulation tools. Bioresour. Technol. 2014, 160, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Garrido, I. Microalgae immobilization: Current techniques and uses. Bioresour. Technol. 2008, 99, 3949–3964. [Google Scholar] [CrossRef]
- He, T.; Li, Q.; Lin, T.; Li, J.; Bai, S.; An, S.; Kong, X.; Song, Y.-F. Recent progress on highly efficient removal of heavy metals by layered double hydroxides. Chem. Eng. J. 2023, 462, 142041. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Bomis, G.; Kosheleva, R.I.; Efthimiadou, E.K.; Favvas, E.P.; Kostoglou, M.; Mitropoulos, A.C. Nanobubbles effect on heavy metal ions adsorption by activated carbon. Chem. Eng. J. 2019, 356, 91–97. [Google Scholar] [CrossRef]
- Pejman Hadi, M.-H.T.; Hui, C.-W.; Lin, C.S.K.; McKay, G. Aqueous mercury adsorption by activated carbons. Water Res. 2015, 73, 37–55. [Google Scholar] [CrossRef]
- Teker, M.; Glu, I.; Saltabas, O. Adsorption of Copper and Cadmium lons by Activated Carbon From Rice Hulls. Turk. J. Chem. 1999, 23, 185–192. [Google Scholar]
- Khosravi, R.; Azizi, A.; Ghaedrahmati, R.; Gupta, V.K.; Agarwal, S. Adsorption of gold from cyanide leaching solution onto activated carbon originating from coconut shell—Optimization, kinetics and equilibrium studies. J. Ind. Eng. Chem. 2017, 54, 464–471. [Google Scholar] [CrossRef]
- Kobya, M.; Demirbas, E.; Senturk, E.; Ince, M. Adsorption of heavy metal ions from aqueous solutions by activated carbon prepared from apricot stone. Bioresour. Technol. 2005, 96, 1518–1521. [Google Scholar] [CrossRef] [PubMed]
- Enniya, I.; Rghioui, L.; Jourani, A. Adsorption of hexavalent chromium in aqueous solution on activated carbon prepared from apple peels. Sustain. Chem. Pharm. 2018, 7, 9–16. [Google Scholar] [CrossRef]
- Bansode, R.R.; Losso, J.N.; Marshall, W.E.; Rao, R.M.; Portier, R.J. Adsorption of metal ions by pecan shell-based granular activated carbons. Bioresour. Technol. 2003, 89, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, C. Chitosan-based biosorbents: Modification and application for biosorption of heavy metals and radionuclides. Bioresour. Technol. 2014, 160, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Ohto, K.; Yoshizuka, K.; Shinbaru, R.; Kina, K. Adsorption behaviors of some metal ions on chitosan modified with EDTA-type ligand. Bunseki Kagaku 1995, 44, 283–287. [Google Scholar] [CrossRef][Green Version]
- Ngah, W.S.W.; Fatinathan, S. Adsorption of Cu(II) ions in aqueous solution using chitosan beads, chitosan–GLA beads and chitosan–alginate beads. Chem. Eng. J. 2008, 143, 62–72. [Google Scholar] [CrossRef]
- Liu, B.; Wang, D.; Yu, G.; Meng, X. Adsorption of heavy metal ions, dyes and proteins by chitosan composites and derivatives—A review. J. Ocean Univ. China 2013, 12, 500–508. [Google Scholar] [CrossRef]
- Crini, G.; Badot, P.-M. Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: A review of recent literature. Prog. Polym. Sci. 2008, 33, 399–447. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, J.; Wang, J. Competitive adsorption of Pb(II), Cu(II) and Zn(II) onto xanthate-modified magnetic chitosan. J. Hazard. Mater. 2012, 221–222, 155–161. [Google Scholar] [CrossRef]
- Nan, L.; Bai, R. Copper adsorption on chitosan–cellulose hydrogel beads: Behaviors and mechanisms. Sep. Purif. Technol. 2005, 42, 237–247. [Google Scholar]
- Alvarez, A.; Saez, J.M.; Davila Costa, J.S.; Colin, V.L.; Fuentes, M.S.; Cuozzo, S.A.; Benimeli, C.S.; Polti, M.A.; Amoroso, M.J. Actinobacteria: Current research and perspectives for bioremediation of pesticides and heavy metals. Chemosphere 2017, 166, 41–62. [Google Scholar] [CrossRef]
- Talaiekhozani, A.; Rezania, S. Application of photosynthetic bacteria for removal of heavy metals, macro-pollutants and dye from wastewater: A review. J. Water Process. Eng. 2017, 19, 312–321. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Yun, Y.S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar] [CrossRef]
- Gupta, P.; Diwan, B. Bacterial Exopolysaccharide mediated heavy metal removal: A Review on biosynthesis, mechanism and remediation strategies. Biotechnol. Rep. 2017, 13, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H. Bacterial mediated alleviation of heavy metal stress and decreased accumulation of metals in plant tissues: Mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 147, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Zeng, G.M.; Niu, Q.Y.; Liu, Y.; Zhou, L.; Jiang, L.H.; Tan, X.F.; Xu, P.; Zhang, C.; Cheng, M. Bioremediation mechanisms of combined pollution of PAHs and heavy metals by bacteria and fungi: A mini review. Bioresour. Technol. 2017, 224, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.-H.; Kwon, Y.-J.; So, J.-S. Bioremediation of heavy metals by using bacterial mixtures. Ecol. Eng. 2016, 89, 64–69. [Google Scholar] [CrossRef]
- Feng, Y.; Yu, Y.; Wang, Y.; Lin, X. Biosorption and bioreduction of trivalent aurum by photosynthetic bacteria Rhodobacter capsulatus. Curr. Microbiol. 2007, 55, 402. [Google Scholar] [CrossRef]
- Martinez-Juarez, V.M.; Cardenas-Gonzalez, J.F.; Torre-Bouscoulet, M.E.; Acosta-Rodriguez, I. Biosorption of Mercury (II) from Aqueous Solutions onto Fungal Biomass. Bioinorg. Chem. Appl. 2012, 2012, 156190. [Google Scholar] [CrossRef]
- Xiao, H.; Zhong, J.-J. Production of Useful Terpenoids by Higher-Fungus Cell Factory and Synthetic Biology Approaches. Trends Biotechnol. 2016, 34, 242–255. [Google Scholar] [CrossRef]
- Bayramoğlu, G.; Tuzun, I.; Celik, G.; Yilmaz, M.; Arica, M.Y. Biosorption of mercury(II), cadmium(II) and lead(II) ions from aqueous system by microalgae Chlamydomonas reinhardtii immobilized in alginate beads. Int. J. Miner. Process. 2006, 81, 35–43. [Google Scholar] [CrossRef]
- Chang, H.X.; Fu, Q.; Huang, Y.; Xia, A.; Liao, Q.; Zhu, X.; Zheng, Y.P.; Sun, C.H. An annular photobioreactor with ion-exchange-membrane for non-touch microalgae cultivation with wastewater. Bioresour. Technol. 2016, 219, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, W.M. Biosorption of heavy metal ions from aqueous solution by red macroalgae. J. Hazard. Mater. 2011, 192, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cao, Q.; Luo, F.; Chen, J. Biosorption of Cd2+, Cu2+, Ni2+ and Zn2+ ions from aqueous solutions by pretreated biomass of brown algae. J. Hazard. Mater. 2009, 163, 931–938. [Google Scholar] [CrossRef]
- Vilar, V.J.; Botelho, C.M.; Loureiro, J.M.; Boaventura, R.A. Biosorption of copper by marine algae Gelidium and algal composite material in a packed bed column. Bioresour. Technol. 2008, 99, 5830–5838. [Google Scholar] [CrossRef]
- Jabar, J.M.; Adebayo, M.A.; Taleat, T.A.A.; Yılmaz, M.; Rangabhashiyam, S. Ipoma batatas (Sweet potato) leaf and leaf-based biochar as potential adsorbents for procion orange MX-2R removal from aqueous solution. J. Anal. Appl. Pyrolysis 2025, 185, 106876. [Google Scholar] [CrossRef]
- Chen, S. Collision-induced breakage of agglomerates in homogenous isotropic turbulence laden with adhesive particles. J. Fluid Mech. 2020, 902, A28. [Google Scholar] [CrossRef]
- Akhtar, N.; Iqbal, M.; Zafar, S.I.; Iqbal, J. Biosorption characteristics of unicellular green alga Chlorella sorokiniana immobilized in loofa sponge for removal of Cr(III). J. Environ. Sci. 2008, 20, 231–239. [Google Scholar] [CrossRef]
- Sheng, P.X.; Wee, K.H.; Ting, Y.P.; Chen, J.P. Biosorption of copper by immobilized marine algal biomass. Chem. Eng. J. 2008, 136, 156–163. [Google Scholar] [CrossRef]
- Arıca, M.Y.; Arpa, Ç.; Kaya, B.; Bektaş, S.; Denizli, A.; Genç, Ö. Comparative biosorption of mercuric ions from aquatic systems by immobilized live and heat-inactivated Trametes versicolor and Pleurotus sajur-caju. Bioresour. Technol. 2003, 89, 145–154. [Google Scholar] [CrossRef]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.C.; Thom, D. Biological interactions between polysaccharides and divalent cations: The egg-box model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef]
- Bulgariu, D.; Bulgariu, L. Equilibrium and kinetics studies of heavy metal ions biosorption on green algae waste biomass. Bioresour. Technol. 2012, 103, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Sarı, A.; Uluozlü, Ö.D.; Tüzen, M. Equilibrium, thermodynamic and kinetic investigations on biosorption of arsenic from aqueous solution by algae (Maugeotia genuflexa) biomass. Chem. Eng. J. 2011, 167, 155–161. [Google Scholar] [CrossRef]
- Khataee, A.R.; Vafaei, F.; Jannatkhah, M. Biosorption of three textile dyes from contaminated water by filamentous green algal Spirogyra sp.: Kinetic, isotherm and thermodynamic studies. Int. Biodeterior. Biodegrad. 2013, 83, 33–40. [Google Scholar] [CrossRef]
- Plazinski, W. Equilibrium and kinetic modeling of metal ion biosorption: On the ways of model generalization for the case of multicomponent systems. Adsorption 2013, 19, 659–666. [Google Scholar] [CrossRef]
- Huang, Y.H.; Peddi, P.K.; Tang, C.; Zeng, H.; Teng, X. Hybrid zero-valent iron process for removing heavy metals and nitrate from flue-gas-desulfurization wastewater. Sep. Purif. Technol. 2013, 118, 690–698. [Google Scholar] [CrossRef]
- Hadavifar, M.; Bahramifar, N.; Younesi, H.; Li, Q. Adsorption of mercury ions from synthetic and real wastewater aqueous solution by functionalized multi-walled carbon nanotube with both amino and thiolated groups. Chem. Eng. J. 2014, 237, 217–228. [Google Scholar] [CrossRef]
- Zia, K.M.; Zia, F.; Zuber, M.; Rehman, S.; Ahmad, M.N. Alginate based polyurethanes: A review of recent advances and perspective. Int. J. Biol. Macromol. 2015, 79, 377–387. [Google Scholar] [CrossRef]
- Li, X.; Shen, Q.; Su, Y.; Tian, F.; Zhao, Y.; Wang, D. Structure−Function Relationship of Calcium Alginate Hydrogels: A Novel Crystal-Forming Engineering. Cryst. Growth Des. 2009, 9, 3470–3476. [Google Scholar] [CrossRef]
- Diaz-Rodriguez, P.; Garcia-Triñanes, P.; Echezarreta López, M.M.; Santoveña, A.; Landin, M. Mineralized alginate hydrogels using marine carbonates for bone tissue engineering applications. Carbohydr. Polym. 2018, 195, 235–242. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, J.; Guo, S.; Shi, J.; Du, W.; Wang, Z.; Ye, L.; Gu, W. Biomimetic mineralization of nano-sized, needle-like hydroxyapatite with ultrahigh capacity for lysozyme adsorption. Mater. Sci. Eng. C 2016, 68, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; He, X.; Wang, L.; Tian, J.; Wan, C.; Jiang, C. Addition of NH4HCO3 as pore-former in membrane electrode assembly for PEMFC. Int. J. Hydrogen Energy 2007, 32, 380–384. [Google Scholar] [CrossRef]
- Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Influence of pH, ionic strength and temperature on lead biosorption by Gelidium and agar extraction algal waste. Process Biochem. 2005, 40, 3267–3275. [Google Scholar] [CrossRef]
- Ponce, S.C.; Prado, C.; Pagano, E.; Prado, F.E.; Rosa, M. Effect of solution pH on the dynamic of biosorption of Cr(VI) by living plants of Salvinia minima. Ecol. Eng. 2015, 74, 33–41. [Google Scholar] [CrossRef]
- Bakatula, E.N.; Cukrowska, E.M.; Weiersbye, I.M.; Mihaly-Cozmuta, L.; Peter, A.; Tutu, H. Biosorption of trace elements from aqueous systems in gold mining sites by the filamentous green algae (Oedogonium sp.). J. Geochem. Explor. 2014, 144, 492–503. [Google Scholar] [CrossRef]
- David Kratochvil, B.V. Advances in the biosorption of heavy metals. Trends Biotechnol. 1998, 16, 291–300. [Google Scholar] [CrossRef]
- Qiu, H.; Lv, L.; Pan, B.c.; Zhang, Q.j.; Zhang, W.m.; Zhang, Q.x. Critical review in adsorption kinetic models. J. Zhejiang Univ. Sci. A 2009, 10, 716–724. [Google Scholar] [CrossRef]
- Vilar, V.J.; Botelho, C.M.; Boaventura, R.A. Kinetics and equilibrium modelling of lead uptake by algae Gelidium and algal waste from agar extraction industry. J. Hazard. Mater. 2007, 143, 396–408. [Google Scholar] [CrossRef]
- Weber Walter, J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]



| Sample | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Width (nm) |
|---|---|---|---|
| CAB | 8.201 | 0.013 | 6.361 |
| MCAB-0.5 | 12.972 | 0.009 | 5.992 |
| MCAB-1 | 15.319 | 0.007 | 5.672 |
| MCAB-1.5 | 14.772 | 0.006 | 5.773 |
| CAB | MCAB-0.5 | MCAB-1 | MCAB-1.5 | ||
|---|---|---|---|---|---|
| Pseudo-first-order model | qe (mg/g) | 0.19 ± 0.01 | 0.54 ± 0.03 | 0.77 ± 0.02 | 0.75 ± 0.03 |
| k1 (/min) | 0.065 ± 0.004 | 0.026 ± 0.001 | 0.055 ± 0.003 | 0.050 ± 0.003 | |
| r2 | 0.972 ± 0.004 | 0.989 ± 0.001 | 0.992 ± 0.002 | 0.963 ± 0.007 | |
| Pseudo-second-order model | qe (mg/g) | 0.18 ± 0.01 | 0.57 ± 0.01 | 0.79 ± 0.01 | 0.78 ± 0.02 |
| k2 (gmg−1 min−1) | 3.151 ± 0.003 | 0.419 ± 0.009 | 0.750 ± 0.001 | 0.741 ± 0.005 | |
| r2 | 0.994 ± 0.002 | 0.991 ± 0.004 | 0.993 ± 0.001 | 0.998 ± 0.006 | |
| W-M model | Kid (mg/g·min0.5) | 1.561 ± 0.207 | 4.266 ± 0.248 | 5.794 ± 0.352 | 5.712 ± 1.083 |
| C | 2.444 ± 1.498 | 1.344 ± 0.248 | 7.911 ± 2.485 | 15.571 ± 7.268 | |
| r2 | 0.963 ± 0.017 | 0.951 ± 0.003 | 0.967 ± 0.015 | 0.977 ± 0.024 |
| CAB | MCAB-0.5 | MCAB-1 | MCAB-1.5 | ||
|---|---|---|---|---|---|
| Langmuir | qm (mgg−1) | 18 ± 1 | 32 ± 2 | 48 ± 1 | 46 ± 4 |
| KL (Lmg−1) | 0.76 ± 0.01 | 0.47 ± 0.04 | 0.76 ± 0.01 | 0.92 ± 0.01 | |
| R2 | 0.996 ± 0.002 | 0.995 ± 0.004 | 0.998 ± 0.003 | 0.999 ± 0.006 | |
| Freundlich | KF (Lng−1mg1−n) | 1.6 ± 0.2 | 4.0 ± 0.1 | 10.0 ± 0.5 | 11.8 ± 0.3 |
| 1/n | 0.52 ± 0.07 | 0.61 ± 0.02 | 0.54 ± 0.06 | 0.52 ± 0.01 | |
| R2 | 0.970 ± 0.006 | 0.968 ± 0.003 | 0.965 ± 0.003 | 0.960 ± 0.009 | |
| Temkin model | KT (Lmg−1) | 0.181 ± 0.051 | 0.082 ± 0.022 | 0.103 ± 0.021 | 0.121 ± 0.022 |
| b (Jmg−1) | 331.819 ± 34.816 | 61.494 ± 6.500 | 39.462 ± 2.855 | 40.429 ± 2.390 | |
| R2 | 0.964 ± 0.007 | 0.981 ± 0.009 | 0.987 ± 0.003 | 0.989 ± 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Zhang, C.; Li, X.; Feng, T.; Gong, X. Mercury Adsorption by Ca-Based Shell-Type Polymers Synthesized by Self-Assembly Mineralization. Polymers 2024, 16, 3454. https://doi.org/10.3390/polym16243454
Peng Y, Zhang C, Li X, Feng T, Gong X. Mercury Adsorption by Ca-Based Shell-Type Polymers Synthesized by Self-Assembly Mineralization. Polymers. 2024; 16(24):3454. https://doi.org/10.3390/polym16243454
Chicago/Turabian StylePeng, Yang, Chuxuan Zhang, Xiaomin Li, Tianyi Feng, and Xun Gong. 2024. "Mercury Adsorption by Ca-Based Shell-Type Polymers Synthesized by Self-Assembly Mineralization" Polymers 16, no. 24: 3454. https://doi.org/10.3390/polym16243454
APA StylePeng, Y., Zhang, C., Li, X., Feng, T., & Gong, X. (2024). Mercury Adsorption by Ca-Based Shell-Type Polymers Synthesized by Self-Assembly Mineralization. Polymers, 16(24), 3454. https://doi.org/10.3390/polym16243454





