Biomimetic 3D Hydrogels with Aligned Topography for Neural Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hydrogel Synthesis
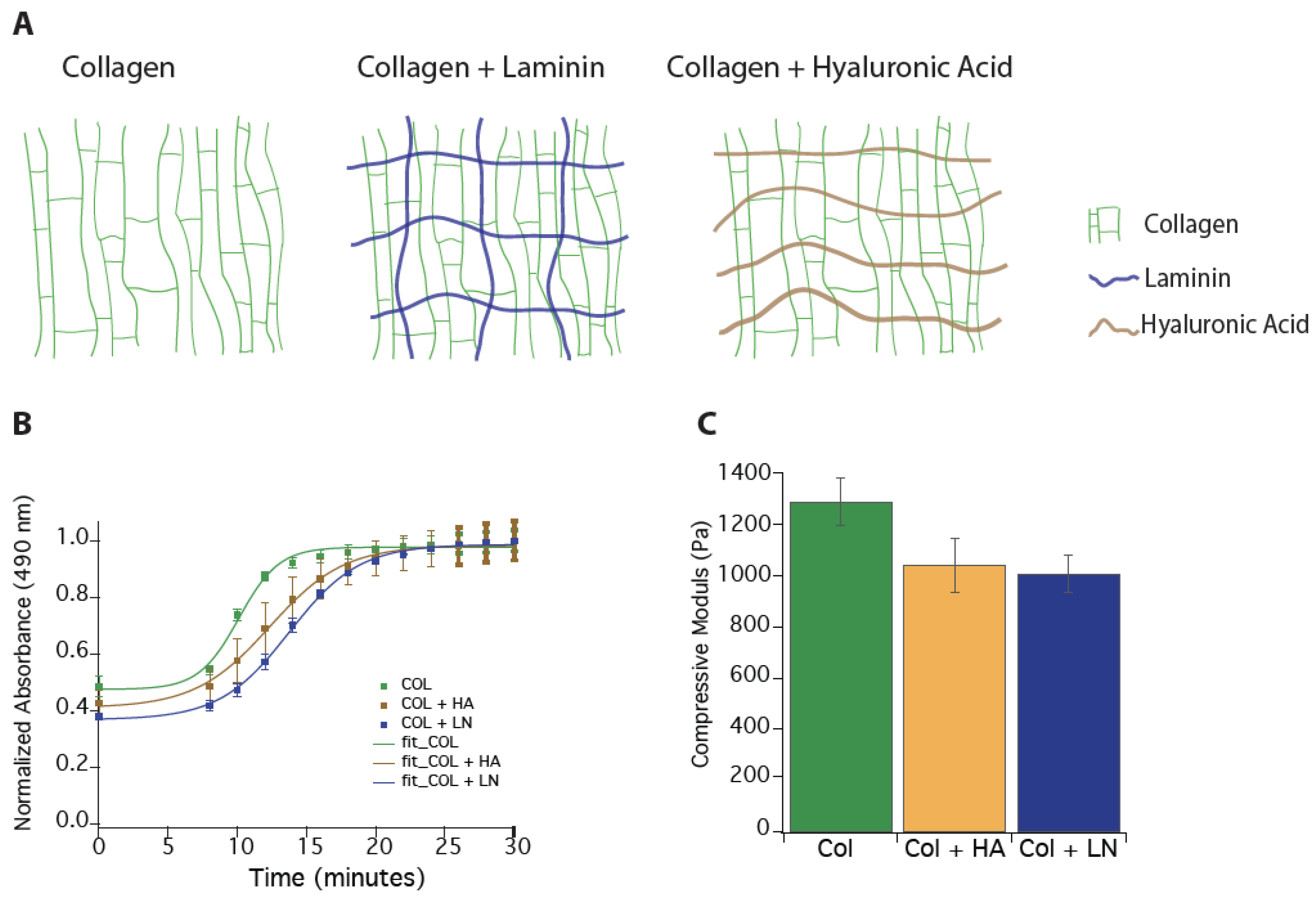
2.2. Gelation
2.3. Compressive Modulus
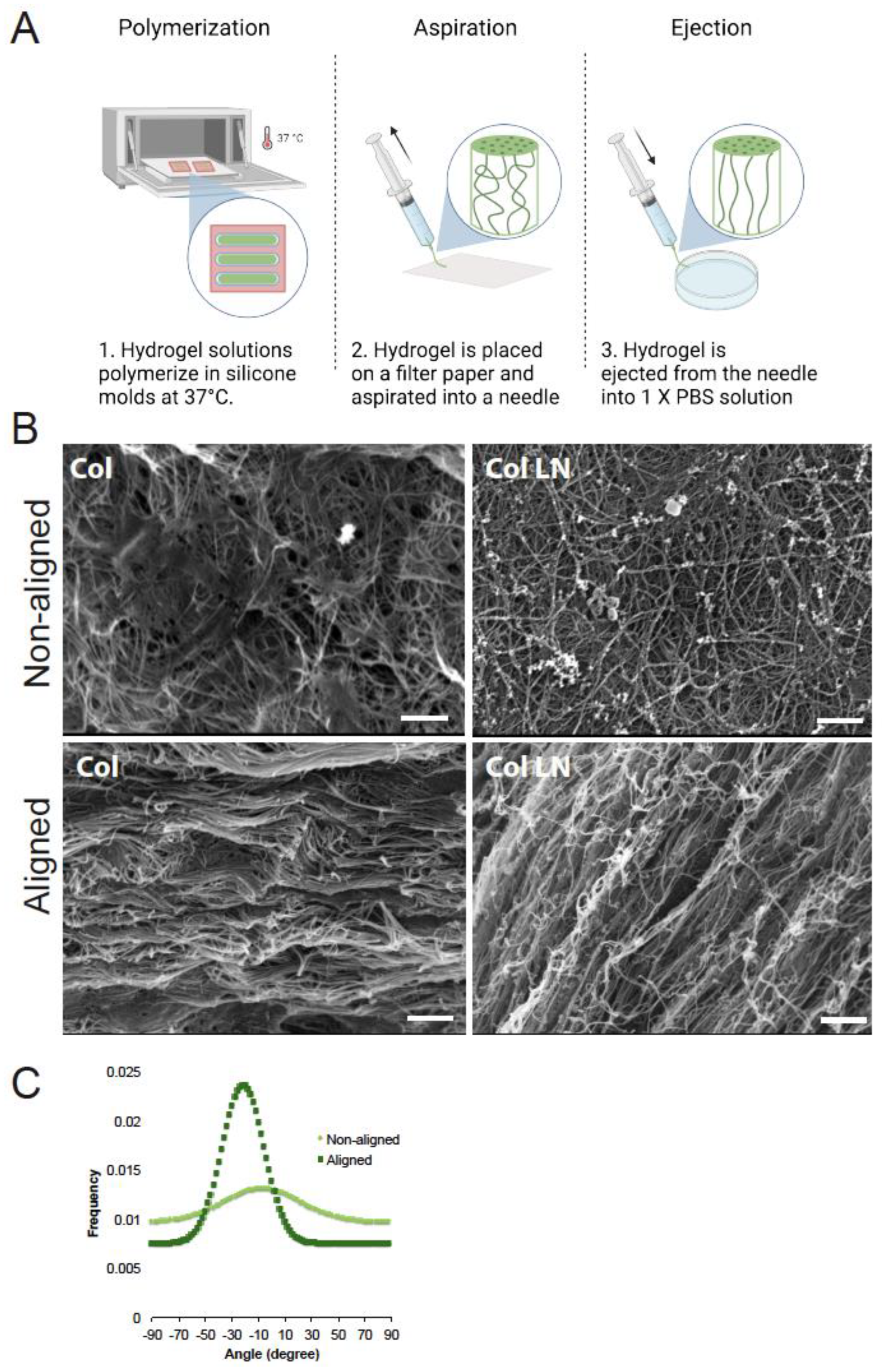
2.4. Fabrication of Aligned Hydrogels
2.5. Scanning Electron Microscopy and Image Analysis
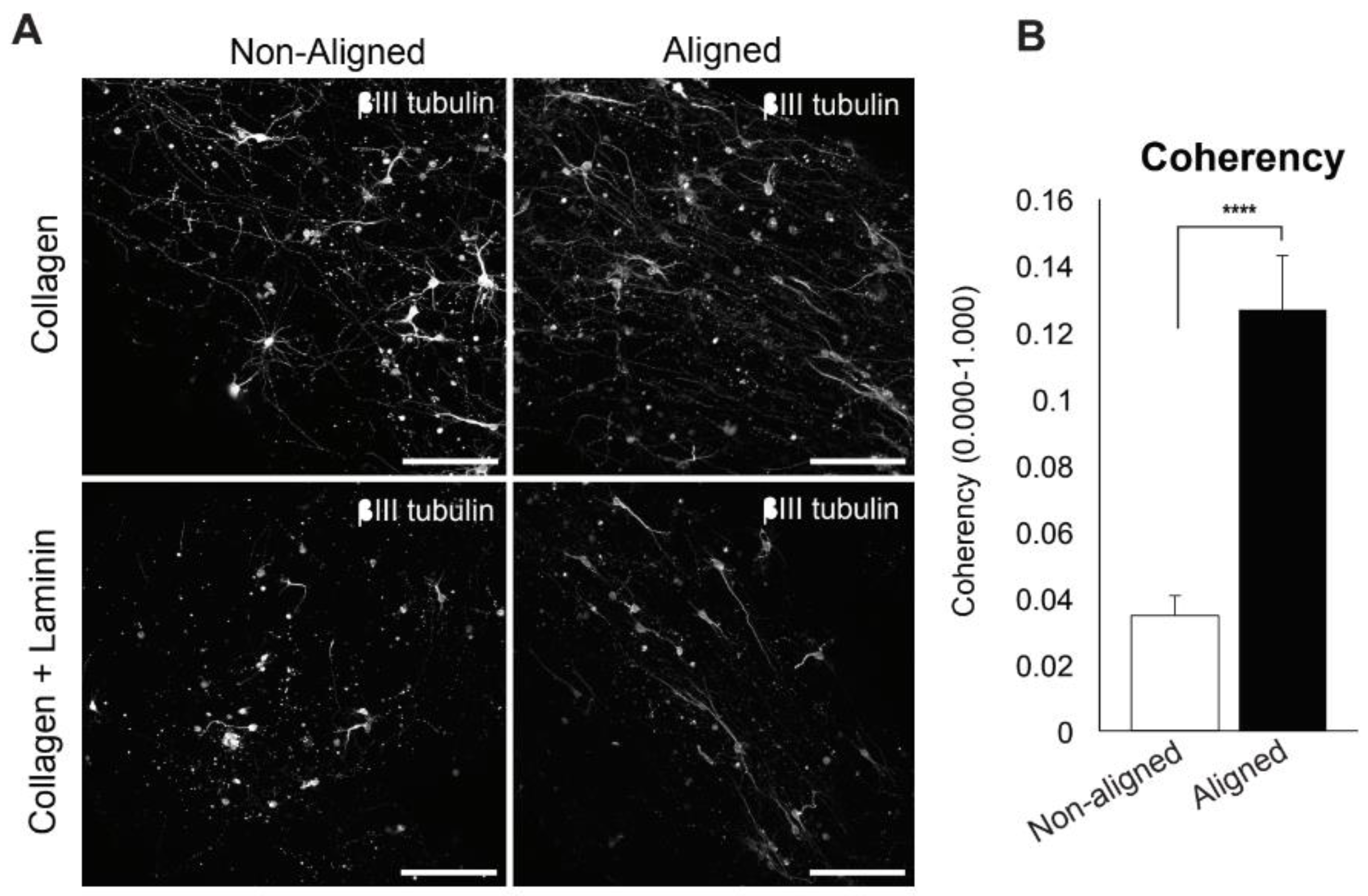
2.6. Embryonic Spinal Cord Neuron Cultures
2.7. Immunocytochemistry
2.8. Spinal Cord Injury and Hydrogel Implantation
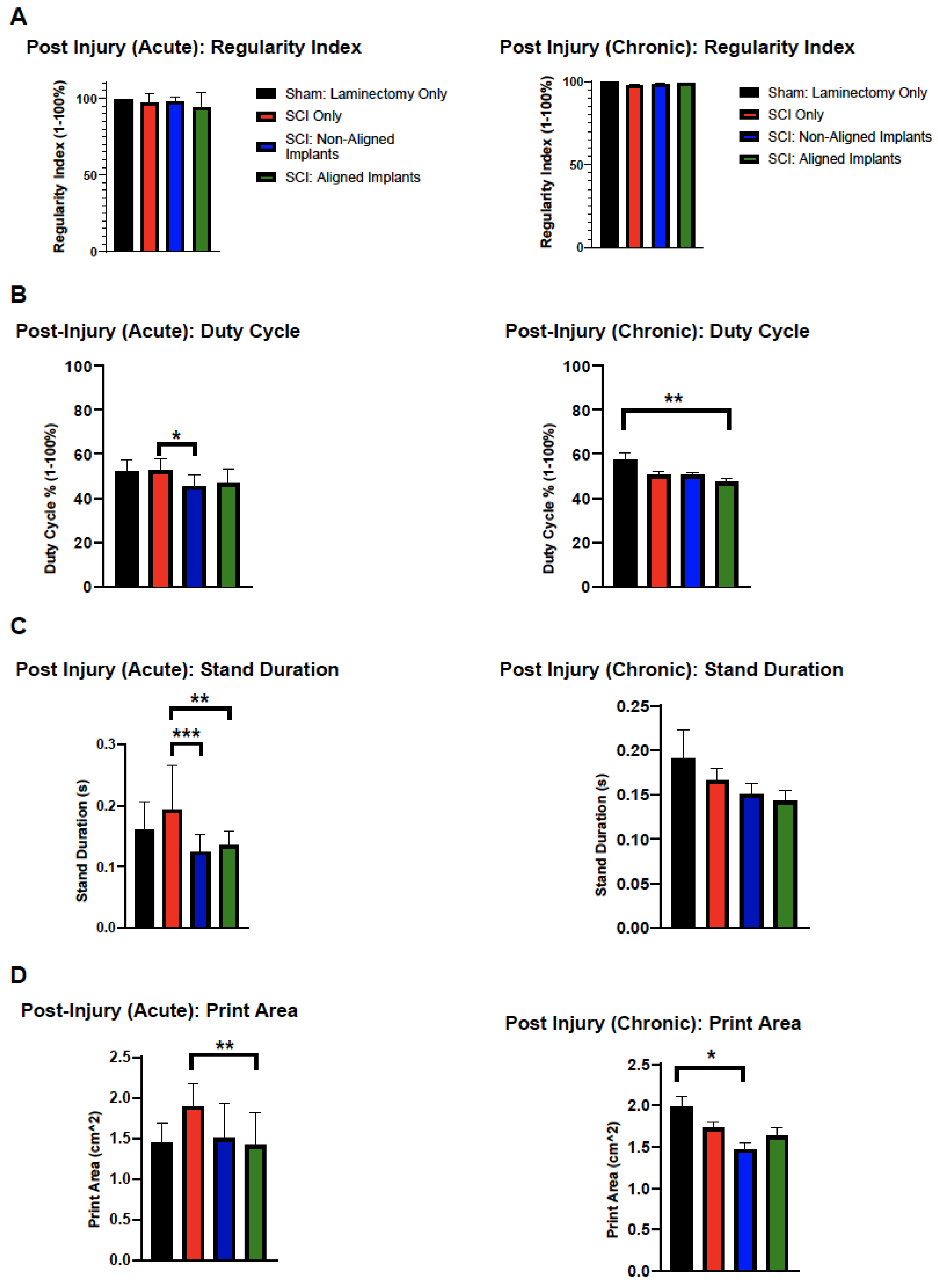
2.9. Behavioral Analysis
2.10. Histological Analysis of Spinal Cord Tissue
2.11. Statistics
3. Results
3.1. Producing Alignment Within Hydrogels
3.2. Embryonic Spinal Cord Neurons in Aligned Scaffolds
3.3. Functional Evaluation
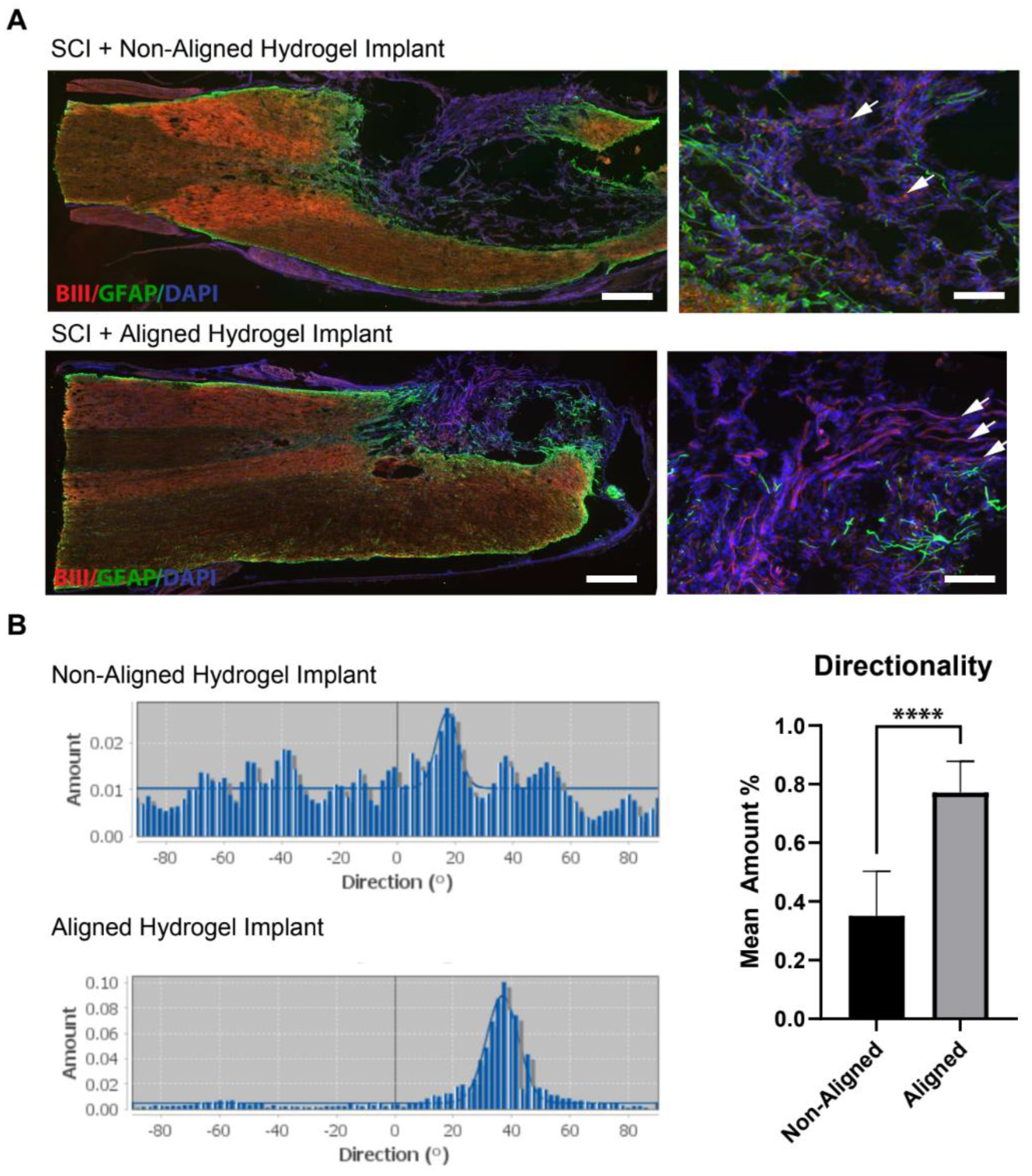
3.4. Histological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dell’Anno, M.T.; Strittmatter, S.M. Rewiring the spinal cord: Direct and indirect strategies. Neurosci. Lett. 2017, 652, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Teng, Z.Q.; Liu, C.M. Extrinsic and Intrinsic Regulation of Axon Regeneration by MicroRNAs after Spinal Cord Injury. Neural Plast. 2016, 2016, 1279051. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, R.; Ueno, M.; Yamashita, T. Intrinsic regenerative mechanisms of central nervous system neurons. Biosci. Trends 2009, 3, 179–183. [Google Scholar]
- Murray, A.J. Axon regeneration: What needs to be overcome? Methods Mol. Biol. 2014, 1162, 3–14. [Google Scholar] [PubMed]
- Ruff, R.L.; McKerracher, L.; Selzer, M.E. Repair and neurorehabilitation strategies for spinal cord injury. Ann. N. Y. Acad. Sci. 2008, 1142, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Khaing, Z.Z.; Ehsanipour, A.; Hofstetter, C.P.; Seidlits, S.K. Injectable Hydrogels for Spinal Cord Repair: A Focus on Swelling and Intraspinal Pressure. Cells Tissues Organs 2016, 202, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Spivey, E.C.; Khaing, Z.Z.; Shear, J.B.; Schmidt, C.E. The fundamental role of subcellular topography in peripheral nerve repair therapies. Biomaterials 2012, 33, 4264–4276. [Google Scholar] [CrossRef] [PubMed]
- Dalamagkas, K.; Tsintou, M.; Seifalian, A.; Seifalian, A.M. Translational Regenerative Therapies for Chronic Spinal Cord Injury. Int. J. Mol. Sci. 2018, 19, 1776. [Google Scholar] [CrossRef] [PubMed]
- Kameda, T.; Imamura, T.; Nakashima, K. Epigenetic regulation of neural stem cell differentiation towards spinal cord regeneration. Cell Tissue Res. 2018, 371, 189–199. [Google Scholar] [CrossRef]
- Liu, S.; Schackel, T.; Weidner, N.; Puttagunta, R. Biomaterial-Supported Cell Transplantation Treatments for Spinal Cord Injury: Challenges and Perspectives. Front. Cell. Neurosci. 2017, 11, 430. [Google Scholar] [CrossRef]
- Maldonado-Lasuncion, I.; Verhaagen, J.; Oudega, M. Mesenchymal Stem Cell-Macrophage Choreography Supporting Spinal Cord Repair. Neurotherapeutics 2018, 15, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Nagoshi, N.; Okano, H. iPSC-derived neural precursor cells: Potential for cell transplantation therapy in spinal cord injury. Cell. Mol. Life Sci. 2018, 75, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, O.; Sugai, K.; Yamaguchi, R.; Tashiro, S.; Nagoshi, N.; Kohyama, J.; Iida, T.; Ohkubo, T.; Itakura, G.; Isoda, M.; et al. Concise Review: Laying the Groundwork for a First-In-Human Study of an Induced Pluripotent Stem Cell-Based Intervention for Spinal Cord Injury. Stem Cells 2019, 37, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Z.; Sakiyama-Elbert, S.E. Matrices, scaffolds & carriers for cell delivery in nerve regeneration. Exp. Neurol. 2019, 319, 112837. [Google Scholar]
- Yu, B.; Gu, X. Combination of biomaterial transplantation and genetic enhancement of intrinsic growth capacities to promote CNS axon regeneration after spinal cord injury. Front. Med. 2019, 13, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, E.J.; Khemani, S.; Von, R.; King; Priestley, J.V.; McMahon, S.B. NT-3 promotes growth of lesioned adult rat sensory axons ascending in the dorsal columns of the spinal cord. Eur. J. Neurosci. 1999, 11, 3873–3883. [Google Scholar] [CrossRef] [PubMed]
- Storer, P.D.; Dolbeare, D.; Houle, J.D. Treatment of chronically injured spinal cord with neurotrophic factors stimulates betaII-tubulin and GAP-43 expression in rubrospinal tract neurons. J. Neurosci. Res. 2003, 74, 502–511. [Google Scholar] [CrossRef]
- Widenfalk, J.; Lundstromer, K.; Jubran, M.; Brene, S.; Olson, L. Neurotrophic factors and receptors in the immature and adult spinal cord after mechanical injury or kainic acid. J. Neurosci. 2001, 21, 3457–3475. [Google Scholar] [CrossRef]
- Fawcett, J.W.; Schwab, M.E.; Montani, L.; Brazda, N.; Muller, H.W. Defeating inhibition of regeneration by scar and myelin components. Handb. Clin. Neurol. 2012, 109, 503–522. [Google Scholar] [PubMed]
- Lauren, J.; Hu, F.; Chin, J.; Liao, J.; Airaksinen, M.S.; Strittmatter, S.M. Characterization of myelin ligand complexes with neuronal Nogo-66 receptor family members. J. Biol. Chem. 2007, 282, 5715–5725. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Kim, J.E.; Budel, S.; Hampton, T.G.; Strittmatter, S.M. Transgenic inhibition of Nogo-66 receptor function allows axonal sprouting and improved locomotion after spinal injury. Mol. Cell. Neurosci. 2005, 29, 26–39. [Google Scholar] [CrossRef]
- Ma, Z.; Cao, Q.; Zhang, L.; Hu, J.; Howard, R.M.; Lu, P.; Whittemore, S.R.; Xu, X.M. Oligodendrocyte precursor cells differentially expressing Nogo-A but not MAG are more permissive to neurite outgrowth than mature oligodendrocytes. Exp. Neurol. 2009, 217, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Khaing, Z.Z.; Li, N.; Hall, B.; Schmidt, C.E.; Ellington, A.D. Aptamer antagonists of myelin-derived inhibitors promote axon growth. PLoS ONE 2010, 5, e9726. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Giger, R.J. A new role for RPTPsigma in spinal cord injury: Signaling chondroitin sulfate proteoglycan inhibition. Sci. Signal 2010, 3, pe6. [Google Scholar] [CrossRef]
- Lang, B.T.; Cregg, J.M.; DePaul, M.A.; Tran, A.P.; Xu, K.; Dyck, S.M.; Madalena, K.M.; Brown, B.P.; Weng, Y.L.; Li, S.; et al. Modulation of the proteoglycan receptor PTPsigma promotes recovery after spinal cord injury. Nature 2015, 518, 404–408. [Google Scholar] [CrossRef]
- Sharma, T.; Pereira Alves, G.C.; Kuo, J.S. “Inhibiting the Inhibitors” to Support Axonal Regeneration. Neurosurgery 2016, 78, N14–N16. [Google Scholar] [CrossRef]
- Shen, Y.; Tenney, A.P.; Busch, S.A.; Horn, K.P.; Cuascut, F.X.; Liu, K.; He, Z.; Silver, J.; Flanagan, J.G. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science 2009, 326, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, C.S.; Nori, S.; Tetreault, L.; Wilson, J.; Kwon, B.; Harrop, J.; Choi, D.; Fehlings, M.G. Traumatic Spinal Cord Injury-Repair and Regeneration. Neurosurgery 2017, 80, S9–S22. [Google Scholar] [CrossRef] [PubMed]
- Altinova, H.; Mollers, S.; Fuhrmann, T.; Deumens, R.; Bozkurt, A.; Heschel, I.; Damink, L.H.; Schugner, F.; Weis, J.; Brook, G.A. Functional improvement following implantation of a microstructured, type-I collagen scaffold into experimental injuries of the adult rat spinal cord. Brain Res. 2014, 1585, 37–50. [Google Scholar] [CrossRef]
- Anderson, M.A.; O’Shea, T.M.; Burda, J.E.; Ao, Y.; Barlatey, S.L.; Bernstein, A.M.; Kim, J.H.; James, N.D.; Rogers, A.; Kato, B.; et al. Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature 2018, 561, 396–400. [Google Scholar] [CrossRef]
- Kumar, P.; Choonara, Y.E.; Modi, G.; Naidoo, D.; Pillay, V. Multifunctional therapeutic delivery strategies for effective neuro-regeneration following traumatic spinal cord injury. Curr. Pharm. Des. 2015, 21, 1517–1528. [Google Scholar] [CrossRef]
- Liu, S.; Sandner, B.; Schackel, T.; Nicholson, L.; Chtarto, A.; Tenenbaum, L.; Puttagunta, R.; Muller, R.; Weidner, N.; Blesch, A. Regulated viral BDNF delivery in combination with Schwann cells promotes axonal regeneration through capillary alginate hydrogels after spinal cord injury. Acta Biomater. 2017, 60, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Pawar, K.; Cummings, B.J.; Thomas, A.; Shea, L.D.; Levine, A.; Pfaff, S.; Anderson, A.J. Biomaterial bridges enable regeneration and re-entry of corticospinal tract axons into the caudal spinal cord after SCI: Association with recovery of forelimb function. Biomaterials 2015, 65, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Wang, Z.; Shen, J.; Xu, S.; Hu, Z. Nerve growth factor delivery by ultrasound-mediated nanobubble destruction as a treatment for acute spinal cord injury in rats. Int. J. Nanomed. 2017, 12, 1717–1729. [Google Scholar] [CrossRef] [PubMed]
- Wilems, T.S.; Pardieck, J.; Iyer, N.; Sakiyama-Elbert, S.E. Combination therapy of stem cell derived neural progenitors and drug delivery of anti-inhibitory molecules for spinal cord injury. Acta Biomater. 2015, 28, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Khaing, Z.Z.; Schmidt, C.E. Advances in natural biomaterials for nerve tissue repair. Neurosci. Lett. 2012, 519, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Corey, J.M.; Gertz, C.C.; Wang, B.S.; Birrell, L.K.; Johnson, S.L.; Martin, D.C.; Feldman, E.L. The design of electrospun PLLA nanofiber scaffolds compatible with serum-free growth of primary motor and sensory neurons. Acta Biomater. 2008, 4, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, T.; Anandakumaran, P.N.; Shoichet, M.S. Combinatorial Therapies After Spinal Cord Injury: How Can Biomaterials Help? Adv. Healthc. Mater. 2017, 6, 1601130. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Q.; Zhang, B.; Ban, D.X.; Feng, S.Q. Multifunctional biomimetic spinal cord: New approach to repair spinal cord injuries. World J. Exp. Med. 2017, 7, 78–83. [Google Scholar] [CrossRef]
- Perale, G.; Rossi, F.; Sundstrom, E.; Bacchiega, S.; Masi, M.; Forloni, G.; Veglianese, P. Hydrogels in spinal cord injury repair strategies. ACS Chem. Neurosci. 2011, 2, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Golland, B.; Tipper, J.L.; Hall, R.M.; Tronci, G.; Russell, S.J. A Biomimetic Nonwoven-Reinforced Hydrogel for Spinal Cord Injury Repair. Polymers 2022, 14, 4376. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.A.; Eltaher, H.M.; Sottile, V.; Yang, J. 3D bioprinting of a stem cell-laden, multi-material tubular composite: An approach for spinal cord repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111707. [Google Scholar] [CrossRef] [PubMed]
- Koffler, J.; Zhu, W.; Qu, X.; Platoshyn, O.; Dulin, J.N.; Brock, J.; Graham, L.; Lu, P.; Sakamoto, J.; Marsala, M.; et al. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat. Med. 2019, 25, 263–269. [Google Scholar] [CrossRef]
- Sha, Q.; Wang, Y.; Zhu, Z.; Wang, H.; Qiu, H.; Niu, W.; Li, X.; Qian, J. A hyaluronic acid/silk fibroin/poly-dopamine-coated biomimetic hydrogel scaffold with incorporated neurotrophin-3 for spinal cord injury repair. Acta Biomater. 2023, 167, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.A.; Salgado, A.J.; Sousa, R.A.; Oliveira, J.T.; Pedro, A.J.; Leite-Almeida, H.; Cerqueira, R.; Almeida, A.; Mastronardi, F.; Mano, J.F.; et al. Development and characterization of a novel hybrid tissue engineering-based scaffold for spinal cord injury repair. Tissue Eng. Part A 2010, 16, 45–54. [Google Scholar] [CrossRef]
- Sun, Z.; Luan, X.; Sun, Z.; Li, D.; Hu, H.; Xue, Q.; Liu, B.; Yu, Q.; Wei, G.; Zhang, X.; et al. Bioactive Peptide Hydrogel Scaffold with High Fluidity, Thermosensitivity, and Neurotropism in 3D Spatial Structure for Promoted Repair of Spinal Cord Injury. Small 2024, e2406990. [Google Scholar] [CrossRef]
- Geissler, S.A.; Sabin, A.L.; Besser, R.R.; Gooden, O.M.; Shirk, B.D.; Nguyen, Q.M.; Khaing, Z.Z.; Schmidt, C.E. Biomimetic hydrogels direct spinal progenitor cell differentiation and promote functional recovery after spinal cord injury. J. Neural Eng. 2018, 15, 025004. [Google Scholar] [CrossRef]
- Khaledian, S.; Mohammadi, G.; Abdoli, M.; Fatahian, A.; Fatahian, A.; Fatahian, R. Recent Advances in Implantable 3D-Printed Scaffolds for Repair of Spinal Cord Injury. Adv. Pharm. Bull. 2024, 14, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; He, J. Hydrogel-based therapeutic strategies for spinal cord injury repair: Recent advances and future prospects. Int. J. Biol. Macromol. 2024, 277 Pt 4, 134591. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, R.; Hao, J.; Yang, Y.; Xu, S.; Zhang, F.; Zhang, F.; Yao, Y. Design and preparation of hydrogel microspheres for spinal cord injury repair. J. Biomed. Mater. Res. A 2024, 112, 2358–2371. [Google Scholar] [CrossRef]
- Fan, C.; Li, X.; Xiao, Z.; Zhao, Y.; Liang, H.; Wang, B.; Han, S.; Li, X.; Xu, B.; Wang, N.; et al. A modified collagen scaffold facilitates endogenous neurogenesis for acute spinal cord injury repair. Acta Biomater. 2017, 51, 304–316. [Google Scholar] [CrossRef]
- Kaneko, A.; Matsushita, A.; Sankai, Y. A 3D nanofibrous hydrogel and collagen sponge scaffold promotes locomotor functional recovery, spinal repair, and neuronal regeneration after complete transection of the spinal cord in adult rats. Biomed. Mater. 2015, 10, 015008. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fan, C.; Xiao, Z.; Zhao, Y.; Zhang, H.; Sun, J.; Zhuang, Y.; Wu, X.; Shi, J.; Chen, Y.; et al. A collagen microchannel scaffold carrying paclitaxel-liposomes induces neuronal differentiation of neural stem cells through Wnt/beta-catenin signaling for spinal cord injury repair. Biomaterials 2018, 183, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Sun, W.; Lin, H.; Zhao, W.; Gao, Y.; Zhao, Y.; Chen, B.; Xiao, Z.; Hu, W.; Li, Y.; et al. Linear ordered collagen scaffolds loaded with collagen-binding brain-derived neurotrophic factor improve the recovery of spinal cord injury in rats. Tissue Eng. Part A 2009, 15, 2927–2935. [Google Scholar] [CrossRef]
- Rocha, L.B.; Goissis, G.; Rossi, M.A. Biocompatibility of anionic collagen matrix as scaffold for bone healing. Biomaterials 2002, 23, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed]
- Achilli, M.; Lagueux, J.; Mantovani, D. On the effects of UV-C and pH on the mechanical behavior, molecular conformation and cell viability of collagen-based scaffold for vascular tissue engineering. Macromol. Biosci. 2010, 10, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Grimpe, B.; Silver, J. The extracellular matrix in axon regeneration. Prog. Brain Res. 2002, 137, 333–349. [Google Scholar]
- Hausmann, B.; Sievers, J.; Hermanns, J.; Berry, M. Regeneration of axons from the adult rat optic nerve: Influence of fetal brain grafts, laminin, and artificial basement membrane. J. Comp. Neurol. 1989, 281, 447–466. [Google Scholar] [CrossRef]
- Hunter, D.D.; Llinas, R.; Ard, M.; Merlie, J.P.; Sanes, J.R. Expression of s-laminin and laminin in the developing rat central nervous system. J. Comp. Neurol. 1992, 323, 238–251. [Google Scholar] [CrossRef]
- Galtrey, C.M.; Kwok, J.C.; Carulli, D.; Rhodes, K.E.; Fawcett, J.W. Distribution and synthesis of extracellular matrix proteoglycans, hyaluronan, link proteins and tenascin-R in the rat spinal cord. Eur. J. Neurosci. 2008, 27, 1373–1390. [Google Scholar] [CrossRef] [PubMed]
- Seidlits, S.K.; Khaing, Z.Z.; Petersen, R.R.; Nickels, J.D.; Vanscoy, J.E.; Shear, J.B.; Schmidt, C.E. The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials 2010, 31, 3930–3940. [Google Scholar] [CrossRef] [PubMed]
- Marelli, B.; Ghezzi, C.E.; James-Bhasin, M.; Nazhat, S.N. Fabrication of injectable, cellular, anisotropic collagen tissue equivalents with modular fibrillar densities. Biomaterials 2015, 37, 183–193. [Google Scholar] [CrossRef]
- Gingras, M.; Gagnon, V.; Minotti, S.; Durham, H.D.; Berthod, F. Optimized protocols for isolation of primary motor neurons, astrocytes and microglia from embryonic mouse spinal cord. J. Neurosci. Methods 2007, 163, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 1996, 139, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Clemons, T.D.; Bradshaw, M.; Toshniwal, P.; Chaudhari, N.; Stevenson, A.W.; Lynch, J.; Fear, M.W.; Wood, F.M.; Iyer, K.S. Coherency image analysis to quantify collagen architecture: Implications in scar assessment. Rsc Adv. 2018, 8, 9661–9669. [Google Scholar] [CrossRef]
- Couppe, C.; Svensson, R.B.; Heinemeier, K.M.; Thomsen, E.W.; Bayer, M.L.; Christensen, L.; Kjaer, M.; Magnusson, S.P.; Schjerling, P. Quantification of cell density in rat Achilles tendon: Development and application of a new method. Histochem. Cell Biol. 2017, 147, 97–102. [Google Scholar] [CrossRef]
- Elsdale, T.; Bard, J. Collagen substrata for studies on cell behavior. J. Cell Biol. 1972, 54, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Orban, J.M.; Wilson, L.B.; Kofroth, J.A.; El-Kurdi, M.S.; Maul, T.M.; Vorp, D.A. Crosslinking of collagen gels by transglutaminase. J. Biomed. Mater. Res. A 2004, 68, 756–762. [Google Scholar] [CrossRef]
- Bancelin, S.; Aime, C.; Gusachenko, I.; Kowalczuk, L.; Latour, G.; Coradin, T.; Schanne-Klein, M.C. Determination of collagen fibril size via absolute measurements of second-harmonic generation signals. Nat. Commun. 2014, 5, 4920. [Google Scholar] [CrossRef] [PubMed]
- Joddar, B.; Kitajima, T.; Ito, Y. The effects of covalently immobilized hyaluronic acid substrates on the adhesion, expansion, and differentiation of embryonic stem cells for in vitro tissue engineering. Biomaterials 2011, 32, 8404–8415. [Google Scholar] [CrossRef] [PubMed]
- Back, S.A.; Tuohy, T.M.; Chen, H.; Wallingford, N.; Craig, A.; Struve, J.; Luo, N.L.; Banine, F.; Liu, Y.; Chang, A.; et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat. Med. 2005, 11, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Baier, C.; Baader, S.L.; Jankowski, J.; Gieselmann, V.; Schilling, K.; Rauch, U.; Kappler, J. Hyaluronan is organized into fiber-like structures along migratory pathways in the developing mouse cerebellum. Matrix Biol. 2007, 26, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Khaing, Z.Z.; Milman, B.D.; Vanscoy, J.E.; Seidlits, S.K.; Grill, R.J.; Schmidt, C.E. High molecular weight hyaluronic acid limits astrocyte activation and scar formation after spinal cord injury. J. Neural Eng. 2011, 8, 046033. [Google Scholar] [CrossRef]
- Hou, S.; Tian, W.; Xu, Q.; Cui, F.; Zhang, J.; Lu, Q.; Zhao, C. The enhancement of cell adherence and inducement of neurite outgrowth of dorsal root ganglia co-cultured with hyaluronic acid hydrogels modified with Nogo-66 receptor antagonist in vitro. Neuroscience 2006, 137, 519–529. [Google Scholar] [CrossRef]
- Tarus, D.; Hamard, L.; Caraguel, F.; Wion, D.; Szarpak-Jankowska, A.; van der Sanden, B.; Auzely-Velty, R. Design of Hyaluronic Acid Hydrogels to Promote Neurite Outgrowth in Three Dimensions. ACS Appl. Mater. Interfaces 2016, 8, 25051–25059. [Google Scholar] [CrossRef]
- Horn, E.M.; Beaumont, M.; Shu, X.Z.; Harvey, A.; Prestwich, G.D.; Horn, K.M.; Gibson, A.R.; Preul, M.C.; Panitch, A. Influence of cross-linked hyaluronic acid hydrogels on neurite outgrowth and recovery from spinal cord injury. J. Neurosurg. Spine 2007, 6, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.K.; Jelacic, S.; Maier, R.V.; Stayton, P.S.; Hoffman, A.S. Anti-inflammatory drug delivery from hyaluronic acid hydrogels. J. Biomater. Sci. Polym. Ed. 2004, 15, 1111–1119. [Google Scholar] [CrossRef]
- Molofsky, A.V.; Glasgow, S.M.; Chaboub, L.S.; Tsai, H.H.; Murnen, A.T.; Kelley, K.W.; Fancy, S.P.; Yuen, T.J.; Madireddy, L.; Baranzini, S.; et al. Expression profiling of Aldh1l1-precursors in the developing spinal cord reveals glial lineage-specific genes and direct Sox9-Nfe2l1 interactions. Glia 2013, 61, 1518–1532. [Google Scholar] [CrossRef] [PubMed]
- Contreras, J.E.; Sanchez, H.A.; Eugenin, E.A.; Speidel, D.; Theis, M.; Willecke, K.; Bukauskas, F.F.; Bennett, M.V.; Saez, J.C. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc. Natl. Acad. Sci. USA 2002, 99, 495–500. [Google Scholar] [CrossRef]
- Allaman, I.; Belanger, M.; Magistretti, P.J. Astrocyte-neuron metabolic relationships: For better and for worse. Trends Neurosci. 2011, 34, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Stogsdill, J.A.; Ramirez, J.; Liu, D.; Kim, Y.H.; Baldwin, K.T.; Enustun, E.; Ejikeme, T.; Ji, R.R.; Eroglu, C. Astrocytic neuroligins control astrocyte morphogenesis and synaptogenesis. Nature 2017, 551, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef]
- Lukovic, D.; Stojkovic, M.; Moreno-Manzano, V.; Jendelova, P.; Sykova, E.; Bhattacharya, S.S.; Erceg, S. Concise review: Reactive astrocytes and stem cells in spinal cord injury: Good guys or bad guys? Stem Cells 2015, 33, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Bartus, K.; James, N.D.; Didangelos, A.; Bosch, K.D.; Verhaagen, J.; Yanez-Munoz, R.J.; Rogers, J.H.; Schneider, B.L.; Muir, E.M.; Bradbury, E.J. Large-scale chondroitin sulfate proteoglycan digestion with chondroitinase gene therapy leads to reduced pathology and modulates macrophage phenotype following spinal cord contusion injury. J. Neurosci. 2014, 34, 4822–4836. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Severs, L.J.; Katta, A.; Cates, L.N.; Dewees, D.M.; Hoagland, R.T.; Horner, P.J.; Hofstetter, C.P.; Khaing, Z.Z. Biomimetic 3D Hydrogels with Aligned Topography for Neural Tissue Engineering. Polymers 2024, 16, 3556. https://doi.org/10.3390/polym16243556
Severs LJ, Katta A, Cates LN, Dewees DM, Hoagland RT, Horner PJ, Hofstetter CP, Khaing ZZ. Biomimetic 3D Hydrogels with Aligned Topography for Neural Tissue Engineering. Polymers. 2024; 16(24):3556. https://doi.org/10.3390/polym16243556
Chicago/Turabian StyleSevers, Liza J., Anjali Katta, Lindsay N. Cates, Dane M. Dewees, Riana T. Hoagland, Philip J. Horner, Christoph P. Hofstetter, and Zin Z. Khaing. 2024. "Biomimetic 3D Hydrogels with Aligned Topography for Neural Tissue Engineering" Polymers 16, no. 24: 3556. https://doi.org/10.3390/polym16243556
APA StyleSevers, L. J., Katta, A., Cates, L. N., Dewees, D. M., Hoagland, R. T., Horner, P. J., Hofstetter, C. P., & Khaing, Z. Z. (2024). Biomimetic 3D Hydrogels with Aligned Topography for Neural Tissue Engineering. Polymers, 16(24), 3556. https://doi.org/10.3390/polym16243556





