The Effect of Processing Conditions on the Microstructure of Homopolymer High-Density Polyethylene Blends: A Multivariate Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- Lupolen 5021 DX from LyondellBasell (Houston, TX, USA), selected as a high-MW polymer and hereinafter named HMW (melt flow rate (190 °C/2.16 kg) = 0.25 g/10 min; density = 0.950 g/cm3);
- Eraclene MP90U from Versalis (San Donato Milanese, Italy), used as a low-MW polymer and hereinafter named LMW (melt flow rate (190 °C/2.16 kg) = 7 g/10 min; density = 0.960 g/cm3).
2.2. Processing
2.3. Characterization
2.4. Data Analysis
2.4.1. Design of Experiments (DoEs)
2.4.2. Principal Component Analysis (PCA)
3. Results and Discussion
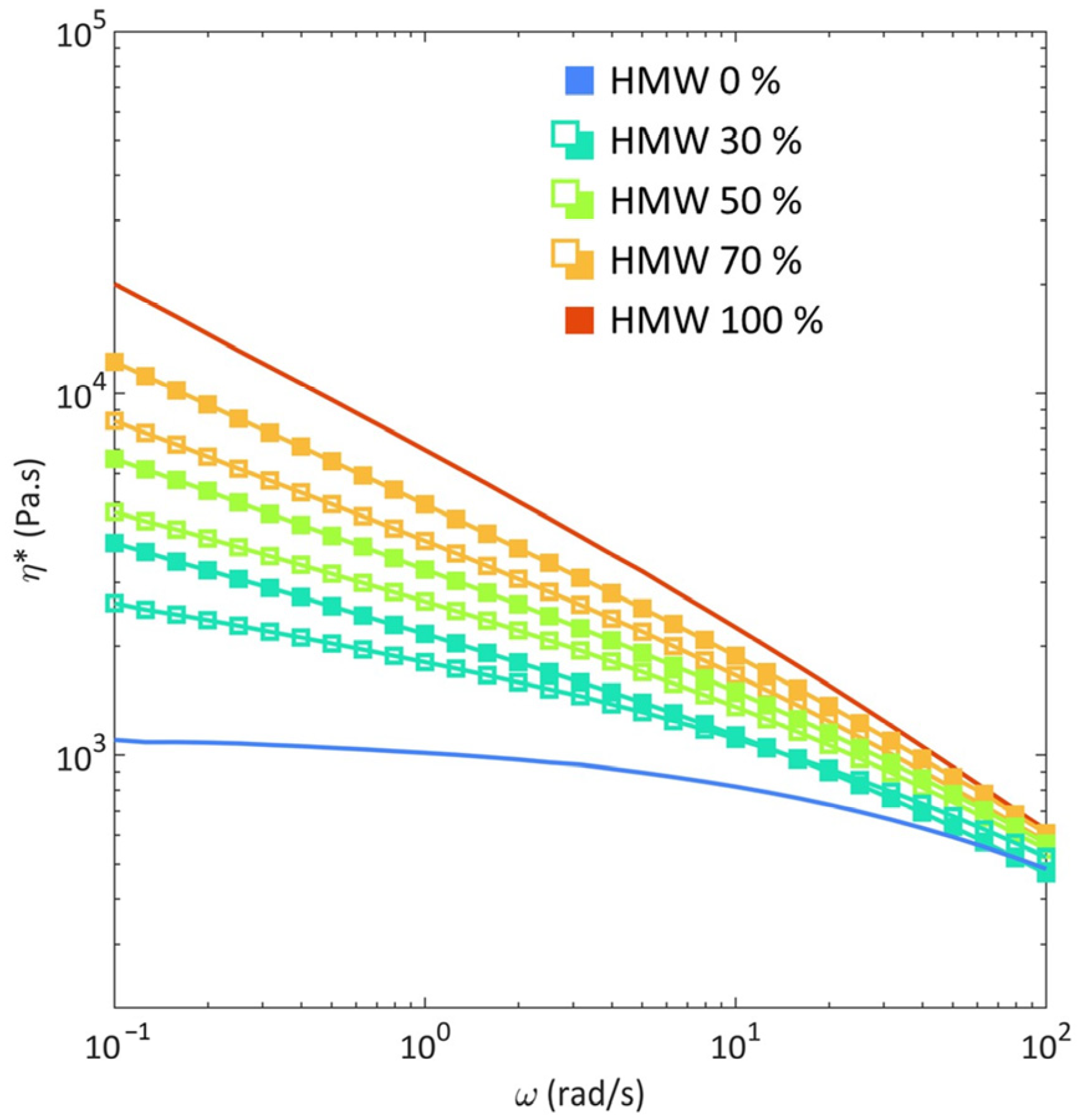
3.1. Rheological Behavior
3.2. PCA Analysis
3.3. DSC Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kissin, Y.V. Polyethylene—End-Use Properties and Their Physical Meaning, 2nd ed.; Hanser Publishers: Cincinnati, OH, USA, 2020. [Google Scholar]
- Mufarrij, F.; Ashrafi, O.; Navarri, P.; Khojasteh, Y. Development and lifecycle assessment of various low- and high-density polyethylene production processes based on CO2 capture and utilization. J. Clean. Prod. 2023, 414, 137624. [Google Scholar] [CrossRef]
- Fitri, M.; Mahzanb, S.; Anggaraa, F. The Mechanical Properties Requirement for Polymer Composite Automotive Parts—A Review. Int. J. Mech. Mechatron. Eng. 2020, 1, 125–133. [Google Scholar] [CrossRef]
- Du, Z.C.; Yang, H.; Luo, X.H.; Xie, Z.X.; Fu, Q.; Gao, X.Q. The Role of Mold Temperature on Morphology and Mechanical Properties of PE Pipe Produced by Rotational Shear. Chin. J. Polym. Sci. 2020, 38, 653–664. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, X.; Lu, Y.; Lv, J.; Zhu, N.; Hu, L. Effect of transverse flow on flame spread and extinction over polyethylene-insulated wires. Proc. Combust. Inst. 2021, 38, 4727–4735. [Google Scholar] [CrossRef]
- Subramanian, M.N. Polymer blends. In Polymer Blends and Composites. Chemistry and Technology; Subramanian, M.N., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Zhao, L.; Choi, P. A Review of the Miscibility of Polyethylene Blends. Mater. Manuf. 2006, 21, 135–142. [Google Scholar] [CrossRef]
- Bai, L.; Li, Y.; Yang, W.; Yang, M. Rheological Behavior and Mechanical Properties of High-Density Polyethylene Blends with Different Molecular Weights. J. Appl. Polym. Sci. 2010, 118, 1356–1363. [Google Scholar] [CrossRef]
- Oliveira, A.D.B.; Freitas, D.M.G.; Araújo, J.P.; Cavalcanti, S.N.; Câmara, D.S.; Agrawal, P.; Mélo, T.J.A. HDPE/LLDPE blends: Rheological, thermal, and mechanical properties. Mater. Res. Innov. 2020, 24, 289–294. [Google Scholar] [CrossRef]
- Cecon, V.S.; Da Silva, P.F.; Vorst, K.L.; Curtzwiler, G.W. The effect of post-consumer recycled polyethylene (PCRPE) on the properties of polyethylene blends of different densities. Polym. Degrad. Stab. 2021, 190, 109627. [Google Scholar] [CrossRef]
- Agrawal, P.; Silva, M.H.A.; Cavalcanti, S.N.; Freitas, D.M.G.; Araújo, J.P.; Oliveira, A.D.B.; Mélo, T.J.A. Rheological properties of high-density polyethylene/linear low-density polyethylene and high-density polyethylene/low-density polyethylene blends. Polym. Bull. 2022, 79, 2321–2343. [Google Scholar] [CrossRef]
- Salakhov, I.I.; Shaidullin, N.M.; Chalykh, A.E.; Matsko, M.A.; Shapagin, A.V.; Batyrshin, A.Z.; Shandryuk, G.A.; Nifant’ev, I.E. Low-Temperature Mechanical Properties of High-Density and Low-Density Polyethylene and Their Blends. Polymers 2021, 13, 1821. [Google Scholar] [CrossRef]
- Sarkhel, G.; Banerjee, A.; Bhattacharya, P. Rheological and Mechanical Properties of LDPE/HDPE Blends. Polym. Plast. Technol. Eng. 2006, 45, 713–718. [Google Scholar] [CrossRef]
- Juan, R.; Domínguez, C.; Robledo, N.; Paredes, B.; García-Munoz, R.A. Incorporation of recycled high-density polyethylene to polyethylene pipe grade resins to increase close-loop recycling and Underpin the circular economy. J. Clean. Prod. 2020, 276, 124081. [Google Scholar] [CrossRef]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Biodegradable compatibilized polymer blends for packaging applications: A literature review. J. Appl. Polym. Sci. 2018, 135, 45726. [Google Scholar] [CrossRef]
- Munioz-Escalona, A.; Lafuente, P.; Vega, J.F.; Mufioz, M.E.; Santamaria, A. Rheological behaviour of metallocene catalysed high density polytheylene blends. Polymer 1997, 38, 589–594. [Google Scholar] [CrossRef]
- Hay, J.N.; Zhou, X. The effect of mixing on the properties of polyethylene blends. Polymer 1993, 34, 2282–2288. [Google Scholar] [CrossRef]
- Vadhar, P.; Kyu, T. Effects of mixing on morphology, rheology, and mechanical properties of blends of ultra-high molecular weight polyethylene with linear low-density polyethylene. Polym. Eng. Sci. 1987, 27, 202–210. [Google Scholar] [CrossRef]
- Tinçer, T.; Coşkun, M. Melt blending of ultra high molecular weight and high density polyethylene: The effect of mixing rate on thermal, mechanical, and morphological properties. Polym. Eng. Sci. 1993, 33, 1243–1250. [Google Scholar] [CrossRef]
- Zhang, J.; Hirschberg, V.; Rodrigue, D. Blending Recycled High-Density Polyethylene HDPE (rHDPE) with Virgin (vHDPE) as an Effective Approach to Improve the Mechanical Properties. Recycling 2023, 8, 1. [Google Scholar] [CrossRef]
- Krishnaswamy, R.K.; Yang, Q. Influence of phase segregation on the mechanical properties of binary blends of polyethylenes that differ considerably in molecular weight. Polymer 2007, 48, 5348–5354. [Google Scholar] [CrossRef]
- Gao, C.; Yu, L.; Liu, H.; Chen, L. Development of self-reinforced polymer composites. Prog. Polym. Sci. 2012, 37, 767–780. [Google Scholar] [CrossRef]
- Hees, T.; Zhong, F.; Stürzel, M.; Mülhaupt, R. Tailoring Hydrocarbon Polymers and All-Hydrocarbon Composites for Circular Economy. Macromol. Rapid Commun. 2019, 40, 1800608. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chen, F.; Li, Z.; Fu, Q. Control of the hierarchical structure of polymer articles via “structuring” processing. Prog. Polym. Sci. 2014, 39, 891–920. [Google Scholar] [CrossRef]
- Zhong, F.; Schwabe, J.; Hofmann, D.; Meier, J.; Thomann, R.; Enders, M.; Mülhaupt, R. All-polyethylene composites reinforced via extended-chain UHMWPE nanostructure formation during melt processing. Polymer 2018, 140, 107–116. [Google Scholar] [CrossRef]
- Pan, X.; Huang, Y.; Zhang, Y.; Liu, B.; He, X. Improved performance and crystallization behaviors of bimodal HDPE/UHMWPE blends assisted by ultrasonic oscillations. Mater. Res. Express. 2019, 6, 035306. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, S.; Hsiao, B.S.; Norman, A.; Tsou, A.H.; Throckmorton, J.; Doufas, A.; Zhang, Y. Shear induced crystallization of bimodal and unimodal high density polyethylene. Polymer 2018, 153, 223–231. [Google Scholar] [CrossRef]
- De Santis, F.; Boragno, L.; Jeremic, D.; Albunia, A.R. Trimodal polyethylene polymer design for more sustainable packaging applications. Polymer 2024, 295, 126740. [Google Scholar] [CrossRef]
- Leardi, R. Experimental design in chemistry: A tutorial. Anal. Chim. Acta 2009, 652, 161–172. [Google Scholar] [CrossRef]
- Ballabio, D. A MATLAB toolbox for Principal Component Analysis and unsupervised exploration of data structure. Chemom. Intell. Lab. Syst. 2015, 149, 1–9. [Google Scholar] [CrossRef]
- Sergent, M.; Mathieu, D.; Phan-Tan-Luu, R.; Drava, G. Correct and incorrect use of multilinear regression. Chemom. Intell. Lab. Syst. 1995, 27, 153–162. [Google Scholar] [CrossRef]
- Leardi, R.; Melzi, C.; Polotti, G. CAT (Chemometric Agile Tool). Available online: https://gruppochemiometria.it/index.php/software (accessed on 26 September 2023).
- Bro, R.; Smilde, A. Principal component analysis. Anal. Methods 2014, 6, 2812–2831. [Google Scholar] [CrossRef]
- Yan, C.; Huang, W.; Lin, P.; Zhang, Y.; Lv, Q. Chemical and rheological evaluation of aging properties of high content SBS polymer modified asphalt. Fuel 2019, 252, 417–426. [Google Scholar] [CrossRef]
- Tian, Y.; Li, H.; Sun, L.; Zhang, H.; Harvey, J.; Yang, J.; Yang, B.; Zuo, X. Laboratory investigation on rheological, chemical and morphological evolution of high content polymer modified bitumen under long-term thermal oxidative aging. Constr. Build. Mater. 2021, 303, 124565. [Google Scholar] [CrossRef]
- Tafuro, G.; Costantini, A.; Baratto, G.; Busata, L.; Semenzato, A. Rheological and Textural Characterization of Acrylic Polymer Water Dispersions for Cosmetic Use. Ind. Eng. Chem. Res. 2019, 58, 23549–23558. [Google Scholar] [CrossRef]
- Liu, Y.; Leng, Y.; Xiao, S.; Zhang, Y.; Ding, W.; Ding, B.; Wu, Y.; Wang, X.; Fu, Y. Effect of inulin with different degrees of polymerization on dough rheology, gelatinization, texture and protein composition properties of extruded flour products. LWT 2022, 159, 113225. [Google Scholar] [CrossRef]
- Raj, A.S.; Boyacioglu, M.H.; Dogan, H.; Siliveru, K. Investigating the Contribution of Blending on the Dough Rheology of Roller-Milled Hard Red Wheat. Foods 2023, 12, 2078. [Google Scholar] [CrossRef]
- Bartkowiak, A.; Lewandowicz, J.; Rojewska, M.; Krüger, K.; Lulek, J.; Prochaska, K. Study of viscoelastic, sorption and mucoadhesive properties of selected polymer blends for biomedical applications. J. Mol. Liq. 2022, 361, 119623. [Google Scholar] [CrossRef]
- Milano Chemometrics and QSAR Research Group, PCA Toolbox for MATLAB. Available online: https://michem.unimib.it/download/matlab-toolboxes/pca-toolbox-for-matlab/ (accessed on 26 September 2023).
- Wood-Adams, P.M.; Dealy, J.M.; deGroot, A.W.; Redwine, O.D. Effect of Molecular Structure on the Linear Viscoelastic Behavior of Polyethylene. Macromolecules 2000, 33, 7489–7499. [Google Scholar] [CrossRef]
- Chen, D.; Hong, L.; Nie, X.; Wang, X.; Tang, X. Study on rheological properties and relaxational behavior of poly(dianilinephosphazene)/low-density polyethylene blends. Eur. Polym. J. 2003, 39, 871–876. [Google Scholar] [CrossRef]
- Shi, D.; Hu, G.; Ke, Z.; Li, R.K.Y.; Yin, J. Relaxation behavior of polymer blends with complex morphologies: Palierne emulsion model for uncompatibilized and compatibilized PP/PA6 blends. Polymer 2006, 47, 4659–4666. [Google Scholar] [CrossRef]
- Xu, L.; Huang, H. Relaxation Behavior of Poly(lactic acid)/Poly(butylenesuccinate) Blend and a New Method for Calculating ItsInterfacial Tension. J. Appl. Polym. Sci. 2012, 125, 272–277. [Google Scholar] [CrossRef]
- Agrawal, P.; Araújo, A.P.M.; Lima, J.C.C.; Cavalcanti, S.N.; Freitas, D.M.G.; Farias, G.M.G.; Ueki, M.M.; Mélo, T.J.A. Rheology, Mechanical Properties and Morphology of Poly(lactic acid)/Ethylene Vinyl Acetate Blends. J. Polym. Environ. 2019, 27, 1439–1448. [Google Scholar] [CrossRef]
- Jozaghkar, M.R.; Jahani, Y.; Arabi, H.; Ziaee, F. Preparation and assessment of phase morphology, rheological properties and thermal behavior of low density polyethylene/polyhexene-1 blends. Polym. Plast. Technol. Eng. 2017, 57, 757–765. [Google Scholar] [CrossRef]
- Hameed, T.; Hussein, I.A. Melt Miscibility and Mechanical Properties of Metallocene LLDPE blends with HDPE: Influence of Mw of LLDPE. Polym. J. 2006, 38, 1114–1126. [Google Scholar] [CrossRef]





| Factors | Code | Levels | ||||
|---|---|---|---|---|---|---|
| −1 | −0.5 | 0 | 0.5 | 1 | ||
| Concentration of HMW (high molecular weight) (wt%) | HMW content | 0 | 30 | 50 | 70 | 100 |
| Compounding temperature (°C) | T | 175 | 190 | |||
| Screw rotation speed (rpm) | rpm | 150 | 400 | |||
| Rheology analysis temperature (°C) | / | 175 | 190 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cravero, F.; Cavallini, N.; Arrigo, R.; Savorani, F.; Frache, A. The Effect of Processing Conditions on the Microstructure of Homopolymer High-Density Polyethylene Blends: A Multivariate Approach. Polymers 2024, 16, 870. https://doi.org/10.3390/polym16070870
Cravero F, Cavallini N, Arrigo R, Savorani F, Frache A. The Effect of Processing Conditions on the Microstructure of Homopolymer High-Density Polyethylene Blends: A Multivariate Approach. Polymers. 2024; 16(7):870. https://doi.org/10.3390/polym16070870
Chicago/Turabian StyleCravero, Fulvia, Nicola Cavallini, Rossella Arrigo, Francesco Savorani, and Alberto Frache. 2024. "The Effect of Processing Conditions on the Microstructure of Homopolymer High-Density Polyethylene Blends: A Multivariate Approach" Polymers 16, no. 7: 870. https://doi.org/10.3390/polym16070870






