Effect of Chitosan and Its Water-Soluble Derivatives on Antioxidant Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Water-Soluble Chitosan Derivatives
2.3. Antioxidant Experiments
2.3.1. DPPH Radical Scavenging Activity
2.3.2. Superoxide Radical Scavenging Activity
2.3.3. Hydroxyl Radical Scavenging Activity
2.3.4. Analysis of Reducing Power
2.4. Animal Experiments
2.5. Statistical Analysis
3. Results
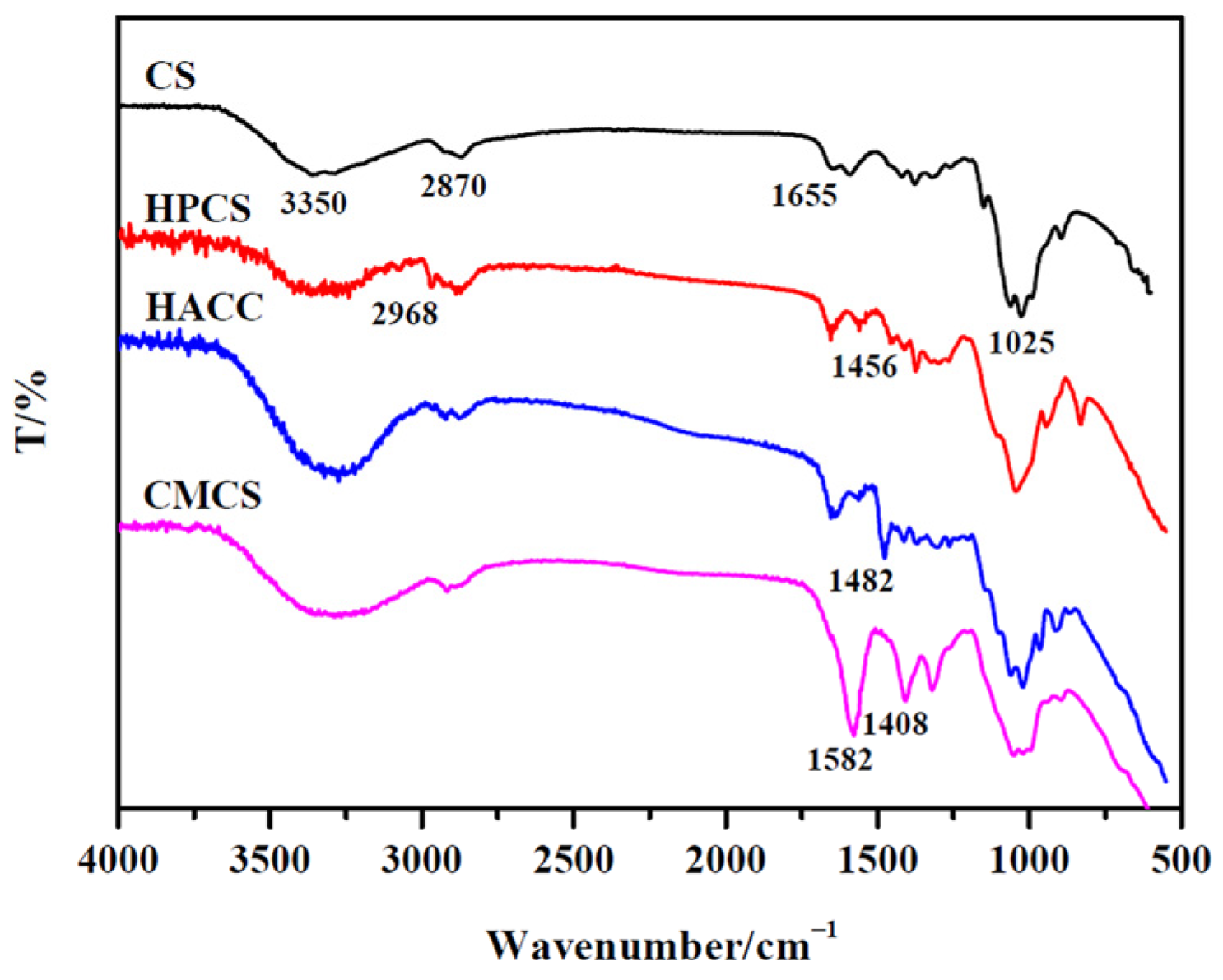
3.1. Structure Analysis of CS and Its Water-Soluble Derivatives
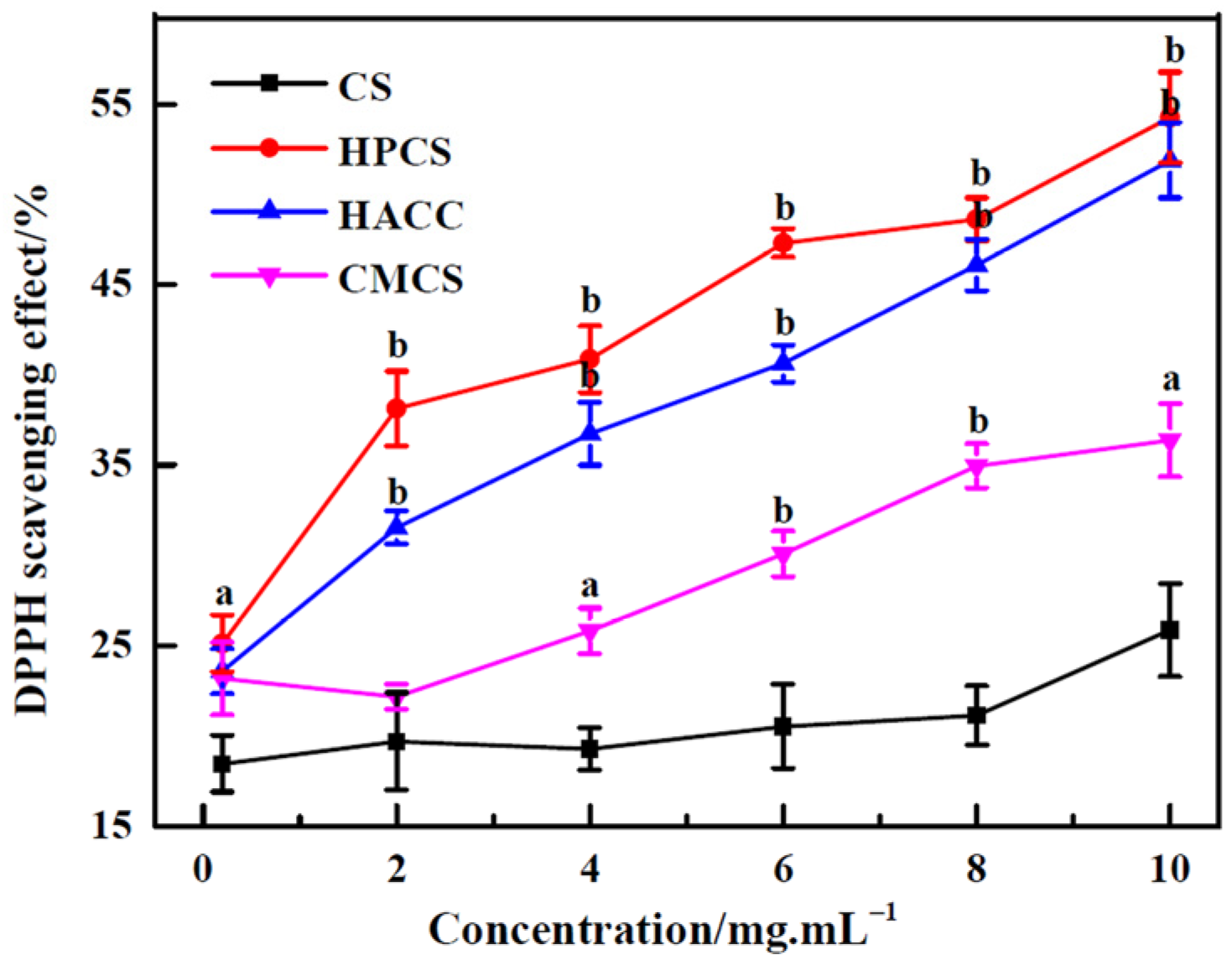
3.2. DPPH Scavenging Activity
3.3. Superoxide Radical (O2−) Scavenging Activity
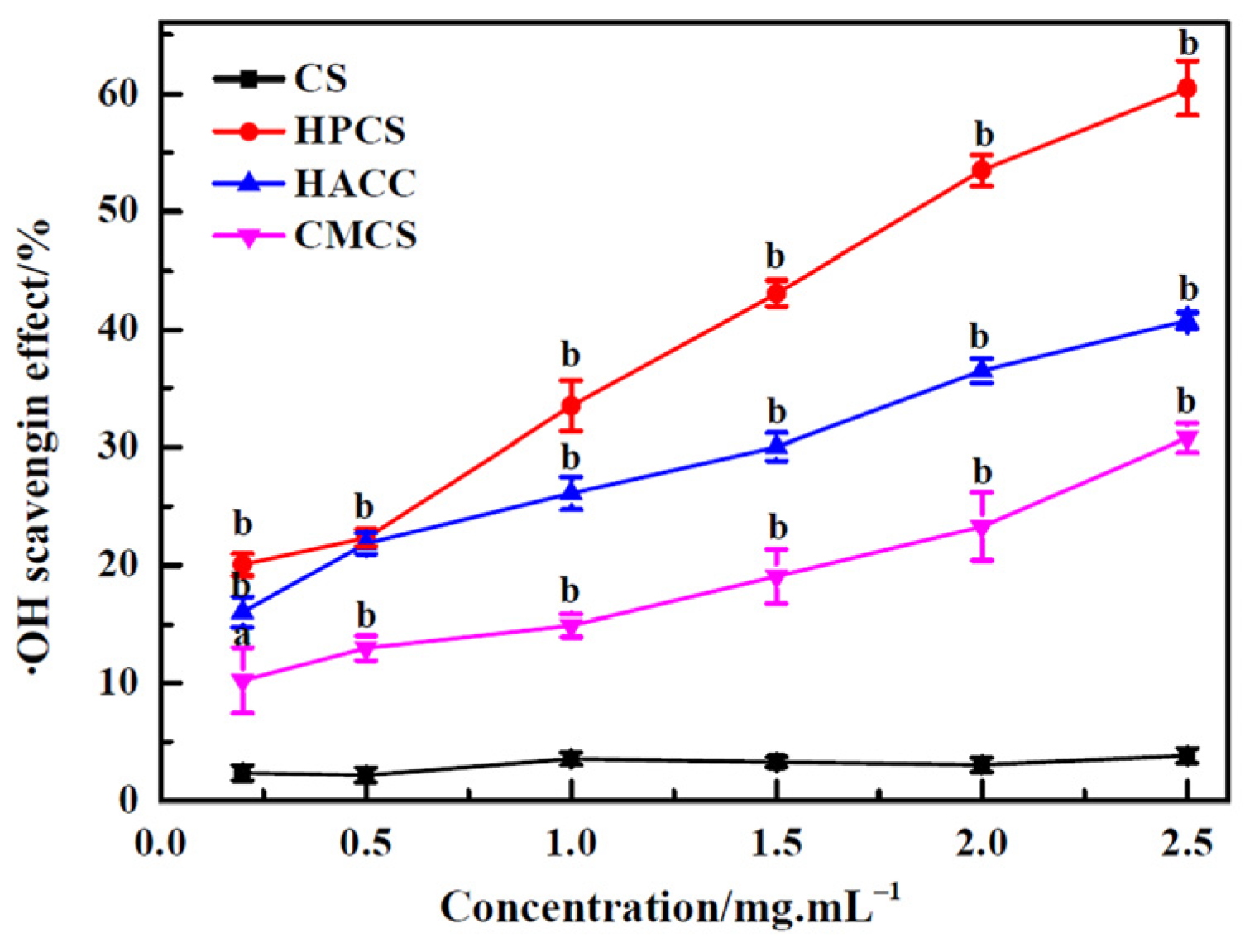
3.4. Hydroxyl Radical (•OH) Scavenging Activity
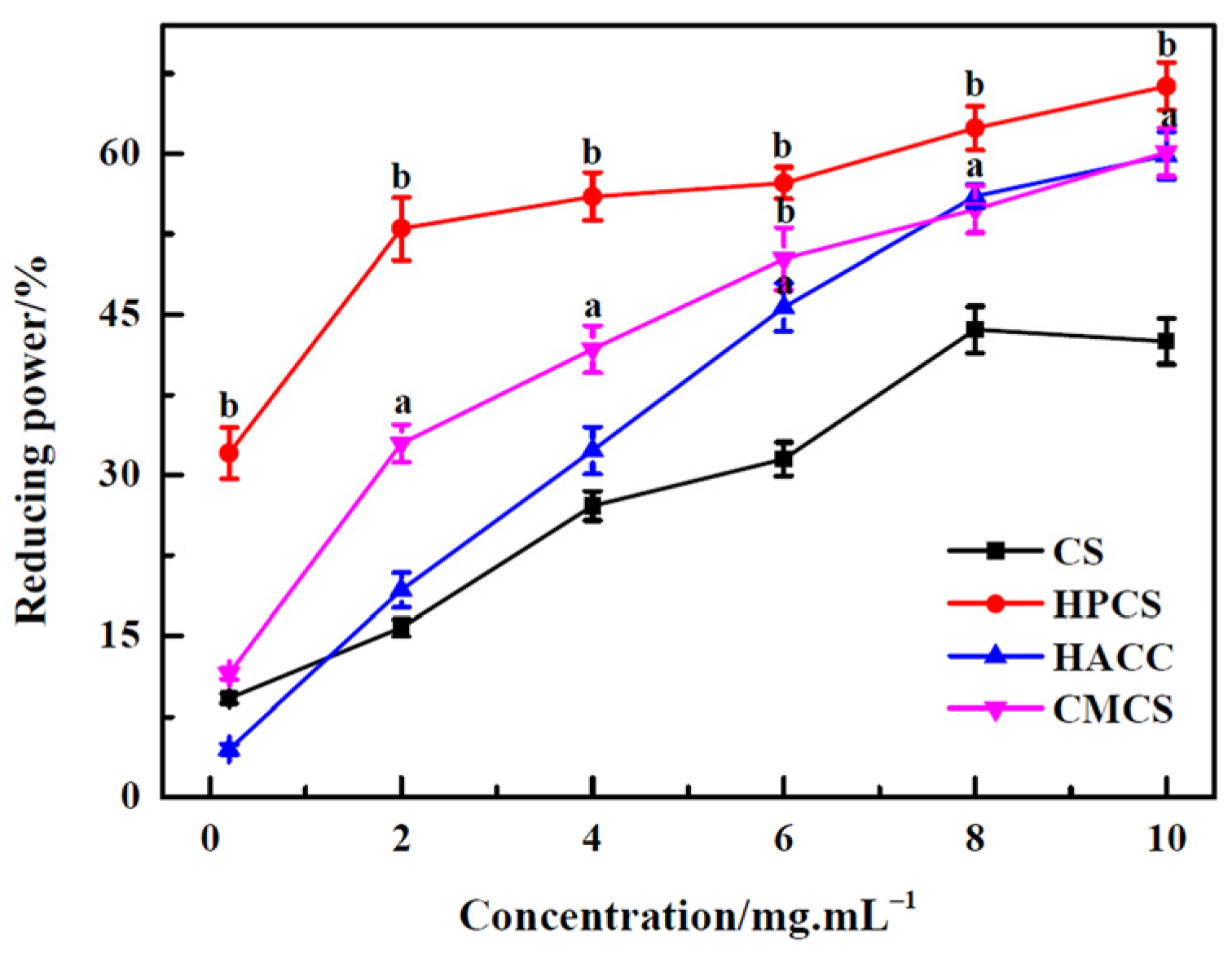
3.5. Reducing Power
3.6. Analysis of the Rat Growth
3.7. Serum Lipid Analysis of the Rats
3.8. Oxidative Stress Analysis of the Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ngo, D.H.; Qian, Z.J.; Vo, T.S.; Ryu, B.M.; Ngo, D.N.; Kim, S.K. Antioxidant activity of gallate-chitooligosaccharides in mouse macrophage RAW264.7 cells. Carbohydr. Polym. 2011, 84, 1282–1288. [Google Scholar] [CrossRef]
- Kerch, G. The potential of chitosan and its derivatives in prevention and treatment of age-related diseases. Mar. Drugs 2015, 13, 2158–2182. [Google Scholar] [CrossRef] [PubMed]
- Oluwasina, O.O.; Igbe, F.O.; Olagboye, A.S.; Abe, T.O. Zinc impacts on carboxymethyl chitosan antioxidant activity. Int. J. Sci. Res. Multidiscip. Stud. 2021, 7, 14–18. [Google Scholar]
- Liu, J.L.; Sun, H.L.; Dong, F.; Xue, Q.Z.; Wang, G.; Qin, S.; Guo, Z.Y. The influence of the cation of quaternized chitosans on antioxidant activity. Carbohyd. Polym. 2009, 78, 439–443. [Google Scholar] [CrossRef]
- Li, S.; Chen, G.W.; Zhang, C.; Wu, M.; Wu, S.Y.; Liu, Q. Research progress of natural antioxidants in foods for the treatment of diseases. Food Sci. Hum. Wellness 2014, 3, 110–116. [Google Scholar] [CrossRef]
- Du, Y.; Guo, H.F.; Lou, H.X. Grape seed polyphenols protect cardiac cells from apoptosis via induction of endogenous antioxidant enzymes. J. Agric. Food Chem. 2007, 55, 1695–1701. [Google Scholar] [CrossRef]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan hydrogels for sustained drug delivery. J. Control. Release 2020, 326, 150–163. [Google Scholar] [CrossRef]
- Qin, Y.K.; Li, P.C. Antimicrobial chitosan conjugates: Current synthetic strategies and potential applications. Int. J. Mol. Sci. 2020, 21, 499–517. [Google Scholar] [CrossRef]
- Varlamov, V.P.; Il’ina, A.V.; Shagdarova, B.T.; Lunkov, A.P.; Mysyakina, I.S. Chitin/Chitosan and its derivatives: Fundamental problems and practical approaches. Biochemistry. 2020, 85, 154–176. [Google Scholar] [CrossRef]
- Liu, B.Q.; Che, C.C.; Liu, J.F.; Si, M.R.; Gong, Z.J.; Li, Y.; Zhang, J.M.; Yang, G. Fabrication and antitumor mechanism of a nanoparticle drug delivery system: Graphene oxide/chitosan oligosaccharide/γ-polyglutamic acid composites for anticancer drug delivery. ChemistrySelect 2019, 4, 12491–12502. [Google Scholar] [CrossRef]
- Chi, J.H.; Jiang, Z.W.; Qiao, J.; Zhang, W.; Peng, Y.F.; Liu, W.S.; Han, B.Q. Antitumor eval uation of carboxymethyl chitosan based norcantharidin conjugates against gastric cancer as novel polymer therapeutics. Int. J. Biol. Macromol. 2019, 136, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Xue, C.H.; Mao, X.Z. Chitosan: Structural modification, biologicalactivity and application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Wu, C.H.; Tsai, G.J. Effects of chitosan molecular weight on its antioxidant and antimutagenic properties. Carbohydr. Polym. 2018, 181, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Tomida, H.; Fujii, T.; Furutani, N.; Michihara, A.; Yasufuku, T.; Akasaki, K.; Maruyama, T.; Otagiri, M.; Gebicki, J.M.; Anraku, M. Antioxidant properties of some different molecular weight chitosans. Carbohydr. Res. 2009, 344, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Park, P.J.; Je, J.Y.; Kim, S.K. Free radical scavenging activities of differently deacetylated chitosans using an ESR spectrometer. Carbohydr. Polym. 2004, 55, 17–22. [Google Scholar] [CrossRef]
- Hromiš, N.M.; Lazić, V.L.; Popović, S.Z.; Šuput, D.Z.; Bulut, S.N. Antioxidative activity of chitosan and chitosan based biopolymer film. Food Feed. Res. 2017, 44, 91–100. [Google Scholar] [CrossRef]
- Liu, J.; Lu, J.F.; Kan, J.; Tang, Y.Q.; Jin, C.H. Preparation, characterization and antioxidant activity of phenolic acids grafted carboxymethyl chitosan. Int. J. Biol. Macromol. 2013, 62, 85–93. [Google Scholar] [CrossRef]
- Xing, R.E.; Yu, H.H.; Liu, S.; Zhang, W.W.; Zhang, Q.B.; Li, Z.E.; Li, P.C. Antioxidant activity of differently regioselective chitosan sulfates in vitro. Bioorg. Med. Chem. 2005, 13, 1387–1392. [Google Scholar] [CrossRef]
- Yan, Y.B.; Wang, Z.H.; Yu, X.H.; Li, W.; Qin, C.Q. Preparation and Characterization of Waterborne Polyurethane/Hydroxypropyl Chitosan Nanocomposites. J. Biobased Mater. Bioenergy 2015, 9, 463–470. [Google Scholar] [CrossRef]
- Peng, Y.F.; Han, B.Q.; Liu, W.S.; Xu, X.J. Preparation and antimicrobial activity of hydroxypropyl chitosan. Carbohydr. Res. 2005, 340, 1846–1851. [Google Scholar] [CrossRef]
- Loubaki, E.; Ourevitch, M.; Sicsic, S. Chemical modification of chitosan byglycidyl trimethylammonium chloride. Characterization of modified chitosanby13C- and1H-NMR spectroscopy. Eur. Polym. J. 1991, 27, 311–317. [Google Scholar] [CrossRef]
- Wang, Z.H.; Zhang, F.; Yan, Y.B.; Zhang, Z.M.; Wang, L.H.; Qin, C.Q. Lipid-lowering activities of chitosan and its quaternary ammonium salt for the hyperlipidemia rats induced by high-fat diets. Int. J. Biol. Macromol. 2019, 132, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.G.; Park, H.J. Chemical characteristics of O-carboxymethyl chitosans related to the preparation conditions. Carbohydr. Polym. 2003, 53, 355–359. [Google Scholar] [CrossRef]
- Guo, Z.Y.; Xing, R.E.; Liu, S.; Yu, H.H.; Wang, P.B.; Li, C.P.; Li, P.C. The synthesis and antioxidant activity of the Schiff bases of chitosan and carboxymethyl chitosan. Bioorg. Med. Chem. Lett. 2005, 15, 4600–4603. [Google Scholar] [CrossRef] [PubMed]
- Zhong, K.; Lin, W.J.; Wang, Q.; Zhou, S.M. Extraction and radicals scavenging activity of polysaccharides with microwave extraction from mung bean hulls. Int. J. Biol. Macromol. 2012, 51, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Du, Y.M.; Li, J.; Hu, Y.; Kennedy, J.F. Enhancement of antioxidant activity of chitosan by irradiation. Carbohydr. Polym. 2008, 73, 126–132. [Google Scholar] [CrossRef]
- Liu, J.; Luo, J.G.; Ye, H.; Zeng, X.X. Preparation, antioxidant and antitumor activities in vitro of different derivatives of levan from endophytic bacterium paenibacillus polymyxa EJS-3. Food Chem. Toxicol. 2012, 50, 767–772. [Google Scholar] [CrossRef]
- Guzman, J.; Saucedo, I.; Revilla, J.; Navarro, R.; Guibal, E. Copper sorption by chitosan in the presence of citrate ions: Influence of metal speciation on sorption mechanism and uptake capacities. Int. J. Biol. Macromol. 2003, 33, 57–65. [Google Scholar] [CrossRef]
- Xing, R.E.; Liu, S.; Guo, Z.Y.; Yu, H.H.; Zhong, Z.M.; Ji, X.; Li, P.C. Relevance of molecular weight of chitosan-N-2-hydroxypropyl trimethyl ammonium chloride and their antioxidant activities. Eur. J. Med. Chem. 2008, 43, 336–340. [Google Scholar] [CrossRef]
- Chatterjee, N.S.; Panda, S.K.; Navitha, M.; Asha, K.K.; Anandan, R.; Mathew, S. Vanillic acid and coumaric acid grafted chitosan derivatives: Improved grafting ratio and potential application in functional food. J. Food Sci. Technol. 2015, 52, 7153–7162. [Google Scholar] [CrossRef]
- Schreiber, S.B.; Bozell, J.J.; Hayes, D.G.; Zivanovic, S. Introduction of primary antioxidant activity to chitosan for application as a multifunctional food packaging material. Food Hydrocoll. 2013, 33, 207–214. [Google Scholar] [CrossRef]
- Marín, A.C.; Culcasi, M.; Cassien, M.; Stocker, P.; Thétiot-Laurent, S.; Robillard, B.; Chinnici, F.; Pietri, S. Chitosan as an antioxidant alternative to sulphites in oenology: EPR investigation of inhibitory mechanisms. Food Chem. 2019, 285, 67–76. [Google Scholar] [CrossRef]
- Xie, W.M.; Xu, P.X.; Liu, Q. Antioxidant activity of water-soluble chitosan derivatives. Bioorg. Med. Chem. Lett. 2001, 11, 1699–1701. [Google Scholar] [CrossRef]
- Guo, Z.Y.; Liu, H.Y.; Chen, X.L.; Ji, X.; Li, P.C. Hydroxyl radicals scavenging activity of N-substituted chitosan and quaternized chitosan. Bioorg. Med. Chem. Lett. 2006, 16, 6348–6350. [Google Scholar] [CrossRef]
- Yang, R.L.; Shi, Y.H.; Hao, G.; Li, W.; Le, G.W. Increasing oxidative stress with progressive hyperlipidemia in human: Relation between malondialdehyde and atherogenic index. J. Clin. Biochem. Nutr. 2008, 43, 154–158. [Google Scholar] [CrossRef]
- Liu, X.F.; Yang, F.; Song, T.; Zeng, A.R.; Wang, Q.; Sun, Z.; Shen, J. Effects of chitosan, O-carboxymethyl chitosan and N-[(2-hydroxy-3-N,N-dimethylhexadecyl ammonium)propyl] chitosan chloride on lipid metabolism enzymes and low-density-lipoprotein receptor in a murine diet-induced obesity. Carbohydr. Polym. 2011, 85, 334–340. [Google Scholar] [CrossRef]
- Pan, H.T.; Yang, Q.Y.; Huang, G.D.; Ding, C.; Cao, P.Q.; Huang, L.L.; Xiao, T.C.; Guo, J.; Su, Z.Q. Hypolipidemic effects of chitosan and its derivatives in hyperlipidemic rats induced by a high-fat diet. Food Nutr. Res. 2016, 60, 31137. [Google Scholar] [CrossRef] [PubMed]
- Anraku, M.; Fujii, T.; Furutani, N.; Kadowaki, D.; Maruyama, T.; Otagiri, M.; Gebicki, J.M.; Tomida, H. Antioxidant effects of a dietary supplement: Reduction of indices of oxidative stress in normal subjects by water-soluble chitosan. Food Chem. Toxicol. 2009, 47, 104–109. [Google Scholar] [CrossRef]
- Zhang, W.; Xia, W.S. Effect of media milling on lipid-lowering and antioxidant activities of chitosan. Int. J. Biol. Macromol. 2015, 72, 1402–1405. [Google Scholar] [CrossRef] [PubMed]


| Group | Initial Weight (g) | Final Weight (g) | BW Gain (g) | Food Intake (g/d) |
|---|---|---|---|---|
| NC | 169.4 ± 11.7 | 324.5 ± 13.4 | 155.1 ± 11.2 | 19.9 ± 2.1 |
| HF | 171.2 ± 12.5 | 381.5 ± 15.5 * | 210.3 ±14.3 * | 20.7 ± 2.9 |
| CS | 170.1 ± 12.6 | 344.0 ±14.6 a | 173.9 ±11.2 | 21.1± 1.9 |
| HPCS | 176.4 ±11.8 | 333.2 ± 15.2 a | 156.8 ±12.0 a | 20.9 ± 3.3 |
| HACC | 173.1 ± 10.6 | 329.1 ± 16.4 b | 156.0 ±11.9 a | 20.1 ± 1.5 |
| CMCS | 172.2 ±11.3 | 356.6 ± 16.9 * | 181.1 ±13.5 | 19.7± 2.8 |
| Group | TG (mmol/L) | TC (mmol/L) | HDL-C(mmol/L) | LDL-C (mmol/L) |
|---|---|---|---|---|
| NC | 0.37 ± 0.06 | 2.65 ± 0.71 | 0.89 ± 0.16 | 1.47 ± 0.45 |
| HF | 0.85 ± 0.14 ** | 7.77 ± 0.67 ** | 0.79 ± 0.28 | 6.68 ± 0.77 ** |
| CS | 0.64 ± 0.09 ** | 6.11 ± 0.90 **a | 0.65± 0.11 * | 4.08 ± 0.59 **b |
| HPCS | 0.34 ± 0.04 b | 3.45 ±0.81 *b | 0.86 ± 0.17 | 3.34± 0.73 **b |
| HACC | 0.35 ± 0.06 b | 4.65 ±0.59 **b | 0.85 ± 0.21 | 4.06 ± 0.83 **b |
| CMCS | 0.40 ± 0.07 b | 6.32 ± 0.38 **a | 0.72 ± 0.18 | 3.51 ± 0.62 **b |
| Group | FFA (μmol/L) | LPO (μmol/L) | SOD (U/mL) |
|---|---|---|---|
| NC | 158.1 ± 25.3 | 5.64 ± 0.74 | 366.8 ± 37.9 |
| HF | 758.5 ± 48.5 ** | 18.05 ± 3.69 ** | 255.6 ± 16.4 ** |
| CS | 742.8 ± 66.4 ** | 17.33 ±4.34 ** | 230.3 ± 13.4 ** |
| HPCS | 269.6 ± 25.4 *b | 7.89 ±1.36 **b | 314.7 ± 19.2 a |
| HACC | 495.7 ± 65.6 **b | 12.98 ±1.79 ** | 280.4 ± 37.9 **a |
| CMCS | 689.4 ± 77.0 **a | 17.65 ± 1.64 ** | 265.5 ± 35.6 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Yan, Y.; Zhang, Z.; Li, C.; Mei, L.; Hou, R.; Liu, X.; Jiang, H. Effect of Chitosan and Its Water-Soluble Derivatives on Antioxidant Activity. Polymers 2024, 16, 867. https://doi.org/10.3390/polym16070867
Wang Z, Yan Y, Zhang Z, Li C, Mei L, Hou R, Liu X, Jiang H. Effect of Chitosan and Its Water-Soluble Derivatives on Antioxidant Activity. Polymers. 2024; 16(7):867. https://doi.org/10.3390/polym16070867
Chicago/Turabian StyleWang, Zhihua, Yongbin Yan, Zhengmao Zhang, Changchun Li, Lanfei Mei, Ruyi Hou, Xiaodan Liu, and Hongxia Jiang. 2024. "Effect of Chitosan and Its Water-Soluble Derivatives on Antioxidant Activity" Polymers 16, no. 7: 867. https://doi.org/10.3390/polym16070867
APA StyleWang, Z., Yan, Y., Zhang, Z., Li, C., Mei, L., Hou, R., Liu, X., & Jiang, H. (2024). Effect of Chitosan and Its Water-Soluble Derivatives on Antioxidant Activity. Polymers, 16(7), 867. https://doi.org/10.3390/polym16070867







