Extraction of Natural-Based Raw Materials Towards the Production of Sustainable Man-Made Organic Fibres
Abstract
1. Introduction
2. Bioresources: Biomass and Biowaste
2.1. Terrestrial Vegetables
2.2. Aquatic Vegetables
2.3. Fungi
2.4. Animalia
2.5. Agroforestry Residues (Hardwood, Softwood, and Agricultural Residues)
2.6. Industrial Waste
3. Pre-Treatments and Extraction Methods: Insights, Main Advantages, and Disadvantages
3.1. Physical Methods
3.2. Chemical Methods
3.3. Physicochemical Methods
3.4. Biological Methods
4. Man-Made Organic Fibres
4.1. Production Methods
4.2. Bio-Based Man-Made Fibres
4.2.1. Polysaccharides
4.2.2. Proteins
4.2.3. Lignin and Other Bioactive Compounds
4.2.4. Lipids
| Composition | Spinning Method | Properties | Application | References |
|---|---|---|---|---|
| Chitosan + Graphene Chitosan + PVA + Graphene Chitosan + Cotton + Polyester + Tencel Chitin + Cellulose | Wet-spinning Electrospinning Wet-spinning Wet-, dry-spinning | Softness, biodegradability, thermal conductivity, antibacterial properties | Antibacterial and technical fibres | [292] |
| [293] | ||||
| [294] | ||||
| [257] | ||||
| Bacterial cellulose + Silica | Wet-spinning | Thermal insulation | Technical fibres | [342] |
| Starch + PVA + Glycerol | Wet-spinning | Biodegradability, skin friendly | Functional fibres | [295] |
| Starch + PLA Starch + PLGA Starch + Nanocellulose | Electrospinning | Hydrophobicity, biodegradability | Functional nanofibres | [296] |
| Keratin + Cellulose | Dry-jet wet-spinning | Flexibility and mechanical resistance | Precursor for carbon fibres | [312] |
| Keratin + Cellulose nanocrystals | Wet-spinning | Hierarchically structured fibres with shape-memory features | Functional fibres | [313] |
| Viscose + Zein | Wet-spinning | High mechanical performance, biodegradability | Technical fibres | [309] |
| Polyurethane + Keratin + AgNPs | Electrospinning | Biocompatibility and antibacterial properties | Nanofibrous mats for wound dressing | [314] |
| Poly(hydroxybutylate-co-hydroxyvalerate) + Keratin | Electrospinning | Biocompatibility, bioadhesiveness, biodegradability | Nanofibrous mats for wound dressing | [315] |
| Collagen + Nanohydroxyapatite | Electrospinning | Biocompatibility, bioadhesiveness, biodegradability | Nanofibrous mats for bone regeneration | [316] |
| PCL + Collagen | Electrospinning | Biocompatibility, bioadhesiveness, vascularisation | Biofunctionalised nanofibrous mats for tissue regeneration | [317] |
| PLGA + Collagen | Electrospinning | Biocompatibility, bioadhesiveness | Nanofibrous structures for tissue regeneration | [318] |
| Gelatin + Tyrosine | Electrospinning | Biocompatibility, bioadhesiveness | Nanofibrous mats for cartilage tissue regeneration | [319] |
| Zein | Electrospinning | Core–shell structure | Drug-loaded nanofibrous mats | [248] |
| Soybean protein | Wet-spinning | Controlled drug load and release delivery | Biofunctional fibres for drug delivery | [311] |
| Zein/Gliadin/Hordein | Electrospinning | Good mechanical properties, biocompatibility | Ultrafine fibres for biomedical applications | [253] |
| Alginate + Pectin + Gelatin + Glycerol | Wet-spinning | Monofilament is bioabsorbable and capable of drug delivery | Suture for biomedical applications | [298] |
| Keratin + Alginate | Wet-spinning | Dual crosslinked fibres suitable for complex braid forms | Flexible fibres | [299] |
| Zein + Alginate + Betanin + TiO2NPs | Electrospinning | Good mechanical performance, hydrophobicity, antibacterial properties | Nanofibres for food packaging | [300] |
| Regenerated cellulose + Cellulose diacetate/Cellulose acetate propionate/Cellulose acetate butyrate | Wet-spinning | Transparency, thermal and chemical stability | Biopolymeric optical fibres | [343] |
| Bio-based polyamide 56 | Electrospinning | Good mechanical performance, antibacterial properties | Bionylon nanofibres for functional textiles | [336] |
| Bio-based polyamide 56 | Melt-spinning | Good mechanical and thermal performance, flame-retardancy, biodegradability | Bionylon fibres for functional textiles | [337] |
| Bio-polyurethane + Triclosan + Cyclodextrin | Electrospinning | Good mechanical properties and antibacterial | Antibacterial nanofibrous materials | [329] |
| Zein + Hordein + Lignin | Electrospinning | Good electrochemical properties, hierarchical porous texture | Supercapacitors precursors for carbon fibres with flame-retardancy | [310] |
| Cellulose | Electrospinning | Reinforced mechanical and thermal properties | Biodegradable nanofibrous composites | [344] |
| Cellulose/cellulose acetate Cellulose acetate/polyurethane | Electrospinning | Light transparency and improved mechanical properties | Light transparent nanofibrous composites | [345,346,347,348] |
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gunaalan, K.; Fabbri, E.; Capolupo, M. The Hidden Threat of Plastic Leachates: A Critical Review on Their Impacts on Aquatic Organisms. Water Res. 2020, 184, 116170. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L.; Neal, M.A. Applications and Societal Benefits of Plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef]
- PlasticsEurope. Plastics–The Fast Facts 2023. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2023/ (accessed on 2 February 2024).
- Franzellitti, S.; Canesi, L.; Auguste, M.; Wathsala, R.H.G.R.; Fabbri, E. Microplastic Exposure and Effects in Aquatic Organisms: A Physiological Perspective. Environ. Toxicol. Pharmacol. 2019, 68, 37–51. [Google Scholar] [CrossRef]
- Gallo, F.; Fossi, C.; Weber, R.; Santillo, D.; Sousa, J.; Ingram, I.; Nadal, A.; Romano, D. Marine Litter Plastics and Microplastics and Their Toxic Chemicals Components: The Need for Urgent Preventive Measures. Environ. Sci. Eur. 2018, 30, 13. [Google Scholar] [CrossRef]
- Xue, B.; Zhang, L.; Li, R.; Wang, Y.; Guo, J.; Yu, K.; Wang, S. Underestimated Microplastic Pollution Derived from Fishery Activities and “Hidden” in Deep Sediment. Environ. Sci. Technol. 2020, 54, 2210–2217. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Q.; Yan, X.; Wang, Y.; Liao, C.; Jiang, G. A Review of Organophosphate Flame Retardants and Plasticizers in the Environment: Analysis, Occurrence and Risk Assessment. Sci. Total Environ. 2020, 731, 139071. [Google Scholar] [CrossRef]
- Schiavo, S.; Oliviero, M.; Chiavarini, S.; Manzo, S. Adverse Effects of Oxo-Degradable Plastic Leachates in Freshwater Environment. Environ. Sci. Pollut. Res. 2020, 27, 8586–8595. [Google Scholar] [CrossRef] [PubMed]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An Overview of Chemical Additives Present in Plastics: Migration, Release, Fate and Environmental Impact during Their Use, Disposal and Recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Quintana, E.; Valls, C.; Roncero, M.B. Dissolving-Grade Pulp: A Sustainable Source for Fiber Production. Wood Sci. Technol. 2024, 58, 23–85. [Google Scholar] [CrossRef]
- Shen, H.; Sun, T.; Zhou, J. Recent Progress in Regenerated Cellulose Fibers by Wet Spinning. Macromol. Mater. Eng. 2023, 308, 2300089. [Google Scholar] [CrossRef]
- Sayyed, A.J.; Deshmukh, N.A.; Pinjari, D.V. A Critical Review of Manufacturing Processes Used in Regenerated Cellulosic Fibres: Viscose, Cellulose Acetate, Cuprammonium, LiCl/DMAc, Ionic Liquids, and NMMO Based Lyocell. Cellulose 2019, 26, 2913–2940. [Google Scholar] [CrossRef]
- Aswathi Mohan, A.; Robert Antony, A.; Greeshma, K.; Yun, J.-H.; Ramanan, R.; Kim, H.-S. Algal Biopolymers as Sustainable Resources for a Net-Zero Carbon Bioeconomy. Bioresour. Technol. 2022, 344, 126397. [Google Scholar] [CrossRef] [PubMed]
- Moura, P.; Henriques, J.; Alexandre, J.; Oliveira, A.C.; Abreu, M.; Gírio, F.; Catarino, J. Sustainable Value Methodology to Compare the Performance of Conversion Technologies for the Production of Electricity and Heat, Energy Vectors and Biofuels from Waste Biomass. Clean. Waste Syst. 2022, 3, 100029. [Google Scholar] [CrossRef]
- European Union. Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009 on the Promotion of the Use of Energy from Renewable Sources and Amending and Subsequently Repealing Directives 2001/77/EC and 2003/30/EC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32009L0028&from=EN (accessed on 30 January 2024).
- U.S. Energy Information Administration. Biomass Explained. Available online: https://www.eia.gov/energyexplained/biomass/ (accessed on 30 January 2024).
- Gold, S.; Seuring, S. Supply Chain and Logistics Issues of Bio-Energy Production. J. Clean. Prod. 2011, 19, 32–42. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, J.; Yao, Y.; Peters, G.; Macdonald, B.; La Rosa, A.D.; Wang, Z.; Scherer, L. Environmental Impacts of Cotton and Opportunities for Improvement. Nat. Rev. Earth Environ. 2023, 4, 703–715. [Google Scholar] [CrossRef]
- Promhuad, K.; Srisa, A.; San, H.; Laorenza, Y.; Wongphan, P.; Sodsai, J.; Tansin, K.; Phromphen, P.; Chartvivatpornchai, N.; Ngoenchai, P.; et al. Applications of Hemp Polymers and Extracts in Food, Textile and Packaging: A Review. Polymers 2022, 14, 4274. [Google Scholar] [CrossRef]
- Tutek, K.; Masek, A. Hemp and Its Derivatives as a Universal Industrial Raw Material (with Particular Emphasis on the Polymer Industry)—A Review. Materials 2022, 15, 2565. [Google Scholar] [CrossRef] [PubMed]
- Singha, S.; Mahmutovic, M.; Zamalloa, C.; Stragier, L.; Verstraete, W.; Svagan, A.J.; Das, O.; Hedenqvist, M.S. Novel Bioplastic from Single Cell Protein as a Potential Packaging Material. ACS Sustain. Chem. Eng. 2021, 9, 6337–6346. [Google Scholar] [CrossRef]
- Moscariello, C.; Matassa, S.; Esposito, G.; Papirio, S. From Residue to Resource: The Multifaceted Environmental and Bioeconomy Potential of Industrial Hemp (Cannabis sativa L.). Resour. Conserv. Recycl. 2021, 175, 105864. [Google Scholar] [CrossRef]
- Mouro, C.; Gomes, A.P.; Gouveia, I.C. From Hemp Waste to Bioactive Nanofiber Composites: Deep Eutectic Solvents and Electrospinning in Upcycling Endeavors. Gels 2023, 10, 1. [Google Scholar] [CrossRef]
- Ahmed, B.; Wu, Q.; Lin, H.; Gwon, J.; Negulescu, I.; Cameron, B. Degumming of Hemp Fibers Using Combined Microwave Energy and Deep Eutectic Solvent Treatment. Ind. Crops Prod. 2022, 184, 115046. [Google Scholar] [CrossRef]
- Palanikumar, K.; Natarajan, E.; Markandan, K.; Ang, C.K.; Franz, G. Targeted Pre-Treatment of Hemp Fibers and the Effect on Mechanical Properties of Polymer Composites. Fibers 2023, 11, 43. [Google Scholar] [CrossRef]
- Ma, J.; Ma, Q.; Fu, J.; Shen, G.; Meng, C. Deep Eutectic Solvent Degumming of Hemp Fiber: Key Factors Influencing Fiber Property and Its Mechanism. Ind. Crops Prod. 2023, 203, 117125. [Google Scholar] [CrossRef]
- Viscusi, G.; Lamberti, E.; Rosaria Acocella, M.; Gorrasi, G. Production of Electrospun Hybrid Membranes Based on Polyamide 6 Reinforced with Hemp Fibers Dissolved in 1-Ethyl-3-Methylimidazolium Dicyanamide Ionic Liquid. J. Mol. Liq. 2023, 387, 122656. [Google Scholar] [CrossRef]
- Pacaphol, K.; Aht-Ong, D. Preparation of Hemp Nanofibers from Agricultural Waste by Mechanical Defibrillation in Water. J. Clean. Prod. 2017, 142, 1283–1295. [Google Scholar] [CrossRef]
- Dhali, K.; Daver, F.; Cass, P.; Adhikari, B. Isolation and Characterization of Cellulose Nanomaterials from Jute Bast Fibers. J. Environ. Chem. Eng. 2021, 9, 106447. [Google Scholar] [CrossRef]
- Biswas, A.; Dey, S.; Huang, S.; Deng, Y.; Birhanie, Z.M.; Zhang, J.; Akhter, D.; Liu, L.; Li, D. A Comprehensive Review of C. capsularis and C. olitorius: A Source of Nutrition, Essential Phytoconstituents and Pharmacological Activities. Antioxidants 2022, 11, 1358. [Google Scholar] [CrossRef] [PubMed]
- Shahinur, S.; Sayeed, M.M.A.; Hasan, M.; Sayem, A.S.M.; Haider, J.; Ura, S. Current Development and Future Perspective on Natural Jute Fibers and Their Biocomposites. Polymers 2022, 14, 1445. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Sarkar, D.; Satya, P.; Karmakar, P.G.; Singh, N.K. Pathways Associated with Lignin Biosynthesis in Lignomaniac Jute Fibres. Mol. Genet. Genom. 2015, 290, 1523–1542. [Google Scholar] [CrossRef]
- Eichhorn, S.J.; Davies, G.R. Modelling the Crystalline Deformation of Native and Regenerated Cellulose. Cellulose 2006, 13, 291–307. [Google Scholar] [CrossRef]
- Guan, Y.; Li, F.; Wang, Y.; Guo, M.; Hou, J. “Reservoir-Law” Synergistic Reinforcement of Electrostatic Spun Polylactic Acid Composites with Cellulose Nanocrystals and 2-Hydroxypropyl-β-Cyclodextrin for Intelligent Bioactive Food Packaging. Int. J. Biol. Macromol. 2024, 274, 133405. [Google Scholar] [CrossRef]
- Das, K.; Ray, D.; Banerjee, C.; Bandyopadhyay, N.R.; Sahoo, S.; Mohanty, A.K.; Misra, M. Physicomechanical and Thermal Properties of Jute-Nanofiber-Reinforced Biocopolyester Composites. Ind. Eng. Chem. Res. 2010, 49, 2775–2782. [Google Scholar] [CrossRef]
- Maiti, S.; Ray, D.; Mitra, D.; Misra, M. Study of Compostable Behavior of Jute Nano Fiber Reinforced Biocopolyester Composites in Aerobic Compost Environment. J. Appl. Polym. Sci. 2012, 123, 2952–2958. [Google Scholar] [CrossRef]
- Khan, G.M.A.; Haque, M.A.; Terano, M.; Alam, M.S. Graft Polycondensation of Microfibrillated Jute Cellulose with Oligo (L-lactic acid) and Its Properties. J. Appl. Polym. Sci. 2014, 131, 40139. [Google Scholar] [CrossRef]
- Meshram, J.H.; Palit, P. On the Role of Cell Wall Lignin in Determining the Fineness of Jute Fibre. Acta Physiol. Plant 2013, 35, 1565–1578. [Google Scholar] [CrossRef]
- Del Río, J.C.; Rencoret, J.; Marques, G.; Li, J.; Gellerstedt, G.; Jiménez-Barbero, J.; Martínez, A.T.; Gutiérrez, A. Structural Characterization of the Lignin from Jute (Corchorus capsularis) Fibers. J. Agric. Food Chem. 2009, 57, 10271–10281. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Li, M.; Zheng, Y. Lignin Biorefinery: Lignin Source, Isolation, Characterization, and Bioconversion. In Advances in Bioenergy; Eleviser: Amsterdam, The Netherlands, 2022; pp. 211–270. [Google Scholar]
- Sfiligoj Smole, M.; Hribernik, S.; Kurečič, M.; Urbanek Krajnc, A.; Kreže, T.; Stana Kleinschek, K. Preparation of Cellulose Nanocrystals CNC from Nettle, Weeping Willow, Balm-Leaved Archangel, Lucerne and Spanish Broom. In Surface Properties of Non-Conventional Cellulose Fibres; Springer: Berlin/Heidelberg, Germany, 2019; pp. 73–86. [Google Scholar]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and Application. Carbon Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Kregiel, D.; Pawlikowska, E.; Antolak, H. Urtica spp.: Ordinary Plants with Extraordinary Properties. Molecules 2018, 23, 1664. [Google Scholar] [CrossRef]
- Grauso, L.; de Falco, B.; Lanzotti, V.; Motti, R. Stinging Nettle, Urtica dioica L.: Botanical, Phytochemical and Pharmacological Overview. Phytochem. Rev. 2020, 19, 1341–1377. [Google Scholar] [CrossRef]
- Upton, R. Stinging Nettles Leaf (Urtica dioica L.): Extraordinary Vegetable Medicine. J. Herb. Med. 2013, 3, 9–38. [Google Scholar] [CrossRef]
- Bogard, F.; Bach, T.; Abbes, B.; Bliard, C.; Maalouf, C.; Bogard, V.; Beaumont, F.; Polidori, G. A Comparative Review of Nettle and Ramie Fiber and Their Use in Biocomposites, Particularly with a PLA Matrix. J. Nat. Fibers 2022, 19, 8205–8229. [Google Scholar] [CrossRef]
- Kassab, Z.; Syafri, E.; Tamraoui, Y.; Hannache, H.; Qaiss, A.E.K.; El Achaby, M. Characteristics of Sulfated and Carboxylated Cellulose Nanocrystals Extracted from Juncus Plant Stems. Int. J. Biol. Macromol. 2020, 154, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Kassab, Z.; Mansouri, S.; Tamraoui, Y.; Sehaqui, H.; Hannache, H.; Qaiss, A.E.K.; El Achaby, M. Identifying Juncus Plant as Viable Source for the Production of Micro- and Nano-Cellulose Fibers: Application for PVA Composite Materials Development. Ind. Crops Prod. 2020, 144, 112035. [Google Scholar] [CrossRef]
- Kassab, Z.; Daoudi, H.; Salim, M.H.; El Idrissi El Hassani, C.; Abdellaoui, Y.; El Achaby, M. Process-Structure-Property Relationships of Cellulose Nanocrystals Derived from Juncus Effusus Stems on ҡ-Carrageenan-Based Bio-Nanocomposite Films. Int. J. Biol. Macromol. 2024, 265, 130892. [Google Scholar] [CrossRef] [PubMed]
- Benali, M.; Oulmekki, A.; Toyir, J. The Impact of the Alkali-Bleaching Treatment on the Isolation of Natural Cellulosic Fibers from Juncus effesus L. Plant. Fibers Polym. 2023, 25, 525–533. [Google Scholar] [CrossRef]
- Naili, H.; Jelidi, A.; Limam, O.; Khiari, R. Extraction Process Optimization of Juncus Plant Fibers for Its Use in a Green Composite. Ind. Crops Prod. 2017, 107, 172–183. [Google Scholar] [CrossRef]
- Ortega, Z.; Bolaji, I.; Suárez, L.; Cunningham, E. A Review of the Use of Giant Reed (Arundo donax L.) in the Biorefineries Context. Rev. Chem. Eng. 2023, 40, 305–328. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, Q.; Liang, D.; Huang, S.; Liao, J. The Potential Application of Giant Reed (Arundo donax) in Ecological Remediation. Front. Environ. Sci. 2021, 9, 652367. [Google Scholar] [CrossRef]
- Di Fidio, N.; Fulignati, S.; De Bari, I.; Antonetti, C.; Raspolli Galletti, A.M. Optimisation of Glucose and Levulinic Acid Production from the Cellulose Fraction of Giant Reed (Arundo donax L.) Performed in the Presence of Ferric Chloride under Microwave Heating. Bioresour. Technol. 2020, 313, 123650. [Google Scholar] [CrossRef] [PubMed]
- Eroğlu, Ö.; Çetin, N.S.; Narlıoğlu, N.; Yan, W. Plastic/Fiber Composite Using Recycled Polypropylene and Fibers from Sorghum halepense L. Bioresources 2023, 18, 3109–3122. [Google Scholar] [CrossRef]
- Iqbal, A.; Badshah, S.L.; Alves, J.L.F.; da Silva, J.C.G.; Di Domenico, M. An Insight into the Thermokinetics of the Pyrolysis of Invasive Grass Sorghum Halepense towards Its Bioenergy Potential. Biomass Convers. Biorefin. 2022, 14, 5305–5318. [Google Scholar] [CrossRef]
- Peter, A.; Žlabur, J.Š.; Šurić, J.; Voća, S.; Purgar, D.D.; Pezo, L.; Voća, N. Invasive Plant Species Biomass—Evaluation of Functional Value. Molecules 2021, 26, 3814. [Google Scholar] [CrossRef] [PubMed]
- Scordia, D.; Testa, G.; Cosentino, S.L. Perennial Grasses as Lignocellulosic Feedstock for Second-Generation Bioethanol Production in Mediterranean Environment. Ital. J. Agron. 2014, 9, 84. [Google Scholar] [CrossRef]
- Scordia, D.; Testa, G.; Copani, V.; Patanè, C.; Cosentino, S.L. Lignocellulosic Biomass Production of Mediterranean Wild Accessions (Oryzopsis Miliacea, Cymbopogon Hirtus, Sorghum Halepense and Saccharum Spontaneum) in a Semi-Arid Environment. Field Crops Res. 2017, 214, 56–65. [Google Scholar] [CrossRef]
- Zamboi, A.; Fraterrigo Garofalo, S.; Tommasi, T.; Fino, D. Optimization of Ultrasounds Assisted Extraction of Polysaccharides from Cladodes of Opuntia Ficus-Indica Using Response Surface Methodology. Sustain. Chem. Pharm. 2024, 37, 101348. [Google Scholar] [CrossRef]
- Albergamo, A.; Potortí, A.G.; Di Bella, G.; Amor, N.B.; Lo Vecchio, G.; Nava, V.; Rando, R.; Ben Mansour, H.; Lo Turco, V. Chemical Characterization of Different Products from the Tunisian Opuntia Ficus-Indica (L.) Mill. Foods 2022, 11, 155. [Google Scholar] [CrossRef]
- De Andrade Vieira, É.; Tribuzy de Magalhães Cordeiro, A.M. Bioprospecting and Potential of Cactus Mucilages: A Bibliometric Review. Food Chem. 2023, 401, 134121. [Google Scholar] [CrossRef]
- Sevgi, A.; Özçelik, M.; Yılmaz, T. Extraction, Characterization, and Rheology of Opuntia ficus indica Cladode Polysaccharides. J. Food Process Preserv. 2022, 46, e16196. [Google Scholar] [CrossRef]
- Bezazi, A.; Belaadi, A.; Bourchak, M.; Scarpa, F.; Boba, K. Novel Extraction Techniques, Chemical and Mechanical Characterisation of Agave americana L. Natural Fibres. Compos. B Eng. 2014, 66, 194–203. [Google Scholar] [CrossRef]
- Sathiamurthi, P.; Karthi Vinith, K.S.; Sathishkumar, T.P.; Arunkumar, S.; Anaamalaai, A.S. Fiber Extraction and Mechanical Properties of Agave Americana/Kenaf Fiber Reinforced Hybrid Epoxy Composite. Mater. Today Proc. 2021, 46, 8594–8601. [Google Scholar] [CrossRef]
- Evdokimova, O.L.; Alves, C.S.; Krsmanović Whiffen, R.M.; Ortega, Z.; Tomás, H.; Rodrigues, J. Cytocompatible Cellulose Nanofibers from Invasive Plant Species Agave americana L. and Ricinus communis L.: A Renewable Green Source of Highly Crystalline Nanocellulose. J. Zhejiang Univ. Sci. B 2021, 22, 450–461. [Google Scholar] [CrossRef]
- Gebretsadik, T.T.; Tesfay, A.H.; Gebru, A.G.; Assayehegn, E.; Desta, Y.H.; Gebremedhin, K.H.; Gebrehiwet, H.; Teklemedhin, T.B. Characterization and Comparative Insights on Agave americana and Agave sisalana Leaf Fibers for High-Performance Applications. J. Nat. Fibers 2023, 20, 2246648. [Google Scholar] [CrossRef]
- Saha, J.; Mondal, M.I.H.; Ahmed, F.; Rahman, M. Extraction, Characterization and Functionality Assessment of Aloe Vera, Chitosan and Silk Sericin. Arab. J. Chem. 2023, 16, 105087. [Google Scholar] [CrossRef]
- Dehouche, N.; Idres, C.; Kaci, M.; Zembouai, I.; Bruzaud, S. Effects of Various Surface Treatments on Aloe Vera Fibers Used as Reinforcement in Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) (PHBHHx) Biocomposites. Polym. Degrad. Stab. 2020, 175, 109131. [Google Scholar] [CrossRef]
- Balaji, A.N.; Nagarajan, K.J. Characterization of Alkali Treated and Untreated New Cellulosic Fiber from Saharan Aloe Vera Cactus Leaves. Carbohydr. Polym. 2017, 174, 200–208. [Google Scholar] [CrossRef]
- Tennakoon, P.; Chandika, P.; Yi, M.; Jung, W.-K. Marine-Derived Biopolymers as Potential Bioplastics, an Eco-Friendly Alternative. iScience 2023, 26, 106404. [Google Scholar] [CrossRef] [PubMed]
- Bose, I.; Nousheen; Roy, S.; Yaduvanshi, P.; Sharma, S.; Chandel, V.; Biswas, D. Unveiling the Potential of Marine Biopolymers: Sources, Classification, and Diverse Food Applications. Materials 2023, 16, 4840. [Google Scholar] [CrossRef]
- Zanchetta, E.; Damergi, E.; Patel, B.; Borgmeyer, T.; Pick, H.; Pulgarin, A.; Ludwig, C. Algal Cellulose, Production and Potential Use in Plastics: Challenges and Opportunities. Algal Res. 2021, 56, 102288. [Google Scholar] [CrossRef]
- Ross, I.L.; Shah, S.; Hankamer, B.; Amiralian, N. Microalgal Nanocellulose–Opportunities for a Circular Bioeconomy. Trends Plant Sci. 2021, 26, 924–939. [Google Scholar] [CrossRef]
- Klassen, V.; Blifernez-Klassen, O.; Bax, J.; Kruse, O. Wastewater-Borne Microalga Chlamydomonas sp.: A Robust Chassis for Efficient Biomass and Biomethane Production Applying Low-N Cultivation Strategy. Bioresour. Technol. 2020, 315, 123825. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Feng, S.; Xu, Z.; Qin, L.; Shang, C.; Feng, P.; Wang, Z.; Yuan, Z. Cultivation of Chlorella Vulgaris on Unsterilized Dairy-Derived Liquid Digestate for Simultaneous Biofuels Feedstock Production and Pollutant Removal. Bioresour. Technol. 2019, 285, 121353. [Google Scholar] [CrossRef] [PubMed]
- Kothari, R.; Pathak, V.V.; Kumar, V.; Singh, D.P. Experimental Study for Growth Potential of Unicellular Alga Chlorella Pyrenoidosa on Dairy Waste Water: An Integrated Approach for Treatment and Biofuel Production. Bioresour. Technol. 2012, 116, 466–470. [Google Scholar] [CrossRef]
- Aguirre, A.; Bassi, A. Investigation of Biomass Concentration, Lipid Production, and Cellulose Content in Chlorella Vulgaris Cultures Using Response Surface Methodology. Biotechnol. Bioeng. 2013, 110, 2114–2122. [Google Scholar] [CrossRef] [PubMed]
- Viotti, C.; Albrecht, K.; Amaducci, S.; Bardos, P.; Bertheau, C.; Blaudez, D.; Bothe, L.; Cazaux, D.; Ferrarini, A.; Govilas, J.; et al. Nettle, a Long-Known Fiber Plant with New Perspectives. Materials 2022, 15, 4288. [Google Scholar] [CrossRef]
- Mannai, F.; Ammar, M.; Yanez, J.G.; Elaloui, E.; Moussaoui, Y. Alkaline Delignification of Cactus Fibres for Pulp and Papermaking Applications. J. Polym. Environ. 2018, 26, 798–806. [Google Scholar] [CrossRef]
- Northcote, D.H.; Goulding, K.J.; Horne, R.W. The Chemical Composition and Structure of the Cell Wall of Hydrodictyon africanum Yaman. Biochem. J. 1960, 77, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Cronshaw, J.; Myers, A.; Preston, R.D. A Chemical and Physical Investigation of the Cell Walls of Some Marine Algae. Biochim. Biophys. Acta 1958, 27, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Moral, A.; Aguado, R.; Castelló, R.; Tijero, A.; Ballesteros, M. Potential Use of Green Alga Ulva sp. for Papermaking. Bioresources 2019, 14, 6851–6862. [Google Scholar] [CrossRef]
- Zhou, W.; Zhu, D.; Langdon, A.; Li, L.; Liao, S.; Tan, L. The Structure Characterization of Cellulose Xanthogenate Derived from the Straw of Eichhornia crassipes. Bioresour. Technol. 2009, 100, 5366–5369. [Google Scholar] [CrossRef]
- Sari, N.H.; Suteja; Rangappa, S.M.; Siengchin, S. A Review on Cellulose Fibers from Eichornia crassipes: Synthesis, Modification, Properties and Their Composites. J. Nat. Fibers 2023, 20, 2162179. [Google Scholar] [CrossRef]
- Gallegos, D.; Wedwitschka, H.; Moeller, L.; Weinrich, S.; Zehnsdorf, A.; Nelles, M.; Stinner, W. Mixed Silage of Elodea and Wheat Straw as a Substrate for Energy Production in Anaerobic Digestion Plants. Energy Sustain. Soc. 2018, 8, 7. [Google Scholar] [CrossRef]
- Poorter, H.; Bergkotte, M. Chemical Composition of 24 Wild Species Differing in Relative Growth Rate. Plant Cell Environ. 1992, 15, 221–229. [Google Scholar] [CrossRef]
- Anbu, P.; Gopinath, S.C.B.; Salimi, M.N.; Letchumanan, I.; Subramaniam, S. Green Synthesized Strontium Oxide Nanoparticles by Elodea Canadensis Extract and Their Antibacterial Activity. J. Nanostruct. Chem. 2022, 12, 365–373. [Google Scholar] [CrossRef]
- Thiripura Sundari, M.; Ramesh, A. Isolation and Characterization of Cellulose Nanofibers from the Aquatic Weed Water Hyacinth—Eichhornia crassipes. Carbohydr. Polym. 2012, 87, 1701–1705. [Google Scholar] [CrossRef]
- Ajithram, A.; Winowlin Jappes, J.T.; Brintha, N.C. Water Hyacinth (Eichhornia crassipes) Natural Composite Extraction Methods and Properties—A Review. Mater. Today Proc. 2021, 45, 1626–1632. [Google Scholar] [CrossRef]
- Din, S.; Hamid, S.; Yaseen, A.; Yatoo, A.M.; Ali, S.; Shamim, K.; Mahdi, W.A.; Alshehri, S.; Rehman, M.U.; Shah, W.A. Isolation and Characterization of Flavonoid Naringenin and Evaluation of Cytotoxic and Biological Efficacy of Water Lilly (Nymphaea mexicana Zucc.). Plants 2022, 11, 3588. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Fang, S.-C.; Yen, G.-C. Anti-Inflammatory Effects of Phenolic Compounds Isolated from the Flowers of Nymphaea mexicana Zucc. Food Funct. 2013, 4, 1216. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Su, F.; Zhang, W.; Kuang, H. A Systematic Review on the Research Progress on Polysaccharides from Fungal Traditional Chinese Medicine. Molecules 2023, 28, 6816. [Google Scholar] [CrossRef]
- Wang, B.; Shi, Y.; Lu, H.; Chen, Q. A Critical Review of Fungal Proteins: Emerging Preparation Technology, Active Efficacy and Food Application. Trends Food Sci. Technol. 2023, 141, 104178. [Google Scholar] [CrossRef]
- Wang, W.; Tan, J.; Nima, L.; Sang, Y.; Cai, X.; Xue, H. Polysaccharides from Fungi: A Review on Their Extraction, Purification, Structural Features, and Biological Activities. Food Chem. X 2022, 15, 100414. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Hisham, F.; Maziati Akmal, M.H.; Ahmad, F.; Ahmad, K.; Samat, N. Biopolymer Chitosan: Potential Sources, Extraction Methods, and Emerging Applications. Ain Shams Eng. J. 2024, 15, 102424. [Google Scholar] [CrossRef]
- Muñoz, G.; Valencia, C.; Valderruten, N.; Ruiz-Durántez, E.; Zuluaga, F. Extraction of Chitosan from Aspergillus niger mycelium and Synthesis of Hydrogels for Controlled Release of Betahistine. React. Funct. Polym. 2015, 91, 1–10. [Google Scholar] [CrossRef]
- Perrin, N.; Mohammadkhani, G.; Homayouni Moghadam, F.; Delattre, C.; Zamani, A. Biocompatible Fibers from Fungal and Shrimp Chitosans for Suture Application. Curr. Res. Biotechnol. 2022, 4, 530–536. [Google Scholar] [CrossRef]
- Svensson, S.E.; Ferreira, J.A.; Hakkarainen, M.; Adolfsson, K.H.; Zamani, A. Fungal Textiles: Wet Spinning of Fungal Microfibers to Produce Monofilament Yarns. Sustain. Mater. Technol. 2021, 28, e00256. [Google Scholar] [CrossRef]
- Xu, C.; Wang, F.; Guan, S.; Wang, L. β-Glucans Obtained from Fungus for Wound Healing: A Review. Carbohydr. Polym. 2024, 327, 121662. [Google Scholar] [CrossRef]
- Arslan, N.P.; Dawar, P.; Albayrak, S.; Doymus, M.; Azad, F.; Esim, N.; Taskin, M. Fungi-Derived Natural Antioxidants. Crit. Rev. Food Sci. Nutr. 2023, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, T.; Zhao, Y.; Jiang, L.; Sui, X. Structural, Extraction and Safety Aspects of Novel Alternative Proteins from Different Sources. Food Chem. 2024, 436, 137712. [Google Scholar] [CrossRef]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional Composition of Black Soldier Fly (Hermetia illucens) Prepupae Reared on Different Organic Waste Substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef]
- Mohan, K.; Ganesan, A.R.; Muralisankar, T.; Jayakumar, R.; Sathishkumar, P.; Uthayakumar, V.; Chandirasekar, R.; Revathi, N. Recent Insights into the Extraction, Characterization, and Bioactivities of Chitin and Chitosan from Insects. Trends Food Sci. Technol. 2020, 105, 17–42. [Google Scholar] [CrossRef]
- Queiroz, L.S.; Nogueira Silva, N.F.; Jessen, F.; Mohammadifar, M.A.; Stephani, R.; Fernandes de Carvalho, A.; Perrone, Í.T.; Casanova, F. Edible Insect as an Alternative Protein Source: A Review on the Chemistry and Functionalities of Proteins under Different Processing Methods. Heliyon 2023, 9, e14831. [Google Scholar] [CrossRef] [PubMed]
- Van Huis, A. Edible Insects Are the Future? Proc. Nutr. Soc. 2016, 75, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Santiago, L.A.; Queiroz, L.S.; Tavares, G.M.; Feyissa, A.H.; Silva, N.F.; Casanova, F. Edible Insect Proteins: How Can They Be a Driver for Food Innovation? Curr. Opin. Food Sci. 2024, 58, 101195. [Google Scholar] [CrossRef]
- Saenz-Mendoza, A.I.; Zamudio-Flores, P.B.; García-Anaya, M.C.; Velasco, C.R.; Acosta-Muñiz, C.H.; Espino-Díaz, M.; Tirado-Gallegos, J.M.; Hernández-González, M.; Vela-Gutiérrez, G.; Salgado-Delgado, R.; et al. Insects as a Potential Source of Chitin and Chitosan: Physicochemical, Morphological and Structural Characterization—A Review. Emir. J. Food Agric. 2023, 5, 388–407. [Google Scholar] [CrossRef]
- Philibert, T.; Lee, B.H.; Fabien, N. Current Status and New Perspectives on Chitin and Chitosan as Functional Biopolymers. Appl. Biochem. Biotechnol. 2017, 181, 1314–1337. [Google Scholar] [CrossRef]
- Machado, S.S.N.; da Silva, J.B.A.; Nascimento, R.Q.; Lemos, P.V.F.; de Jesus Assis, D.; Marcelino, H.R.; de Souza Ferreira, E.; Cardoso, L.G.; Pereira, J.D.; Santana, J.S.; et al. Insect Residues as an Alternative and Promising Source for the Extraction of Chitin and Chitosan. Int. J. Biol. Macromol. 2024, 254, 127773. [Google Scholar] [CrossRef]
- Hahn, T.; Tafi, E.; Paul, A.; Salvia, R.; Falabella, P.; Zibek, S. Current State of Chitin Purification and Chitosan Production from Insects. J. Chem. Technol. Biotechnol. 2020, 95, 2775–2795. [Google Scholar] [CrossRef]
- Brigode, C.; Hobbi, P.; Jafari, H.; Verwilghen, F.; Baeten, E.; Shavandi, A. Isolation and Physicochemical Properties of Chitin Polymer from Insect Farm Side Stream as a New Source of Renewable Biopolymer. J. Clean. Prod. 2020, 275, 122924. [Google Scholar] [CrossRef]
- Rehman, K.U.; Hollah, C.; Wiesotzki, K.; Heinz, V.; Aganovic, K.; Rehman, R.U.; Petrusan, J.-I.; Zheng, L.; Zhang, J.; Sohail, S.; et al. Insect-Derived Chitin and Chitosan: A Still Unexploited Resource for the Edible Insect Sector. Sustainability 2023, 15, 4864. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Loureiro, L.M.E.F.; Sá, L.C.R.; Silva, H.F.C. Waste Recovery through Thermochemical Conversion Technologies: A Case Study with Several Portuguese Agroforestry By-Products. Clean. Technol. 2020, 2, 377–391. [Google Scholar] [CrossRef]
- Gaspar, M.C.; Mendes, C.V.T.; Pinela, S.R.; Moreira, R.; Carvalho, M.G.V.S.; Quina, M.J.; Braga, M.E.M.; Portugal, A.T. Assessment of Agroforestry Residues: Their Potential within the Biorefinery Context. ACS Sustain. Chem. Eng. 2019, 7, 17154–17165. [Google Scholar] [CrossRef]
- Gupta, J.; Kumari, M.; Mishra, A.; Swati; Akram, M.; Thakur, I.S. Agro-Forestry Waste Management—A Review. Chemosphere 2022, 287, 132321. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Zhao, H.; Xie, J.; Qi, J.; Shupe, T.F. Agricultural and Forest Residues towards Renewable Chemicals and Materials Using Microwave Liquefaction. Int. J. Polym. Sci. 2019, 2019, 7231263. [Google Scholar] [CrossRef]
- Bangar, S.P.; Whiteside, W.S.; Kajla, P.; Tavassoli, M. Value Addition of Rice Straw Cellulose Fibers as a Reinforcer in Packaging Applications. Int. J. Biol. Macromol. 2023, 243, 125320. [Google Scholar] [CrossRef]
- Thorenz, A.; Wietschel, L.; Stindt, D.; Tuma, A. Assessment of Agroforestry Residue Potentials for the Bioeconomy in the European Union. J. Clean. Prod. 2018, 176, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Klinghoffer, N.B.; ElGhamrawy, I.; Berruti, F. Thermochemical Conversion of Agroforestry Biomass and Solid Waste Using Decentralized and Mobile Systems for Renewable Energy and Products. Renew. Sustain. Energy Rev. 2021, 149, 111372. [Google Scholar] [CrossRef]
- Caetano, N.S.; Caldeira, D.; Martins, A.A.; Mata, T.M. Valorisation of Spent Coffee Grounds: Production of Biodiesel via Enzymatic Catalysis with Ethanol and a Co-Solvent. Waste Biomass Valorization 2017, 8, 1981–1994. [Google Scholar] [CrossRef]
- Liu, C.; Luan, P.; Li, Q.; Cheng, Z.; Xiang, P.; Liu, D.; Hou, Y.; Yang, Y.; Zhu, H. Biopolymers Derived from Trees as Sustainable Multifunctional Materials: A Review. Adv. Mater. 2021, 33, 2001654. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, F.; Striani, R.; Fico, D.; Alam, M.M.; Greco, A.; Esposito Corcione, C. An Overview on Wood Waste Valorization as Biopolymers and Biocomposites: Definition, Classification, Production, Properties and Applications. Polymers 2022, 14, 5519. [Google Scholar] [CrossRef] [PubMed]
- Sirviö, J.A.; Mikola, M.; Ahola, J.; Heiskanen, J.P.; Filonenko, S.; Ämmälä, A. Highly Effective Fractionation Chemistry to Overcome the Recalcitrance of Softwood Lignocellulose. Carbohydr. Polym. 2023, 312, 120815. [Google Scholar] [CrossRef] [PubMed]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic Biomass: A Sustainable Platform for the Production of Bio-Based Chemicals and Polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Cai, J.; He, Y.; Yu, X.; Banks, S.W.; Yang, Y.; Zhang, X.; Yu, Y.; Liu, R.; Bridgwater, A.V. Review of Physicochemical Properties and Analytical Characterization of Lignocellulosic Biomass. Renew. Sustain. Energy Rev. 2017, 76, 309–322. [Google Scholar] [CrossRef]
- Plakantonaki, S.; Roussis, I.; Bilalis, D.; Priniotakis, G. Dietary Fiber from Plant-Based Food Wastes: A Comprehensive Approach to Cereal, Fruit, and Vegetable Waste Valorization. Processes 2023, 11, 1580. [Google Scholar] [CrossRef]
- Subhedar, P.B.; Ray, P.; Gogate, P.R. Intensification of Delignification and Subsequent Hydrolysis for the Fermentable Sugar Production from Lignocellulosic Biomass Using Ultrasonic Irradiation. Ultrason. Sonochem. 2018, 40, 140–150. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S. Recent Updates on Different Methods of Pretreatment of Lignocellulosic Feedstocks: A Review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Tapangnoi, P.; Sae-Oui, P.; Naebpetch, W.; Siriwong, C. Preparation of Purified Spent Coffee Ground and Its Reinforcement in Natural Rubber Composite. Arab. J. Chem. 2022, 15, 103917. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, Functional, and Structural Properties of Spent Coffee Grounds and Coffee Silverskin. Food Bioproc Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Singh, T.A.; Pal, N.; Sharma, P.; Passari, A.K. Spent Coffee Ground: Transformation from Environmental Burden into Valuable Bioactive Metabolites. Rev. Environ. Sci. Biotechnol. 2023, 22, 887–898. [Google Scholar] [CrossRef]
- Toushik, S.H.; Lee, K.; Lee, J.; Kim, K. Functional Applications of Lignocellulolytic Enzymes in the Fruit and Vegetable Processing Industries. J. Food Sci. 2017, 82, 585–593. [Google Scholar] [CrossRef]
- Álvarez, A.; Cachero, S.; González-Sánchez, C.; Montejo-Bernardo, J.; Pizarro, C.; Bueno, J.L. Novel Method for Holocellulose Analysis of Non-Woody Biomass Wastes. Carbohydr. Polym. 2018, 189, 250–256. [Google Scholar] [CrossRef]
- Nawirska, A.; Kwaśniewska, M. Dietary Fibre Fractions from Fruit and Vegetable Processing Waste. Food Chem. 2005, 91, 221–225. [Google Scholar] [CrossRef]
- Prameela, K.; Venkatesh, K.; Vani, K.D.; Sudesh Kumar, E.; Mohan, C.M. Eco-Friendly Extraction of Biopolymer Chitin and Carotenoids from Shrimp Waste. IOP Conf. Ser. Mater. Sci. Eng. 2017, 225, 012266. [Google Scholar] [CrossRef]
- Kaya, M.; Baran, T.; Karaarslan, M. A New Method for Fast Chitin Extraction from Shells of Crab, Crayfish and Shrimp. Nat. Prod. Res. 2015, 29, 1477–1480. [Google Scholar] [CrossRef]
- Pakizeh, M.; Moradi, A.; Ghassemi, T. Chemical Extraction and Modification of Chitin and Chitosan from Shrimp Shells. Eur. Polym. J. 2021, 159, 110709. [Google Scholar] [CrossRef]
- Premasudha, P.; Vanathi, P.; Abirami, M. Extraction and Characterization of Chitosan from Crustacean Waste: A Constructive Waste Management Approach. Int. J. Sci. Res. 2017, 6, 1194–1198. [Google Scholar] [CrossRef]
- Ifuku, S.; Nogi, M.; Abe, K.; Yoshioka, M.; Morimoto, M.; Saimoto, H.; Yano, H. Preparation of Chitin Nanofibers with a Uniform Width as α-Chitin from Crab Shells. Biomacromolecules 2009, 10, 1584–1588. [Google Scholar] [CrossRef]
- Ideia, P.; Pinto, J.; Ferreira, R.; Figueiredo, L.; Spínola, V.; Castilho, P.C. Fish Processing Industry Residues: A Review of Valuable Products Extraction and Characterization Methods. Waste Biomass Valorization 2020, 11, 3223–3246. [Google Scholar] [CrossRef]
- Senthilkumar, N.; Chowdhury, S.; Sanpui, P. Extraction of Keratin from Keratinous Wastes: Current Status and Future Directions. J. Mater. Cycles Waste Manag. 2023, 25, 1–16. [Google Scholar] [CrossRef]
- Arunmozhivarman, K. Extraction and Molecular Characterization of Collagen from Poultry Meat Processing By-Product (Chicken Skin). Int. J. Pure Appl. Biosci. 2017, 5, 1085–1091. [Google Scholar] [CrossRef]
- Matinong, A.M.E.; Chisti, Y.; Pickering, K.L.; Haverkamp, R.G. Collagen Extraction from Animal Skin. Biology 2022, 11, 905. [Google Scholar] [CrossRef]
- Manimegalai, N.P.; Ramanathan, G.; Gunasekaran, D.; Jeyakumar, G.F.S.; Sivagnanam, U.T. Cardinal Acuity on the Extraction and Characterization of Soluble Collagen from the Underutilized Abattoir Junks for Clinical Demands. Process Biochem. 2022, 122, 29–37. [Google Scholar] [CrossRef]
- Sockalingam, K.; Abdullah, H.Z. Extraction and Characterization of Gelatin Biopolymer from Black Tilapia (Oreochromis mossambicus) Scales. AIP Conf. Proc. 2015, 1669, 020053. [Google Scholar]
- Rather, J.A.; Akhter, N.; Ashraf, Q.S.; Mir, S.A.; Makroo, H.A.; Majid, D.; Barba, F.J.; Khaneghah, A.M.; Dar, B.N. A Comprehensive Review on Gelatin: Understanding Impact of the Sources, Extraction Methods, and Modifications on Potential Packaging Applications. Food Packag. Shelf Life 2022, 34, 100945. [Google Scholar] [CrossRef]
- Alipal, J.; Mohd Pu’ad, N.A.S.; Lee, T.C.; Nayan, N.H.M.; Sahari, N.; Basri, H.; Idris, M.I.; Abdullah, H.Z. A Review of Gelatin: Properties, Sources, Process, Applications, and Commercialisation. Mater. Today Proc. 2021, 42, 240–250. [Google Scholar] [CrossRef]
- Vineis, C.; Varesano, A.; Varchi, G.; Aluigi, A. Extraction and Characterization of Keratin from Different Biomasses. In Keratin as a Protein Biopolymer; Springer: Berlin/Heidelberg, Germany, 2019; pp. 35–76. [Google Scholar]
- Chukwunonso Ossai, I.; Shahul Hamid, F.; Hassan, A. Valorisation of Keratinous Wastes: A Sustainable Approach towards a Circular Economy. Waste Manag. 2022, 151, 81–104. [Google Scholar] [CrossRef] [PubMed]
- Anbesaw, M.S. Bioconversion of Keratin Wastes Using Keratinolytic Microorganisms to Generate Value-Added Products. Int. J. Biomater. 2022, 2022, 2048031. [Google Scholar] [CrossRef]
- Yukesh Kannah, R.; Dinesh Kumar, M.; Kavitha, S.; Rajesh Banu, J.; Kumar Tyagi, V.; Rajaguru, P.; Kumar, G. Production and Recovery of Polyhydroxyalkanoates (PHA) from Waste Streams—A Review. Bioresour. Technol. 2022, 366, 128203. [Google Scholar] [CrossRef] [PubMed]
- Titz, M.; Kettl, K.-H.; Shahzad, K.; Koller, M.; Schnitzer, H.; Narodoslawsky, M. Process Optimization for Efficient Biomediated PHA Production from Animal-Based Waste Streams. Clean. Technol. Environ. Policy 2012, 14, 495–503. [Google Scholar] [CrossRef]
- Muhr, A.; Rechberger, E.M.; Salerno, A.; Reiterer, A.; Malli, K.; Strohmeier, K.; Schober, S.; Mittelbach, M.; Koller, M. Novel Description of Mcl-PHA Biosynthesis by Pseudomonas Chlororaphis from Animal-Derived Waste. J. Biotechnol. 2013, 165, 45–51. [Google Scholar] [CrossRef]
- Sar, T.; Harirchi, S.; Ramezani, M.; Bulkan, G.; Akbas, M.Y.; Pandey, A.; Taherzadeh, M.J. Potential Utilization of Dairy Industries By-Products and Wastes through Microbial Processes: A Critical Review. Sci. Total Environ. 2022, 810, 152253. [Google Scholar] [CrossRef]
- Ahmad, T.; Aadil, R.M.; Ahmed, H.; Rahman, U.U.; Soares, B.C.V.; Souza, S.L.Q.; Pimentel, T.C.; Scudino, H.; Guimarães, J.T.; Esmerino, E.A.; et al. Treatment and Utilization of Dairy Industrial Waste: A Review. Trends Food Sci. Technol. 2019, 88, 361–372. [Google Scholar] [CrossRef]
- Wu, Z.; Yin, H.; Liu, W.; Huang, D.; Hu, N.; Yang, C.; Zhao, X. Xanthan Gum Assisted Foam Fractionation for the Recovery of Casein from the Dairy Wastewater. Prep. Biochem. Biotechnol. 2020, 50, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Cancella, M.J.; Cerqueira, A.F.L.W.; da Costa Teodoro, L.; Pereira, J.R.; da Costa Ludwig, Z.M.; de Carvalho Anjos, V.; Denadai, Â.M.L.; Húngaro, H.M.; Rodarte, M.P. Xanthan Gum Produced from Milk Permeate and Deproteinized Cheese Whey: A Comparative Analysis with Commercial Xanthan Gums. Biocatal. Agric. Biotechnol. 2024, 56, 103053. [Google Scholar] [CrossRef]
- Costa, C.; Azoia, N.G.; Coelho, L.; Freixo, R.; Batista, P.; Pintado, M. Proteins Derived from the Dairy Losses and By-Products as Raw Materials for Non-Food Applications. Foods 2021, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Galante, R.; Cunha, F.; Fangueiro, R. Extraction and Properties of Casein Biopolymer from Milk. In Handbook of Natural Polymers, Volume 1; Elsevier: Amsterdam, The Netherlands, 2023; pp. 471–487. [Google Scholar]
- Palaniraj, A.; Jayaraman, V. Production, Recovery and Applications of Xanthan Gum by Xanthomonas Campestris. J. Food Eng. 2011, 106, 1–12. [Google Scholar] [CrossRef]
- Colombo, B.; Pepè Sciarria, T.; Reis, M.; Scaglia, B.; Adani, F. Polyhydroxyalkanoates (PHAs) Production from Fermented Cheese Whey by Using a Mixed Microbial Culture. Bioresour. Technol. 2016, 218, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Pais, J.; Serafim, L.S.; Freitas, F.; Reis, M.A.M. Conversion of Cheese Whey into Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) by Haloferax Mediterranei. New Biotechnol. 2016, 33, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, P. Biermann’s Handbook of Pulp and Paper; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128142400. [Google Scholar]
- Reyes, L.; Nikitine, C.; Vilcocq, L.; Fongarland, P. Green Is the New Black—A Review of Technologies for Carboxylic Acid Recovery from Black Liquor. Green Chem. 2020, 22, 8097–8115. [Google Scholar] [CrossRef]
- Viel, M.; Collet, F.; Lanos, C. Effect of Compaction on Multi-Physical Properties of Hemp-Black Liquor Composites. J. Mater. Res. Technol. 2020, 9, 2487–2494. [Google Scholar] [CrossRef]
- Morya, R.; Kumar, M.; Tyagi, I.; Kumar Pandey, A.; Park, J.; Raj, T.; Sirohi, R.; Kumar, V.; Kim, S.-H. Recent Advances in Black Liquor Valorization. Bioresour. Technol. 2022, 350, 126916. [Google Scholar] [CrossRef] [PubMed]
- Stanescu, M.D. State of the Art of Post-Consumer Textile Waste Upcycling to Reach the Zero Waste Milestone. Environ. Sci. Pollut. Res. 2021, 28, 14253–14270. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Salmon, S. Progress toward Circularity of Polyester and Cotton Textiles. Sustain. Chem. 2022, 3, 376–403. [Google Scholar] [CrossRef]
- Dissanayake, D.G.K.; Weerasinghe, D.U. Fabric Waste Recycling: A Systematic Review of Methods, Applications, and Challenges. Mater. Circ. Econ. 2021, 3, 24. [Google Scholar] [CrossRef]
- Pensupa, N.; Leu, S.-Y.; Hu, Y.; Du, C.; Liu, H.; Jing, H.; Wang, H.; Lin, C.S.K. Recent Trends in Sustainable Textile Waste Recycling Methods: Current Situation and Future Prospects. Top. Curr. Chem. 2017, 375, 76. [Google Scholar] [CrossRef]
- Costa, C.; Viana, A.; Silva, C.; Marques, E.F.; Azoia, N.G. Recycling of Textile Wastes, by Acid Hydrolysis, into New Cellulosic Raw Materials. Waste Manag. 2022, 153, 99–109. [Google Scholar] [CrossRef]
- Wang, H.; Kaur, G.; Pensupa, N.; Uisan, K.; Du, C.; Yang, X.; Lin, C.S.K. Textile Waste Valorization Using Submerged Filamentous Fungal Fermentation. Process Saf. Environ. Prot. 2018, 118, 143–151. [Google Scholar] [CrossRef]
- Navone, L.; Moffitt, K.; Hansen, K.-A.; Blinco, J.; Payne, A.; Speight, R. Closing the Textile Loop: Enzymatic Fibre Separation and Recycling of Wool/Polyester Fabric Blends. Waste Manag. 2020, 102, 149–160. [Google Scholar] [CrossRef]
- Li, X.; Hu, Y.; Du, C.; Lin, C.S.K. Recovery of Glucose and Polyester from Textile Waste by Enzymatic Hydrolysis. Waste Biomass Valorization 2019, 10, 3763–3772. [Google Scholar] [CrossRef]
- Kaabel, S.; Arciszewski, J.; Borchers, T.H.; Therien, J.P.D.; Friščić, T.; Auclair, K. Solid-State Enzymatic Hydrolysis of Mixed PET/Cotton Textiles. ChemSusChem 2023, 16, e202201613. [Google Scholar] [CrossRef]
- Zebec, Ž.; Poberžnik, M.; Lobnik, A. Enzymatic Hydrolysis of Textile and Cardboard Waste as a Glucose Source for the Production of Limonene in Escherichia coli. Life 2022, 12, 1423. [Google Scholar] [CrossRef]
- Li, X.; Zhang, M.; Luo, J.; Zhang, S.; Yang, X.; Igalavithana, A.D.; Ok, Y.S.; Tsang, D.C.W.; Lin, C.S.K. Efficient Succinic Acid Production Using a Biochar-Treated Textile Waste Hydrolysate in an in Situ Fibrous Bed Bioreactor. Biochem. Eng. J. 2019, 149, 107249. [Google Scholar] [CrossRef]
- Haslinger, S.; Hummel, M.; Anghelescu-Hakala, A.; Määttänen, M.; Sixta, H. Upcycling of Cotton Polyester Blended Textile Waste to New Man-Made Cellulose Fibers. Waste Manag. 2019, 97, 88–96. [Google Scholar] [CrossRef]
- Rao, J.; Gao, H.; Guan, Y.; Li, W.; Liu, Q. Fabrication of Hemicelluloses Films with Enhanced Mechanical Properties by Graphene Oxide for Humidity Sensing. Carbohydr. Polym. 2019, 208, 513–520. [Google Scholar] [CrossRef]
- Kun, D.; Pukánszky, B. Polymer/Lignin Blends: Interactions, Properties, Applications. Eur. Polym. J. 2017, 93, 618–641. [Google Scholar] [CrossRef]
- Schlee, P.; Hosseinaei, O.; O’ Keefe, C.A.; Mostazo-López, M.J.; Cazorla-Amorós, D.; Herou, S.; Tomani, P.; Grey, C.P.; Titirici, M.-M. Hardwood versus Softwood Kraft Lignin–Precursor-Product Relationships in the Manufacture of Porous Carbon Nanofibers for Supercapacitors. J. Mater. Chem. A Mater. 2020, 8, 23543–23554. [Google Scholar] [CrossRef]
- Worku, L.A.; Bachheti, A.; Bachheti, R.K.; Rodrigues Reis, C.E.; Chandel, A.K. Agricultural Residues as Raw Materials for Pulp and Paper Production: Overview and Applications on Membrane Fabrication. Membranes 2023, 13, 228. [Google Scholar] [CrossRef]
- Ranganathan, S.; Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Utilization of Food Waste Streams for the Production of Biopolymers. Heliyon 2020, 6, e04891. [Google Scholar] [CrossRef] [PubMed]
- Arun, K.B.; Madhavan, A.; Sindhu, R.; Binod, P.; Pandey, A.; Reshmy, R.; Sirohi, R. Remodeling Agro-Industrial and Food Wastes into Value-Added Bioactives and Biopolymers. Ind. Crops Prod. 2020, 154, 112621. [Google Scholar] [CrossRef]
- Shewry, P.R.; Tatham, A.S. The Prolamin Storage Proteins of Cereal Seeds: Structure and Evolution. Biochem. J. 1990, 267, 1–12. [Google Scholar] [CrossRef]
- Szymańska-Chargot, M.; Chylińska, M.; Gdula, K.; Kozioł, A.; Zdunek, A. Isolation and Characterization of Cellulose from Different Fruit and Vegetable Pomaces. Polymers 2017, 9, 495. [Google Scholar] [CrossRef]
- Tapia-Blácido, D.R.; Maniglia, B.C.; Martelli-Tosi, M. Biopolymers from Sugarcane and Soybean Lignocellulosic Biomass. In Sustainable Polymers from Biomass; Wiley: Hoboken, NJ, USA, 2017; pp. 227–253. [Google Scholar]
- Obruca, S.; Petrik, S.; Benesova, P.; Svoboda, Z.; Eremka, L.; Marova, I. Utilization of Oil Extracted from Spent Coffee Grounds for Sustainable Production of Polyhydroxyalkanoates. Appl. Microbiol. Biotechnol. 2014, 98, 5883–5890. [Google Scholar] [CrossRef]
- Aslan, A.K.H.N.; Ali, M.D.M.; Morad, N.A.; Tamunaidu, P. Polyhydroxyalkanoates Production from Waste Biomass. IOP Conf. Ser. Earth Environ. Sci. 2016, 36, 012040. [Google Scholar] [CrossRef]
- Karmee, S.K. A Spent Coffee Grounds Based Biorefinery for the Production of Biofuels, Biopolymers, Antioxidants and Biocomposites. Waste Manag. 2018, 72, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Amiri, H.; Aghbashlo, M.; Sharma, M.; Gaffey, J.; Manning, L.; Moosavi Basri, S.M.; Kennedy, J.F.; Gupta, V.K.; Tabatabaei, M. Chitin and Chitosan Derived from Crustacean Waste Valorization Streams Can Support Food Systems and the UN Sustainable Development Goals. Nat. Food 2022, 3, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO). World Food and Agriculture—Statistical Yearbook 2020; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [Google Scholar]
- Chokshi, K.; Pancha, I.; Ghosh, A.; Mishra, S. Microalgal Biomass Generation by Phycoremediation of Dairy Industry Wastewater: An Integrated Approach towards Sustainable Biofuel Production. Bioresour. Technol. 2016, 221, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Porwal, H.J.; Mane, A.V.; Velhal, S.G. Biodegradation of Dairy Effluent by Using Microbial Isolates Obtained from Activated Sludge. Water Resour. Ind. 2015, 9, 1–15. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Passarini, L. Valorization of Biomass Residues from Forest Operations and Wood Manufacturing Presents a Wide Range of Sustainable and Innovative Possibilities. Curr. For. Rep. 2020, 6, 172–183. [Google Scholar] [CrossRef]
- Kasavan, S.; Yusoff, S.; Guan, N.C.; Zaman, N.S.K.; Fakri, M.F.R. Global Trends of Textile Waste Research from 2005 to 2020 Using Bibliometric Analysis. Environ. Sci. Pollut. Res. 2021, 28, 44780–44794. [Google Scholar] [CrossRef]
- Tang, K.H.D. State of the Art in Textile Waste Management: A Review. Textiles 2023, 3, 454–467. [Google Scholar] [CrossRef]
- Tomovska, E.; Jordeva, S.; Trajković, D.; Zafirova, K. Attitudes towards Managing Post-Industrial Apparel Cuttings Waste. J. Text. Inst. 2016, 108, 172–177. [Google Scholar] [CrossRef][Green Version]
- Shukla, A.; Kumar, D.; Girdhar, M.; Kumar, A.; Goyal, A.; Malik, T.; Mohan, A. Strategies of Pretreatment of Feedstocks for Optimized Bioethanol Production: Distinct and Integrated Approaches. Biotechnol. Biofuels Bioprod. 2023, 16, 44. [Google Scholar] [CrossRef]
- Padhi, S.; Singh, A.; Routray, W. Nanocellulose from Agro-Waste: A Comprehensive Review of Extraction Methods and Applications. Rev. Environ. Sci. Biotechnol. 2023, 22, 1–27. [Google Scholar] [CrossRef]
- Basbasan, A.J.; Hararak, B.; Winotapun, C.; Wanmolee, W.; Leelaphiwat, P.; Boonruang, K.; Chinsirikul, W.; Chonhenchob, V. Emerging Challenges on Viability and Commercialization of Lignin in Biobased Polymers for Food Packaging: A Review. Food Packag. Shelf Life 2022, 34, 100969. [Google Scholar] [CrossRef]
- Michelin, M.; Gomes, D.G.; Romaní, A.; Polizeli, M.D.L.T.; Teixeira, J.A. Nanocellulose Production: Exploring the Enzymatic Route and Residues of Pulp and Paper Industry. Molecules 2020, 25, 3411. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Liu, L.; Esquivel, M.; Tardy, B.L.; Huan, S.; Niu, X.; Liu, S.; Yang, G.; Fan, Y.; Rojas, O.J. Nanochitin: Chemistry, Structure, Assembly, and Applications. Chem. Rev. 2022, 122, 11604–11674. [Google Scholar] [CrossRef] [PubMed]
- Giteru, S.G.; Ramsey, D.H.; Hou, Y.; Cong, L.; Mohan, A.; Bekhit, A.E.A. Wool Keratin as a Novel Alternative Protein: A Comprehensive Review of Extraction, Purification, Nutrition, Safety, and Food Applications. Compr. Rev. Food Sci. Food Saf. 2023, 22, 643–687. [Google Scholar] [CrossRef]
- Liu, D.; Smagghe, G.; Liu, T.-X. Interactions between Entomopathogenic Fungi and Insects and Prospects with Glycans. J. Fungi 2023, 9, 575. [Google Scholar] [CrossRef]
- Guo, X.; Jiang, Z.; Li, H.; Li, W. Production of Recycled Cellulose Fibers from Waste Paper via Ultrasonic Wave Processing. J. Appl. Polym. Sci. 2015, 132, 41962. [Google Scholar] [CrossRef]
- Calcio Gaudino, E.; Grillo, G.; Tabasso, S.; Stevanato, L.; Cravotto, G.; Marjamaa, K.; Pihlajaniemi, V.; Koivula, A.; Aro, N.; Uusitalo, J.; et al. Optimization of Ultrasound Pretreatment and Enzymatic Hydrolysis of Wheat Straw: From Lab to Semi-Industrial Scale. J. Clean. Prod. 2022, 380, 134897. [Google Scholar] [CrossRef]
- González-Balderas, R.M.; Orta Ledesma, M.T.; Santana, I.; Felix, M.; Bengoechea, C. Desmodesmus sp. from Biowaste to Produce Electrospinning Membranes: Effect of Ultrasounds and Ozone Pre-Treatments. J. Environ. Chem. Eng. 2023, 11, 110621. [Google Scholar] [CrossRef]
- Han, W.; Geng, Y. Optimization and Characterization of Cellulose Extraction from Olive Pomace. Cellulose 2023, 30, 4889–4903. [Google Scholar] [CrossRef]
- Avelino, F.; da Silva, K.T.; de Souza Filho, M.D.S.M.; Mazzetto, S.E.; Lomonaco, D. Microwave-Assisted Organosolv Extraction of Coconut Shell Lignin by Brønsted and Lewis Acids Catalysts. J. Clean. Prod. 2018, 189, 785–796. [Google Scholar] [CrossRef]
- Marciano, S.J.; Avelino, F.; da Silva, L.R.R.; Mazzetto, S.E.; Lomonaco, D. Microwave-Assisted Phosphorylation of Organosolv Lignin: New Bio-Additives for Improvement of Epoxy Resins Performance. Biomass Convers. Biorefin. 2022, 12, 619–631. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, C.; Lin, Q.; Wang, X.; Cheng, B.; Li, H.; Ren, J. Microwave-Assisted Oxalic Acid Pretreatment for the Enhancing of Enzyme Hydrolysis in the Production of Xylose and Arabinose from Bagasse. Molecules 2018, 23, 862. [Google Scholar] [CrossRef]
- Avelino, F.; Silva, K.T.; Mazzetto, S.E.; Lomonaco, D. Tailor-Made Organosolv Lignins from Coconut Wastes: Effects of Green Solvents in Microwave-Assisted Processes upon Their Structure and Antioxidant Activities. Bioresour. Technol. Rep. 2019, 7, 100219. [Google Scholar] [CrossRef]
- Guo, Y.; Zhou, J.; Wang, Y.; Zhang, L.; Lin, X. An Efficient Transformation of Cellulose into Cellulose Carbamates Assisted by Microwave Irradiation. Cellulose 2010, 17, 1115–1125. [Google Scholar] [CrossRef]
- Baksi, S.; Saha, D.; Saha, S.; Sarkar, U.; Basu, D.; Kuniyal, J.C. Pre-Treatment of Lignocellulosic Biomass: Review of Various Physico-Chemical and Biological Methods Influencing the Extent of Biomass Depolymerization. Int. J. Environ. Sci. Technol. 2023, 20, 13895–13922. [Google Scholar] [CrossRef]
- Prasetyo, I.; Permatasari, P.R.; Laksmana, W.T.; Rochmadi, R.; Oh, W.-C.; Ariyanto, T. Lignin Refinery Using Organosolv Process for Nanoporous Carbon Synthesis. Molecules 2020, 25, 3428. [Google Scholar] [CrossRef] [PubMed]
- Vieira, F.; Santana, H.E.P.; Silva, D.P.; Ruzene, D.S. A Bibliometric Description of Organosolv Pretreatment for Coconut Waste Valorization. Bioenergy Res. 2023, 16, 2115–2130. [Google Scholar] [CrossRef]
- Sidiras, D.; Politi, D.; Giakoumakis, G.; Salapa, I. Simulation and Optimization of Organosolv Based Lignocellulosic Biomass Refinery: A Review. Bioresour. Technol. 2022, 343, 126158. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.A.; Taherzadeh, M.J. Improving the Economy of Lignocellulose-Based Biorefineries with Organosolv Pretreatment. Bioresour. Technol. 2020, 299, 122695. [Google Scholar] [CrossRef]
- Joy, S.P.; Krishnan, C. Modified Organosolv Pretreatment for Improved Cellulosic Ethanol Production from Sorghum Biomass. Ind. Crops Prod. 2022, 177, 114409. [Google Scholar] [CrossRef]
- Ding, R.; Wu, H.; Thunga, M.; Bowler, N.; Kessler, M.R. Processing and Characterization of Low-Cost Electrospun Carbon Fibers from Organosolv Lignin/Polyacrylonitrile Blends. Carbon 2016, 100, 126–136. [Google Scholar] [CrossRef]
- Chopda, R.; Ferreira, J.A.; Taherzadeh, M.J. Biorefining Oat Husks into High-Quality Lignin and Enzymatically Digestible Cellulose with Acid-Catalyzed Ethanol Organosolv Pretreatment. Processes 2020, 8, 435. [Google Scholar] [CrossRef]
- Sar, T.; Arifa, V.H.; Hilmy, M.R.; Ferreira, J.A.; Wikandari, R.; Millati, R.; Taherzadeh, M.J. Organosolv Pretreatment of Oat Husk Using Oxalic Acid as an Alternative Organic Acid and Its Potential Applications in Biorefinery. Biomass Convers. Biorefin. 2022, 1–10. [Google Scholar] [CrossRef]
- Kim, T.H.; Kwak, H.; Kim, T.H.; Oh, K.K. Extraction Behaviors of Lignin and Hemicellulose-Derived Sugars During Organosolv Fractionation of Agricultural Residues Using a Bench-Scale Ball Milling Reactor. Energies 2020, 13, 352. [Google Scholar] [CrossRef]
- Kosan, B.; Michels, C.; Meister, F. Dissolution and Forming of Cellulose with Ionic Liquids. Cellulose 2008, 15, 59–66. [Google Scholar] [CrossRef]
- Gough, C.R.; Rivera-Galletti, A.; Cowan, D.A.; Salas-de la Cruz, D.; Hu, X. Protein and Polysaccharide-Based Fiber Materials Generated from Ionic Liquids: A Review. Molecules 2020, 25, 3362. [Google Scholar] [CrossRef]
- Mohd, N.; Draman, S.F.S.; Salleh, M.S.N.; Yusof, N.B. Dissolution of Cellulose in Ionic Liquid: A Review. AIP Conf. Proc. 2017, 1809, 020035. [Google Scholar]
- Taokaew, S.; Kriangkrai, W. Recent Progress in Processing Cellulose Using Ionic Liquids as Solvents. Polysaccharides 2022, 3, 671–691. [Google Scholar] [CrossRef]
- Willberg-Keyriläinen, P.; Hiltunen, J.; Ropponen, J. Production of Cellulose Carbamate Using Urea-Based Deep Eutectic Solvents. Cellulose 2018, 25, 195–204. [Google Scholar] [CrossRef]
- Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Green and Sustainable Pretreatment Methods for Cellulose Extraction from Lignocellulosic Biomass and Its Applications: A Review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100396. [Google Scholar] [CrossRef]
- Rao, J.; Lv, Z.; Chen, G.; Peng, F. Hemicellulose: Structure, Chemical Modification, and Application. Prog. Polym. Sci. 2023, 140, 101675. [Google Scholar] [CrossRef]
- Chen, W.-H.; Nižetić, S.; Sirohi, R.; Huang, Z.; Luque, R.; Papadopoulos, A.M.; Sakthivel, R.; Phuong Nguyen, X.; Tuan Hoang, A. Liquid Hot Water as Sustainable Biomass Pretreatment Technique for Bioenergy Production: A Review. Bioresour. Technol. 2022, 344, 126207. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Peng, K.; Zhang, Y.; Wang, M.; Yong, C.; Chen, L.; Qu, P.; Huang, H.; Sun, E.; Pan, M. Lignocellulose Dissociation with Biological Pretreatment towards the Biochemical Platform: A Review. Mater. Today Bio 2022, 16, 100445. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Y.; Qin, C.; Yang, S.; Song, X.; Wang, S.; Li, K. Enzyme-Assisted Mechanical Grinding for Cellulose Nanofibers from Bagasse: Energy Consumption and Nanofiber Characteristics. Cellulose 2018, 25, 7065–7078. [Google Scholar] [CrossRef]
- Ferdeș, M.; Dincă, M.N.; Moiceanu, G.; Zăbavă, B.Ș.; Paraschiv, G. Microorganisms and Enzymes Used in the Biological Pretreatment of the Substrate to Enhance Biogas Production: A Review. Sustainability 2020, 12, 7205. [Google Scholar] [CrossRef]
- Andlar, M.; Rezić, T.; Marđetko, N.; Kracher, D.; Ludwig, R.; Šantek, B. Lignocellulose Degradation: An Overview of Fungi and Fungal Enzymes Involved in Lignocellulose Degradation. Eng. Life Sci. 2018, 18, 768–778. [Google Scholar] [CrossRef]
- Pavithra, S.; Jayaprakash, J.; Gummadi, S.N.; Giri Dev, V.R. Assessment of Process Integration Approach for Coir Biosoftening and Lignin-modifying Enzyme Production from Agro Residues. Biofuels Bioprod. Biorefin. 2023, 17, 921–932. [Google Scholar] [CrossRef]
- Sánchez-Corzo, L.D.; Álvarez-Gutiérrez, P.E.; Meza-Gordillo, R.; Villalobos-Maldonado, J.J.; Enciso-Pinto, S.; Enciso-Sáenz, S. Lignocellulolytic Enzyme Production from Wood Rot Fungi Collected in Chiapas, Mexico, and Their Growth on Lignocellulosic Material. J. Fungi 2021, 7, 450. [Google Scholar] [CrossRef]
- Hufenus, R.; Yan, Y.; Dauner, M.; Kikutani, T. Melt-Spun Fibers for Textile Applications. Materials 2020, 13, 4298. [Google Scholar] [CrossRef]
- Chen, L.; Pan, D.; He, H. Morphology Development of Polymer Blend Fibers along Spinning Line. Fibers 2019, 7, 35. [Google Scholar] [CrossRef]
- Jin, Y.; Lin, J.; Cheng, Y.; Lu, C. Lignin-Based High-Performance Fibers by Textile Spinning Techniques. Materials 2021, 14, 3378. [Google Scholar] [CrossRef]
- DeFrates, K.G.; Moore, R.; Borgesi, J.; Lin, G.; Mulderig, T.; Beachley, V.; Hu, X. Protein-Based Fiber Materials in Medicine: A Review. Nanomaterials 2018, 8, 457. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.-E.; Inai, R.; Ramakrishna, S. Technological Advances in Electrospinning of Nanofibers. Sci. Technol. Adv. Mater. 2011, 12, 013002. [Google Scholar] [CrossRef]
- Abdu, M.T.; Abuhasel, K.A.; Alquraish, M.; Nagy, S.; Khodir, S.; Ali, A.A. Selected Natural Fibers and Their Electrospinning. J. Polym. Res. 2023, 30, 340. [Google Scholar] [CrossRef]
- Salas, C.; Ago, M.; Lucia, L.A.; Rojas, O.J. Synthesis of Soy Protein–Lignin Nanofibers by Solution Electrospinning. React. Funct. Polym. 2014, 85, 221–227. [Google Scholar] [CrossRef]
- Huang, W.; Zou, T.; Li, S.; Jing, J.; Xia, X.; Liu, X. Drug-Loaded Zein Nanofibers Prepared Using a Modified Coaxial Electrospinning Process. AAPS PharmSciTech 2013, 14, 675–681. [Google Scholar] [CrossRef]
- Ruiz-Rosas, R.; Bedia, J.; Lallave, M.; Loscertales, I.G.; Barrero, A.; Rodríguez-Mirasol, J.; Cordero, T. The Production of Submicron Diameter Carbon Fibers by the Electrospinning of Lignin. Carbon 2010, 48, 696–705. [Google Scholar] [CrossRef]
- Suen, D.W.-S.; Chan, E.M.-H.; Lau, Y.-Y.; Lee, R.H.-P.; Tsang, P.W.-K.; Ouyang, S.; Tsang, C.-W. Sustainable Textile Raw Materials: Review on Bioprocessing of Textile Waste via Electrospinning. Sustainability 2023, 15, 11638. [Google Scholar] [CrossRef]
- Refate, A.; Mohamed, Y.; Mohamed, M.; Sobhy, M.; Samhy, K.; Khaled, O.; Eidaroos, K.; Batikh, H.; El-Kashif, E.; El-Khatib, S.; et al. Influence of Electrospinning Parameters on Biopolymers Nanofibers, with Emphasis on Cellulose & Chitosan. Heliyon 2023, 9, e17051. [Google Scholar] [CrossRef]
- Dallmeyer, I.; Lin, L.T.; Li, Y.; Ko, F.; Kadla, J.F. Preparation and Characterization of Interconnected, Kraft Lignin-Based Carbon Fibrous Materials by Electrospinning. Macromol. Mater. Eng. 2014, 299, 540–551. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L. Electrospinning of Prolamin Proteins in Acetic Acid: The Effects of Protein Conformation and Aggregation in Solution. Macromol. Mater. Eng. 2012, 297, 902–913. [Google Scholar] [CrossRef]
- Liu, L.; Xu, W.; Ding, Y.; Agarwal, S.; Greiner, A.; Duan, G. A Review of Smart Electrospun Fibers toward Textiles. Compos. Commun. 2020, 22, 100506. [Google Scholar] [CrossRef]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.; Beck Tan, N.C. The Effect of Processing Variables on the Morphology of Electrospun Nanofibers and Textiles. Polymers 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Zhang, M.; Ogale, A.A. Carbon Fibers from Dry-Spinning of Acetylated Softwood Kraft Lignin. Carbon 2014, 69, 626–629. [Google Scholar] [CrossRef]
- Ota, A.; Beyer, R.; Hageroth, U.; Müller, A.; Tomasic, P.; Hermanutz, F.; Buchmeiser, M.R. Chitin/Cellulose Blend Fibers Prepared by Wet and Dry-Wet Spinning. Polym. Adv. Technol. 2021, 32, 335–342. [Google Scholar] [CrossRef]
- Hauru, L.K.J.; Hummel, M.; Michud, A.; Sixta, H. Dry Jet-Wet Spinning of Strong Cellulose Filaments from Ionic Liquid Solution. Cellulose 2014, 21, 4471–4481. [Google Scholar] [CrossRef]
- Yoo, M.K.; Reza, M.S.; Kim, I.M.; Kim, K.J. Physical Properties and Fibrillation Tendency of Regenerated Cellulose Fiber Dry Jet-Wet Spun from High-Molecular Weight Cotton Linter Pulp/NMMO Solution. Fibers Polym. 2015, 16, 1618–1628. [Google Scholar] [CrossRef]
- Cai, J.; Kimura, S.; Wada, M.; Kuga, S.; Zhang, L. Cellulose Aerogels from Aqueous Alkali Hydroxide–Urea Solution. ChemSusChem 2008, 1, 149–154. [Google Scholar] [CrossRef]
- Wilkes, A.G. The Viscose Process. In Regenerated Cellulose Fibres; Elsevier: Amsterdam, The Netherlands, 2001; pp. 37–61. [Google Scholar]
- Kreze, T.; Malej, S. Structural Characteristics of New and Conventional Regenerated Cellulosic Fibers. Text. Res. J. 2003, 73, 675–684. [Google Scholar] [CrossRef]
- Michud, A.; Tanttu, M.; Asaadi, S.; Ma, Y.; Netti, E.; Kääriainen, P.; Persson, A.; Berntsson, A.; Hummel, M.; Sixta, H. Ioncell-F: Ionic Liquid-Based Cellulosic Textile Fibers as an Alternative to Viscose and Lyocell. Text. Res. J. 2016, 86, 543–552. [Google Scholar] [CrossRef]
- Jiang, G.; Huang, W.; Li, L.; Wang, X.; Pang, F.; Zhang, Y.; Wang, H. Structure and Properties of Regenerated Cellulose Fibers from Different Technology Processes. Carbohydr. Polym. 2012, 87, 2012–2018. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, C.; Duan, C.; Hu, H.; Li, H.; Li, J.; Liu, Y.; Ma, X.; Stavik, J.; Ni, Y. Regenerated Cellulose by the Lyocell Process, a Brief Review of the Process and Properties. Bioresources 2018, 13, 4577–4592. [Google Scholar] [CrossRef]
- Pocienė, R.; Žemaitaitienė, R.; Vitkauskas, A. Mechanical Properties and a Physical-Chemical Analysis of Acetate Yarns. Mater. Sci. 2004, 10, 75–79. [Google Scholar]
- Klemm, D.; Heublein, B.; Fink, H.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Yu, G.; Fu, Y.; Yin, C. The Preparation and Study of Regenerated Cellulose Fibers by Cellulose Carbamate Pathway. Int. J. Biol. Macromol. 2018, 107, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Erdal, N.B.; Hakkarainen, M. Degradation of Cellulose Derivatives in Laboratory, Man-Made, and Natural Environments. Biomacromolecules 2022, 23, 2713–2729. [Google Scholar] [CrossRef] [PubMed]
- Muhammed, N.; Govindan, N. Chemical Modification of Cotton Cellulose by Carbamation with Urea and Its Dyeability with Reactive Dyes without the Use of Electrolyte. J. Nat. Fibers 2022, 19, 1402–1418. [Google Scholar] [CrossRef]
- Fu, F.; Yang, Q.; Zhou, J.; Hu, H.; Jia, B.; Zhang, L. Structure and Properties of Regenerated Cellulose Filaments Prepared from Cellulose Carbamate–NaOH/ZnO Aqueous Solution. ACS Sustain. Chem. Eng. 2014, 2, 2604–2612. [Google Scholar] [CrossRef]
- Gan, S.; Zakaria, S.; Syed Jaafar, S.N. Enhanced Mechanical Properties of Hydrothermal Carbamated Cellulose Nanocomposite Film Reinforced with Graphene Oxide. Carbohydr. Polym. 2017, 172, 284–293. [Google Scholar] [CrossRef]
- Paunonen, S.; Kamppuri, T.; Katajainen, L.; Hohenthal, C.; Heikkilä, P.; Harlin, A. Environmental Impact of Cellulose Carbamate Fibers from Chemically Recycled Cotton. J. Clean. Prod. 2019, 222, 871–881. [Google Scholar] [CrossRef]
- Hong, W.; Li, Q.; Di, Y.; Sun, J.; Jiao, T.; Xu, M.; Zhao, Z.; Xing, G. Preliminary Study on the Preparation of Bamboo Cellulose Carbamate. J. Nanosci. Nanotechnol. 2013, 13, 6741–6747. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Qiu, C.; Lu, A.; Luo, L.; Guo, J.; Cong, H.; Chen, F.; Liu, X.; Zhang, X.; Wang, H.; et al. Mechanically Strong Multifilament Fibers Spun from Cellulose Solution via Inducing Formation of Nanofibers. ACS Sustain. Chem. Eng. 2018, 6, 5314–5321. [Google Scholar] [CrossRef]
- Qiu, C.; Zhu, K.; Zhou, X.; Luo, L.; Zeng, J.; Huang, R.; Lu, A.; Liu, X.; Chen, F.; Zhang, L.; et al. Influences of Coagulation Conditions on the Structure and Properties of Regenerated Cellulose Filaments via Wet-Spinning in LiOH/Urea Solvent. ACS Sustain. Chem. Eng. 2018, 6, 4056–4067. [Google Scholar] [CrossRef]
- Qi, H.; Cai, J.; Zhang, L.; Nishiyama, Y.; Rattaz, A. Influence of Finishing Oil on Structure and Properties of Multi-Filament Fibers from Cellulose Dope in NaOH/Urea Aqueous Solution. Cellulose 2008, 15, 81–89. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, L.; Zhou, J.; Qi, H.; Chen, H.; Kondo, T.; Chen, X.; Chu, B. Multifilament Fibers Based on Dissolution of Cellulose in NaOH/Urea Aqueous Solution: Structure and Properties. Adv. Mater. 2007, 19, 821–825. [Google Scholar] [CrossRef]
- Fu, F.; Zhou, J.; Zhou, X.; Zhang, L.; Li, D.; Kondo, T. Green Method for Production of Cellulose Multifilament from Cellulose Carbamate on a Pilot Scale. ACS Sustain. Chem. Eng. 2014, 2, 2363–2370. [Google Scholar] [CrossRef]
- Fink, H.-P.; Weigel, P.; Purz, H.J.; Ganster, J. Structure Formation of Regenerated Cellulose Materials from NMMO-Solutions. Prog. Polym. Sci. 2001, 26, 1473–1524. [Google Scholar] [CrossRef]
- Woodings, C.R. The Development of Advanced Cellulosic Fibres. Int. J. Biol. Macromol. 1995, 17, 305–309. [Google Scholar] [CrossRef]
- Jia, B.; Yu, L.; Fu, F.; Li, L.; Zhou, J.; Zhang, L. Preparation of Helical Fibers from Cellulose–Cuprammonium Solution Based on Liquid Rope Coiling. RSC Adv. 2014, 4, 9112. [Google Scholar] [CrossRef]
- Kamide, K.; Nishiyama, K. Cuprammonium Processes. In Regenerated Cellulose Fibres; Elsevier: Amsterdam, The Netherlands, 2001; pp. 88–155. [Google Scholar]
- Hong, Y.-K.; Chung, K.-H.; Lee, W.-S. Structure of Regenerated Cellulose Fibers from DMAc/LiCl Solution. Text. Res. J. 1998, 68, 65–69. [Google Scholar] [CrossRef]
- Dawsey, T.R.; McCormick, C.L. The Lithium Chloride/Dimethylacetamide Solvent for Cellulose: A Literature Review. J. Macromol. Sci. Part C 1990, 30, 405–440. [Google Scholar] [CrossRef]
- White, P. Lyocell: The Production Process and Market Development. In Regenerated Cellulose Fibres; Series in Textiles; Woodings, C., Ed.; Woodhead Publishing: Cambridge, UK, 2001; pp. 62–87. [Google Scholar]
- Sayyed, A.J.; Mohite, L.V.; Deshmukh, N.A.; Pinjari, D.V. Structural Characterization of Cellulose Pulp in Aqueous NMMO Solution under the Process Conditions of Lyocell Slurry. Carbohydr. Polym. 2019, 206, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Gavillon, R.; Budtova, T. Kinetics of Cellulose Regeneration from Cellulose–NaOH–Water Gels and Comparison with Cellulose-N.-Methylmorpholine-N.-Oxide–Water Solutions. Biomacromolecules 2007, 8, 424–432. [Google Scholar] [CrossRef]
- Medronho, B.; Lindman, B. Competing Forces during Cellulose Dissolution: From Solvents to Mechanisms. Curr. Opin. Colloid. Interface Sci. 2014, 19, 32–40. [Google Scholar] [CrossRef]
- Sixta, H.; Michud, A.; Hauru, L.; Asaadi, S.; Ma, Y.; King, A.W.T.; Kilpeläinen, I.; Hummel, M. Ioncell-F: A High-Strength Regenerated Cellulose Fibre. Nord. Pulp Pap. Res. J. 2015, 30, 43–57. [Google Scholar] [CrossRef]
- Orange Fiber. Available online: https://orangefiber.it/process/ (accessed on 25 September 2024).
- Li, J.; Tian, X.; Hua, T.; Fu, J.; Koo, M.; Chan, W.; Poon, T. Chitosan Natural Polymer Material for Improving Antibacterial Properties of Textiles. ACS Appl. Bio Mater. 2021, 4, 4014–4038. [Google Scholar] [CrossRef]
- Favatela, M.F.; Otarola, J.; Ayala-Peña, V.B.; Dolcini, G.; Perez, S.; Torres Nicolini, A.; Alvarez, V.A.; Lassalle, V.L. Development and Characterization of Antimicrobial Textiles from Chitosan-Based Compounds: Possible Biomaterials Against SARS-CoV-2 Viruses. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1473–1486. [Google Scholar] [CrossRef] [PubMed]
- Kaurin, T.; Pušić, T.; Čurlin, M. Biopolymer Textile Structure of Chitosan with Polyester. Polymers 2022, 14, 3088. [Google Scholar] [CrossRef]
- Afzal, A.; Azam, F.; Ahmad, S.; Khaliq, Z.; Shahzad, A.; Qadir, M.B.; Hai, A.M. Development and Characterization of Biodegradable Starch-Based Fibre by Wet Extrusion. Cellulose 2021, 28, 2039–2051. [Google Scholar] [CrossRef]
- Temesgen, S.; Rennert, M.; Tesfaye, T.; Nase, M. Review on Spinning of Biopolymer Fibers from Starch. Polymers 2021, 13, 1121. [Google Scholar] [CrossRef] [PubMed]
- Hibbert, R. What Textile Fibres Are Applicable for the Layering System for the Active Ageing? In Textile-Led Design for the Active Ageing Population; Elsevier: Amsterdam, The Netherlands, 2015; pp. 329–359. [Google Scholar]
- De la Harpe, K.M.; Marimuthu, T.; Kondiah, P.P.D.; Kumar, P.; Ubanako, P.; Choonara, Y.E. Synthesis of a Novel Monofilament Bioabsorbable Suture for Biomedical Applications. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 2189–2210. [Google Scholar] [CrossRef]
- Mukherjee, A.; Kabutare, Y.H.; Ghosh, P. Dual Crosslinked Keratin-Alginate Fibers Formed via Ionic Complexation of Amide Networks with Improved Toughness for Assembling into Braids. Polym. Test. 2020, 81, 106286. [Google Scholar] [CrossRef]
- Amjadi, S.; Almasi, H.; Ghorbani, M.; Ramazani, S. Preparation and Characterization of TiO2NPs and Betanin Loaded Zein/Sodium Alginate Nanofibers. Food Packag. Shelf Life 2020, 24, 100504. [Google Scholar] [CrossRef]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, Production, Recent Developments and Applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Pakalapati, H.; Chang, C.-K.; Show, P.L.; Arumugasamy, S.K.; Lan, J.C.-W. Development of Polyhydroxyalkanoates Production from Waste Feedstocks and Applications. J. Biosci. Bioeng. 2018, 126, 282–292. [Google Scholar] [CrossRef]
- Taib, N.-A.A.B.; Rahman, M.R.; Huda, D.; Kuok, K.K.; Hamdan, S.; Bakri, M.K.B.; Julaihi, M.R.M.B.; Khan, A. A Review on Poly Lactic Acid (PLA) as a Biodegradable Polymer. Polym. Bull. 2023, 80, 1179–1213. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Qian, S.; Liu, Z.; Weng, Y.; Zhang, Y. Depolymerization and Re/Upcycling of Biodegradable PLA Plastics. ACS Omega 2024, 9, 13509–13521. [Google Scholar] [CrossRef] [PubMed]
- Auerbach George, H.; Stenton, M.; Kapsali, V.; Blackburn, R.S.; Houghton, J.A. Referencing Historical Practices and Emergent Technologies in the Future Development of Sustainable Textiles: A Case Study Exploring “Ardil”, a UK-Based Regenerated Protein Fibre. Sustainability 2022, 14, 8414. [Google Scholar] [CrossRef]
- QMILK. Available online: https://www.qmilkfiber.eu/?lang=en (accessed on 25 September 2024).
- UMORFIL® Beauty Fiber®. Available online: https://www.umorfil.com/product.html (accessed on 25 September 2024).
- Babysoy. Available online: https://babysoyusa.com/pages/about-azlon-from-soy-protein-fiber (accessed on 25 September 2024).
- Chi, F.; Chen, H. Fabrication and Characterization of Zein/Viscose Textibe Fibers. J. Appl. Polym. Sci. 2010, 118, 3364–3370. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Luo, J.; Chen, L. Facile Preparation of Self-Standing Hierarchical Porous Nitrogen-Doped Carbon Fibers for Supercapacitors from Plant Protein–Lignin Electrospun Fibers. ACS Omega 2018, 3, 4647–4656. [Google Scholar] [CrossRef]
- Xu, W.; Yang, Y. Drug Sorption onto and Release from Soy Protein Fibers. J. Mater. Sci. Mater. Med. 2009, 20, 2477–2486. [Google Scholar] [CrossRef]
- Zahra, H.; Selinger, J.; Sawada, D.; Ogawa, Y.; Orelma, H.; Ma, Y.; Kumagai, S.; Yoshioka, T.; Hummel, M. Evaluation of Keratin–Cellulose Blend Fibers as Precursors for Carbon Fibers. ACS Sustain. Chem. Eng. 2022, 10, 8314–8325. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, Z.; Li, M.; Su, Y.; Zhang, Q.; Zhang, S.; Hu, J. Reconstructed Hierarchically Structured Keratin Fibers with Shape-Memory Features Based on Reversible Secondary-Structure Transformation. Adv. Mater. 2023, 35, 2304725. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P.; Xiang, P.; Lu, J.; Yuan, J.; Shen, J. Electrospun Polyurethane/Keratin/AgNP Biocomposite Mats for Biocompatible and Antibacterial Wound Dressings. J. Mater. Chem. B 2016, 4, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Xing, Z.-C.; Park, S.-W.; Geng, J.; Kang, I.-K.; Yuan, J.; Shen, J.; Meng, W.; Shim, K.-J.; Han, I.-S.; et al. Fabrication of PHBV/Keratin Composite Nanofibrous Mats for Biomedical Applications. Macromol. Res. 2009, 17, 850–855. [Google Scholar] [CrossRef]
- Ribeiro, N.; Sousa, S.R.; van Blitterswijk, C.A.; Moroni, L.; Monteiro, F.J. A Biocomposite of Collagen Nanofibers and Nanohydroxyapatite for Bone Regeneration. Biofabrication 2014, 6, 035015. [Google Scholar] [CrossRef] [PubMed]
- Ekaputra, A.K.; Prestwich, G.D.; Cool, S.M.; Hutmacher, D.W. The Three-Dimensional Vascularization of Growth Factor-Releasing Hybrid Scaffold of Poly (ɛ-Caprolactone)/Collagen Fibers and Hyaluronic Acid Hydrogel. Biomaterials 2011, 32, 8108–8117. [Google Scholar] [CrossRef]
- Guzmán-Soria, A.; Moreno-Serna, V.; Canales, D.A.; García-Herrera, C.; Zapata, P.A.; Orihuela, P.A. Effect of Electrospun PLGA/Collagen Scaffolds on Cell Adhesion, Viability, and Collagen Release: Potential Applications in Tissue Engineering. Polymers 2023, 15, 1079. [Google Scholar] [CrossRef]
- Agheb, M.; Dinari, M.; Rafienia, M.; Salehi, H. Novel Electrospun Nanofibers of Modified Gelatin-Tyrosine in Cartilage Tissue Engineering. Mater. Sci. Eng. C 2017, 71, 240–251. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, M.; Xu, Y.; Wang, W.; Wang, Z.; Zhang, L. Bio-Based Polyesters: Recent Progress and Future Prospects. Prog. Polym. Sci. 2021, 120, 101430. [Google Scholar] [CrossRef]
- Câmara, J.S.; Perestrelo, R.; Ferreira, R.; Berenguer, C.V.; Pereira, J.A.M.; Castilho, P.C. Plant-Derived Terpenoids: A Plethora of Bioactive Compounds with Several Health Functions and Industrial Applications—A Comprehensive Overview. Molecules 2024, 29, 3861. [Google Scholar] [CrossRef] [PubMed]
- Firdaus, M.; Montero de Espinosa, L.; Meier, M.A.R. Terpene-Based Renewable Monomers and Polymers via Thiol–Ene Additions. Macromolecules 2011, 44, 7253–7262. [Google Scholar] [CrossRef]
- Nsengiyumva, O.; Miller, S.A. Synthesis, Characterization, and Water-Degradation of Biorenewable Polyesters Derived from Natural Camphoric Acid. Green Chem. 2019, 21, 973–978. [Google Scholar] [CrossRef]
- Zeng, C.; Seino, H.; Ren, J.; Hatanaka, K.; Yoshie, N. Bio-Based Furan Polymers with Self-Healing Ability. Macromolecules 2013, 46, 1794–1802. [Google Scholar] [CrossRef]
- Avantium. Available online: https://avantium.com/ (accessed on 2 October 2024).
- Ma, J.; Pang, Y.; Wang, M.; Xu, J.; Ma, H.; Nie, X. The Copolymerization Reactivity of Diols with 2,5-Furandicarboxylic Acid for Furan-Based Copolyester Materials. J. Mater. Chem. 2012, 22, 3457. [Google Scholar] [CrossRef]
- Ryu, Y.S.; Oh, K.W.; Kim, S.H. Furan-Based Self-Healing Breathable Elastomer Coating on Polylactide Fabric. Text. Res. J. 2019, 89, 814–824. [Google Scholar] [CrossRef]
- Sousa, A.F.; Vilela, C.; Fonseca, A.C.; Matos, M.; Freire, C.S.R.; Gruter, G.-J.M.; Coelho, J.F.J.; Silvestre, A.J.D. Biobased Polyesters and Other Polymers from 2,5-Furandicarboxylic Acid: A Tribute to Furan Excellency. Polym. Chem. 2015, 6, 5961–5983. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, S.H.; Kim, S.H. Fabrication of Bio-Based Polyurethane Nanofibers Incorporated with a Triclosan/Cyclodextrin Complex for Antibacterial Applications. RSC Adv. 2020, 10, 3450–3458. [Google Scholar] [CrossRef]
- Choi, K.K.; Park, S.H.; Oh, K.W.; Kim, S.H. Effect of Castor Oil/Polycaprolactone Hybrid Polyols on the Properties of Biopolyurethane. Macromol. Res. 2015, 23, 333–340. [Google Scholar] [CrossRef]
- Wang, X.C.; Zheng, Q.; Yang, G.S. Influence of Preparation Methods on Structure and Properties of PA6/PA66 Blends: A Comparison of Melt-mixing and in Situ Blending. J. Polym. Sci. B Polym. Phys. 2007, 45, 1176–1186. [Google Scholar] [CrossRef]
- Brehmer, B.; Boom, R.M.; Sanders, J. Maximum Fossil Fuel Feedstock Replacement Potential of Petrochemicals via Biorefineries. Chem. Eng. Res. Des. 2009, 87, 1103–1119. [Google Scholar] [CrossRef]
- Yang, H.; Wentao, L. Bio-based Polyamide 56: Recent Advances in Basic and Applied Research. Polym. Eng. Sci. 2023, 63, 2484–2497. [Google Scholar] [CrossRef]
- Mutlu, H.; Meier, M.A.R. Castor Oil as a Renewable Resource for the Chemical Industry. Eur. J. Lipid Sci. Technol. 2010, 112, 10–30. [Google Scholar] [CrossRef]
- Ogunniyi, D.S. Castor Oil: A Vital Industrial Raw Material. Bioresour. Technol. 2006, 97, 1086–1091. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Hsu, K.-M.; Chiu, C.-Y.; Chang, Y.-K.; Ng, I.-S. Fabrication of Bio-Based Polyamide 56 and Antibacterial Nanofiber Membrane from Cadaverine. Chemosphere 2021, 266, 128967. [Google Scholar] [CrossRef]
- Luo, K.; Liu, J.; Abbay, K.; Mei, Y.; Guo, X.; Song, Y.; Guan, Q.; You, Z. The Relationships between the Structure and Properties of PA56 and PA66 and Their Fibers. Polymers 2023, 15, 2877. [Google Scholar] [CrossRef]
- Bio-Based Rilsan® PA11. Available online: https://www.arkema.com/global/en/products/product-finder/product/technicalpolymers/rilsan-family-products/rilsan-pa11/ (accessed on 30 September 2024).
- Radilon®. Available online: https://www.radicigroup.com/en/products/plastics/pa6-pa66-pa6-10-pa6-12-radilon (accessed on 30 September 2024).
- Bio-Based Yarns-EVO Fulgar®. Available online: https://www.fulgar.com/en/feature/39/bio-based-yarns-evo#:~:text=What%20is%20EVO%C2%AE%20made%20of?%20The%20biomass%20from%20which%20EVO%C2%AE (accessed on 30 September 2024).
- Bio-Based Dyneema® Fiber. Available online: https://www.dyneema.com/sustainability/bio-based-dyneema-fiber (accessed on 25 September 2024).
- Sai, H.; Wang, M.; Miao, C.; Song, Q.; Wang, Y.; Fu, R.; Wang, Y.; Ma, L.; Hao, Y. Robust Silica-Bacterial Cellulose Composite Aerogel Fibers for Thermal Insulation Textile. Gels 2021, 7, 145. [Google Scholar] [CrossRef]
- Reimer, M.; Van Opdenbosch, D.; Zollfrank, C. Fabrication of Cellulose-Based Biopolymer Optical Fibers and Their Theoretical Attenuation Limit. Biomacromolecules 2021, 22, 3297–3312. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, M.; Piorkowska, E.; Kulpinski, P.; Pracella, M. Mechanical and Thermal Properties of PLA Composites with Cellulose Nanofibers and Standard Size Fibers. Compos. Part A Appl. Sci. Manuf. 2011, 42, 1509–1514. [Google Scholar] [CrossRef]
- Tang, C.; Wu, M.; Wu, Y.; Liu, H. Effects of Fiber Surface Chemistry and Size on the Structure and Properties of Poly(Vinyl Alcohol) Composite Films Reinforced with Electrospun Fibers. Compos. Part A Appl. Sci. Manuf. 2011, 42, 1100–1109. [Google Scholar] [CrossRef]
- Chen, G.; Liu, H. Electrospun Cellulose Nanofiber Reinforced Soybean Protein Isolate Composite Film. J. Appl. Polym. Sci. 2008, 110, 641–646. [Google Scholar] [CrossRef]
- Liao, H.; Wu, Y.; Wu, M.; Liu, H. Effects of Fiber Surface Chemistry and Roughness on Interfacial Structures of Electrospun Fiber Reinforced Epoxy Composite Films. Polym. Compos. 2011, 32, 837–845. [Google Scholar] [CrossRef]
- Liao, H.; Wu, Y.; Wu, M.; Zhan, X.; Liu, H. Aligned Electrospun Cellulose Fibers Reinforced Epoxy Resin Composite Films with High Visible Light Transmittance. Cellulose 2012, 19, 111–119. [Google Scholar] [CrossRef]
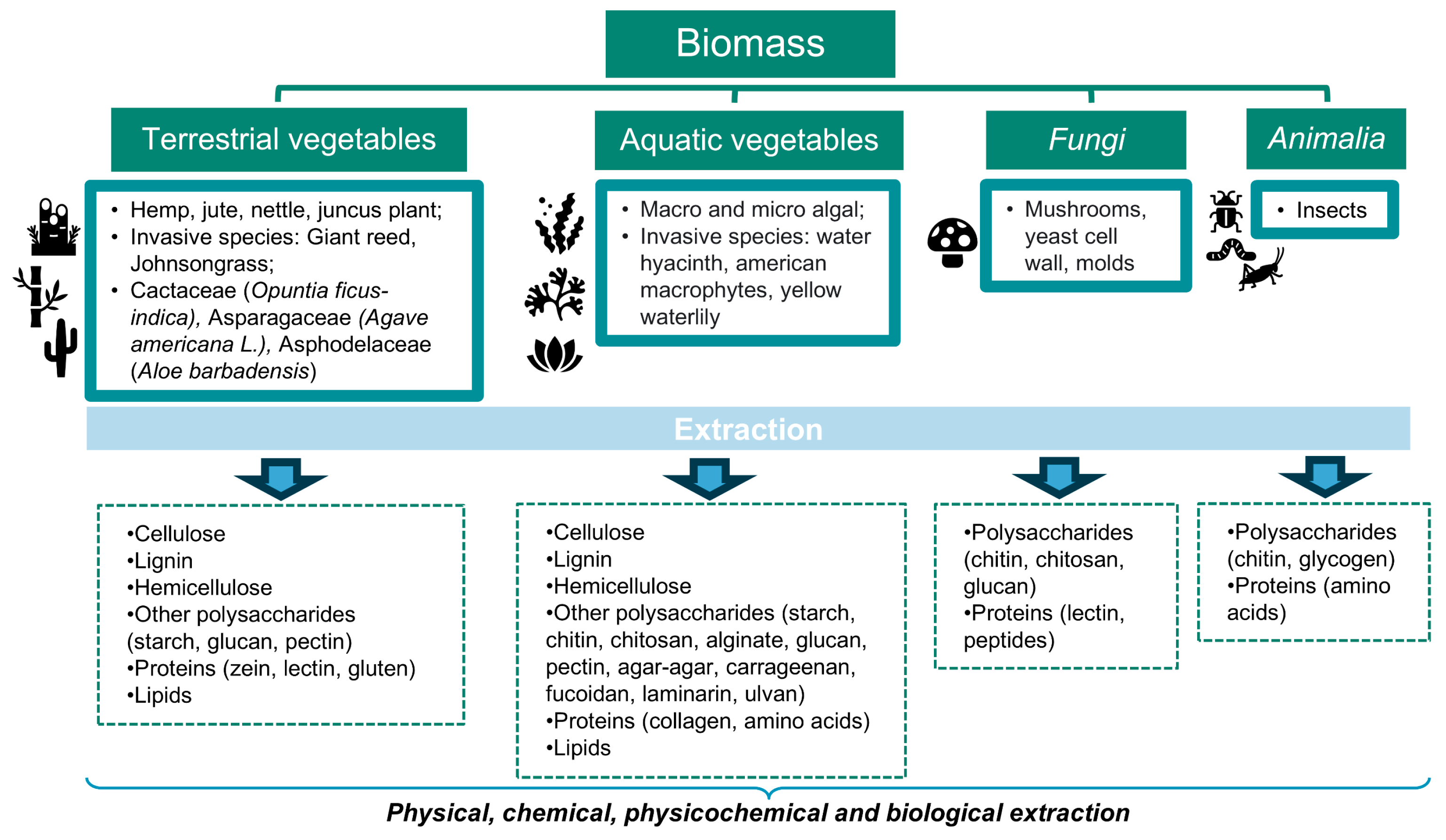
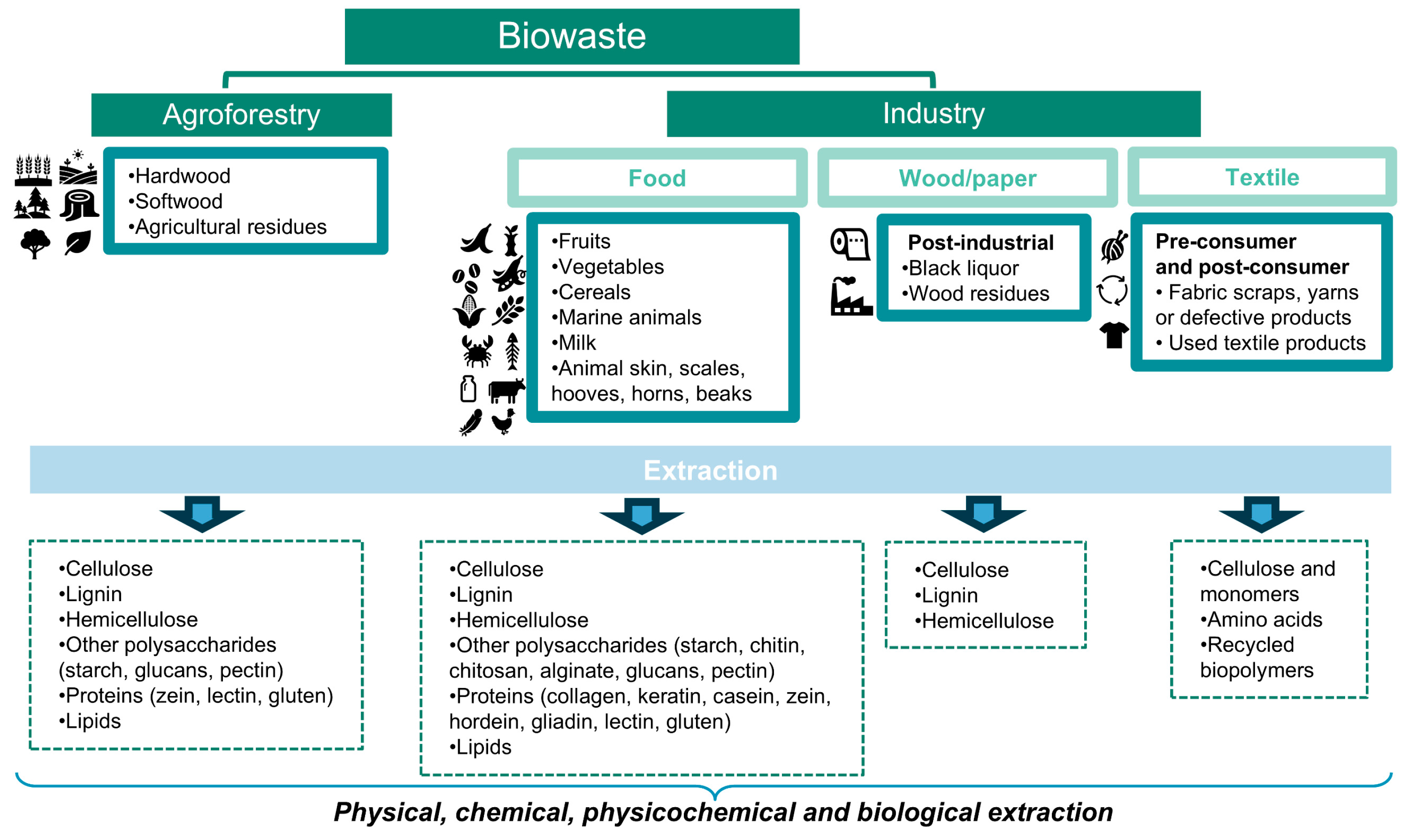
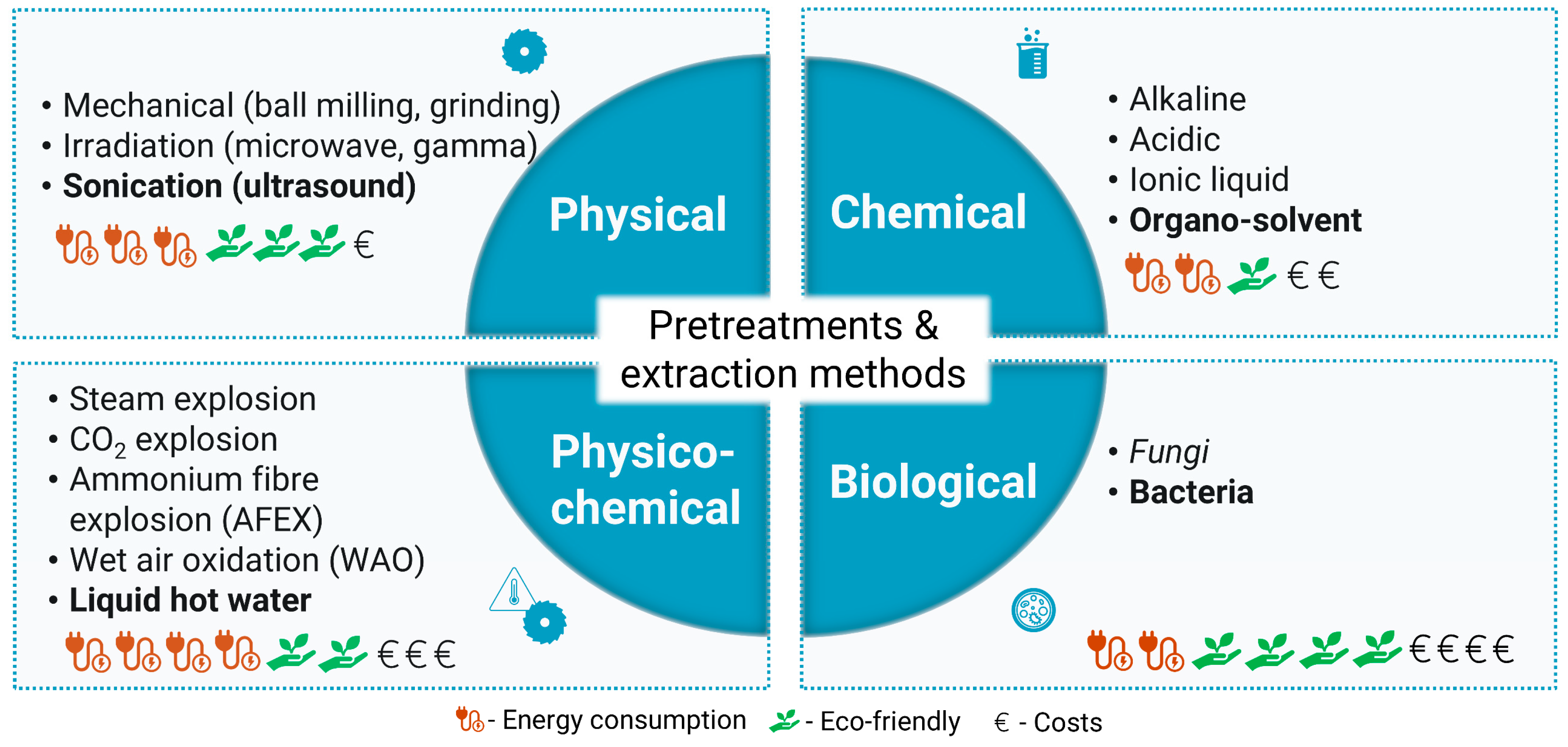
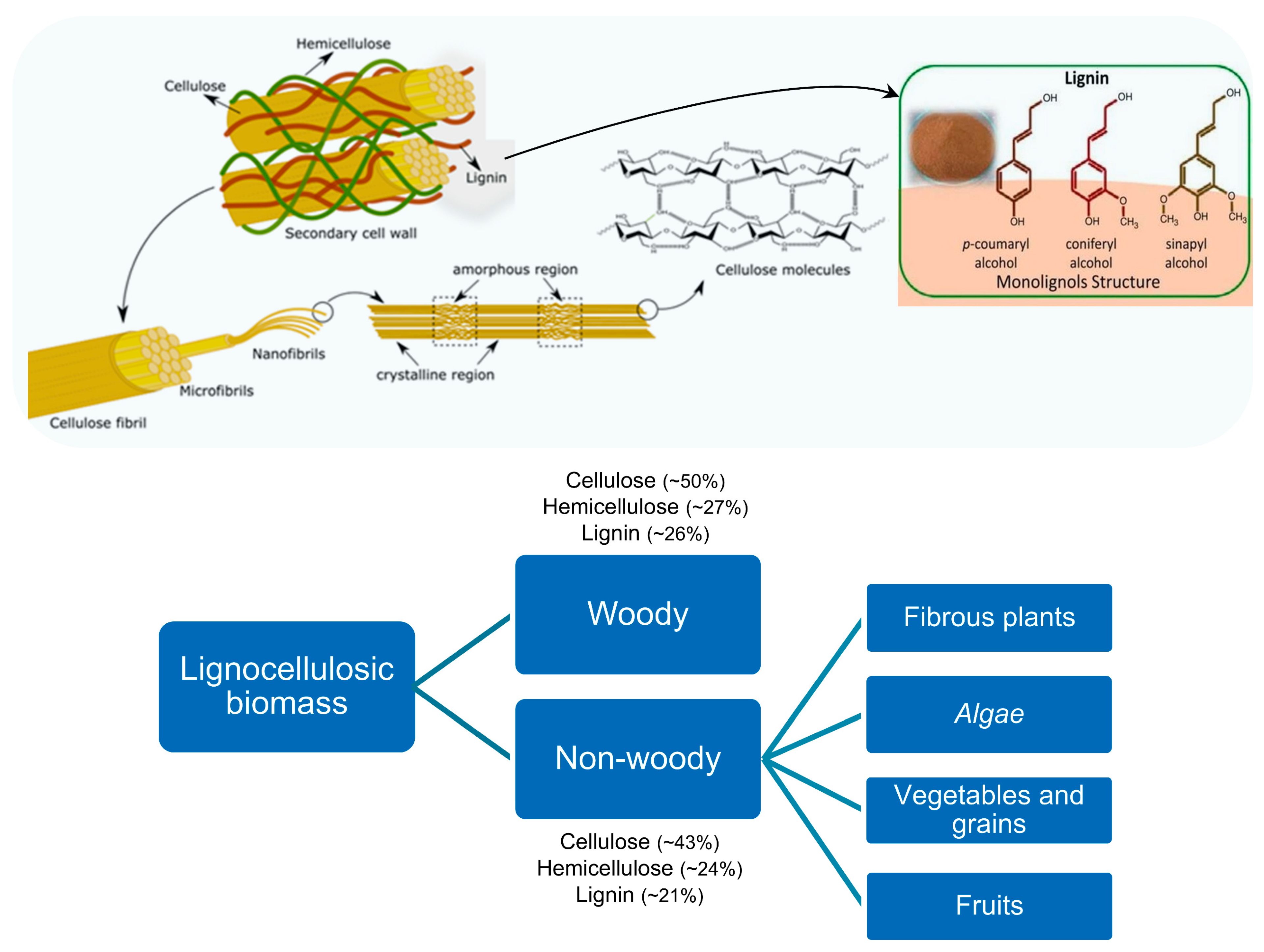
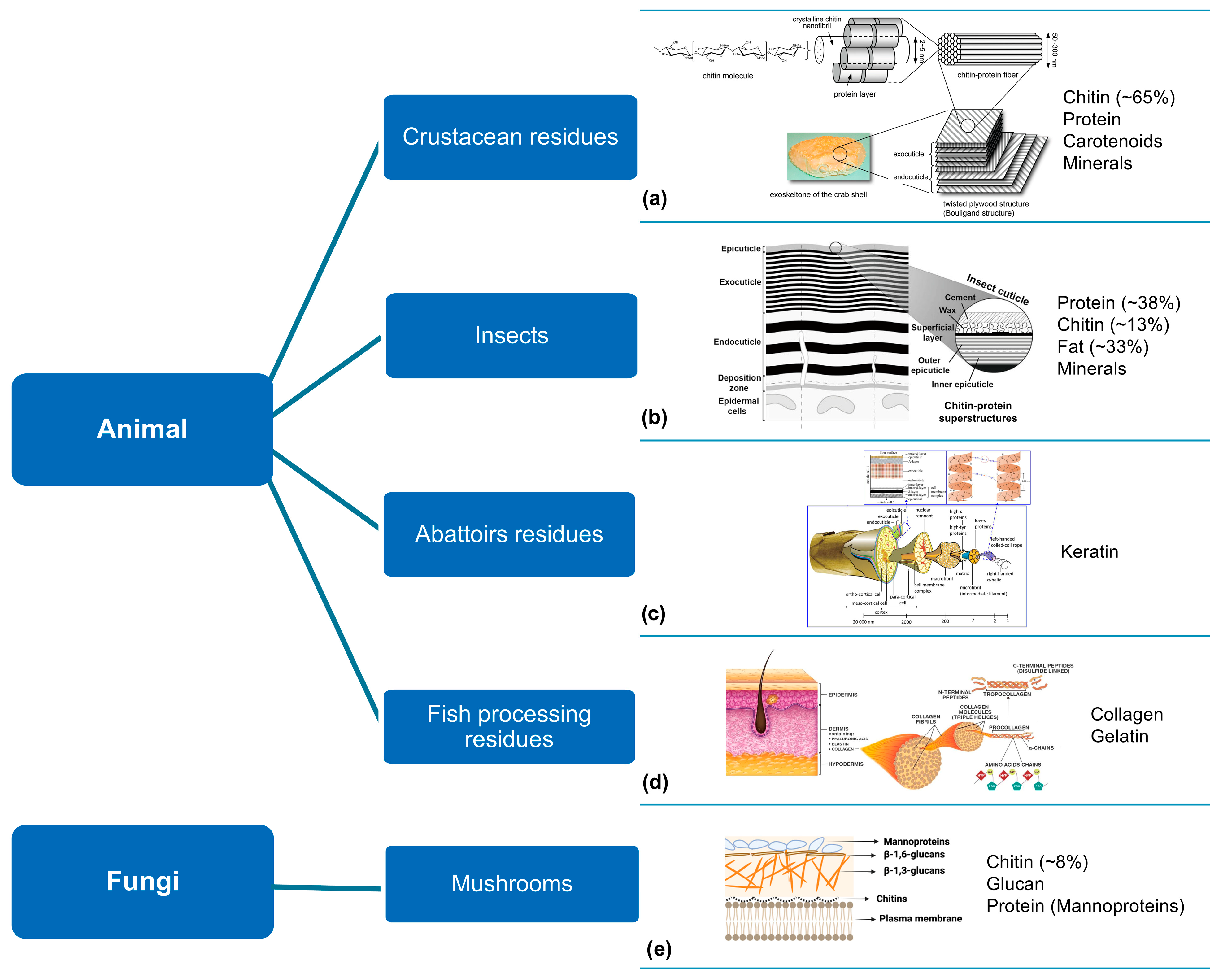
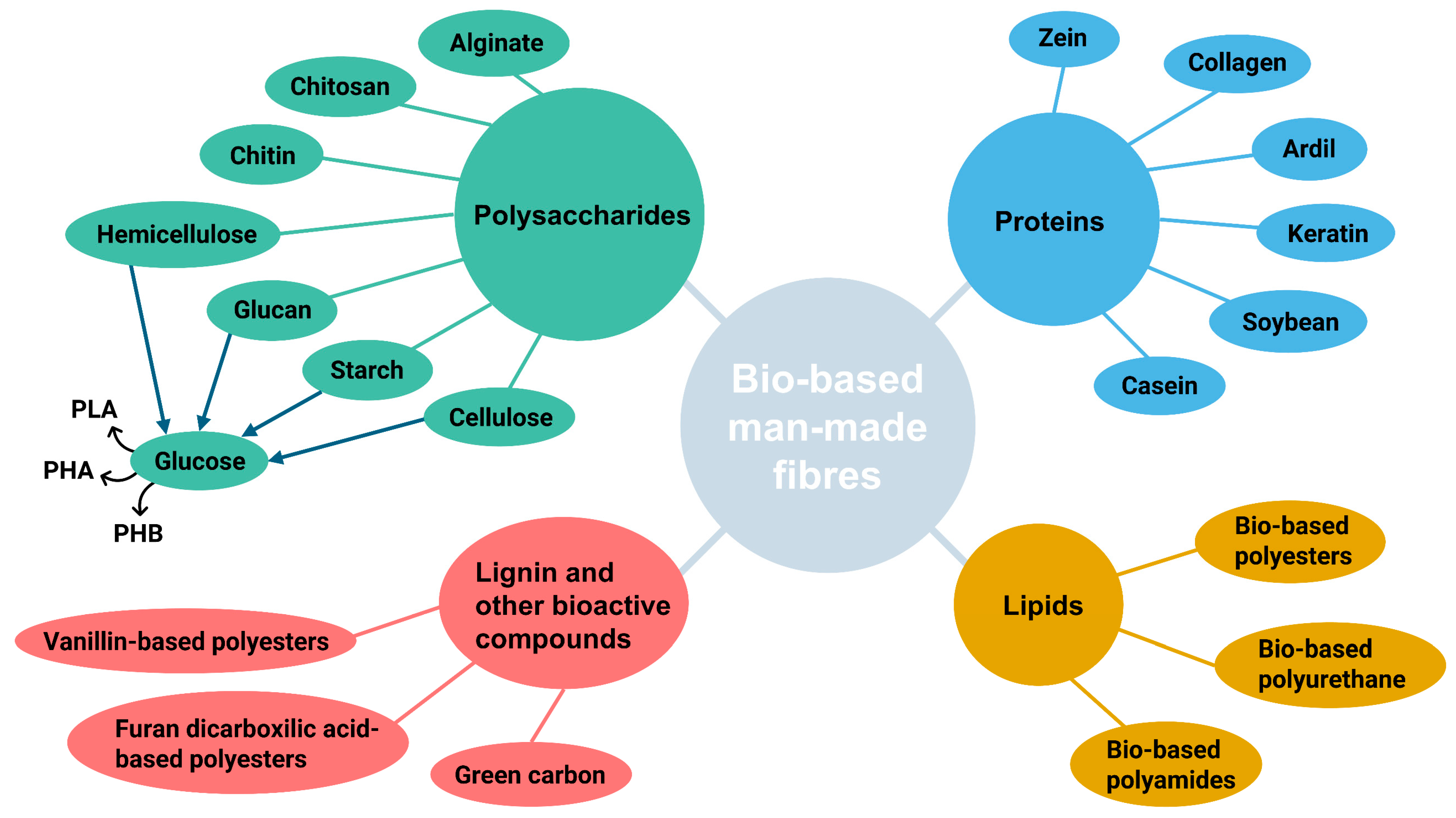

| Biomass Resource | Cellulose (%) | Hemicellulose (%) | Pectin (%) | Lignin (%) | Applications | References |
|---|---|---|---|---|---|---|
| Hemp (stalks) | 67–76 | 12–18 | 3–18 | 3–6 | Textile, paper, food, cosmetics, packaging, biodiesel | [19] |
| Jute (stalks) | 61–73 | 14–23 | n.d. | 12–16 | Textile, packaging, biocomposites | [31] |
| Nettle (leaves, stem, roots) | 54–88 | 4–10 | 1–4.1 | 5–9 | Textile, food, biocomposites, biomedical | [79] |
| Juncus (stem) | 40–64 | 20–28 | n.d. | 6–19 | Biocomposites | [51] |
| Giant reed (leaves, stem, rhizome) | 21–38 | 11–42 | n.d. | 13–32 | Paper, biorefineries, bioplastics, biocomposites | [52] |
| Johnsongrass (leaves, stem) | 40 | 36 | n.d. | 10 | Bioenergy, biorefineries, biocomposites | [59] |
| Opuntia ficus-indica L. (stem) | 53 | 11 | n.d. | 4.8 | Paper, food, pharmaceutical biocomposites | [80] |
| Agave americana L. (leaves) | 59.5 | 17.4 | 6.80 | 13.6 | Biocomposites | [67] |
| Aloe vera (leaves) | 64.9 | 25.1 | n.d. | 4.1 | Textile, cosmetic, pharmaceutical, biocomposites | [69] |
| Chlorella vulgaris (microalgae) | 10–47.5 | n.d. | n.d. | n.d. | Biorefinery, bioplastics, biocomposites | [78] |
| Chlorella pyrenoidosa (microalgae) | 15.4 | 31 | n.d. | n.d. | Biorefinery, bioplastics, biocomposites | [81] |
| Cladophora rupestris (macroalgae) | 28.5 | abs | abs | n.d. | Biorefinery, bioplastics, biocomposites | [82] |
| Chaetomorpha melagonium (macroalgae) | 41 | n.d. | n.d. | n.d. | Biorefinery, bioplastics, biocomposites | [82] |
| Laminaria digitata (macroalgae) | 20 | n.d | n.d. | n.d. | Biorefinery, bioplastics, biocomposites | [82] |
| Halidrys siliquosa (macroalgae) | 14 | n.d. | n.d | n.d. | Biorefinery, bioplastics, biocomposites | [82] |
| Ulva sp. | 40.7 | 7.1 | n.d. | 7.9 | Paper, biorefinery | [83] |
| Water hyacinth | 12.8–14.9 | 24–27.5 | n.d. | 5.9–14.3 | Textile, paper, biocomposites | [84,85] |
| Waterweeds | 18.8 | 5 | n.d. | 2.3 | Biosynthesis of nanoparticles | [86,87,88] |
| Biowaste Product | Cellulose (%) | Hemicellulose (%) | Pectin (%) | Lignin (%) | Applications | References | |
|---|---|---|---|---|---|---|---|
| Hardwood | Poplar | 50–53 | 26–29 | - | 15.5–16 | Paper, biorefineries, biocomposites | [126,127] |
| Eucalyptus | 54 | 18 | - | 21.5 | [126] | ||
| Softwood | Pine | 45–50 | 25–35 | - | 25–35 | Biorefineries, biocomposites | [126,127] |
| Spruce | 46 | 23 | - | 28 | [126] | ||
| Cereals | Wheat straw | 35–39 | 23–30 | - | 12–16 | Biorefineries, PHA | [120,126,127] |
| Corn stalk | 35–40 | 17–35 | - | 7–18 | |||
| Barley straw | 36–43 | 24–33 | - | 6.3–10 | |||
| Fruits | Apple pomace | 40–44 | 19–24 | 9–12 | 15–20 | Biorefineries, bio-fertiliser, biofiller, PHA, xanthan gum | [128] |
| Orange pulp | 25.3 | 5.3 | 15.7–16.3 | 2.2–3.0 | [128] | ||
| Peach pomace | 29–30 | 19–20 | 21–24 | 5–6 | [128] | ||
| Coconut coir | 44 | 22 | - | 33 | [129] | ||
| Banana waste | 13 | 15 | - | 14 | [130] | ||
| Spent coffee ground | 12 | 39 | - | 24 | [131,132,133] | ||
| Vegetables | Soybean straw | 25 | 12 | - | 18 | Biorefineries, bio-fertiliser, biofiller, PHA, PLA, xanthan gum | [120] |
| Tomato pomace | 9–19 | 5–12 | 7.5 | 3–36 | [128,134] | ||
| Potato pulp | 17–22 | 14 | 2.2 | 2.6 | [128,134] | ||
| Olive pomace | 19–37 | 22–27 | 16–17 | 26–40 | [134,135] | ||
| Carrot pomace | 28–52 | 7–12 | 2–4 | 18–32 | [134,136] | ||
| Marine animals | Crustacean shells | - | - | - | - | Chitin and chitosan extraction | [137,138,139,140,141] |
| Abattoir | Meat and poultry waste | - | - | - | - | Collagen, gelatine, keratin, PHA | [142,143,144,145,146,147,148,149,150,151,152,153,154,155] |
| Dairy | Milk and cheese waste | - | - | - | - | PHA, xanthan gum | [156,157,158,159,160,161,162,163,164] |
| Eucalyptus black liquor | Paper industry | - | 1–2 | - | 40–42 | Biorefineries, biogas, PHA, carboxylic acids | [165,166,167,168] |
| Discarded textile | Pre-consumer and post-consumer | - | - | - | - | Extraction of cellulose derivatives, polyester monomers, glucose and amino acids, biorefineries, fillers | [169,170,171,172,173,174,175,176,177,178,179,180] |
| Fabrication Method | Composition | Process |
|---|---|---|
| Melt-spinning | Polymer molecular structure Polydispersity Crystallinity Melting temperature Glass transition Decomposition temperature Enhancing and functional additives | Screw extruder (type and velocity) Spin pack design (spinneret) Heating temperature Extrusion speed Draw ratio Coagulation bath (composition and temperature) Take-up speed Environmental temperature and humidity |
| Electrospinning | Polymer concentration Viscosity Conductivity Solvent evaporation rate Molecular weight | Flow rate Applied voltage Tip to collector distance Collector types Environmental temperature and humidity |
| Dry-spinning | Polymer concentration Molecular weight Solvent | Spinneret size Drawing ratio Take-up speed Environmental temperature and humidity |
| Wet/Dry-jet-spinning | Polymer concentration Molecular weight Solvent Viscosity | Spinneret size Air gap Coagulation bath Drawing ratio Take-up speed Environmental temperature and humidity |
| Process | Spinning Method | Tenacity (cN/dTex) | Elongation (%) | References |
|---|---|---|---|---|
| Viscose (NaOH/CS2) | Wet-spinning | 1.80 (dry) | 18.00 (dry) | [262] |
| 1.00 (wet) | 21.60 (wet) | |||
| 2.07 (dry) | 19.20 (dry) | [263] | ||
| 0.75 (wet) | 12.50 (wet) | |||
| 2.15 | 22.60 | [264] | ||
| 1.77–2.30 (dry) | 17.00–25.00 (dry) | [258] | ||
| 0.88–1.32 (wet) | 21.00–30.00 (wet) | [265] | ||
| Cellulose acetate | Air-jet spinning | 1.06–1.24 (dry) | 25.00–35.00 (dry) | [266] |
| 0.57–0.66 (wet) | 35.00–45.00 (wet) | |||
| Alkali/urea | Wet-spinning | 3.50 (dry) | 8.00 (dry) | [275] |
| 2.50 (wet) | 8.70 (wet) | |||
| 3.43 | 10.20 | [276] | ||
| 2.20 | 1.90 | [277] | ||
| 1.90 | 2.00 | [278] | ||
| NaOH/ZnO | Wet-spinning | 2.36 (dry) | 15.90 (dry) | [271] |
| 0.73 (wet) | 17.80 (wet) | |||
| 2.58 | 12.10 | [279] | ||
| Cuprammonium | Wet-spinning | 2.00 (dry) | 10.00 (dry) | [280] |
| 1.00 (wet) | 20.00 (wet) | |||
| 1.50–2.39 (dry) | 12.00–13.00 (dry) | |||
| 1.41–1.50 (wet) | 26.00–27.00 (wet) | |||
| LiCl/DMAc | Wet-spinning | 4.23 | 7.76 | [284] |
| 1.68–3.27 (dry) | 6.00–12.00 (dry) | [285] | ||
| 0.53–2.30 (wet) | 8.00–18.00 (wet) | |||
| Ionic liquids | Dry-jet wet-spinning | 3.60 (dry) | 10.20 (dry) | [290] |
| 2.70 (wet) | 12.40 (wet) | |||
| 3.85 | 7.90 | [258] | ||
| 3.09–3.62 (dry) | 7.00–8.00 (dry) | [227] | ||
| 2.03–2.74 (wet) | 9.00–12.00 (wet) | [264] | ||
| NMMO | Dry-jet wet-spinning | 4.90 | 4.00 | [259] |
| 4.30 | 13.10 | [264] | ||
| 3.97–4.42 (dry) | 11.00–16.00 (dry) | [286] | ||
| 3.44–3.53 (wet) | 16.00–18.00 (wet) | [258] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vale, A.C.; Leite, L.; Pais, V.; Bessa, J.; Cunha, F.; Fangueiro, R. Extraction of Natural-Based Raw Materials Towards the Production of Sustainable Man-Made Organic Fibres. Polymers 2024, 16, 3602. https://doi.org/10.3390/polym16243602
Vale AC, Leite L, Pais V, Bessa J, Cunha F, Fangueiro R. Extraction of Natural-Based Raw Materials Towards the Production of Sustainable Man-Made Organic Fibres. Polymers. 2024; 16(24):3602. https://doi.org/10.3390/polym16243602
Chicago/Turabian StyleVale, Ana Catarina, Liliana Leite, Vânia Pais, João Bessa, Fernando Cunha, and Raul Fangueiro. 2024. "Extraction of Natural-Based Raw Materials Towards the Production of Sustainable Man-Made Organic Fibres" Polymers 16, no. 24: 3602. https://doi.org/10.3390/polym16243602
APA StyleVale, A. C., Leite, L., Pais, V., Bessa, J., Cunha, F., & Fangueiro, R. (2024). Extraction of Natural-Based Raw Materials Towards the Production of Sustainable Man-Made Organic Fibres. Polymers, 16(24), 3602. https://doi.org/10.3390/polym16243602







