The Influence of Initiators, Particle Size and Composition on the Electrokinetic Potential of N-(Isopropyl)acrylamide Derivatives
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Particle Synthesis
2.2.2. Hydrodynamic Diameter Measurements
2.2.3. Zeta Potential and pH Measurements
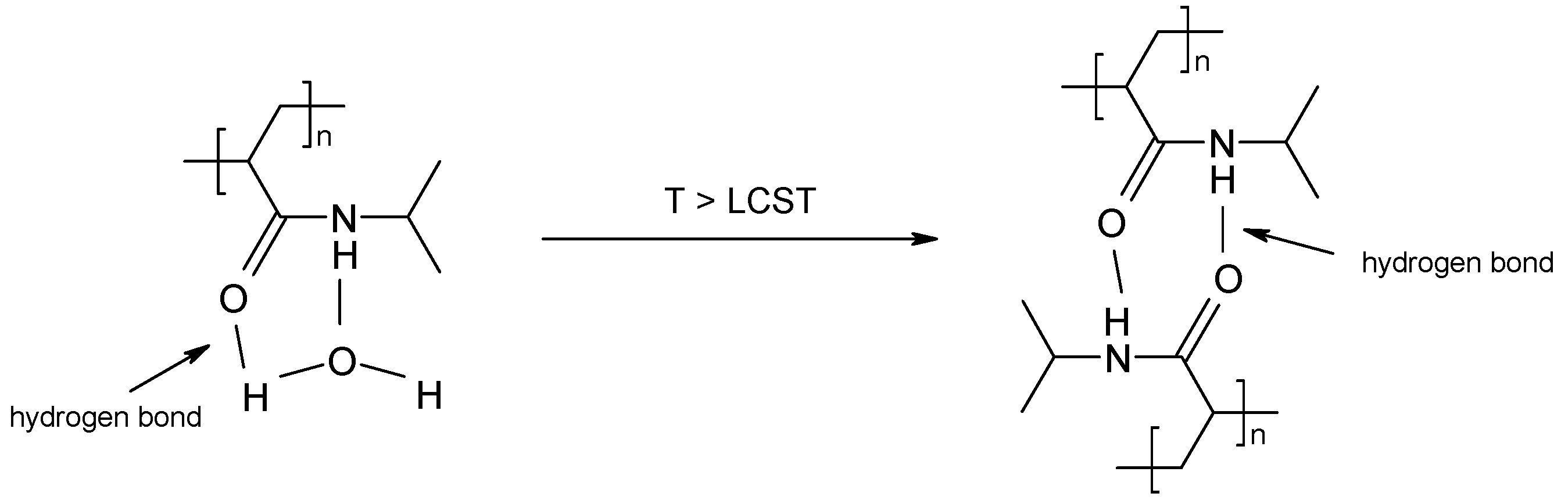
2.2.4. Volume Phase Transition Temperature
3. Results
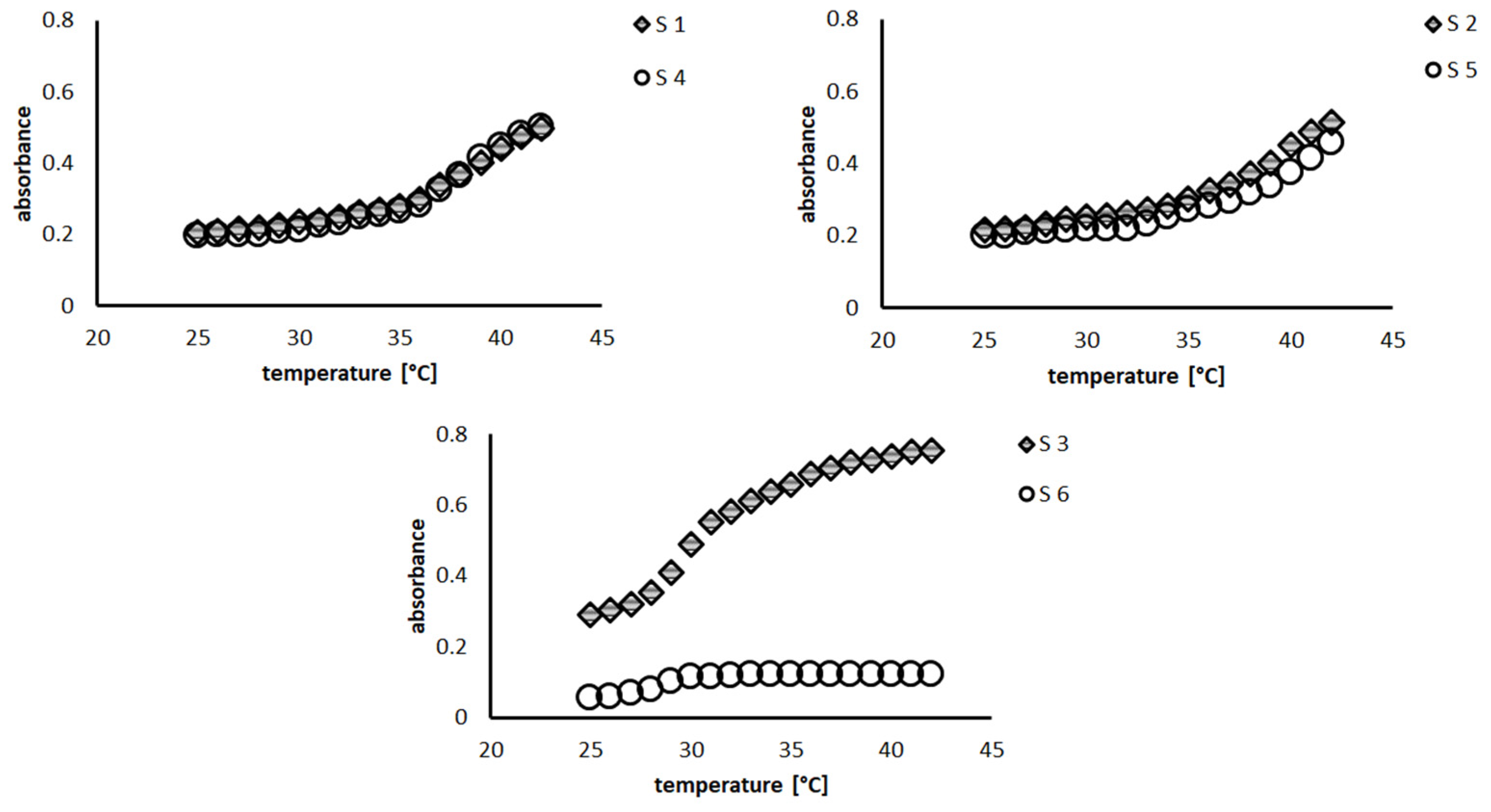
3.1. Hydrodynamic Diameter and Volume Phase Transition Temperature Evaluations
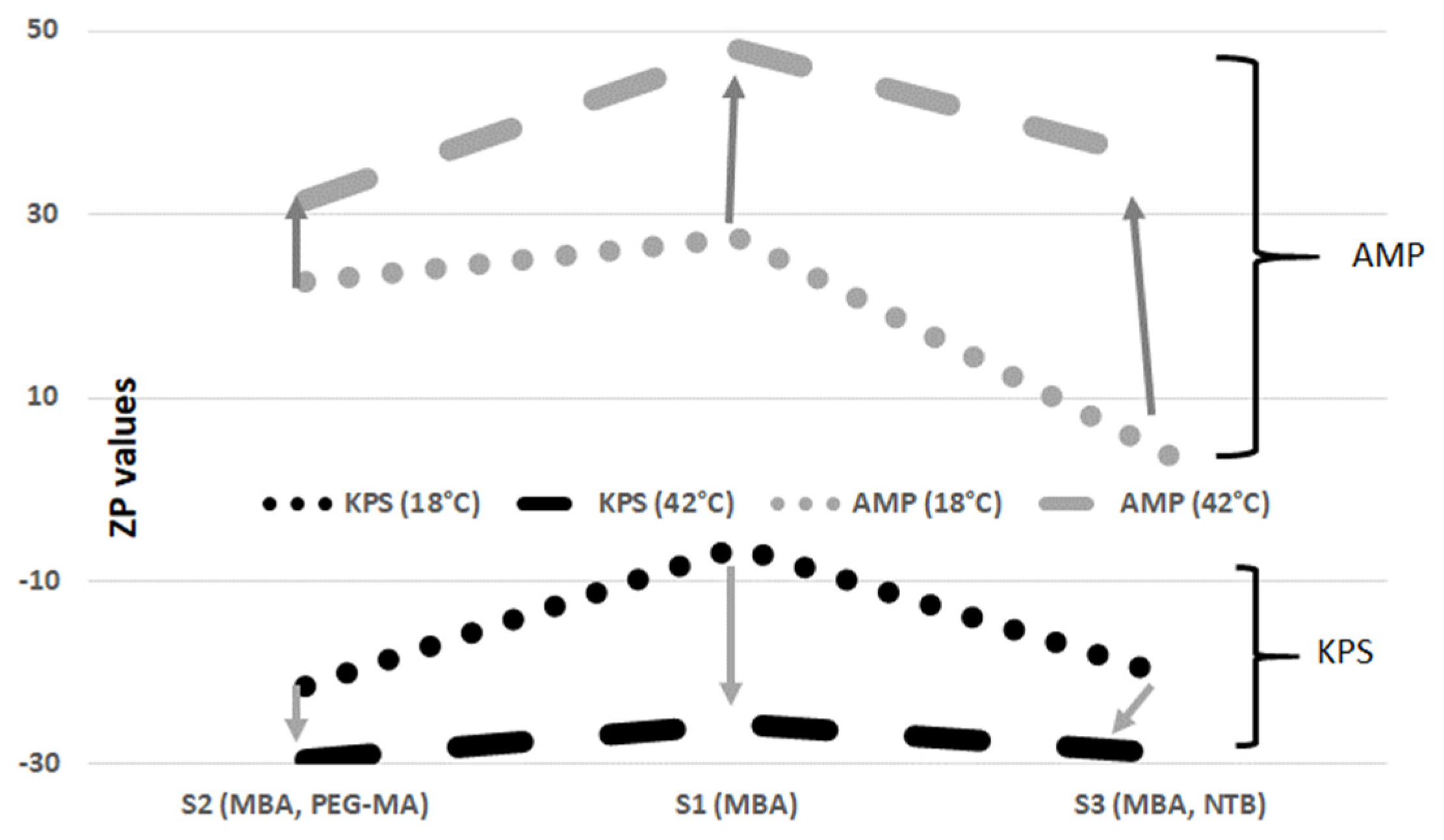
3.2. Zeta Potential and pH Measurements
4. Discussion
- z—Zeta potential;
- UE—Electrophoretic mobility;
- Ɛ—Dielectric constant;
- η—Viscosity.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Priya James, H.; Rijo, J.; Anju, A.; Anoop, K.R. Smart polymers for the controlled delivery of drugs—A concise overview. Acta Pharm. Sin. B 2014, 4, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.; Shen, J.; Duan, P.; Xia, X.; Chen, R.; Jin, B. Temperature-Sensitive Poly(N-isopropylacrylamide)/Konjac Glucomannan/Graphene Oxide Composite Membranes with Improved Mechanical Property, Swelling Capability, and Degradability. Int. J. Polym. Sci. 2018, 10, 7906747. [Google Scholar] [CrossRef]
- Bordat, A.; Boissenot, T.; Nicolas, J.; Tsapis, N. Thermoresponsive polymer nanocarriers for biomedical applications. Adv. Drug Deliv. Rev. 2019, 138, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Rok, J.; Kim, J.H. Biodegradable Thermo-and pH-Respon-sive Hydrogels Based on Amphiphilic Poly-aspartamide Derivatives Containing N,N-Diisopropylamine Pendants. Macromol. Res. 2008, 16, 489–491. [Google Scholar]
- Tamer, T.M.; Omer, A.M.; Hassan, M.A.; Hassan, M.E.; Sabet, M.M.; Mohy Eldin, M.S. Development of thermo-sensitive poly N-isopropyl acrylamide grafted chitosan derivatives. J. Appl. Pharm. Sci. 2015, 5, 001–006. [Google Scholar] [CrossRef]
- Cardoso, A.M.; Calejo, T.; Morais, C.M.; Cardoso, A.L.; Cruz, R.; Zhu, K.; Pedroso de Lima, M.C.; Jurado, A.S.; Nyström, B. Application of Thermoresponsive PNIPAAM-b-PAMPTMA Diblock Copolymers in siRNA Delivery. Mol. Pharm. 2014, 11, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-L.; Yang, X.-L.; Xu, F.; Chen, Y.S.; Zhang, B. Thermosensitive PNIPAM-b-HTPB block copolymer micelles: Molecular architectures and camptothecin drug release. Colloids Surf. B 2014, 114, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Krakovský, I.; Kouřilová, H.; Hrubovský, M.; Labuta, J.; Hanyková, L. Thermoresponsive double network hydrogels composed of poly(N-isopropylacrylamide) and polyacrylamide. Eur. Polym. J. 2019, 116, 415–424. [Google Scholar] [CrossRef]
- Bawa, P.; Pillay, V.; Choonara, Y.E.; Du Toit, L.C. Stimuli-responsive polymers and their applications in drug delivery. Biomed. Mater. 2009, 4, 022001. [Google Scholar] [CrossRef]
- Wei, M.; Gao, Y.; Li, X.; Serpe, M.J. Stimuli-responsive polymers and their applications. Polym. Chem. 2017, 8, 127–143. [Google Scholar] [CrossRef]
- Chu, L.Y.; Niitsuma, T.; Yamaguchi, T.; Nakao, S. Thermoresponsive transport through porous membranes with grafted PNIPAM gates. AIChE J. 2003, 49, 896–909. [Google Scholar] [CrossRef]
- Abdelaty, M.S.A.; Kuckling, D. Poly (N-Isopropyl Acrylamide-Co-Vanillin Acrylate) Dual Responsive Functional Copolymers for Grafting Biomolecules by Schiff’s Base Click Reaction. Open J. Org. Polym. Mater. 2018, 8, 15–32. [Google Scholar] [CrossRef]
- Su, G.; Zhou, T.; Liu, X.; Zhang, Y. Two-step volume phase transition mechanism of poly(N-vinylcaprolactam) hydrogel online-tracked by two-dimensional correlation spectroscopy. Phys. Chem. Chem. Phys. 2017, 19, 27221–27232. [Google Scholar] [CrossRef] [PubMed]
- Sanson, N.; Rieger, J. Synthesis of nanogels/microgels by conventional and controlled radical crosslinking copolymerization. Polym. Chem. 2010, 7, 965–977. [Google Scholar] [CrossRef]
- Cors, M.; Wiehemeier, L.; Oberdisse, J.; Hellweg, T. Deuteration-Induced Volume Phase Transition Temperature Shift of PNIPMAM Microgels. Polymers 2019, 11, 620. [Google Scholar] [CrossRef] [PubMed]
- Shymborska, Y.; Budkowski, A.; Raczkowska, J.; Donchak, V.; Melnyk, Y.; Vasiichuk, V.; Stetsyshyn, Y. Switching it Up: The Promise of Stimuli-Responsive Polymer Systemsin Biomedical Science. Chem. Rec. 2024, 24, e202300217. [Google Scholar] [CrossRef] [PubMed]
- Işikver, Y.; Saraydin, D. Environmentally sensitive hydrogels: N-isopropyl acrylamide/Acrylamide/ Mono-, Di-, Tricarboxylic acid crosslinked polymers. Polym. Eng. Sci. 2015, 55, 843–851. [Google Scholar] [CrossRef]
- Su, W.; Yang, M.; Zhao, K.; Ngai, T. Influence of Charged Groups on the Structure of Microgel and Volume Phase Transition by Dielectric Analysis. Macromolecules 2016, 49, 7997–8008. [Google Scholar] [CrossRef]
- Jia, D.; Muthukumar, M.; Cheng, H.; Han, C.C.; Hammouda, B. Concentration Fluctuations near Lower Critical Solution Temperature in Ternary Aqueous Solutions. Macromolecules 2017, 50, 7291–7298. [Google Scholar] [CrossRef]
- Ma, X.; Xi, J.; Huang, X.; Zhao, X.; Tang, X. Novel hydrophobically modified temperature-sensitive microgels with tunable volume-phase transition temperature. Mater. Lett. 2004, 58, 3400–3404. [Google Scholar] [CrossRef]
- Constantin, M.; Cristea, M.; Ascenzi, P.; Fundueanu, G. Lower critical solution temperature versus volume phase transition temperature in thermoresponsive drug delivery systems. Express Polym. Lett. 2011, 5, 839–848. [Google Scholar] [CrossRef]
- Burmistrova, A.; Steitz, R.; Von Klitzing, R. Temperature Response of PNIPAM Derivatives at Planar Surfaces: Comparison between Polyelectrolyte Multilayers and Adsorbed Microgels. Chem. Phys. Chem. 2010, 11, 3571–3579. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.L.; Wu, G.S.; Liao, B.Q. Zeta potential of shape-controlled TiO2 nanoparticles with surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2009, 348, 270–275. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Choi, S.M.; Kline, S.R. Polymerized Rodlike Nanoparticles with Controlled Surface Charge Density. Langmuir 2006, 22, 2844–2850. [Google Scholar] [CrossRef] [PubMed]
- Yalçın, T.; Alemdar, A.; Ece, Ö.; Güngör, N. The viscosity and zeta potential of bentonite dispersions in presence of anionic surfactants. Mater. Lett. 2002, 57, 420–424. [Google Scholar] [CrossRef]
- Carneiro-Da-Cunha, M.G.; Cerqueira, M.A.; Souza, B.W.S.; Teixeira, J.A.; Vicente, A.A. Influence of concentration, ionic strength and pH on zeta potential and mean hydrodynamic diameter of edible polysaccharide solutions envisaged for multinanolayered films production. Carbohydr. Polym. 2011, 85, 522–528. [Google Scholar] [CrossRef]
- Tian, H.; Li, F.; Chen, J.; Huang, Y.; Chen, X. N-Isopropylacrylamide-Modified Polyethylenimines as Effective Gene Carriers. Macromol. Biosci. 2012, 12, 1680–1688. [Google Scholar] [CrossRef] [PubMed]
- Gasztych, M.; Valh, J.V.; Kokol, V.; Szumny, A.J.; Gola, A.; Musiał, W. Synthesis, evaluation and release studies of NIPA nanopolymers presumed for temperature-controlled drug delivery. J. Sol-Gel Sci. Technol. 2016, 79, 466–474. [Google Scholar] [CrossRef]
- Gasztych, M.; Gola, A.; Kobryń, J.; Musiał, W. Synthesis and Formulation of Thermosensitive Drug Carrier for Temperature Triggered Delivery of Naproxen Sodium. Molecules 2016, 21, 1473. [Google Scholar] [CrossRef]
- Gola, A.; Gasztych, M.; Kokol, V.; Malamis, A.; Mucha, I.; Musia∤, W. Effect of Selected Comonomers on the Transition Temperature of Thermosensitive NIPA Derivatives Synthesized with an Anionic Initiator. J. Nanosci. Nanotechnol. 2019, 19, 3049–3056. [Google Scholar] [CrossRef]
- Gasztych, M.; Kotowska, A.; Musial, W. Application of Polymerization Activator in the Course of Synthesis of N-Isopropylacrylamide Derivatives for Thermally Triggered Release of Naproxen Sodium. Materials 2018, 11, 261. [Google Scholar] [CrossRef] [PubMed]
- Musial, W.; Pluta, J.; Michalek, J. Thermosensitive microgels of poly-N-isopropyloacrylamide for drug carriers—Practical approach to synthesis. Acta Pol. Pharm 2015, 72, 409–422. [Google Scholar] [PubMed]
- Gola, A.; Knysak, T.; Musial, W. The Influence of Anionic Initiator on the Selected Properties of Poly-N-Isopropyl Acrylamide Evaluated for Controlled Drug Delivery. Molecules 2017, 22, 23. [Google Scholar] [CrossRef] [PubMed]
- Gola, A.; Bernardi, A.; Pasut, G.; Musiał, W. The Influence of Initiator Concentration on Selected Properties of Thermosensitive Poly(Acrylamide-co-2-Acrylamido-2-Methyl-1-Propanesulfonic Acid) Microparticles. Polymers 2021, 13, 996. [Google Scholar] [CrossRef] [PubMed]
- Gola, A.; Niżniowska, A.; Musiał, W. The Influence of Initiator Concentration on Selected Properties on Poly- N-Vinylcaprolactam Nanoparticles. Nanomaterials 2019, 9, 1577. [Google Scholar] [CrossRef] [PubMed]
- Németh, Z.; Csoka, I.; Jazani, R.S.; Sipos, B.; Haspel, H.; Kozma, G.; Konya, Z.; Dobo, D.G. Quality by Design-Driven Zeta Potential Optimisation Study of Liposomes with Charge Imparting Membrane Additives. Pharmaceutics 2022, 26, 1798. [Google Scholar] [CrossRef] [PubMed]
- Ramaye, Y.; Dabrio, M.; Roebben, G.; Kestens, V. Development and Validation of Optical Methods for Zeta Potential Determination of Silica and Polystyrene Particles in Aqueous Suspensions. Materials 2021, 14, 290. [Google Scholar] [CrossRef] [PubMed]
- Kaszuba, M.; Corbett, J.; Mcneil, F.; Jones, A. High-concentration zeta potential measurements using light-scattering techniques. Trans. R. Soc. A 2010, 368, 4439–4451. [Google Scholar] [CrossRef]
- Danaei, M.; Kalantari, M.; Raji, M.; Samareh Fekri, H.; Saber, R.; Asnani, G.P.; Mortazavi, S.M.; Mozafari, M.R.; Rasti, B.; Taheriazam, A. Probing nanoliposomes using single particle analytical techniques: Effect of excipients, solvents, phase transition and zeta potential. Heliyon 2018, 4, e01088. [Google Scholar] [CrossRef]
- Rahmat, D.; Müller, C.; Shahnaz, G.; Leithner, K.; Laffleur, F.; Khan, M.I.; Martien, R.; Bernkop Schnürch, A. HEC-cysteamine particles: Influence of particlesize, zeta potential, morphology and sulfhydrylgroups on permeation enhancing properties. Drug Dev. Ind. Pharm. 2013, 39, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.A.; Abdelsadig, M.S.; Conway, B.R.; Merchant, H.A. Using zeta potential to study the ionisation behaviour of polymers employed in modified-release dosage forms and estimating their pKa. Int. J. Pharm. 2019, 1, 100024. [Google Scholar] [CrossRef] [PubMed]
- Musiał, W.; Pluta, J.; Byrski, T.; Valh, J.V. The Conductivity and pH Values of Dispersions of Nanospheres for Targeted Drug Delivery in the Course of Forced Equilibrium. Adv. Clin. Exp. Med. 2015, 24, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yadav, S.; Wang, Y.J.; Mozziconacci, O.; Schöneich, C. Dual Effect of Histidine on Polysorbate 20 Stability: Mechanistic Studies. Pharm. Res. 2018, 16, 33. [Google Scholar] [CrossRef] [PubMed]
- Werber, J.; Wang, Y.J.; Milligan, M.; Li, X.; Ji, J.A. Analysis of 2,2′-Azobis (2-Amidinopropane) Dihydrochloride Degradation and Hydrolysis in Aqueous Solutions. J. Pharm. Sci. 2011, 100, 3307–3315. [Google Scholar] [CrossRef]
- Fuciños, C.; Fuciños, P.; Míguez, M.; Katime, I.; Pastrana, L.M.; Rúa, M.L. Temperature- and pH-sensitive nanohydrogels of poly(N-isopropylacrylamide) for food packaging applications: Modelling the swelling-collapse behaviour. PLoS ONE 2014, 9, e87190. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Furuta, R.; Kawai, Y. Effect of Initiator Charge on Dispersion Stability of Polymer Particles Formed by Soap-free Emulsion Polymerization of 4-Vinylaniline or 4-Vinylpyridine. Chem. Lett. 2019, 48, 208–210. [Google Scholar] [CrossRef]
- Zhao, X.J.; Gao, Z.F. Synthesis and characterization of hydrophilic polymer nanoparticles using n-isopropylacrylamide (NIPAM) via emulsion polymerization technique You may also like Effects of thiocyanate anions on switching and structure of poly(N-isopropylacrylamide) brushes. Conf. Ser. Mater. Sci. Eng. 2018, 440, 012008. [Google Scholar]

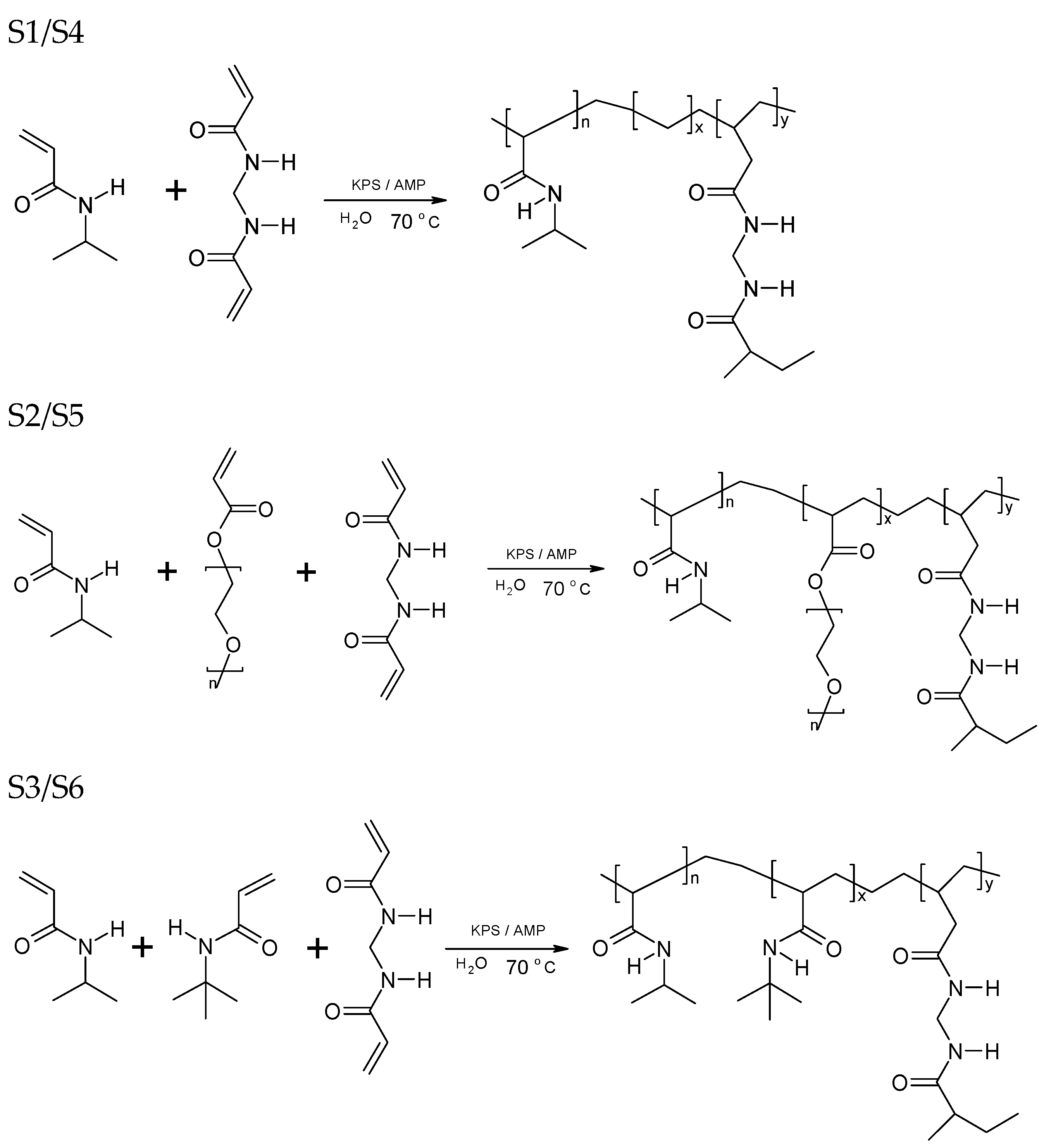


| Substrates % (w/w) | Monomer | Initiator | Crosslinker | Comonomer | Solvent | Bibliography * | ||
|---|---|---|---|---|---|---|---|---|
| Anionic | Cationic | Short Chain | Hydrophilic | Lipophilic | ||||
| Type of Polymer | NIPA | KPS | AMP | MBA | PEG-MA | NTB | H2O | |
| S1 | 0.5 | 0.05 | 0.05 | 99.40 | [29] | |||
| S2 | 0.5 | 0.05 | 0.05 | 0.05 | 99.35 | |||
| S3 | 0.5 | 0.05 | 0.05 | 0.05 | 99.35 | |||
| S4 | 0.5 | 0.05 | 0.05 | 99.40 | [30] | |||
| S5 | 0.5 | 0.05 | 0.05 | 0.05 | 99.35 | |||
| S6 | 0.5 | 0.05 | 0.05 | 0.05 | 99.35 | |||
| Type of Polymer | Monomer (mol) | Anionic Initiator (mol) | Cationic Initiator (mol) | NIPA to KPS/AMP Radical Molar Ratio |
|---|---|---|---|---|
| NIPA | KPS | AMP | ||
| S1–S6 | 4.4 × 10−2 | 1.85 × 10−3 | 1.84 × 10−3 | 1:0.1 |
| Type of Initiator | Type of Polymer | 18 °C | 42 °C | VPTT | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DH [nm] | SD | PI | SD | DH [nm] | SD | PI | SD | °C | ||
| anionic initiator | S1 | 525.00 | 8.61 | 0.21 | 0.02 | 256.60 | 0.20 | 1.31 | 0.01 | 34 |
| S2 | 838.20 | 10.44 | 0.32 | 0.03 | 361.24 | 2.17 | 0.02 | 0.01 | 35–36 | |
| S3 | 859.40 | 17.23 | 0.03 | 0.02 | 276.74 | 1.06 | 0.02 | 0.01 | 29 | |
| cationic initiator | S4 | 724.68 | 4.60 | 0.04 | 0.01 | 413.20 | 3.79 | 0.02 | 0.01 | 35 |
| S5 | 917.27 | 15.81 | 0.35 | 0.01 | 344.20 | 4.18 | 0.04 | 0.03 | 35–37 | |
| S6 | 312.00 | 12.48 | 0.49 | 0.01 | 100.70 | 8.24 | 0.35 | 0.02 | 26 | |
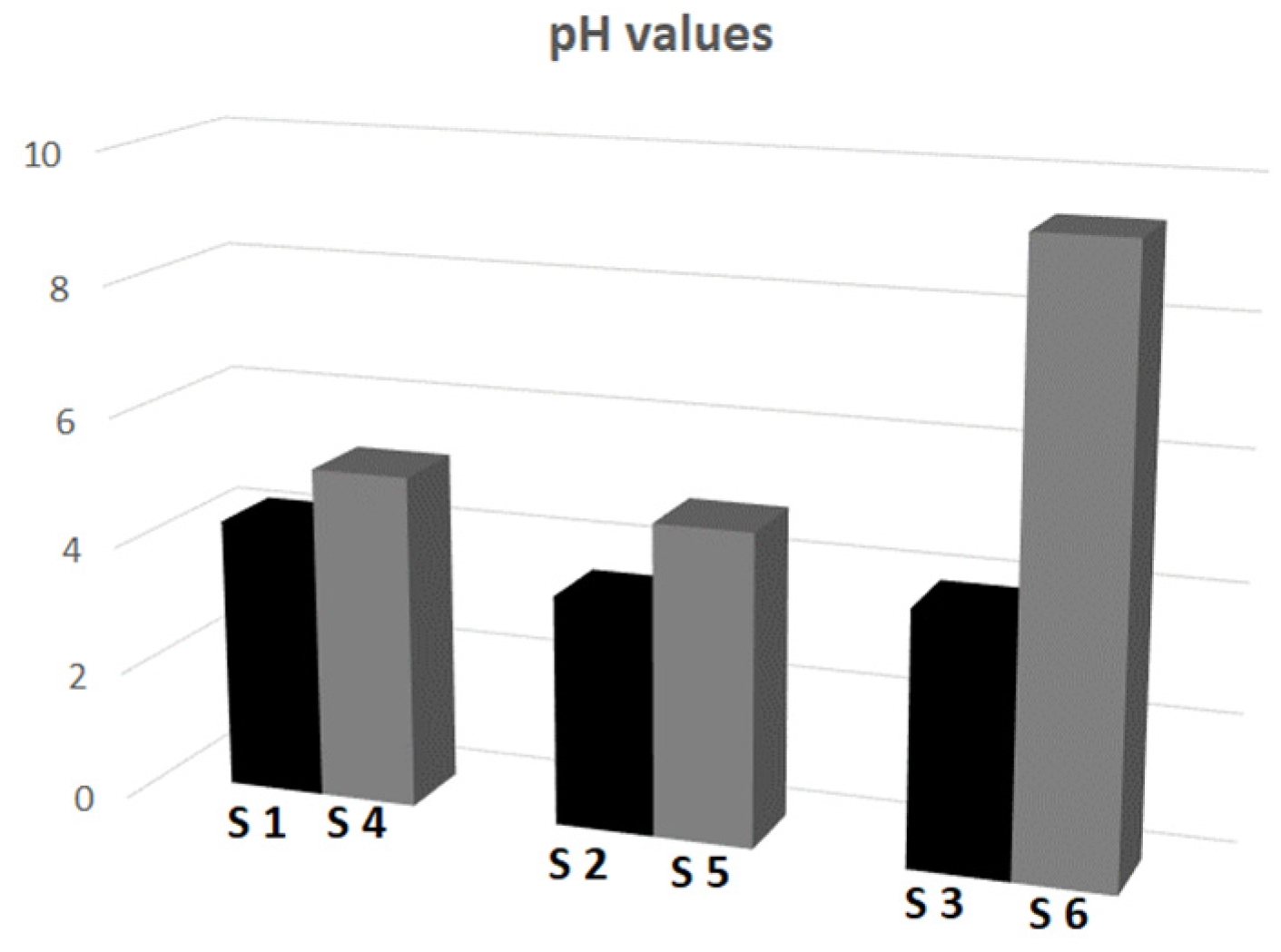
| Type of Initiator | Type of Polymer | 18 °C | 42 °C | pHBPP | SD | pHAPP | SD | ||
|---|---|---|---|---|---|---|---|---|---|
| ZP [mV] | SD | ZP [mV] | SD | ||||||
| anionic initiator | S1 | −6.41 | 0.23 | −25.63 | 0.14 | 2.58 | 0.08 | 4.22 | 0.07 |
| S2 | −21.40 | 0.44 | −29.38 | 0.36 | 2.24 | 0.07 | 3.57 | 0.10 | |
| S3 | −20.33 | 0.47 | −28.87 | 0.90 | 2.38 | 0.03 | 3.94 | 0.12 | |
| cationic initiator | S4 | 27.40 | 0.53 | 47.97 | 0.23 | 5.11 | 0.11 | 5.17 | 0.09 |
| S5 | 22.70 | 0.80 | 31.50 | 0.26 | 4.51 | 0.09 | 4.83 | 0.05 | |
| S6 | 3.70 | 0.01 | 35.73 | 0.99 | 5.51 | 0.04 | 9.43 | 0.30 | |
| Substrate | NIPA | KPS | AMP | MBA | PEG-MA | NTB |
|---|---|---|---|---|---|---|
| pH | 6.23 | 4.23 | 5.86 | 5.15 | 4.25 | 4.14 |
| SD | 0.32 | 0.05 | 0.59 | 0.07 | 0.01 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gasztych, M.; Malamis, A.; Musiał, W. The Influence of Initiators, Particle Size and Composition on the Electrokinetic Potential of N-(Isopropyl)acrylamide Derivatives. Polymers 2024, 16, 907. https://doi.org/10.3390/polym16070907
Gasztych M, Malamis A, Musiał W. The Influence of Initiators, Particle Size and Composition on the Electrokinetic Potential of N-(Isopropyl)acrylamide Derivatives. Polymers. 2024; 16(7):907. https://doi.org/10.3390/polym16070907
Chicago/Turabian StyleGasztych, Monika, Aleksandra Malamis, and Witold Musiał. 2024. "The Influence of Initiators, Particle Size and Composition on the Electrokinetic Potential of N-(Isopropyl)acrylamide Derivatives" Polymers 16, no. 7: 907. https://doi.org/10.3390/polym16070907
APA StyleGasztych, M., Malamis, A., & Musiał, W. (2024). The Influence of Initiators, Particle Size and Composition on the Electrokinetic Potential of N-(Isopropyl)acrylamide Derivatives. Polymers, 16(7), 907. https://doi.org/10.3390/polym16070907





