Thermal-Robust Phenoxyimine Titanium Catalysts Bearing Bulky Sidearms for High Temperature Ethylene Homo-/Co- Polymerizations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
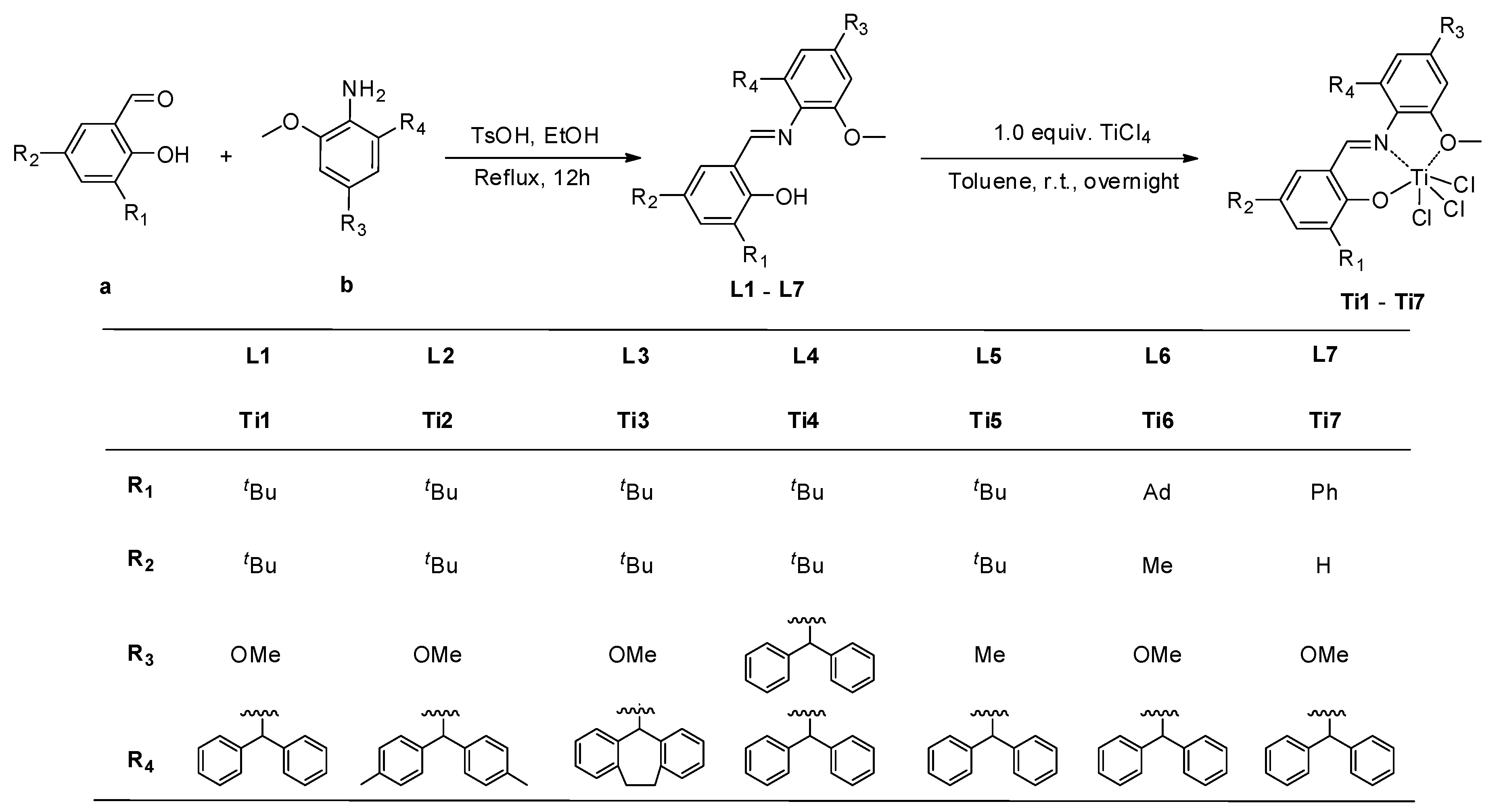
2.2. Synthesis of the Ligands and Complexes
2.2.1. Synthesis of Ligand L1
2.2.2. Synthesis of Ligand L2
2.2.3. Synthesis of Ligand L3
2.2.4. Synthesis of Ligand L4
2.2.5. Synthesis of Ligand L5
2.2.6. Synthesis of Ligand L6
2.2.7. Synthesis of Ligand L7
2.2.8. Synthesis of Ligand Ti1
2.2.9. Synthesis of Ligand Ti2
2.2.10. Synthesis of Ligand Ti3
2.2.11. Synthesis of Ligand Ti4
2.2.12. Synthesis of Ligand Ti5
2.2.13. Synthesis of Ligand Ti6
2.2.14. Synthesis of Ligand Ti7
2.3. DFT Calculation Details
3. Results and Discussion
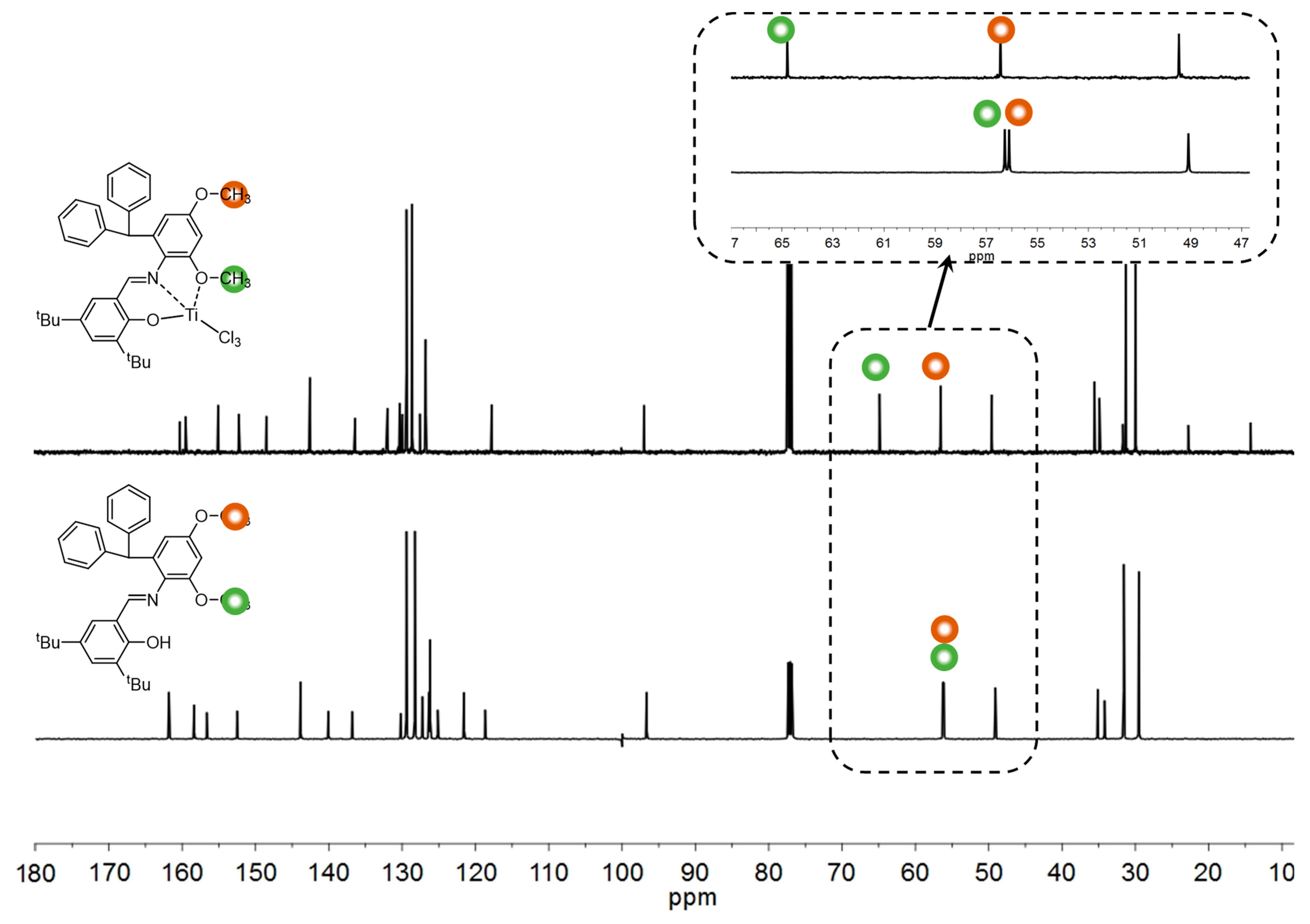
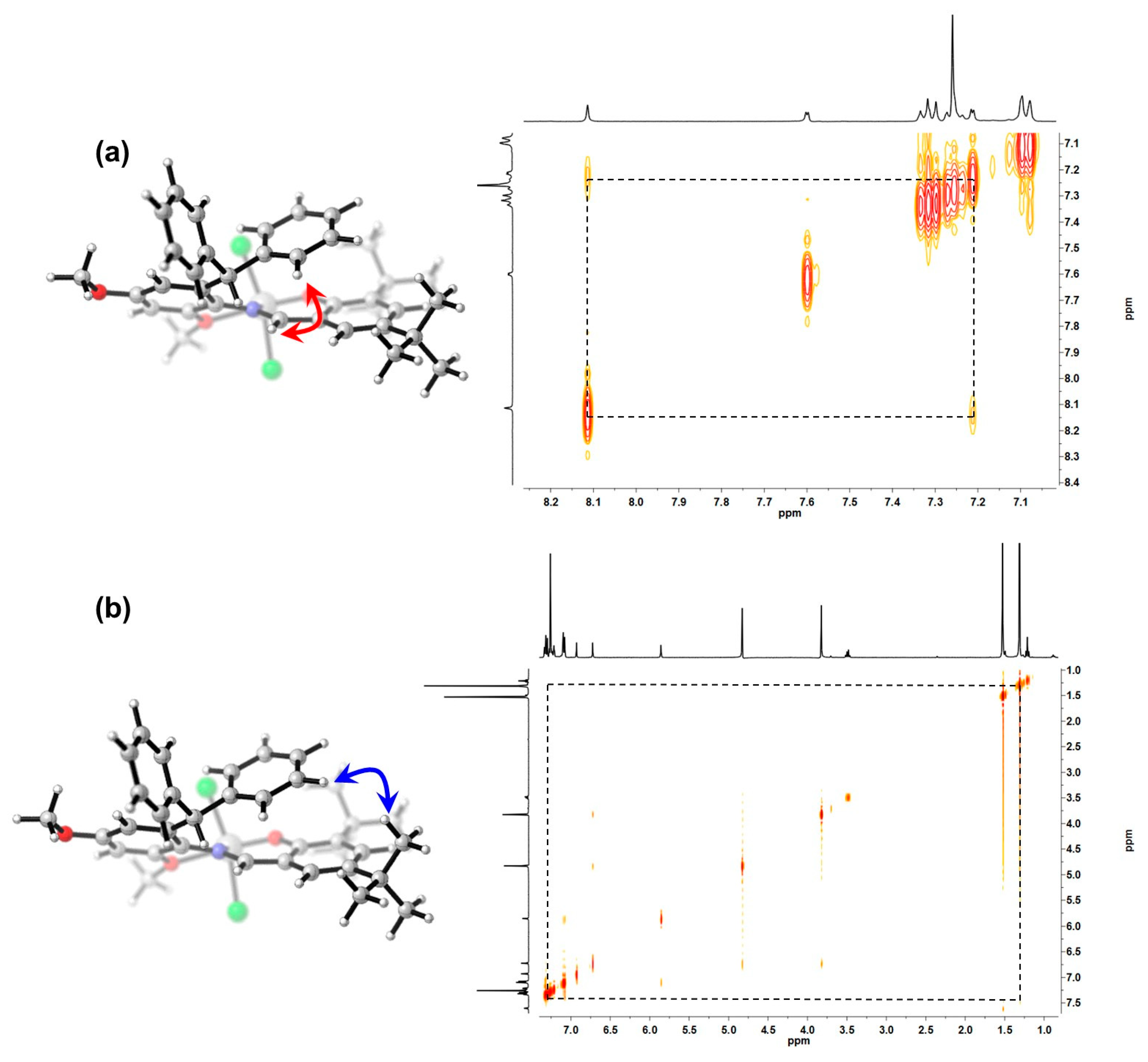
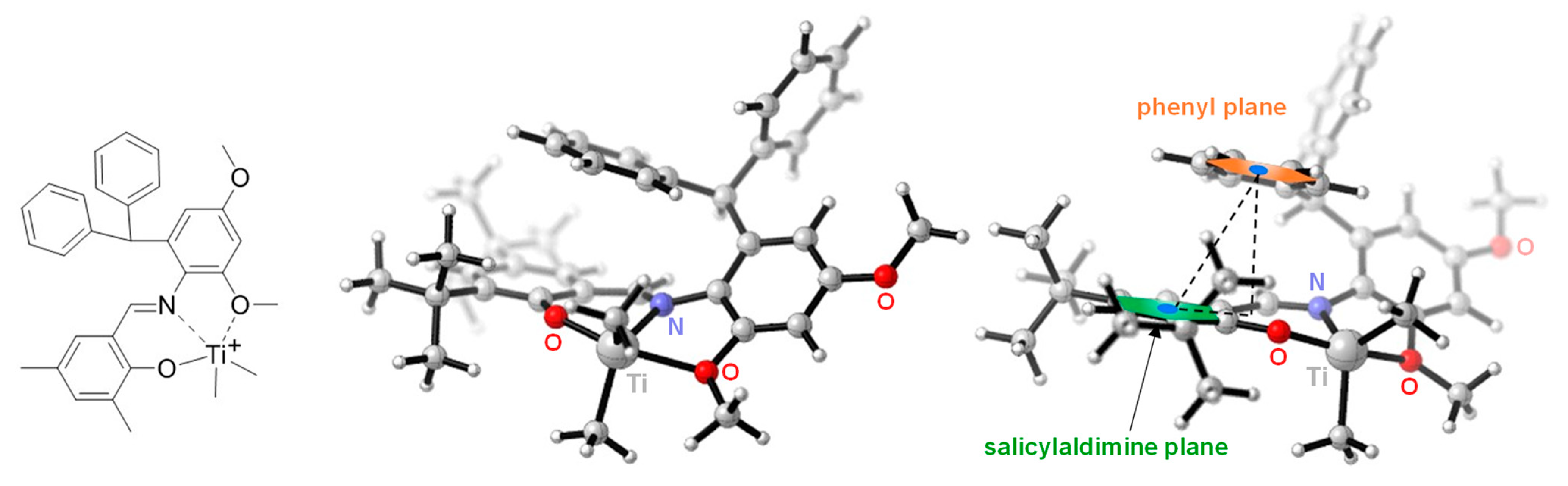
3.1. Synthesis and Characterization of the Titanium Complexes
3.2. High-Temperature Ethylene Polymerizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chum, P.S.; Swogger, K.W. Olefin polymer technologies—History and recent progress at The Dow Chemical Company. Prog. Polym. Sci. 2008, 33, 797–819. [Google Scholar] [CrossRef]
- Li, F.; Liu, W. Progress in the catalyst for ethylene/α-olefin copolymerization at high temperature. Can. J. Chem. Eng. 2023, 101, 4992–5019. [Google Scholar] [CrossRef]
- Park, J.H.; Do, S.H.; Cyriac, A.; Yun, H.; Lee, B.Y. Preparation of half-metallocenes of thiophene-fused and tetrahydroquinoline-linked cyclopentadienyl ligands for ethylene/α-olefin copolymerization. Dalton Trans. 2010, 39, 9994–10002. [Google Scholar] [CrossRef] [PubMed]
- Senda, T.; Hanaoka, H.; Nakahara, S.; Oda, Y.; Tsurugi, H.; Mashima, K. Rational design of silicon-bridged fluorenyl−phenoxy group 4 metal complexes as catalysts for producing high molecular weight copolymers of ethylene and 1-hexene at elevated temperature. Macromolecules 2010, 43, 2299–2306. [Google Scholar] [CrossRef]
- Chen, Y.-X.; Fu, P.-F.; Stern, C.L.; Marks, T.J. A Novel Phenolate “Constrained Geometry” Catalyst System. Efficient Synthesis, Structural Characterization, and α-Olefin Polymerization Catalysis. Organometallics 1997, 16, 5958–5963. [Google Scholar] [CrossRef]
- Cho, D.J.; Wu, C.J.; Sujith, S.; Han, W.-S.; Kang, S.O.; Lee, B.Y. o-Phenylene-Bridged Cp/Amido Titanium Complexes for Ethylene/1-Hexene Copolymerizations. Organometallics 2006, 25, 2133–2134. [Google Scholar] [CrossRef]
- Nabika, M.; Katayama, H.; Watanabe, T.; Kawamura-Kuribayashi, H.; Yanagi, K.; Imai, A. ansa-Cyclopentadienyl-Phenoxy Titanium(IV) Complexes (PHENICS): Synthesis, Characterization, and Catalytic Behavior in Olefin Polymerization. Organometallics 2009, 28, 3785–3792. [Google Scholar] [CrossRef]
- Wu, C.J.; Lee, S.H.; Yun, H.; Lee, B.Y. Ortho Lithiation of Tetrahydroquinoline Derivatives and Its Use for the Facile Construction of Polymerization Catalysts. Organometallics 2007, 26, 6685–6687. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, W.; Tao, X.; Wu, Q.; Mu, Y.; Ye, L. New Chromium(III) Complexes with Imine−Cyclopentadienyl Ligands: Synthesis, Characterization, and Catalytic Properties for Ethylene Polymerization. Organometallics 2011, 30, 433–440. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Liu, S.-R.; Li, B.-X.; Li, Y.-G.; Li, Y.-S. Synthesis and Characterization of Novel Half-Metallocene-Type Group IV Complexes Containing Phosphine Oxide–Phenolate Chelating Ligands and Their Application to Ethylene Polymerization. Organometallics 2011, 30, 4052–4059. [Google Scholar] [CrossRef]
- Wannaborworn, M.; Praserthdam, P.; Jongsomjit, B.; Cai, Z.; Yano, H.; Shiono, T. Copolymerization of Ethylene and 1-Hexene with Ansa-Dimethylsilylene(fluorenyl) (t-butylamido)Dimethyltitanium Complexes Activated by Modified Methylaluminoxane. Macromol. Chem. Phys. 2013, 214, 2584–2590. [Google Scholar] [CrossRef]
- Zhang, Y.; Mu, Y.; Lü, C.; Li, G.; Xu, J.; Zhang, Y.; Zhu, D.; Feng, S. Constrained Geometry Tetramethylcyclopentadienyl-phenoxytitanium Dichlorides: Template Synthesis, Structures, and Catalytic Properties for Ethylene Polymerization. Organometallics 2004, 23, 540–546. [Google Scholar] [CrossRef]
- Huang, W.; Li, B.; Wang, Y.; Zhang, W.; Wang, L.; Li, Y.; Sun, W.-H.; Redshaw, C. Synthesis, characterization and ethylene (co-)polymerization behavior of half-titanocene 2-(1-(arylimino)ethyl)quinolin-8-olate chlorides. Catal. Sci. Technol. 2011, 1, 1208–1215. [Google Scholar] [CrossRef]
- Braunschweig, H.; Breitling, F.M. Constrained geometry complexes—Synthesis and applications. Coord. Chem. Rev. 2006, 250, 2691–2720. [Google Scholar] [CrossRef]
- Stevens, J.C. Constrained geometry and other single site metallocene polyolefin catalysts: A revolution in olefin polymerization. In Studies in Surface Science and Catalysis; Hightower, J.W., Nicholas Delgass, W., Iglesia, E., Bell, A.T., Eds.; Elsevier: Amsterdam, The Netherlands, 1996; Volume 101, pp. 11–20. [Google Scholar]
- Cano, J.; Kunz, K. How to synthesize a constrained geometry catalyst (CGC)—A survey. J. Organomet. Chem. 2007, 692, 4411–4423. [Google Scholar] [CrossRef]
- Wang, W.-J.; Kolodka, E.; Zhu, S.; Hamielec, A.E. Continuous solution copolymerization of ethylene and octene-1 with constrained geometry metallocene catalyst. J. Polym. Sci. Polym. Chem. 1999, 37, 2949–2957. [Google Scholar] [CrossRef]
- Wang, W.-j.; Yan, D.; Charpentier, P.A.; Zhu, S.; Hamielec, A.E.; Sayer, B.G. Long chain branching in ethylene polymerization using constrained geometry metallocene catalyst. Macromol. Chem. Phys. 1998, 199, 2409–2416. [Google Scholar] [CrossRef]
- van Doremaele, G.; van Duin, M.; Valla, M.; Berthoud, A. On the development of titanium κ1-Amidinate Complexes, Commercialized as Keltan ACE™ Technology, Enabling the Production of an Unprecedented Large Variety of EPDM Polymer Structures. J. Polym. Sci. Polym. Chem. 2017, 55, 2877–2891. [Google Scholar] [CrossRef]
- Fontaine, P.P.; Klosin, J.; McDougal, N.T. Hafnium Amidoquinoline Complexes: Highly Active Olefin Polymerization Catalysts with Ultrahigh Molecular Weight Capacity. Organometallics 2012, 31, 6244–6251. [Google Scholar] [CrossRef]
- Fontaine, P.P.; Ueligger, S.; Klosin, J.; Hazari, A.; Daller, J.; Hou, J. Development of Improved Amidoquinoline Polyolefin Catalysts with Ultrahigh Molecular Weight Capacity. Organometallics 2015, 34, 1354–1363. [Google Scholar] [CrossRef]
- De Waele, P.; Jazdzewski, B.A.; Klosin, J.; Murray, R.E.; Theriault, C.N.; Vosejpka, P.C.; Petersen, J.L. Synthesis of Hafnium and Zirconium Imino−Amido Complexes from Bis-imine Ligands. A New Family of Olefin Polymerization Catalysts. Organometallics 2007, 26, 3896–3899. [Google Scholar] [CrossRef]
- Froese, R.D.J.; Jazdzewski, B.A.; Klosin, J.; Kuhlman, R.L.; Theriault, C.N.; Welsh, D.M.; Abboud, K.A. Imino-Amido Hf and Zr Complexes: Synthesis, Isomerization, and Olefin Polymerization. Organometallics 2011, 30, 251–262. [Google Scholar] [CrossRef]
- Figueroa, R.; Froese, R.D.; He, Y.; Klosin, J.; Theriault, C.N.; Abboud, K.A. Synthesis of Imino-Enamido Hafnium and Zirconium Complexes: A New Family of Olefin Polymerization Catalysts with Ultrahigh-Molecular-Weight Capabilities. Organometallics 2011, 30, 1695–1709. [Google Scholar] [CrossRef]
- Fontaine, P.P.; Figueroa, R.; McCann, S.D.; Mort, D.; Klosin, J. Synthesis and Scale-up of Imino–Enamido Hafnium and Zirconium Olefin Polymerization Catalysts. Organometallics 2013, 32, 2963–2972. [Google Scholar] [CrossRef]
- Szuromi, E.; Klosin, J.; Abboud, K.A. Aminotroponiminato Hafnium and Zirconium Complexes: Synthesis and Ethylene/1-Octene Copolymerization Study. Organometallics 2011, 30, 4589–4597. [Google Scholar] [CrossRef]
- Frazier, K.A.; Boone, H.; Vosejpka, P.C.; Stevens, J.C. High Activity Transition Metal Complex Polymerization Catalyst and process of Polymerizing Olefins. U.S. Patent US20040220050, 4 November 2004. [Google Scholar]
- Boussie, T.R.; Diamond, G.M.; Goh, C.; Hall, K.A.; LaPointe, A.M.; Leclerc, M.K.; Murphy, V.; Shoemaker, J.A.W.; Turner, H.; Rosen, R.K.; et al. Nonconventional Catalysts for Isotactic Propene Polymerization in Solution Developed by Using High-Throughput-Screening Technologies. Angew. Chem. Int. Ed. 2006, 45, 3278–3283. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, S.; Yang, W.; Liu, S. Hafnium and Zirconium Complexes Bearing SNN-Ligands Enhancing Catalytic Performances toward Ethylene/1-Octene Copolymerization. Organometallics 2022, 41, 3724–3731. [Google Scholar] [CrossRef]
- Froese, R.D.J.; Hustad, P.D.; Kuhlman, R.L.; Wenzel, T.T. Mechanism of Activation of a Hafnium Pyridyl−Amide Olefin Polymerization Catalyst: Ligand Modification by Monomer. J. Am. Chem. Soc. 2007, 129, 7831–7840. [Google Scholar] [CrossRef]
- Domski, G.J.; Lobkovsky, E.B.; Coates, G.W. Polymerization of α-Olefins with Pyridylamidohafnium Catalysts: Living Behavior and Unexpected Isoselectivity from a Cs-Symmetric Catalyst Precursor. Macromolecules 2007, 40, 3510–3513. [Google Scholar] [CrossRef]
- Shang, R.; Gao, H.; Luo, F.; Li, Y.; Wang, B.; Ma, Z.; Pan, L.; Li, Y. Functional Isotactic Polypropylenes via Efficient Direct Copolymerizations of Propylene with Various Amino-Functionalized α-Olefins. Macromolecules 2019, 52, 9280–9290. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Shi, X.; Liu, J.; Chen, C.; Li, Y. Syntheses of Well-Defined Functional Isotactic Polypropylenes via Efficient Copolymerization of Propylene with ω-Halo-α-alkenes by Post-metallocene Hafnium Catalyst. Macromolecules 2014, 47, 552–559. [Google Scholar] [CrossRef]
- Zhao, R.; Liu, T.; Wang, L.; Ma, H. High temperature ethylene polymerization catalyzed by titanium(iv) complexes with tetradentate aminophenolate ligands in cis-O, N, N chelating mode. Dalton Trans. 2014, 43, 12663–12677. [Google Scholar] [CrossRef]
- Wan, D.-W.; Chen, Z.; Gao, Y.-S.; Shen, Q.; Sun, X.-L.; Tang, Y. Synthesis and characterization of tridentate [O−N(H)X] titanium complexes and their applications in olefin polymerization. J. Polym. Sci. Polym. Chem. 2013, 51, 2495–2503. [Google Scholar] [CrossRef]
- Li, A.; Ma, H.; Huang, J. Highly Thermally Stable Eight-Coordinate Dichloride Zirconium Complexes Supported by Tridentate [ONN] Ligands: Syntheses, Characterization, and Ethylene Polymerization Behavior. Organometallics 2013, 32, 7460–7469. [Google Scholar] [CrossRef]
- Ishii, S.-I.; Mitani, M.; Saito, J.; Matsuura, S.; Furuyama, R.; Fujita, T. 6 Ethylene polymerization behavior of polymethylene-bridged bis (phenoxy-imine) Zr complexes. In Studies in Surface Science and Catalysis; Anpo, M., Onaka, M., Yamashita, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 145, pp. 49–54. [Google Scholar]
- Wang, C.; Ma, Z.; Sun, X.-L.; Gao, Y.; Guo, Y.-H.; Tang, Y.; Shi, L.-P. Synthesis and Characterization of Titanium(IV) Complexes Bearing Monoanionic [O-NX] (X = O, S, Se) Tridentate Ligands and Their Behaviors in Ethylene Homo- and Copolymerizaton with 1-Hexene. Organometallics 2006, 25, 3259–3266. [Google Scholar] [CrossRef]
- Arriola, D.J.; Carnahan, E.M.; Hustad, P.D.; Kuhlman, R.L.; Wenzel, T.T. Catalytic production of olefin block copolymers via chain shuttling polymerization. Science 2006, 312, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, T.; Arriola, D.; Carnahan, E.; Hustad, P.; Kuhlman, R. Chain shuttling catalysis and olefin block copolymers (OBCs). In Metal Catalysts in Olefin Polymerization; Guan, Z., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 26, pp. 65–104. [Google Scholar]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Dolg, M.; Wedig, U.; Stoll, H.; Preuss, H. Energy-adjusted ab initio pseudopotentials for the first row transition elements. J. Chem. Phys. 1987, 86, 866–872. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Akkurt, M.; Karaca, S.; Jarrahpour, A.A.; Rezaei, S.; Buyukgungor, O. (E)-2-[1-(2,4-Dimethoxyphenylimino)ethyl]phenol. Acta Crystallogr., Sect. E Struct. Rep. Online 2007, 63, o3597. [Google Scholar] [CrossRef]
- Taneda, M.; Amimoto, K.; Koyama, H.; Kawato, T. Photochromism of polymorphic 4,4′-methylenebis(N-salicylidene-2,6-diisopropylaniline) crystals. Org. Biomol. Chem. 2004, 2, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Filarowski, A.; Glowiaka, T.; Koll, A.; Mukherjee, S. Strengthening of the intramolecular hydrogen bond in 7-ethylsalicylidene aniline due to steric repulsion. J. Mol. Struct. 2002, 577, 153–159. [Google Scholar] [CrossRef]
- Sutradhar, M.; Pombeiro, A.J.L. π–π Interaction Directed Applications of Metal Complexes. In Non-Covalent Interactions in the Synthesis and Design of New Compounds; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 101–114. [Google Scholar]
- Cordier, A.; Breuil, P.-A.; Michel, T.; Magna, L.; Olivier-Bourbigou, H.; Raynaud, J.; Boisson, C.; Monteil, V. Titanium-based phenoxy-imine catalyst for selective ethylene trimerization: Effect of temperature on the activity, selectivity and properties of polymeric side products. Catal. Sci. Technol. 2020, 10, 1602–1608. [Google Scholar] [CrossRef]
- Wang, W.; Fan, Z.-Q.; Feng, L.-X.; Li, C.-H. Substituent effect of bisindenyl zirconene catalyst on ethylene/1-hexene copolymerization and propylene polymerization. Eur. Polym. J. 2005, 41, 83–89. [Google Scholar] [CrossRef]





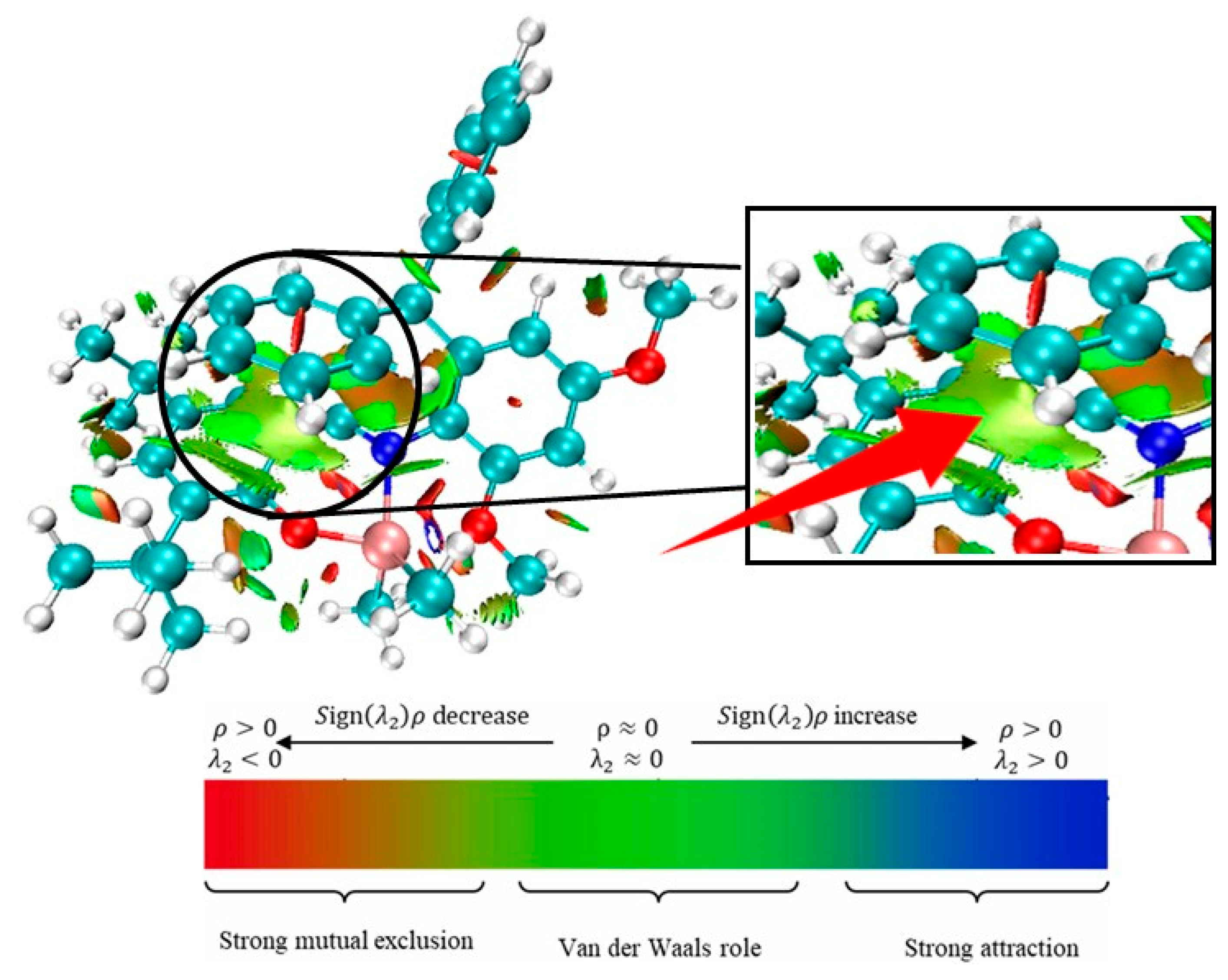
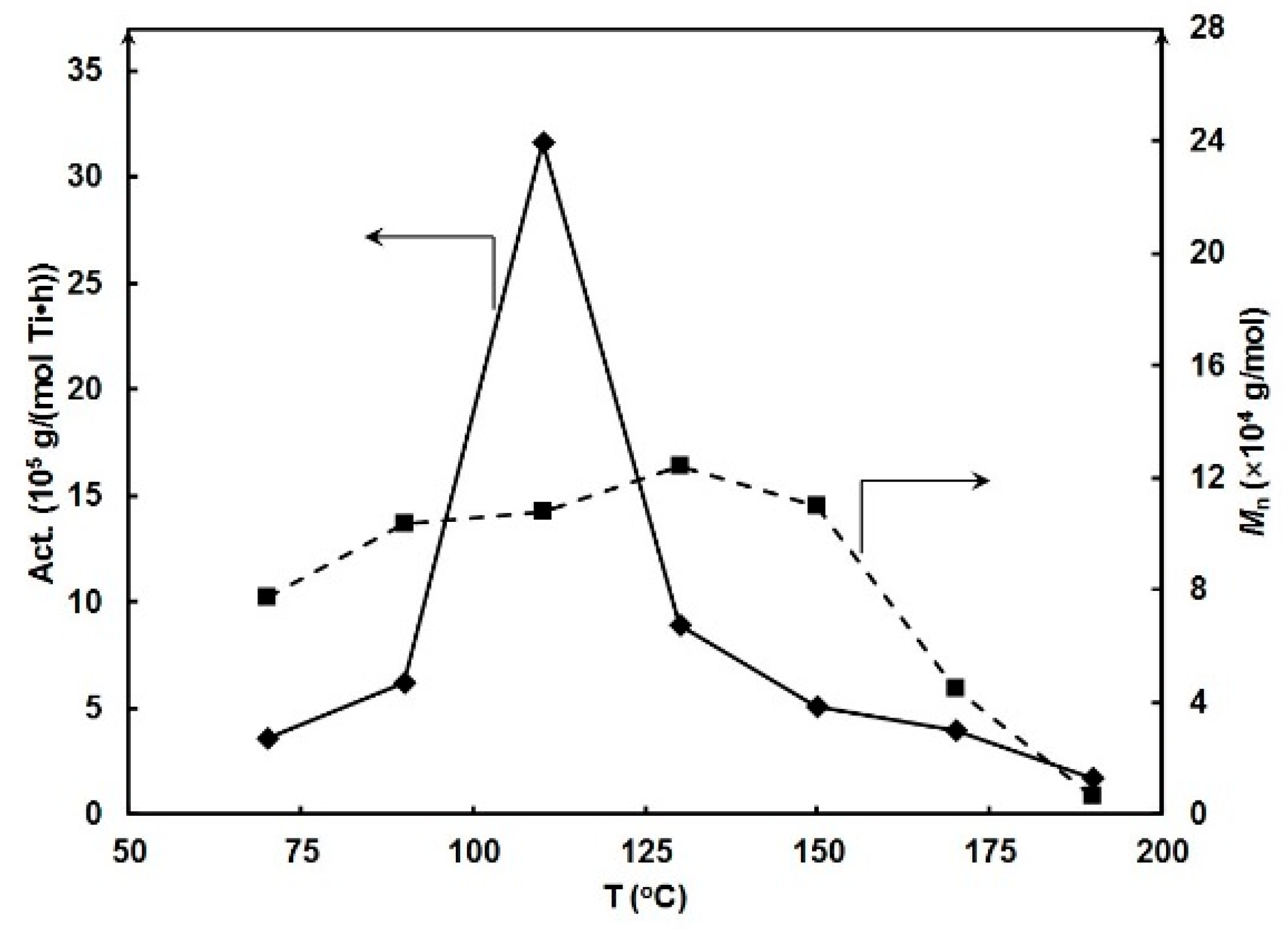

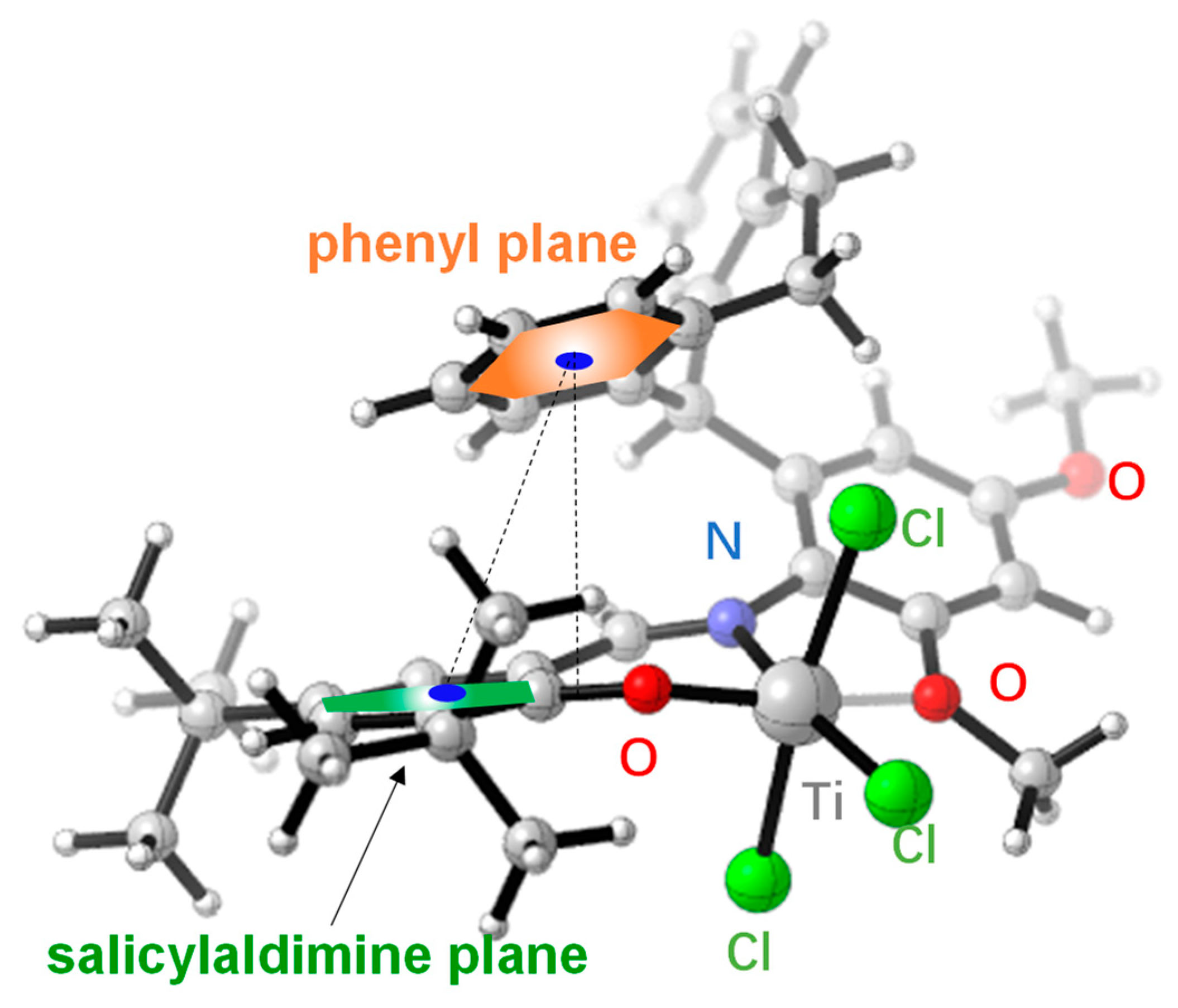

| Entry | T (°C) | Press. (atm) | MAO/Ti | Yield (g) | Act. b | Mn c | Mw c | PDI c | Tm (°C) d |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 70 | 20 | 1000 | 0.045 | 3.60 | 7.74 | 46.79 | 2.86 | 140.0 |
| 2 | 90 | 20 | 1000 | 0.077 | 6.16 | 10.33 | 46.39 | 4.49 | 140.9 |
| 3 | 110 | 20 | 1000 | 0.395 | 31.60 | 10.75 | 47.52 | 4.42 | 140.0 |
| 4 | 130 | 20 | 1000 | 0.112 | 8.96 | 12.44 | 48.55 | 3.90 | 139.5 |
| 5 | 150 | 20 | 1000 | 0.064 | 5.12 | 11.02 | 39.83 | 3.62 | 136.6 |
| 6 | 170 | 20 | 1000 | 0.049 | 3.92 | 4.48 | 42.55 | 4.58 | 133.0 |
| 7 | 190 | 20 | 1000 | 0.022 | 1.76 | 0.63 | 0.73 | 1.17 | 132.6 |
| 8 | 110 | 20 | 500 | 0.005 | 0.40 | - | - | - | 130.6 |
| 9 | 110 | 20 | 800 | 0.084 | 6.72 | 6.75 | 19.04 | 2.82 | 136.0 |
| 10 | 110 | 15 | 1000 | 0.180 | 14.4 | 11.96 | 48.15 | 4.03 | 136.0 |
| 11 | 110 | 10 | 1000 | 0.032 | 2.56 | 4.14 | 11.74 | 2.84 | 134.5 |
| 12 | 110 | 8 | 1000 | 0.029 | 2.32 | 5.57 | 16.52 | 2.96 | 141.4 |
| 13 | 110 | 5 | 1000 | 0.019 | 1.52 | 3.19 | 11.66 | 3.65 | 137.9 |
| Entry | Cat. | T (°C) | MAO/Ti | Yield (g) | Act. b | Mn c | Mw c | PDI c | Tm (°C) d |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Ti1 | 90 | 1000 | 0.077 | 6.16 | 10.33 | 10.33 | 4.49 | 140.9 |
| 2 | 110 | 1000 | 0.395 | 31.60 | 10.75 | 10.75 | 4.42 | 140.0 | |
| 3 | 130 | 1000 | 0.112 | 8.96 | 12.44 | 12.44 | 3.90 | 139.5 | |
| 4 | 150 | 1000 | 0.064 | 5.12 | 11.02 | 11.02 | 3.62 | 136.6 | |
| 5 | Ti2 | 90 | 1000 | 0.047 | 3.76 | 17.94 | 17.94 | 3.94 | 136.4 |
| 6 | 110 | 1000 | 0.065 | 5.20 | 10.60 | 46.12 | 4.35 | 136.1 | |
| 7 | 130 | 1000 | 0.048 | 3.84 | 8.56 | 37.00 | 4.32 | 132.6 | |
| 8 | 150 | 1000 | 0.044 | 3.52 | 3.01 | 26.18 | 8.47 | 132.1 | |
| 9 | Ti3 | 90 | 1000 | 0.045 | 3.60 | 11.48 | 30.41 | 2.65 | 138.7 |
| 10 | 110 | 1000 | 0.105 | 8.40 | 10.20 | 35.02 | 3.44 | 138.6 | |
| 11 | 130 | 1000 | 0.038 | 3.04 | 12.39 | 40.21 | 3.25 | 138.1 | |
| 12 | 150 | 1000 | 0.022 | 1.76 | 5.62 | 46.15 | 8.21 | 138.8 | |
| 13 | Ti4 | 90 | 1000 | 0.147 | 11.76 | 9.24 | 18.16 | 1.97 | 141.1 |
| 14 | 110 | 1000 | 0.394 | 31.52 | 6.16 | 16.05 | 2.60 | 140.1 | |
| 15 | 130 | 1000 | 0.055 | 4.40 | 6.78 | 34.21 | 5.05 | 138.5 | |
| 16 | 150 | 1000 | 0.037 | 2.96 | 7.29 | 34.20 | 4.69 | 137.6 | |
| 17 | Ti5 | 90 | 1000 | 0.100 | 8.00 | 10.12 | 34.46 | 3.41 | 139.9 |
| 18 | 110 | 1000 | 0.416 | 33.28 | 21.59 | 60.03 | 2.78 | 139.7 | |
| 19 | 130 | 1000 | 0.188 | 15.04 | 14.54 | 45.41 | 3.12 | 138.7 | |
| 20 | 150 | 1000 | 0.130 | 10.04 | 15.38 | 47.74 | 3.11 | 138.1 | |
| 21 | Ti6 | 90 | 1000 | 0.042 | 3.36 | 1.01 | 1.26 | 1.12 | 138.7 |
| 22 | 110 | 1000 | 0.122 | 9.76 | 8.53 | 37.76 | 4.42 | 138.7 | |
| 23 | 130 | 1000 | 0.109 | 8.72 | 6.69 | 28.09 | 4.25 | 138.1 | |
| 24 | 150 | 1000 | 0.088 | 7.04 | 5.17 | 27.32 | 5.28 | 137.9 | |
| 25 | Ti7 | 90 | 1000 | 0.035 | 2.80 | 8.96 | 28.31 | 3.16 | 139.4 |
| 26 | 110 | 1000 | 0.160 | 12.8 | 15.87 | 39.04 | 2.46 | 138.8 | |
| 27 | 130 | 1000 | 0.091 | 7.28 | 8.74 | 32.92 | 3.77 | 138.1 | |
| 28 | 150 | 1000 | 0.016 | 1.28 | 1.81 | 16.59 | 9.15 | 138.0 |
| Entry | Cat. | 1-Oct. (mol/L) | Yield (g) | Act. b | Mn c | Mw c | PDI c | Tm (°C) d | χ (%) e |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Ti1 | 0.254 | 0.126 | 10.08 | 6.61 | 17.68 | 2.67 | 131.4 | 4.1 |
| 2 | Ti2 | 0.254 | 0.160 | 12.80 | 6.45 | 18.26 | 2.83 | 129.9 | 4.9 |
| 3 | Ti3 | 0.254 | 0.040 | 3.20 | 4.99 | 16.21 | 3.25 | 127.9 | 5.3 |
| 4 | Ti4 | 0.254 | 0.158 | 12.6 | 5.91 | 18.68 | 3.16 | 130.5 | 4.5 |
| 5 | Ti5 | 0.254 | 0.118 | 9.44 | 11.79 | 65.68 | 5.57 | 130.5 | 4.5 |
| 6 | Ti6 | 0.254 | 0.134 | 10.72 | 6.20 | 17.20 | 2.77 | 133.9 | 4.0 |
| 7 | Ti7 | 0.254 | 0.040 | 3.20 | 17.07 | 96.30 | 5.64 | 128.9 | 5.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Ma, L.; Wang, X.; Zhao, W.; Liu, H.; Zhang, X.; Wang, F. Thermal-Robust Phenoxyimine Titanium Catalysts Bearing Bulky Sidearms for High Temperature Ethylene Homo-/Co- Polymerizations. Polymers 2024, 16, 902. https://doi.org/10.3390/polym16070902
Wang X, Ma L, Wang X, Zhao W, Liu H, Zhang X, Wang F. Thermal-Robust Phenoxyimine Titanium Catalysts Bearing Bulky Sidearms for High Temperature Ethylene Homo-/Co- Polymerizations. Polymers. 2024; 16(7):902. https://doi.org/10.3390/polym16070902
Chicago/Turabian StyleWang, Xin, Lishuang Ma, Xiaohua Wang, Wenpeng Zhao, Heng Liu, Xuequan Zhang, and Feng Wang. 2024. "Thermal-Robust Phenoxyimine Titanium Catalysts Bearing Bulky Sidearms for High Temperature Ethylene Homo-/Co- Polymerizations" Polymers 16, no. 7: 902. https://doi.org/10.3390/polym16070902






