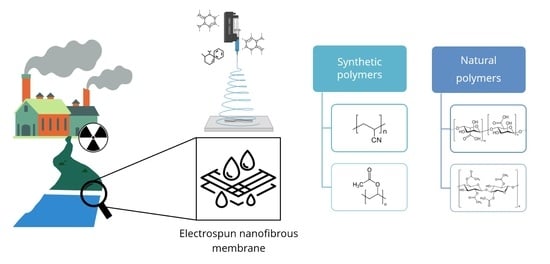
The Potential of Electrospun Membranes in the Treatment of Textile Wastewater: A Review
Abstract
1. Introduction
2. Textile Wastewater: Composition and Purification Requirements
3. Electrospinning—Process Technology and Operation
3.1. Processing Parameters
3.1.1. Solution Parameters
- -
- Concentration and viscosity
- -
- Molecular weight
- -
- Conductivity
3.1.2. Process Parameters
- -
- Applied voltage
- -
- Distance between the needle and collector
- -
- Flow rate
- -
- Needle diameter
- -
- Collector
3.1.3. Environmental Parameters
- -
- Temperature
- -
- Humidity
4. Electrospun Membranes for Water Treatment
4.1. Synthetic Polymers
4.2. Natural Polymers
4.2.1. Cellulose Acetate
4.2.2. Chitosan

4.2.3. Alginate
4.2.4. β-Cyclodextrin
5. Limitations of Textile Wastewater Filtering Structures by Electrospinning
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sanaeepur, H.; Ebadi Amooghin, A.; Shirazi, M.M.A.; Pishnamazi, M.; Shirazian, S. Water desalination and ion removal using mixed matrix electrospun nanofibrous membranes: A critical review. Desalination 2022, 521, 115350. [Google Scholar] [CrossRef]
- Peydayesh, M.; Mezzenga, R. Protein nanofibrils for next generation sustainable water purification. Nat. Commun. 2021, 12, 3248. [Google Scholar] [PubMed]
- Adam, M.R.; Othman, M.H.D.; Kurniawan, T.A.; Puteh, M.H.; Ismail, A.F.; Khongnakorn, W.; Rahman, M.A.; Jaafar, J. Advances in adsorptive membrane technology for water treatment and resource recovery applications: A critical review. J. Environ. Chem. Eng. 2022, 10, 107633. [Google Scholar]
- Sultana, M.; Rownok, M.H.; Sabrin, M.; Rahaman, M.H.; Alam, S.M.N. A review on experimental chemically modified activated carbon to enhance dye and heavy metals adsorption. Clean. Eng. Technol. 2022, 6, 100382. [Google Scholar] [CrossRef]
- Wang, X.; Hsiao, B.S. Electrospun nanofiber membranes. Curr. Opin. Chem. Eng. 2016, 12, 62–81. [Google Scholar] [CrossRef]
- Kugarajah, V.; Ojha, A.K.; Ranjan, S.; Dasgupta, N.; Ganesapillai, M.; Dharmalingam, S.; Elmoll, A.; Hosseini, S.A.; Muthulakshmi, L.; Vijayakumar, S.; et al. Future applications of electrospun nanofibers in pressure driven water treatment: A brief review and research update. J. Environ. Chem. Eng. 2021, 9, 105107. [Google Scholar] [CrossRef]
- Wang, Z.; Xue, M.; Huang, K.; Liu, Z. Textile Dyeing Wastewater Treatment. In Advances in Treating Textile Effluent; InTech Open: London, UK, 2011; Chapter 5; pp. 91–116. [Google Scholar] [CrossRef]
- Kant, R. Textile dyeing industry an environmental hazard. Nat. Sci. 2012, 4, 22–26. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef]
- Al-Ahmed, Z.A.; Al-Radadi, N.S.; Ahmed, M.K.; Shoueir, K.; El-Kemary, M. Dye removal, antibacterial properties, and morphological behavior of hydroxyapatite doped with Pd ions. Arab. J. Chem. 2020, 13, 8626–8637. [Google Scholar] [CrossRef]
- Siddique, K.; Rizwan, M.; Shahid, M.J.; Ali, S.; Ahmad, R.; Rizvi, H. Textile Wastewater Treatment Options: A Critical Review. Enhancing Cleanup Environ. Pollut. 2017, 2, 183–207. [Google Scholar]
- Li, Y.; Zhu, J.; Cheng, H.; Li, G.; Cho, H.; Jiang, M.; Gao, Q.; Zhang, X. Developments of Advanced Electrospinning Techniques: A Critical Review. Adv. Mater. Technol. 2021, 6, 2100410. [Google Scholar] [CrossRef]
- Patel, K.D.; Kim, H.W.; Knowles, J.C.; Poma, A. Molecularly Imprinted Polymers and Electrospinning: Manufacturing Convergence for Next-Level Applications. Adv. Funct. Mater. 2020, 30, 2001955. [Google Scholar] [CrossRef]
- Nasreen, S.A.A.N.; Sundarrajan, S.; Nizar, S.A.S.; Ramakrishna, S. Nanomaterials: Solutions to water-concomitant challenges. Membranes 2019, 9, 40. [Google Scholar] [CrossRef]
- Subrahmanya, T.M.; Arshad, A.B.; Lin, P.T.; Widakdo, J.; Makari, H.K.; Austria, H.F.M.; Hu, C.C.; Lai, J.Y.; Hung, W.S. A review of recent progress in polymeric electrospun nanofiber membranes in addressing safe water global issues. RSC Adv. 2021, 11, 9638–9663. [Google Scholar]
- Zia, Q.; Tabassum, M.; Lu, Z.; Khawar, M.T.; Song, J.; Gong, H.; Meng, J.; Li, Z.; Li, J. Porous poly(L–lactic acid)/chitosan nanofibres for copper ion adsorption. Carbohydr. Polym. 2020, 227, 115343. [Google Scholar] [CrossRef]
- Zhang, K.; Li, Z.; Deng, N.; Ju, J.; Li, Y.; Cheng, B.; Kang, W.; Yan, J. Tree-like cellulose nanofiber membranes modified by citric acid for heavy metal ion (Cu2+) removal. Cellulose 2019, 26, 945–958. [Google Scholar] [CrossRef]
- Nalbandian, M.J.; Greenstein, K.E.; Shuai, D.; Zhang, M.; Choa, Y.H.; Parkin, G.F.; Myung, N.V.; Cwiertny, D.M. Tailored synthesis of photoactive TiO2 nanofibers and Au/TiO2 nanofiber composites: Structure and reactivity optimization for water treatment applications. Environ. Sci. Technol. 2015, 49, 1654–1663. [Google Scholar] [CrossRef]
- Thyavihalli Girijappa, Y.G.; Mavinkere Rangappa, S.; Parameswaranpillai, J.; Siengchin, S. Natural Fibers as Sustainable and Renewable Resource for Development of Eco-Friendly Composites: A Comprehensive Review. Front. Mater. 2019, 6, 226. [Google Scholar] [CrossRef]
- Yaseen, D.A.; Scholz, M. Textile Dye Wastewater Characteristics and Constituents of Synthetic Effluents: A Critical Review; Springer: Berlin/Heidelberg, Germany, 2019; Volume 16. [Google Scholar]
- Wang, X.; Jiang, J.; Gao, W. Reviewing textile wastewater produced by industries: Characteristics, environmental impacts, and treatment strategies. Water Sci. Technol. 2022, 85, 2076–2096. [Google Scholar] [CrossRef]
- Malik, A.; Hussain, M.; Uddin, F.; Raza, W.; Hussain, S.; Habiba, U.E.; Malik, T.; Ajmal, Z. Investigation of textile dyeing effluent using activated sludge system to assess the removal efficiency. Water Environ. Res. 2021, 93, 2931–2940. [Google Scholar] [CrossRef] [PubMed]
- Dojčinović, B.P.; Obradović, B.M.; Kuraica, M.M.; Pergal, M.V.; Dolić, S.D.; Indić, D.R.; Tosti, T.B.; Manojlović, D.D. Application of non-thermal plasma reactor for degradation and detoxification of high concentrations of dye Reactive Black 5 in water. J. Serbian Chem. Soc. 2016, 81, 829–845. [Google Scholar] [CrossRef]
- Tijing, L.D.; Yao, M.; Ren, J.; Park, C.H.; Kim, C.S.; Shon, H.K. Nanofibers for Water and Wastewater Treatment: Recent Advances and Developments; Springer Nature: Singapore, 2019. [Google Scholar]
- Ma, Z.; Chang, H.; Liang, Y.; Meng, Y.; Ren, L.; Liang, H. Research progress and trends on state-of-the-art membrane technologies in textile wastewater treatment. Sep. Purif. Technol. 2024, 333, 125853. [Google Scholar] [CrossRef]
- Müller, A.K.; Xu, Z.K.; Greiner, A. Filtration of Paint-Contaminated Water by Electrospun Membranes. Macromol. Mater. Eng. 2022, 307, 2200238. [Google Scholar] [CrossRef]
- Nayl, A.A.; Abd-Elhamid, A.I.; Awwad, N.S.; Abdelgawad, M.A.; Wu, J.; Mo, X.; Gomha, S.M.; Aly, A.A.; Bräse, S. Review of the Recent Advances in Electrospun Nanofibers Applications in Water Purification. Polymers 2022, 14, 1594. [Google Scholar] [CrossRef]
- Mingjun, C.; Youchen, Z.; Haoyi, L.; Xiangnan, L.; Yumei, D.; Bubakir, M.M.; Weimin, Y. An example of industrialization of melt electrospinning: Polymer melt differential electrospinning. Adv. Ind. Eng. Polym. Res. 2019, 2, 110–115. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Dadras Chomachayi, M.; Solouk, A.; Akbari, S.; Sadeghi, D.; Mirahmadi, F.; Mirzadeh, H. Electrospun nanofibers comprising of silk fibroin/gelatin for drug delivery applications: Thyme essential oil and doxycycline monohydrate release study. J. Biomed. Mater. Res. Part A 2018, 106, 1092–1103. [Google Scholar] [CrossRef]
- Kalantari, K.; Afifi, A.M.; Jahangirian, H.; Webster, T.J. Biomedical applications of chitosan electrospun nanofibers as a green polymer—Review. Carbohydr. Polym. 2019, 207, 588–600. [Google Scholar] [CrossRef]
- Kyselica, R.; Enikov, E.T.; Polyvas, P.; Anton, R. Electrostatic focusing of electrospun Polymer(PEO) nanofibers. J. Electrostat. 2018, 94, 21–29. [Google Scholar] [CrossRef]
- Chen, X.; Xu, C.; He, H. Electrospinning of silica nanoparticles-entrapped nanofibers for sustained gentamicin release. Biochem. Biophys. Res. Commun. 2019, 516, 1085–1089. [Google Scholar] [CrossRef]
- Martins, A.; Reis, R.L.; Neves, N.M. Electrospinning: Processing technique for tissue engineering scaffolding. Int. Mater. Rev. 2008, 53, 257–274. [Google Scholar] [CrossRef]
- Zeyrek Ongun, M.; Paralı, L.; Oğuzlar, S.; Pechousek, J. Characterization of β-PVDF-based nanogenerators along with Fe2O3 NPs for piezoelectric energy harvesting. J. Mater. Sci. Mater. Electron. 2020, 31, 19146–19158. [Google Scholar] [CrossRef]
- Mirjalili, M.; Zohoori, S. Review for application of electrospinning and electrospun nanofibers technology in textile industry. J. Nanostruct. Chem. 2016, 6, 207–213. [Google Scholar] [CrossRef]
- Aruna, S.T.; Balaji, L.S.; Kumar, S.S.; Prakash, B.S. Electrospinning in solid oxide fuel cells—A review. Renew. Sustain. Energy Rev. 2017, 67, 673–682. [Google Scholar] [CrossRef]
- Pisani, S.; Dorati, R.; Conti, B.; Modena, T.; Bruni, G.; Genta, I. Design of copolymer PLA-PCL electrospun matrix for biomedical applications. React. Funct. Polym. 2018, 124, 77–89. [Google Scholar] [CrossRef]
- Costa, R.G.F.; De Oliveira, J.E.; De Paula, G.F.; De Picciani, P.H.S.; De Medeiros, E.S.; Ribeiro, C.; Mattoso, L.H.C. Eletrofiação de polímeros em solução. Parte I: Fundamentação teórica. Polimeros 2012, 22, 170–177. [Google Scholar] [CrossRef]
- Leidy, R.; Maria Ximena, Q.C. Use of electrospinning technique to produce nanofibres for food industries: A perspective from regulations to characterisations. Trends Food Sci. Technol. 2019, 85, 92–106. [Google Scholar] [CrossRef]
- Mirtič, J.; Balažic, H.; Zupančič, Š.; Kristl, J. Effect of Solution Composition Variables on Electrospun Alginate Nanofibers: Response Surface Analysis. Polymers 2019, 11, 692. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Krishnan, U.M.; Sethuraman, S. Fabrication and characterization of chitosan-gelatin blend nanofibers for skin tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 94, 264–272. [Google Scholar] [CrossRef]
- Nezarati, R.M.; Eifert, M.B.; Cosgriff-Hernandez, E. Effects of Humidity and Solution Viscosity on Electrospun Fiber Morphology. Tissue Eng. Part C Methods 2013, 19, 810–819. [Google Scholar] [CrossRef]
- Li, Z.; Wang, C. One-Dimensional Nanostructures Electrospinning Technique and Unique Nanofibers; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Cheng, Y.-L.; Lee, C.-Y.; Huang, Y.-L.; Buckner, C.A.; Lafrenie, R.M.; Dénommée, J.A.; Caswell, J.M.; Want, D.A.; Gan, G.G.; Leong, Y.C.; et al. Electrospinning and Drug Delivery; Intech: Vienna, Austria, 2016; Volume 11, p. 13. [Google Scholar]
- Sohi, A.N.; Naderi-Manesh, H.; Soleimani, M.; Mirzaei, S.; Delbari, M.; Dodel, M. Influence of Chitosan Molecular Weight and Poly(ethylene oxide): Chitosan Proportion on Fabrication of Chitosan Based Electrospun Nanofibers. Polym. Sci. Ser. A 2018, 60, 471–482. [Google Scholar] [CrossRef]
- Colmenares-Roldán, G.J.; Quintero-Martínez, Y.; Agudelo-Gómez, L.M.; Rodríguez-Vinasco, L.F.; Hoyos-Palacio, L.M. Influence of the molecular weight of polymer, solvents and operational condition in the electrospinning of polycaprolactone. Rev. Fac. Ing. 2017, 2017, 35–45. [Google Scholar] [CrossRef]
- Akduman, Ç.; Perrin, E.; Kumabasar, A.; Çay, A. Effect of Molecular Weight on the Morphology of Electrospun Poly(Vinyl Alcohol) Nanofibers. In Proceedings of the XIIIth International Izmir Textile and Apparel Symposium, Izmir, Turkey, 2–5 April 2014; pp. 127–134. [Google Scholar]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef]
- Zuo, W.; Zhu, M.; Yang, W.; Yu, H.; Chen, Y.; Zhang, Y. Experimental Study on Relationship Between Jet Instability and Formation of Beaded Fibers During Electrospinning. Polym. Eng. Sci. 2005, 45, 704–709. [Google Scholar] [CrossRef]
- Mahmud, M.M.; Perveen, A.; Matin, M.A.; Arafat, M.T. Effects of binary solvent mixtures on the electrospinning behavior of poly (vinyl alcohol). Mater. Res. Express 2018, 5, 115407. [Google Scholar] [CrossRef]
- Gazquez, G.C.; Smulders, V.; Veldhuis, S.A.; Wieringa, P.; Moroni, L.; Boukamp, B.A.; Ten Elshof, J.E. Influence of Solution Properties and Process Parameters on the Formation and Morphology of YSZ and NiO Ceramic Nanofibers by Electrospinning. Nanomaterials 2017, 7, 16. [Google Scholar] [CrossRef]
- Abid, S.; Hussain, T. Materials Science & Engineering C Current applications of electrospun polymeric nano fi bers in cancer therapy. Mater. Sci. Eng. C 2019, 97, 966–977. [Google Scholar]
- Haghju, S.; Bari, M.R.; Khaled-Abad, M.A. Affecting parameters on fabrication of β-D-galactosidase immobilized chitosan/poly (vinyl alcohol) electrospun nanofibers. Carbohydr. Polym. 2018, 200, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Reda, R.I.; Wen, M.M.; El-Kamel, A.H. Ketoprofen-loaded Eudragit electrospun nanofibers for the treatment of oral mucositis. Int. J. Nanomed. 2017, 12, 2335–2351. [Google Scholar] [CrossRef] [PubMed]
- Megelski, S.; Stephens, J.S.; Bruce Chase, D.; Rabolt, J.F. Micro- and nanostructured surface morphology on electrospun polymer fibers. Macromolecules 2002, 35, 8456–8466. [Google Scholar] [CrossRef]
- Fallah, M.; Bahrami, S.H.; Ranjbar-Mohammadi, M. Fabrication and characterization of PCL/gelatin/curcumin nanofibers and their antibacterial properties. J. Ind. Text. 2016, 46, 562–577. [Google Scholar] [CrossRef]
- Doshi, J.; Reneker, D.H. Electrospinning process and applications of electrospun fibers. J. Electrostat. 1995, 35, 151–160. [Google Scholar] [CrossRef]
- Patil, J.V.; Mali, S.S.; Kamble, A.S.; Hong, C.K.; Kim, J.H.; Patil, P.S. Electrospinning: A versatile technique for making of 1D growth of nanostructured nanofibers and its applications: An experimental approach. Appl. Surf. Sci. 2017, 423, 641–674. [Google Scholar] [CrossRef]
- Zargham, S.; Bazgir, S.; Tavakoli, A.; Rashidi, A.S.; Damerchely, R. The Effect of Flow Rate on Morphology and Deposition Area of Electrospun Nylon 6 Nanofiber. J. Eng. Fiber. Fabr. 2012, 7, 42–49. [Google Scholar] [CrossRef]
- Hekmati, A.H.; Rashidi, A.; Ghazisaeidi, R.; Drean, J.Y. Effect of needle length, electrospinning distance, and solution concentration on morphological properties of polyamide-6 electrospun nanowebs. Text. Res. J. 2013, 83, 1452–1466. [Google Scholar] [CrossRef]
- Kuchi, C.; Harish, G.S.; Reddy, P.S. Effect of polymer concentration, needle diameter and annealing temperature on TiO2-PVP composite nanofibers synthesized by electrospinning technique. Ceram. Int. 2018, 44, 5266–5272. [Google Scholar] [CrossRef]
- Abunahel, B.M.; Azman, N.Z.N.; Jamil, M. Effect of Needle Diameter on the Morphological Structure of Electrospun n-Bi2O3/Epoxy-PVA Nanofiber Mats. Int. J. Chem. Mater. Eng. 2018, 12, 296–299. [Google Scholar]
- Wang, X.; Um, I.C.; Fang, D.; Okamoto, A.; Hsiao, B.S.; Chu, B. Formation of water-resistant hyaluronic acid nanofibers by blowing-assisted electro-spinning and non-toxic post treatments. Polymer 2005, 46, 4853–4867. [Google Scholar] [CrossRef]
- Sundaray, B.; Subramanian, V.; Natarajan, T.S.; Xiang, R.Z.; Chang, C.C.; Fann, W.S. Electrospinning of continuous aligned polymer fibers. Appl. Phys. Lett. 2004, 84, 1222–1224. [Google Scholar] [CrossRef]
- Anindyajati, A.; Boughton, P.; Ruys, A. The Effect of Rotating Collector Design on Tensile Properties and Morphology of Electrospun Polycaprolactone Fibres. MATEC Web Conf. 2015, 27. [Google Scholar] [CrossRef]
- Ghobeira, R.; Asadian, M.; Vercruysse, C.; Declercq, H.; De Geyter, N.; Morent, R. Wide-ranging diameter scale of random and highly aligned PCL fibers electrospun using controlled working parameters. Polymer 2018, 157, 19–31. [Google Scholar] [CrossRef]
- De Vrieze, S.; Van Camp, T.; Nelvig, A.; Hagström, B.; Westbroek, P.; De Clerck, K. The effect of temperature and humidity on electrospinning. J. Mater. Sci. 2009, 44, 1357–1362. [Google Scholar] [CrossRef]
- Supaphol, P.; Mit-uppatham, C.; Nithitanakul, M. Ultrafine Electrospun Polyamide-6 Fibers: Effects of Solvent System and Emitting Electrode Polarity on Morphology and Average Fiber Diameter. Macromol. Mater. Eng. 2005, 290, 933–942. [Google Scholar] [CrossRef]
- Yang, G.Z.; Li, H.P.; Yang, J.H.; Wan, J.; Yu, D.G. Influence of Working Temperature on The Formation of Electrospun Polymer Nanofibers. Nanoscale Res. Lett. 2017, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Casper, C.L.; Stephens, J.S.; Tassi, N.G.; Chase, D.B.; Rabolt, J.F. Controlling Surface Morphology of Electrospun Polystyrene Fibers: Effect of Humidity and MolecularWweight in the Electrospinning Process. Macromolecules 2004, 37, 573–578. [Google Scholar] [CrossRef]
- Medeiros, E.S.; Mattoso, L.H.C.; Offeman, R.D.; Wood, D.F.; Orts, W.J. Effect of relative humidity on the morphology of electrospun polymer fibers. Can. J. Chem. 2008, 86, 590–599. [Google Scholar] [CrossRef]
- Long, Y.Z.; Yan, X.; Wang, X.X.; Zhang, J.; Yu, M. Electrospinning: The Setup and Procedure. In Electrospinning: Nanofabrication and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 21–52. [Google Scholar]
- Herrero-Herreo, M. Role of Electrospinning Parameters on Poly(Lactic-co-Glycolic Acid) and Poly(Caprolactone-co-Glycolic Acid) Membranes. Polymers 2021, 13, 695. [Google Scholar] [CrossRef]
- Tang, Y.; Cai, Z.; Sun, X.; Chong, C.; Yan, X.; Li, M.; Xu, J. Electrospun Nanofiber-Based Membranes for Water Treatment. Polymers 2022, 14, 2004. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Ma, H.; Hsiao, B.S.; Chu, B. Functionalized electrospun nanofibrous microfiltration membranes for removal of bacteria and viruses. J. Membr. Sci. 2014, 452, 446–452. [Google Scholar] [CrossRef]
- Makaremi, M.; De Silva, R.T.; Pasbakhsh, P. Electrospun nanofibrous membranes of polyacrylonitrile/halloysite with superior water filtration ability. J. Phys. Chem. C 2015, 119, 7949–7958. [Google Scholar] [CrossRef]
- Cao, X.; Huang, M.; Ding, B.; Yu, J.; Sun, G. Robust polyacrylonitrile nanofibrous membrane reinforced with jute cellulose nanowhiskers for water purification. Desalination 2013, 316, 120–126. [Google Scholar] [CrossRef]
- Haider, S.; Binagag, F.F.; Haider, A.; Mahmood, A.; Al Masry, W.A.; Alhoshan, M.; Khan, S.U.D. Fabrication of the diethylenetriamine grafted polyacrylonitrile electrospun nanofibers membrane for the aqueous removal of cationic dyes. Sci. Adv. Mater. 2015, 7, 309–318. [Google Scholar] [CrossRef]
- Jin, L.; Ye, J.; Wang, Y.; Qian, X.; Dong, M. Electrospinning Synthesis of ZIF-67/PAN Fibrous Membrane with High-capacity Adsorption for Malachite Green. Fibers Polym. 2019, 20, 2070–2077. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, Y.; Lan, W.; Sameen, D.E.; Ahmed, S.; Dai, J. Enhanced photocatalytic degradation of organic dyes by ultrasonic-assisted electrospray TiO2/graphene oxide on polyacrylonitrile/β-cyclodextrin nanofibrous membranes. Ultrason. Sonochem. 2021, 70, 105343. [Google Scholar] [CrossRef]
- Mohamed, A.; Yousef, S.; Ali Abdelnaby, M.; Osman, T.A.; Hamawandi, B.; Toprak, M.S.; Muhammed, M.; Uheida, A. Photocatalytic degradation of organic dyes and enhanced mechanical properties of PAN/CNTs composite nanofibers. Sep. Purif. Technol. 2017, 182, 219–223. [Google Scholar] [CrossRef]
- Du, F.; Sun, L.; Huang, Z.; Chen, Z.; Xu, Z.; Ruan, G.; Zhao, C. Electrospun reduced graphene oxide/TiO2/poly (acrylonitrile-co-maleic acid) composite nanofibers for efficient adsorption and photocatalytic removal of malachite green and leucomalachite green. Chemosphere 2020, 239, 124764. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.; Liang, B.; Liu, Y.; Xu, T.; Wang, L.; Cao, B.; Pan, K. Graphene Oxide as an Effective Barrier on a Porous Nanofibrous Membrane for Water Treatment. ACS Appl. Mater. Interfaces 2016, 8, 6211–6218. [Google Scholar] [CrossRef]
- Feng, Q.; Wu, D.; Zhao, Y.; Wei, A.; Wei, Q.; Fong, H. Electrospun AOPAN/RC blend nanofiber membrane for efficient removal of heavy metal ions from water. J. Hazard. Mater. 2018, 344, 819–828. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, Y.; Sun, Z.; Yang, C.; Tang, D. Effective strategy to fabricate ZIF-8@ZIF-8/polyacrylonitrile nanofibers with high loading efficiency and improved removing of Cr(VI). Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125292. [Google Scholar] [CrossRef]
- Mohammad, N.; Atassi, Y. Enhancement of removal efficiency of heavy metal ions by polyaniline deposition on electrospun polyacrylonitrile membranes. Water Sci. Eng. 2021, 14, 129–138. [Google Scholar] [CrossRef]
- Akduman, C.; Akçakoca Kumbasar, E.P.; Morsunbul, S. Electrospun nanofiber membranes for adsorption of dye molecules from textile wastewater. IOP Conf. Ser. Mater. Sci. Eng. 2017, 254, 102001. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Vossoughi, M.; Mahmoodi, N.M.; Sadrzadeh, M. Efficient dye removal from aqueous solution by high-performance electrospun nanofibrous membranes through incorporation of SiO2 nanoparticles. J. Clean. Prod. 2018, 183, 1197–1206. [Google Scholar] [CrossRef]
- Teng, M.; Li, F.; Zhang, B.; Taha, A.A. Electrospun cyclodextrin-functionalized mesoporous polyvinyl alcohol/SiO2 nanofiber membranes as a highly efficient adsorbent for indigo carmine dye. Colloids Surf. A Physicochem. Eng. Asp. 2011, 385, 229–234. [Google Scholar] [CrossRef]
- Karim, M.R.; Aijaz, M.O.; Alharth, N.H.; Alharbi, H.F.; Al-Mubaddel, F.S.; Awual, M.R. Composite nanofibers membranes of poly(vinyl alcohol)/chitosan for selective lead(II) and cadmium(II) ions removal from wastewater. Ecotoxicol. Environ. Saf. 2019, 169, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Wang, R.; Tang, G.; Mou, Z.; Lei, J.; Han, J.; De Smedt, S.; Xiong, R.; Huang, C. Ecofriendly Electrospun Membranes Loaded with Visible-Light-Responding Nanoparticles for Multifunctional Usages: Highly Efficient Air Filtration, Dye Scavenging, and Bactericidal Activity. ACS Appl. Mater. Interfaces 2019, 11, 12880–12889. [Google Scholar] [CrossRef] [PubMed]
- Sitinjak, E.M.; Masmur, I.; Marbun, N.V.M.D.; Hutajulu, P.E.; Gultom, G.; Sitanggang, Y. Direct Z-scheme of n-type CuS/p-type ZnS@electrospun PVP nanofiber for the highly efficient catalytic reduction of 4-nitrophenol and mixed dyes. RSC Adv. 2022, 12, 16165–16173. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Han, P. Electrospinning preparation of g-C3N4/Nb2O5 nanofibers heterojunction for enhanced photocatalytic degradation of organic pollutants in water. Sci. Rep. 2021, 11, 22950. [Google Scholar] [CrossRef]
- Xing, Y.; Que, W.; Yin, X.; He, Z.; Liu, X.; Yang, Y.; Shao, J.; Kong, L.B. Electrospun ZnO/Bi2O3 Nanofibers with Enhanced Photocatalytic Cctivity. Nanomaterials 2014, 2014, 130539. [Google Scholar]
- Lu, J.; Liu, M.; Zhou, S.; Zhou, X.; Yang, Y. Electrospinning fabrication of ZnWO4 nanofibers and photocatalytic performance for organic dyes. Dye. Pigment. 2017, 136, 1–7. [Google Scholar] [CrossRef]
- Kolak, S.; Birhanlı, E.; Boran, F.; Bakar, B.; Ulu, A.; Yeşilada, Ö.; Ateş, B. Tailor-made novel electrospun polycaprolactone/polyethyleneimine fiber membranes for laccase immobilization: An all-in-one material to biodegrade textile dyes and phenolic compounds. Chemosphere 2023, 313, 137478. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghafri, B.; Lau, W.J.; Al-Abri, M.; Goh, P.S.; Ismail, A.F. Titanium dioxide-modified polyetherimide nanofiber membrane for water treatment. J. Water Process Eng. 2019, 32, 100970. [Google Scholar] [CrossRef]
- De Farias, L.M.S.; Ghislandi, M.G.; De Aguiar, M.F.; Silva, B.R.S.; Leal, A.N.R.; Silva, F.D.A.O.; Fraga, T.J.M.; De Melo, C.P.; Kleber, G.; Alves, B. Electrospun polystyrene/graphene oxide fibers applied to the remediation of dye wastewater. Mater. Chem. Phys. 2022, 276, 125356. [Google Scholar] [CrossRef]
- Li, X.; Yi, K.; Ran, Q.; Fan, Z.; Liu, C.; Liu, X.; Jia, K. Selective removal of cationic organic dyes via electrospun nanofibrous membranes derived from polyarylene ethers containing pendent nitriles and sulfonates. Sep. Purif. Technol. 2022, 301, 121942. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Lu, Y.; Zeng, H. Studies of the preparation, structure, and properties of an acrylic chelating fiber containing amidoxime groups. J. Appl. Polym. Sci. 1993, 47, 45–52. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, J.; Menkhaus, T.J.; Varadaraju, H.; Sun, Y.; Fong, H. Antimicrobial nano-fibrous membranes developed from electrospun polyacrylonitrile nanofibers. J. Membr. Sci. 2011, 369, 499–505. [Google Scholar] [CrossRef]
- Huang, F.; Xu, Y.; Liao, S.; Yang, D.; Hsieh, Y.L.; Wei, Q. Preparation of amidoxime polyacrylonitrile chelating nanofibers and their application for adsorption of metal ions. Materials 2013, 6, 969–980. [Google Scholar] [CrossRef]
- Saeed, K.; Haider, S.; Oh, T.J.; Park, S.Y. Preparation of amidoxime-modified polyacrylonitrile (PAN-oxime) nanofibers and their applications to metal ions adsorption. J. Membr. Sci. 2008, 322, 400–405. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, Y.; Wang, B.; Qian, G.; Tao, T. Synthesis of palladium dendritic nanostructures on amidoxime modified polyacrylonitrile fibers through a complexing-reducing method. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2013, 178, 923–929. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, X.; Yu, M.; Wang, Z.; Zhang, B.; Ma, H.; Wang, M.; Li, J. A study on the degree of amidoximation of polyacrylonitrile fibers and its effect on their capacity to adsorb uranyl ions. Ind. Eng. Chem. Res. 2015, 54, 3101–3106. [Google Scholar] [CrossRef]
- Jatoi, A.W.; Gianchandani, P.K.; Kim, I.S.; Ni, Q.Q. Sonication induced effective approach for coloration of compact polyacrylonitrile (PAN) nanofibers. Ultrason. Sonochem. 2019, 51, 399–405. [Google Scholar] [CrossRef]
- Hu, X.Q.; Ye, D.Z.; Tang, J.B.; Zhang, L.J.; Zhang, X. From waste to functional additives: Thermal stabilization and toughening of PVA with lignin. RSC Adv. 2016, 6, 13797–13802. [Google Scholar] [CrossRef]
- Lin, G.; Bai, Z.; Liu, C.; Liu, S.; Han, M.; Huang, Y.; Liu, X. Mechanically robust, nonflammable and surface cross-linking composite membranes with high wettability for dendrite-proof and high-safety lithium-ion batteries. J. Membr. Sci. 2022, 647, 120262. [Google Scholar] [CrossRef]
- Phan, D.N.; Khan, M.Q.; Nguyen, N.T.; Phan, T.T.; Ullah, A.; Khatri, M.; Kien, N.N.; Kim, I.S. A review on the fabrication of several carbohydrate polymers into nanofibrous structures using electrospinning for removal of metal ions and dyes. Carbohydr. Polym. 2021, 252, 117175. [Google Scholar] [CrossRef]
- Mokhena, T.C.; Jacobs, V.; Luyt, A.S. A review on electrospun bio-based polymers for water treatment. Express Polym. Lett. 2015, 9, 839–880. [Google Scholar] [CrossRef]
- Frey, M.W. Electrospinning cellulose and cellulose derivatives. Polym. Rev. 2008, 48, 378–391. [Google Scholar] [CrossRef]
- Sato, A.; Wang, R.; Ma, H.; Hsiao, B.S.; Chu, B. Novel nanofibrous scaffolds for water filtration with bacteria and virus removal capability. J. Electron Microsc. 2011, 60, 201–209. [Google Scholar] [CrossRef]
- Chu, B.; Brook, S.; Hsiao, B.S.; Ma, H. High Flux High Efficiency Nanofiber Membranes and Methods of Production Thereof. No. 61/103,479. 2009. Available online: https://patentimages.storage.googleapis.com/e1/3a/7e/5f49ad5f2ac2dd/WO2010042647A2.pdf (accessed on 10 March 2024).
- Ma, Z.; Kotaki, M.; Ramakrishna, S. Electrospun cellulose nanofiber as affinity membrane. J. Membr. Sci. 2005, 265, 115–123. [Google Scholar] [CrossRef]
- Wenten, I.G. Recent development in membrane science and its industrial applications. J. Sci. Technol. 2003, 24, 1009–1024. [Google Scholar]
- Ma, H.; Burger, C.; Hsiao, B.S.; Chu, B. Highly permeable polymer membranes containing directed channels for water purification. ACS Macro Lett. 2012, 1, 723–726. [Google Scholar] [CrossRef]
- Ji, F.; Li, C.; Tang, B.; Xu, J.; Lu, G.; Liu, P. Preparation of cellulose acetate/zeolite composite fiber and its adsorption behavior for heavy metal ions in aqueous solution. Chem. Eng. J. 2012, 209, 325–333. [Google Scholar] [CrossRef]
- Taha, A.A.; Wu, Y.; Wang, H.; Li, F. Preparation and application of functionalized cellulose acetate/silica composite nanofibrous membrane via electrospinning for Cr(VI) ion removal from aqueous solution. J. Environ. Manag. 2012, 112, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Bódalo, A.; Gómez, J.L.; Gómez, E.; León, G.; Tejera, M. Ammonium removal from aqueous solutions by reverse osmosis using cellulose acetate membranes. Desalination 2005, 184, 149–155. [Google Scholar] [CrossRef]
- Konwarh, R.; Karak, N.; Misra, M. Electrospun cellulose acetate nanofibers: The present status and gamut of biotechnological applications. Biotechnol. Adv. 2013, 31, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Ismail, I.I.N.; Zayadi, R.A.; Ho, K.C.; Soo, J.Z.; Idris, M.S.; Tay, K.Y. Electrospun Nano-Palm Frond Titania Fiber (Nano-PFTF) Membrane for Industrial Wastewater Treatment. Solid State Sci. Technol. 2020, 28, 87–102. [Google Scholar]
- Cheng, J.; Zhan, C.; Wu, J.; Cui, Z.; Si, J.; Wang, Q.; Peng, X.; Turng, L.S. Highly Efficient Removal of Methylene Blue Dye from an Aqueous Solution Using Cellulose Acetate Nanofibrous Membranes Modified by Polydopamine. ACS Omega 2020, 5, 5389–5400. [Google Scholar] [CrossRef]
- Akduman, Ç. Fabrication and characterization of diatomite functionalized cellulose acetate nanofibers. AATCC J. Res. 2019, 6, 28–36. [Google Scholar] [CrossRef]
- San, N.O.; Celebioglu, A.; Tümtaş, Y.; Uyar, T.; Tekinay, T. Reusable bacteria immobilized electrospun nanofibrous webs for decolorization of methylene blue dye in wastewater treatment. RSC Adv. 2014, 4, 32249–32255. [Google Scholar] [CrossRef]
- Schiffman, J.D.; Schauer, C.L. A review: Electrospinning of biopolymer nanofibers and their applications. Polym. Rev. 2008, 48, 317–352. [Google Scholar] [CrossRef]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.V.; Tamura, H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Potential of chitin/chitosan-bearing materials for uranium recovery: An interdisciplinary review. Carbohydr. Polym. 2011, 84, 54–63. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Teong, L.C.; Hanafiah, M.A.K.M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- Li, C.; Lou, T.; Yan, X.; Long, Y.; Cui, G.; Wang, X. Fabrication of pure chitosan nanofibrous membranes as effective absorbent for dye removal. Int. J. Biol. Macromol. 2018, 106, 768–774. [Google Scholar] [CrossRef]
- Haider, S.; Park, S.Y. Preparation of the electrospun chitosan nanofibers and their applications to the adsorption of Cu(II) and Pb(II) ions from an aqueous solution. J. Membr. Sci. 2009, 328, 90–96. [Google Scholar] [CrossRef]
- Razzaz, A.; Ghorban, S.; Hosayni, L.; Irani, M.; Aliabadi, M. Chitosan nanofibers functionalized by TiO2 nanoparticles for the removal of heavy metal ions. J. Taiwan Inst. Chem. Eng. 2016, 58, 333–343. [Google Scholar] [CrossRef]
- Mokhena, T.C.; Luyt, A.S. Development of multifunctional nano/ultrafiltration membrane based on a chitosan thin film on alginate electrospun nanofibres. J. Clean. Prod. 2017, 156, 470–479. [Google Scholar] [CrossRef]
- Alsberg, E.; Anderson, K.W.; Albeiruti, A.; Franceschi, R.T.; Mooney, D.J. Cell-interactive alginate hydrogels for bone tissue engineering. J. Dent. Res. 2001, 80, 2025–2029. [Google Scholar] [CrossRef]
- Dar, A.; Shachar, M.; Leor, J.; Cohen, S. Optimization of cardiac cell seeding and distribution in 3D porous alginate scaffolds. Biotechnol. Bioeng. 2002, 80, 305–312. [Google Scholar] [CrossRef]
- Hashimoto, T.; Suzuki, Y.; Tanihara, M.; Kakimaru, Y.; Suzuki, K. Development of alginate wound dressings linked with hybrid peptides derived from laminin and elastin. Biomaterials 2004, 25, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.A.; Volesky, B.; Mucci, A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 2003, 37, 4311–4330. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Katsaros, F.K.; Kouvelos, E.P.; Kanellopoulos, N.K. Prediction of binary adsorption isotherms of Cu2+, Cd2+ and Pb2+ on calcium alginate beads from single adsorption data. J. Hazard. Mater. 2009, 162, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Liu, H.; Lu, Y.; Zhang, L. Preparation and Physical Properties of Blend Films from Sodium Alginate and Polyacrylamide Solutions. J. Macromol. Sci. Part A Pure Appl. Chem. 2000, 37, 1663–1675. [Google Scholar] [CrossRef]
- Çaykara, T.; Demirci, S.; Eroǧlu, M.S.; Güven, O. Poly(ethylene oxide) and its blends with sodium alginate. Polymer 2005, 46, 10750–10757. [Google Scholar] [CrossRef]
- Omidian, H.; Rocca, J.G.; Park, K. Elastic, superporous hydrogel hybrids of polyacrylamide and sodium alginate. Macromol. Biosci. 2006, 6, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Mokhena, T.C.; Jacobs, N.V.; Luyt, A.S. Electrospun alginate nanofibres as potential bio-sorption agent of heavy metals in water treatment. Express Polym. Lett. 2017, 11, 652–663. [Google Scholar] [CrossRef]
- Fang, D.; Liu, Y.; Jiang, S.; Nie, J.; Ma, G. Effect of intermolecular interaction on electrospinning of sodium alginate. Carbohydr. Polym. 2011, 85, 276–279. [Google Scholar] [CrossRef]
- Li, W.; Li, X.; Chen, Y.; Li, X.; Deng, H.; Wang, T.; Huang, R.; Fan, G. Poly(vinyl alcohol)/sodium alginate/layered silicate based nanofibrous mats for bacterial inhibition. Carbohydr. Polym. 2013, 92, 2232–2238. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Sadeghizadeh, A.; Neysan, F.; Heydari, M. Fabrication of nanofibers using sodium alginate and Poly(Vinyl alcohol) for the removal of Cd2+ ions from aqueous solutions: Adsorption mechanism, kinetics and thermodynamics. Heliyon 2019, 5, 1–10. [Google Scholar] [CrossRef]
- Wang, Q.; Ju, J.; Tan, Y.; Hao, L.; Ma, Y.; Wu, Y.; Zhang, H.; Xia, Y.; Sui, K. Controlled synthesis of sodium alginate electrospun nanofiber membranes for multi-occasion adsorption and separation of methylene blue. Carbohydr. Polym. 2019, 205, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Crini, G. Review: A history of cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Liu, H.; Cai, X.; Wang, Y.; Chen, J. Adsorption mechanism-based screening of cyclodextrin polymers for adsorption and separation of pesticides from water. Water Res. 2011, 45, 3499–3511. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, G.; Liu, W. Preparation of water-soluble β-cyclodextrin/poly(acrylic acid)/graphene oxide nanocomposites as new adsorbents to remove cationic dyes from aqueous solutions. Chem. Eng. J. 2014, 257, 299–308. [Google Scholar] [CrossRef]
- Connors, K.A. The stability of cyclodextrin complexes in solution. Chem. Rev. 1997, 97, 1325–1357. [Google Scholar] [CrossRef]
- Sherje, A.P.; Dravyakar, B.R.; Kadam, D.; Jadhav, M. Cyclodextrin-based nanosponges: A critical review. Carbohydr. Polym. 2017, 173, 37–49. [Google Scholar] [CrossRef]
- Liu, F.; Sun, Y.; Gu, J.; Gao, Q.; Sun, D.; Zhang, X.; Pan, B.; Qian, J. Highly efficient photodegradation of various organic pollutants in water: Rational structural design of photocatalyst via thiol-ene click reaction. Chem. Eng. J. 2020, 381, 122631. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.; Lu, J.; Zhou, Y. Novel cyclodextrin-based adsorbents for removing pollutants from wastewater: A critical review. Chemosphere 2020, 241, 125043. [Google Scholar] [CrossRef]
- Alsbaiee, A.; Smith, B.J.; Xiao, L.; Ling, Y.; Helbling, D.E.; Dichtel, W.R. Rapid removal of organic micropollutants from water by a porous β-cyclodextrin polymer. Nature 2016, 529, 190–194. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Winterton, P.; Fourmentin, S.; Wilson, L.D.; Fenyvesi, É.; Crini, G. Water-insoluble β-cyclodextrin–epichlorohydrin polymers for removal of pollutants from aqueous solutions by sorption processes using batch studies: A review of inclusion mechanisms. Prog. Polym. Sci. 2018, 78, 1–23. [Google Scholar] [CrossRef]
- Jeong, D.; Joo, S.W.; Shinde, V.V.; Jung, S. Triple-crosslinkedβ-cyclodextrin oligomer self-healing hydrogel showing high mechanical strength, enhanced stability and pH responsiveness. Carbohydr. Polym. 2018, 198, 563–574. [Google Scholar] [CrossRef] [PubMed]
- San Keskin, N.O.; Celebioglu, A.; Sarioglu, O.F.; Uyar, T.; Tekinay, T. Encapsulation of living bacteria in electrospun cyclodextrin ultrathin fibers for bioremediation of heavy metals and reactive dye from wastewater. Colloids Surf. B Biointerfaces 2018, 161, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Wang, Y.; Li, X.; Sun, B.; Wang, C. Synthesis of β-cyclodextrin-based electrospun nanofiber membranes for highly efficient adsorption and separation of methylene blue. ACS Appl. Mater. Interfaces 2015, 7, 26649–26657. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Chen, C.; Qian, J. Film-like bacterial cellulose/cyclodextrin oligomer composites with controllable structure for the removal of various persistent organic pollutants from water. J. Hazard. Mater. 2021, 405, 124122. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Huang, M.; Liu, Y.; Meng, L.; Ma, M. Functionalized electrospun nanofiber membranes for water treatment: A review. Sci. Total Environ. 2020, 739, 139944. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.D.; Dalton, P.D.; Hutmacher, D.W. Melt electrospinning today: An opportune time for an emerging polymer process. Prog. Polym. Sci. 2016, 56, 116–166. [Google Scholar] [CrossRef]
- Jian, S.; Zhu, J.; Jiang, S.; Chen, S.; Fang, H.; Song, Y.; Duan, G.; Zhang, Y.; Hou, H. Nanofibers with diameter below one nanometer from electrospinning. RSC Adv. 2018, 8, 4794–4802. [Google Scholar] [CrossRef]
- Xue, J.; Xie, J.; Liu, W.; Xia, Y. Electrospun Nanofibers: New Concepts, Materials, and Applications. Acc. Chem. Res. 2017, 50, 1976–1987. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Sundarrajan, S.; Rana, D.; Matsuura, T.; Ramakrishna, S. Influence of electrospun fiber size on the separation efficiency of thin film nanofiltration composite membrane. J. Membr. Sci. 2012, 392–393, 101–111. [Google Scholar] [CrossRef]
- Dobosz, K.M.; Kuo-Leblanc, C.A.; Martin, T.J.; Schiffman, J.D. Ultrafiltration Membranes Enhanced with Electrospun Nanofibers Exhibit Improved Flux and Fouling Resistance. Ind. Eng. Chem. Res. 2017, 56, 5724–5733. [Google Scholar] [CrossRef]
- Park, M.J.; Gonzales, R.R.; Abdel-Wahab, A.; Phuntsho, S.; Shon, H.K. Hydrophilic polyvinyl alcohol coating on hydrophobic electrospun nanofiber membrane for high performance thin film composite forward osmosis membrane. Desalination 2018, 426, 50–59. [Google Scholar] [CrossRef]
- Shokrollahzadeh, S.; Tajik, S. Fabrication of thin film composite forward osmosis membrane using electrospun polysulfone/polyacrylonitrile blend nanofibers as porous substrate. Desalination 2018, 425, 68–76. [Google Scholar] [CrossRef]
- Zhou, T.; Li, J.; Guo, X.; Yao, Y.; Zhu, P.; Xiang, R. Freestanding PTFE electrospun tubular membrane for reverse osmosis brine concentration by vacuum membrane distillation. Desalin. Water Treat. 2019, 165, 63–72. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, Y.; Fan, X.; Liu, Z.; Song, Y.; Wang, Y.; Tao, P.; Song, C.; Shao, M. In-situ silica nanoparticle assembly technique to develop an omniphobic membrane for durable membrane distillation. Desalination 2021, 499, 114832. [Google Scholar] [CrossRef]
- Gontarek-Castro, E.; Castro-Muñoz, R.; Lieder, M. New insights of nanomaterials usage toward superhydrophobic membranes for water desalination via membrane distillation: A review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 2104–2149. [Google Scholar] [CrossRef]
- Ağtaş, M.; Yılmaz, Ö.; Dilaver, M.; Alp, K.; Koyuncu, İ. Pilot-scale ceramic ultrafiltration/nanofiltration membrane system application for caustic recovery and reuse in textile sector. Environ. Sci. Pollut. Res. 2021, 28, 41029–41038. [Google Scholar] [CrossRef]
- Sahinkaya, E.; Tuncman, S.; Koc, I.; Guner, A.R.; Ciftci, S.; Aygun, A.; Sengul, S. Performance of a pilot-scale reverse osmosis process for water recovery from biologically-treated textile wastewater. J. Environ. Manag. 2019, 249, 109382. [Google Scholar] [CrossRef]
- Kurt, E.; Koseoglu-Imer, D.Y.; Dizge, N.; Chellam, S.; Koyuncu, I. Pilot-scale evaluation of nanofiltration and reverse osmosis for process reuse of segregated textile dyewash wastewater. Desalination 2012, 302, 24–32. [Google Scholar] [CrossRef]


















| Membrane Composition | Results | Ref. |
|---|---|---|
| Polyacrylonitrile (PAN) | Retention of 99.99% of bacteriophages and 99.9999% of bacteria. | [78] |
| PAN Haloisite nanotubes (HNTs) | The incorporation of HNTs, especially 1% w/w, improved the mechanical and thermal properties of the membranes. Rejection rate of 99.5% of oil/water for membranes with 3% w/w HNTs, and removal efficiency of 31.1% of heavy metal ions. | [79] |
| PAN TIME Jute cellulose nanowhiskers | Good mechanical properties, efficient nanoparticle filtration capacity, and good oil/water separation (with a rejection rate of over 99%). | [80] |
| PAN Diethylenetriamine (DETA) | Maximum adsorption capacities: methylene blue—184.84 mg/g; rhodamine B—367.65 mg/g; safratin T—195.7 mg/g. The membrane showed higher maximum adsorption capacities when compared to conventional adsorbents. | [81] |
| PAN Structure of zeolitic imidazole-67 (ZIF-67) | Maximum adsorption capacities of malachite green: ZIF-67 membranes—2545 mg/g; ZIF-67/PAN membrane—1305 mg/g. After four regeneration cycles, the ZIF-67/PAN membrane showed more than 92% of its original capacity. It also showed good adsorption abilities for Congo red (849 mg/g) and fuchsin (730 mg/g). The membrane can be reused by washing it with ethanol. | [82] |
| PAN Graphene oxide (GO) Titanium dioxide (TiO2) β-cyclodextrin (β-CD) | In 5 h, the degradation efficiency for methyl orange and methylene blue was around 93.52% and 90.92%, respectively. The membranes’ MB and MO degradation efficiency was 80% for the first three cycles, but dropped to around 68.42% and 65.13% in the fifth cycle, respectively. Antibacterial properties against E. coli and S. aureus. | [83] |
| PAN Carbon nanotubes (CNTs) | Almost complete degradation after 120 min and 60 min for methylene blue and indigo carmine, respectively. Improvements of 38% and 84% in tensile strength and elastic modulus, respectively, with just 0.05 wt% CNTs. | [84] |
| Polyacrylonitrile-co-maleic acid (PANCMA) GO TiO2 | Under optimized conditions, by the E-spun RGO/TiO2/PANCMA NFs, 90.6% of malachite green and 93.7% of leucomalachite green were adsorbed in 2 min, and subsequently 91.4% and 95.2% adsorbed were degraded in 60 min under UV irradiation, respectively. Good recyclability. Before the 14th cycle, the removal efficiencies of malachite green and leucomalachite were over 91%. | [85] |
| PAN GO | High rejection performance (almost 100% rejection of Congo red, 56.7% for Na2SO4, and 9.8% for NaCl). The water flow under extremely low external pressure (1.0 bar) increased significantly due to the structure of the graphene oxide layer and the nanofibrous support. | [86] |
| PAN Cellulose acetate (CA) | The optimum solution pH values for the adsorption of Fe(III), Cu(II), and Cd(II) ions were 2, 5, and 6, respectively, and the adsorption equilibria were obtained in 5, 20, and 60 min. The amount of saturation adsorption of the nanofibrous membranes (at 25 °C) for Fe(III), Cu(II), and Cd(II) was 7.47, 4.26, and 1.13 mmol/g, respectively. After five consecutive adsorption and desorption tests, the desorption rate of the metal ions maintained more than 80% of their first desorption rate. The AOPAN/RC nanofibers showed excellent regeneration capacity. | [87] |
| PAN ZIF-8 | With relatively larger surface areas (of 871.0 m2/g) and adequate pore sizes (from around 0.6 to 0.8 nm), the nanofibers exhibited greater Cr(VI) adsorption capacity (with qmax of 39.68 mg/g) and good recyclability. | [88] |
| PAN Polyaniline (PANI) | The maximum adsorption capacities for lead and Cr2O72− on the PANI-coated membranes were 290.12 and 1202.53 mg/g, respectively. Greater removal of lead ions (99%) compared to chromium (VI) ions (90%) at 5 mg/L. The PAN/PANI membrane retained almost 58% and 60% of its initial adsorption capacity after four cycles for Cr2O72− and Pb(II). | [89] |
| Polyvinyl acetate (PVA) 1,2,3,4 butanetetracarboxylic acid (BTAC) crosslinked | Good performance in adsorbing the dye Reactive red 141. The maximum adsorption capacity reached 88.31 mg/g. If the temperature is increased from 110 °C to 130 °C, the adsorption capacity decreases. | [90] |
| PVA Silica (SiO2) Chitosan | The addition of 1.0% wt SiO2 resulted in a significant improvement in dye rejection and water permeability. Under 0.4 bar transmembrane pressure, the improved nanocomposite membrane yielded 98% Direct Red 23 rejection with a water flux value as high as 1711 L/m2h. It was discovered that the membranes were reusable and antifouling. | [91] |
| PVA SiO2 Cyclodextrin | The maximum adsorption capacity for the indigo carmine dye reached 495 mg/g and adsorption equilibrium was reached in less than 40 min. Recycled through acidification. | [92] |
| PVA Chitosan | The maximum adsorption capacity was 266.12 mg/g (Pb(II)) and 148.79 mg/g (Cd(II)). Detailed adsorption studies were carried out at pH 8 and 6 for Cd(II) and Pb(II), respectively. It is a simpler and more sustainable process than conventional methods. | [93] |
| PVA Konjac glucomannan (KGM) Zinc oxide nanoparticles (ZnO NPs) | Filtration efficiency for ultrafine particles (300 nm) of over 99.99%, superior to commercial HEPA filters. Methyl orange removal efficiency of over 98%, with an initial concentration of 20 mg/L, during 120 min of solar irradiation. Antibacterial activity (E. coli and Bacillus subtilis). | [94] |
| Polyvinylpyrrolidone (PVP) Copper (II) acetate hydrate Zinc (II) acetate | A total of 100% degradation of mixed dyes (methylene blue, rhodamine B, and methyl orange, 10 ppm each) in 90 min. Good reusability (94.1% after five cycles). | [95] |
| PVP Graphitic carbon nitride (g-C3N4) Niobium pentoxide (Nb2O5) | After 120 min in visible light, 98.1% degradation was recorded for rhodamine B and phenol (10 mg/L each). No obvious change in the performance of the nanofibers was recorded after four cycles (remained ≈ 98%). | [96] |
| PVP Zinc oxide (ZnO) Bismuth oxide (Bi2O3) | The compound with a molar ratio of 23:1 (ZnO/Bi2O3) showed the best activity under both excitations (UV and visible light). Approximately 95% degradation of rhodamine B (1.0 × 10−5 M, 60 mL) was reported after 90 min. | [97] |
| PVP Zinc tungstate (ZnWO4) | The degradation efficiency of rhodamine B (10 mg/L) was over 90% in about 90 min of irradiation. There was no decline in photocatalytic activity after five photodegradation cycles. | [98] |
| Lacase Polyetherimide (PEI) Polycaprolactone (PCL) | After ten cycles, PCL/PEI/TTL and PCL/PEI/TVL had residual activities of 33.2 ± 0.2% and 26.0 ± 0.9%, respectively. At 50 °C and pH 5, PCL/PEI/TTL demonstrated the highest decolorization efficiency of orange II and malachite green, reaching over 86% and 46%, respectively, after eight continuous uses. PCL/PEI/TTL and PCL/PEI/TVL had maximum removal efficiencies of 64.5 ± 7.6% and 52.6 ± 0.1%, respectively, and successfully decomposed 2,6-dichlorophenol. Environmentally friendly, sustainable materials. | [99] |
| PEI TiO2 | The PEI membrane modified with 0.2% TiO2 achieved a significant removal rate of E. coli (99%) and humic acid (≈80%). Degradation of 85% of methylene blue during the photocatalytic process. | [100] |
| Polystyrene (PS) GO | PSGO films had a removal capacity ≈ 2.3 times higher than that of pure PS membranes. After 120 min, the equilibrium value of the adsorption capacity (qe = 114 mg/g) was reached for all of the methylene blue concentrations that were examined. After the first cycle, the removal capacity was reduced to ≈65%, a value that became constant during subsequent cycles (up to a maximum of five cycles). | [101] |
| PEN Bisphenol A (BPA) Hydroquinone methanesulfonic acid potassium salt (HQS) 2,6-difluorobenzonitrile (DFBN) | Methylene blue exhibited a high adsorption capacity of 796.25 mg/g. Even after eight separation–regeneration cycles, the optimized membrane achieved a 99% selective removal efficiency of cationic dyes. Good recyclability and stability at high temperatures. | [102] |
| Sample | Surface Area (m2/g) | Pore Volume (cm3/g) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|
| Non-crosslinked | 13.97 | 0.0256 | 3.8 | 9.8 |
| CaCl2 crosslinked | 13.56 | 0.0450 | 10.4 | 9.9 |
| GA vapor crosslinked | 11.86 | 0.0185 | 3.7 | 11.2 |
| TFA crosslinked | 15.26 | 0.0455 | 3.6 | 12.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, J.M.; Sousa, R.P.C.L.; Fangueiro, R.; Ferreira, D.P. The Potential of Electrospun Membranes in the Treatment of Textile Wastewater: A Review. Polymers 2024, 16, 801. https://doi.org/10.3390/polym16060801
Rocha JM, Sousa RPCL, Fangueiro R, Ferreira DP. The Potential of Electrospun Membranes in the Treatment of Textile Wastewater: A Review. Polymers. 2024; 16(6):801. https://doi.org/10.3390/polym16060801
Chicago/Turabian StyleRocha, Joana M., Rui P. C. L. Sousa, Raul Fangueiro, and Diana P. Ferreira. 2024. "The Potential of Electrospun Membranes in the Treatment of Textile Wastewater: A Review" Polymers 16, no. 6: 801. https://doi.org/10.3390/polym16060801
APA StyleRocha, J. M., Sousa, R. P. C. L., Fangueiro, R., & Ferreira, D. P. (2024). The Potential of Electrospun Membranes in the Treatment of Textile Wastewater: A Review. Polymers, 16(6), 801. https://doi.org/10.3390/polym16060801