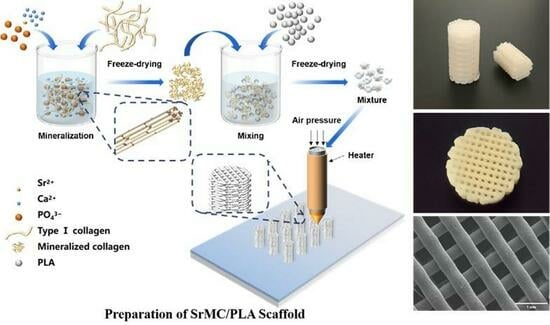
Three-Dimensional Bioprinting of Strontium-Modified Controlled Assembly of Collagen Polylactic Acid Composite Scaffold for Bone Repair
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the MC and SrMC Powder
2.3. Preparation of the MC/PLA Powder and SrMC/PLA Powder
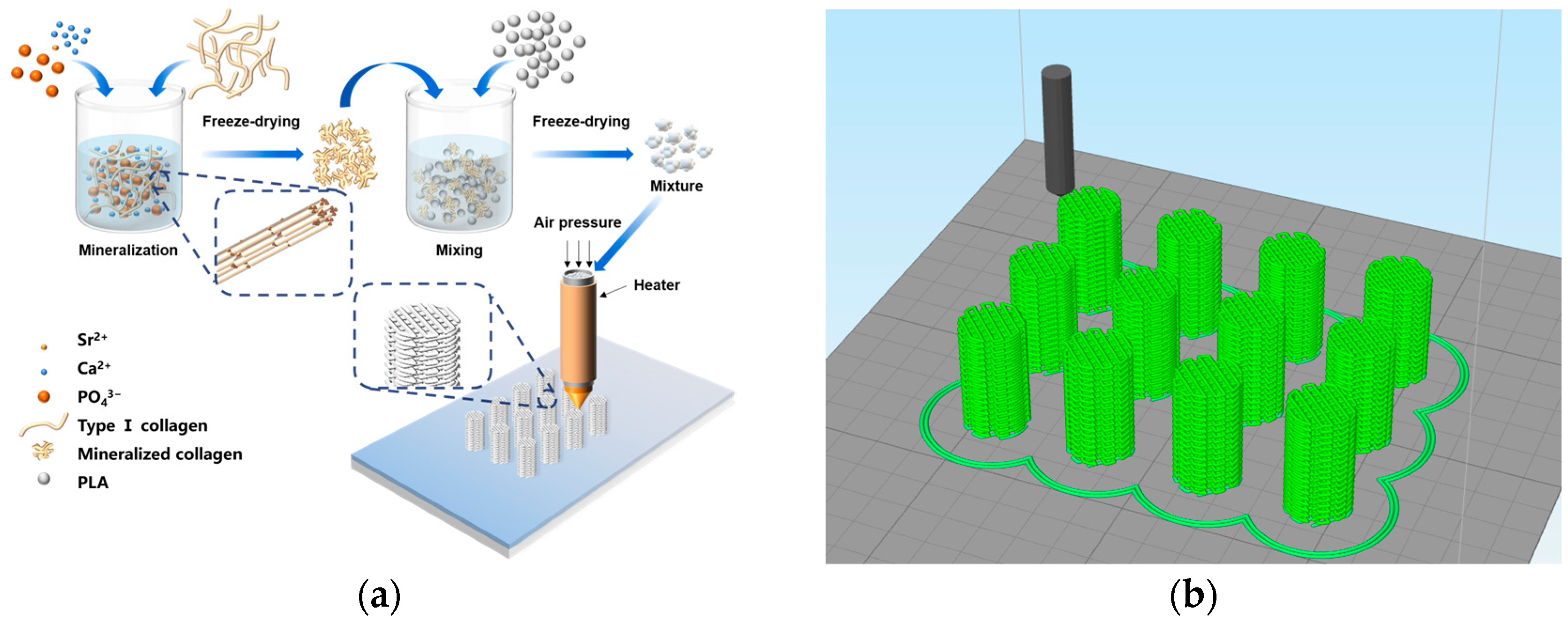
2.4. Preparation of the MC/PLA Scaffold and SrMC/PLA Scaffold
2.5. Material Characterization
2.6. In Vitro Cell Toxicity
2.7. Biocompatibility Analysis
2.8. Impact on Osteoclast Differentiation
2.9. Statistical Analysis
3. Results and Discussion
3.1. Formation Mechanism
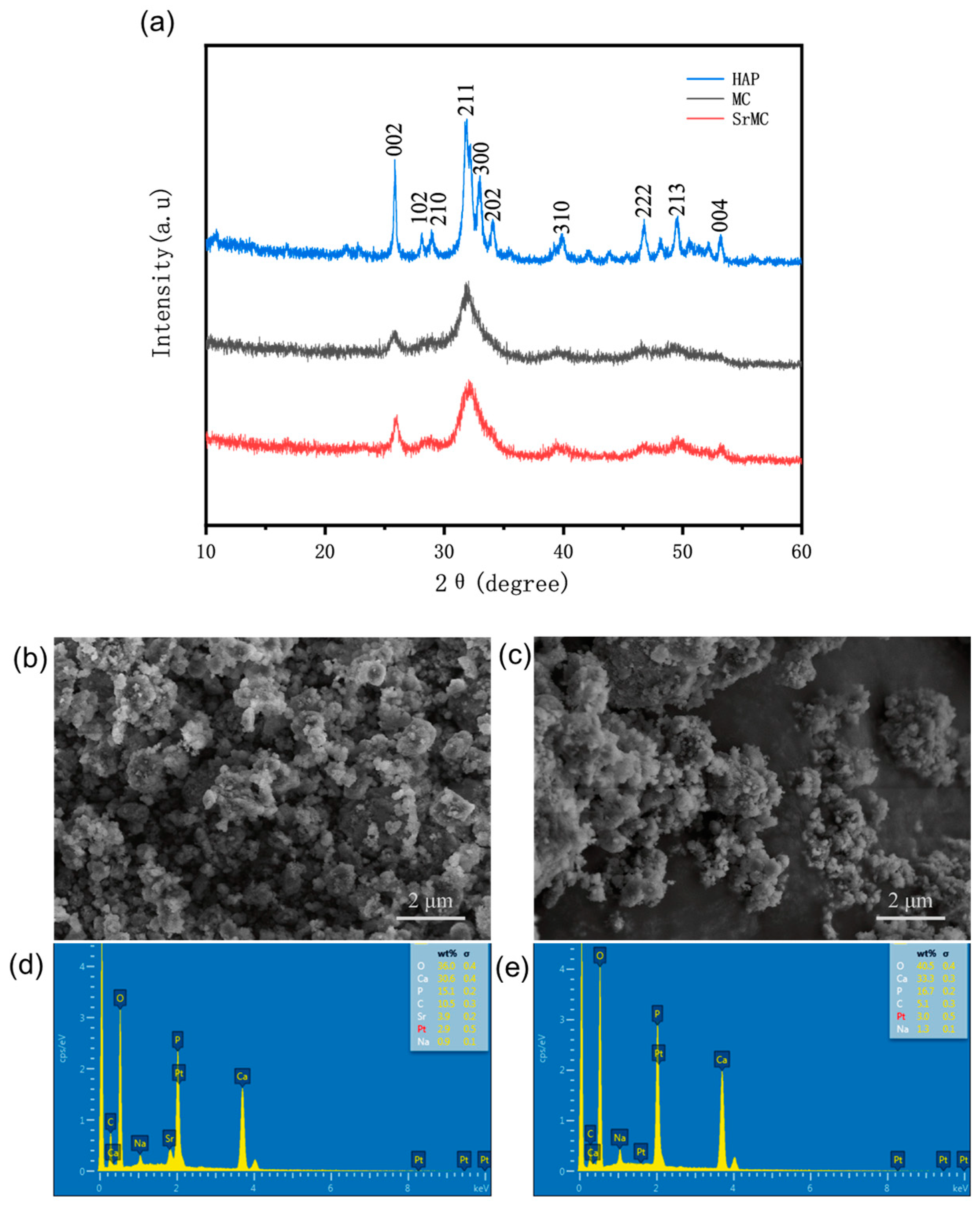
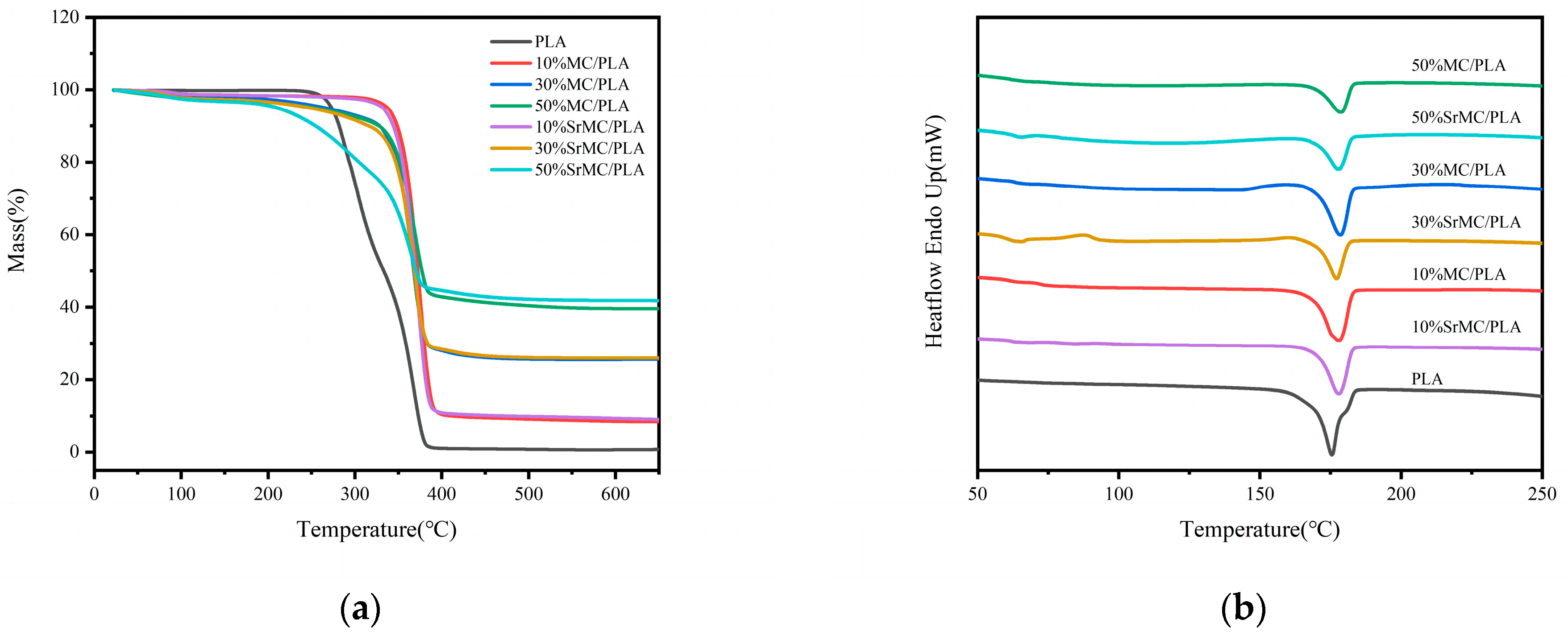
3.2. Characterization Analysis
3.3. Mechanical Properties
3.4. In Vitro Cell Toxicity
3.5. Cell Biocompatibility
3.6. Impact on Osteoclast Differentiation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C 2020, 110, 110698. [Google Scholar] [CrossRef]
- Han, Y.; Gomez, J.; Hua, R.; Xiao, P.W.; Gao, W.; Jiang, J.X.; Wang, X.D. Removal of glycosaminoglycans affects the in situ mechanical behavior of extrafibrillar matrix in bone. J. Mech. Behav. Biomed. Mater. 2021, 123, 104766. [Google Scholar] [CrossRef]
- Wang, X.D.; Xu, J.; Ge, X.Y. Regulation of Mineralized Collagen and Microstructure on Differentiation of Bone Marrow Mesenchymal Stem Cells. Indian J. Pharm. Sci. 2020, 82, 14. [Google Scholar]
- Ma, Y.F.; Hu, N.; Liu, J.; Zhai, X.Y.; Wu, M.M.; Hu, C.S.; Li, L.; Lai, Y.X.; Pan, H.B.; Lu, W.W.; et al. Three-Dimensional Printing of Biodegradable Piperazine-Based Polyurethane-Urea Scaffolds with Enhanced Osteogenesis for Bone Regeneration. ACS Appl. Mater. Inter. 2019, 11, 9415–9424. [Google Scholar] [CrossRef] [PubMed]
- He, Y.Z.; Jin, Y.H.; Ying, X.X.; Wu, Q.; Yao, S.L.; Li, Y.Y.; Liu, H.Y.; Ma, G.W.; Wang, X.M. Development of an antimicrobial peptide-loaded mineralized collagen bone scaffold for infective bone defect repair. Regen. Biomater. 2020, 7, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yuan, Y.; Liu, L.; Leung, Y.-S.; Chen, Y.; Guo, Y.; Chai, Y.; Chen, Y. 3D printing of hydroxyapatite/tricalcium phosphate scaffold with hierarchical porous structure for bone regeneration. Bio-Des. Manuf. 2020, 3, 15–29. [Google Scholar] [CrossRef]
- Fayyazbakhsh, F.; Solati-Hashjin, M.; Keshtkar, A.; Shokrgozar, M.A.; Dehghan, M.M.; Larijani, B. Novel layered double hydroxides-hydroxyapatite/gelatin bone tissue engineering scaffolds: Fabrication, characterization, and in vivo study. Mater. Sci. Eng. C 2017, 76, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef]
- Fu, S.Z.; Ni, P.Y.; Wang, B.Y.; Chu, B.; Peng, J.; Zheng, L.; Zhao, X.; Luo, F.; Wei, Y.; Qian, Z. In vivo biocompatibility and osteogenesis of electrospun poly (ε-caprolactone)–poly (ethylene glycol)–poly (ε-caprolactone)/nano-hydroxyapatite composite scaffold. Biomaterials 2012, 33, 8363–8371. [Google Scholar] [CrossRef]
- Ma, Z.; Li, S.; Zhu, X. Anisotropic Bone Remodelling Model Based on (Wolff’s) Law. Chin. J. Biomed. Eng. 2007, 26, 908–911. [Google Scholar]
- Li, Z.; Du, T.; Ruan, C.; Niu, X. Bioinspired mineralized collagen scaffolds for bone tissue engineering. Bioact. Mater. 2021, 6, 1491–1511. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.J.; Qiu, Z.Y.; Wu, J.J.; Kong, X.D.; Weng, X.S.; Cui, F.Z.; Wang, X.M. Osteogenic Differentiation Gene Expression Profiling of hMSCs on Hydroxyapatite and Mineralized Collagen. Tissue Eng. Part A 2016, 22, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.-Z.; Li, Y.; Ge, J. Self-assembly of mineralized collagen composites. Mater. Sci. Eng. R Rep. 2007, 57, 1–27. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.M.; Tian, L.L.; Cheng, Z.J.; Cui, F.Z. In situ remineralizaiton of partially demineralized human dentine mediated by a biomimetic non-collagen peptide. Soft Matter 2011, 7, 9673–9680. [Google Scholar] [CrossRef]
- Liao, S.S.; Cui, F.Z. In vitro and in vivo degradation of mineralized collagen-based composite scaffold: Nanohydroxyapatite/collagen/poly(L-lactide). Tissue Eng. 2004, 10, 73–80. [Google Scholar] [CrossRef]
- Turekian, K.K.; Kulp, J.L. Strontium content of human bones. Science 1956, 124, 405–407. [Google Scholar] [CrossRef]
- Marx, D.; Yazdi, A.R.; Papini, M.; Towler, M. A review of the latest insights into the mechanism of action of strontium in bone. Bone Rep. 2020, 12, 100273. [Google Scholar] [CrossRef]
- Shorr, E.; Carter, A. The Usefulness of Strontium as an Adjunct to Calcium in Remineralisation. Bull. Hosp. Jt. Dis. 1952, 86, 59–66. [Google Scholar]
- Zhang, J.; Zhao, S.; Zhu, Y.; Huang, Y.; Zhu, M.; Tao, C.; Zhang, C. Three-dimensional printing of strontium-containing mesoporous bioactive glass scaffolds for bone regeneration. Acta Biomater. 2014, 10, 2269–2281. [Google Scholar] [CrossRef]
- Zhao, F.; Lei, B.; Li, X.; Mo, Y.; Wang, R.; Chen, D.; Chen, X. Promoting in vivo early angiogenesis with sub-micrometer strontium-contained bioactive microspheres through modulating macrophage phenotypes. Biomaterials 2018, 178, 36–47. [Google Scholar] [CrossRef]
- Lin, K.; Xia, L.; Li, H.; Jiang, X.; Pan, H.; Xu, Y.; Lu, W.W.; Zhang, Z.; Chang, J. Enhanced osteoporotic bone regeneration by strontium-substituted calcium silicate bioactive ceramics. Biomaterials 2013, 34, 10028–10042. [Google Scholar] [CrossRef]
- Tovani, C.B.; Oliveira, T.M.; Soares, M.P.R.; Nassif, N.; Fukada, S.Y.; Ciancaglini, P.; Gloter, A.; Ramos, A.P. Strontium calcium phosphate nanotubes as bioinspired building blocks for bone regeneration. ACS Appl. Mater. Interfaces 2020, 12, 43422–43434. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, S.; Zhao, W.; Yang, L.; Yuan, B.; Ioan, V.S.; Iulian, A.V.; Yang, X.; Zhu, X.; Zhang, X. A bioceramic scaffold composed of strontium-doped three-dimensional hydroxyapatite whiskers for enhanced bone regeneration in osteoporotic defects. Theranostics 2020, 10, 1572. [Google Scholar] [CrossRef]
- Ye, Z.; Qi, Y.; Zhang, A.; Karels, B.J.; Aparicio, C. Biomimetic mineralization of fibrillar collagen with strontium-doped hydroxyapatite. ACS Macro Lett. 2023, 12, 408–414. [Google Scholar] [CrossRef]
- Nashchekina, Y.A.; Alexandrova, S.A.; Nikonov, P.O.; Ivankova, E.I.; Yudin, V.E.; Blinova, M.I.; Mikhailova, N.A. Study of the Osteoindictive Properties of Protein-Modified Polylactide Scaffolds. Bull. Exp. Biol. Med. 2019, 167, 164–168. [Google Scholar] [CrossRef]
- Birhanu, G.; Akbari Javar, H.; Seyedjafari, E.; Zandi-Karimi, A.; Telgerd, M.D. An improved surface for enhanced stem cell proliferation and osteogenic differentiation using electrospun composite PLLA/P123 scaffold. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Kosowska, K.; Domalik-Pyzik, P.; Krok-Borkowicz, M.; Chłopek, J. Polylactide/hydroxyapatite nonwovens incorporated into chitosan/graphene materials hydrogels to form novel hierarchical scaffolds. Int. J. Mol. Sci. 2020, 21, 2330. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, B.; Owen, R.; Bahmaee, H.; Wally, Z.; Rao, P.S.; Reilly, G.C. Composite porous scaffold of PEG/PLA support improved bone matrix deposition in vitro compared to PLA-only scaffolds. J. Biomed. Mater. Res. Part A 2018, 106, 1334–1340. [Google Scholar] [CrossRef] [PubMed]
- GB/T 16886.5-2011; National Food Safety Standard—Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. National Medical Products Administration: Beijing, China, 2011.
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H.; Shima, N.; Nakagawa, N.; Mochizuki, S.-I.; Yano, K.; Fujise, N.; Sato, Y.; Goto, M.; Yamaguchi, K.; Kuriyama, M.; et al. Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): A mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology 1998, 139, 1329–1337. [Google Scholar] [CrossRef]
- Khalaf, R.M.; Almudhi, A.A. The effect of vitamin D deficiency on the RANKL/OPG ratio in rats. J. Oral Biol. Craniofacial Res. 2022, 12, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Gómez, S.; Vlad, M.D.; López, J.; Fernández, E. Design and properties of 3D scaffolds for bone tissue engineering. Acta Biomater. 2016, 42, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Quade, M.; Vater, C.; Schlootz, S.; Bolte, J.; Langanke, R.; Bretschneider, H.; Gelinsky, M.; Goodman, S.B.; Zwingenberger, S. Strontium enhances BMP-2 mediated bone regeneration in a femoral murine bone defect model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Pallela, R.; Bhatnagar, I.; Kim, S.K. Chitosan-amylopectin/hydroxyapatite and chitosan-chondroitin sulphate/hydroxyapatite composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2012, 51, 1033–1042. [Google Scholar] [CrossRef]
- Won, J.E.; Yun, Y.R.; Jang, J.H.; Yang, S.H.; Kim, J.H.; Chrzanowski, W.; Wall, I.B.; Knowles, J.C.; Kim, H.-W. Multifunctional and stable bone mimic proteinaceous matrix for bone tissue engineering. Biomaterials 2015, 56, 46–57. [Google Scholar] [CrossRef]






| Cell Culture Duration | Group | OD Value | RGR Value | Cytotoxicity Classification |
|---|---|---|---|---|
| 1 | blank control | 0.6003 | 100.0% | 0 |
| 10% MC/PLA | 0.7113 | 118.5% | 0 | |
| 30% MC/PLA | 0.6452 | 107.5% | 0 | |
| 50% MC/PLA | 0.6240 | 104.0% | 0 | |
| 10% SrMC/PLA | 0.6382 | 106.3% | 0 | |
| 30% SrMC/PLA | 0.5697 | 94.9% | 1 | |
| 50% SrMC/PLA | 0.5415 | 90.2% | 1 | |
| 3 | blank control | 0.8661 | 100.0% | 0 |
| 10% MC/PLA | 0.8913 | 102.9% | 0 | |
| 30% MC/PLA | 0.7468 | 86.2% | 1 | |
| 50% MC/PLA | 0.6928 | 80.0% | 1 | |
| 10% SrMC/PLA | 0.7968 | 92.0% | 1 | |
| 30% SrMC/PLA | 0.7461 | 86.1% | 1 | |
| 50% SrMC/PLA | 0.7969 | 92.0% | 1 | |
| 5 | blank control | 1.4585 | 100.0% | 0 |
| 10% MC/PLA | 2.1571 | 147.9% | 0 | |
| 30% MC/PLA | 1.7979 | 123.3% | 0 | |
| 50% MC/PLA | 1.6432 | 112.7% | 0 | |
| 10% SrMC/PLA | 1.4741 | 101.1% | 0 | |
| 30% SrMC/PLA | 1.7311 | 118.7% | 0 | |
| 50% SrMC/PLA | 1.4585 | 100.0% | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, W.; Xie, W.; Hu, K.; Yang, Z.; Han, L.; Li, L.; Qi, Y.; Wei, Y. Three-Dimensional Bioprinting of Strontium-Modified Controlled Assembly of Collagen Polylactic Acid Composite Scaffold for Bone Repair. Polymers 2024, 16, 498. https://doi.org/10.3390/polym16040498
Sun W, Xie W, Hu K, Yang Z, Han L, Li L, Qi Y, Wei Y. Three-Dimensional Bioprinting of Strontium-Modified Controlled Assembly of Collagen Polylactic Acid Composite Scaffold for Bone Repair. Polymers. 2024; 16(4):498. https://doi.org/10.3390/polym16040498
Chicago/Turabian StyleSun, Weiwei, Wenyu Xie, Kun Hu, Zongwen Yang, Lu Han, Luhai Li, Yuansheng Qi, and Yen Wei. 2024. "Three-Dimensional Bioprinting of Strontium-Modified Controlled Assembly of Collagen Polylactic Acid Composite Scaffold for Bone Repair" Polymers 16, no. 4: 498. https://doi.org/10.3390/polym16040498
APA StyleSun, W., Xie, W., Hu, K., Yang, Z., Han, L., Li, L., Qi, Y., & Wei, Y. (2024). Three-Dimensional Bioprinting of Strontium-Modified Controlled Assembly of Collagen Polylactic Acid Composite Scaffold for Bone Repair. Polymers, 16(4), 498. https://doi.org/10.3390/polym16040498