Application of Infrared Pyrolysis and Chemical Post-Activation in the Conversion of Polyethylene Terephthalate Waste into Porous Carbons for Water Purification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Porous Carbon Preparation
2.2. Porous Carbon Characterization
2.3. Adsorption Study
3. Results
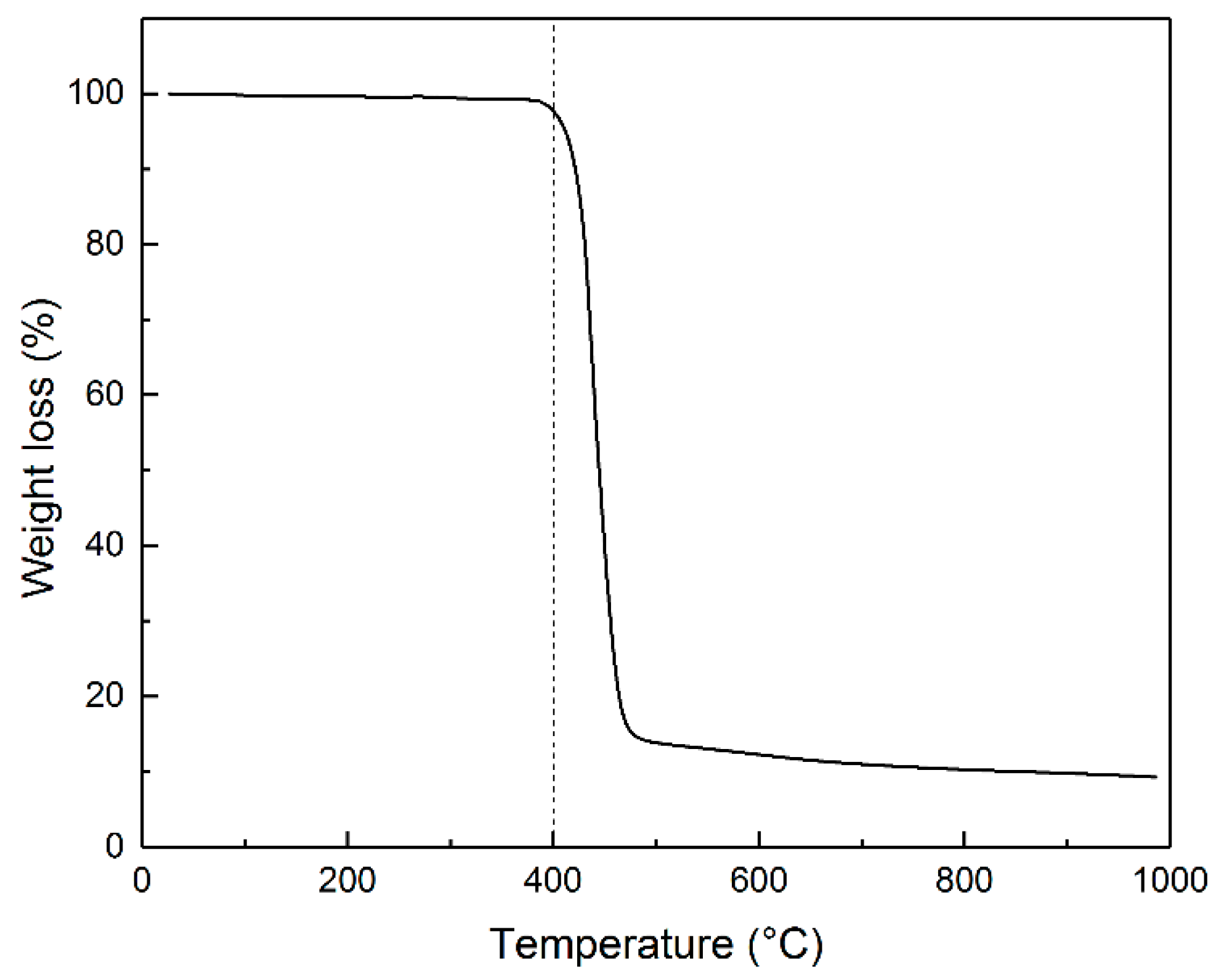
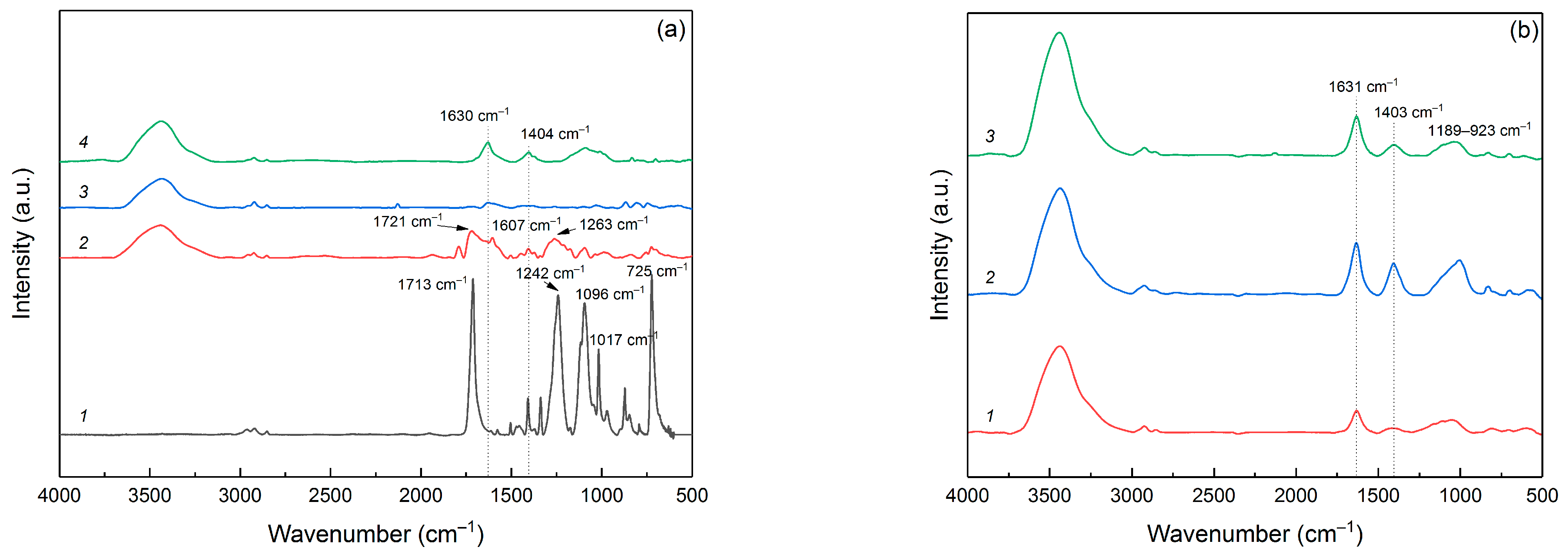
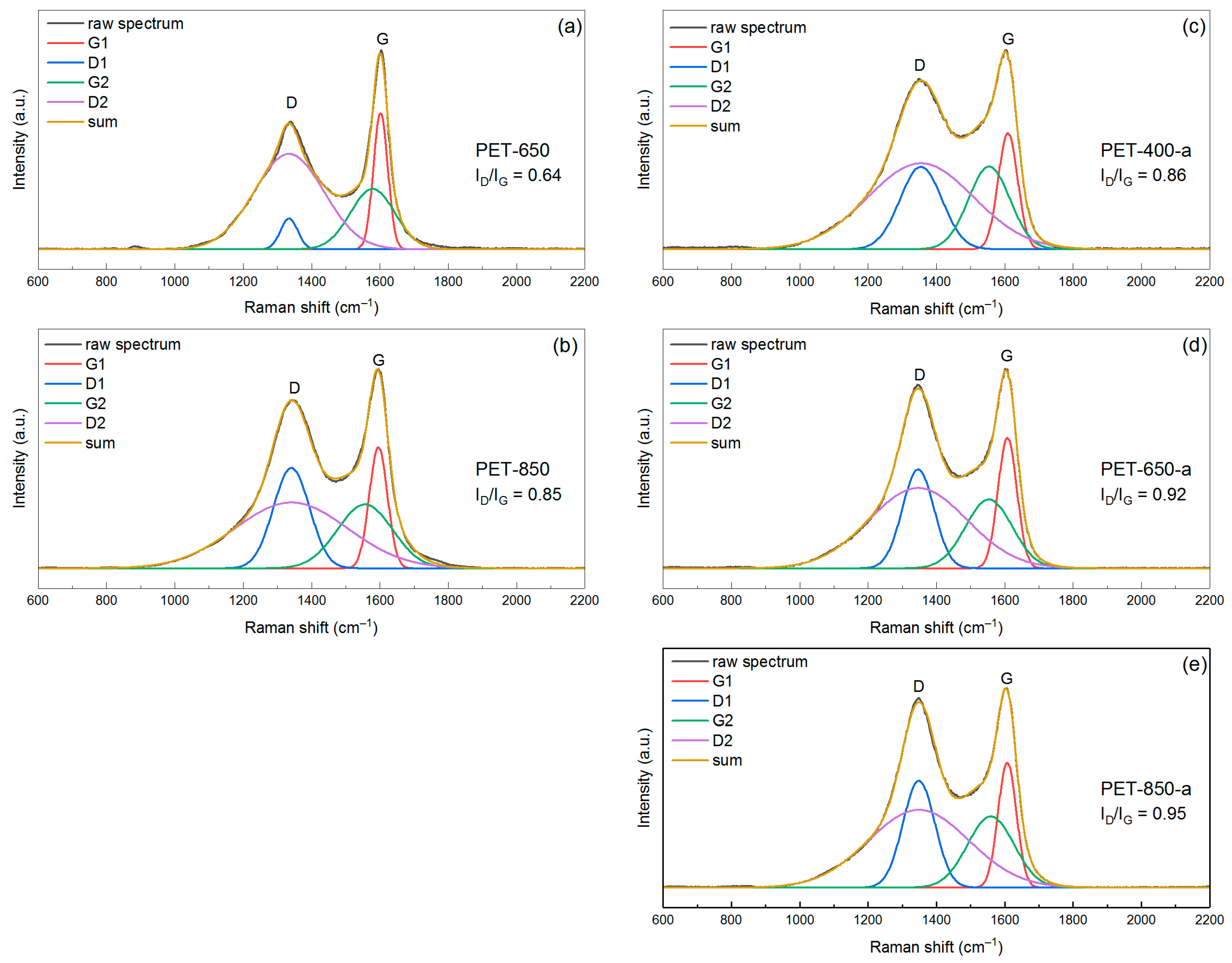
3.1. Structural Characteristics of Pyrolyzed and Post-Activated PET Samples
3.2. Adsorption Properties of Pyrolyzed and Post-Activated PET Samples
3.3. Comparison with other Materials and their Adsorption Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2023, 3, e1700782. [Google Scholar] [CrossRef]
- Williams, A.T.; Rangel-Buitrago, N. The Past, Present, and Future of Plastic Pollution. Mar. Pollut. Bull. 2022, 176, 113429. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.M.; Ni, B.J. Microplastics in Wastewater Treatment Plants: Detection, Occurrence and Removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, G.; Uchida, N.; Tuyen, L.H.; Tanaka, K.; Matsukami, H.; Kunisue, T.; Takahashi, S.; Viet, P.H.; Kuramochi, H.; Osako, M. Mechanical Recycling of Plastic Waste as a Point Source of Microplastic Pollution. Environ. Pollut. 2022, 303, 119114. [Google Scholar] [CrossRef] [PubMed]
- Suhaimi, N.A.S.; Muhamad, F.; Abd Razak, N.A.; Zeimaran, E. Recycling of Polyethylene Terephthalate Wastes: A Review of Technologies, Routes, and Applications. Polym. Eng. Sci. 2022, 62, 2355–2375. [Google Scholar] [CrossRef]
- Damayanti; Wu, H.-S. Strategic Possibility Routes of Recycled PET. Polymers 2021, 13, 1475. [Google Scholar] [CrossRef] [PubMed]
- Kirshanov, K.A.; Toms, R.V.; Balashov, M.S.; Golubkov, S.S.; Melnikov, P.V.; Gervald, A.Y. Modeling of Poly(Ethylene Terephthalate) Homogeneous Glycolysis Kinetics. Polymers 2023, 15, 3146. [Google Scholar] [CrossRef]
- Yang, W.; Liu, R.; Li, C.; Song, Y.; Hu, C. Hydrolysis of Waste Polyethylene Terephthalate Catalyzed by Easily Recyclable Terephthalic Acid. Waste Manag. 2021, 135, 267–274. [Google Scholar] [CrossRef]
- Laldinpuii, Z.T.; Khiangte, V.; Lalhmangaihzuala, S.; Lalmuanpuia, C.; Pachuau, Z.; Lalhriatpuia, C.; Vanlaldinpuia, K. Methanolysis of PET Waste Using Heterogeneous Catalyst of Bio-Waste Origin. J. Polym. Environ. 2022, 30, 1600–1614. [Google Scholar] [CrossRef]
- Kárpáti, L.; Fogarassy, F.; Kovácsik, D.; Vargha, V. One-Pot Depolymerization and Polycondensation of PET Based Random Oligo- and Polyesters. J. Polym. Environ. 2019, 27, 2167–2181. [Google Scholar] [CrossRef]
- Zhu, M.; Li, S.; Li, Z.; Lu, X.; Zhang, S. Investigation of Solid Catalysts for Glycolysis of Polyethylene Terephthalate. Chem. Eng. J. 2012, 185–186, 168–177. [Google Scholar] [CrossRef]
- Nabgan, W.; Nabgan, B.; Tuan Abdullah, T.A.; Ngadi, N.; Jalil, A.A.; Hassan, N.S.; Izan, S.M.; Luing, W.S.; Abdullah, S.N.; Majeed, F.S.A. Conversion of Polyethylene Terephthalate Plastic Waste and Phenol Steam Reforming to Hydrogen and Valuable Liquid Fuel: Synthesis Effect of Ni–Co/ZrO2 Nanostructured Catalysts. Int. J. Hydrogen Energy 2020, 45, 6302–6317. [Google Scholar] [CrossRef]
- Nabgan, W.; Nabgan, B.; Tuan Abdullah, T.A.; Jalil, A.A.; Ul-Hamid, A.; Ikram, M.; Nordin, A.H.; Coelho, A. Production of Hydrogen and Valuable Fuels from Polyethylene Terephthalate Waste Dissolved in Phenol Reforming and Cracking Reactions via Ni-Co/CeO2 Nano-Catalyst. J. Anal. Appl. Pyrolysis 2021, 154, 105018. [Google Scholar] [CrossRef]
- Choi, M.-J.; Jeong, Y.-S.; Kim, J.-S. Air Gasification of Polyethylene Terephthalate Using a Two-Stage Gasifier with Active Carbon for the Production of H2 and CO. Energy 2021, 223, 120122. [Google Scholar] [CrossRef]
- Fernández-Morales, I.; Almazán-Almazán, M.C.; Pérez-Mendoza, M.; Domingo-García, M.; López-Garzón, F.J. PET as Precursor of Microporous Carbons: Preparation and Characterization. Microporous Mesoporous Mater. 2005, 80, 107–115. [Google Scholar] [CrossRef]
- Sharifian, S.; Asasian-Kolur, N. Polyethylene Terephthalate (PET) Waste to Carbon Materials: Theory, Methods and Applications. J. Anal. Appl. Pyrolysis 2022, 163, 105496. [Google Scholar] [CrossRef]
- Yang, I.; Mok, J.H.; Jung, M.; Yoo, J.; Kim, M.-S.; Choi, D.; Jung, J.C. Polyethylene-Derived Activated Carbon Materials for Commercially Available Supercapacitor in an Organic Electrolyte System. Macromol. Rapid Commun. 2022, 43, 2200006. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Duan, X.; Zhang, P.; Niu, Q.; Dai, S. Processing Poly (Ethylene Terephthalate) Waste into Functional Carbon Materials by Mechanochemical Extrusion. ChemSusChem 2022, 15, e202201576. [Google Scholar] [CrossRef] [PubMed]
- Efimov, M.N.; Vasilev, A.A.; Muratov, D.G.; Dzidziguri, E.L.; Sheverdiyev, K.A.; Karpacheva, G.P. Conversion of Polyethylene Terephthalate Waste in the Presence of Cobalt Compound into Highly-Porous Metal-Carbon Nanocomposite (c-PET-Co). Compos. Commun. 2022, 33, 101200. [Google Scholar] [CrossRef]
- Mendoza-Carrasco, R.; Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernández-González, C.; Gómez-Serrano, V. Preparation of High-Quality Activated Carbon from Polyethyleneterephthalate (PET) Bottle Waste. Its Use in the Removal of Pollutants in Aqueous Solution. J. Environ. Manag. 2016, 181, 522–535. [Google Scholar] [CrossRef]
- de Castro, C.S.; Viau, L.N.; Andrade, J.T.; Mendonça, T.A.P.; Gonçalves, M. Mesoporous Activated Carbon from Polyethyleneterephthalate (PET) Waste: Pollutant Adsorption in Aqueous Solution. New J. Chem. 2018, 42, 14612–14619. [Google Scholar] [CrossRef]
- Efimov, M.N.; Vasilev, A.A.; Muratov, D.G.; Kostev, A.I.; Kolesnikov, E.A.; Kiseleva, S.G.; Karpacheva, G.P. Conversion of Polyethylene Terephthalate Waste into High-Yield Porous Carbon Adsorbent via Pyrolysis of Dipotassium Terephthalate. Waste Manag. 2023, 162, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Efimov, M.N.; Vasilev, A.A.; Muratov, D.G.; Baranchikov, A.E.; Karpacheva, G.P. IR Radiation Assisted Preparation of KOH-Activated Polymer-Derived Carbon for Methylene Blue Adsorption. J. Environ. Chem. Eng. 2019, 7, 103514. [Google Scholar] [CrossRef]
- Nolasco Cruz, J.; Donjuan Martínez, K.; Álvaro Zavariz, D.; Hernández, I.P. Review of the Thermochemical Degradation of PET: An Alternative Method of Recycling. J. Ecol. Eng. 2022, 23, 319–330. [Google Scholar] [CrossRef]
- Liew, R.K.; Chai, C.; Yek, P.N.Y.; Phang, X.Y.; Chong, M.Y.; Nam, W.L.; Su, M.H.; Lam, W.H.; Ma, N.L.; Lam, S.S. Innovative Production of Highly Porous Carbon for Industrial Effluent Remediation via Microwave Vacuum Pyrolysis plus Sodium-Potassium Hydroxide Mixture Activation. J. Clean. Prod. 2019, 208, 1436–1445. [Google Scholar] [CrossRef]
- Ibor, O.R.; Mpama, N.O.L.; Okoli, C.P.; Ogarekpe, D.M.; Edet, U.O.; Ajang, R.O.; Onyezobi, C.E.; Anyanti, J.; Idogho, O.; Aizobu, D.; et al. Occurrence, Identification and Characterization of Plastic Pollution from an Open Solid Waste Dumpsite in Calabar, Southern Nigeria. Environ. Adv. 2023, 11, 100338. [Google Scholar] [CrossRef]
- Perret, E.; Braun, O.; Sharma, K.; Tritsch, S.; Muff, R.; Hufenus, R. High-Resolution 2D Raman Mapping of Mono- and Bicomponent Filament Cross-Sections. Polymer 2021, 229, 124011. [Google Scholar] [CrossRef]
- Jia, H.; Ben, H.; Luo, Y.; Wang, R. Catalytic Fast Pyrolysis of Poly (Ethylene Terephthalate) (PET) with Zeolite and Nickel Chloride. Polymers 2020, 12, 705. [Google Scholar] [CrossRef]
- Epure, E.-L.; Cojocaru, F.D.; Aradoaei, M.; Ciobanu, R.C.; Dodi, G. Exploring the Surface Potential of Recycled Polyethylene Terephthalate Composite Supports on the Collagen Contamination Level. Polymers 2023, 15, 776. [Google Scholar] [CrossRef]
- Brems, A.; Baeyens, J.; Vandecasteele, C.; Dewil, R. Polymeric Cracking of Waste Polyethylene Terephthalate to Chemicals and Energy. J. Air Waste Manag. Assoc. 2011, 61, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Suebsaeng, T.; Wilkie, C.A.; Burger, V.T.; Carter, J.; Brown, C.E. Solid Products from Thermal Decomposition of Polyethylene Terephthalate: Investigation by CP/MAS 13C-NMR and Fourier Transform-IR Spectroscopy. J. Polym. Sci. Polym. Chem. Ed. 1984, 22, 945–957. [Google Scholar] [CrossRef]
- Diaz-Silvarrey, L.S.; McMahon, A.; Phan, A.N. Benzoic Acid Recovery via Waste Poly(Ethylene Terephthalate) (PET) Catalytic Pyrolysis Using Sulphated Zirconia Catalyst. J. Anal. Appl. Pyrolysis 2018, 134, 621–631. [Google Scholar] [CrossRef]
- Gale, M.; Nguyen, P.M.; Gilliard-AbdulAziz, K.L. Synergistic and Antagonistic Effects of the Co-Pyrolysis of Plastics and Corn Stover to Produce Char and Activated Carbon. ACS Omega 2023, 8, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Poulose, A.M.; Elnour, A.Y.; Kumar, N.S.; Alhamidi, A.; George, J.; Al-Ghurabi, E.H.; Boumaza, M.; Al-Zahrani, S. Utilization of Polyethylene Terephthalate Waste as a Carbon Filler in Polypropylene Matrix: Investigation of Mechanical, Rheological, and Thermal Properties. J. Appl. Polym. Sci. 2021, 138, 50292. [Google Scholar] [CrossRef]
- Kaur, B.; Singh, J.; Gupta, R.K.; Bhunia, H. Porous Carbons Derived from Polyethylene Terephthalate (PET) Waste for CO2 Capture Studies. J. Environ. Manag. 2019, 242, 68–80. [Google Scholar] [CrossRef]
- Olam, M. Production of Activated Carbon from Waste PET’ Chars. Int. J. Environ. Monit. Anal. 2022, 10, 39–44. [Google Scholar] [CrossRef]
- Dhahak, A.; Hild, G.; Rouaud, M.; Mauviel, G.; Burkle-Vitzthum, V. Slow Pyrolysis of Polyethylene Terephthalate: Online Monitoring of Gas Production and Quantitative Analysis of Waxy Products. J. Anal. Appl. Pyrolysis 2019, 142, 104664. [Google Scholar] [CrossRef]
- Warren, B.E. X-ray Diffraction in Random Layer Lattices. Phys. Rev. 1941, 59, 693–698. [Google Scholar] [CrossRef]
- Cançado, L.G.; Takai, K.; Enoki, T.; Endo, M.; Kim, Y.A.; Mizusaki, H.; Jorio, A.; Coelho, L.N.; Magalhães-Paniago, R.; Pimenta, M.A. General Equation for the Determination of the Crystallite Size La of Nanographite by Raman Spectroscopy. Appl. Phys. Lett. 2006, 88, 163106. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman Spectra of Disordered and Amorphous Carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Kostina, Y.V.; Rusakova, O.Y.; Mikhalitsyn, L.A.; Bondarenko, G.N. Use of Raman Spectroscopy in Analysis of Crude Oils, Petroleum Products, Oil-Bearing Rocks, and Petrochemical Process Catalysts (A Review). Pet. Chem. 2023, 63, 1–30. [Google Scholar] [CrossRef]
- Shimodaira, N.; Masui, A. Raman Spectroscopic Investigations of Activated Carbon Materials. J. Appl. Phys. 2002, 92, 902–909. [Google Scholar] [CrossRef]
- Wei, Z.; Pan, R.; Hou, Y.; Yang, Y.; Liu, Y. Graphene-Supported Pd Catalyst for Highly Selective Hydrogenation of Resorcinol to 1, 3-Cyclohexanedione through Giant π-Conjugate Interactions. Sci. Rep. 2015, 5, 15664. [Google Scholar] [CrossRef] [PubMed]
- Mallet-Ladeira, P.; Puech, P.; Toulouse, C.; Cazayous, M.; Ratel-Ramond, N.; Weisbecker, P.; Vignoles, G.L.; Monthioux, M. A Raman Study to Obtain Crystallite Size of Carbon Materials: A Better Alternative to the Tuinstra–Koenig Law. Carbon N. Y. 2014, 80, 629–639. [Google Scholar] [CrossRef]
- Thomsen, C.; Reich, S. Double Resonant Raman Scattering in Graphite. Phys. Rev. Lett. 2000, 85, 5214–5217. [Google Scholar] [CrossRef]
- Efimov, M.N.; Muratov, D.G.; Klyuev, A.L.; Zhilyaeva, N.A.; Vasilev, A.A.; Ozkan, S.Z.; Karpacheva, G.P. Ultrasonic Treatment Duration: A Nuanced Parameter in Synthesis Affecting Structural Properties and ORR Performance of KOH-Activated Carbon. Diam. Relat. Mater. 2024, 142, 110804. [Google Scholar] [CrossRef]
- Thue, P.S.; dos Reis, G.S.; Lima, E.C.; Sieliechi, J.M.; Dotto, G.L.; Wamba, A.G.N.; Dias, S.L.P.; Pavan, F.A. Activated Carbon Obtained from Sapelli Wood Sawdust by Microwave Heating for O-Cresol Adsorption. Res. Chem. Intermed. 2017, 43, 1063–1087. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, L.; Liu, Y.; Feng, R.; Zou, T.; Zhang, Y.; Kang, Y.; Zhou, P. Efficient Removal of Methylene Blue Using the Mesoporous Activated Carbon Obtained from Mangosteen Peel Wastes: Kinetic, Equilibrium, and Thermodynamic Studies. Microporous Mesoporous Mater. 2021, 315, 110904. [Google Scholar] [CrossRef]
- Üner, O.; Geçgel, Ü.; Bayrak, Y. Adsorption of Methylene Blue by an Efficient Activated Carbon Prepared from Citrullus Lanatus Rind: Kinetic, Isotherm, Thermodynamic, and Mechanism Analysis. Water Air Soil Pollut. 2016, 227, 247. [Google Scholar] [CrossRef]
- Timofeeva, E.A.; Karavanova, E.I. The Evaluation of the Ecological State of the Moscow River near the Kuryanovo Water Treatment Facilities. Urban Stud. Pract. 2020, 3, 99–110. [Google Scholar] [CrossRef]
- Ezzat, M.N.; Ali, Z.T.A. Green Approach for Fabrication of Graphene from Polyethylene Terephthalate (PET) Bottle Waste as Reactive Material for Dyes Removal from Aqueous Solution: Batch and Continuous Study. Sustain. Mater. Technol. 2022, 32, e00404. [Google Scholar] [CrossRef]
- Zhang, F.S.; Itoh, H. Adsorbents Made from Waste Ashes and Post-Consumer PET and Their Potential Utilization in Wastewater Treatment. J. Hazard. Mater. 2003, 101, 323–337. [Google Scholar] [CrossRef]
- Novelo, F.J.X.; Pareja-Rodríguez, R.; Martínez-Flores, R.; Gattorno, G.R. Synthesis of Graphene Oxide from Post-Consumer PET Bottles by a One-Step Thermal Treatment in Air Atmosphere for Removal of Methylene Blue. J. Environ. Chem. Eng. 2024, 12, 112244. [Google Scholar] [CrossRef]
- Pham, T.H.T. Synthesis of Activated Carbon from Polyethylene Terephthalate (PET) Plastic Waste and Its Application for Removal of Organic Dyes from Water. Non-Met. Mater. Sci. 2023, 5, 27–37. [Google Scholar] [CrossRef]
- Tabrizi, N.S.; Yavari, M. Methylene Blue Removal by Carbon Nanotube-Based Aerogels. Chem. Eng. Res. Des. 2015, 94, 516–523. [Google Scholar] [CrossRef]
- Ceroni, L.; Benazzato, S.; Pressi, S.; Calvillo, L.; Marotta, E.; Menna, E. Enhanced Adsorption of Methylene Blue Dye on Functionalized Multi-Walled Carbon Nanotubes. Nanomaterials 2024, 14, 522. [Google Scholar] [CrossRef]
- Jedynak, K.; Charmas, B. Application of Activated Carbons Obtained from Polymer Waste for the Adsorption of Dyes from Aqueous Solutions. Materials 2024, 17, 748. [Google Scholar] [CrossRef]
- Melo, A.L.; Carneiro, M.T.; Morais, A.Í.; Viana, B.C.; Santos, F.E.; Osajima, J.A.; Bezerra, R.D.S.; Peña-Garcia, R.R.; Almeida, L.C.; Carrasco, S.M.; et al. Using Activated Biochar from Caryocar Brasiliense Pequi Almonds for Removing Methylene Blue Dye in an Aqueous Solution. Water 2023, 15, 4006. [Google Scholar] [CrossRef]
- Li, H.; Li, T.; Zhang, T.; Zhu, J.; Deng, W.; He, D. Construction and Adsorption Performance Study of GO-CNT/Activated Carbon Composites for High Efficient Adsorption of Pollutants in Wastewater. Polymers 2022, 14, 4951. [Google Scholar] [CrossRef]
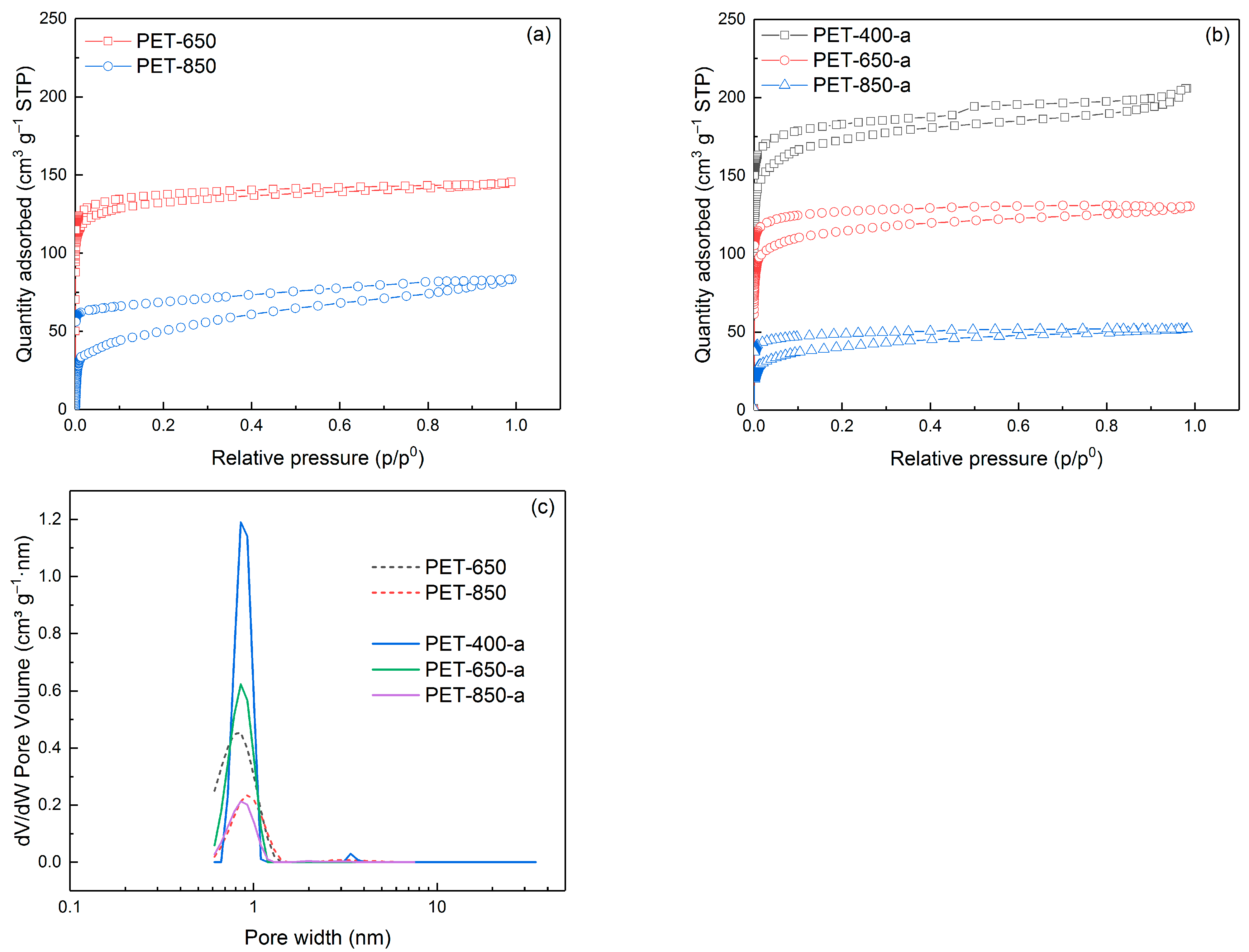


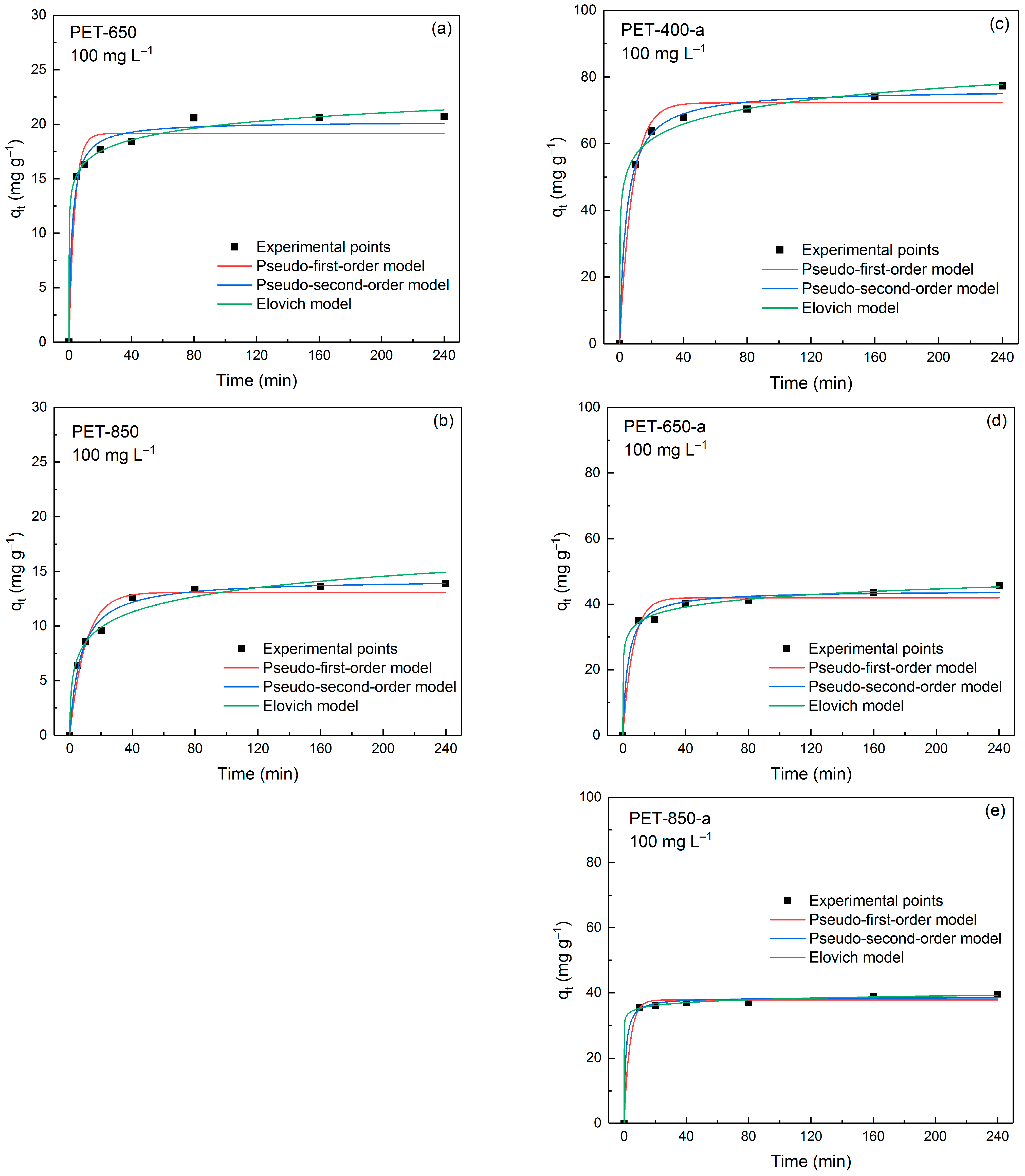

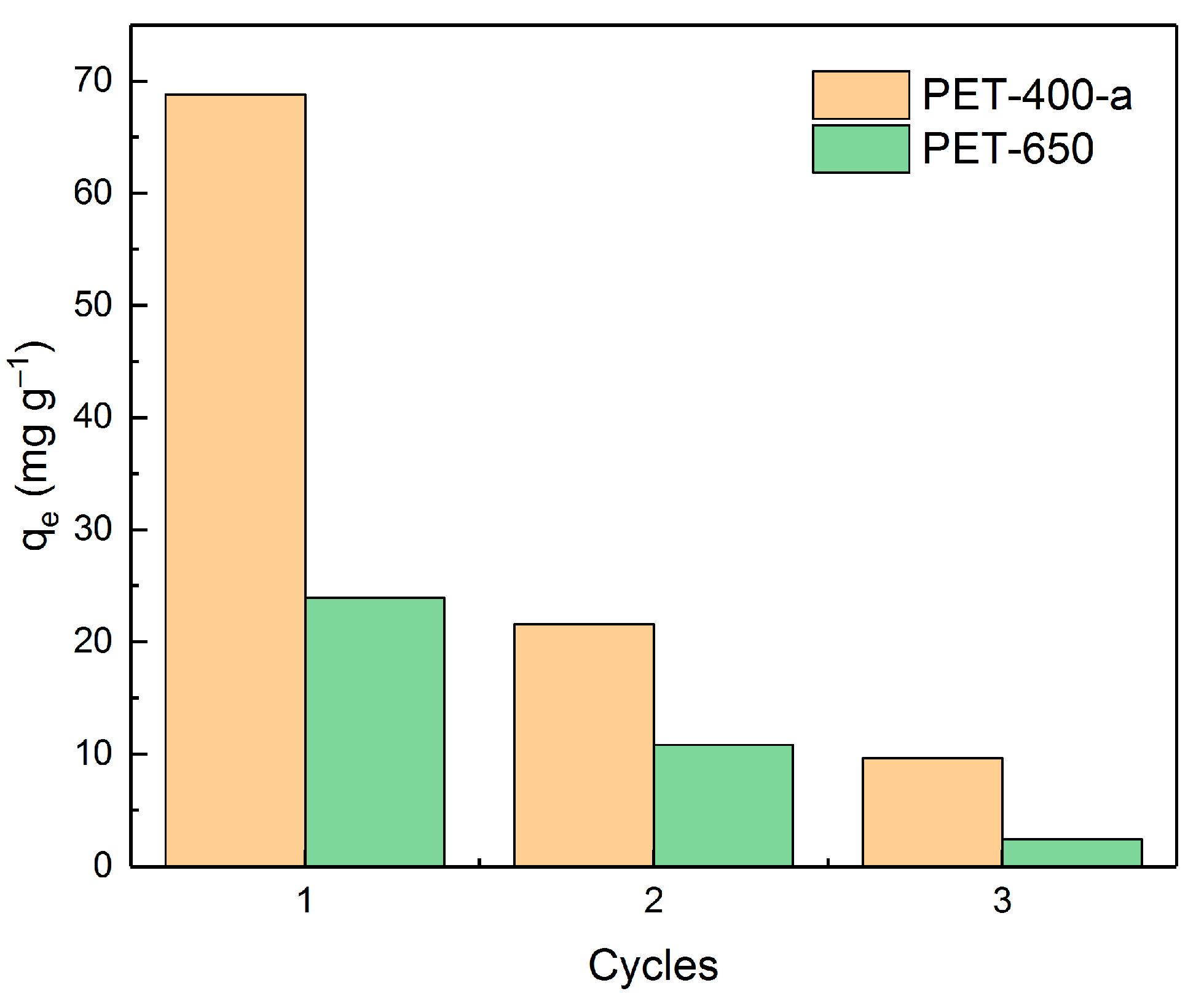
| Sample | SSA, m2 g−1 | V, cm3 g−1 | C, wt% | O, wt% | H, wt% |
|---|---|---|---|---|---|
| PET-400 | - | - | 73.8 | 22.3 | 3.9 |
| PET-550 | 521.5 | 0.257 | 93.0 | 3.3 | 3.7 |
| PET-650 | 504.2 | 0.204 | 92.1 | 5.4 | 2.5 |
| PET-750 | 414.1 | 0.197 | 87.7 | 11.3 | 1.0 |
| PET-850 | 221.0 | 0.119 | 85.3 | 13.8 | 0.9 |
| PET-400-a | 617.7 | 0.289 | 89.3 | 10.3 | 0.4 |
| PET-650-a | 439.0 | 0.187 | 87.4 | 12.2 | 0.4 |
| PET-850-a | 166.0 | 0.074 | 87.0 | 12.7 | 0.3 |
| Sample | a DXRD, nm | b DRaman, nm |
|---|---|---|
| PET-400 | 1.7 | - |
| PET-450 | 1.7 | - |
| PET-550 | 1.7 | - |
| PET-650 | 1.7 | 5.2 |
| PET-750 | 2.0 | - |
| PET-800 | 2.2 | - |
| PET-850 | 2.4 | 3.8 |
| PET-400-a | 2.6 | 3.5 |
| PET-650-a | 2.7 | 4.3 |
| PET-850-a | 2.9 | 4.0 |
| Parameters | PET-650 | PET-850 | PET-400-a | PET-650-a | PET-850-a |
|---|---|---|---|---|---|
| Pseudo-first-order | |||||
| q1 (mg g−1) | 19.2 | 13.1 | 72.3 | 41.9 | 37.8 |
| k1 (min−1) | 0.2731 | 0.1002 | 0.1260 | 0.1549 | 0.2706 |
| R2 | 0.9663 | 0.9637 | 0.9863 | 0.9664 | 0.9934 |
| ARE (%) | 7.00 | 6.96 | 3.18 | 4.52 | 2.06 |
| Pseudo-second-order | |||||
| q2 (mg g−1) | 20.3 | 14.3 | 76.4 | 44.2 | 38.6 |
| k2 (g mg−1 min−1) | 0.0246 | 0.0101 | 0.0031 | 0.0069 | 0.0245 |
| R2 | 0.9895 | 0.9903 | 0.9972 | 0.9893 | 0.9969 |
| ARE (%) | 3.84 | 2.89 | 1.17 | 2.74 | 1.51 |
| Elovich | |||||
| α (mg g–1 min–1) | 6.86 × 103 | 12.98 | 3.2 × 103 | 8.38 × 103 | 1.63 |
| β (g mg–1) | 0.6515 | 0.4911 | 0.1496 | 0.2934 | 0.7915 |
| R2 | 0.9954 | 0.9726 | 0.9921 | 0.9952 | 0.9994 |
| ARE (%) | 1.78 | 5.03 | 1.83 | 1.3 | 0.53 |
| Models | PET-650 | PET-850 | PET-400-a | PET-650-a | PET-850-a |
|---|---|---|---|---|---|
| Langmuir | |||||
| qm (mg g−1) | 20.4 | 13.5 | 105.1 | 72.9 | 60.3 |
| KL (L mg−1) | 1.35 | 1.68 | 8.87 | 0.04 | 0.03 |
| R2 | 0.9989 | 0.9972 | 0.8668 | 0.9708 | 0.9864 |
| ARE (%) | 1.85 | 2.39 | 15.24 | 16.57 | 10.72 |
| Freundlich | |||||
| KF((mg g−1)(L mg−1)1/n) | 10.31 | 7.1 | 59.6 | 9.9 | 7.5 |
| n | 5.59 | 6.02 | 7.25 | 2.60 | 2.57 |
| R2 | 0.9268 | 0.9658 | 0.9400 | 0.9968 | 0.9937 |
| ARE (%) | 18.97 | 10.54 | 10.42 | 3.61 | 3.71 |
| Carbonaceous Precursor | Synthesis Conditions | SSA (m2 g−1) | Adsorption Value (mg g−1) | Ref |
|---|---|---|---|---|
| PET | Post-activation 850 °C, 50 °C min−1, exposure time: 2 min, N2 atmosphere | 617.7 | 127.7 | This study |
| PET | 800 °C, 8 °C min−1, exposure time: 90 min, autoclave | 723.7 | 125 | [52] |
| PET + waste ash | 850 °C, 10 °C min−1, exposure time: 60 min, N2 atmosphere | 485.0 | 92.3 | [53] |
| PET | 500 °C, 10 °C min−1, exposure time: 15 min, air atmosphere | 378.8 | 43.9 | [54] |
| PET | Post-activation 850 °C, exposure time: 25 min CO2 atmosphere | 703.4 | 18.3 | [55] |
| MWCNT | 1050 °C, exposure time: 180 min, N2 atmosphere | 537 | 62.5 | [56] |
| MWCNT-S | - | 233.0 | 150.2 | [57] |
| Compact disc | Post-activation 940 °C, 1 °C min−1, exposure time: 480 min CO2 atmosphere | 1136.0 | 357.0 | [58] |
| Pequi almonds | Post-activation 800 °C, 5 °C min−1, | 1923.0 | 500.0 | [59] |
| GO-CNT/AC | Complex preparation (see [60]) | 1361.9 | 174.8 | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efimov, M.; Vasilev, A.; Muratov, D.; Panin, A.; Malozovskaya, M.; Karpacheva, G. Application of Infrared Pyrolysis and Chemical Post-Activation in the Conversion of Polyethylene Terephthalate Waste into Porous Carbons for Water Purification. Polymers 2024, 16, 891. https://doi.org/10.3390/polym16070891
Efimov M, Vasilev A, Muratov D, Panin A, Malozovskaya M, Karpacheva G. Application of Infrared Pyrolysis and Chemical Post-Activation in the Conversion of Polyethylene Terephthalate Waste into Porous Carbons for Water Purification. Polymers. 2024; 16(7):891. https://doi.org/10.3390/polym16070891
Chicago/Turabian StyleEfimov, Mikhail, Andrey Vasilev, Dmitriy Muratov, Alexander Panin, Maria Malozovskaya, and Galina Karpacheva. 2024. "Application of Infrared Pyrolysis and Chemical Post-Activation in the Conversion of Polyethylene Terephthalate Waste into Porous Carbons for Water Purification" Polymers 16, no. 7: 891. https://doi.org/10.3390/polym16070891
APA StyleEfimov, M., Vasilev, A., Muratov, D., Panin, A., Malozovskaya, M., & Karpacheva, G. (2024). Application of Infrared Pyrolysis and Chemical Post-Activation in the Conversion of Polyethylene Terephthalate Waste into Porous Carbons for Water Purification. Polymers, 16(7), 891. https://doi.org/10.3390/polym16070891







