Lignocellulosic Membranes Grafted with N-Vinylcaprolactam Using Radiation Chemistry: Load and Release Capacity of Vancomycin
Abstract
1. Introduction
2. Materials and Methods
2.1. Grafting of Membranes (MS-g-NVCL)
2.2. Contact Angle
2.3. Study of Swelling: Thermal-Response
2.4. Mechanical Tests
2.5. Load and Release of Vancomycin
2.6. Instruments
- Vacuum Drying:
- Contact Angle Measurement:
- FTIR-ATR Analysis:
- Nuclear Magnetic Resonance Spectroscopy (CP-MAS 13C-NMR):
- Scanning Electron Microscopy (SEM):
- Atomic Force Microscopy (AFM):
- X-Ray Photoelectron Spectroscopy (XPS):
- Mechanical Tests:
- Ultraviolet–Visible (UV-Vis):
3. Results and Discussion
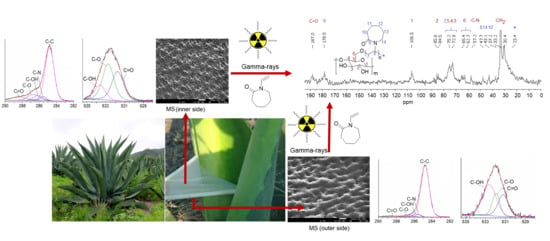
3.1. Synthesis of MS-g-NVCL Membranes
3.2. Water Contact Angle and Thermal Responsiveness
3.3. FTIR-ATR Study
3.4. CP-MAS 13C-NMR and XPS Analysis
3.5. Mechanical Properties
3.6. SEM and AFM Study
3.7. Loading and Release of Vancomycin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akilbekova, D.; Shaimerdenova, M.; Adilov, S.; Berillo, D. Biocompatible Scaffolds Based on Natural Polymers for Regenerative Medicine. Int. J. Biol. Macromol. 2018, 114, 324–333. [Google Scholar] [CrossRef]
- De Moraes Porto, I.C.C. Polymer Biocompatibility. In Polymerization; De Souza Gomes, A., Ed.; InTech: London, UK, 2012; ISBN 978-953-51-0745-3. [Google Scholar]
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Almasi, H. Biodegradable Polymers. In Biodegradation—Life of Science; Chamy, R., Ed.; InTech: London, UK, 2013; ISBN 978-953-51-1154-2. [Google Scholar]
- Halake, K.; Kim, H.J.; Birajdar, M.; Kim, B.S.; Bae, H.; Lee, C.; Kim, Y.J.; Kim, S.; Ahn, S.; An, S.Y.; et al. Recently Developed Applications for Natural Hydrophilic Polymers. J. Ind. Eng. Chem. 2016, 40, 16–22. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Hatakeyama, T. Interaction between Water and Hydrophilic Polymers. Thermochim. Acta 1998, 308, 3–22. [Google Scholar] [CrossRef]
- Ko, H.-F.; Sfeir, C.; Kumta, P.N. Novel Synthesis Strategies for Natural Polymer and Composite Biomaterials as Potential Scaffolds for Tissue Engineering. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2010, 368, 1981–1997. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.; Wang, H. Eco Polymeric Materials and Natural Polymer. Polymers 2023, 15, 4021. [Google Scholar] [CrossRef]
- Thakur, V.K.; Singha, A.S.; Thakur, M.K. Surface Modification of Natural Polymers to Impart Low Water Absorbency. Int. J. Polym. Anal. Charact. 2012, 17, 133–143. [Google Scholar] [CrossRef]
- Silva, A.C.Q.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Natural Polymers-Based Materials: A Contribution to a Greener Future. Molecules 2021, 27, 94. [Google Scholar] [CrossRef]
- Ponnusamy, P.G.; Mani, S. Material and Environmental Properties of Natural Polymers and Their Composites for Packaging Applications—A Review. Polymers 2022, 14, 4033. [Google Scholar] [CrossRef]
- Teixeira-Costa, B.E.; Andrade, C.T. Natural Polymers Used in Edible Food Packaging—History, Function and Application Trends as a Sustainable Alternative to Synthetic Plastic. Polysaccharides 2021, 3, 32–58. [Google Scholar] [CrossRef]
- Gao, D.; Lv, J.; Lee, P.S. Natural Polymer in Soft Electronics: Opportunities, Challenges, and Future Prospects. Adv. Mater. 2022, 34, 2105020. [Google Scholar] [CrossRef]
- Niu, Q.; Huang, X.; Lv, S.; Yao, X.; Fan, S.; Zhang, Y. Natural Polymer-Based Bioabsorbable Conducting Wires for Implantable Bioelectronic Devices. J. Mater. Chem. A 2020, 8, 25323–25335. [Google Scholar] [CrossRef]
- Muthukumaran, P.; Suresh Babu, P.; Karthikeyan, S.; Kamaraj, M.; Aravind, J. Tailored Natural Polymers: A Useful Eco-Friendly Sustainable Tool for the Mitigation of Emerging Pollutants: A Review. Int. J. Environ. Sci. Technol. 2021, 18, 2491–2510. [Google Scholar] [CrossRef]
- Sivasankarapillai, V.S.; Das, S.S.; Sabir, F.; Sundaramahalingam, M.A.; Colmenares, J.C.; Prasannakumar, S.; Rajan, M.; Rahdar, A.; Kyzas, G.Z. Progress in Natural Polymer Engineered Biomaterials for Transdermal Drug Delivery Systems. Mater. Today Chem. 2021, 19, 100382. [Google Scholar] [CrossRef]
- Arredondo, C.H.; Aguilar-Lira, G.; Perez-Silva, I.; Rodriguez, J.A.; Islas, G.; Hernandez, P. Characterization and Application of Agave salmiana Cuticle as Bio-Membrane in Low-Temperature Electrolyzer and Fuel Cells. Appl. Sci. 2019, 9, 4461. [Google Scholar] [CrossRef]
- Xiao, F.; Jin, S.; Zhang, W.; Zhang, Y.; Zhou, H.; Huang, Y. Wearable Pressure Sensor Using Porous Natural Polymer Hydrogel Elastomers with High Sensitivity over a Wide Sensing Range. Polymers 2023, 15, 2736. [Google Scholar] [CrossRef]
- Flores-Rojas, G.G.; Vázquez, E.; López-Saucedo, F.; Buendía-González, L.; Vera-Graziano, R.; Mendizabal, E.; Bucio, E. Lignocellulosic Membrane Grafted with 4-Vinylpiridine Using Radiation Chemistry: Antimicrobial Activity of Loaded Vancomycin. Cellulose 2023, 30, 3853–3868. [Google Scholar] [CrossRef]
- Pires, P.C.; Mascarenhas-Melo, F.; Pedrosa, K.; Lopes, D.; Lopes, J.; Macário-Soares, A.; Peixoto, D.; Giram, P.S.; Veiga, F.; Paiva-Santos, A.C. Polymer-Based Biomaterials for Pharmaceutical and Biomedical Applications: A Focus on Topical Drug Administration. Eur. Polym. J. 2023, 187, 111868. [Google Scholar] [CrossRef]
- Pereira, G.G.; Detoni, C.B.; Balducci, A.G.; Rondelli, V.; Colombo, P.; Guterres, S.S.; Sonvico, F. Hyaluronate Nanoparticles Included in Polymer Films for the Prolonged Release of Vitamin E for the Management of Skin Wounds. Eur. J. Pharm. Sci. 2016, 83, 203–211. [Google Scholar] [CrossRef]
- Mansour, O.Y.; Nagaty, A. Grafting of Synthetic Polymers to Natural Polymers by Chemical Processes. Prog. Polym. Sci. 1985, 11, 91–165. [Google Scholar] [CrossRef]
- Mehra, S.; Nisar, S.; Chauhan, S.; Singh, G.; Singh, V.; Rattan, S. A Dual Stimuli Responsive Natural Polymer Based Superabsorbent Hydrogel Engineered through a Novel Cross-Linker. Polym. Chem. 2021, 12, 2404–2420. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.-L. Review of Stimuli-Responsive Polymers in Drug Delivery and Textile Application. Molecules 2019, 24, 2547. [Google Scholar] [CrossRef]
- Haag, R.; Kratz, F. Polymer Therapeutics: Concepts and Applications. Angew. Chem. Int. Ed. 2006, 45, 1198–1215. [Google Scholar] [CrossRef] [PubMed]
- Pino-Ramos, V.H.; Flores-Rojas, G.G.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Graft Copolymerization by Ionization Radiation, Characterization, and Enzymatic Activity of Temperature-Responsive SR-g-PNVCL Loaded with Lysozyme. React. Funct. Polym. 2018, 126, 74–82. [Google Scholar] [CrossRef]
- Winninger, J.; Iurea, D.M.; Atanase, L.I.; Salhi, S.; Delaite, C.; Riess, G. Micellization of Novel Biocompatible Thermo-Sensitive Graft Copolymers Based on Poly(ε-Caprolactone), Poly(N-Vinylcaprolactam) and Poly(N-Vinylpyrrolidone). Eur. Polym. J. 2019, 119, 74–82. [Google Scholar] [CrossRef]
- Mohammed, M.N.; Yusoh, K.B.; Shariffuddin, J.H.B.H. Poly(N-Vinyl Caprolactam) Thermoresponsive Polymer in Novel Drug Delivery Systems: A Review. Mater. Express 2018, 8, 21–34. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Di, Y.; Liu, J.; Zhou, H. The Effects of Clothing Thermal Resistance and Operative Temperature on Human Skin Temperature. J. Therm. Biol. 2013, 38, 233–239. [Google Scholar] [CrossRef]
- Indulekha, S.; Arunkumar, P.; Bahadur, D.; Srivastava, R. Thermoresponsive Polymeric Gel as an On-Demand Transdermal Drug Delivery System for Pain Management. Mater. Sci. Eng. C 2016, 62, 113–122. [Google Scholar] [CrossRef]
- Ashfaq, A.; Clochard, M.-C.; Coqueret, X.; Dispenza, C.; Driscoll, M.S.; Ulański, P.; Al-Sheikhly, M. Polymerization Reactions and Modifications of Polymers by Ionizing Radiation. Polymers 2020, 12, 2877. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Ballesteros, A.; Pino-Ramos, V.H.; López-Saucedo, F.; Flores-Rojas, G.G.; Bucio, E. γ-Rays and Ions Irradiation. In Surface Modification of Polymers; Pinson, J., Thiry, D., Eds.; Wiley: Hoboken, NJ, USA, 2019; pp. 185–209. ISBN 978-3-527-34541-0. [Google Scholar]
- Flores-Rojas, G.G.; López-Saucedo, F.; Vázquez, E.; Hernández-Mecinas, E.; Huerta, L.; Cedillo, G.; Concheiro, A.; Alvarez-Lorenzo, C.; Bucio, E. Synthesis of Polyamide-6@cellulose Microfilms Grafted with N-Vinylcaprolactam Using Gamma-Rays and Loading of Antimicrobial Drugs. Cellulose 2020, 27, 2785–2801. [Google Scholar] [CrossRef]
- Flores-Rojas, G.G.; López-Saucedo, F.; Vera-Graziano, R.; Magaña, H.; Mendizábal, E.; Bucio, E. Silver Nanoparticles Loaded on Polyethylene Terephthalate Films Grafted with Chitosan. Polymers 2022, 15, 125. [Google Scholar] [CrossRef] [PubMed]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef]
- Lindberg, G.C.J.; Lim, K.S.; Soliman, B.G.; Nguyen, A.; Hooper, G.J.; Narayan, R.J.; Woodfield, T.B.F. Biological Function Following Radical Photo-Polymerization of Biomedical Polymers and Surrounding Tissues: Design Considerations and Cellular Risk Factors. Appl. Phys. Rev. 2021, 8, 011301. [Google Scholar] [CrossRef]
- Álvarez-Chávez, J.; Villamiel, M.; Santos-Zea, L.; Ramírez-Jiménez, A.K. Agave By-Products: An Overview of Their Nutraceutical Value, Current Applications, and Processing Methods. Polysaccharides 2021, 2, 720–743. [Google Scholar] [CrossRef]
- Elmas, A.; Akyüz, G.; Bergal, A.; Andaç, M.; Andaç, Ö. Mathematical Modelling of Drug Release. Res. Eng. Struct. Mater. 2020, 6, 327–350. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Mathematical Modeling of Drug Delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Azadi, S.; Ashrafi, H.; Azadi, A. Mathematical Modeling of Drug Release from Swellable Polymeric Nanoparticles. J. Appl. Pharm. Sci. 2017, 7, 125–133. [Google Scholar] [CrossRef]
- Grassi, M.; Grassi, G. Application of Mathematical Modeling in Sustained Release Delivery Systems. Expert Opin. Drug Deliv. 2014, 11, 1299–1321. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of Solute Release from Porous Hydrophilic Polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Jeffree, C.E. The Fine Structure of the Plant Cuticle. In Biology of the Plant Cuticle; Riederer, M., Müller, C., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2006; pp. 11–125. ISBN 978-0-470-98871-8. [Google Scholar]
- Liu, L.-Y.; Cho, M.; Sathitsuksanoh, N.; Chowdhury, S.; Renneckar, S. Uniform Chemical Functionality of Technical Lignin Using Ethylene Carbonate for Hydroxyethylation and Subsequent Greener Esterification. ACS Sustain. Chem. Eng. 2018, 6, 12251–12260. [Google Scholar] [CrossRef]
- Deshmukh, A.P.; Simpson, A.J.; Hadad, C.M.; Hatcher, P.G. Insights into the Structure of Cutin and Cutan from Agave americana Leaf Cuticle Using HRMAS NMR Spectroscopy. Org. Geochem. 2005, 36, 1072–1085. [Google Scholar] [CrossRef]
- España, V.H.P.; Parra, J.A.C.; Burgos, J.E.A.; Ovando, M.A.M.; Cortes, T.R. Importancia de La Capa Cuticular Durante La Colonización Del Hongo Causante de La Negrilla En Agave salmiana Otto Ex Salm-Dyck ssp. salmiana. Rev. Mex. Cienc. For. 2022, 13, 166–176. [Google Scholar] [CrossRef]
- Perez-Espana, V.H.; Cuervo-Parra, J.A.; Paz-Camacho, C.; Morales-Ovando, M.A.; Gomez-Aldapa, C.A.; Rodriguez-Jimenes, G.C.; Robles-Olvera, V.J.; Lopez-Perez, P.A.; Romero-Cortes, T. General Characterization of Cuticular Membrane Isolated from Agave salmiana. Int. J. Bio-Resour. Stress Manag. 2019, 10, 46–52. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A Simple Equation for the Description of Solute Release. III. Coupling of Diffusion and Relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Colpo, J.C.; Pigatto, C.; Brizuela, N.; Aragón, J.; Dos Santos, L.A.L. Antibiotic and Anesthetic Drug Release from Double-Setting α-TCP Cements. J. Mater. Sci. 2018, 53, 7112–7124. [Google Scholar] [CrossRef]











| Elemental Composition (%) * | Chemical Group Concentration (%) * | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C1s | O1s | ||||||||||
| Membrane | C1s | N1s | O1s | C=O | C–O–C | C–OH | C–N | C–C | C=O | –OH | C–O–C |
| PNVCL | 81.1 | 6.9 | 11.9 | 14.7 | 0 | 0 | 22.6 | 62.6 | 56.3 | 43.6 | 0 |
| MS (a) | 75.5 | 3.4 | 20.7 | 12.4 | 13.8 | 11.6 | 9.9 | 52.0 | 34.6 | 28.5 | 36.8 |
| MS (b) | 90.1 | 0.8 | 9.9 | 6.9 | 6.9 | 8.3 | 7.4 | 70.3 | 32.6 | 35.6 | 31.7 |
| MS-g-NVCL (13.2%-a) | 73.6 | 4.3 | 22.1 | 14.0 | 10.0 | 9.1 | 15.0 | 51.8 | 41.2 | 25.5 | 33.1 |
| MS-g-NVCL (13.2%-b) | 69.9 | 3.5 | 26.4 | 13.9 | 10.3 | 8.8 | 15.3 | 51.4 | 42.0 | 26.6 | 31.2 |
| Membrane | Young’s Module (MPa) | Tensile Break (MPa) | Elongation Break (%) |
|---|---|---|---|
| MS | 48 ± 2.26 | 3.7 ± 0.24 | 16.8 ± 1.16 |
| MS-g-NVCL 0.8% | 36 ± 3.74 | 3.6 ± 0.58 | 20 ± 3.78 |
| MS-g-NVCL 4.6% | 45 ± 3.82 | 3.8 ± 0.87 | 18.5 ± 4.96 |
| MS-g-NVCL 13.2% | 48 ± 4.74 | 3.7 ± 0.68 | 20.2 ± 2.55 |
| Membrane | Higuchi | Peppas–Sahlin | Korsmeyer–Peppas | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25 °C | 40 °C | 25 °C | 40 °C | 25 °C | 40 °C | |||||||||||||
| R2 | Kh a | R2 | Kh a | R2 | K1 | K2 | n b | R2 | K1 | K2 | n b | R2 | K1 | n b | R2 | K1 | n b | |
| MS | 0.54 | 3.1 | 0.55 | 3.2 | 0.96 | 12.04 | -0.59 | 0.33 | 0.97 | 11.88 | −0.57 | 0.32 | 0.96 | 15.52 | 0.21 | 0.97 | 15.35 | 0.21 |
| MS-g-NVCL 0.8% | 0.93 | 4.1 | 0.91 | 4.2 | 0.99 | 3.28 | -0.04 | 0.63 | 0.99 | 3.46 | −0.04 | 0.62 | 0.97 | 7.74 | 0.37 | 0.98 | 7.71 | 0.36 |
| MS-g-NVCL 4.6% | 0.97 | 4.3 | 0.98 | 4.3 | 0.99 | 4.91 | -0.07 | 0.51 | 0.99 | 5.18 | −0.07 | 0.50 | 0.99 | 6.43 | 0.42 | 0.98 | 6.31 | 0.42 |
| MS-g-NVCL 13.2% | 0.97 | 3.8 | 0.97 | 3.8 | 0.98 | 5.65 | 0.64 | 0.33 | 0.98 | 5.05 | 1.25 | 0.30 | 0.98 | 5.19 | 0.44 | 0.98 | 5.16 | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rentería-Urquiza, M.; Flores-Rojas, G.G.; Gómez-Lázaro, B.; López-Saucedo, F.; Vera-Graziano, R.; Mendizabal, E.; Bucio, E. Lignocellulosic Membranes Grafted with N-Vinylcaprolactam Using Radiation Chemistry: Load and Release Capacity of Vancomycin. Polymers 2024, 16, 551. https://doi.org/10.3390/polym16040551
Rentería-Urquiza M, Flores-Rojas GG, Gómez-Lázaro B, López-Saucedo F, Vera-Graziano R, Mendizabal E, Bucio E. Lignocellulosic Membranes Grafted with N-Vinylcaprolactam Using Radiation Chemistry: Load and Release Capacity of Vancomycin. Polymers. 2024; 16(4):551. https://doi.org/10.3390/polym16040551
Chicago/Turabian StyleRentería-Urquiza, Maite, Guadalupe Gabriel Flores-Rojas, Belén Gómez-Lázaro, Felipe López-Saucedo, Ricardo Vera-Graziano, Eduardo Mendizabal, and Emilio Bucio. 2024. "Lignocellulosic Membranes Grafted with N-Vinylcaprolactam Using Radiation Chemistry: Load and Release Capacity of Vancomycin" Polymers 16, no. 4: 551. https://doi.org/10.3390/polym16040551
APA StyleRentería-Urquiza, M., Flores-Rojas, G. G., Gómez-Lázaro, B., López-Saucedo, F., Vera-Graziano, R., Mendizabal, E., & Bucio, E. (2024). Lignocellulosic Membranes Grafted with N-Vinylcaprolactam Using Radiation Chemistry: Load and Release Capacity of Vancomycin. Polymers, 16(4), 551. https://doi.org/10.3390/polym16040551