Electrospun MoS2-CNTs-PVA/PVA Hybrid Separator for High-Performance Li/FeS2 Batteries
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Preparation of MoS2-CNTs-PVA (MCP)/PVA Separator
2.3. Characterizations
2.4. Electrochemical Measurements
3. Results and Discussion
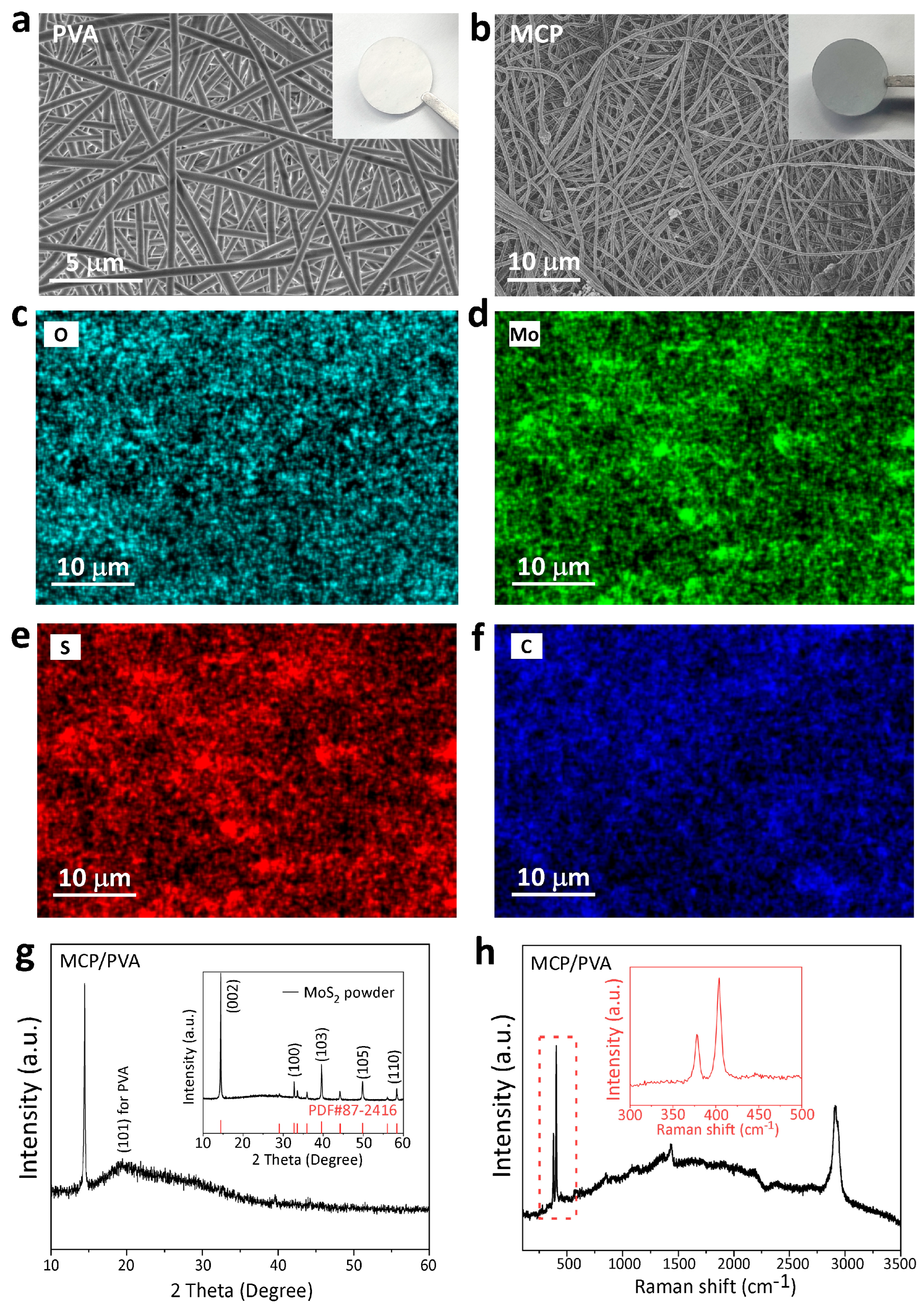
3.1. Characterization of the Hybrid Separator
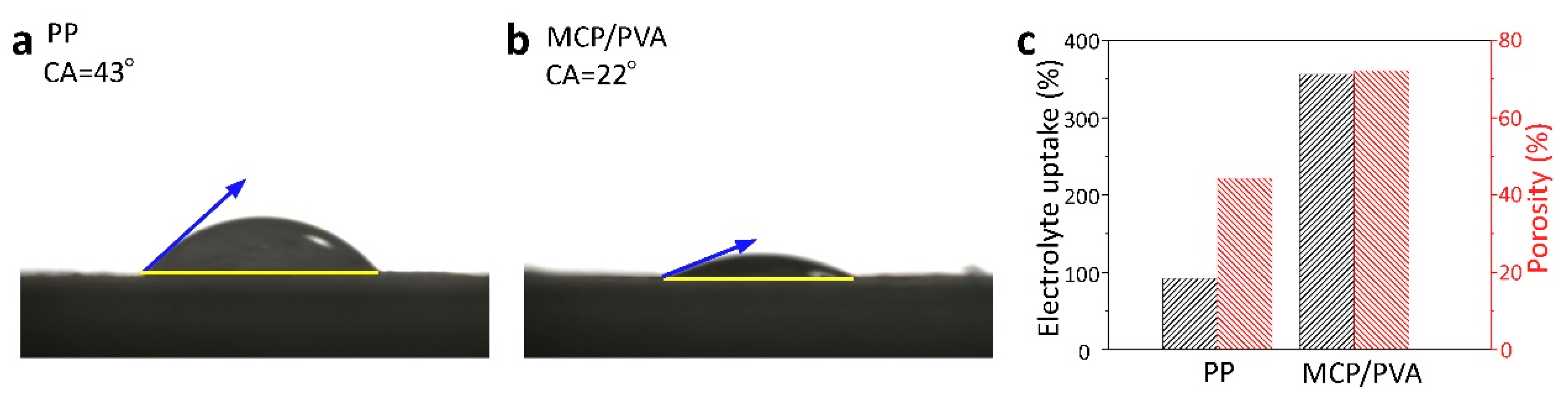
3.2. Electrolyte Wettability
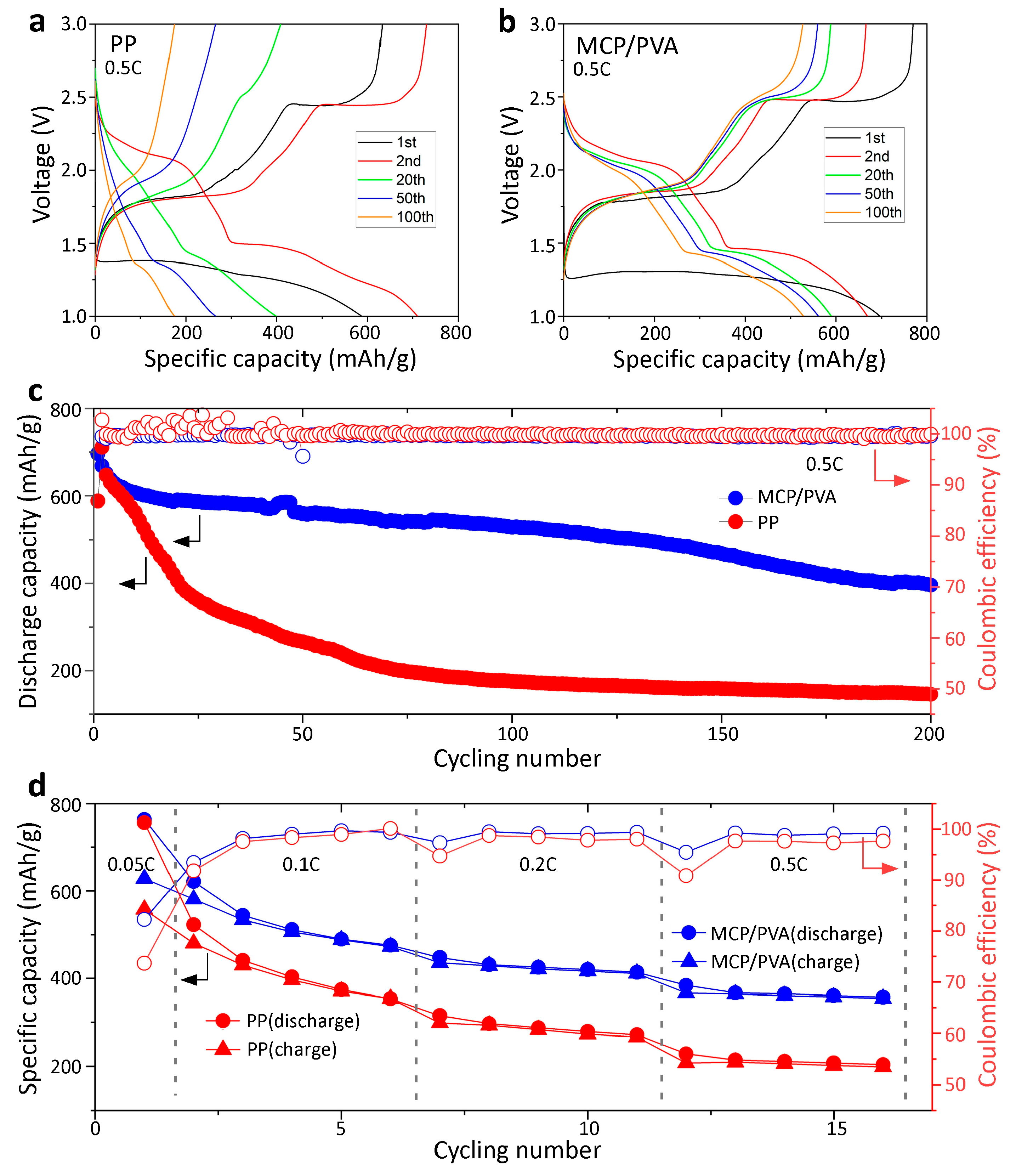
3.3. Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jiao, S.C.; Wang, J.Y.; Hu, Y.S.; Yu, X.Q.; Li, H. High-capacity oxide cathode beyond 300 mAh/g focus review. Acs Energy Lett. 2023, 8, 3025–3037. [Google Scholar] [CrossRef]
- Yan, D.; Yang, H.Y.; Bai, Y. Tactics to optimize conversion-type metal fluoride/sulfide/oxide cathodes toward advanced lithium metal batteries. Nano Res. 2023, 16, 8173–8190. [Google Scholar] [CrossRef]
- Wu, F.X.; Yushin, G. Conversion cathodes for rechargeable lithium and lithium-ion batteries. Energ. Environ. Sci. 2017, 10, 435–459. [Google Scholar] [CrossRef]
- Wang, L.P.; Wu, Z.R.; Zou, J.; Gao, P.; Niu, X.B.; Li, H.; Chen, L.Q. Li-free cathode materials for high energy density lithium batteries. Joule 2019, 3, 2086–2102. [Google Scholar] [CrossRef]
- Ma, W.Q.; Liu, X.Z.; Lei, X.F.; Yuan, Z.H.; Ding, Y. Micro/nano-structured FeS for high energy efficiency rechargeable Li-FeS battery. Chem. Eng. J. 2018, 334, 725–731. [Google Scholar] [CrossRef]
- Ashby, D.S.; Horner, J.S.; Whang, G.; Lapp, A.S.; Roberts, S.A.; Dunn, B.; Kolesnichenko, I.V.; Lambert, T.N.; Talin, A.A. Understanding the electrochemical performance of FeS conversion cathodes. Acs Appl. Mater. Inter. 2022, 14, 26604–26611. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.J.; Fan, X.L.; Suo, L.M.; Luo, C.; Gao, T.; Wang, C.S. Electrospun FeS@Carbon fiber electrode as a high energy density cathode for rechargeable lithium batteries. Acs Nano 2016, 10, 1529–1538. [Google Scholar] [CrossRef]
- Zou, J.; Zhao, J.; Wang, B.J.; Chen, S.L.; Chen, P.Y.; Ran, Q.W.; Li, L.; Wang, X.; Yao, J.M.; Li, H.; et al. Unraveling the reaction mechanism of FeS as a Li-Ion battery cathode. Acs Appl. Mater. Inter. 2020, 12, 44850–44857. [Google Scholar] [CrossRef] [PubMed]
- Whang, G.; Ashby, D.S.; Lapp, A.S.; Hsieh, Y.C.; Butts, D.M.; Kolesnichenko, I.V.; Wu, P.W.; Lambert, T.N.; Talin, A.A.; Dunn, B.S. Temperature-dependent reaction pathways in FeS: Reversibility and the electrochemical formation of FeS. Chem. Mater. 2022, 34, 5422–5432. [Google Scholar] [CrossRef]
- Zhang, S.S. The redox mechanism of FeS in non-aqueous electrolytes for lithium and sodium batteries. J. Mater. Chem. A 2015, 3, 7689–7694. [Google Scholar] [CrossRef]
- Jing, P.; Wang, Q.; Wang, B.Y.; Gao, X.; Zhang, Y.; Wu, H. Encapsulating yolk-shell FeS@carbon microboxes into interconnected graphene framework for ultrafast lithium/sodium storage. Carbon 2020, 159, 366–377. [Google Scholar] [CrossRef]
- Jin, X.Z.; Gao, S.; Wu, A.M.; Zhao, J.J.; Huang, H.; Cao, G.Z. Dual-constrained sulfur in FeS@C nanostructured lithium-sulfide batteries. Acs Appl. Energ. Mater. 2020, 3, 10950–10960. [Google Scholar] [CrossRef]
- Su, Q.F.; Lu, Y.H.; Liu, S.H.; Zhang, X.C.; Lin, Y.H.; Fu, R.W.; Wu, D.C. Nanonetwork-structured yolk-shell FeS@C as high-performance cathode materials for Li-ion batteries. Carbon 2018, 140, 433–440. [Google Scholar] [CrossRef]
- Wang, J.; Fang, J.J.; Zhao, H.L.; Zhang, Z.J.; Li, Z.L. Raspberry-like hierarchical structure FeS decorated by dual-carbon framework as high-performance cathode for rechargeable lithium batteries. Carbon 2021, 171, 171–178. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, S.; Li, L.E.; Jia, L.J.; Wu, J.; Li, X.R.; Guan, Q.H.; Hu, H.M.; Zhang, J.; Lin, H.Z. Robust interfacial engineering construction to alleviate polysulfide shuttling in metal sulfide electrodes for achieving fast-charge high-capacity lithium storages. Chem. Eng. J. 2022, 446, 137291. [Google Scholar] [CrossRef]
- Chen, K.Y.; Zhang, Y.; Li, C.L. High-rate nanostructured pyrite cathodes enabled by fluorinated surface and compact grain stacking sulfuration of ionic liquid coated fluorides. Acs Nano 2018, 12, 12444–12455. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.D.; Zhou, G.; Li, S.; Liu, H.Y.; Wang, L.P.; Li, H. Unlocking cycling longevity in micro-sized conversion-type FeS2 cathodes. Joule 2023, 7, 2609–2621. [Google Scholar] [CrossRef]
- Zhu, Y.B.; Chen, Z.; Chen, H.; Fu, X.G.; Awuye, D.E.; Yin, X.C.; Zhao, Y.X. Breaking the barrier: Strategies for mitigating shuttle effect in lithium-sulfur batteries using advanced separators. Polymers 2023, 15, 3955. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Shao, M.M.; Jin, J.T.; Cui, L.F.; Tu, H.R.; Fu, X.W. Configurational and structural design of separators toward shuttling-free and dendrite-free lithium-sulfur batteries: A review. Energy Storage Mater. 2022, 47, 629–648. [Google Scholar] [CrossRef]
- Fu, X.W.; Wang, Y.; Scudiero, L.; Zhong, W.H. A polymeric nanocomposite interlayer as ion-transport-regulator for trapping polysulfides and stabilizing lithium metal. Energy Storage Mater. 2018, 15, 447–457. [Google Scholar] [CrossRef]
- Shi, C.M.; Yu, M.P. Flexible solid-state lithium-sulfur batteries based on structural designs. Energy Storage Mater. 2023, 57, 429–459. [Google Scholar] [CrossRef]
- Shi, C.M.; Hamann, T.; Takeuchi, S.; Alexander, G.V.; Nolan, A.M.; Limpert, M.; Fu, Z.Z.; O’Neill, J.; Godbey, G.; Dura, J.A.; et al. 3D Asymmetric bilayer garnet-hybridized high-energy-density lithium-sulfur batteries. Acs Appl. Mater. Inter. 2023, 15, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, Y.G.; Zheng, T.T.; Zhang, K.; She, X.J.; Xu, H.; Fang, Z.; Xie, K.Y. Facile fabrication of permselective g-CN separator for improved lithium-sulfur batteries. Electrochim. Acta 2018, 272, 60–67. [Google Scholar] [CrossRef]
- Wu, J.Y.; Zeng, H.X.; Li, X.W.; Xiang, X.; Liao, Y.G.; Xue, Z.G.; Ye, Y.S.; Xie, X.L. Ultralight layer-by-layer self-assembled mos-polymer modified separator for simultaneously trapping polysulfides and suppressing lithium dendrites. Adv. Energy Mater. 2018, 8, 1802430. [Google Scholar] [CrossRef]
- Li, M.L.; Wan, Y.; Huang, J.K.; Assen, A.H.; Hsiung, C.E.; Jiang, H.; Han, Y.; Eddaoudi, M.; Lai, Z.P.; Ming, J.; et al. Metal-organic framework-based separators for enhancing Li-S battery stability: Mechanism of mitigating polysulfide diffusion. Acs Energy Lett. 2017, 2, 2362–2367. [Google Scholar] [CrossRef]
- Wang, J.N.; Yi, S.S.; Liu, J.W.; Sun, S.Y.; Liu, Y.P.; Yang, D.W.; Xi, K.; Gao, G.X.; Abdelkader, A.; Yan, W.; et al. Suppressing the shuttle effect and dendrite growth in lithium-sulfur batteries. Acs Nano 2020, 14, 9819–9831. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Li, Q.H.; Tan, Z.C. A review of electrospun nanofiber-based separators for rechargeable lithium-ion batteries. J. Power Sources 2019, 443, 227262. [Google Scholar] [CrossRef]
- Xiao, W.; Zhao, L.N.; Gong, Y.Q.; Liu, J.G.; Yan, C.W. Preparation and performance of poly(vinyl alcohol) porous separator for lithium-ion batteries. J. Membr. Sci. 2015, 487, 221–228. [Google Scholar] [CrossRef]
- Xiao, W.; Song, J.; Huang, L.; Yang, Z.X.; Qiao, Q.D. PVA-ZrO multilayer composite separator with enhanced electrolyte property and mechanical strength for lithium-ion batteries. Ceram. Int. 2020, 46, 29212–29221. [Google Scholar] [CrossRef]
- Jiang, K.; Gao, S.; Wang, R.X.; Jiang, M.; Han, J.; Gu, T.T.; Liu, M.Y.; Cheng, S.J.; Wang, K.L. Lithium sulfonate/carboxylate-anchored polyvinyl alcohol separators for lithium sulfur batteries. Acs Appl. Mater. Inter. 2018, 10, 18310–18315. [Google Scholar] [CrossRef]
- Fridrikh, S.V.; Yu, J.H.; Brenner, M.P.; Rutledge, G.C. Controlling the fiber diameter during electrospinning. Phys. Rev. Lett. 2003, 90, 144502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.J.; Li, Q.; Zhang, L.F.; Wang, S.Q.; Harper, D.P.; Wu, Q.; Young, T.M. Preparation of electrospun nanofibrous poly(vinyl alcohol)/cellulose nanocrystals air filter for efficient particulate matter removal with repetitive usage capability via facile heat treatment. Chem. Eng. J. 2020, 399, 125768. [Google Scholar] [CrossRef]
- Dou, Y.; Wang, S.J.; Gibril, M.E.; Kong, F.G. Electrospun of polyvinyl alcohol composite hydrogel nanofibers prepared by in-situ polymerization: A novel approach to fabricate hydrogel nanofiber membrane for lithium-ion batteries. Chem. Eng. J. 2024, 481, 148435. [Google Scholar] [CrossRef]
- Liu, J.; Wen, Y.R.; Wang, Y.; van Aken, P.A.; Maier, J.; Yu, Y. Carbon-encapsulated pyrite as stable and earth-abundant high energy cathode material for rechargeable lithium batteries. Adv. Mater. 2014, 26, 6025–6030. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.Q.; Zhang, Q.; Huang, J.Q.; Tian, G.L.; Nie, J.Q.; Peng, H.J.; Wei, F. Unstacked double-layer templated graphene for high-rate lithium-sulphur batteries. Nat. Commun. 2014, 5, 3410. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, K.; Zhu, Z.Q.; Tao, Z.L.; Chen, J. FeS microspheres with an ether-based electrolyte for high-performance rechargeable lithium batteries. J. Mater. Chem. A 2015, 3, 12898–12904. [Google Scholar] [CrossRef]
- Lu, J.H.; Lian, F.; Guan, L.L.; Zhang, Y.X.; Ding, F. Adapting FeS micron particles as an electrode material for lithium-ion batteries via simultaneous construction of CNT internal networks and external cages. J. Mater. Chem. A 2019, 7, 991–997. [Google Scholar] [CrossRef]
- Zhang, D.; Tu, J.P.; Xiang, J.Y.; Qiao, Y.Q.; Xia, X.H.; Wang, X.L.; Gu, C.D. Influence of particle size on electrochemical performances of pyrite FeS for Li-ion batteries. Electrochim. Acta 2011, 56, 9980–9985. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Liu, Q.; Zhang, W.; Wu, R.; Tang, H.; Ma, Y.; Xu, W.; Jiang, S. Electrospun MoS2-CNTs-PVA/PVA Hybrid Separator for High-Performance Li/FeS2 Batteries. Polymers 2024, 16, 921. https://doi.org/10.3390/polym16070921
Wu S, Liu Q, Zhang W, Wu R, Tang H, Ma Y, Xu W, Jiang S. Electrospun MoS2-CNTs-PVA/PVA Hybrid Separator for High-Performance Li/FeS2 Batteries. Polymers. 2024; 16(7):921. https://doi.org/10.3390/polym16070921
Chicago/Turabian StyleWu, Sheng, Qian Liu, Wei Zhang, Ruizhe Wu, Hongping Tang, Yulin Ma, Wenqiang Xu, and Shufang Jiang. 2024. "Electrospun MoS2-CNTs-PVA/PVA Hybrid Separator for High-Performance Li/FeS2 Batteries" Polymers 16, no. 7: 921. https://doi.org/10.3390/polym16070921
APA StyleWu, S., Liu, Q., Zhang, W., Wu, R., Tang, H., Ma, Y., Xu, W., & Jiang, S. (2024). Electrospun MoS2-CNTs-PVA/PVA Hybrid Separator for High-Performance Li/FeS2 Batteries. Polymers, 16(7), 921. https://doi.org/10.3390/polym16070921





