Structure–Property Relationships in PVDF/SrTiO3/CNT Nanocomposites for Optoelectronic and Solar Cell Applications
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. Preparation of SrTiO3/CNTs Nanocomposite
2.3. Preparation of PVDF/SrTiO3/CNTs Polymer Films
2.4. Characterization Techniques
3. Results and Discussion
3.1. X-ray Diffraction Analysis
3.2. FTIR Spectroscopy
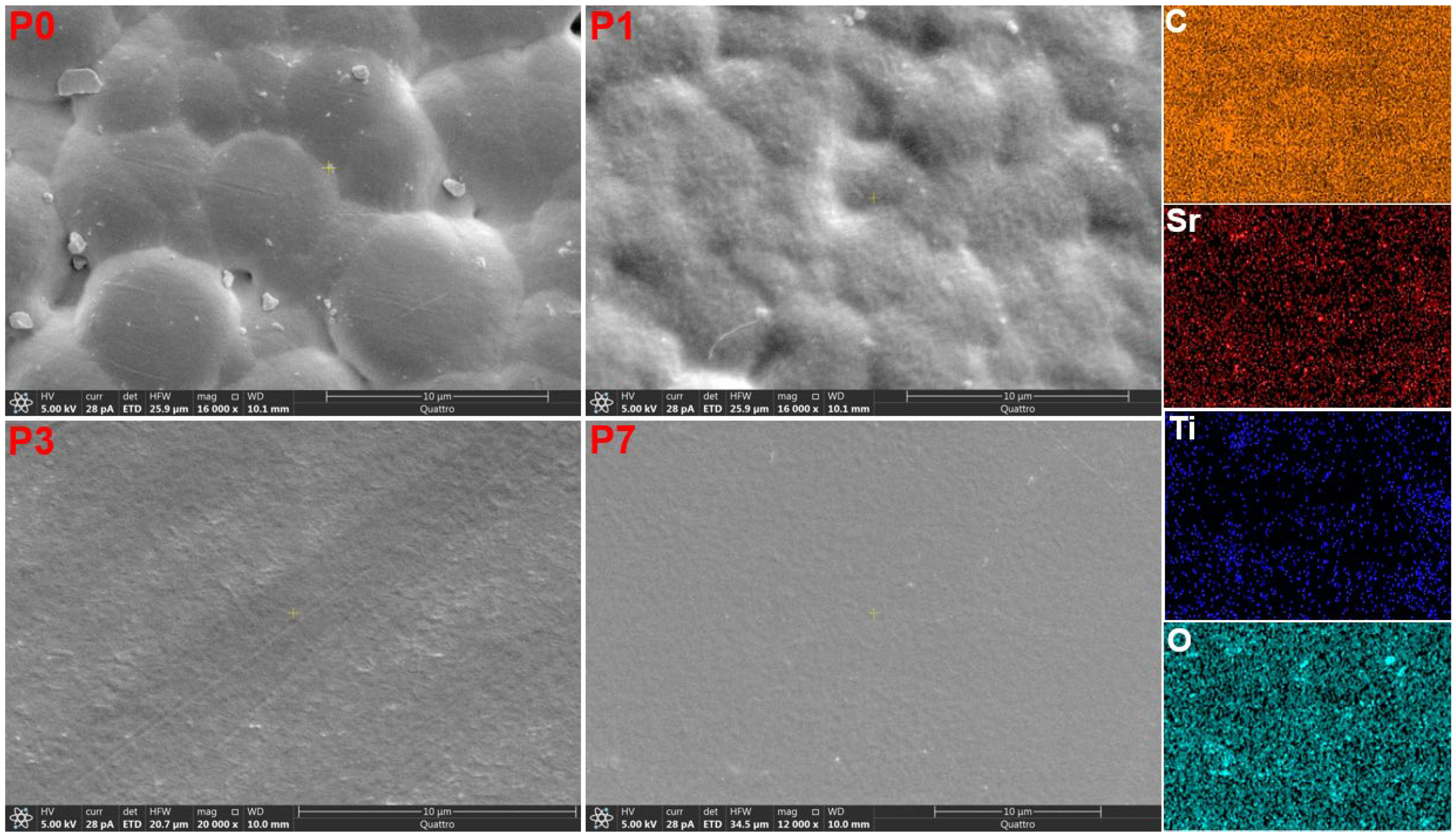
3.3. Scanning Electron Microscopy (SEM)
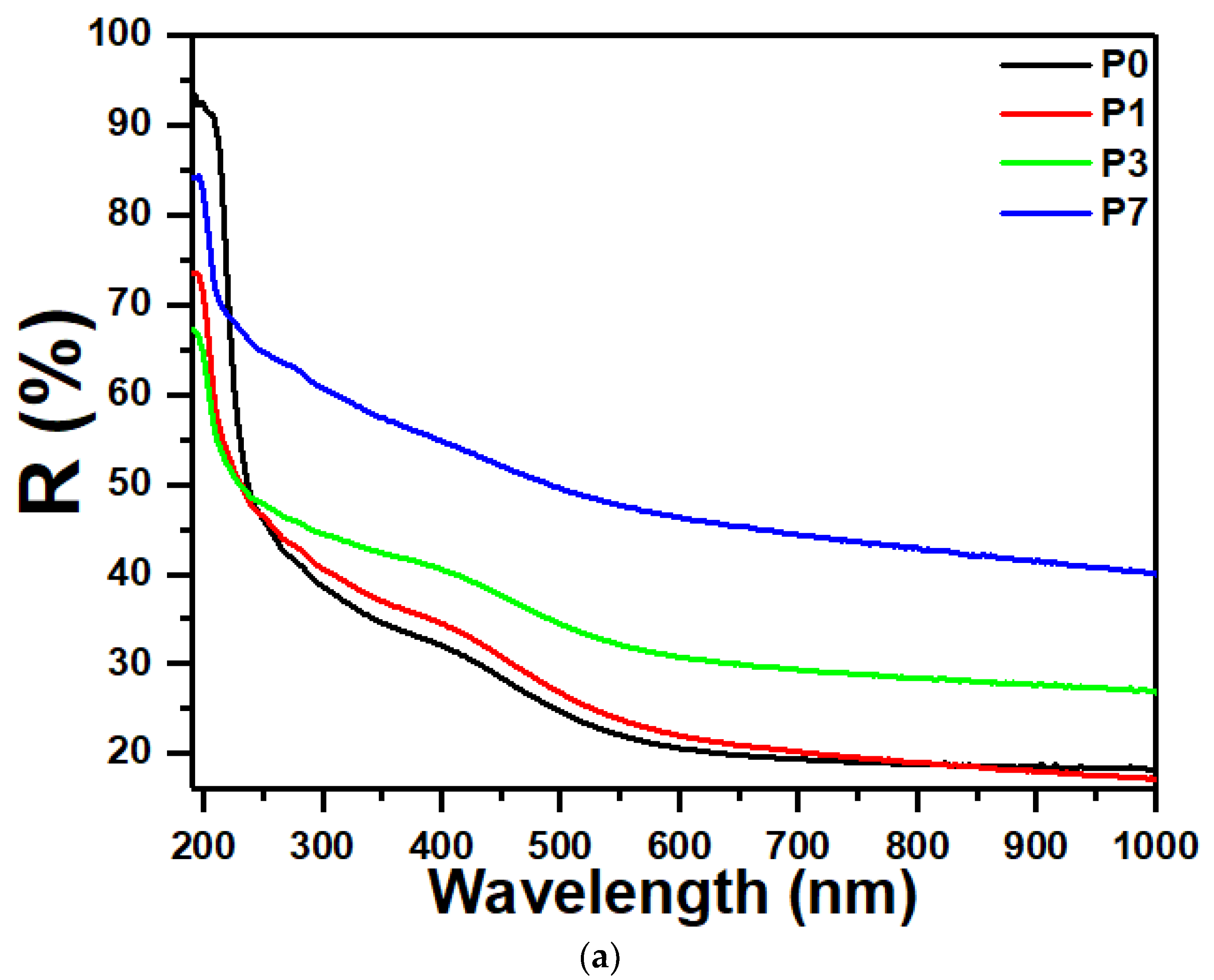
3.4. UV-Vis Optical Absorption Spectroscopy
3.5. Optical Band Gap Investigations
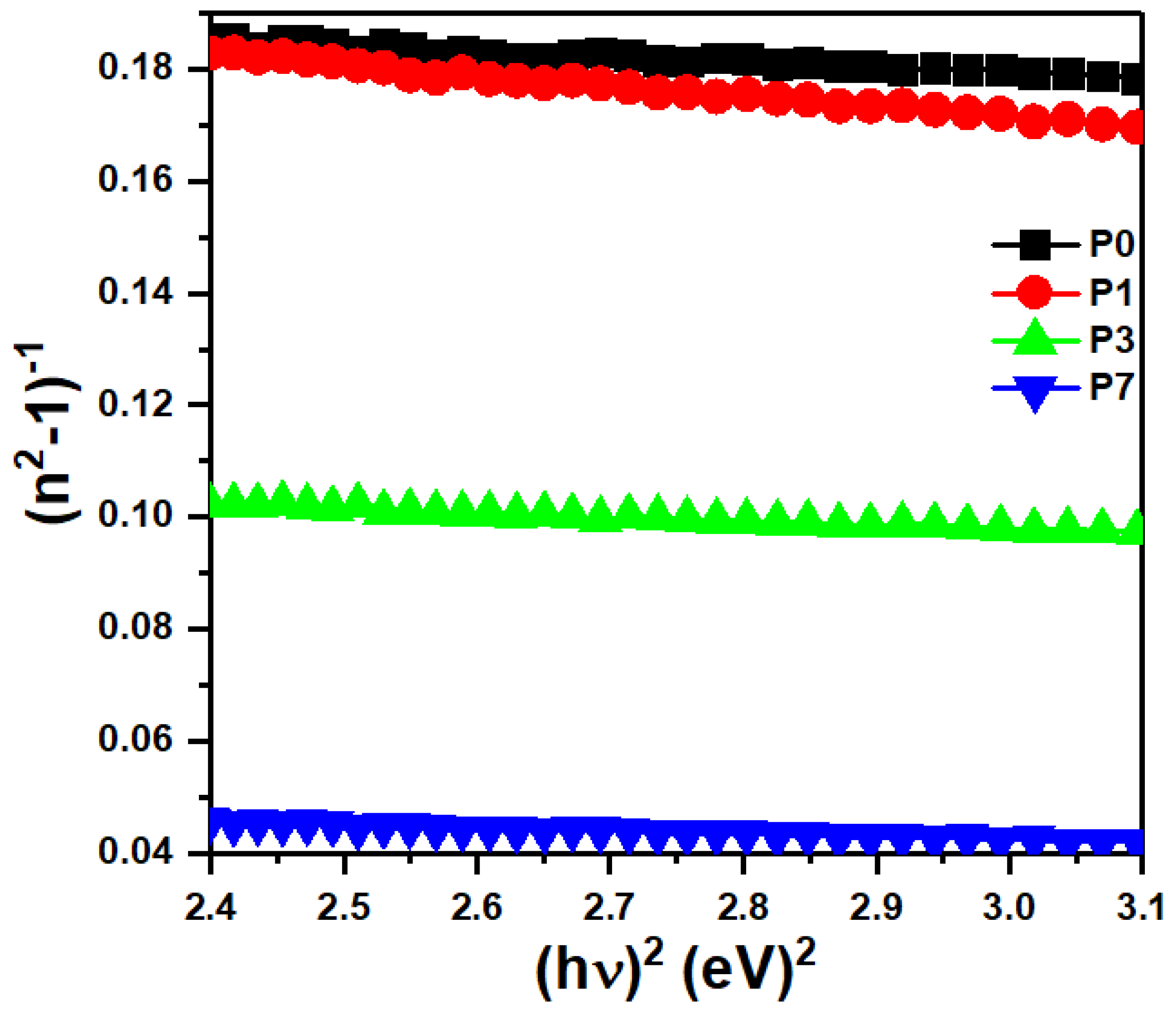
3.6. Linear Refractive Index
3.7. Nonlinear Optical Parameters
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darwish, M.S.; Mostafa, M.H.; Al-Harbi, L.M. Polymeric nanocomposites for environmental and industrial applications. Int. J. Mol. Sci. 2022, 23, 1023. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xue, J.P.; Zhang, T.D.; Chi, Q.G.; Li, J.L.; Chen, Q.G.; Wang, J.J.; Chen, L.Q. Double-gradients design of polymer nanocomposites with high energy density. Energy Storage Mater. 2022, 44, 73–81. [Google Scholar] [CrossRef]
- Cheikh, D.; Majdoub, H.; Darder, M. An overview of clay-polymer nanocomposites containing bioactive compounds for food packaging applications. Appl. Clay Sci. 2022, 216, 106335. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, J.; Liu, M. Interphase in polymer nanocomposites. JACS Au 2022, 2, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Ming, Y.; Zhou, Z.; Hao, T.; Nie, Y. Polymer Nanocomposites: Role of modified filler content and interfacial interaction on crystallization. Eur. Polym. J. 2022, 162, 110894. [Google Scholar] [CrossRef]
- Ishizuka, F.; Kim, H.J.; Kuchel, R.P.; Yao, Y.; Chatani, S.; Niino, H.; Zetterlund, P.B. Nano-dimensional spheres and worms as fillers in polymer nanocomposites: Effect of filler morphology. Polym. Chem. 2022, 13, 1818–1823. [Google Scholar] [CrossRef]
- Sudharsan, J.; Khare, S.K. Role of nanocomposite additives in well bore stability during shale formation drilling with water based mud—A comprehensive review. Mater. Today Proc. 2022, 62, 6412–6419. [Google Scholar] [CrossRef]
- Mazari, S.A.; Ali, E.; Abro, R.; Khan, F.S.A.; Ahmed, I.; Ahmed, M.; Nizamuddin, S.; Siddiqui, T.H.; Hossain, N.; Mubarak, N.M.; et al. Nanomaterials: Applications, waste-handling, environmental toxicities, and future challenges–A review. J. Environ. Chem. Eng. 2021, 9, 105028. [Google Scholar] [CrossRef]
- Kulkarni, N.D.; Kumari, P. Development of highly flexible PVDF-TiO2 nanocomposites for piezoelectric nanogenerator applications. Mater. Res. Bull. 2023, 157, 112039. [Google Scholar] [CrossRef]
- Prateek; Bhunia, R.; Siddiqui, S.; Garg, A.; Gupta, R.K. Significantly enhanced energy density by tailoring the interface in hierarchically structured TiO2–BaTiO3–TiO2 nanofillers in PVDF-based thin-film polymer nanocomposites. ACS Appl. Mater. Interfaces 2019, 11, 14329–14339. [Google Scholar] [CrossRef]
- Taha, T.A.; Mahmoud, M.H.; Hamdeh, H.H. Development, thermal and dielectric investigations of PVDF-Y2O3 polymer nanocomposite films. J. Polym. Res. 2021, 28, 148. [Google Scholar] [CrossRef]
- Singh, D.; Singh, N.; Garg, A.; Gupta, R.K. Engineered thiol anchored Au-BaTiO3/PVDF polymer nanocomposite as efficient dielectric for electronic applications. Compos. Sci. Technol. 2019, 174, 158–168. [Google Scholar]
- Ahmed, I.; Khan, A.N.; Jan, R.; Gul, I.H. Structure–properties relationships of graphene and spinel nickel ferrites based poly (vinylidene fluoride) hybrid polymer nanocomposites for improved dielectric and EMI shielding characteristics. Mater. Res. Bull. 2022, 148, 111687. [Google Scholar] [CrossRef]
- Sapkota, B.; Martin, A.; Lu, H.; Mahbub, R.; Ahmadi, Z.; Azadehranjbar, S.; Mishra, E.; Shield, J.E.; Jeelani, S.; Rangari, V. Changing the polarization and mechanical response of flexible PVDF-nickel ferrite films with nickel ferrite additives. Mater. Sci. Eng. B 2022, 283, 115815. [Google Scholar] [CrossRef]
- Kum-onsa, P.; Putasaeng, B.; Manyam, J.; Thongbai, P. Significantly improved dielectric properties of poly (vinylidene fluoride) polymer nanocomposites by the addition of nAu−LaFeO3 hybrid particles. Mater. Res. Bull. 2022, 146, 111603. [Google Scholar] [CrossRef]
- Sengwa, R.J.; Dhatarwal, P. Crystalline phases thermal behaviour and radio frequencies dielectric properties of PVDF/PEO/metal oxides hybrid polymer nanocomposite films. J. Polym. Res. 2022, 29, 186. [Google Scholar] [CrossRef]
- Tripathy, A.; Raj, N.P.M.J.; Saravanakumar, B.; Kim, S.J.; Ramadoss, A. Tuning of highly piezoelectric bismuth ferrite/PVDF-copolymer flexible films for efficient energy harvesting performance. J. Alloys Compd. 2023, 932, 167569. [Google Scholar] [CrossRef]
- Ponraj, B.; Deepa, S. Enhancement of dielectric and magnetic properties of electroactive LaNiO3 based PVDF films by inclusion of magnetic Sn0.2Fe2.8O4 nanofiller. Mater. Chem. Phys. 2023, 297, 127259. [Google Scholar]
- Mohammed, M.I. Optical properties of ZnO nanoparticles dispersed in PMMA/PVDF blend. J. Mol. Struct. 2018, 1169, 9–17. [Google Scholar] [CrossRef]
- Huang, P.; Xu, S.; Zhong, W.; Fu, H.; Luo, Y.; Xiao, Z.; Zhang, M. Carbon quantum dots inducing formation of β phase in PVDF-HFP to improve the piezoelectric performance. Sens. Actuators A Phys. 2021, 330, 112880. [Google Scholar] [CrossRef]
- Huyen, D.N. Carbon nanotubes and semiconducting polymer nanocomposites. Carbon Nanotub.-Synth. Charact. Appl. 2021. [Google Scholar] [CrossRef]
- Wang, D.; Ye, J.; Kako, T.; Kimura, T. Photophysical and photocatalytic properties of SrTiO3 doped with Cr cations on different sites. J. Phys. Chem. B 2006, 110, 15824–15830. [Google Scholar] [CrossRef]
- Botelho, C.N.; Falcão, S.S.; Soares, R.E.P.; Pereira, S.R.; de Menezes, A.S.; Kubota, L.T.; Damos, F.S.; Luz, R.C. Evaluation of a photoelectrochemical platform based on strontium titanate, sulfur doped carbon nitride and palladium nanoparticles for detection of SARS-CoV-2 spike glycoprotein S1. Biosens. Bioelectron. X 2022, 11, 100167. [Google Scholar] [CrossRef] [PubMed]
- Sattar, M.A. Interface Structure and Dynamics in Polymer-Nanoparticle Hybrids: A Review on Molecular Mechanisms Underlying the Improved Interfaces. ChemistrySelect 2021, 6, 5068–5096. [Google Scholar] [CrossRef]
- Scotland, K.M.; Strong, O.K.; Parnis, J.M.; Vreugdenhil, A.J. DFT modeling of polyaniline: A computational investigation into the structure and band gap of polyaniline. Can. J. Chem. 2022, 100, 162–167. [Google Scholar] [CrossRef]
- Xu, P.; Lu, T.; Ju, L.; Tian, L.; Li, M.; Lu, W. Machine learning aided design of polymer with targeted band gap based on DFT computation. J. Phys. Chem. B 2021, 125, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Chen, P.; Wang, H.; Koh, C.W.; Uddin, M.A.; Liu, B.; Liao, Q.; Feng, K.; Woo, H.Y.; Xiao, G.; et al. Ultranarrow Bandgap Naphthalenediimide-Dialkylbifuran-Based Copolymers with High-Performance Organic Thin-Film Transistors and All-Polymer Solar Cells. Macromol. Rapid Commun. 2020, 41, 2000144. [Google Scholar] [CrossRef] [PubMed]
- Tipirneni, P.; Jindal, V.; Janik, M.J.; Milner, S.T. Tight binding models accurately predict band structures for copolymer semiconductors. Phys. Chem. Chem. Phys. 2020, 22, 19659–19671. [Google Scholar] [CrossRef]
- Brosseau, C. Generalized effective medium theory and dielectric relaxation in particle-filled polymeric resins. J. Appl. Phys. 2002, 91, 3197–3204. [Google Scholar] [CrossRef]
- Panda, M.; Sultana, N.; Singh, A.K. Structural and optical properties of PVDF/GO nanocomposites. Fuller. Nanotub. Carbon Nanostruct. 2022, 30, 559–570. [Google Scholar] [CrossRef]
- Indolia, A.P.; Gaur, M.S. Optical properties of solution grown PVDF-ZnO nanocomposite thin films. J. Polym. Res. 2013, 20, 43. [Google Scholar] [CrossRef]
- Gaur, A.M.; Rana, D.S. Structural, optical and electrical properties of MgCl2 doped polyvinylidene fluoride (PVDF) composites. J. Mater. Sci. Mater. Electron. 2015, 26, 1246–1251. [Google Scholar] [CrossRef]
- El-Masry, M.M.; Ramadan, R. The effect of CoFe2O4, CuFe2O4 and Cu/CoFe2O4 nanoparticles on the optical properties and piezoelectric response of the PVDF polymer. Appl. Phys. A 2022, 128, 110. [Google Scholar] [CrossRef]
- Hu, L.; Yao, J.; You, F.; Jiang, X.; Li, Z.; Yan, L.; Qi, Y. Preparation and dielectric properties of cysteine modified nano Ag/PYDF composite. J. Funct. Mater. Gongneng Cailiao 2018, 49, 5151–5155. [Google Scholar]
- Teow, Y.H.; Ooi, B.S.; Ahmad, A.L. Study on PVDF-TiO2 mixed-matrix membrane behaviour towards humic acid adsorption. J. Water Process Eng. 2017, 15, 99–106. [Google Scholar] [CrossRef]
- Alhassan, S.; Alshammari, M.; Alshammari, K.; Alotaibi, T.; Alshammari, A.H.; Fawaz, Y.; Taha, T.A.M.; Henini, M. Preparation and Optical Properties of PVDF-CaFe2O4 Polymer Nanocomposite Films. Polymers 2023, 15, 2232. [Google Scholar] [CrossRef]
- Taha, T.A.; Alzara, M.A.A. Synthesis, thermal and dielectric performance of PVA-SrTiO3 polymer nanocomposites. J. Mol. Struct. 2021, 1238, 130401. [Google Scholar] [CrossRef]
- Konstas, P.S.; Konstantinou, I.; Petrakis, D.; Albanis, T. Synthesis, characterization of g-C3N4/SrTiO3 heterojunctions and photocatalytic activity for organic pollutants degradation. Catalysts 2018, 8, 554. [Google Scholar] [CrossRef]
- Campos, J.S.D.C.; Ribeiro, A.A.; Cardoso, C.X. Preparation and characterization of PVDF/CaCO3 composites. Mater. Sci. Eng. B 2007, 136, 123–128. [Google Scholar] [CrossRef]
- Prasad, G.; Sathiyanathan, P.; Prabu, A.A.; Kim, K.J. Piezoelectric characteristics of electrospun PVDF as a function of phase-separation temperature and metal salt content. Macromol. Res. 2017, 25, 981–988. [Google Scholar] [CrossRef]
- Taha, T.A.; Saad, R.; Zayed, M.; Shaban, M.; Ahmed, A.M. Tuning the surface morphologies of ZnO nanofilms for enhanced sensitivity and selectivity of CO2 gas sensor. Appl. Phys. A 2023, 129, 115. [Google Scholar] [CrossRef]
- Manzoor, S.; Abid, A.G.; Aman, S.; Abdullah, M.; Rashid, A.R.; Ali, H.M.; Ali, T.E.; Assiri, M.A.; Ashiq, M.N.; Taha, T.A. Facile synthesis of CoFePO4 on eggshell membrane for oxygen evolution reaction and supercapacitor applications. Ceram. Int. 2022, 48, 36975–36982. [Google Scholar] [CrossRef]
- Badatya, S.; Bharti, D.K.; Srivastava, A.K.; Gupta, M.K. Solution processed high performance piezoelectric eggshell membrane–PVDF layer composite nanogenerator via tuning the interfacial polarization. J. Alloys Compd. 2021, 863, 158406. [Google Scholar] [CrossRef]
- Inamuddin; Abbas Kashmery, H. Polyvinylidene fluoride/sulfonated graphene oxide blend membrane coated with polypyrrole/platinum electrode for ionic polymer metal composite actuator applications. Sci. Rep. 2019, 9, 9877. [Google Scholar] [CrossRef]
- Luo, B.; Wang, X.; Wang, Y.; Li, L. Fabrication, characterization, properties and theoretical analysis of ceramic/PVDF composite flexible films with high dielectric constant and low dielectric loss. J. Mater. Chem. A 2014, 2, 510–519. [Google Scholar] [CrossRef]
- Hao, Y.N.; Wang, X.H.; O’Brien, S.; Lombardi, J.; Li, L.T. Flexible BaTiO3/PVDF gradated multilayer nanocomposite film with enhanced dielectric strength and high energy density. J. Mater. Chem. C 2015, 3, 9740–9747. [Google Scholar] [CrossRef]
- Abdullah, I.Y.; Yahaya, M.; Jumali, M.H.H.; Shanshool, H.M. Effect of annealing process on the phase formation in poly (vinylidene fluoride) thin films. In AIP Conference Proceedings; American Institute of Physic: College Park, MD, USA, 2014; Volume 1614, pp. 147–151. [Google Scholar]
- Shari’ati, Y.; Vura-Weis, J. Polymer thin films as universal substrates for extreme ultraviolet absorption spectroscopy of molecular transition metal complexes. J. Synchrotron Radiat. 2021, 28, 1850–1857. [Google Scholar] [CrossRef]
- Hajduk, B.; Bednarski, H.; Trzebicka, B. Temperature-dependent spectroscopic ellipsometry of thin polymer films. J. Phys. Chem. B 2020, 124, 3229–3251. [Google Scholar] [CrossRef]
- Alshammari, A.H.; Alshammari, M.; Ibrahim, M.; Alshammari, K.; Taha, T.A.M. Processing polymer film nanocomposites of polyvinyl chloride–Polyvinylpyrrolidone and MoO3 for optoelectronic applications. Opt. Laser Technol. 2024, 168, 109833. [Google Scholar] [CrossRef]
- Zaidi, S.M.A.; Kalyar, M.A.; Raza, Z.A.; Shoukat, A.; Waseem, R.; Aslam, M. Effect of laser irradiance on opto-electrical properties of PVA embedded graphene copper ferrite nanocomposite strips. Opt. Mater. 2024, 147, 114590. [Google Scholar] [CrossRef]
- Shanshool, H.M.; Yahaya, M.; Yunus WM, M.; Abdullah, I.Y. Investigation of energy band gap in polymer/ZnO nanocomposites. J. Mater. Sci. Mater. Electron. 2016, 27, 9804–9811. [Google Scholar] [CrossRef]
- Muthupandeeswari, A.; Kalyani, P.; Nehru, L.C. On the effects of high loading of ZnO nanofiller on the structural, optical, impedance and dielectric features of PVA@ZnO nanocomposite films. Polymer Bulletin 2021, 78, 7071–7088. [Google Scholar] [CrossRef]
- Heiba, Z.K.; Mohamed, M.B.; Ahmed, S.I. Exploring the physical properties of PVA/PEG polymeric material upon doping with nano gadolinium oxide. Alex. Eng. J. 2022, 61, 3375–3383. [Google Scholar] [CrossRef]
- Taha, T.A.; Abouhaswa, A.S. Structure, optical and magnetic properties of barium sodium borate/cobalt oxide glass structures. Opt. Quantum Electron. 2023, 55, 483. [Google Scholar] [CrossRef]
- Alshammari, A.H.; Alshammari, K.; Alshammari, M.; Taha, T.A.M. Structural and optical characterization of g-C3N4 nanosheet integrated PVC/PVP polymer nanocomposites. Polymers 2023, 15, 871. [Google Scholar] [CrossRef] [PubMed]
- Alhassan, S.; Alshammari, K.; Alshammari, M.; Alotaibi, T.; Alshammari, A.H.; Fawaz, Y.; Taha, T.A.; Henini, M. Synthesis and optical properties of polyvinylidene difluoride nanocomposites comprising MoO3/g-C3N4. Results Phys. 2023, 48, 106403. [Google Scholar] [CrossRef]
- El-naggar, A.M.; Heiba, Z.K.; Mohamed, M.B.; Kamal, A.M.; Osman, M.M.; Albassam, A.A.; Lakshminarayana, G. Improvement of the optical characteristics of PVA/PVP blend with different concentrations of SnS2/Fe. J. Vinyl Addit. Technol. 2022, 28, 82–93. [Google Scholar] [CrossRef]
- Yasir, M.; Sai, T.; Sicher, A.; Scheffold, F.; Steiner, U.; Wilts, B.D.; Dufresne, E.R. Enhancing the Refractive Index of Polymers with a Plant-Based Pigment. Small 2021, 17, 2103061. [Google Scholar] [CrossRef]
- Al Orainy, R.H. Single oscillator model and refractive index dispersion properties of ternary ZnO films by sol gel method. J. Sol-Gel Sci. Technol. 2014, 70, 47–52. [Google Scholar] [CrossRef]
- Štrbac, D.D.; Lukić, S.R.; Petrović, D.M.; Gonzalez-Leal, J.M.; Srinivasan, A. Single oscillator energy and dispersion energy of uniform thin chalcogenide films from Cu–As–S–Se system. J. Non-Cryst. Solids 2007, 353, 1466–1469. [Google Scholar] [CrossRef]
- Stachewicz, U.; Li, S.; Bilotti, E.; Barber, A.H. Dependence of surface free energy on molecular orientation in polymer films. Appl. Phys. Lett. 2012, 100, 094104. [Google Scholar] [CrossRef]
- Connolly, T.; Smith, R.C.; Hernandez, Y.; Gun’ko, Y.; Coleman, J.N.; Carey, J.D. Carbon-nanotube–polymer nanocomposites for field-emission cathodes. Small 2009, 5, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Dutcher, J.R.; Dalnoki-Veress, K.; Nickel, B.G.; Roth, C.B. Instabilities in thin polymer films: From pattern formation to rupture. In Macromolecular Symposia; Weinheim: WILEY-VCH: Hoboken, NJ, USA, 2000; Volume 159, pp. 143–150. [Google Scholar]
- Taha, T.A.; Hendawy, N.; El-Rabaie, S.; Esmat, A.; El-Mansy, M.K. Effect of NiO NPs doping on the structure and optical properties of PVC polymer films. Polym. Bull. 2019, 76, 4769–4784. [Google Scholar] [CrossRef]
- Higashihara, T.; Ueda, M. Recent Progress in High Refractive Index Polymers. Macromolecules 2015, 48, 1915–1929. [Google Scholar] [CrossRef]
- Tang, Y.; Cabrini, S.; Nie, J.; Pina-Hernandez, C. High-refractive index acrylate polymers for applications in nanoimprint lithography. Chin. Chem. Lett. 2020, 31, 256–260. [Google Scholar] [CrossRef]
- Nalwa, H.S. Organic materials for third-order nonlinear optics. Adv. Mater. 1993, 5, 341–358. [Google Scholar] [CrossRef]
- Mondal, R.; Biswas, D.; Paul, S.; Das, A.S.; Chakrabarti, C.; Roy, D.; Bhattacharya, S.; Kabi, S. Investigation of microstructural, optical, physical properties and dielectric relaxation process of sulphur incorporated selenium–tellurium ternary glassy systems. Mater. Chem. Phys. 2021, 257, 123793. [Google Scholar] [CrossRef]
- Keru, G.; Ndungu, P.G.; Nyamori, V.O. A review on carbon nanotube/polymer composites for organic solar cells. Int. J. Energy Res. 2014, 38, 1635–1653. [Google Scholar] [CrossRef]
- Divya, R.; Manikandan, N.; Girisun, T.S.; Vinitha, G. Investigations on the structural, morphological, linear and third order nonlinear optical properties of manganese doped zinc selenide nanoparticles for optical limiting application. Opt. Mater. 2020, 100, 109641. [Google Scholar] [CrossRef]
- Bredas, J.L.; Adant, C.; Tackx, P.; Persoons, A.; Pierce, B.M. Third-order nonlinear optical response in organic materials: Theoretical and experimental aspects. Chem. Rev. 1994, 94, 243–278. [Google Scholar] [CrossRef]
- Innocenzi, P.; Lebeau, B. Organic–inorganic hybrid materials for non-linear optics. J. Mater. Chem. 2005, 15, 3821–3831. [Google Scholar] [CrossRef]
- Dhatarwal, P.; Sengwa, R.J. Investigation on the optical properties of (PVP/PVA)/Al2O3 nanocomposite films for green disposable optoelectronics. Phys. B Condens. Matter 2021, 613, 412989. [Google Scholar] [CrossRef]
- Ismail, A.M.; Mohammed, M.I.; Fouad, S.S. Optical and structural properties of polyvinylidene fluoride (PVDF)/reduced graphene oxide (RGO) nanocomposites. J. Mol. Struct. 2018, 1170, 51–59. [Google Scholar] [CrossRef]
- Sabira, K.; Saheeda, P.; Divyasree, M.C.; Jayalekshmi, S. Impressive nonlinear optical response exhibited by Poly (vinylidene fluoride)(PVDF)/reduced graphene oxide (RGO) nanocomposite films. Opt. Laser Technol. 2017, 97, 77–83. [Google Scholar] [CrossRef]
- El-Metwally, E.G.; Nasrallah, D.A.; Fadel, M. The effect of Li4Ti5O12 nanoparticles on structural, linear and third order nonlinear optical properties of PVDF films. Mater. Res. Express 2019, 6, 085312. [Google Scholar] [CrossRef]



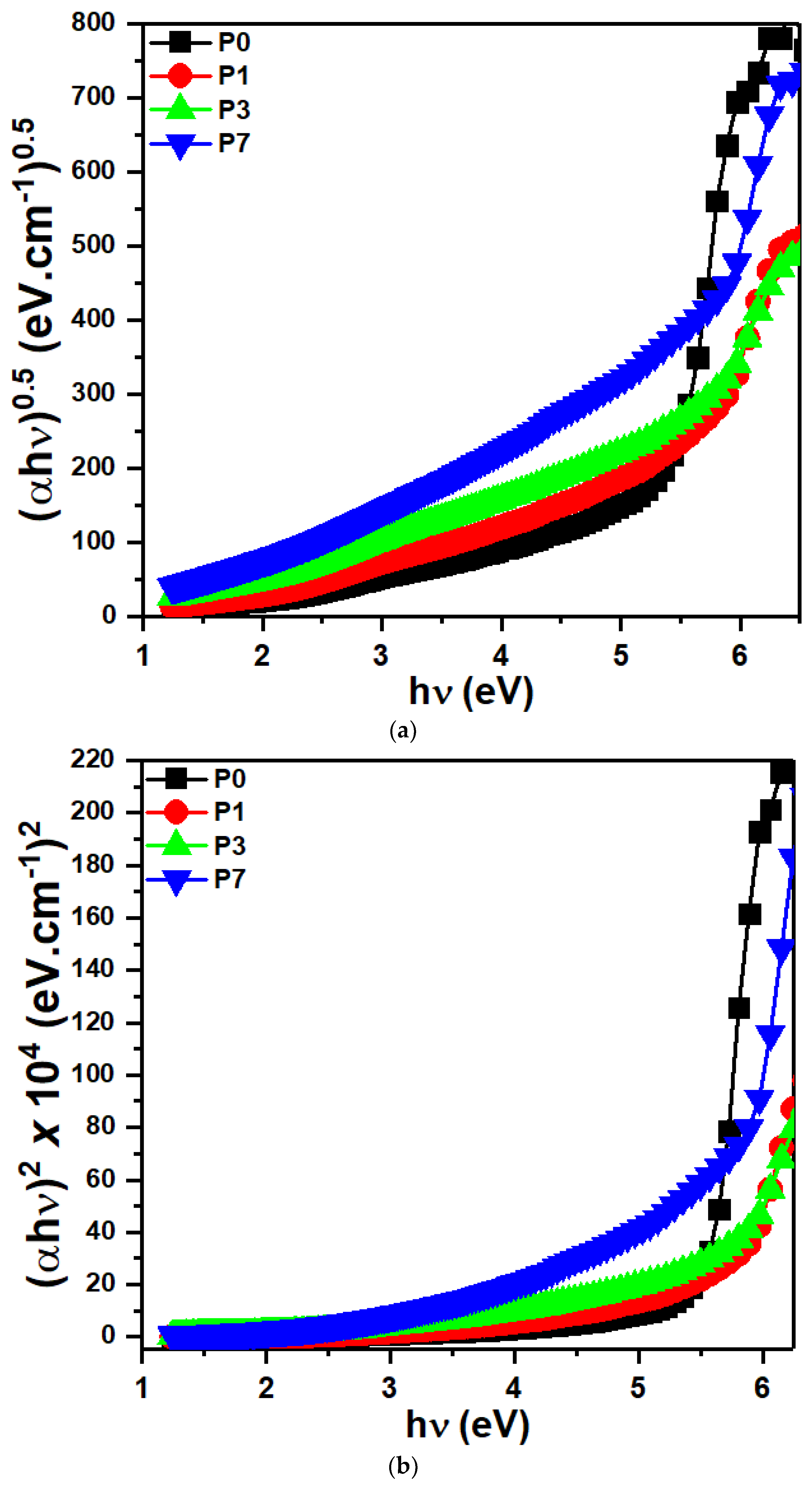
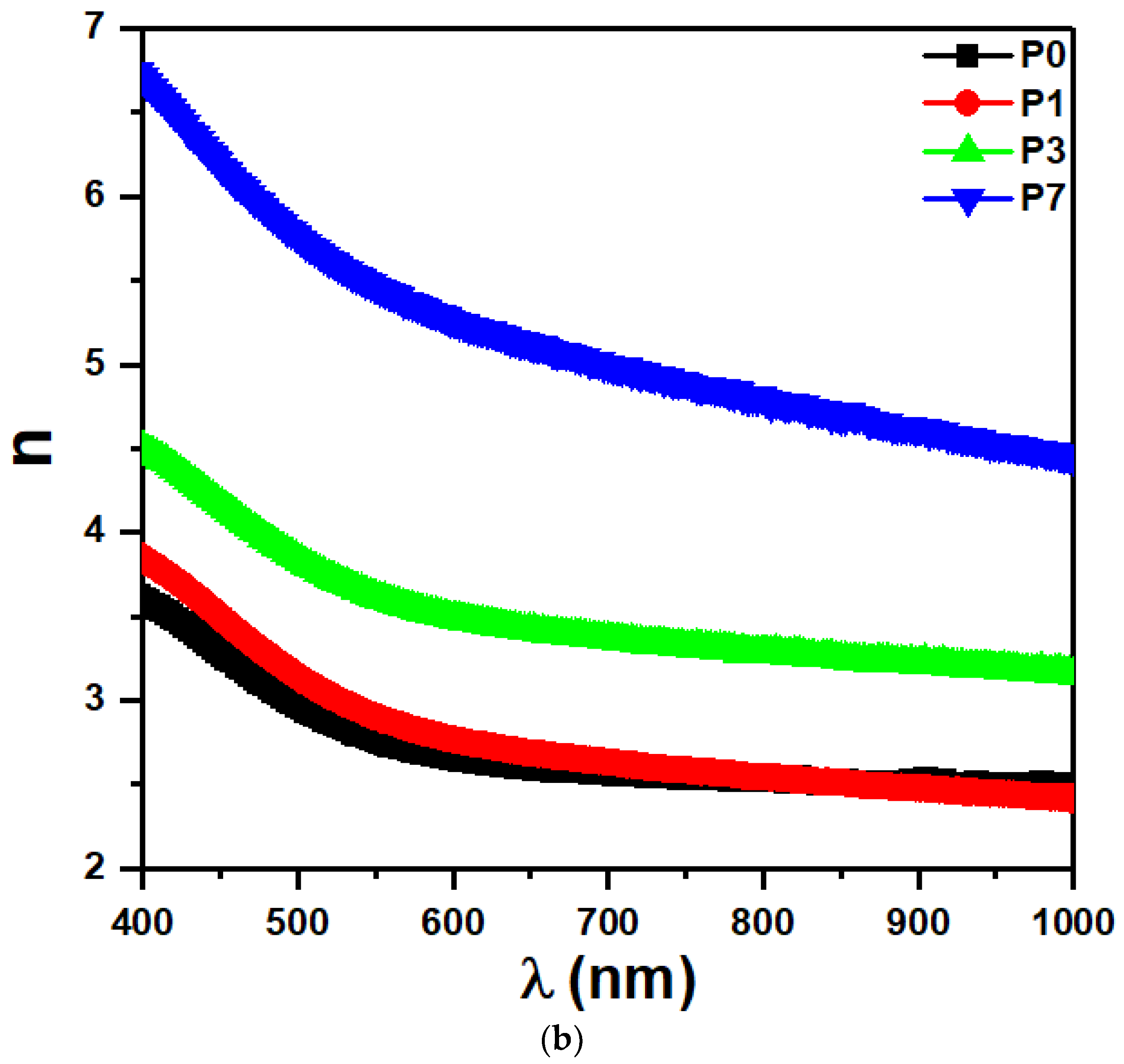
| SrTiO3/CNTs (wt%) | Eg(ind) (eV) | Eg(dir) (eV) | E0 (eV) | Ed (eV) | n0 |
|---|---|---|---|---|---|
| 0.0 | 5.27 | 5.56 | 4.77 | 23.08 | 2.42 |
| 0.1 | 5.50 | 5.70 | 3.48 | 15.29 | 2.32 |
| 0.3 | 5.40 | 5.60 | 4.06 | 33.95 | 3.06 |
| 0.7 | 5.30 | 5.53 | 3.70 | 63.63 | 4.27 |
| SrTiO3/CNTs (wt%) | χ(1) (esu) | χ(3) × 10−12 (esu) | n2 × 10−11 (esu) |
|---|---|---|---|
| 0.0 | 0.38 | 3.73 | 5.82 |
| 0.1 | 0.35 | 2.53 | 4.12 |
| 0.3 | 0.66 | 33.26 | 40.98 |
| 0.7 | 1.37 | 597.70 | 528.09 |
| Polymer Nanocomposite | Direct Band Gap (eV) | Indirect Band Gap (eV) | χ(3) (esu) | n2 (esu) | Ref. |
|---|---|---|---|---|---|
| PVDF/CaFe2O4 | 5.15 | 4.18 | 870 × 10−9 | 3.17 × 10−6 | [36] |
| PVDF/MoO3/g-C3N4 | 4.5 | 4.0 | 2.5 × 10−10 | 2.5 × 10−9 | [57] |
| PVDF/ZnO | 4.95 | 3.35 | - | - | [31] |
| PVDF/RGO | 4.3 | 3.2 | - | - | [75] |
| PVDF/RGO | - | - | 12.96 × 10−11 | 6.27 × 10−10 | [76] |
| PVDF/Li4Ti5O12 | 4.858 | 1.005 | 1.0 × 10−6 | 6.0 × 10−6 | [77] |
| PVDF/SrTiO3/CNTS | 5.53 | 5.30 | 597.7 × 10−12 | 528.09 × 10−11 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taha, T.A.M.; Alanazi, S.S.; El-Nasser, K.S.; Alshammari, A.H.; Ismael, A. Structure–Property Relationships in PVDF/SrTiO3/CNT Nanocomposites for Optoelectronic and Solar Cell Applications. Polymers 2024, 16, 736. https://doi.org/10.3390/polym16060736
Taha TAM, Alanazi SS, El-Nasser KS, Alshammari AH, Ismael A. Structure–Property Relationships in PVDF/SrTiO3/CNT Nanocomposites for Optoelectronic and Solar Cell Applications. Polymers. 2024; 16(6):736. https://doi.org/10.3390/polym16060736
Chicago/Turabian StyleTaha, Taha Abdel Mohaymen, Sultan Saud Alanazi, Karam S. El-Nasser, Alhulw H. Alshammari, and Ali Ismael. 2024. "Structure–Property Relationships in PVDF/SrTiO3/CNT Nanocomposites for Optoelectronic and Solar Cell Applications" Polymers 16, no. 6: 736. https://doi.org/10.3390/polym16060736







