A Review of the Strategic Use of Sodium Alginate Polymer in the Immobilization of Microorganisms for Water Recycling
Abstract
:1. Introduction
2. Materials for Microorganism Immobilization
3. Intrinsic and Specific Properties of Polymers
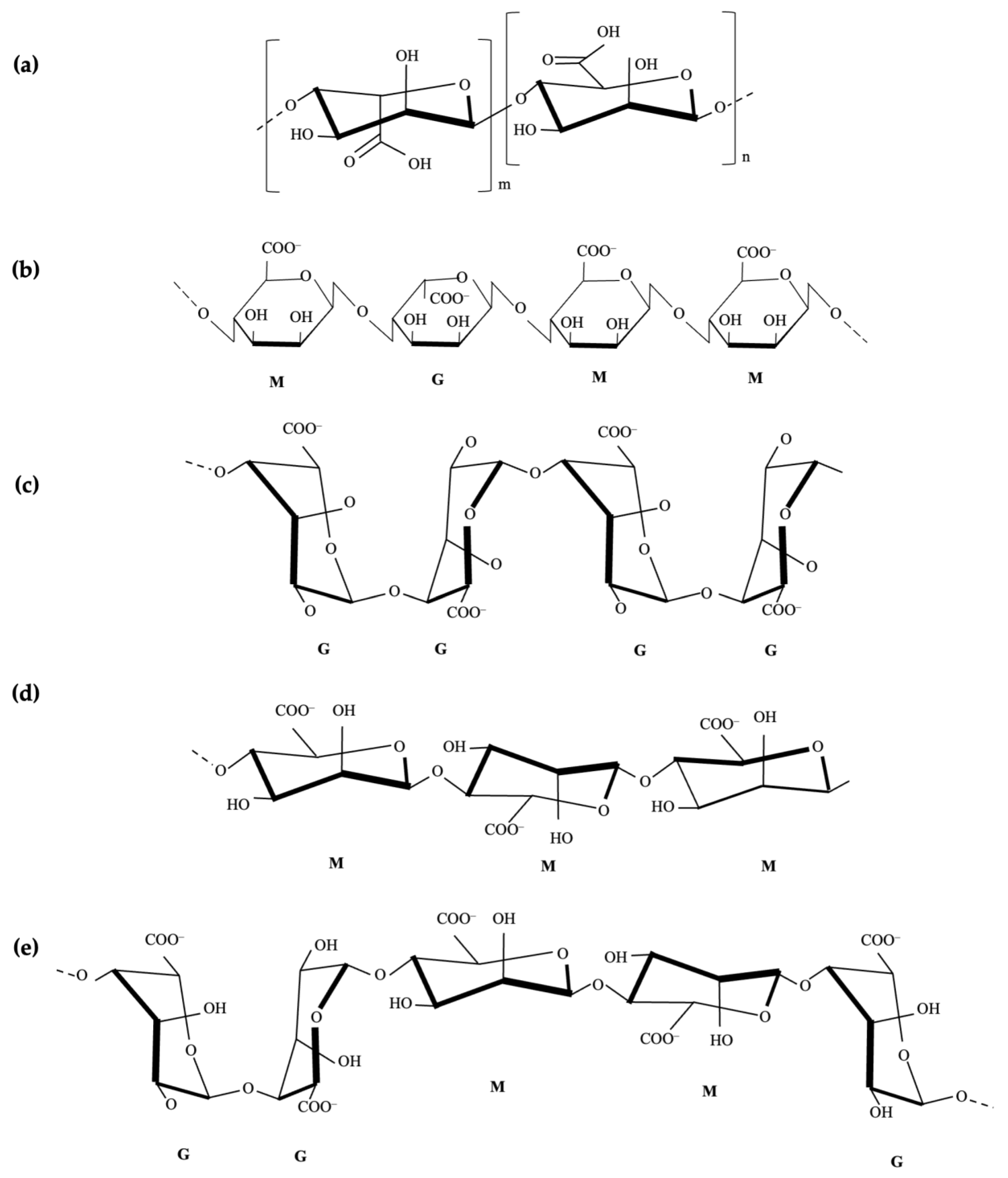
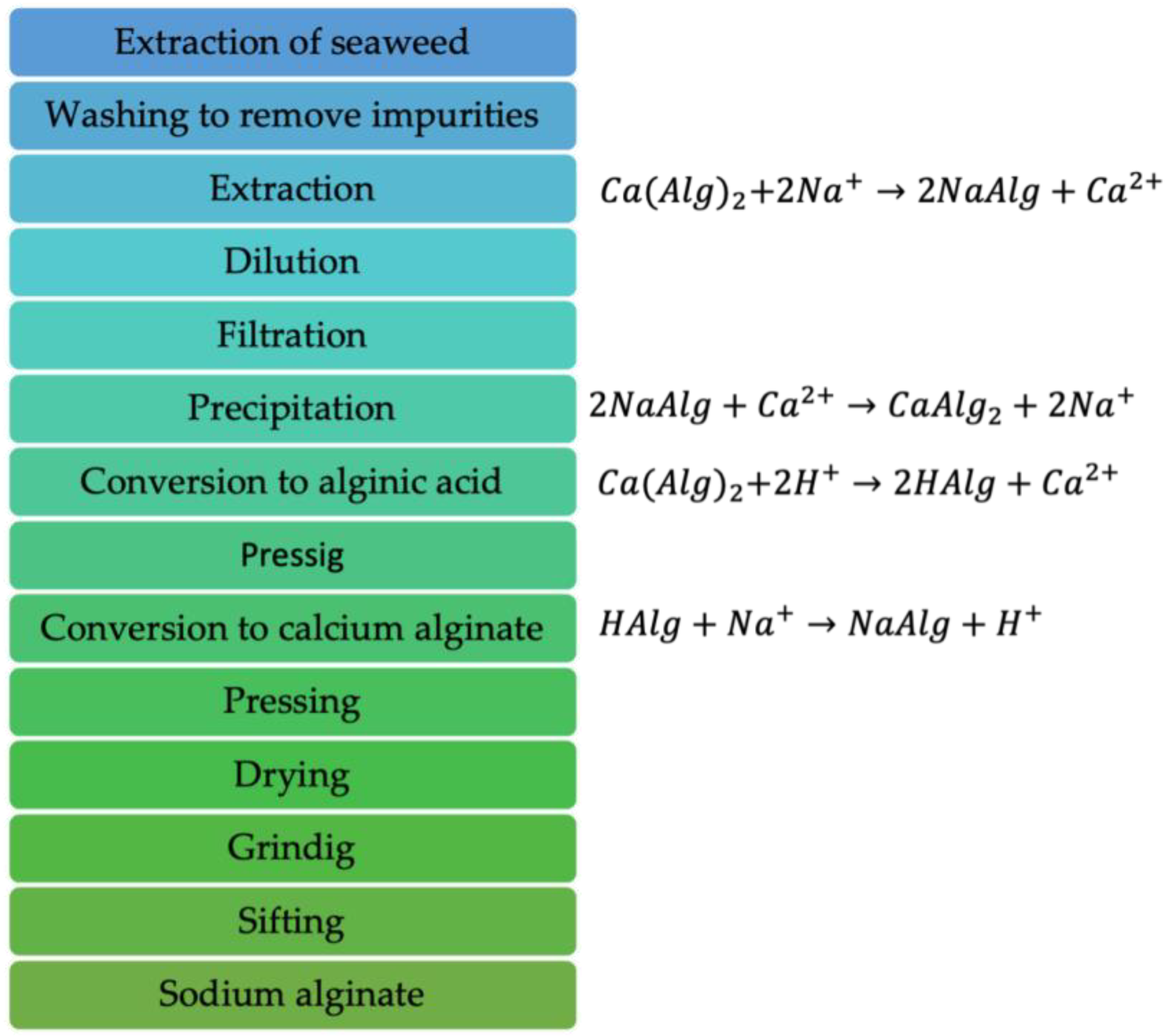
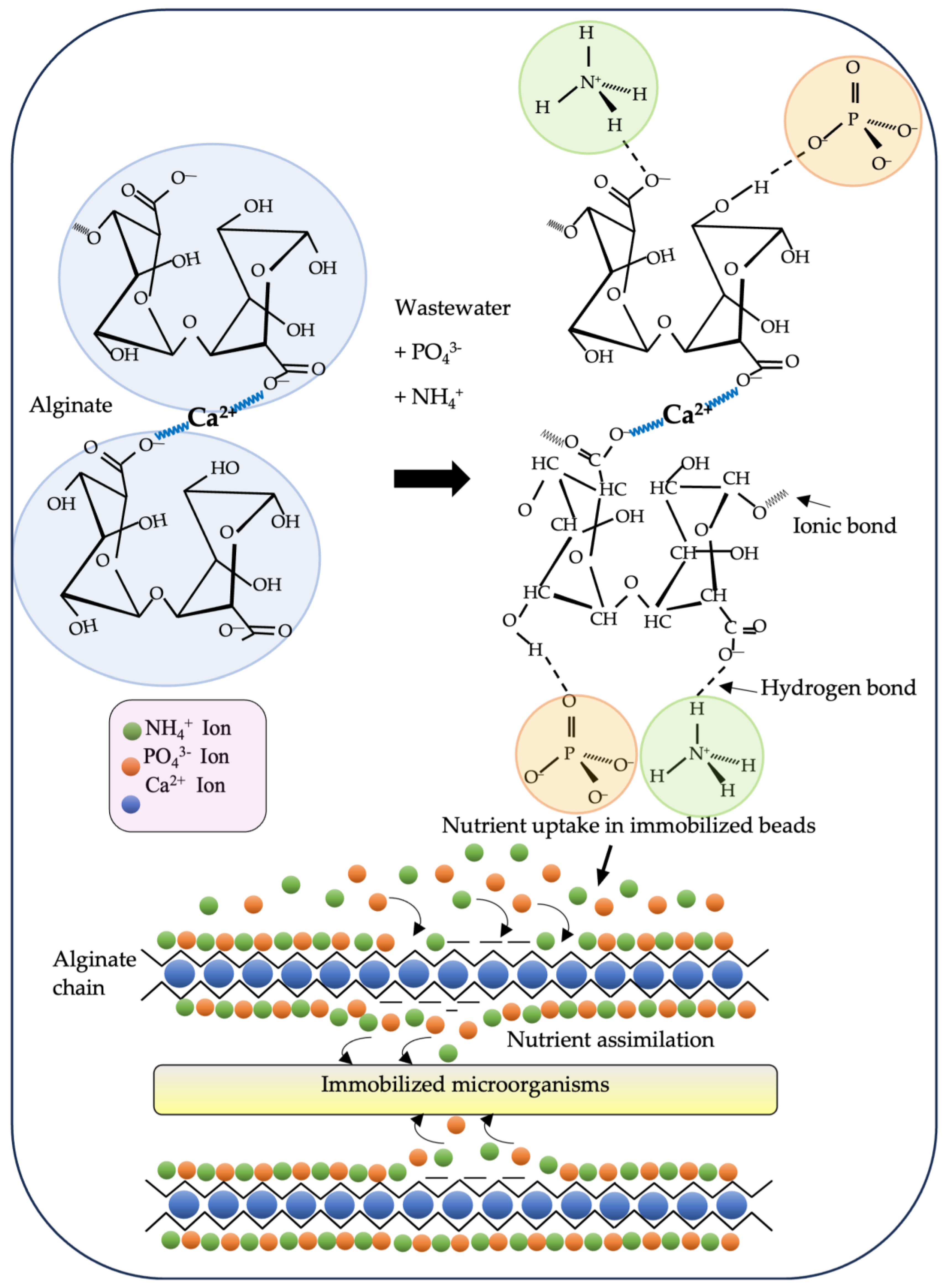
Uses and Properties of Sodium Alginate
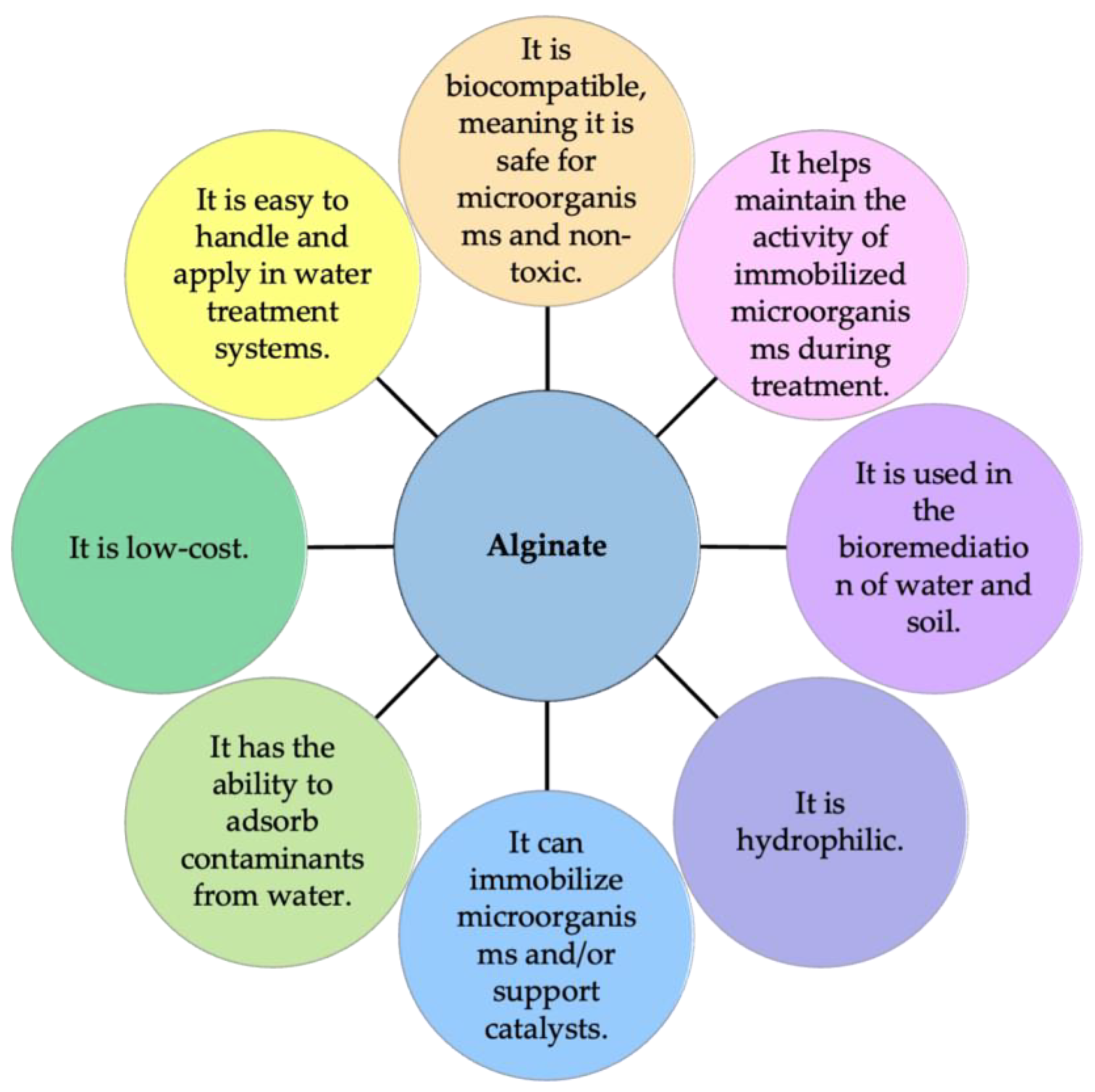
4. Sodium Alginate as an Adsorbent
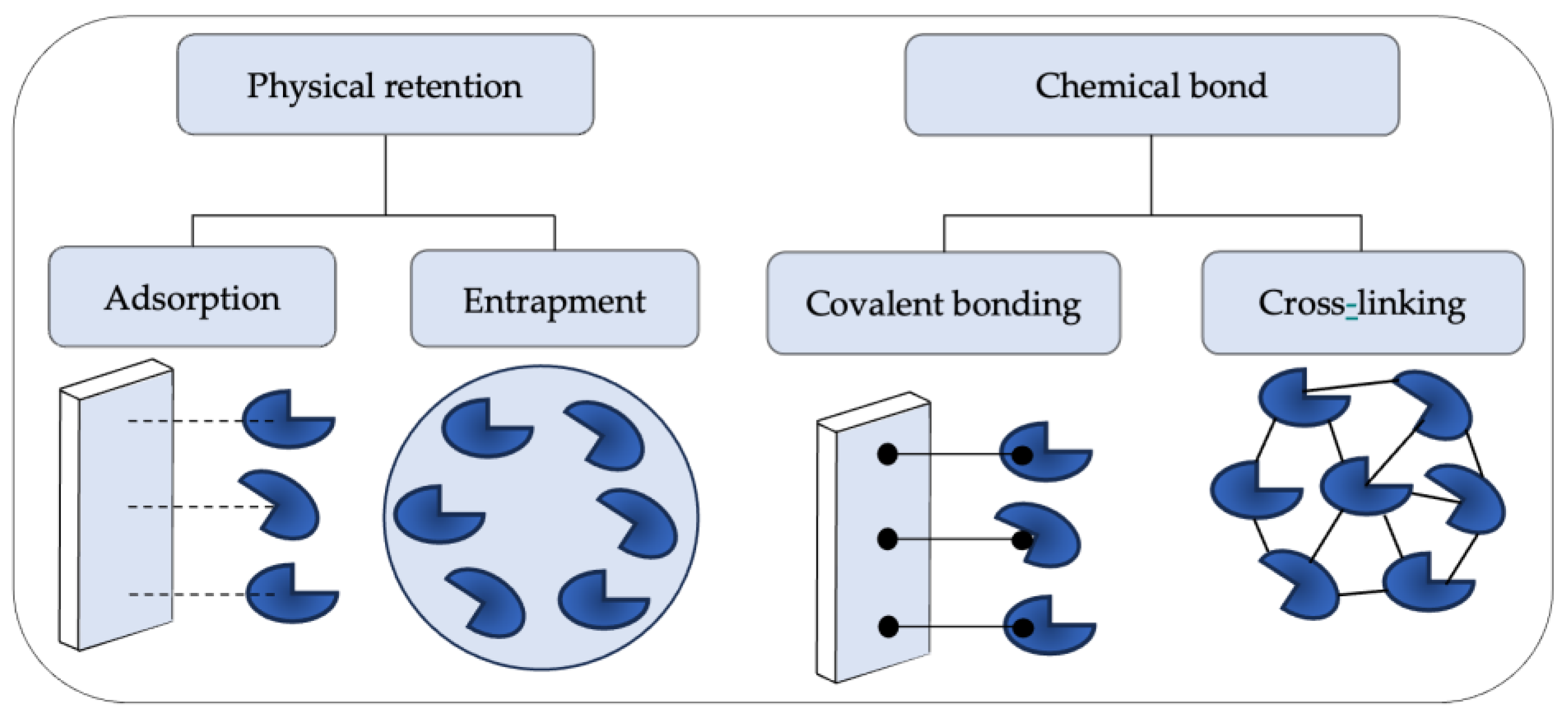
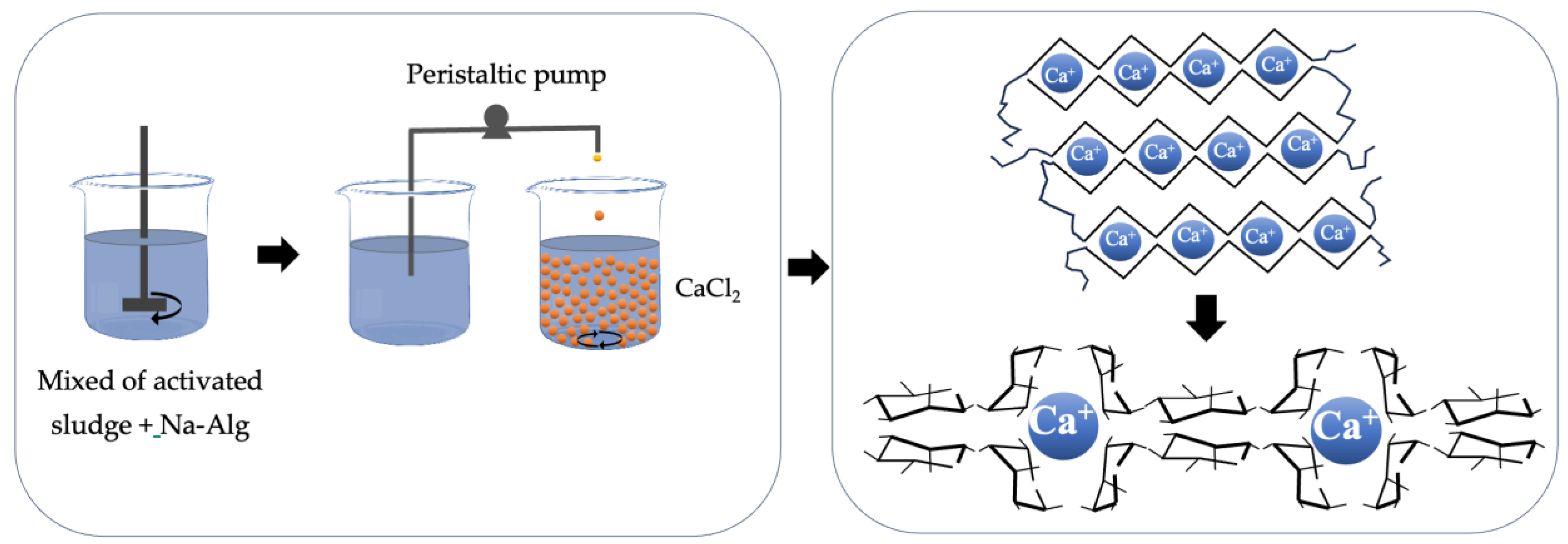
5. Immobilization Methods
6. Types of Immobilized Microorganisms
Factors Affecting Immobilized Microorganisms
7. Contaminants Removed by Immobilized Microorganisms
Degradation of Nutrients by Immobilized Microorganisms
8. Residence Times Using Immobilized Microorganisms
9. Cycles in the Use of Alginate and Immobilized Microorganisms
10. Degradation Kinetics of Contaminants Using Na-Alg
10.1. Isotherm of Adsorption
10.2. Kinetics of Adsorption
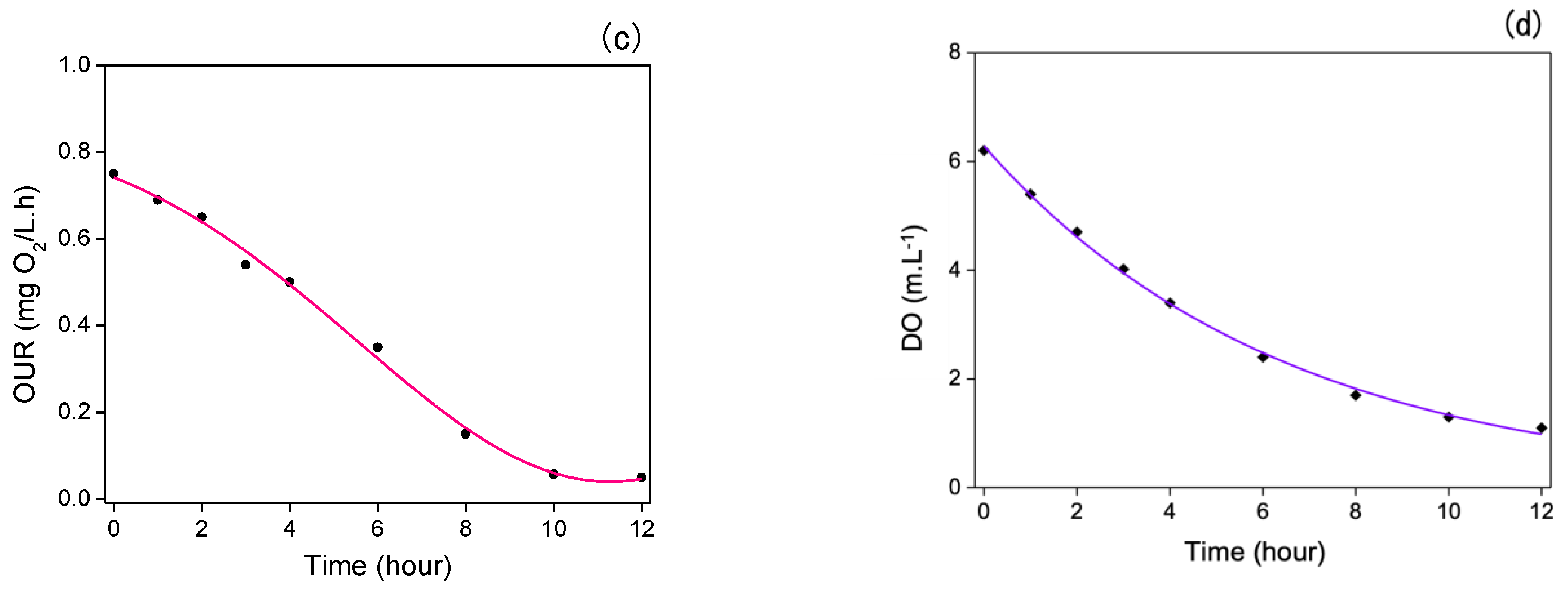
11. Dissolved Oxygen Consumption Rate of Immobilized Microorganisms
12. Case Study
13. Challenges and Future Investigations
14. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bustos-Terrones, Y.A.; Bandala, E.R.; Moeller-Chávez, G.E.; Bustos-Terrones, V. Enhanced biological wastewater treatment using sodium alginate-immobilized microorganisms in a fluidized bed reactor. Polym. Int. 2023, 72, 984–996. [Google Scholar] [CrossRef]
- Bustos-Terrones, Y.A.; Hermosillo-Nevárez, J.J.; Ramírez-Pereda, B.; Vaca, M.; Rangel-Peraza, J.G.; Bustos-Terrones, V.; Rojas-Valencia, M.N. Removal of BB9 textile dye by biological, physical, chemical, and electrochemical treatments. J. Taiwan Inst. Chem. Eng. 2021, 121, 29–37. [Google Scholar] [CrossRef]
- Wasito, H.; Fatoni, A.; Hermawan, D.; Susilowati, S.S. Immobilized bacterial biosensor for rapid and effective monitoring of acute toxicity in water. Ecotoxicol. Environ. Saf. 2019, 170, 205–209. [Google Scholar] [CrossRef]
- Tang, K.H.D.; Lock, S.S.M.; Yap, P.S.; Cheah, K.W.; Chan, Y.H.; Yiin, C.L.; Chai, Y.H. Immobilized enzyme/microorganism complexes for degradation of microplastics: A review of recent advances, feasibility and future prospects. Sci. Total Environ. 2022, 832, 154868. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Wang, K.; Bai, B. A critical review of sodium alginate-based composites in water treatment. Carb. Polym. 2024, 331, 121850. [Google Scholar] [CrossRef] [PubMed]
- Naseem, K.; Tahir, M.H.; Farooqi, F.; Manzoor, S.; Khan, S.U. Strategies adopted for the preparation of sodium alginate–based nanocomposites and their role as catalytic, antibacterial, and antifungal agents. Rev. Chem. Eng. 2023, 39, 1359–1391. [Google Scholar] [CrossRef]
- Hernández-Carmona, G.; Rodríguez-Montesinos, Y.E.; Arvizu-Higuera, D.L.; Reyes-Tisnado, R.; Murillo-Álvarez, J.I.; Muñoz-Ochoa, M. Technological advance for alginate production in Mexico. Ing. Investig. Y Tecnol. 2012, 13, 155–168. [Google Scholar]
- Ünsal, S.B.E.; Tufan, H.N.G.; Canatar, M.; Yatmaz, H.A.; Turhan, İ.; Yatmaz, E. Ethanol production by immobilized Saccharomyces cerevisiae cells on 3D spheres designed by different lattice structure types. Process Biochem. 2023, 125, 104–112. [Google Scholar] [CrossRef]
- Costa, I.O.; Morais, J.R.F.; de Medeiros Dantas, J.M.; Gonçalves, L.R.B.; Dos Santos, E.S.; Rios, N.S. Enzyme immobilization technology as a tool to innovate in the production of biofuels: A special review of the Cross-Linked Enzyme Aggregates (CLEAs) strategy. Enzym. Microb. Technol. 2023, 170, 110300. [Google Scholar] [CrossRef]
- Sam, S.P.; Adnan, R.; Ng, S.L. Statistical optimization of immobilization of activated sludge in PVA/alginate cryogel beads using response surface methodology for p-nitrophenol biodegradation. J. Water Process Eng. 2021, 39, 101725. [Google Scholar] [CrossRef]
- Sivakumar, M.; Senthamarai, R.; Rajendran, L.; Lyons, M.E.G. Reaction and Kinetic Studies of Immobilized Enzyme Systems: Part-I Without External Mass Transfer Resistance. Int. J. Electrochem. Sci. 2022, 17, 221159. [Google Scholar] [CrossRef]
- Li, J.; Jia, Y.; Zhong, J.; Liu, Q.; Li, H.; Agranovski, I. Use of calcium alginate/biochar microsphere immobilized bacteria Bacillus sp. for removal of phenol in water. Environ. Chall. 2022, 9, 100599. [Google Scholar] [CrossRef]
- Yu, X.; Shi, J.; Khan, A.; Yun, H.; Zhang, P.; Zhang, P.; Li, X. Immobilized-microbial bioaugmentation protects aerobic denitrification from heavy metal shock in an activated-sludge reactor. Bioresour. Technol. 2020, 307, 123185. [Google Scholar] [CrossRef]
- Saravanan, A.; Swaminaathan, P.; Kumar, P.S.; Yaashikaa, P.R.; Kamalesh, R.; Rangasamy, G. A comprehensive review on immobilized microbes-biochar and their environmental remediation: Mechanism, challenges and future perspectives. Environ. Res. 2023, 236, 116723. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhi, D.; Yao, B.; Zhou, Y.; Yang, Y.; Zhou, Y. Immobilization of microbes on biochar for water and soil remediation: A review. Environ. Res. 2022, 212, 113226. [Google Scholar] [CrossRef]
- Vishakar, V.V.; Haran, N.H.; Vidya, C.; Mohamed, M.A. Removal of ammonia in water systems using cell immobilization technique in surrounding environment. Mater. Today Proc. 2021, 43, 1513–1518. [Google Scholar] [CrossRef]
- Ozyurek, S.B. A comparative study for petroleum removal capacities of the bacterial consortia entrapped in sodium alginate, sodium alginate/poly (vinyl alcohol), and bushnell haas agar. Pet. Sci. 2024, 21, 705–715. [Google Scholar] [CrossRef]
- Woo, W.X.; Koh, H.S.; Tan, J.P.; Yeap, S.K.; Abdul, P.M.; Luthfi, A.A.I.; Manaf, S.F.A. An overview on cell and enzyme immobilization for enhanced biohydrogen production from lignocellulosic biomass. Int. J. Hydrogen Energy 2022, 47, 40714–40730. [Google Scholar] [CrossRef]
- Bustos-Terrones, Y.A.; Estrada-Vázquez, R.; Ramírez-Pereda, B.; Bustos-Terrones, V.; Rangel-Peraza, J.G. Kinetics of a fixed bed reactor with immobilized microorganisms for the removal of organic matter and phosphorous. Water Environ. Res. 2020, 92, 1956–1965. [Google Scholar] [CrossRef]
- Katam, K.; Bhattacharyya, D. Simultaneous treatment of domestic wastewater and bio-lipid synthesis using immobilized and suspended cultures of microalgae and activated sludge. J. Ind. Eng. Chem. 2019, 69, 295–303. [Google Scholar] [CrossRef]
- Chwastowski, J.; Staroń, P. Immobilization of Phaffia rhodozyma cells in biopolymer for enhanced Cr (VI) bioremediation. Colloids Surf. A: Physicochem. Eng. Asp. 2023, 672, 131698. [Google Scholar] [CrossRef]
- Bouabidi, Z.B.; El-Naas, M.H.; Zhang, Z. Immobilization of microbial cells for the biotreatment of wastewater: A review. Environ. Chem. Lett. 2019, 17, 241–257. [Google Scholar] [CrossRef]
- Gong, Y.Z.; Niu, Q.Y.; Liu, Y.G.; Dong, J.; Xia, M.M. Development of multifarious carrier materials and impact conditions of immobilised microbial technology for environmental remediation: A review. Environ. Pollut. 2022, 314, 120232. [Google Scholar] [CrossRef]
- Girijan, S.; Kumar, M. Immobilized biomass systems: An approach for trace organics removal from wastewater and environmental remediation. Curr. Opin. Environ. Sci. Health 2019, 12, 18–29. [Google Scholar] [CrossRef]
- Ouyang, X.; Yin, H.; Yu, X.; Guo, Z.; Zhu, M.; Lu, G.; Dang, Z. Enhanced bioremediation of 2, 3′, 4, 4′, 5-pentachlorodiphenyl by consortium GYB1 immobilized on sodium alginate-biochar. Sci. Total Environ. 2021, 788, 147774. [Google Scholar] [CrossRef]
- Perez, C.L.; Pereira, L.P.D.C.; Milessi, T.S.; Sandri, J.P.; Demeke, M.; Foulquié-Moreno, M.R.; Zangirolami, T.C. Towards a practical industrial 2G ethanol production process based on immobilized recombinant S. cerevisiae: Medium and strain selection for robust integrated fixed-bed reactor operation. Renew. Energy 2022, 185, 363–375. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, Y.; Su, R.; An, N.; Zhang, Y.; Wei, Y.; Ma, B. A novel method for immobilizing anammox bacteria in polyurethane foam carriers through dewatering. J. Water Process Eng. 2023, 53, 103738. [Google Scholar] [CrossRef]
- Li, R.; Wang, B.; Niu, A.; Cheng, N.; Chen, M.; Zhang, X.; Wang, S. Application of biochar immobilized microorganisms for pollutants removal from wastewater: A review. Sci. Total Environ. 2022, 837, 155563. [Google Scholar] [CrossRef] [PubMed]
- Zamel, D.; Khan, A.U. Bacterial immobilization on cellulose acetate-based nanofibers for methylene blue removal from wastewater: Mini-review. Inorg. Chem. Commun. 2021, 131, 108766. [Google Scholar] [CrossRef]
- Brányik, T.; Kuncová, G.; Páca, J.; Demnerová, K. Encapsulation of microbial cells into silica gel. J. Sol-Gel Sci. Technol. 1998, 13, 283–287. [Google Scholar] [CrossRef]
- Mehrotra, T.; Dev, S.; Banerjee, A.; Chatterjee, A.; Singh, R.; Aggarwal, S. Use of immobilized bacteria for environmental bioremediation: A review. J. Environ. Chem. Eng. 2021, 9, 105920. [Google Scholar] [CrossRef]
- Mark, J.E. (Ed.) Physical Properties of Polymers Handbook; Springer: New York, NY, USA, 2007; Volume 1076, p. 825. [Google Scholar]
- Lapointe, M.; Barbeau, B. Understanding the roles and characterizing the intrinsic properties of synthetic vs. natural polymers to improve clarification through interparticle Bridging: A review. Sep. Purif. Technol. 2020, 231, 115893. [Google Scholar] [CrossRef]
- Ahmad, A.; Mubarak, N.M.; Jannat, F.T.; Ashfaq, T.; Santulli, C.; Rizwan, M.; Ali, S. A critical review on the synthesis of natural sodium alginate based composite materials: An innovative biological polymer for biomedical delivery applications. Processes 2021, 9, 137. [Google Scholar] [CrossRef]
- Akbar, M.; Yaqoob, A.; Ahmad, A.; Luque, R. Sodium alginate: An overview. In Sodium Alginate-Based Nanomaterials for Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–17. [Google Scholar]
- Badita, C.R.; Aranghel, D.; Burducea, C.; Mereuta, P.J.R.J.P. Characterization of sodium alginate based films. Rom. J. Phys. 2020, 65, 602. [Google Scholar]
- Guo, H.; Qin, Q.; Chang, J.S.; Lee, D.J. Modified alginate materials for wastewater treatment: Application prospects. Bioresour. Technol. 2023, 387, 129639. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, R.; Ghobril, C.; Perrin, P.; Sanson, N. Adsorption of sodium alginate on calcium carbonate microparticles: Effect of molar mass and composition. Colloids Surf. A: Physicochem. Eng. Asp. 2024, 682, 132782. [Google Scholar] [CrossRef]
- Sipos, B.; Benei, M.; Katona, G.; Csóka, I. Optimization and Characterization of Sodium Alginate Beads Providing Extended Release for Antidiabetic Drugs. Molecules 2023, 28, 6980. [Google Scholar] [CrossRef] [PubMed]
- Jadach, B.; Świetlik, W.; Froelich, A. Sodium alginate as a pharmaceutical excipient: Novel applications of a well-known polymer. J. Pharm. Sci. 2022, 111, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Azizeh, N.; Karam, A.; Heer, A.; Najlah, M.; Singer, R.; Alany, R.G.; Khoder, M. Efficient removal of antibiotics from water by highly crosslinked metal-alginate particles: Preparation, isotherms, kinetics, and microbiological assay. Carbohydr. Polym. 2024, 326, 121604. [Google Scholar] [CrossRef]
- Alkhursani, S.A.; Barai, H.R.; Alshangiti, D.M.; Al-Gahtany, S.A.; Ghobashy, M.M.; Atia, G.A.N.; Joo, S.W. pH-Sensitive Hydrogel Membrane-Based Sodium Alginate/Poly (vinyl alcohol) Cross-Linked by Freeze–Thawing Cycles for Dye Water Purification. ACS ES&T Water 2023, 4, 509–519. [Google Scholar]
- Chen, Q.; Zuo, W.; Xie, Z.; Liu, W.; Lu, M.; Qiu, X.; Zhu, J. Design of sodium alginate/PVA based high-efficiency recycled rewritten film by water-soluble-regeneration. Cellulose 2023, 30, 7865–7875. [Google Scholar] [CrossRef]
- Stachowiak, N.; Kowalonek, J.; Kozlowska, J.; Burkowska-But, A. Stability Studies, Biodegradation Tests, and Mechanical Properties of Sodium Alginate and Gellan Gum Beads Containing Surfactant. Polymers 2023, 15, 2568. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Lin, Z.; Fu, Q.; Xu, Y.; Chen, Y.; Lu, L. An eco-friendly versatile superabsorbent hydrogel based on sodium alginate and urea for soil improvement with a synchronous chemical loading strategy. Carbohydr. Polym. 2024, 327, 121676. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Nosheen, A.; Atiq, M.S.; Mushtaq, B.; Ahmad, S.; Azam, F.; Nawab, Y. An eco-friendly hydroentangled cotton non-woven membrane with alginate hydrogel for water filtration. Int. J. Biol. Macromol. 2024, 256, 128422. [Google Scholar] [CrossRef] [PubMed]
- Bairagi, S.; Banerjee, S.; Mulvihill, D.M.; Ahmed, S.; Ali, S.W. Extraction, structural properties, and applications of sodium alginate. In Natural Gums; Elsevier: Amsterdam, The Netherlands, 2023; pp. 599–618. [Google Scholar]
- Shaikh, M.A.J.; Gupta, G.; Goyal, A.; Gupta, M.M.; Afzal, O.; Altamimi, A.S.A.; Dua, K. Sodium alginate-based drug delivery for diabetes management: A review. Int. J. Biol. Macromol. 2023, 236, 123986. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Velu, G.; Palaniappan, M.; Jackson, K.M. Decolourization of azo dyes using immobilized bacterial isolates from termite mound ecosystem. Total Environ. Res. Themes 2023, 6, 100041. [Google Scholar] [CrossRef]
- Chen, S.; Wen, H.; Zheng, T.; Liu, X.; Wang, Z.; Tian, S.; Wang, Y. Engineering sodium alginate-SiO2 composite beads for efficient removal of methylene blue from water. Int. J. Biol. Macromol. 2023, 239, 124279. [Google Scholar] [CrossRef]
- Yan, P.; Lan, W.; Xie, J. Modification on sodium alginate for food preservation: A review. Trends Food Sci. Technol. 2023, 143, 104217. [Google Scholar] [CrossRef]
- Wu, P.H.; Hsieh, T.M.; Wu, H.Y.; Yu, C.P. Characterization of the immobilized algae-based bioreactor with external ceramic ultrafiltration membrane to remove nutrients from the synthetic secondary wastewater effluent. Int. Biodeterior. Biodegrad. 2021, 164, 105309. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Y. Sodium alginate-based functional materials toward sustainable applications: Water treatment and energy storage. Ind. Eng. Chem. Res. 2023, 62, 11279–11304. [Google Scholar] [CrossRef]
- Asif, A.; Mubeen, S.; Ahmad, A.; Luque, R.; Verpoort, F. Sodium alginate base nanocomposite for wastewater treatment. In Sodium Alginate-Based Nanomaterials for Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2023; pp. 183–198. [Google Scholar]
- Han, M.; Zhang, C.; Ho, S.H. Immobilized microalgal system: An achievable idea for upgrading current microalgal wastewater treatment. Environ. Sci. Ecotechnol. 2022, 14, 100227. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chen, C.; Chen, W.; Jiang, J.; Chen, B.; Zheng, F. Effective immobilization of Bacillus subtilis in chitosan-sodium alginate composite carrier for ammonia removal from anaerobically digested swine wastewater. Chemosphere 2021, 284, 131266. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, Y.; Fu, X.; Li, N.; Sun, J.; Qiao, Y. Study on degradation characteristics and bacterial community structure changes of immobilized cells in straw-alginate beads in marine environment. Bioresour. Technol. Rep. 2020, 10, 100402. [Google Scholar] [CrossRef]
- Raza, S.; Ghasali, E.; Hayat, A.; Zhang, P.; Orooji, Y.; Lin, H. Sodium alginate hydrogel-encapsulated trans-anethole based polymer: Synthesis and applications as an eradicator of metals and dyes from wastewater. Int. J. Biol. Macromol. 2024, 254, 127153. [Google Scholar] [CrossRef]
- Kamacı, U.D.; Kamacı, M. Hydrogel beads based on sodium alginate and quince seed nanoparticles for the adsorption of methylene blue. Inorg. Chem. Commun. 2024, 160, 111919. [Google Scholar] [CrossRef]
- Idrissi, A.; Dardari, O.; Metomo, F.N.; Essamlali, Y.; Akil, A.; Amadine, O.; Zahouily, M. Effect of sodium alginate-based superabsorbent hydrogel on tomato growth under different water deficit conditions. Int. J. Biol. Macromol. 2023, 253, 127229. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, H.; Xi, Y.; Huang, Y.; Su, Z.; Zhang, Z.; Li, X. Adsorption of lead ions by activated carbon doped sodium alginate/sodium polyacrylate hydrogel beads and their in-situ recycle as sustainable photocatalysts. J. Colloid Interface Sci. 2023, 645, 133–145. [Google Scholar] [CrossRef]
- Ma, Y.; Qi, P.; Ju, J.; Wang, Q.; Hao, L.; Wang, R.; Tan, Y. Gelatin/alginate composite nanofiber membranes for effective and even adsorption of cationic dyes. Composites Part B Eng. 2019, 162, 671–677. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, X.; Lou, T. Preparation of fibrous chitosan/sodium alginate composite foams for the adsorption of cationic and anionic dyes. J. Hazard. Mater. 2021, 403, 124054. [Google Scholar] [CrossRef]
- Zhao, H.; Ouyang, X.K.; Yang, L.Y. Adsorption of lead ions from aqueous solutions by porous cellulose nanofiber–sodium alginate hydrogel beads. J. Mol. Liq. 2021, 324, 115122. [Google Scholar] [CrossRef]
- Zhu, C.; Feng, Z.; Meng, Y.; Wang, M.; Wang, Z. Effective removal of lead and copper ions from water using a novel sodium alginate–streptomycin sulfate composite aerogel. N. J. Chem. 2023, 47, 6739–6748. [Google Scholar] [CrossRef]
- Yaacoubi, F.E.; Sekkouri, C.; Ennaciri, K.; Rabichi, I.; Izghri, Z.; Baçaoui, A.; Yaacoubi, A. Synthesis of composites from activated carbon based on olive stones and sodium alginate for the removal of methylene blue. Int. J. Biol. Macromol. 2024, 254, 127706. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Guo, C.; Hao, J.; Zhao, Z.; Long, H.; Li, M. Adsorption of heavy metal ions by sodium alginate-based adsorbent—A review and new perspectives. Int. J. Biol. Macromol. 2020, 164, 4423–4434. [Google Scholar] [CrossRef]
- Banerjee, S.; Tiwade, P.B.; Sambhav, K.; Banerjee, C.; Bhaumik, S.K. Effect of alginate concentration in wastewater nutrient removal using alginate-immobilized microalgae beads: Uptake kinetics and adsorption studies. Biochem. Eng. J. 2019, 149, 107241. [Google Scholar] [CrossRef]
- Phiri, I.; Ko, J.M.; Mushonga, P.; Kugara, J.; Opiyo Onani, M.; Msamadya, S.; Madzvamuse, A. Simultaneous removal of cationic, anionic and organic pollutants in highly acidic water using magnetic nanocomposite alginate beads. J. Water Process Eng. 2019, 31, 100884. [Google Scholar] [CrossRef]
- Lu, D.; Liu, C.; Zhu, F.; Liu, Y.; Lin, Y.; Yang, Q.; Han, S. Fabrication and performance of novel multifunctional sodium alginate/polyvinylpyrrolidone hydrogels. Chemosphere 2024, 348, 140758. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Kamani, H.; Esrafili, A.; Badi, M.Y.; Gholami, M. Removal of Malathion by Sodium Alginate/Biosilicate/Magnetite Nanocomposite as a Novel Adsorbent: Kinetics, Isotherms, and Thermodynamic. Study Health Scope 2019, 8, 11. [Google Scholar] [CrossRef]
- Khiavi, N.M.N.; Khiabani, M.S.; Mokarram, R.R.; Kafil, H.S. Reduction of aflatoxin M1 using mixture of Saccharomyces cerevisiae and Candida albicans cell walls immobilized on silica nanoparticles entrapped in alginate gel. J. Environ. Chem. Eng. 2020, 8, 103635. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.; Yu, D.; Wang, Y.; Wang, W.; Wu, M. Hyperbranched polyamide–functionalized sodium alginate microsphere as a novel adsorbent for the removal of antimony(III) in wastewater. Environ. Sci. Pollut. Res. Int. 2019, 26, 27372–27384. [Google Scholar] [CrossRef]
- Ablouh, E.; Essaghraoui, A.; Eladlani, N.; Rhazi, M.; Taourirte, M. Uptake of Pb(II) Onto Nanochitosan/Sodium Alginate Hybrid Beads: Mechanism and kinetics Study. Water Environ. Res. 2019, 91, 239–249. [Google Scholar] [CrossRef]
- Aziz, F.; El Achaby, M.; Lissaneddine, A.; Aziz, K.; Ouazzani, N.; Mamouni, R.; Mandi, L. Composites with alginate beads: A novel design of nano-adsorbents impregnation for large-scale continuous flow wastewater treatment pilots. Saudi J. Biol. Sci. 2019, 27, 2499–2508. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Z. Preparation of composite aerogels based on sodium alginate, and its application in removal of Pb2+ and Cu2+ from water. Int. J. Biol. Macromol. 2018, 107, 741–747. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, M.; Chen, S.; Jasmi, I.I.; Tang, Y.; Lin, S. Enhanced Adsorption and Slow Release of Phosphate by Dolomite-Alginate Composite Beads as Potential Fertilizer. Water Environ. Res. 2019, 91, 797–804. [Google Scholar] [CrossRef]
- Li, L.; Zhao, J.; Sun, Y.; Yu, F.; Ma, J. Ionically cross-linked sodium alginate/ĸ-carrageenan double-network gel beads with low-swelling, enhanced mechanical properties, and excellent adsorption performance. Chem. Eng. J. 2019, 372, 1091–1103. [Google Scholar] [CrossRef]
- Ociński, D.; Jacukowicz-Sobala, I.; Kociołek-Balawejder, E. Alginate beads containing water treatment residuals for arsenic removal from water—Formation and adsorption studies. Environ. Sci. Pollut. Res. Int. 2016, 23, 24527–24539. [Google Scholar] [CrossRef]
- Shen, C.; Zhao, Y.; Liu, R.; Mao, Y.; Morgan, D. Adsorption of phosphorus with calcium alginate beads containing drinking water treatment residual. Water Sci. Technol. 2018, 78, 1980–1989. [Google Scholar] [CrossRef]
- Zhou, A.; Zhu, C.; Chen, W.; Wan, J.; Tao, T.; Zhang, T.C.; Xie, P. Phosphorus recovery from water by lanthanum hydroxide embedded interpenetrating network poly (vinyl alcohol)/sodium alginate hydrogel beads. Colloids Surf. A Physicochem. Eng. Asp. 2018, 554, 237–244. [Google Scholar] [CrossRef]
- Godiya, C.B.; Xiao, Y.; Lu, X. Amine functionalized sodium alginate hydrogel for efficient and rapid removal of methyl blue in water. Int. J. Biol. Macromol. 2020, 144, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Shelar-Lohar, G.; Joshi, S. Amidoximated functionalized sodium alginate graft copolymer: An effective adsorbent for rapid removal of cationic dyes. Mater. Today Proc. 2020, 26, 3357–3362. [Google Scholar] [CrossRef]
- Wang, B.; Gao, B.; Zimmerman, A.R.; Zheng, Y.; Lyu, H. Novel biochar-impregnated calcium alginate beads with improved water holding and nutrient retention properties. J. Environ. Manag. 2018, 209, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ren, X.; He, J.; Zhang, Q.; Qiu, C.; Fan, B. Application of natural mixed bacteria immobilized carriers to different kinds of organic wastewater treatment and microbial community comparison. J. Hazard. Mater. 2019, 377, 113–123. [Google Scholar] [CrossRef]
- Thakre, P.N.; Mukherjee, S.; Samanta, S.; Barman, S.; Halder, G. A mechanistic insight into defluoridation of simulated wastewater applying bio-inspired sodium alginate bead. Appl. Water Sci. 2020, 10, 65. [Google Scholar] [CrossRef]
- Xu, X.; Wang, B.; Tang, H.; Jin, Z.; Mao, Y.; Huang, T. Removal of phosphate from wastewater by modified bentonite entrapped in ca-alginate beads. J. Environ. Manag. 2020, 260, 110130. [Google Scholar] [CrossRef]
- Mujtaba, G.; Lee, K. Treatment of real wastewater using co-culture of immobilized chlorella vulgaris and suspended activated sludge. Water Res. 2017, 120, 174–184. [Google Scholar] [CrossRef]
- Milojković, J.V.; Lopičić, Z.R.; Anastopoulos, I.P.; Petrović, J.T.; Milićević, S.Z.; Petrović, M.S.; Stojanović, M.D. Performance of aquatic weed-Waste Myriophyllum spicatum immobilized in alginate beads for the removal of Pb (II). J. Environ. Manag. 2019, 232, 97–109. [Google Scholar] [CrossRef]
- Sujitha, R.; Ravindhranath, K. Extraction of phosphate from polluted waters using calcium alginate beads doped with active carbon derived from A. aspera plant as adsorbent. J. Anal. Methods Chem. 2017, 2017, 3610878. [Google Scholar] [CrossRef]
- Solé, A.; Matamoros, V. Removal of endocrine disrupting compounds from wastewater by microalgae co-immobilized in alginate beads. Chemosphere 2016, 164, 516–523. [Google Scholar] [CrossRef]
- Jung, K.W.; Hwang, M.J.; Jeong, T.U.; Chau, D.M.; Kim, K.; Ahn, K.H. Entrapment of powdered drinking water treatment residues in calcium-alginate beads for fluoride removal from actual industrial wastewater. J. Ind. Eng. Chem. 2016, 39, 101–111. [Google Scholar] [CrossRef]
- Bang, S.; Choi, J.W.; Cho, K.; Chung, C.; Kang, H.; Hong, S.W. Simultaneous reduction of copper and toxicity in semiconductor wastewater using protonated alginate beads. Chem. Eng. J. 2018, 288, 525–531. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J. Uranium removal by novel graphene oxide-immobilized Saccharomyces cerevisiae gel beads. J. Environ. Radioact. 2016, 162, 134–145. [Google Scholar] [CrossRef]
- Nordin, A.H.; Ahmad, K.; Xin, L.K.; Syieluing, W.; Ngadi, N. Efficient adsorptive removal of methylene blue from synthetic dye wastewater by green alginate modified with pandan. Mater. Today Proc. 2021, 39, 979–982. [Google Scholar]
- Bilal, M.; Iqbal, H.M. Lignin peroxidase immobilization on Ca-alginate beads and its dye degradation performance in a packed bed reactor system. Biocatal. Agric. Biotechnol. 2019, 20, 101205. [Google Scholar] [CrossRef]
- Martinez, S.A.; Bustos, Y. Biodegradation of wastewater pollutants by activated sludge encapsulated inside calcium-alginate beads in a tubular packed bed reactor. Biodegradation 2009, 20, 709–715. [Google Scholar]
- Ashikin, N.A.L.N.; Fuzi, S.F.Z.M.; Manaf, S.A.A.; Manas, N.H.A.; Shaarani, S.M.; Nawi, M.; Illias, R.M. Optimization and characterization of immobilized E. coli for engineered thermostable xylanase excretion and cell viability. Arab. J. Chem. 2022, 15, 103803. [Google Scholar] [CrossRef]
- Elmerhi, N.; Al-Maqdi, K.; Athamneh, K.; Mohammed, A.K.; Skorjanc, T.; Gándara, F.; Ashraf, S.S. Enzyme-immobilized hierarchically porous covalent organic framework biocomposite for catalytic degradation of broad-range emerging pollutants in water. J. Hazard. Mater. 2023, 459, 132261. [Google Scholar] [CrossRef]
- Metin, A.Ü.; Doğan, D.; Can, M. Novel magnetic gel beads based on ionically crosslinked sodium alginate and polyanetholesulfonic acid: Synthesis and application for adsorption of cationic dyes. Mater. Chem. Phys. 2020, 256, 123659. [Google Scholar] [CrossRef]
- Li, Z.; Guo, J.; Guan, F.; Yin, J.; Yang, Q.; Zhang, S.; Wang, W. Oxidized sodium alginate cross-linked calcium alginate/antarctic krill protein composite fiber for improving strength and water resistance. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130317. [Google Scholar] [CrossRef]
- Gao, Z.; Gao, C.; Jiang, W.; Xu, L.; Hu, B.; Yao, X.; Wu, Y. In situ crosslinking sodium alginate on oil-water interface to stabilize the O/W emulsions. Food Hydrocoll. 2023, 135, 108233. [Google Scholar] [CrossRef]
- Murshid, S.; Dhakshinamoorthy, G.P. Application of an immobilized microbial consortium for the treatment of pharmaceutical wastewater: Batch-wise and continuous studies. Chin. J. Chem. Eng. 2021, 29, 391–400. [Google Scholar] [CrossRef]
- Liu, D.; Yang, X.; Zhang, L.; Tang, Y.; He, H.; Liang, M.; Zhu, H. Immobilization of biomass materials for removal of refractory organic pollutants from wastewater. Int. J. Environ. Res. Public Health 2022, 19, 13830. [Google Scholar] [CrossRef]
- Li, L.; Zhang, M.; Jiang, W.; Yang, P. Study on the efficacy of sodium alginate gel particles immobilized microorganism SBBR for wastewater treatment. J. Environ. Chem. Eng. 2022, 10, 107134. [Google Scholar] [CrossRef]
- Rachpirom, M.; Pichayakorn, W.; Puttarak, P. Box-Behnken design to optimize the cross-linked sodium alginate/mucilage/Aloe vera film: Physical and mechanical studies. Int. J. Biol. Macromol. 2023, 246, 125568. [Google Scholar] [CrossRef]
- Jun, L.Y.; Yon, L.S.; Mubarak, N.M.; Bing, C.H.; Pan, S.; Danquah, M.K.; Khalid, M. An overview of immobilized enzyme technologies for dye and phenolic removal from wastewater. J. Environ. Chem. Eng. 2019, 7, 102961. [Google Scholar] [CrossRef]
- Gan, Y.; Ye, Z.; Zhao, Q.; Li, L.; Lu, X. Spatial denitrification performance and microbial community composition in an up-flow immobilized biofilter for nitrate micro-polluted water treatment. J. Clean. Prod. 2020, 258, 120913. [Google Scholar] [CrossRef]
- Gao, Y.; Shah, K.; Kwok, I.; Wang, M.; Rome, L.H.; Mahendra, S. Immobilized fungal enzymes: Innovations and potential applications in biodegradation and biosynthesis. Biotechnol. Adv. 2022, 57, 107936. [Google Scholar] [CrossRef]
- Rishi, S.; Kaur, I.; Naseem, M.; Gaur, V.K.; Mishra, S.; Srivastava, S.; Srivastava, P.K. Development of immobilized novel fungal consortium for the efficient remediation of cyanide-contaminated wastewaters. Bioresour. Technol. 2023, 373, 128750. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, J.; Massey, I.Y.; Peng, T.; Yang, F. Immobilization of Microbes for Biodegradation of Microcystins: A Mini Review. Toxins 2022, 14, 573. [Google Scholar] [CrossRef]
- Zhuang, L.L.; Li, M.; Ngo, H.H. Non-suspended microalgae cultivation for wastewater refinery and biomass production. Bioresour. Technol. 2020, 308, 123320. [Google Scholar] [CrossRef]
- Wang, J.; Yang, H.; Zhang, F.; Su, Y.; Wang, S. Activated sludge under free ammonia treatment using gel immobilization technology for long-term partial nitrification with different initial biomass. Process Biochem. 2020, 99, 282–289. [Google Scholar] [CrossRef]
- Popović, N.; Pržulj, D.; Mladenović, M.; Prodanović, O.; Ece, S.; Đurđić, K.I.; Prodanović, R. Immobilization of yeast cell walls with surface displayed laccase from Streptomyces cyaneus within dopamine-alginate beads for dye decolorization. Int. J. Biol. Macromol. 2021, 181, 1072–1080. [Google Scholar] [CrossRef]
- De Souza, W.F.C.; Pereira, I.; de Lucena, F.A.; Martins, L.P.; Furtado, R.F.; de Castro, R.J.S.; Sato, H.H. A new system of Erwinia sp. D12 cells immobilized in a matrix of alginate and algaroba gum (Prosopis juliflora): An efficient way to improve isomaltulose production. Process Biochem. 2022, 114, 52–58. [Google Scholar] [CrossRef]
- López-Menchero, J.R.; Ogawa, M.; Mauricio, J.C.; Moreno, J.; Moreno-García, J. Effect of calcium alginate coating on the cell retention and fermentation of a fungus-yeast immobilization system. LWT 2021, 144, 111250. [Google Scholar] [CrossRef]
- Xue, J.; Wu, Y.; Shi, K.; Xiao, X.; Gao, Y.; Li, L.; Qiao, Y. Study on the degradation performance and kinetics of immobilized cells in straw-alginate beads in marine environment. Bioresour. Technol. 2019, 280, 88–94. [Google Scholar] [CrossRef]
- Ng, S.L.; Yong, K.J.L.; Pushpamalar, J.; Sam, S.P. Carboxymethyl sago pulp/chitosan hydrogel as an immobilization medium for activated sludge for p-nitrophenol biodegradation. J. Appl. Polym. Sci. 2019, 136, 47531. [Google Scholar] [CrossRef]
- Shin, D.C.; Kim, J.S.; Park, C.H. Study on physical and chemical characteristics of microorganism immobilized media for advanced wastewater treatment. J. Water Process Eng. 2019, 29, 100784. [Google Scholar] [CrossRef]
- Bai, F.; Liu, S.; Ma, J.; Zhang, Y. Biodegradation of sulfate and elimination of heavy metals by immobilized-microbial bioaugmentation coupled with anaerobic membrane bioreactor. Chem. Eng. J. 2023, 473, 145196. [Google Scholar] [CrossRef]
- Kiran, M.G.; Pakshirajan, K.; Das, G. Heavy metal removal from aqueous solution using sodium alginate immobilized sulfate reducing bacteria: Mechanism and process optimization. J. Environ. Manag. 2018, 218, 486–496. [Google Scholar] [CrossRef]
- Abed, M.F.; Faisal, A.A. Calcium/iron-layered double hydroxides-sodium alginate for removal of tetracycline antibiotic from aqueous solution. Alex. Eng. J. 2023, 63, 127–142. [Google Scholar] [CrossRef]
- Mokeddem, A.; Benykhlef, S.; Bendaoudi, A.A.; Boudouaia, N.; Mahmoudi, H.; Bengharez, Z.; Topel, Ö. Sodium Alginate-Based Composite Films for Effective Removal of Congo Red and Coralene Dark Red 2B Dyes: Kinetic, Isotherm and Thermodynamic Analysis. Water 2023, 15, 1709. [Google Scholar] [CrossRef]
- Kurade, M.B.; Waghmode, T.R.; Xiong, J.; Govindwar, S.P.; Jeon, B. Decolorization of textile industry effluent using immobilized consortium cells in upflow fixed bed reactor. J. Cleaner Prod. 2019, 213, 884–891. [Google Scholar] [CrossRef]
- Osadebe, A.U.; Ogugbue, C.J.; Okpokwasili, G.C. Biochar and Iron oxide nanoparticle-impregnated alginate beads as adsorbents for enhanced ex situ bioremediation of petroleum-contaminated freshwater. Environ. Chem. Ecotoxicol. 2024, 6, 42–50. [Google Scholar] [CrossRef]
- Pan, Y.; Ding, Q.; Xu, H.; Shi, C.; Singh, A.; Kumar, A.; Liu, J. A new Zn (ii)-based 3D metal–organic framework with uncommon sev topology and its photocatalytic properties for the degradation of organic dyes. Cryst. Eng. Comm. 2019, 21, 4578–4585. [Google Scholar] [CrossRef]
- Wang, J.; Rao, C.; Lu, L.; Zhang, S.; Muddassir, M.; Liu, J. Efficient photocatalytic degradation of methyl violet using two new 3D MOFs directed by different carboxylate spacers. Cryst. Eng. Comm. 2021, 23, 741–747. [Google Scholar] [CrossRef]
- Li, S.; Lu, W.; Tang, Q.; Xiao, Q.; Kang, Y.; Hu, L.; Yang, H. Preparation of superhydrophilic sodium alginate/chitosan-Ag composite membranes with antibacterial activity for effective oil–water emulsion separation. Chem. Eng. Sci. 2024, 285, 119547. [Google Scholar] [CrossRef]
- Lee, H.; Jeong, D.; Im, S.; Jang, A. Optimization of alginate bead size immobilized with Chlorella vulgaris and Chlamydomonas reinhardtii for nutrient removal. Bioresour. Technol. 2020, 302, 122891. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, S.; Yang, Y.; Li, L.; Wang, D. Study on degradation of oily wastewater by immobilized microorganisms with biodegradable polyacrylamide and sodium alginate mixture. ACS Omega 2019, 4, 15149–15157. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhang, Y.; Tu, B. Immobilization of ammonia-oxidizing bacteria by polyvinyl alcohol and sodium alginate. Brazil. J. Microbiol. 2017, 48, 515–521. [Google Scholar] [CrossRef]
- Guo, Q.; Bandala, E.R.; Goonetilleke, A.; Hong, N.; Li, Y.; Liu, A. Application of Chlorella pyrenoidosa embedded biochar beads for water treatment. J. Water Process Eng. 2021, 40, 101892. [Google Scholar] [CrossRef]
- Feng, Q.; Chen, M.; Wu, P.; Zhang, X.; Wang, S.; Yu, Z.; Wang, B. Simultaneous reclaiming phosphate and ammonium from aqueous solutions by calcium alginate-biochar composite: Sorption performance and governing mechanisms. Chem. Eng. J. 2022, 429, 132166. [Google Scholar] [CrossRef]
- Han, Z.; Guo, N.; Yan, H.; Xu, Y.; Wang, J.; Zhao, Y.; Tucker, M.E. Recovery of phosphate, magnesium and ammonium from eutrophic water by struvite biomineralization through free and immobilized Bacillus cereus MRR2. J. Cleaner Prod. 2021, 320, 128796. [Google Scholar] [CrossRef]
- Mannacharaju, M.; Somasundaram, S.; Ganesan, S. Treatment of refractory organics in secondary biological treated post tanning wastewater using bacterial cell immobilized fluidized reactor. J. Water Process Eng. 2021, 43, 102213. [Google Scholar] [CrossRef]
- Mannacharaju, M.; Ganesan, S.; Lee, J.K.; Rajagopal, R.; Chang, S.W.; Ravindran, B. Bacterial cell immobilized packed bed reactor for the elimination of dissolved organics from biologically treated post-tanning wastewater and its microbial community profile. Chemosphere 2023, 320, 138022. [Google Scholar] [CrossRef]
- Mujtaba, G.; Rizwan, M.; Kim, G.; Lee, K. Removal of nutrients and COD through co-culturing activated sludge and immobilized Chlorella vulgaris. Chem. Eng. J. 2018, 343, 155–162. [Google Scholar] [CrossRef]
- Noreen, R.; Ahmad, A. Hydrogels reaction with sodium alginate over pollutant degradation. In Sodium Alginate-Based Nanomaterials for Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2023; pp. 199–223. [Google Scholar]
- Jaafari, J.; Barzanouni, H.; Mazloomi, S.; Farahani, N.A.A.; Sharafi, K.; Soleimani, P.; Haghighat, G.A. Effective adsorptive removal of reactive dyes by magnetic chitosan nanoparticles: Kinetic, isothermal studies and response surface methodology. Int. J. Biol. Macromol. 2020, 164, 344–355. [Google Scholar] [CrossRef]
- Chu, K.H.; Hashim, M.A.; da Costa Santos, Y.T.; Debord, J.; Harel, M.; Bollinger, J.C. The Redlich–Peterson isotherm for aqueous phase adsorption: Pitfalls in data analysis and interpretation. Chem. Eng. Sci. 2024, 285, 119573. [Google Scholar] [CrossRef]
- Sabbagh, N.; Tahvildari, K.; Sharif, A.A.M. Highly Efficient and Rapid Removal of Malathion Using Crosslinked Chitosan-Alginate Nanocomposites and Optimization of Parameters by Box–Behnken Design: Isotherms and Kinetic Studies. J. Polym. Environ. 2023, 31, 2595–2611. [Google Scholar] [CrossRef]
- Wu, F.C.; Liu, B.L.; Wu, K.T.; Tseng, R.L. A new linear form analysis of Redlich–Peterson isotherm equation for the adsorptions of dyes. Chem. Eng. J. 2010, 162, 21–27. [Google Scholar] [CrossRef]
- Belhachemi, M.; Addoun, F. Comparative adsorption isotherms and modeling of methylene blue onto activated carbons. Appl. Water Sci. 2011, 1, 111–117. [Google Scholar] [CrossRef]
- Bonifácio, E.; Facchi, D.P.; Souza, P.R.; Monteiro, J.P.; Popat, K.C.; Kipper, M.J.; Martins, A.F. A tannin-polymer adsorbent created from the freezing-thawing method for removal of metal-complex acid black 172 and methylene blue from aqueous solutions. J. Mol. Liq. 2022, 351, 118682. [Google Scholar] [CrossRef]
- Puccia, V.; Avena, M.J. On the use of the Dubinin-Radushkevich equation to distinguish between physical and chemical adsorption at the solid-water interface. Colloid Interface Sci. Commun. 2021, 41, 100376. [Google Scholar] [CrossRef]
- Liu, L.; Luo, X.B.; Ding, L.; Luo, S.L. Application of nanotechnology in the removal of heavy metal from water. In Nanomaterials for the Removal of Pollutants and Resource Reutilization; Elsevier: Amsterdam, The Netherlands, 2019; pp. 83–147. [Google Scholar]
- Kumar, K.V.; Gadipelli, S.; Howard, C.A.; Kwapinski, W.; Brett, D.J. Probing adsorbent heterogeneity using Toth isotherms. J. Mater. Chem. A 2021, 9, 944–962. [Google Scholar] [CrossRef]
- İsmail, O.; Gökçe Kocabay, Ö. Absorption and adsorption studies of polyacrylamide/sodium alginate hydrogels. Colloid Polym. Sci. 2021, 299, 783–796. [Google Scholar] [CrossRef]
- Dong, K.; Xu, K.; Wei, N.; Fang, Y.; Qin, Z. Three-dimensional porous sodium alginate/gellan gum environmentally friendly aerogel: Preparation, characterization, adsorption, and kinetics studies. Chem. Eng. Res. Des. 2022, 179, 227–236. [Google Scholar] [CrossRef]
- Hashem, A.; Aniagor, C.O.; Nasr, M.F.; Abou-Okeil, A. Efficacy of treated sodium alginate and activated carbon fibre for Pb (II) adsorption. Int. J. Biol. Macromol. 2021, 176, 201–216. [Google Scholar] [CrossRef]
- Marzban, N.; Moheb, A.; Filonenko, S.; Hosseini, S.H.; Nouri, M.J.; Libra, J.A.; Farru, G. Intelligent modeling and experimental study on methylene blue adsorption by sodium alginate-kaolin beads. Int. J. Biol. Macromol. 2021, 186, 79–91. [Google Scholar] [CrossRef] [PubMed]
- De la Morena, S.; Santos, V.E.; García-Ochoa, F. Influence of oxygen transfer and uptake rates on dihydroxyacetone production from glycerol by Gluconobacter oxydans in resting cells operation. Biochem. Eng. J. 2019, 147, 20–28. [Google Scholar] [CrossRef]
- Castillo, T.; García, A.; Padilla-Córdova, C.; Díaz-Barrera, A.; Peña, C. Respiration in Azotobacter vinelandii and its relationship with the synthesis of biopolymers. Electron. J. Biotechnol. 2020, 48, 36–45. [Google Scholar] [CrossRef]
- Garcia-Ochoa, F.; Gomez, E.; Santos, V.E.; Merchuk, J.C. Oxygen uptake rate in microbial processes: An overview. Biochem. Eng. J. 2010, 49, 289–307. [Google Scholar] [CrossRef]
- Ponce, B.; Urtuvia, V.; Maturana, N.; Peña, C.; Díaz-Barrera, A. Increases in alginate production and transcription levels of alginate lyase (alyA1) by control of the oxygen transfer rate in Azotobacter vinelandii cultures under diazotrophic conditions. Electron. J. Biotechnol. 2021, 52, 35–44. [Google Scholar] [CrossRef]
- Hussain, F.; Yu, H.W.; Chon, K.; Lee, Y.G.; Eom, H.; Chae, K.J.; Oh, S.E. Real-time biomonitoring of oxygen uptake rate and biochemical oxygen demand using a novel optical biogas respirometric system. J. Environ. Manag. 2021, 277, 111467. [Google Scholar] [CrossRef]
- Bandaiphet, C.; Prasertsan, P. Effect of aeration and agitation rates and scale-up on oxygen transfer coefficient, kLa in exopolysaccharide production from Enterobacter cloacae WD7. Carbohydr. Polym. 2006, 66, 216–228. [Google Scholar] [CrossRef]
- Liu, J.Q.; Luo, Z.D.; Pan, Y.; Singh, A.K.; Trivedi, M.; Kumar, A. Recent developments in luminescent coordination polymers: Designing strategies, sensing application and theoretical evidences. Coord. Chem. Rev. 2020, 406, 213145. [Google Scholar] [CrossRef]
- Zhao, J.; Dang, Z.; Muddassir, M.; Raza, S.; Zhong, A.; Wang, X.; Jin, J. A New Cd(II)-Based Coordination Polymer for Efficient Photocatalytic Removal of Organic Dyes. Molecules 2023, 28, 6848. [Google Scholar] [CrossRef]
- Ye, D.; Liu, L.; Peng, Q.; Qiu, J.; Gong, H.; Zhong, A.; Liu, S. Effect of Controlling Thiophene Rings on D-A Polymer Photocatalysts Accessed via Direct Arylation for Hydrogen Production. Molecules 2023, 28, 4507. [Google Scholar] [CrossRef]
- Huang, X.M.; Chen, N.; Ye, D.N.; Zhong, A.G.; Liu, H.; Li, Z.; Liu, S.Y. Structurally Complementary Star-shaped Unfused Ring Electron Acceptors with Simultaneously Enhanced Device Parameters for Ternary Organic Solar Cells. Sol. RRL 2023, 7, 2300143. [Google Scholar] [CrossRef]

| Immobilization Support | Adsorbate | Adsorption | pH | Temperature | References |
|---|---|---|---|---|---|
| Hyperbranched polyamide/alginate | Sb(III) | 195.7 mg/g | 7 | room temperature | [73] |
| Sodium alginate/biosilicate/magnetite | Malathion | 36.76 mg/g | 7 | 45 °C | [71] |
| Nanochitosan/sodium alginate | Pb(II) | 178.57 mg/g | 6.65 | 45 °C | [74] |
| Sodium alginate/activated charcoal | Cd (II) | 137 mg/g | 7 | room temperature | [75] |
| Ethylenediamine/sodium alginate | Pb2+ | 219.3 mg/g | 3–7 | room temperature | [76] |
| Ethylenediamine/sodium alginate | Cu2+ | 87.8 mg/g | 3–7 | room temperature | [76] |
| Dolomite/sodium alginate | Phosphorous | 28.45 mg/g | 7–10 | 20 °C | [77] |
| Sodium alginate/ĸ-carrageenan | Antibiotic | 291.6 mg/g | 5.1 | room temperature | [78] |
| Calcium alginate | Arsenic (V) | 3.4 mg/g | 4.5 | room temperature | [79] |
| Calcium alginate | Phosphorus | 19.42 mg/g | 7.43 | 20 °C | [80] |
| Poly(vinyl alcohol)/sodium alginate | Phosphorus | 107.53 mg/g | 4 | 25 °C | [81] |
| Sodium alginate/polyethyleneimine | Methylene Blue | 400 mg/g | 5.5 | 60 °C | [82] |
| Acrylonitrile/sodium alginate | Methylene Blue | 20.31 mg/g | 7 | 70 °C | [83] |
| Poly(vinyl alcohol)-sodium Alginate/chitosan/montmorillonite | Methylene Blue | 137.15 mg/g | 8 | room temperature | [84] |
| Contaminant Removed | Na-Alg (W/V) | CaCl2 | pH | Temperature | Removal | Reference |
|---|---|---|---|---|---|---|
| Phenol | 2% | - | 7 | 30 °C | 100% | [85] |
| P-nitrophenol | 1.4% | 3% | 5.5 | 55 °C | 92% | [10] |
| Methyl blue | 3% | 5% w/v | 5.5 | 30 °C | 99% | [82] |
| Fluoride | 1% | 1% w/v | 4.0 | 30 °C | 92% | [86] |
| Phosphate | 2% | 3% w/v | 3.0 | 30 °C | 98.4% | [87] |
| Nutrient | 3% | 5.5% | 7.0 | 27 °C | 98% | [68] |
| Nutrient | 2% | 2% w/v | 8.9 | 25 °C | 95% | [88] |
| Pb2+ | 2% | 2% w/v | 5.0 | 25 °C | 80% | [89] |
| Pb2+, Cu2+ | 1% | 0.2 mol/L | 4.5 | 25 °C | 75.3% | [76] |
| Methyl blue | 1% | 0.1 mol/L | 8.0 | 30 °C | 90% | [84] |
| Phosphate | 2% | 2% w/v | 4.5 | 45 °C | 76% | [90] |
| Arsenic | 1% | 0.1 mol/L | 4.5 | 25 °C | 53.6% | [79] |
| Phosphorous | 2% | 2% w/v | 8.5 | 23 °C | 96% | [91] |
| Fluoride | 2% | 2% w/v | 6.0 | 20 °C | 90% | [92] |
| Copper | 4% | 4% w/v | 3.0 | 21 °C | - | [93] |
| Uranium | 1% | 2% w/v | 3.0 | - | 91% | [94] |
| Methyl blue | 1% | 22% | 5.0 | - | 61% | [95] |
| Dyes | 2% | 2.2% | 3.0 | 30 °C | 95.4% | [96] |
| Ammonium | 2% | 5% | 7.0 | 25 °C | 70% | [97] |
| Degraded Pollutant | %Removal | Microorganism | Immobilizing Matrix | References |
|---|---|---|---|---|
| Oil | 99.73 | E. Coli and aureus | Sodium alginate/chitosan–Ag | [128] |
| Nutrients | 89 | Chlorella vulgaris | Sodium alginate | [68] |
| Phenol | 99.5 | Bacillus cereus | Alginate/biochar | [12] |
| Diesel | 68.68 | Halomonas and Aneurinibacillus | Sodium alginate | [117] |
| Cadmium, arsenic, mercury, chromium | 100 | E. coli | Sodium alginate | [3] |
| COD and TP | 94.26 | Activated sludge | Sodium alginate | [19] |
| Ammonia | 90 | Activated sludge | Polyvinyl alcohol | [113] |
| Nutrients | 95 | Chlorella vulgaris and Chlamydomonas reinhardtii | Sodium alginate | [129] |
| P-nitrophenol | 92 | Activated sludge | Polyvinyl alcohol/alginate | [10] |
| Oil | 70 | Pseudomonas, Bacillus, Acinetobacter | Polyacrylamide/sodium alginate | [130] |
| Sulfate | 99.4 | Sulfate-reducing bacteria | Sodium alginate | [120] |
| Nitrogen | 88 | Denitrifying bacteria and anaerobic bacteria | Sodium alginate | [105] |
| Ammonia | 96.5 | Bacillus subtilis | Chitosan/sodium alginate | [56] |
| Nitrate and ammonia nitrogen | 90.3 | Ammonia-oxidizing bacteria | Polyvinyl alcohol/alginate | [131] |
| 2,3′,4,4′,5-pentachlorodiphenyl | 50.5 | Pseudomonas and Stenotrophomonas | Sodium alginate | [25] |
| Nitrate | 95 | Activated sludge | Sodium alginate/kaolin | [13] |
| Ammoniacal nitrogen | 89 | Nitrifying bacteria | Sodium alginate | [16] |
| Nutrients and total organic carbon | 96.5 | Chlorella pyrenoidosa | Sodium alginate/biochar | [132] |
| Isotherm | Terms | References | |
|---|---|---|---|
| Langmuir | qe is the adsorption amount per adsorbent unit mass in equilibrium (mg.g−1); qmax is the maximum adsorption capacity per unit mass of adsorbent (mg g−1); Ce is the pollutant equilibrium concentration in solution (mg.L−1); and KL is the Langmuir’s constant based on the affinity of the adsorbate binding site per adsorbent (L.g−1). KF and n are the Freundlich constants. | [68] [141] | |
| Freundlich | [71] [89] | ||
| Redlich–Peterson | KRP is the Redlich–Peterson isotherm constant (L.g−1), B is also a constant (L.mg−1), ß is an exponent that lies between 0 and 1, Ce is the equilibrium liquid phase concentration of the sorbate (mg.L−1), and qe is the equilibrium sorbate loading by the sorbent (mg.g−1). | [140] [142] | |
| Sips | qe (mmol·g−1) is the amount adsorbed at equilibrium, Ce (mmol·L−1) is the equilibrium concentration of the adsorbate, q0 (mmol·g−1) is the maximum adsorption capacity of Sips, KS is the Sips equilibrium constant, and n is the exponent of the Sips model related to the heterogeneity of the system. | [143] [144] | |
| Temkin | B1 = RT/b1 denotes the Temkin constant (J.mol−1), R is the universal gas constant (equal to 8.314 J/mol.K), T is the absolute temperature (°K), and KT and b1 represent the equilibrium binding constant (L.g−1) and adsorption heat (kJ.mol−1), respectively. | [71] | |
| Dubinin–Radushkevich | qe is the equilibrium adsorbent phase concentration of adsorbate (mg.g−1), qm is the theoretical saturation capacity (mg.g−1), K is the activity coefficient related to the mean free energy of adsorption (mol2.kJ−2), and ɛ is the Polanyi potential (kJ.mol−1). | [145] [146] | |
| Toth | qs is the maximal adsorption, t is the Tóth parameter (reflecting the inhomogeneity of adsorbent), qeq is the equilibrium concentration on the solid phase (reflecting the adsorbate sequestering capacity), Ce is the equilibrium concentration in the bulk fluid phase, qmax is the maximal sequestering capacity in the mono-layer case, and bT is the Tóth constant (characterizing the interaction between the adsorbed and solution molecules). | [147] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bustos-Terrones, Y.A. A Review of the Strategic Use of Sodium Alginate Polymer in the Immobilization of Microorganisms for Water Recycling. Polymers 2024, 16, 788. https://doi.org/10.3390/polym16060788
Bustos-Terrones YA. A Review of the Strategic Use of Sodium Alginate Polymer in the Immobilization of Microorganisms for Water Recycling. Polymers. 2024; 16(6):788. https://doi.org/10.3390/polym16060788
Chicago/Turabian StyleBustos-Terrones, Yaneth A. 2024. "A Review of the Strategic Use of Sodium Alginate Polymer in the Immobilization of Microorganisms for Water Recycling" Polymers 16, no. 6: 788. https://doi.org/10.3390/polym16060788
APA StyleBustos-Terrones, Y. A. (2024). A Review of the Strategic Use of Sodium Alginate Polymer in the Immobilization of Microorganisms for Water Recycling. Polymers, 16(6), 788. https://doi.org/10.3390/polym16060788






