Delivery of Probiotics with Cellulose-Based Films and Their Food Applications
Abstract
1. Introduction
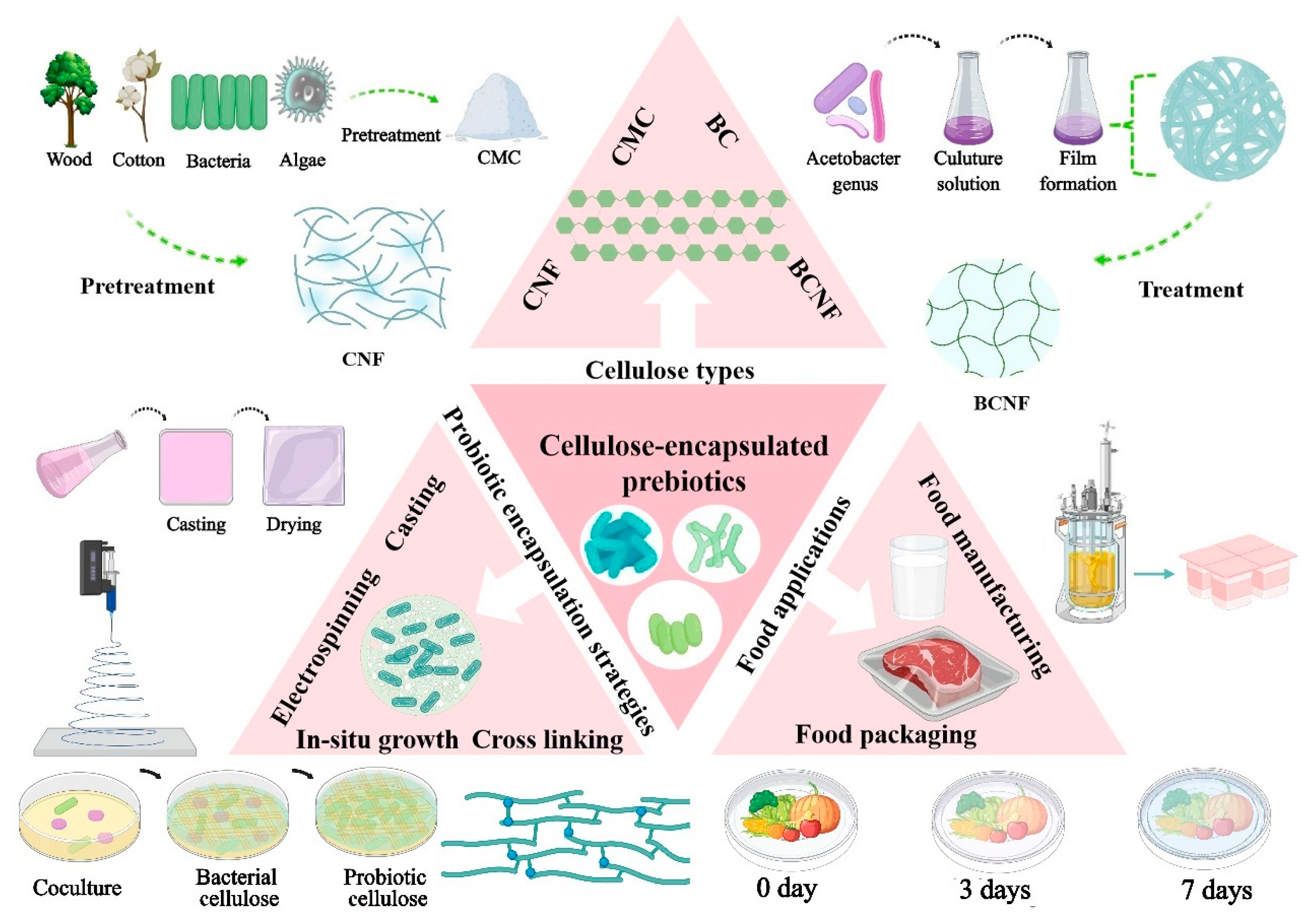
2. Cellulose Types Used for Probiotic Encapsulation
2.1. Bacterial Cellulose (BC)

2.2. Bacterial Cellulose Nanofibers
2.3. Carboxymethyl Cellulose (CMC)
2.4. Cellulose Nanofiber (CNF)
3. Probiotic Encapsulation Strategies with Cellulose-Based Materials
3.1. Electrospinning
3.2. Cross-Linking
3.3. In-Situ Growth
3.4. Casting
4. Cellulose-Based Probiotic Films for Food Applications
4.1. Food Packaging
4.2. Food Manufacturing
5. Conclusions and Perspective
Funding
Conflicts of Interest
References
- FAO/WHO. Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria; Report of the Joint Food and Agriculture (FAO) of the United Nations/World Health Organization (WHO) Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria; Report of a joint FAO/WHO expert Consultation; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- Binda, S.; Hill, C.; Johansen, E.; Obis, D.; Pot, B.; Sanders, M.E.; Tremblay, A.; Ouwehand, A.C. Criteria to qualify microorganisms as “probiotic” in foods and dietary supplements. Front. Microbiol. 2020, 11, 1662. [Google Scholar] [CrossRef]
- Sharma, R.; Mokhtari, S.; Jafari, S.M.; Sharma, S. Barley-based probiotic food mixture: Health effects and future prospects. Crit. Rev. Food Sci. Nutr. 2022, 62, 7961–7975. [Google Scholar] [CrossRef]
- Centurion, F.; Basit, A.W.; Liu, J.; Gaisford, S.; Rahim, M.A.; Kalantar-Zadeh, K. Nanoencapsulation for probiotic delivery. ACS Nano 2021, 15, 18653–18660. [Google Scholar] [CrossRef]
- Xu, C.; Ban, Q.; Wang, W.; Hou, J.; Jiang, Z. Novel nano-encapsulated probiotic agents: Encapsulate materials, delivery, and encapsulation systems. J. Control Release 2022, 349, 184–205. [Google Scholar] [CrossRef]
- Liu, H.; Cui, S.W.; Chen, M.; Li, Y.; Liang, R.; Xu, F.; Zhong, F. Protective approaches and mechanisms of microencapsulation to the survival of probiotic bacteria during processing, storage and gastrointestinal digestion: A review. Crit. Rev. Food Sci. 2019, 59, 2863–2878. [Google Scholar] [CrossRef]
- Li, C.C.; Wang, Z.X.; Xiao, H.N.; Wu, F.G. Intestinal delivery of probiotics: Materials, strategies, and applications. Adv. Mater. 2024; ahead of print. [Google Scholar] [CrossRef]
- Hu, R.; Dong, D.; Hu, J.; Liu, H. Improved viability of probiotics encapsulated in soybean protein isolate matrix microcapsules by coacervation and cross-linking modification. Food Hydrocoll. 2023, 138, 108457. [Google Scholar] [CrossRef]
- Loyeau, P.A.; Spotti, M.J.; Vinderola, G.; Carrara, C.R. Encapsulation of potential probiotic and canola oil through emulsification and ionotropic gelation, using protein/polysaccharides maillard conjugates as emulsifiers. LWT 2021, 150, 111980. [Google Scholar] [CrossRef]
- Su, J.; Wang, X.; Li, W.; Chen, L.; Zeng, X.; Huang, Q.; Hu, B. Enhancing the viability of Lactobacillus plantarum as probiotics through encapsulation with high internal phase emulsions stabilized with whey protein isolate microgels. J. Agric. Food Chem. 2018, 66, 12335–12343. [Google Scholar] [CrossRef]
- Zou, Q.; Liu, X.; Zhao, J.; Tian, F.; Zhang, H.; Zhang, H.; Chen, W. Microencapsulation of Bifidobacterium bifidum F-35 in whey protein-based microcapsules by transglutaminase-induced gelation. J. Food Sci. 2012, 77, 270. [Google Scholar] [CrossRef]
- Doherty, S.; Gee, V.; Ross, R.P.; Stanton, C.; Fitzgerald, G.; Brodkorb, A. Development and characterisation of whey protein micro-beads as potential matrices for probiotic protection. Food Hydrocoll. 2011, 25, 1604–1617. [Google Scholar] [CrossRef]
- Sultana, M.; Chan, E.S.; Pushpamalar, J.; Choo, W.S. Advances in extrusion-dripping encapsulation of probiotics and omega-3 rich oils. Trends Food Sci. Technol. 2022, 123, 69–86. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, C.; Song, X.; Zeng, J.; Zhang, L.; Gong, P. Spray drying co-encapsulation of lactic acid bacteria and lipids: A review. Trends Food Sci. Technol. 2022, 129, 134–143. [Google Scholar] [CrossRef]
- Qin, X.S.; Luo, Z.G.; Li, X.L. An enhanced pH-sensitive carrier based on alginate-Ca-EDTA in a set-type W1/O/W2 double emulsion model stabilized with WPI-EGCG covalent conjugates for probiotics colon-targeted release. Food Hydrocoll. 2021, 113, 106460. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, X.; Pang, Y.; Cheng, S.; Liu, J. Biointerfacial self-assembly generates lipid membrane coated bacteria for enhanced oral delivery and treatment. Nat. Commun. 2019, 10, 5783. [Google Scholar] [CrossRef]
- Sim, K.; Youn, H.J. Preparation of porous sheets with high mechanical strength by the addition of cellulose nanofibrils. Cellulose 2016, 23, 1383–1392. [Google Scholar] [CrossRef]
- Zhang, F.; Ren, H.; Tong, G.; Deng, Y. Ultra-lightweight poly (sodium acrylate) modified TEMPO-oxidized cellulose nanofibril aerogel spheres and their superabsorbent properties. Cellulose 2016, 23, 3665–3676. [Google Scholar] [CrossRef]
- Chitprasert, P.; Sudsai, P.; Rodklongtan, A. Aluminum carboxymethyl cellulose-rice bran microcapsules: Enhancing survival of Lactobacillus reuteri KUB-AC5. Carbohydr. Polym. 2012, 90, 78–86. [Google Scholar] [CrossRef]
- Li, W.; Li, X.; Wang, Q.; Pan, Y.; Wang, T.; Wang, H.; Song, R.; Deng, H. Antibacterial activity of nanofibrous mats coated with lysozyme-layered silicate composites via electrospraying. Carbohydr. Polym. 2014, 99, 218–225. [Google Scholar] [CrossRef]
- Hu, M.X.; Li, J.N.; Guo, Q.; Zhu, Y.Q.; Niu, H.M. Probiotics biofilm-integrated electrospun nanofiber membranes: A new starter culture for fermented milk production. J. Agric. Food Chem. 2019, 67, 3198–3208. [Google Scholar] [CrossRef]
- Dai, L.; Cheng, T.; Duan, C.; Zhao, W.; Zhang, W.P.; Zou, X.J.; Aspler, J.; Ni, Y.H. 3D printing using plant-derived cellulose and its derivatives: A review. Carbohydr. Polym. 2019, 203, 71–86. [Google Scholar] [CrossRef]
- Almeida, A.P.C.; Canejo, J.P.; Fernandes, S.N.; Echeverria, C.; Almeida, P.L.; Godinho, M.H. Cellulose-based biomimetics and their applications. Adv. Mater. 2018, 30, 1703655. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Das, P.P.; Kalyani, P.; Kumar, R.; Khandelwal, M. Cellulose-based natural nanofibers for fresh produce packaging: Current status, sustainability and future outlook. Sustain. Food Packag. Technol. 2023, 1, 528–544. [Google Scholar] [CrossRef]
- Li, T.; Chen, C.; Brozena, A.H.; Zhu, J.Y.; Xu, L.; Driemeier, C.; Dai, J.; Rojas, O.J.; Isogai, A.; Wågberg, L.; et al. Developing fibrillated cellulose as a sustainable technological material. Nature 2021, 590, 47–56. [Google Scholar] [CrossRef]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A review of cellulose and its derivatives in biopolymer-based for food packaging application. Trends Food Sci. Tech. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- Vanderfleet, O.M.; Cranston, E.D. Production routes to tailor the performance of cellulose nanocrystals. Nat. Rev. Mater. 2021, 6, 124–144. [Google Scholar] [CrossRef]
- Moghanjougi, Z.M.; Bari, M.R.; Khaledabad, M.A.; Almasi, H.; Amiri, S. Bio-preservation of white brined cheese (Feta) by using probiotic bacteria immobilized in bacterial cellulose: Optimization by response surface method and characterization. LWT-Food Sci. Technol. 2020, 117, 108603. [Google Scholar] [CrossRef]
- Lappa, I.K.; Kachrimanidou, V.; Alexandri, M.; Papadaki, A.; Kopsahelis, N. Novel probiotic/bacterial cellulose biocatalyst for the development of functional dairy beverage. Foods 2022, 11, 2586. [Google Scholar] [CrossRef]
- Lan, W.T.; Zhang, R.; Ji, T.T.; Sameen, D.E.; Ahmed, S.; Qin, W.; Dai, J.W.; He, L.; Liu, Y.W. Improving nisin production by encapsulated Lactococcus lactis with starch/carboxymethyl cellulose edible films. Carbohyd. Polym. 2021, 251, 117062. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, H.S.; El-Sayed, S.M.; Mabrouk, A.M.M.; Nawwar, G.A.; Youssef, A.M. Development of eco-friendly probiotic edible coatings based on chitosan, alginate and carboxymethyl cellulose for improving the shelf life of UF soft cheese. J. Polym. Environ. 2021, 29, 1941–1953. [Google Scholar] [CrossRef]
- Zabihollahi, N.; Alizadeh, A.; Almasi, H.; Hanifian, S.; Hamishekar, H. Development and characterization of carboxymethyl cellulose based probiotic nanocomposite film containing cellulose nanofiber and inulin for chicken fillet shelf life extension. Int. J. Biol. Macromol. 2020, 160, 409–417. [Google Scholar] [CrossRef]
- Seyedzadeh-Hashemi, S.; Mofid, V.; Hosseini, S.M.; Gharibzahedi, S.M.T.; Mortazavian, A.M.; Shojaee-Aliabadi, S. Characterization of synbiotic films based on carboxymethyl cellulose/β-glucan and development of a shelf life prediction model. Food Biosci. 2023, 51, 102228. [Google Scholar] [CrossRef]
- Salimiraad, S.; Safaeian, S.; Basti, A.A.; Khanjari, A.; Nadoushan, R.M. Characterization of novel probiotic nanocomposite films based on nano chitosan/nano cellulose/gelatin for the preservation of fresh chicken fillets. LWT 2022, 162, 113429. [Google Scholar] [CrossRef]
- Çanga, E.M.; Dudak, F.C. Improved digestive stability of probiotics encapsulated within poly(vinyl alcohol)/cellulose acetate hybrid fibers. Carbohydr. Polym. 2021, 264, 117990. [Google Scholar] [CrossRef]
- Li, W.; Liu, L.M.; Tian, H.F.; Luo, X.G.; Liu, S.L. Encapsulation of Lactobacillus plantarum in cellulose based microgel with controlled release behavior and increased long-term storage stability. Carbohydr. Polym. 2019, 223, 115056. [Google Scholar] [CrossRef]
- Charoenrak, S.; Charumanee, S.; Sirisa-ard, P.; Bovonsombut, S.; Kumdhitiahutsawakul, L.; Kiatkarun, S.; Pathom-Aree, W.; Chitov, T.; Bovonsombut, S. Nanobacterial cellulose from kombucha fermentation as a potential protective carrier of Lactobacillus plantarum under simulated gastrointestinal tract conditions. Polymers 2023, 15, 1356. [Google Scholar] [CrossRef] [PubMed]
- Li, G.H.; Wang, L.; Deng, Y.; Wei, Q.F. Research progress of the biosynthetic strains and pathways of bacterial cellulose. J. Ind. Microbiol. Biot. 2022, 49, kuab071. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhang, Y.; Phillips, G.O.; Yang, G. Utilization of bacterial cellulose in food. Food Hydrocoll. 2014, 35, 539–545. [Google Scholar] [CrossRef]
- Torres, F.G.; Arroyo, J.J.; Troncoso, O.P. Bacterial cellulose nanocomposites: An all-nano type of material. Mat. Sci. Eng. C-Mater. 2019, 98, 1277–1293. [Google Scholar] [CrossRef]
- Wahid, F.; Huang, L.H.; Zhao, X.Q.; Li, W.C.; Wang, Y.Y.; Jia, S.R.; Zhong, C. Bacterial cellulose and its potential for biomedical applications. Biotechnol. Adv. 2021, 53, 107856. [Google Scholar] [CrossRef]
- Selestina, G.; Janja, T. Bacterial cellulose: Production, modification and perspectives in biomedical applications. Nanomaterials 2019, 9, 1352. [Google Scholar] [CrossRef]
- Picheth, G.F.; Pirich, C.L.; Sierakowski, M.R.; Woehl, M.A.; Sakakibara, C.N.; de Souza, C.F.; Martin, A.A.; da Silva, R.; de Freitas, R.A. Bacterial cellulose in biomedical applications: A review. Int. J. Biol. Macromol. 2017, 104, 97–106. [Google Scholar] [CrossRef]
- da Silva, C.J.G.; de Medeiros, A.D.L.M.; de Amorim, J.D.P.; do Nascimento, H.A.; Converti, A.; Costa, A.F.S.; Sarubbo, L.A. Bacterial cellulose biotextiles for the future of sustainable fashion: A review. Environ. Chem. Lett. 2021, 19, 2967–2980. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Bacterial cellulose as a biodegradable food packaging material: A review. Food Hydrocoll. 2021, 113, 106530. [Google Scholar] [CrossRef]
- Caro-Astorga, J.; Walker, K.T.; Herrera, N.; Lee, K.Y.; Ellis, T. Bacterial cellulose spheroids as building blocks for 3D and patterned living materials and for regeneration. Nat. Commun. 2021, 12, 5027. [Google Scholar] [CrossRef]
- de Amorim, J.D.P.; de Souza, K.C.; Duarte, C.R.; da Silva Duarte, I.; de Assis Sales Ribeiro, F.; Silva, G.S.; de Farias, P.M.A.; Stingl, A.; Costa, A.F.S.; Vinhas, G.M.; et al. Plant and bacterial nanocellulose: Production, properties and applications in medicine, food, cosmetics, electronics and engineering. a review. Environ. Chem. Lett. 2020, 18, 851–869. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Saha, N.; Brodnjak, U.V.; Saha, P. Bacterial cellulose based greener packaging material: A bioadhesive polymeric film. Mater. Res. Express 2018, 5, 115405. [Google Scholar] [CrossRef]
- Gregory, D.A.; Tripathi, L.; Fricker, A.T.R.; Asare, E.; Orlando, I.; Raghavendran, V.; Roy, I. Bacterial cellulose: A smart biomaterial with diverse applications. Mat. Sci. Eng. R. 2021, 145, 100623. [Google Scholar] [CrossRef]
- Alizadeh, A.M.; Mohseni, M.; Gerami, K.; Gharavi-nakhjavani, M.; Aminzare, M.; Rastegar, H.; Assadpour, E.; Hashempour-baltork, F.; Jafari, S.M. Electrospun fibers loaded with probiotics: Fundamentals, characterization, and applications. Probiotics Antimicrob. Proteins, 2023; ahead of print. [Google Scholar] [CrossRef]
- Muiruri, J.K.; Yeo, J.C.C.; Zhu, Q.; Ye, E.; Loh, X.J.; Li, Z. Bacterial cellulose: Recent advances in biosynthesis, functionalization strategies and emerging applications. Eur. Polym. J. 2023, 199, 112446. [Google Scholar] [CrossRef]
- Ullah, M.W.; Ul Islam, M.; Khan, S.; Shah, N.; Park, J.K. Recent advancements in bioreactions of cellular and cell-free systems: A study of bacterial cellulose as a model. Korean J. Chem. Eng. 2017, 34, 1591–1599. [Google Scholar] [CrossRef]
- Manan, S.; Ullah, M.W.; Ul-Islam, M.; Shi, Z.; Gauthier, M.; Yang, G. Bacterial cellulose: Molecular regulation of biosynthesis, supramolecular assembly, and tailored structural and functional properties. Prog. Mater. Sci. 2022, 129, 100972. [Google Scholar] [CrossRef]
- Sajadi, E.; Babaipour, V.; Deldar, A.A.; Yakhchali, B.; Fatemi, S.S. Enhancement of crystallinity of cellulose produced by Escherichia coli through heterologous expression of bcsD gene from Gluconacetobacter xylinus. Biotechnol. Lett. 2017, 39, 1395–1401. [Google Scholar] [CrossRef]
- Sajadi, E.; Fatemi, S.S.; Babaeipour, V.; Deldar, A.A.; Yakhchali, B.; Anvar, M.S. Increased cellulose production by heterologous expression of bcsA and B genes from Gluconacetobacterxylinus in E. coli Nissle 1917. Bioprocess Biosyst. Eng. 2019, 42, 2023–2034. [Google Scholar] [CrossRef]
- Wasim, M.; Mushtaq, M.; Khan, S.U.; Farooq, A.; Naeem, M.A.; Khan, M.R.; Salam, A.; Wei, Q. Development of bacterial cellulose nanocomposites: An overview of the synthesis of bacterial cellulose nanocomposites with metallic and metallic-oxide nanoparticles by different methods and techniques for biomedical applications. J. Ind. Text. 2022, 51, 1886S–1915S. [Google Scholar] [CrossRef]
- Silverman, A.D.; Kelley-Loughnane, N.; Lucks, J.B.; Jewett, M.C. Deconstructing cell-free extract preparation for in vitro activation of transcriptional cenetic circuitry. ACS Synth. Biol. 2019, 8, 403–414. [Google Scholar] [CrossRef]
- Ullah, M.W.; Ul-Islam, M.; Khan, S.; Kim, Y.; Park, J.K. Innovative production of bio-cellulose using a cell-free system derived from a single cell line. Carbohydr. Polym. 2015, 132, 286–294. [Google Scholar] [CrossRef]
- Choi, S.M.; Shin, E.J. The nanofication and functionalization of bacterial cellulose and its applications. Nanomaterials 2020, 10, 406. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Catchmark, J.M. Surface area and porosity of acid hydrolyzed cellulose nanowhiskers and cellulose produced by Gluconacetobacter xylinus. Carbohydr. Polym. 2012, 87, 1026–1037. [Google Scholar] [CrossRef]
- Kim, J.; Seo, Y.B. Electro-active paper actuators. Smart Mater. Struct. 2002, 11, 355–360. [Google Scholar] [CrossRef]
- Campano, C.; Merayo, N.; Negro, C.; Blanco, A. Low-fibrillated bacterial cellulose nanofibers as a sustainable additive to enhance recycled paper quality. Int. J. Biol. Macromol. 2018, 114, 1077–1083. [Google Scholar] [CrossRef]
- Jayani, T.; Sanjeev, B.; Marimuthu, S.; Uthandi, S. Bacterial cellulose nano fiber (BCNF) as carrier support for the immobilization of probiotic, Lactobacillus acidophilus 016. Carbohydr. Polym. 2020, 250, 116965. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, F.; Xu, X.; Kuang, Y.; Fu, K.; Hitz, E.; Hu, L. Super-strong, super-stiff macrofibers with aligned, long bacterial cellulose nanofibers. Adv. Mater. 2017, 29, 1702498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, D.; Liu, S.; Tang, J. Bacterial cellulose nanofibril-based Pickering emulsions: Recent trends and applications in the food industry. Foods 2022, 11, 4064. [Google Scholar] [CrossRef]
- Bao, Y.; Ma, J.; Li, N. Synthesis and swelling behaviors of sodium carboxymethyl cellulose-g-poly (AA-co-AM-co-AMPS)/MMT superabsorbent hydrogel. Carbohydr. Polym. 2010, 84, 76–82. [Google Scholar] [CrossRef]
- Tongdeesoontorn, W.; Mauer, L.; Wongruong, S.; Sriburi, P.; Rachtanapun, P. Effect of carboxymethyl cellulose concentration on physical properties of biodegradable cassava starch-based films. Chem. Centr. J. 2011, 5, 6. [Google Scholar] [CrossRef]
- Bisht, S.S.; Pandey, K.; Joshi, G.; Naithani, S. New route for carboxymethylation of cellulose: Synthesis, structural analysis and properties. Cell. Chem. Technol. 2017, 51, 609–619. [Google Scholar]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Edit. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Moussa, I.; Khiari, R.; Moussa, A.; Belgacem, M.N.; Mhenni, M.F. Preparation and characterization of carboxymethyl cellulose with a high degree of substitution from agricultural wastes. Fiber. Polym. 2019, 20, 933–943. [Google Scholar] [CrossRef]
- Akhlaq, M.; Maqsood, H.; Uroos, M.; Iqbal, A. A Comparative study of different methods for cellulose extraction from lignocellulosic wastes and conversion into carboxymethyl cellulose. ChemistrySelect 2022, 7, e202201533. [Google Scholar] [CrossRef]
- Oun, A.A.; Rhim, J.W. Isolation of cellulose nanocrystals from grain straws and their use for the preparation of carboxymethyl cellulose-based nanocomposite films. Carbohydr. Polym. 2016, 150, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Perumal, A.B.; Nambiar, R.B.; Moses, J.A.; Anandharamakrishnan, C. Nanocellulose: Recent trends and applications in the food industry. Food Hydrocoll. 2022, 127, 107484. [Google Scholar] [CrossRef]
- Malucelli, L.C.; Matos, M.; Jordão, C.; Lomonaco, D.; Lacerda, L.G.; Carvalho Filho, M.A.S.; Magalhães, W.L.E. Influence of cellulose chemical pretreatment on energy consumption and viscosity of produced cellulose nanofibers (CNF) and mechanical properties of nanopaper. Cellulose 2019, 26, 1667–1681. [Google Scholar] [CrossRef]
- Copenhaver, K.; Li, K.; Wang, L.; Lamm, M.; Zhao, X.H.; Korey, M.; Neivandt, D.; Dixon, B.; Sultana, S.; Kelly, P.; et al. Pretreatment of lignocellulosic feedstocks for cellulose nanofibril production. Cellulose 2022, 29, 4835–4876. [Google Scholar] [CrossRef]
- Perzon, A.; Jørgensen, B.; Ulvskov, P. Sustainable production of cellulose nanofiber gels and paper from sugar beet waste using enzymatic pre-treatment. Carbohydr. Polym. 2020, 230, 115581. [Google Scholar] [CrossRef]
- Luan, Q.; Zhou, W.; Zhang, H.; Bao, Y.; Zheng, M.; Shi, J.; Tang, H.; Huang, F. Cellulose-based composite macrogels from cellulose fiber and cellulose nanofiber as intestine delivery vehicles for probiotics. J. Agric. Food Chem. 2018, 66, 339–345. [Google Scholar] [CrossRef]
- Gunzburg, W.H.; Aung, M.M.; Toa, P.; Ng, S.; Read, E.; Tan, W.J.; Brandtner, E.M.; Dangerfield, J.; Salmons, B. Efficient protection of microorganisms for delivery to the intestinal tract by cellulose sulphate encapsulation. Microb. Cell Fact. 2020, 19, 216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, C.; Zhou, W.J.; Luan, Q.; Li, W.L.; Deng, Q.C.; Dong, X.Y.; Tang, H.; Huang, F.H. A pH-responsive gel macrosphere based on sodium alginate and cellulose nanofiber for potential intestinal delivery of probiotics. ACS Sustain. Chem. Eng. 2018, 6, 13924–13931. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Z.; Fang, H.; Chang, S.; Ren, G.; Cheng, X.; Pan, Y.; Wu, R.; Liu, H.; Wu, J. Construction of probiotic double-layered multinucleated microcapsules based on sulfhydryl-modified carboxymethyl cellulose sodium for increased intestinal adhesion of probiotics and therapy for intestinal inflammation induced by Escherichia coli O157:H7. ACS Appl. Mater. Interfaces 2023, 15, 18569–18589. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.; Ramasamy, M.; Cho, D.G.; Chung Soo, C.C.; Kapar, S.; Lee, J.Y.; Tam, K.C. A new approach for the encapsulation of saccharomyces cerevisiae using shellac and cellulose nanocrystals. Food Hydrocoll. 2023, 134, 108079. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Liu, K.L.; Yao, G.Q.; Zhang, H.P. Research progress in encapsulation of probiotics based on electrofluid processing technology. J. Food Biotechnol. 2022, 41, 24–31. [Google Scholar]
- Khalf, A.; Madihally, S.V. Recent advances in multiaxial electrospinning for drug delivery. Eur. J. Pharm. Biopharm. 2017, 112, 1–17. [Google Scholar] [CrossRef]
- Wang, X.; Cao, Z.; Zhang, M.; Meng, L.; Ming, Z.; Liu, J. Bioinspired oral delivery of gut microbiota by self-coating with biofilms. Sci. Adv. 2020, 6, eabb1952. [Google Scholar] [CrossRef]
- Meng, X.H.; He, F.; Zhao, Z.S.; Guo, Y.X.; Ma, X.K.; Tu, C.K.; Teng, H.; Chen, Z.X.; Yan, H.; Shao, X. Electrospun nanofibrous membranes accelerate biofilm formation and probiotic enrichment: Enhanced tolerances to pH and antibiotics. ACS Appl. Bio Mater. 2022, 14, 31601–31612. [Google Scholar]
- Singh, P.; Magalhaes, S.; Alves, L.; Antunes, F.; Miguel, M.; Lindman, B.; Medronho, B. Cellulose-based edible films for probiotic entrapment. Food Hydrocoll. 2019, 88, 68–74. [Google Scholar] [CrossRef]
- Sabio, L.; González, A.; Ramírez-Rodríguez, G.B.; Gutiérrez-Fernández, J.; Bañuelo, O.; Olivares, M.; Gálvez, N.; Delgado-López, J.M.; Dominguez-Vera, J.M. Probiotic cellulose: Antibiotic-free biomaterials with enhanced antibacterial activity. Acta Biomater. 2021, 124, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Sabio, L.; Dominguez-Vera, J.M.; de Vicente, J.; Delgado-Lopez, J.M. Living cellulose materials with tunable viscoelasticity through probiotic proliferation. ACS Appl. Bio Mater. 2023, 6, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Alcântara, A.V.; Abreu, A.A.S.; Gonçalves, C.; Fuciños, P.; Cerqueira, M.A.; Gama, F.M.P.; Pastrana, L.M.; Rodrigues, S.; Azeredo, H.M.C. Bacterial cellulose/cashew gum films as probiotic carriers. LWT-Food Sci. Technol. 2020, 130, 109699. [Google Scholar] [CrossRef]
- Mozaffarzogh, M.; Misaghi, A.; Shahbazi, Y.; Kamkar, A. Evaluation of probiotic carboxymethyl cellulose-sodium caseinate films and their application in extending shelf life quality of fresh trout fillets. LWT-Food Sci. Technol. 2020, 126, 109305. [Google Scholar] [CrossRef]
- Pal, K.I.; Kaur, D.P.; Mahendra, B.; Kiran, K.K. Process for Preparation of a Probiotic Encapsulated Formulation. India Patent Application IN201711011030A, 5 October 2018. [Google Scholar]
- Ross, C.; Luz, S.; Ann, A.M. Probiotic Storage and Delivery. U.S. Patent Application US8871266B2, 28 October 2014. [Google Scholar]
- Jinwook, Y.; Hyun, K.J. Probiotics-Delivering Hydrogel Formulation for Protecting Probiotics in Acidic Environment and Composition for Delivering Probiotics Comprising Same. WIPO Patent Application WO2018230939A1, 20 December 2018. [Google Scholar]
- Anil, N.A.; Krishnachandra, L.S.; Dhondiram, S.S.; Sachin, P.S.; Atul, K.A. Process for the Preparation of Powdered Probiotic Formulations for Monogastric Animals. U.S. Patent Application US11571387B2, 5 March 2018. [Google Scholar]
- Illathu, M.K.; Paulose, M.B. Method of Preparing Stable, Water Soluble Probiotic Compositions Based on Millets and Similar Cereals. U.S. Patent Application US10576113B2, 19 October 2017. [Google Scholar]
- Juncai, H.; Jiage, M.; Zhanmei, J.; Cong, X.; Wan, W. Probiotic-Encapsulating Gum Arabic Composite Fiber/Capsule, Preparation Method and Application Thereof. U.S. Patent Application US2023/019354 A1, 22 June 2023. [Google Scholar]
- Porubcan, R.S. Formulations to Increase In Vivo Survival of Probiotic Bacteria and Extend Their Shelf-Life. U.S. Patent Application US2004/0175389 A1, 9 September 2004. [Google Scholar]
- Karimi, N.; Alizadeh, A.; Almasi, H.; Hanifian, S. Preparation and characterization of whey protein isolate/polydextrose-based nanocomposite film incorporated with cellulose nanofiber and L. plantarum: A new probiotic active packaging system. LWT 2020, 121, 108978. [Google Scholar] [CrossRef]
- Rasouli, Y.; Moradi, M.; Tajik, H.; Molaei, R. Fabrication of anti-listeria film based on bacterial cellulose and Lactobacillus sakei derived bioactive metabolites; application in meat packaging. Food Biosci. 2021, 42, 101218. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastro. Hepat. 2020, 17, 687–701. [Google Scholar] [CrossRef] [PubMed]






| Cellulose Type | Probiotic Type | Survival Rate of Probiotics before Cellulose Encapsulation | Survival Rate of Probiotics after Cellulose Encapsulation | Application of the Cellulose-Based Probiotic Films | Ref. |
|---|---|---|---|---|---|
| Bacterial cellulose | Lactobacillus acidophilus, Bifidobacterium animalis | – | – | Bio-preservation | [29] |
| Bacterial cellulose | Lactiplantibacillus pentosus, Lactiplantibacillus plantarum | Less than 80% (After 5 months of storage at 4 °C) | About 90–95% (After 5 months of storage at 4 °C) | Milk fermentation | [30] |
| Bacterial cellulose nanofibers | Lactobacillus plantarum | <60% (Treatment in pH 2.5, 3.5, 4.5 and 6.8 for 3 h) | >150% (Treatment in pH 2.5, 3.5, 4.5 and 6.8 for 3 h) | Milk fermentation | [21] |
| Carboxymethyl cellulose | Lactobacillus lactis | – | – | Improving nisin production | [31] |
| Carboxymethyl cellulose | Bifidobacterium lactis, Lactobacillus acidophilus, Lactobacillus casei | The number of probiotics is less than 7.00 log CFU/g (45 days of storage at 7 °C) | The number of probiotics exceeded 8.00 log CFU/g (45 days of storage at 7 °C) | Food coating | [32] |
| Carboxymethyl cellulose | Lactobacillus plantarum | – | – | Bioactive food packaging | [33] |
| Carboxymethyl cellulose | Lactobacillus acidophilus | About 49% (Digest in simulated gastric juices for 120 min) | About 70% (Digest in simulated gastric juices for 120 min) | Antibacterial food coating | [34] |
| Cellulose nanofiber | Lactobacillus casei, Bacillus coagulans | – | – | Food packaging | [35] |
| Cellulose acetate | Escherichia coli Nissle 1917 | 0% (Digest in a simulated digestive system for 100 min) | About 26% (Digest in a simulated digestive system for 100 min) | – | [36] |
| Cellulose microgels | Lactobacillus plantarum | The number of viable bacteria decreased by 105 (Freeze drying) | The number of viable bacteria decreased by 103 (Freeze drying) | – | [37] |
| Kombucha bacterial cellulose | Lactobacillus plantarum | About 33% (Freeze drying) | About 49% (Freeze drying) | Antibacterial food packaging | [38] |
| Probiotic Type | Cellulose Type | Grafting Method | Function | Ref. |
|---|---|---|---|---|
| Lactobacillus plantarum | Cellulose nanofiber | TEMPO-mediated oxidation to endow cellulose with carboxyl groups | Improving the survival rate and the intestinal retention time of the probiotics | [78] |
| Lactobacillus casei | Cellulose | Sulfation of cellulose to endow it with negatively charged sulfuric acid groups | Improving the survival rate and intestinal delivery rate of the probiotics, realizing their controllable release | [79] |
| Lactobacillus plantarum | Cellulose nanofiber | TEMPO-mediated oxidation | Improving the survival rate of the probiotics and realizing the controllable release of the probiotics | [80] |
| Bifidobacterium adolescentis and Bacillus subtilis | Carboxymethyl cellulose | Obtaining mercaptoylcarboxymethyl cellulose through the EDC (1-ethyl-3(3-dimethylaminopropyl-carbodiimide hydrochloride)/NHS (N-hydroxysuccinimide) chemistry | Improving the survival rate and improving the storage stability of the probiotics and promoting the proliferation, adhesion, and colonization of probiotics | [81] |
| Saccharomyces cerevisiae | Cellulose nanocrystals | The complexation of shellac and cellulose nanocrystals via hydrogen bonding | Improving the survival rate of the probiotics and realizing the controllable release of the probiotics | [82] |
| Encapsulation Strategies | Cellulose Type | Probiotic Type | Advantage | Disadvantage | Refs. |
|---|---|---|---|---|---|
| Electrospinning | Cellulose acetate; Cellulose acetate nanofiber | Escherichia coli Nissle 1917; Lactobacillus paracasei | High preparation efficiency, a variety of fiber structures and shapes can be prepared | Uneven fiber thickness, and complex electrospinning equipment | [36,83,87] |
| Cross-linking | Carboxymethyl cellulose and hydroxyethyl cellulose; Cellulose; TEMPO oxidized cellulose nanofiber | Lactobacillus rhamnosus; Lactobacillus plantarum; Lactobacillus plantarum | Improving mechanical properties and enhancing the stability of the fiber | Fiber aggregation caused by excessive cross-linking | [37,80,88] |
| In-situ growth | Bacterial cellulose; Bacterial cellulose; Kombucha bacterial cellulose | Lactobacillus fermentum and Lactobacillus gasseri; Lactobacillus fermentum; Lactobacillus plantarum | It is easy to operate and does not require the use of toxic chemicals | Long production period, and difficult to remove the microorganisms used for producing cellulose fiber | [38,89,90] |
| Casting | Carboxymethyl cellulose; Carboxymethyl cellulose; Carboxymethyl cellulose and microcrystalline cellulose; Carboxymethyl cellulose | Lactobacillus lactis; Bacillus coagulans; Bifidobacterium lactis, Lactobacillus acidophilus and Lactobacillus casei; Lactobacillus acidophilus, Lactobacillus reuteri, Lactobacillus casei, Lactobacillus rhamnosus and Bifidobacterium bifidum | It can improve the physical properties of materials | Complicated and tedious operation process | [31,32,91,92] |
| Encapsulation Materials | Encapsulation Method | Suggested Application | Patent Number | Ref. |
|---|---|---|---|---|
| Sodium alginates/PEG 4000/methacrylate polymers | Ion gelation | Drugs that promote intestinal health | IN201711011030A | [93] |
| Casein/starch | Spray drying | Probiotic powder supplement | US8871266B2 | [94] |
| 4-(2-hydroxyethyl)-1-piperazine-ethanesulfonic acid/glycine/betaine/carboxymethyl cellulose | Cross-linking | A drug to treat intestinal disorders | WO2018230939A1 | [95] |
| Sodium carboxymethyl cellulose/maltodextrin | Freeze drying | Freeze-dried powder preparation of probiotics | US11571387 B2 | [96] |
| Millet extract powder | Freeze drying or spray drying | Functional food supplements or dietary supplements | US10576113B2 | [97] |
| Gum Arabic/polyvinyl alcohol/polyvinylpyrrolidone/whey protein concentrate or maltodextrin | Electrospinning | Probiotic capsules | US2023/019354 A1 | [98] |
| Monovalent alginate/gelatin or cellulose | Freeze drying or spray drying | Probiotic powder supplements | US2004/0175389 A1 | [99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zhang, J.; Li, C. Delivery of Probiotics with Cellulose-Based Films and Their Food Applications. Polymers 2024, 16, 794. https://doi.org/10.3390/polym16060794
Yang Y, Zhang J, Li C. Delivery of Probiotics with Cellulose-Based Films and Their Food Applications. Polymers. 2024; 16(6):794. https://doi.org/10.3390/polym16060794
Chicago/Turabian StyleYang, Ying, Junze Zhang, and Chengcheng Li. 2024. "Delivery of Probiotics with Cellulose-Based Films and Their Food Applications" Polymers 16, no. 6: 794. https://doi.org/10.3390/polym16060794
APA StyleYang, Y., Zhang, J., & Li, C. (2024). Delivery of Probiotics with Cellulose-Based Films and Their Food Applications. Polymers, 16(6), 794. https://doi.org/10.3390/polym16060794





