Performance of Full-Component Coal Gasification Fine Slag: High-Value Utilization as Reinforcing Material in Styrene-Butadiene Rubber (ESBR) for Replacing Carbon Black
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Preparation of Ultrafine Coal Gasification Slag (HCGS)
2.3. Preparation of HCGS/ESBR Composites
2.4. Characterization
3. Results and Discussion
3.1. Physicochemical Characteristics of CGS
3.1.1. Basic Characteristics of CGS
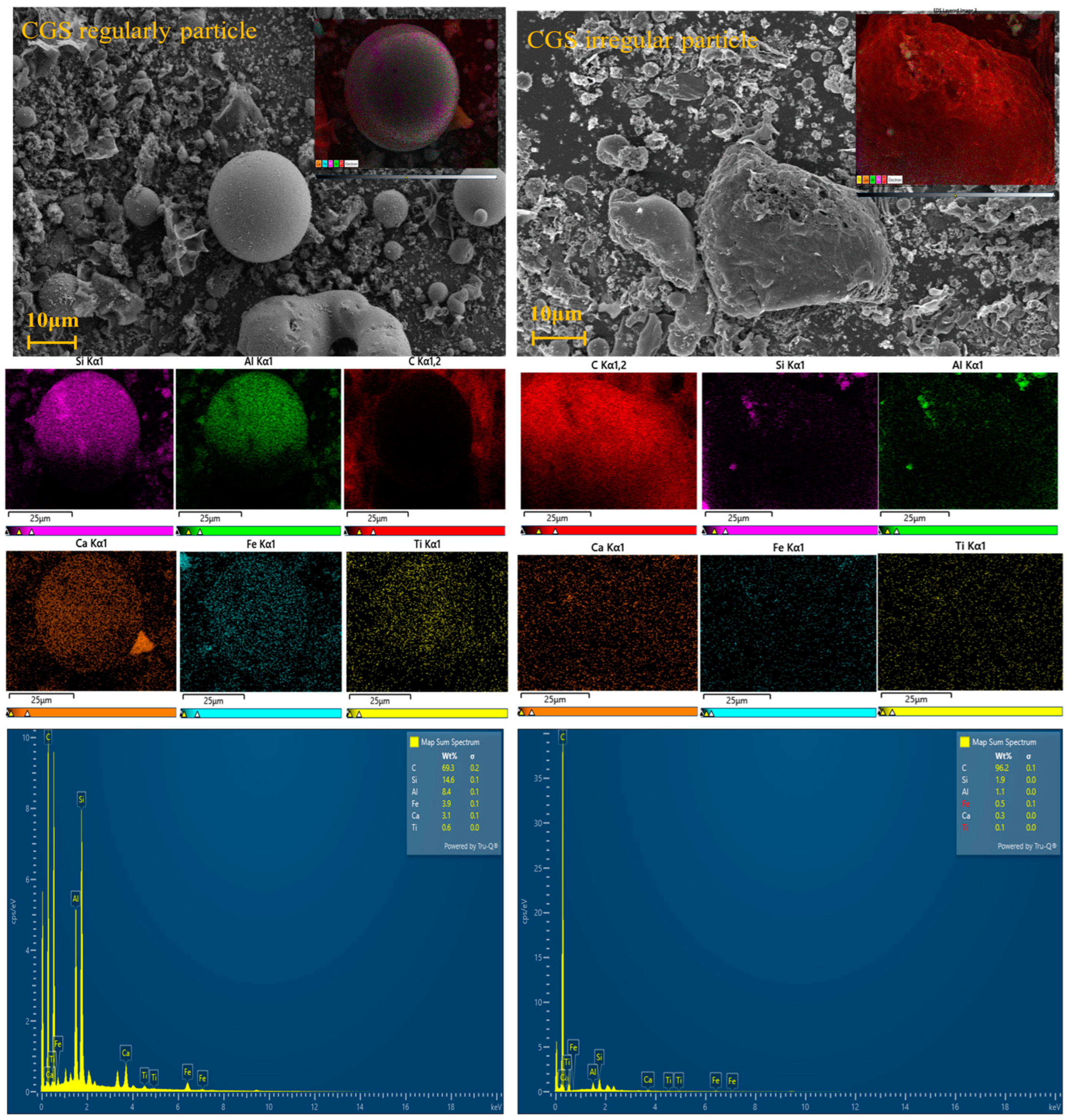
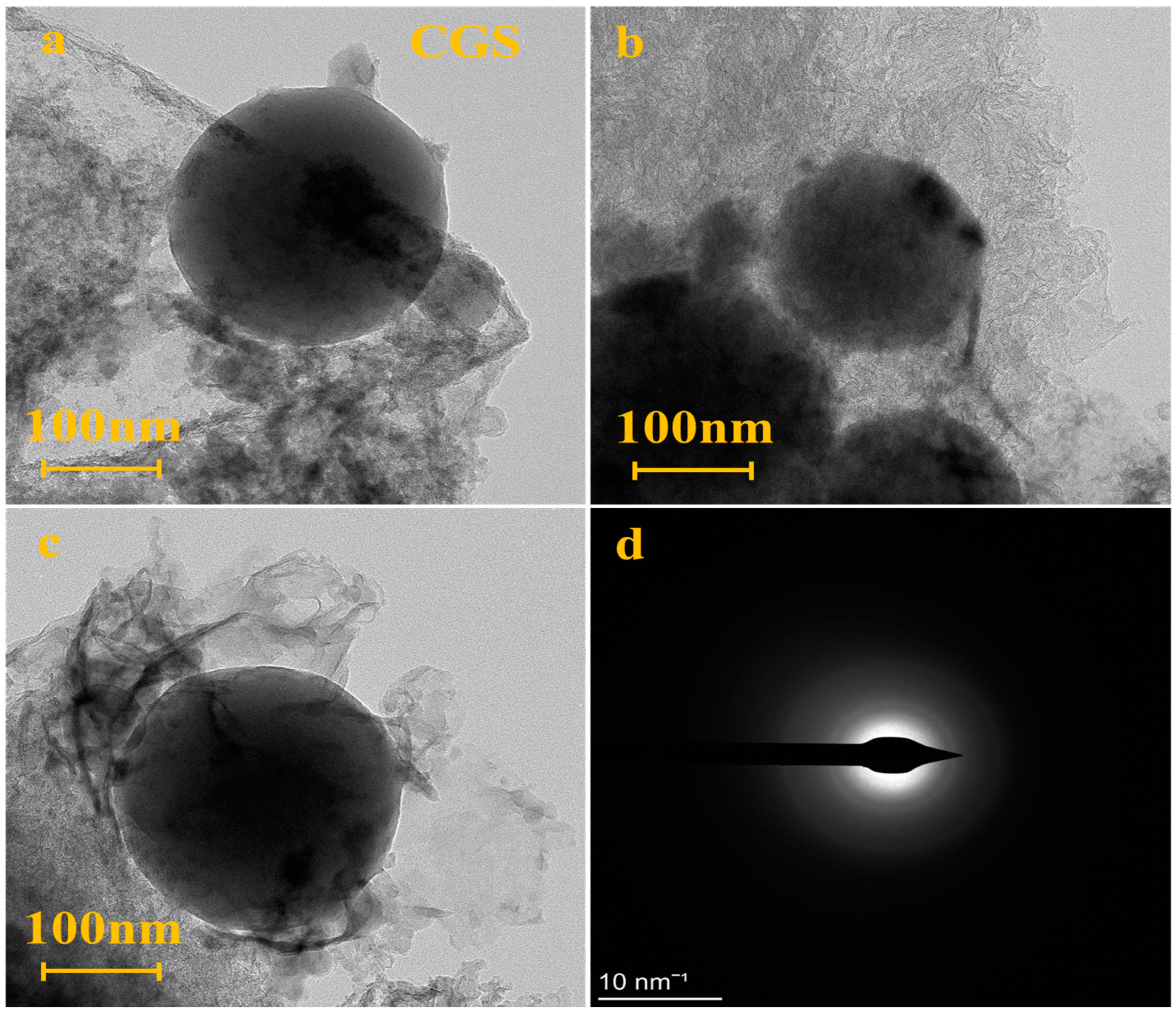
3.1.2. Morphology Analysis
3.2. Orthogonal Experiment for Ultra-Fine, Highly Active Coal Gasification Fine Slag
3.2.1. Design of Orthogonal Experiment
3.2.2. Mechanical Ball Milling Activation and Refinement of Coal Gasification Fine Slag
3.2.3. Range Analysis of Experimental Results
3.2.4. Visual Analysis of Orthogonal Test Efficiency Curve for Mechanical Activation and Refinement by Ball Milling
3.3. Effect of Mechanical Refinement by Ball Milling on Particle Size Distribution and Surface Properties of Highly Active Coal Gasification Slag
3.3.1. Changes in Particle Size Distribution and Surface Morphology before and after Ball Milling
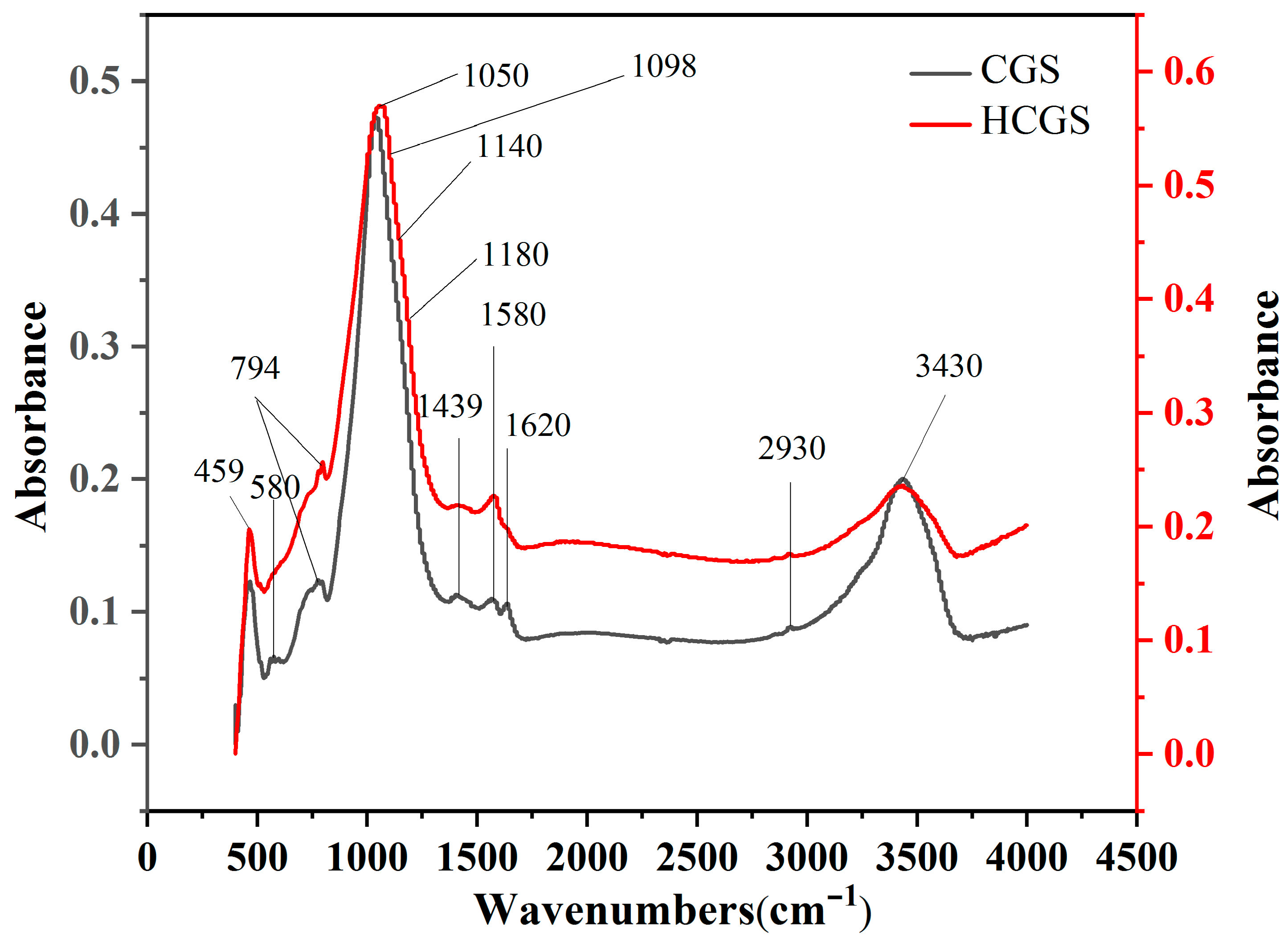
3.3.2. Changes in Surface Properties before and after Ball Milling
3.3.3. Changes in Carbon Morphology and Activity before and after Ball Milling
3.3.4. Stability of Dispersion System after Mechanical Activation by Ball Milling
3.4. Characterization of HCGS-Filled ESBR Composites
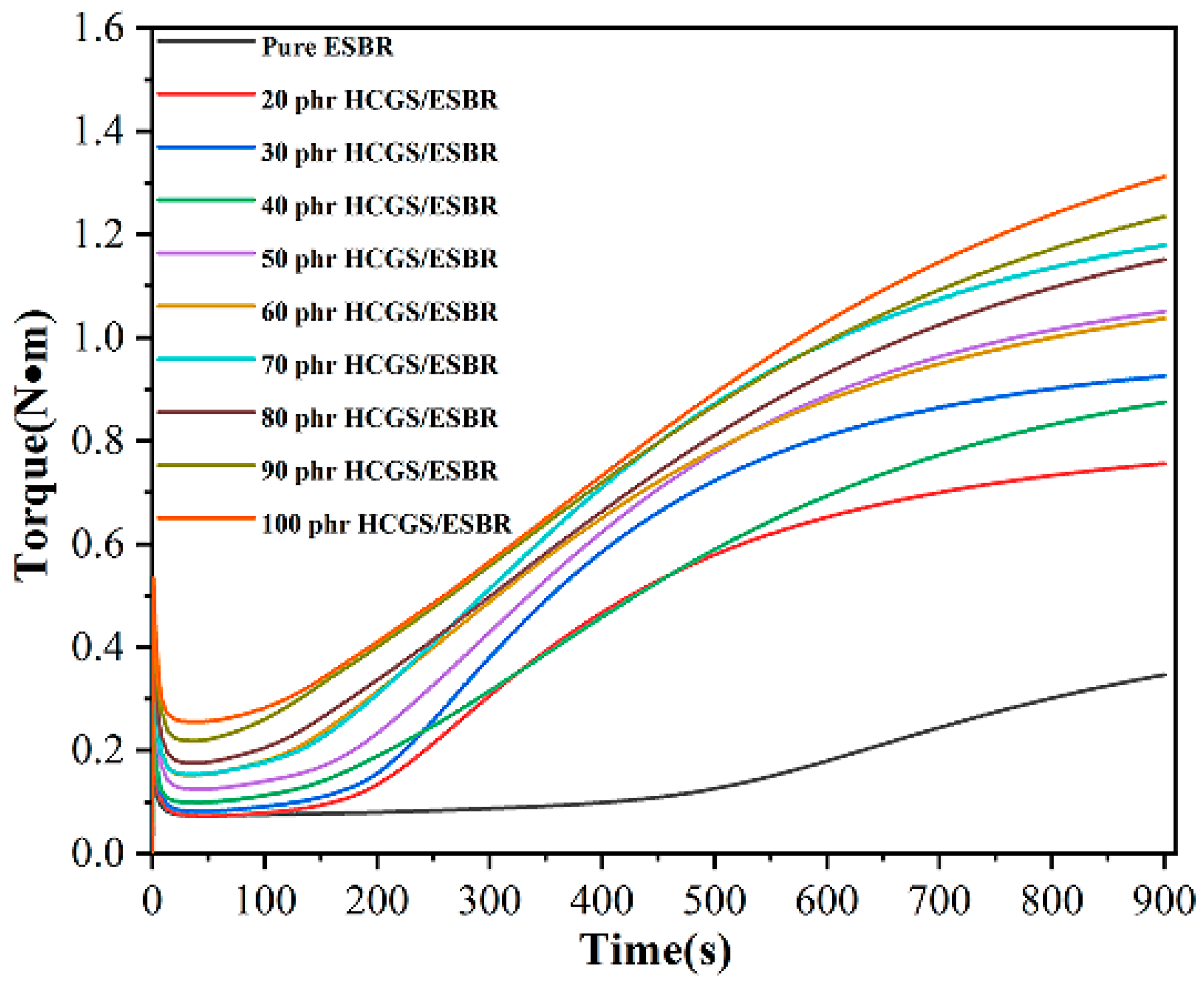
3.4.1. Cure Behaviour
3.4.2. Mechanical Properties of ESBR-Based HCGS
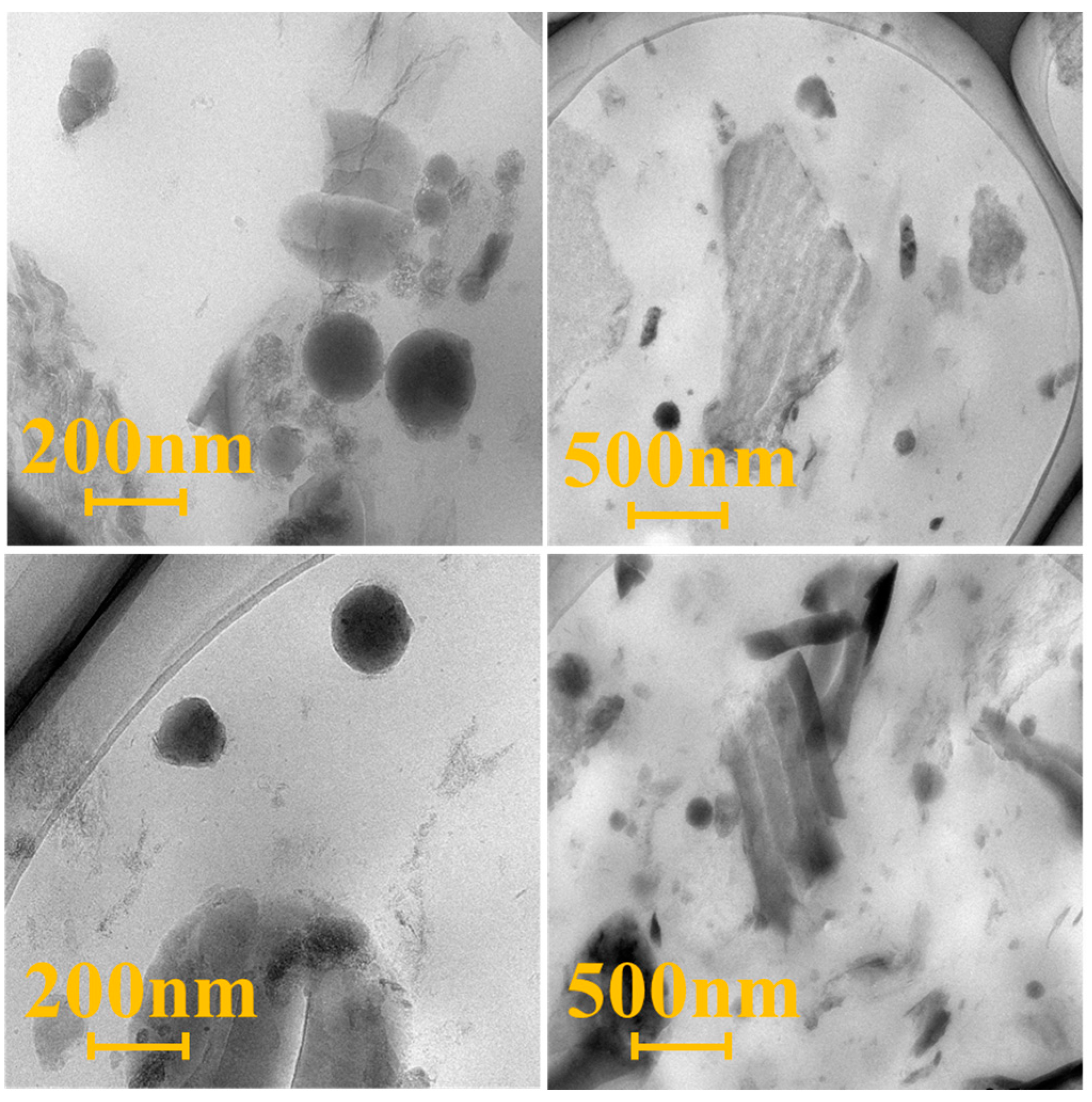
3.4.3. Microstructural Properties of HCGS/ESBR Composite
3.5. Processing Properties and Static Mechanical Properties of HCGS/CB/ESBR Composites with Different Proportions of Carbon Black
3.5.1. Processing Properties of HCGS/CB-Filled ESBR Composites
3.5.2. Mechanical Properties of HCGS/CB-Filled ESBR Composites
3.6. Crosslinking Density and Average Molecular Weight of HCGS/CB Filled SBR Composites
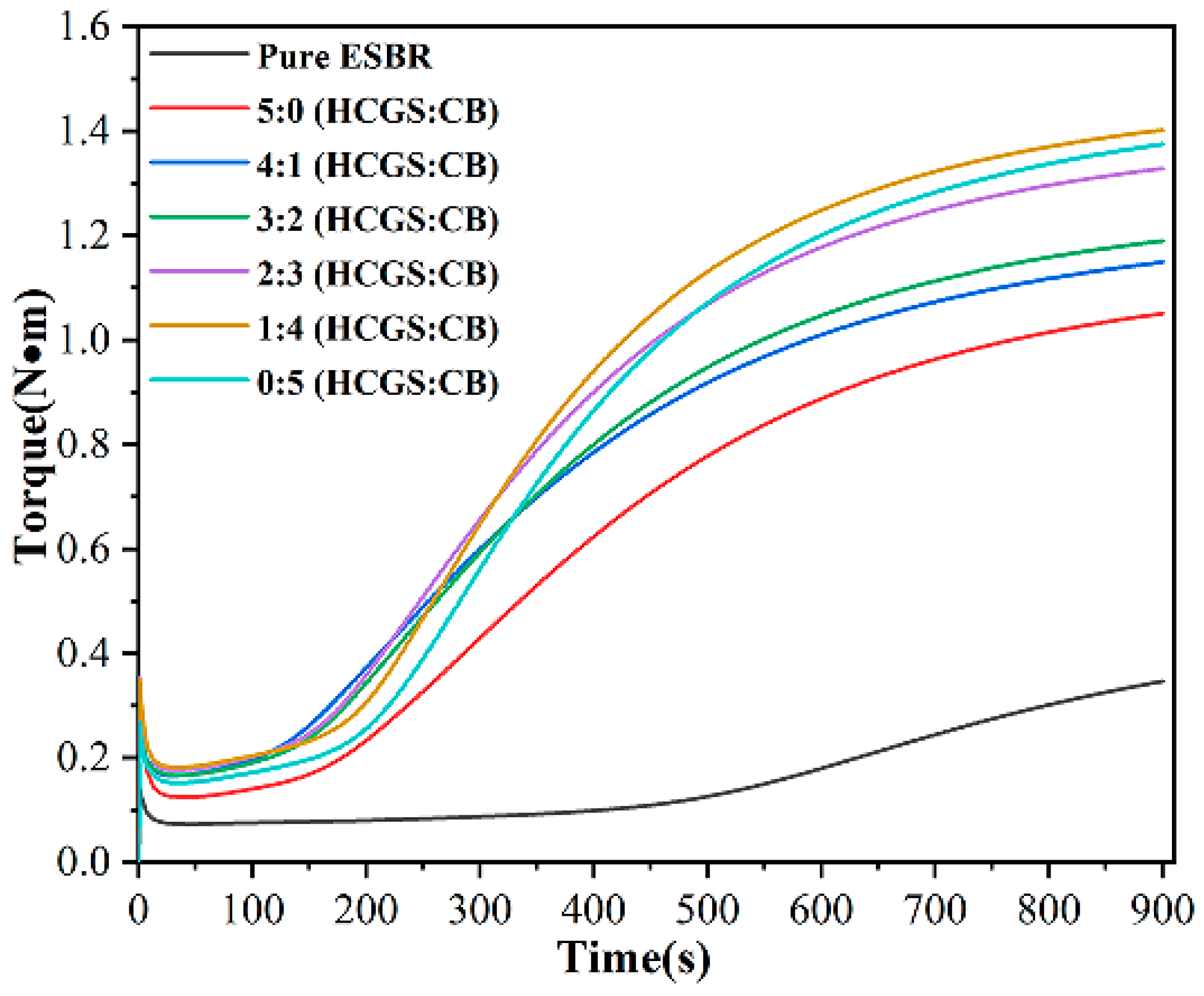
3.7. Vulcanization Kinetics of HCGS/CB Filled SBR Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, Y.; Jiang, J.; Islam, M.A. Elastomers and plastics for resisting erosion attack of abrasive/erosive slurries. Wear 2019, 426, 612–619. [Google Scholar] [CrossRef]
- Pöschl, M.; Vašina, M.; Zádrapa, P.; Měřínská, D.; Žaludek, M. Study of carbon black types in SBR Rubber: Mechanical and vibration damping properties. Materials 2020, 13, 2394. [Google Scholar] [CrossRef] [PubMed]
- Da Costa Labanca, A.R. Carbon black and hydrogen production process analysis. Int. J. Hydrog. Energy 2020, 45, 25698–25707. [Google Scholar] [CrossRef]
- Chen, S.; Chen, J.; Xu, K.; Pan, R.; Peng, Z.; Ma, L.; Wu, S. Effect of coal gangue/carbon black/multiwall carbon nanotubes hybrid fillers on the properties of natural rubber composites. Polym. Comp. 2016, 37, 3083–3092. [Google Scholar] [CrossRef]
- Chai, Z.; Liu, B.; Lv, P.; Bai, Y.; Wang, J.; Song, X.; Su, W.; Yu, G. Recycling of coal gasification fine slag as ultra-high capacity adsorbents for the removal of Rhodamine B dye: Graded synthesis method, kinetics and adsorption mechanism. Fuel 2023, 333, 126318. [Google Scholar] [CrossRef]
- Shi, D.; Zhang, J.; Hou, X.; Li, S.; Li, H.; He, F.; Zhu, G. Occurrence mode and molecular structure model of unburned carbon in coal gasification fine slags. Fuel 2022, 323, 124364. [Google Scholar] [CrossRef]
- Gao, Z.; Han, X.; Wang, G.; Liu, J.; Cui, X.; Zhang, C.; Wang, J. Preparation of activated carbon adsorption materials derived from coal gasification fine slag via low-temperature air activation. Gas Sci. Eng. 2023, 117, 205069. [Google Scholar] [CrossRef]
- Shu, R.; Bai, J.; Guo, F.; Mao, S.; Qiao, Q.; Dong, K.; Qian, L.; Bai, Y. Synthesis of carbon/P-zeolite composites from coal gasification fine slag and studies on adsorption characteristics for methylene blue. Korean J. Chem. Eng. 2023, 40, 1639–1649. [Google Scholar] [CrossRef]
- GB/T 9869-2014; Determination of Vulcanization Characteristics of Rubber Compounds-Disc Oscillating Vulcanization Apparatus Method. China Petroleum and Phemical Industry Association: Beijing, China, 2015.
- GB/T 528-2009; Determination of Tensile Stress-Strain Properties of Vulcanized Rubber or Thermoplastic Rubber. China Petroleum and Phemical Industry Association: Beijing, China, 2009.
- Xiao, K.; Zhang, Y.; Zhang, Y.; Gong, Y. Preparation of stearic acid/halloysite intercalation compound and their reinforcement for styrene butadiene rubber composite. J. Polym. Res. 2022, 29, 451. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J. Statistical mechanics of cross-linked polymer networks I. Rubberlike elasticity. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Wu, J.; Xing, W.; Huang, G.; Li, H.; Tang, M.; Wu, S.; Liu, Y. Vulcanization kinetics of graphene/natural rubber nanocomposites. Polymer 2013, 54, 3314–3323. [Google Scholar] [CrossRef]
- GB/T 212-2008; Industrial Analysis Method of Coal. China Coal Industry Association: Beijing, China, 2008.
- Wu, R.; Lv, P.; Wang, J.; Bai, Y.; Wei, J.; Song, X.; Su, W.; Yu, G. Catalytic upgrading of cow manure pyrolysis vapors over zeolite/carbon composites prepared from coal gasification fine slag: High quality bio-oil obtaining and mechanism investigation. Fuel 2023, 339, 126941. [Google Scholar] [CrossRef]
- Yin, Z.; Peng, Y.; Zhu, Z.; Yu, Z.; Li, T.; Zhao, L.; Xu, J. Experimental study of charge dynamics in a laboratory-scale ball mill. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2017, 232, 3491–3499. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, F.; Yang, H.; Gao, W.; Miao, L. Orthogonal experimental study of soil–rock mixtures under the freeze–thaw cycle environment. Int. J. Pave. Eng. 2019, 22, 1376–1388. [Google Scholar] [CrossRef]
- Ke, C.; Li, X.; Jiang, J. Dynamic response analysis of RC frame against progressive collapse based on orthogonal test. Appl. Sci. 2023, 13, 4317. [Google Scholar] [CrossRef]
- Shu, R.; Qiao, Q.; Guo, F.; Dong, K.; Liu, S.; Xu, L.; Bai, Y.; Zhou, N. Controlled design of Na-P1 zeolite/porous carbon composites from coal gasification fine slag for high-performance adsorbent. Environ. Res. 2023, 217, 114912. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Zhao, P.; Yang, C.; Dong, L.; Bao, W.; Wang, J.; Fan, P. Capture performance and quantum chemistry simulation of composite collectors for coal gasification coarse slag. Energy Sources Part A Recovery Util. Environ. Eff. 2023, 45, 1807–1821. [Google Scholar] [CrossRef]
- Li, M.; Li, P.; Wu, J.; Teng, D.; Zhou, G.; Cao, Y.; Fan, G. Directly application of waste cooking oil on the flotation of coal gasification fine slag. Fuel 2023, 331, 125666. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Shen, G.; Cheng, P.; Zhang, J.; Guo, S. Reduction of graphene oxide via L-ascorbic acid. Chem. Commun. 2010, 46, 1112–1114. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; He, J.; Li, H.; Bai, Y.; Gao, S. ZnFe2O4 nanospheres decorated residual carbon from coal gasification fine slag as an ultra-thin microwave absorber. Fuel 2023, 331, 125811. [Google Scholar] [CrossRef]
- Shen, Y.; Lu, G.; Bai, Y.; Lv, P.; Yong, Z.; Wang, J.; Song, X.; Yan, L.; Yu, G. Structural features of residue carbon formed by gasification of different coal macerals. Fuel 2022, 320, 123918. [Google Scholar] [CrossRef]
- Potgieter-Vermaak, S.; Maledi, N.; Wagner, N.; Van Heerden, J.H.P.; Van Grieken, R.; Potgieter, J.H. Raman spectroscopy for the analysis of coal: A review. J. Raman Spectros. 2011, 42, 123–129. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Reich, S.; Thomsen, C. Raman spectroscopy of graphite. Philos. Trans. A Math. Phys. Eng. Sci. 2004, 362, 2271–2288. [Google Scholar] [CrossRef] [PubMed]
- LÜ, D.-P.; Bai, Y.-H.; Wang, J.-F.; Song, X.-D.; Su, W.-G.; Yu, G.-S.; Zhu, H.; Tang, G.-J. Structural features and combustion reactivity of residual carbon in fine slag from entrained-flow gasification. J. Fuel Chem. Technol. 2021, 49, 129–136. [Google Scholar] [CrossRef]
- Predota, M.; Machesky, M.L.; Wesolowski, D.J. Molecular origins of the zeta potential. Langmuir 2016, 32, 10189–10198. [Google Scholar] [CrossRef] [PubMed]
- Manciu, M.; Manciu, F.S.; Ruckenstein, E. On the surface tension and zeta potential of electrolyte solutions. Adv. Colloid. Interface Sci. 2017, 244, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, Y.; Zhang, Y.; Sun, J. Vulcanization, static mechanical properties, and thermal stability of activated calcium silicate/styrene-butadiene rubber composites prepared via a latex compounding method. J. Appl. Polym. Sci. 2021, 139, 51462. [Google Scholar] [CrossRef]





| Component | ESBR | HCGS/ESBR | ZnO | SA | NS | Sulfur |
|---|---|---|---|---|---|---|
| Content/phr | 100.00 | Variable | 3.00 | 1.00 | 1.00 | 1.75 |
| Sample (mm) | Mad% | Ad% | Vdaf% | Fcad% |
|---|---|---|---|---|
| Raw CGFS | 0.75 | 66.88 | 10.4 | 29.45 |
| Sample | Na2O | MgO | Al2O3 | SiO2 | SO3 | CaO | TiO2 | Fe2O3 | LOI815/% |
|---|---|---|---|---|---|---|---|---|---|
| CGFS | 0.23 | 1.45 | 19.6 | 47.05 | 4.90 | 6.77 | 1.25 | 11.65 | 35.3 |
| Factors | Material:Ball Ratio | Ball Milling Time (h) | Rotation (rpm) |
|---|---|---|---|
| Value level | 1:2 | 1 | 300 |
| 1:3 | 2 | 400 | |
| 1:4 | 3 | 500 | |
| 1:5 | 4 | 600 | |
| 1:6 | 5 | 700 |
| Experimental Scheme | Experimental Results | |||||
|---|---|---|---|---|---|---|
| Serial Number | A Material/Ball Ratio | B (rpm) Rotation | C (h) Ball Milling Time | D10 | D50 | D90 |
| 1 | 1:2 | 300 | 1 | 2.38 | 15.25 | 45.3 |
| 2 | 1:2 | 400 | 2 | 1.53 | 8.56 | 22.86 |
| 3 | 1:2 | 500 | 3 | 0.93 | 4.25 | 13.34 |
| 4 | 1:2 | 600 | 4 | 0.94 | 3.46 | 13.31 |
| 5 | 1:2 | 700 | 5 | 0.89 | 2.99 | 16.02 |
| 6 | 1:3 | 400 | 1 | 1.42 | 9.39 | 25.43 |
| 7 | 1:3 | 500 | 2 | 0.97 | 4.48 | 15.65 |
| 8 | 1:3 | 600 | 3 | 0.89 | 3.02 | 13.32 |
| 9 | 1:3 | 700 | 4 | 0.83 | 2.37 | 19.28 |
| 10 | 1:3 | 300 | 5 | 1.07 | 5.60 | 32.55 |
| 11 | 1:4 | 500 | 1 | 1.01 | 5.34 | 15.12 |
| 12 | 1:4 | 600 | 2 | 0.92 | 3.27 | 14.49 |
| 13 | 1:4 | 700 | 3 | 0.82 | 2.27 | 10.59 |
| 14 | 1:4 | 300 | 4 | 0.91 | 4.37 | 15.05 |
| 15 | 1:4 | 400 | 5 | 0.89 | 2.98 | 13.64 |
| 16 | 1:5 | 600 | 1 | 0.89 | 4.04 | 16.34 |
| 17 | 1:5 | 700 | 2 | 0.92 | 3.44 | 38.77 |
| 18 | 1:5 | 300 | 3 | 0.93 | 4.50 | 15.47 |
| 19 | 1:5 | 400 | 4 | 0.85 | 2.51 | 10.73 |
| 20 | 1:5 | 500 | 5 | 0.84 | 6.65 | 17.51 |
| 21 | 1:6 | 700 | 1 | 0.90 | 4.32 | 22.88 |
| 22 | 1:6 | 300 | 2 | 1.03 | 3.99 | 21.73 |
| 23 | 1:6 | 400 | 3 | 0.87 | 2.89 | 18.26 |
| 24 | 1:6 | 500 | 4 | 0.79 | 2 | 7.1 |
| 25 | 1:6 | 600 | 5 | 0.76 | 1.77 | 12.35 |
| D90 | K1 | 22.17 | 26.02 | 25.01 |
| K2 | 21.25 | 18.18 | 22.7 | |
| K3 | 13.78 | 13.74 | 14.20 | |
| K4 | 19.76 | 13.96 | 13.09 | |
| K5 | 16.46 | 21.51 | 18.41 | |
| Poor R | 8.39 | 12.28 | 11.92 |
| Wavenumber/cm−1 | Peak Assignment |
|---|---|
| 459 | Al-O bending vibration peak [18] |
| 580 | Vibration of C–S–C skeleton [18] |
| 794 | Si–O–Si [19] |
| 1050 | C–O–C [19] |
| 1098 | Stretching vibration of Si–O–Al [20] |
| 1140 | C–O from alkoxides in RC [21] |
| 1180 | Characteristic vibration peak of C–S [18] |
| 1439 | C=O bond stretching vibration peak [7] |
| 1580 | Characteristic C–C peak of the benzene skeleton [18] |
| 1620 | C=C bond stretching vibration peak [20] |
| 2930 | Vibration of C–H [22] |
| 3430 | OH bonds [22] |
| Peak Type | Area (CGS) | Area (HCGS) |
|---|---|---|
| D4 | 38,947.6711 | 32,370.39397 |
| D1 | 328,489.60646 | 152,371.04697 |
| D3 | 47,477.81474 | 29,243.27679 |
| G | 161,914.21121 | 86,728.18662 |
| D2 | 2192.3369 | 6971.39049 |
| ID1/G | 2.029 | 1.757 |
| ID3+D4/IALL | 0.1493 | 0.2003 |
| Sample | Zeta Potential |
|---|---|
| Raw CGS | −21.95 |
| HCGS | −26.05 |
| Vulcanization Index (phr) | ML (dN·m) | MH (dN·m) | MH-ML (dN·m) | t10 (min) | t90 (min) |
|---|---|---|---|---|---|
| Pure ESBR | 0.07 | 0.35 | 0.28 | 6.49 | 13.56 |
| 20 | 0.07 | 0.76 | 0.69 | 3.26 | 11.1 |
| 30 | 0.08 | 0.93 | 0.85 | 3.27 | 10.52 |
| 40 | 0.10 | 0.87 | 0.77 | 3.09 | 12.19 |
| 50 | 0.12 | 1.05 | 0.93 | 3.1 | 11.32 |
| 60 | 0.15 | 1.04 | 0.89 | 2.36 | 11.39 |
| 70 | 0.15 | 1.18 | 1.03 | 2.5 | 11.43 |
| 80 | 0.18 | 1.15 | 0.97 | 2.38 | 12.18 |
| 90 | 0.22 | 1.23 | 1.03 | 2.25 | 12.28 |
| 100 | 0.26 | 1.31 | 1.05 | 2.46 | 12.43 |
| Different Fillers | Tensile Strength/MPa | Tear Strength/(KN/m) | Modulus/MPa | Elongation at Break/% | ||
|---|---|---|---|---|---|---|
| 100% | 300% | 500% | ||||
| Pure ESBR | 1.16 ± 0.133 | 16.57 ± 2.440 | 0.43 ± 0.016 | 0.56 ± 0.005 | 0.60 ± 0.008 | 786.72 ± 139.7 |
| 20 phr | 6.09 ± 1.471 | 34 ± 3.347 | 0.85 ± 0.019 | 1.35 ± 0.013 | 2.06 ± 0.064 | 915.54 ± 82.35 |
| 30 phr | 8 ± 0.578 | 42.25 ± 8.313 | 0.94 ± 0.027 | 1.56 ± 0.061 | 2.49 ± 0.179 | 985.12 ± 54.6 |
| 40 phr | 10.48 ± 0.973 | 47.41 ± 5.897 | 0.98 ± 0.041 | 1.51 ± 0.097 | 2.39 ± 0.27 | 1337.18 ± 148.4 |
| 50 phr | 10.91 ± 1.877 | 49.01 ± 6.341 | 1.17 ± 0.071 | 2.05 ± 0.185 | 3.48 ± 0.483 | 1023.60 ± 144.3 |
| 60 phr | 9.96 ± 0.372 | 57.04 ± 3.307 | 1.22 ± 0.03 | 1.99 ± 0.069 | 3.28 ± 0.187 | 1160.44 ± 63.05 |
| 70 phr | 9.97 ± 0.25 | 58.21 ± 2.981 | 1.4 ± 0.078 | 2.48 ± 0.251 | 4.28 ± 0.593 | 991.52 ± 132.7 |
| 80 phr | 6.96 ± 0.206 | 62.34 ± 2.891 | 1.45 ± 0.079 | 2.33 ± 0.163 | 3.61 ± 0.235 | 999.22 ± 65.3 |
| 90 phr | 7.87 ± 0.276 | 64.92 ± 2.896 | 1.71 ± 0.081 | 2.86 ± 0.11 | 4.54 ± 0.201 | 907.16 ± 17.24 |
| 100 phr | 6.53 ± 0.392 | 63.02 ± 2.272 | 1.83 ± 0.063 | 3.03 ± 0.147 | 4.48 ± 0.305 | 818.10 ± 66.21 |
| Vulcanization Index | ML (dN·m) | MH (dN·m) | MH-ML (dN·m) | t10 (min) | t90 (min) |
|---|---|---|---|---|---|
| pure SBR | 0.07 | 0.35 | 0.28 | 6.49 | 13.56 |
| HCGS:CB 5:0 | 0.18 | 1.05 | 0.87 | 2.54 | 12 |
| HCGS:CB 4:1 | 0.17 | 1.15 | 0.98 | 2.32 | 11.01 |
| HCGS:CB 3:2 | 0.17 | 1.19 | 1.02 | 2.49 | 10.57 |
| HCGS:CB 2:3 | 0.18 | 1.33 | 1.15 | 2.55 | 10.45 |
| HCGS:CB 1:4 | 0.18 | 1.40 | 1.22 | 3.30 | 10.38 |
| HCGS:CB 0:5 | 0.15 | 1.37 | 1.22 | 3.30 | 10.58 |
| Different Fillers | Tensile Strength/MPa | Tear Strength/(KN/m) | Modulus/MPa | Elongation at Break/% | ||
|---|---|---|---|---|---|---|
| 100% | 300% | 500% | ||||
| Pure SBR | 1.16 ± 0.133 | 16.57 ± 2.440 | 0.43 ± 0.016 | 0.56 ± 0.005 | 0.60 ± 0.008 | 782.72 ± 139.7 |
| HCGS:CB 5:0 | 10.91 ± 1.877 | 49.01 ± 6.341 | 1.17 ± 0.071 | 2.05 ± 0.185 | 3.48 ± 0.483 | 1023.60 ± 144.3 |
| HCGS:CB 4:1 | 10.89 ± 0.297 | 64.67 ± 12.82 | 0.96 ± 0.205 | 2.11 ± 0.276 | 3.95 ± 0.266 | 1259.35 ± 70.65 |
| HCGS:CB 3:2 | 11.12 ± 0.77 | 69.74 ± 10.56 | 0.96 ± 0.097 | 1.87 ± 0.109 | 3.4 ± 0.275 | 1286.32 ± 84.89 |
| HCGS:CB 2:3 | 11.89 ± 0.442 | 82.91 ± 6.299 | 1.37 ± 0.121 | 2.58 ± 0.135 | 4.44 ± 0.159 | 1285.45 ± 44.82 |
| HCGS:CB 1:4 | 12.78 ± 0.42 | 90.70 ± 9.996 | 1.14 ± 0.012 | 2.42 ± 0.111 | 4.36 ± 0.259 | 1301.93 ± 88.55 |
| HCGS:CB 0:5 | 12.52 ± 0.007 | 89.55 ± 6.583 | 1.15 ± 0.007 | 2.1 ± 0.106 | 3.79 ± 0.247 | 1289.23 ± 39.28 |
| Type of Modifier | Crosslinking Density (×10−4 mol/cm3) | Swelling Ratio (%) | Available Average Molecular Weight (×10−4 g/mol) |
|---|---|---|---|
| Pure SBR | 0.3330 | 96.4582 | 0.02748 |
| HCGS:CB 5:0 | 2.1678 | 75.6507 | 0.4221 |
| HCGS:CB 4:1 | 2.2885 | 79.1478 | 0.3998 |
| HCGS:CB 3:2 | 2.3506 | 78.7522 | 0.3893 |
| HCGS:CB 2:3 | 2.7835 | 76.6002 | 0.3287 |
| HCGS:CB 1:4 | 2.9364 | 75.2460 | 0.3116 |
| HCGS:CB 0:5 | 2.8975 | 76.3226 | 0.3263 |
| ParpaleCB (phr) | K | m | n | R2 |
|---|---|---|---|---|
| 0 | 0.00384 | 0.68414 | 0.71796 | 0.85805 |
| 10 | 0.00434 | 0.50127 | 0.82108 | 0.96624 |
| 20 | 0.00565 | 0.71001 | 0.92256 | 0.96605 |
| 30 | 0.00702 | 0.83906 | 1.01456 | 0.97042 |
| 40 | 0.01197 | 1.20423 | 1.25499 | 0.94793 |
| 50 | 0.01118 | 1.15232 | 1.22475 | 0.93476 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, X.; Wang, Y.; Li, F.; Hao, Z.; Zhang, Y.; Zhang, Y. Performance of Full-Component Coal Gasification Fine Slag: High-Value Utilization as Reinforcing Material in Styrene-Butadiene Rubber (ESBR) for Replacing Carbon Black. Polymers 2024, 16, 522. https://doi.org/10.3390/polym16040522
Feng X, Wang Y, Li F, Hao Z, Zhang Y, Zhang Y. Performance of Full-Component Coal Gasification Fine Slag: High-Value Utilization as Reinforcing Material in Styrene-Butadiene Rubber (ESBR) for Replacing Carbon Black. Polymers. 2024; 16(4):522. https://doi.org/10.3390/polym16040522
Chicago/Turabian StyleFeng, Xianggang, Yunpeng Wang, Fei Li, Zhifei Hao, Yongfeng Zhang, and Yinmin Zhang. 2024. "Performance of Full-Component Coal Gasification Fine Slag: High-Value Utilization as Reinforcing Material in Styrene-Butadiene Rubber (ESBR) for Replacing Carbon Black" Polymers 16, no. 4: 522. https://doi.org/10.3390/polym16040522
APA StyleFeng, X., Wang, Y., Li, F., Hao, Z., Zhang, Y., & Zhang, Y. (2024). Performance of Full-Component Coal Gasification Fine Slag: High-Value Utilization as Reinforcing Material in Styrene-Butadiene Rubber (ESBR) for Replacing Carbon Black. Polymers, 16(4), 522. https://doi.org/10.3390/polym16040522





