Amorphous Elastomeric Ultra-High Molar Mass Polypropylene in High Yield by Half-Titanocene Catalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Measurements
2.3. Synthesis of (Me3SiC5H4)TiCl2(N=CtBu2) (3)
2.4. Polymerization Procedure
3. Results and Discussion
3.1. Polymerizaton
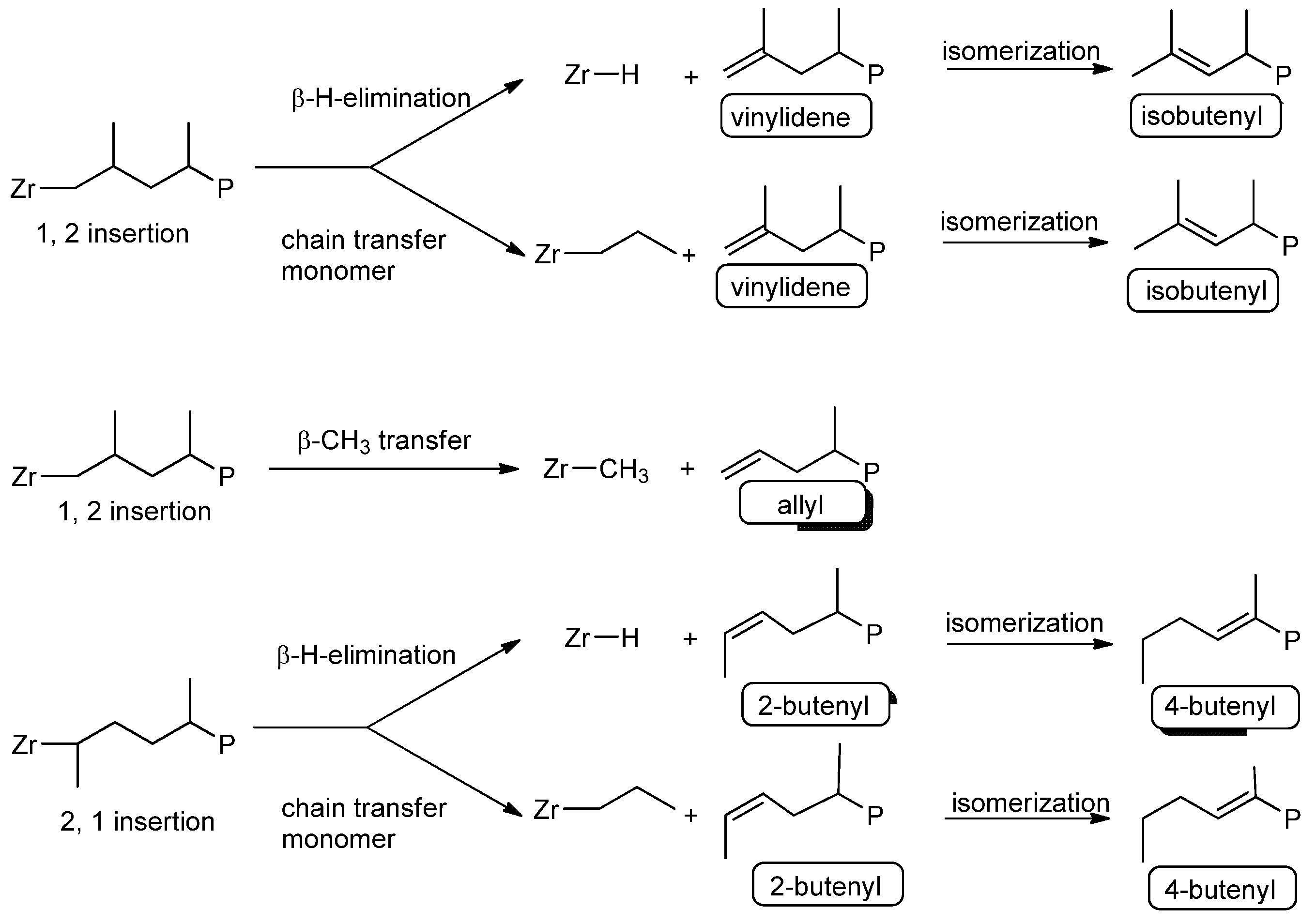
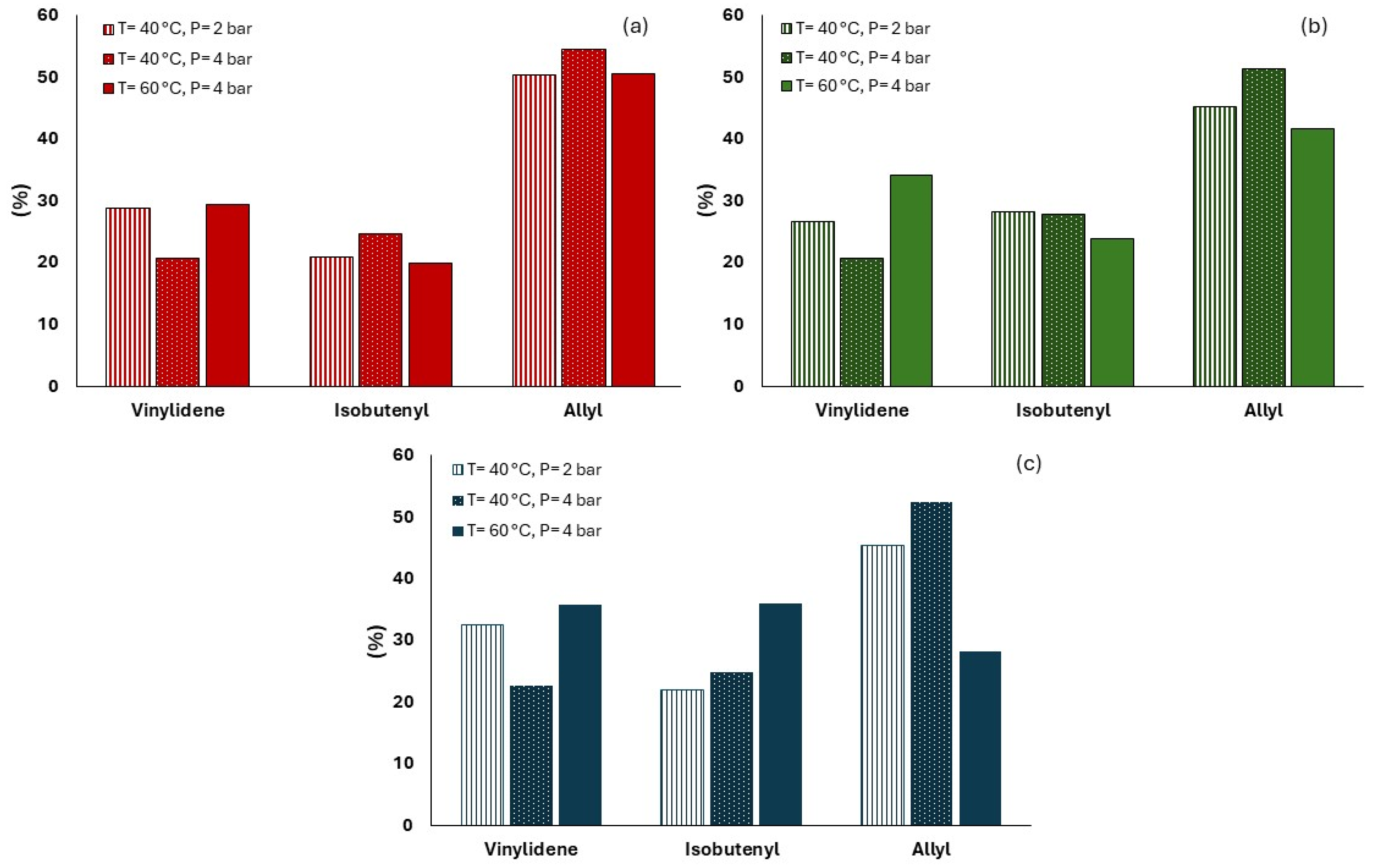
3.2. Microstructure
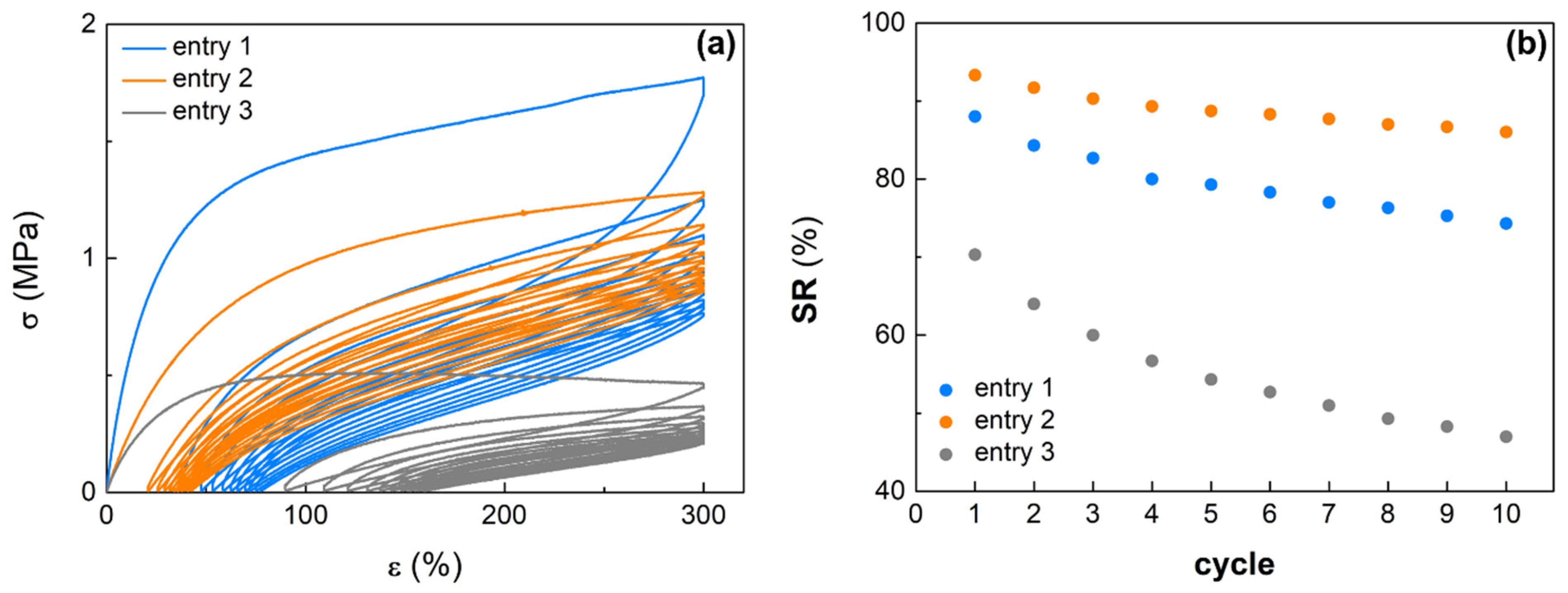
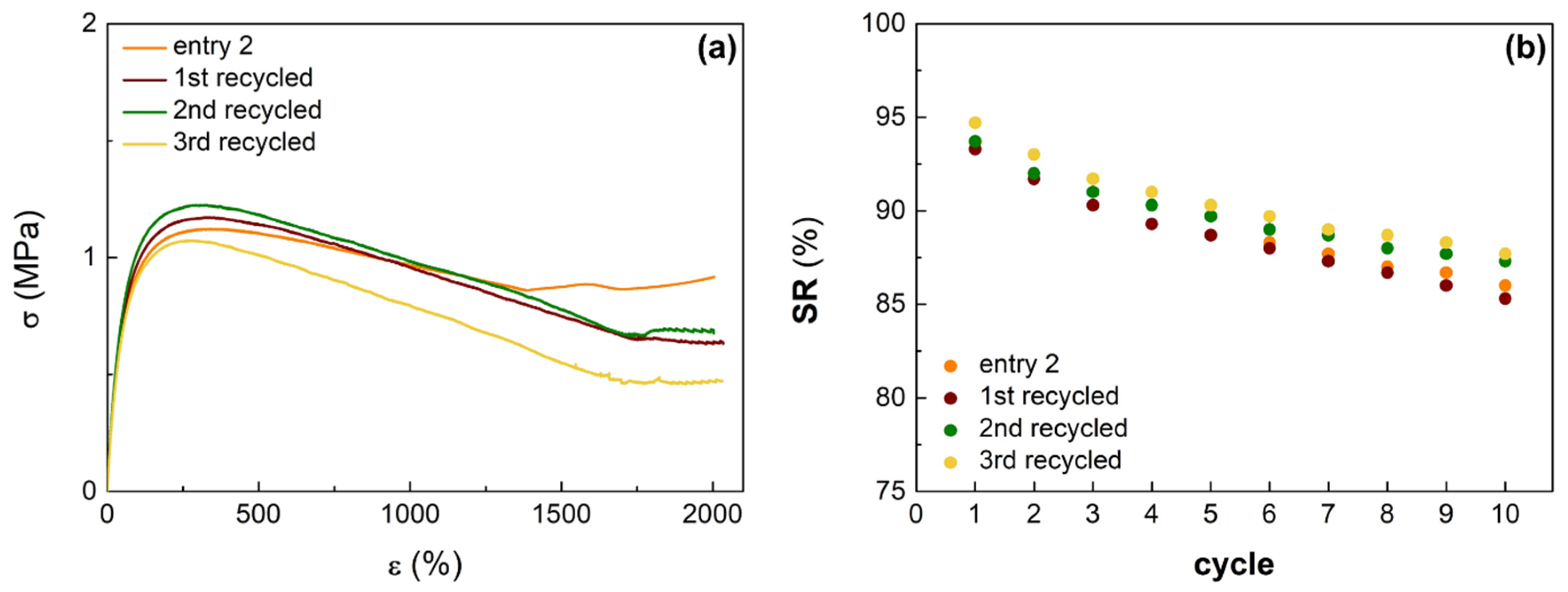
3.3. Mechanical Properties and Recyclibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ewen, J.A. Mechanisms of stereochemical control in propylene polymerizations with soluble Group 4B metallocene/methylalumoxane catalysts. J. Am. Chem. Soc. 1984, 106, 6355–6364. [Google Scholar] [CrossRef]
- Kaminsky, W. Stereoselektive polymerisation von olefinen mit homogenen, chiralen ziegler-natta-katalysatoren. Angew. Makromol. Chem. 1986, 145, 149–160. [Google Scholar] [CrossRef]
- Brintzinger, H.H.; Fischer, D.; Mülhaupt, R.; Rieger, B.; Waymouth, R.M. A Tailor-made metallocene for the copolymerization of ethene with bulky cycloalkenes. Angew. Chem. Int. Ed. Engl. 1995, 34, 1143–1170. [Google Scholar] [CrossRef]
- Kaminsky, W. New polymers by metallocene catalysis. Macromol. Chem. Phys. 1996, 197, 3907–3945. [Google Scholar] [CrossRef]
- Kaminsky, W.; Arndt, M. Metallocenes for polymer catalysis. Adv. Polym. Sci. 1997, 127, 143–187. [Google Scholar]
- Spaleck, W.; Antberg, M.; Rohrmann, J.; Winter, A.; Bachmann, B.; Kiprof, P.; Behm, J.; Hermann, W. High molecular-weight polypropylene through specifically designed zirconocene catalysts. Angew. Chem. Int. Ed. Engl. 1992, 31, 1347–1350. [Google Scholar] [CrossRef]
- Razavi, A.; Atwood, J.L. Preparation and crystal structures of the complexes (η5-C5H4CPh2-η5-C13H8) MCl2 (M = Zr, Hf) and the catalytic formation of high molecular weight high tacticity syndiotactic polypropylene. J. Organomet. Chem. 1993, 459, 117–123. [Google Scholar] [CrossRef]
- Resconi, L.; Cavallo, L.; Fait, A.; Piemontesi, F. Selectivity in propene polymerization with metallocene catalysts. Chem. Rev. 2000, 100, 1253–1346. [Google Scholar] [CrossRef] [PubMed]
- Resconi, L.; Jones, R.L.; Rheingold, A.L.; Yap, G.P.A. High-Molecular-Weight Atactic Polypropylene from Metallocene Catalysts. 1. Me2Si(9-Flu)2ZrX2 (X = Cl, Me). Organometallics 1996, 15, 998–1005. [Google Scholar] [CrossRef]
- Rieger, B.; Troll, C.; Preuschen, J. Ultrahigh Molecular Weight Polypropene Elastomers by High Activity “Dual-Side” Hafnocene Catalysts. Macromolecules 2002, 35, 5742–5743. [Google Scholar] [CrossRef]
- Spaleck, W.; Küber, F.; Winter, A.; Rohrmann, J.; Bachmann, B.; Antberg, M.; Dolle, V.; Paulus, E. The influence of aromatic substituents on the polymerization behaviour of bridged zirconocene catalysts. Organometallics 1994, 13, 954–963. [Google Scholar] [CrossRef]
- McKnight, A.L.; Waymouth, R.M. Group 4 ansa-cyclopentadienyl-amido catalysts for olefin polymerization. Chem. Rev. 1998, 98, 2587–2598. [Google Scholar] [CrossRef]
- Braunschweig, H.; Breitling, F.M. Constrained geometry complexes—Synthesis and applications. Coord. Chem. Rev. 2006, 250, 2691–2720. [Google Scholar] [CrossRef]
- Nomura, K. Half-titanocenes containing anionic ancillary donor ligands as promising new catalysts for precise olefin polymerization. Dalton Trans. 2009, 41, 8811–8823. [Google Scholar] [CrossRef]
- Nomura, K.; Liu, J. Half-titanocenes for precise olefin polymerisation: Effects of ligand substituents and some mechanistic aspects. Dalton Trans. 2011, 40, 7666–7682. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Gibson, V.C.; Wass, D.F. The search for new-generation olefin polymerization catalysts: Life beyond metallocenes. Angew. Chem. Int. Ed. Engl. 1999, 38, 428–447. [Google Scholar] [CrossRef]
- Bolton, P.D.; Mountford, P. Transition metal imido compounds as Ziegler-Natta olefin polymerisation catalysts. Adv. Synth. Catal. 2005, 347, 355–366. [Google Scholar] [CrossRef]
- Nomura, K.; Zhang, S. Design of vanadium complex catalysts for precise olefin polymerization. Chem. Rev. 2011, 111, 2342–2362. [Google Scholar] [CrossRef]
- Makio, H.; Terao, H.; Iwashita, A.; Fujita, T. FI Catalysts for olefin polymerization—A comprehensive treatment. Chem. Rev. 2011, 111, 2363–2449. [Google Scholar] [CrossRef]
- Redshaw, C.; Tang, Y. Tridentate ligands and beyond in group IV metal α-olefin homo-/co-polymerization catalysis. Chem. Soc. Rev. 2012, 41, 4484–4510. [Google Scholar] [CrossRef]
- Wu, Q.; Su, Q.; Ye, L.; Li, G.; Mu, Y. Propylene polymerization to high molecular weight atactic polypropylene and copolymerization with 1-hexene using monocyclopentadienyl titanium catalysts. Dalton Trans. 2010, 39, 2525–2535. [Google Scholar] [CrossRef] [PubMed]
- Collins Rice, C.G.; Buffet, J.-C.; Turner, Z.R.; O’Hare, D. Efficient synthesis of thermoplastic elastomeric amorphous ultra-high molecular weight atactic polypropylene (UHMWaPP). Polym. Chem. 2022, 13, 5597–5603. [Google Scholar] [CrossRef]
- Zhao, W.; Nomura, K. Copolymerizations of Norbornene and Tetracyclododecene with α-Olefins by Half-Titanocene Catalysts: Efficient Synthesis of Highly Transparent, Thermal Resistance Polymers. Macromolecules 2016, 49, 59–70. [Google Scholar] [CrossRef]
- Okabe, M.; Nomura, K. Propylene/Cyclic Olefin Copolymers with Cyclopentene, Cyclohexene, Cyclooctene, Tricyclo[6.2.1.0(2,7)]undeca-4-ene, and Tetracyclododecene: The Synthesis and Effect of Cyclic Structure on Thermal Properties. Macromolecules 2023, 56, 81–91. [Google Scholar] [CrossRef]
- Boggioni, L.; Harakawa, H.; Losio, S.; Nomura, K.; Tritto, I. Synthesis of ethylene-norbornene-1-octene terpolymers with high 1-octene contents, molar masses, and tunable Tg values, in high yields using half-titanocene catalysts. Polym. Chem. 2021, 12, 4372–4383. [Google Scholar] [CrossRef]
- Nomura, K.; Fujita, K.; Fujiki, M. Olefin polymerization by (cyclopentadienyl)(ketimide)-titanium(IV) complexes of the type. J. Mol. Catal. A Chem. 2004, 220, 133–144. [Google Scholar] [CrossRef]
- Miyazawa, A.; Kase, T.; Soga, K. Cis-Specific Living Polymerization of 1,3-Butadiene Catalyzed by Alkyl and Alkylsilyl Substituted Cyclopentadienyltitanium Trichlorides with MAO. Macromolecules 2000, 33, 2796–2800. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F. Structure and physical properties of syndiotactic polypropylene: A highly crystalline thermoplastic elastomer. Prog. Polym. Sci. 2006, 31, 145–237. [Google Scholar] [CrossRef]
- Busico, V.; Cipullo, R. Microstructure of polypropylene. Prog. Polym. Sci. 2001, 26, 443–533. [Google Scholar] [CrossRef]
- Carvill, A.; Zetta, L.; Zannoni, G.; Sacchi, M.C. ansa-Zirconocene-Catalyzed Solution Polymerization of Propene: Influence of Polymerization Conditions on the Unsaturated Chain-End Groups. Macromolecules 1998, 31, 3783–3789. [Google Scholar] [CrossRef]
- Mihailov, M.; Minkova, L. Pecularities of the thermomechanical behaviour of ultra-high molecular weight linear polyethylene and its blends with linear polyethylene of normal molecular weight. Colloid Polym. Sci. 1987, 265, 681–685. [Google Scholar] [CrossRef]
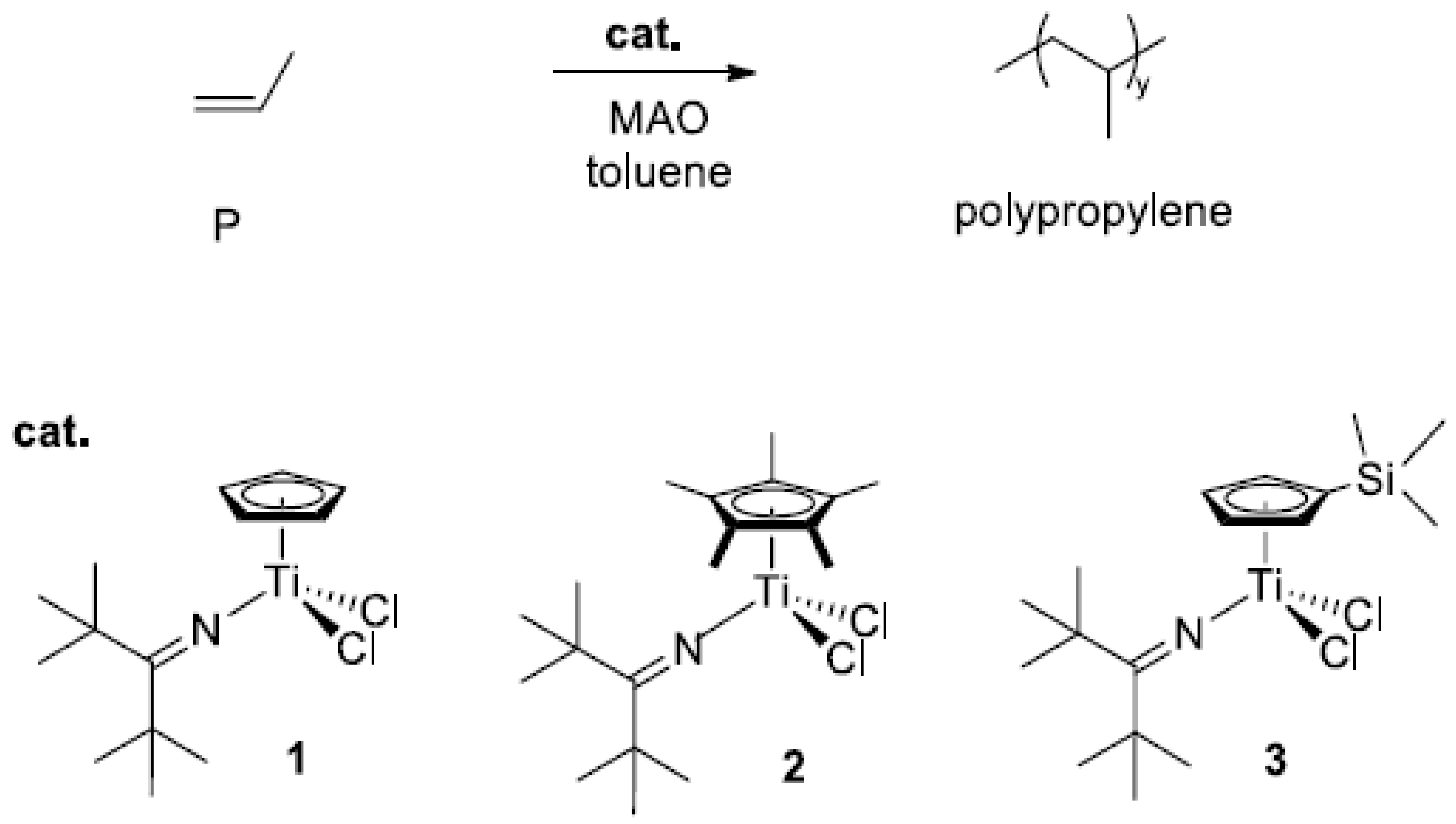


| Entry | Catalyst | T (°C) | P (bar) | Yield (g) | Activity (Kg/(mol-Ti·h) | Mw 2 (Kg/mol) | Ð2 | Tg 3 (°C) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 40 | 2 | 4.06 | 1626 | 1250 | 2.7 | 1 |
| 2 | 40 | 4 | 6.08 | 2433 | 1404 | 1.7 | 1 | |
| 3 | 60 | 4 | 3.85 | 1543 | 663 | 7.3 | -2 | |
| 4 | 2 | 40 | 2 | 1.59 | 627 | 660 | 1.8 | 2 |
| 5 | 40 | 4 | 11.20 | 4480 | 832 | 2.6 | 2 | |
| 6 | 60 | 4 | 12.93 | 5173 | 650 | 1.7 | 0 | |
| 7 | 3 | 40 | 2 | 4.66 | 1862 | 1303 | 4.0 | 0 |
| 8 | 40 | 4 | 9.16 | 3663 | 1240 | 1.5 | 1 | |
| 9 | 60 | 4 | 1.90 | 761 | 627 | 3.7 | -2 |
| Entry | Catalyst | E (MPa) | σmax (MPa) | ε (%) |
|---|---|---|---|---|
| 1 | 1 | 3.2 ± 0.3 | 0.91 ± 0.08 | >2000 |
| 2 | 2.5 ± 0.3 | 1.05 ± 0.10 | >2000 | |
| 3 | 2.3 ± 0.1 | 0.39 ± 0.01 | 915 ± 70 | |
| 4 | 2 | 1.9 ± 0.1 | 0.70 ± 0.06 | 790 ± 99 |
| 5 | 3.1 ± 0.3 | 0.78 ± 0.03 | >2000 | |
| 6 | 2.0 ± 0.3 | 0.56 ± 0.05 | 421 ± 23 | |
| 7 | 3 | 6.0 ± 0.4 | 1.11 ± 0.01 | >2000 |
| 8 | 4.1 ± 0.1 | 1.24 ± 0.08 | >2000 | |
| 9 | 1.3 ± 0.1 | 0.31 ± 0.02 | 483 ± 45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Losio, S.; Bertini, F.; Vignali, A.; Fujioka, T.; Nomura, K.; Tritto, I. Amorphous Elastomeric Ultra-High Molar Mass Polypropylene in High Yield by Half-Titanocene Catalysts. Polymers 2024, 16, 512. https://doi.org/10.3390/polym16040512
Losio S, Bertini F, Vignali A, Fujioka T, Nomura K, Tritto I. Amorphous Elastomeric Ultra-High Molar Mass Polypropylene in High Yield by Half-Titanocene Catalysts. Polymers. 2024; 16(4):512. https://doi.org/10.3390/polym16040512
Chicago/Turabian StyleLosio, Simona, Fabio Bertini, Adriano Vignali, Taiga Fujioka, Kotohiro Nomura, and Incoronata Tritto. 2024. "Amorphous Elastomeric Ultra-High Molar Mass Polypropylene in High Yield by Half-Titanocene Catalysts" Polymers 16, no. 4: 512. https://doi.org/10.3390/polym16040512
APA StyleLosio, S., Bertini, F., Vignali, A., Fujioka, T., Nomura, K., & Tritto, I. (2024). Amorphous Elastomeric Ultra-High Molar Mass Polypropylene in High Yield by Half-Titanocene Catalysts. Polymers, 16(4), 512. https://doi.org/10.3390/polym16040512










