Phosphorus-Based Flame-Retardant Acrylonitrile Butadiene Styrene Copolymer with Enhanced Mechanical Properties by Combining Ultrahigh Molecular Weight Silicone Rubber and Ethylene Methyl Acrylate Copolymer
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
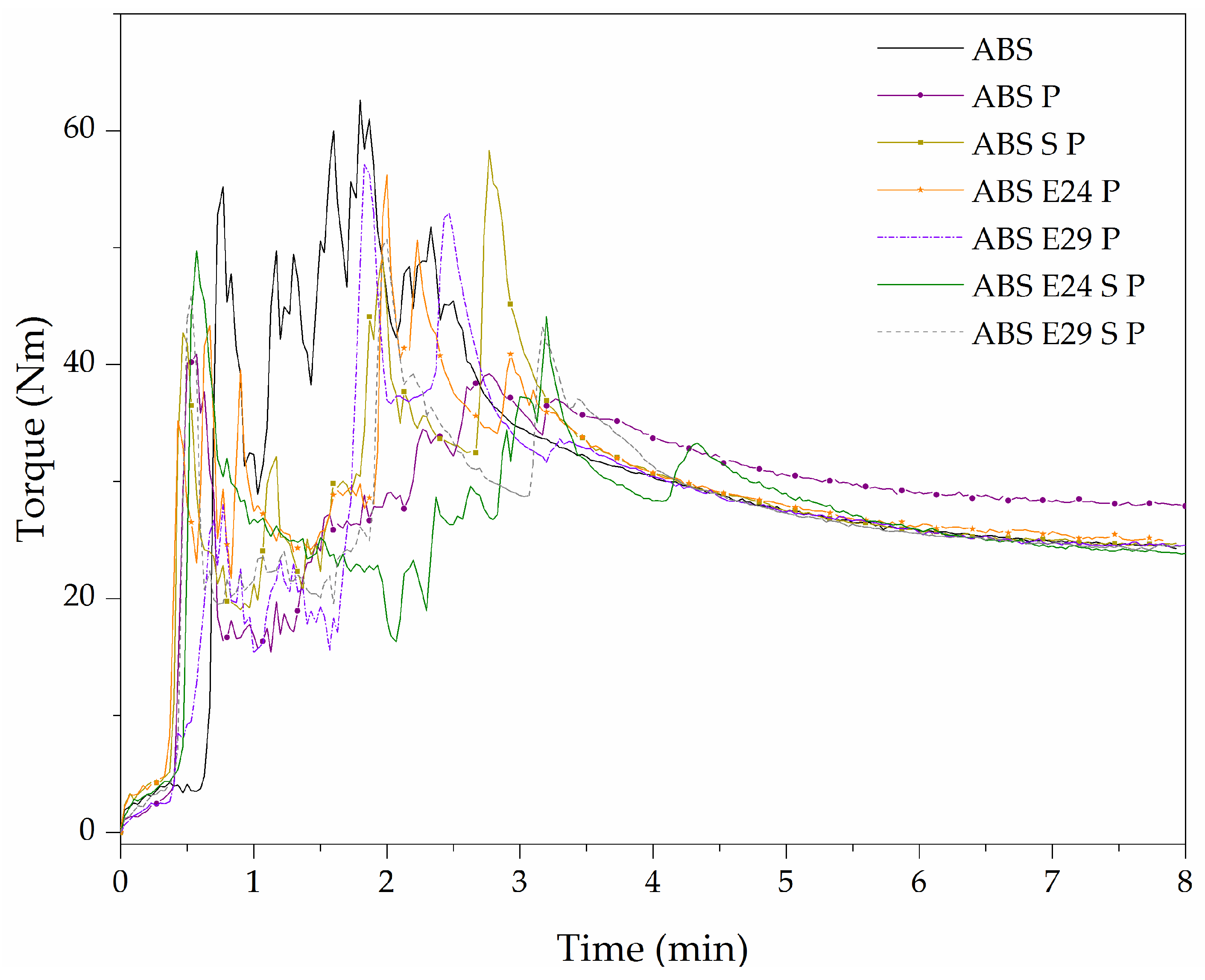
3.1. Melt-Blending Behavior
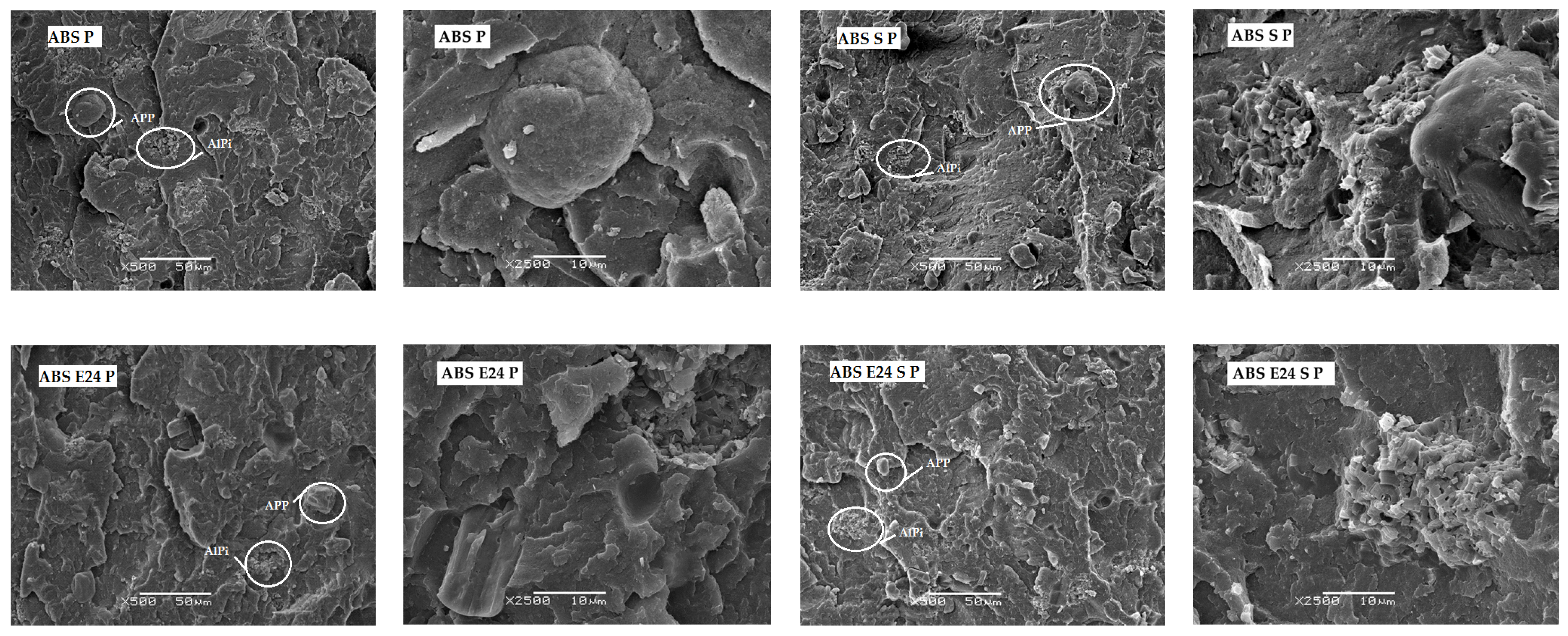
3.2. Morphology of ABS Composites
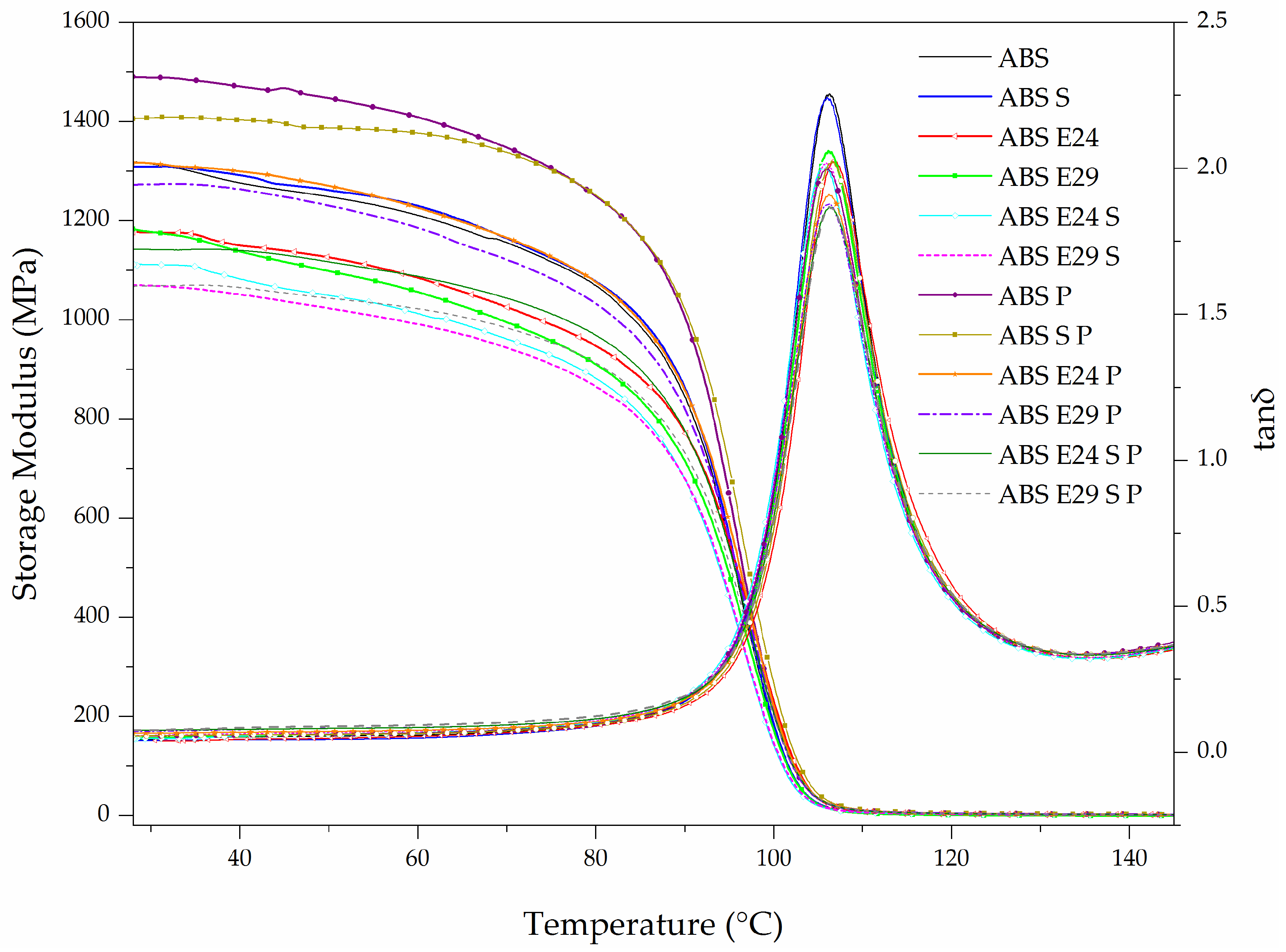
3.3. Dynamic Mechanical Thermal Behavior
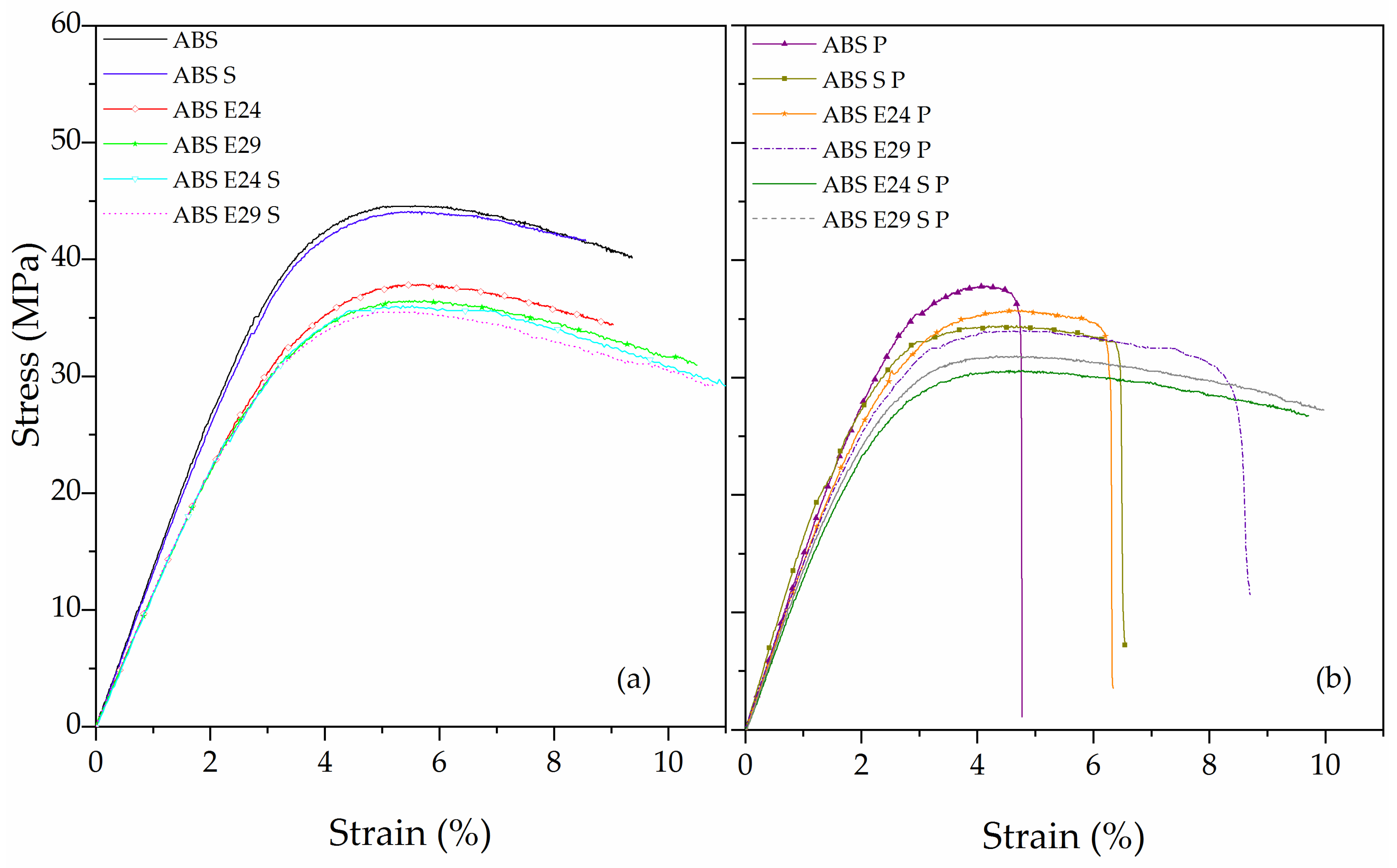
3.4. Flexural Behavior
3.5. Notched Impact Strength
3.6. Thermal Stability
3.7. Fire Behavior
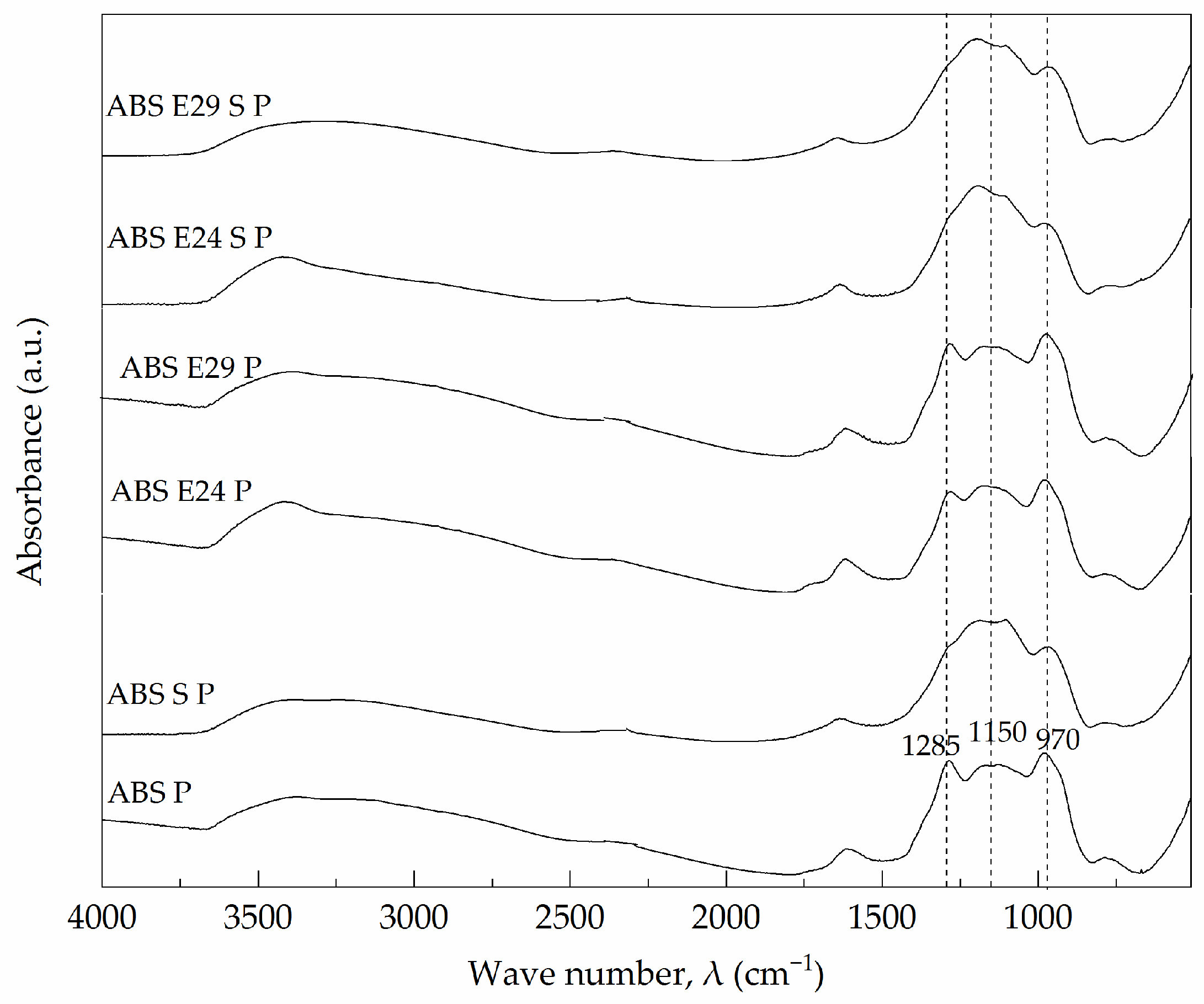
3.8. Morphological and Chemical Analysis of Residue
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, H.; Tong, L.; Xu, Z.; Fang, Z.; Jin, Y.; Lu, F. A novel intumescent flame retardant: Synthesis and application in ABS copolymer. Polym. Degrad. Stab. 2007, 92, 720–726. [Google Scholar] [CrossRef]
- Cao, X.; Yang, Y.; Luo, H.; Cai, X. High efficiency intumescent flame retardancy between Hexakis (4-nitrophenoxy) cyclotriphosphazene and ammonium polyphosphate on ABS. Polym. Degrad. Stab. 2017, 143, 259–265. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Fang, Z. Synergistic effects of expandable graphite and ammonium polyphosphate with a new carbon source derived from biomass in flame retardant ABS. J. Appl. Polym. Sci. 2013, 128, 2424–2432. [Google Scholar] [CrossRef]
- Jian, R.-K.; Chen, L.; Zhao, B.; Yan, Y.-W.; Li, X.-F.; Wang, Y.-Z. Acrylonitrile-Butadiene-Styrene Terpolymer with Metal Hypophosphites: Flame Retardance and Mechanism Research. Ind. Eng. Chem. Res. 2014, 53, 2299–2307. [Google Scholar] [CrossRef]
- Levchik, S.V.; Wei, E.D. New developments in flame retardancy of styrene thermoplastics and foams. Polym. Int. 2008, 57, 431–448. [Google Scholar] [CrossRef]
- Attia, N.F.; Hassan, M.A.; Nour, M.A.; Geckeler, K.E. Flame-retardant materials: Synergistic effect of halloysite nanotubes on the flammability properties of acrylonitrile-butadiene-styrene composites. Polym. Int. 2014, 63, 1168–1173. [Google Scholar] [CrossRef]
- Webster, T.F.; McClean, M.D. Exposure to polybrominated diphenyl ethers in the indoor environment. Fire Technol. 2015, 51, 85–95. [Google Scholar] [CrossRef]
- Kim, J.; Lee, K.; Lee, K.; Bae, J.; Yang, J.; Hong, S. Studies on the thermal stabilization enhancement of ABS; synergistic effect of triphenyl phosphate nanocomposite, epoxy resin, and silane coupling agent mixtures. Polym. Degrad. Stab. 2003, 79, 201–207. [Google Scholar] [CrossRef]
- Jian, R.-K.; Chen, L.; Chen, S.-Y.; Long, J.-W.; Wang, Y.-Z. A novel flame-retardant acrylonitrile-butadiene-styrene system based on aluminum isobutylphosphinate and red phosphorus: Flame retardance, thermal degradation and pyrolysis behavior. Polym. Degrad. Stab. 2014, 109, 184–193. [Google Scholar] [CrossRef]
- Dasari, A.; Yu, Z.Z.; Cai, G.P.; Mai, Y.W. Recent developments in the fire retardancy of polymeric materials. Prog. Polym. Sci. 2013, 38, 1357–1387. [Google Scholar] [CrossRef]
- Hoang, D.; Kim, W.; An, H.; Kim, J. Flame retardancies of novel organo-phosphorus flame retardants based on DOPO derivatives when applied to ABS. Macromol. Res. 2015, 23, 442–448. [Google Scholar] [CrossRef]
- Wawrzyn, E.; Schartel, B.; Ciesielski, M.; Kretzschmar, B.; Braun, U.; Doering, M. Are novel aryl phosphates competitors for bisphenol A bis(diphenyl phosphate) in halogen-free flame-retarded polycarbonate/acrylonitrile-butadiene-styrene blends? Eur. Polym. J. 2012, 48, 1561–1574. [Google Scholar] [CrossRef]
- Xia, Y.; Jian, X.-G.; Li, J.-F.; Wang, X.-H.; Xu, Y.-Y. Synergistic effect of montmorillonite and intumescent flame retardant on flame retardance enhancement of ABS. Polym. Plast. Technol. Eng. 2007, 46, 227–232. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, H.; Cao, X.; Zhou, F.; Kong, W.; Cai, X. The synergistic effects of a novel intumescent flame-retardant poly-(4-nitrophenoxy)-phosphazene and ammonium polyphosphate on ABS systems. J. Therm. Anal. Calorim. 2019, 137, 65–77. [Google Scholar] [CrossRef]
- Bee, S.-T.; Lim, K.-S.; Sin, L.T.; Ratnam, C.T.; Bee, S.L.; Rahmat, A.R. Interactive effect of ammonium polyphosphate and montmorillonite on enhancing flame retardancy of polycarbonate/acrylonitrile butadiene styrene composites. Iran. Polym. J. 2018, 27, 899–911. [Google Scholar] [CrossRef]
- Realinho, V.; Arencon, D.; Antunes, M.; Velasco, J.I. Effects of a Phosphorus Flame Retardant System on the Mechanical and Fire Behavior of Microcellular ABS. Polymers 2019, 11, 30. [Google Scholar] [CrossRef]
- Yuan, Z.; Wen, H.; Liu, Y.; Wang, Q. Synergy between piperazine pyrophosphate and aluminum diethylphosphinate in flame retarded acrylonitrile-butadiene-styrene copolymer. Polym. Degrad. Stab. 2021, 190, 109639. [Google Scholar] [CrossRef]
- Realinho, V.; Haurie, L.; Formosa, J.; Ignacio Velasco, J. Flame retardancy effect of combined ammonium polyphosphate and aluminium diethyl phosphinate in acrylonitrile-butadiene-styrene. Polym. Degrad. Stab. 2018, 155, 208–219. [Google Scholar] [CrossRef]
- Dai, P.-B.; Meng, F.-D.; Wang, X.-L.; Wang, Y.-Z. Effect of an Ultrahigh Rubber ABS Impact Modifier Resin on Mechanical Properties of Intumescent Flame-Retardant ABS Composites. J. Macromol. Sci. Part B-Phys. 2010, 49, 542–551. [Google Scholar] [CrossRef]
- Jaidev, K.; Biswal, M.; Mohanty, S.; Nayak, S.K. The influence of hybrid flame retardant and impact modifier on recycled blends formulated from keyboard waste plastics: A study on its flame retardant, mechanical, thermal, and chemical properties. Polym. Adv. Technol. 2022, 33, 647–657. [Google Scholar] [CrossRef]
- Chiang, W.Y.; Tzeng, G.L. Effect of the compatibilizers on flame-retardant polycarbonate (PC) acrylonitrile-butadiene-styrene (ABS) alloy. J. Appl. Polym. Sci. 1997, 65, 795–805. [Google Scholar] [CrossRef]
- Ozkoc, G.; Bayram, G.; Bayramli, E. Impact essential work of fracture toughness of ABS/polyamide-6 blends compatibilized with olefin based copolymers. J. Mater. Sci. 2008, 43, 2642–2652. [Google Scholar] [CrossRef]
- Lin, J.; Li, J.; Li, X.; Guan, Y.; Wang, G.; Chen, L. Flame retardancy and toughening modification of glass fiber-reinforced polycarbonate composites. Polym. J. 2019, 51, 657–665. [Google Scholar] [CrossRef]
- ISO 179-2 Standard; Plastics—Determination of Charpy Impact Properties—Part 2: Instrumented Impact Test. European Committee for Standardization: Brussels, Belguim, 2021.
- UL-94; Standard for Tests for Flammability of Plastic Materials for Parts in Devices and Appliances. American National Standard: Washington, DC, USA, 2001.
- ISO 4589-2 Standard; Plastics Plastics—Determination of Burning Behaviour by Oxygen Index—Part 2: Ambient-Temperature Test. European Committee for Standardization: Brussels, Belguim, 2017.
- ISO 5660-1 Standard; Reaction-to-Fire Tests—Heat Release, Smoke Production and Mass Loss Rate—Part 1: Heat Release Rate (Cone Calorimeter Method) and Smoke Production Rate (Dynamic Measurement). International Standard: Geneva, Switzerland, 2015.
- Mujtaba, A.; Keller, M.; Ilisch, S.; Radusch, H.J.; Thurn-Albrecht, T.; Saalwaechter, K.; Beiner, M. Mechanical Properties and Cross-Link Density of Styrene-Butadiene Model Composites Containing Fillers with Bimodal Particle Size Distribution. Macromolecules 2012, 45, 6504–6515. [Google Scholar] [CrossRef]
- Aid, S.; Eddhahak, A.; Ortega, Z.; Froelich, D.; Tcharkhtchi, A. Experimental study of the miscibility of ABS/PC polymer blends and investigation of the processing effect. J. Appl. Polym. Sci. 2017, 134, 44975. [Google Scholar] [CrossRef]
- Malekzadeh, Y.l.; Shelesh-Nezhad, K. The effects of HNO3-surface treated carbon fiber and nano-CaCO3 inclusions on dynamic mechanical and heat properties of PA6/ABS-based composites. J. Thermoplast. Compos. Mater. 2019, 32, 867–883. [Google Scholar] [CrossRef]
- Bazli, L.; Khavandi, A.; Boutorabi, M.A.; Karrabi, M. Correlation between viscoelastic behavior and morphology of nanocomposites based on SR/EPDM blends compatibilized by maleic anhydride. Polymer 2017, 113, 156–166. [Google Scholar] [CrossRef]
- Yildiz, C.; Seki, Y.; Kizilkan, E.; Sarikanat, M.; Altay, L. Development of Halogen-Free Flame Retardant Acrylonitrile Butadiene Styrene (ABS) Based Composite Materials. Chemistryselect 2023, 8, e202300989. [Google Scholar] [CrossRef]
- Suzuki, M.; Wilkie, C.A. The thermal degradation of acrylonitrile-butadiene-styrene terpolymer as studied by TGA/FTIR. Polym. Degrad. Stab. 1995, 47, 217–221. [Google Scholar] [CrossRef]
- Dicortemiglia, M.P.L.; Camino, G.; Costa, L.; Guaita, M. Thermal degradation of ABS. Thermochim. Acta 1985, 93, 187–190. [Google Scholar] [CrossRef]
- Basuli, U.; Chaki, T.K.; Chattopadhyay, S. Thermomechanical and rheological behaviour of polymer nanocomposites based on ethylene-methyl acrylate (EMA) and multiwalled carbon nanotube (MWNT). Plast. Rubber Compos. 2011, 40, 213–222. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, S.Y.; Ma, Q.Y. Kinetics of the thermal degradation and thermal stability of conductive silicone rubber filled with conductive carbon black. J. Appl. Polym. Sci. 2003, 89, 1548–1554. [Google Scholar] [CrossRef]
- Wang, F.; Wang, X. Preparation and thermal stability of micro/nano silicone rubber particles. In Proceedings of the International Conference on Chemical, Material and Metallurgical Engineering (ICCMME 2011), Beihai, China, 23–25 December 2012; pp. 718–721. [Google Scholar]
- Atazadeh, A.; Ameri, E. Synthesis of PMHS-PDMS composite membranes embedded with silica nanoparticles and their application to separate of DMSO from aqueous solutions. Polym. Bull. 2021, 78, 5003–5028. [Google Scholar] [CrossRef]
- Schartel, B.; Hull, T.R. Development of fire-retarded materials—Interpretation of cone calorimeter data. Fire Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]
- Dai, P.-B.; Wang, D.-Y.; Wang, Y.-Z. Thermal Degradation and Combustion Behavior of a Modified Intumescent Flame-retardant ABS Composite. J. Thermoplast. Compos. Mater. 2010, 23, 473–486. [Google Scholar] [CrossRef]
- Ke, C.-H.; Li, J.; Fang, K.-Y.; Zhu, Q.-L.; Zhu, J.; Yan, Q.; Wang, Y.-Z. Synergistic effect between a novel hyperbranched charring agent and ammonium polyphosphate on the flame retardant and anti-dripping properties of polylactide. Polym. Degrad. Stab. 2010, 95, 763–770. [Google Scholar] [CrossRef]
- Aljamal, A.; Marosi, G.; Szolnoki, B. Investigation of the modes of action for phosphorous flame retardants in a fully waterborne sugar-based epoxy resin. J. Therm. Anal. Calorim. 2023, 148, 281–292. [Google Scholar] [CrossRef]
- Xu, B.; Shao, L.; Wang, J.; Liu, Y.; Qian, L. Enhancement of the intumescent flame retardant efficiency in polypropylene by synergistic charring effect of a hypophosphite/cyclotetrasiloxane bi-group compound. Polym. Degrad. Stab. 2020, 181, 109281. [Google Scholar] [CrossRef]







| Materials | ABS (wt.%) | P (wt.%) | E24 (wt.%) | E29 (wt.%) | UHMW-SR (wt.%) |
|---|---|---|---|---|---|
| ABS | 100 | - | - | - | - |
| ABS S | 98 | - | - | - | 2 |
| ABS E24 | 95 | - | 5 | - | - |
| ABS E29 | 95 | - | - | 5 | - |
| ABS E24 S | 93 | - | 5 | - | 2 |
| ABS E29 S | 93 | - | - | 5 | 2 |
| ABS P | 80 | 20 | - | - | - |
| ABS S P | 78 | 20 | - | - | 2 |
| ABS E24 P | 75 | 20 | 5 | - | - |
| ABS E29 P | 75 | 20 | - | 5 | - |
| ABS E24 S P | 73 | 20 | 5 | - | 2 |
| ABS E29 S P | 73 | 20 | - | 5 | 2 |
| Sample | Average of Torque (Nm) | Max of Torque (Nm) | Stabilized of Torque (Nm) |
|---|---|---|---|
| ABS | 30 | 63 | 25.5 |
| ABS P | 28 | 48 | 29.0 |
| ABS S P | 26 | 55 | 25.5 |
| ABS E24 P | 26 | 50 | 26.1 |
| ABS E29 P | 26 | 55 | 25.5 |
| ABS E24 S P | 26 | 53 | 25.9 |
| ABS E29 S P | 26 | 49 | 25.3 |
| Sample | E′ at 30 °C (MPa) | Intensity of tan δ | Tg (°C) |
|---|---|---|---|
| Max tan δ | |||
| ABS | 1345 ± 29 | 2.23 ± 0.03 | 107 ± 0.4 |
| ABS S | 1307 ± 2 | 2.18 ± 0.05 | 106 ± 0.2 |
| ABS E24 | 1176 ± 5 | 2.01 ± 0.01 | 107 ± 0.1 |
| ABS E29 | 1175 ± 4 | 2.03 ± 0.04 | 106 ± 0.2 |
| ABS E24 S | 1104 ± 10 | 2.03 ± 0.03 | 106 ± 0.1 |
| ABS E29 S | 1066 ± 3 | 2.01 ± 0.01 | 106 ± 0.1 |
| ABS P | 1478 ± 15 | 2.01 ± 0.02 | 106 ± 0.2 |
| ABS S P | 1371 ± 32 | 2.02 ± 0.01 | 107 ± 0.1 |
| ABS E24 P | 1317 ± 2 | 1.95 ± 0.03 | 106 ± 0.1 |
| ABS E29 P | 1267 ± 9 | 1.88 ±0.01 | 106 ± 0.1 |
| ABS E24 S P | 1163 ± 24 | 1.86 ± 0.01 | 106 ± 0.1 |
| ABS E29 S P | 1067 ± 7 | 1.86 ± 0.03 | 107 ± 0.3 |
| Sample | Flexural Modulus (GPa) | Maximum Flexural Strength (MPa) | Strain at Maximum Strength (%) | Strain at Break (%) |
|---|---|---|---|---|
| ABS | 1.35 ± 0.02 | 44.0 ± 0.8 | 5.5 ± 0.1 | NB * |
| ABS S | 1.30 ± 0.02 | 43.8 ± 0.1 | 4.8 ± 0.4 | NB |
| ABS E24 | 1.10 ± 0.02 | 38.1 ± 0.4 | 5.7 ± 0.1 | NB |
| ABS E29 | 1.11 ± 0.01 | 36.3 ± 0.4 | 5.4 ± 0.1 | NB |
| ABS E24 S | 1.11 ± 0.01 | 36.5 ± 0.1 | 5.5 ± 0.2 | NB |
| ABS E29 S | 1.12 ± 0.01 | 36.2 ± 0.9 | 5.2 ± 0.1 | NB |
| ABS P | 1.72 ± 0.05 | 37.6 ± 3.2 | 4.1 ± 0.6 | 4.7 ± 0.4 |
| ABS S P | 1.64 ± 0.03 | 34.4 ± 0.1 | 4.4 ± 0.2 | 6.5 ± 0.1 |
| ABS E24 P | 1.44 ± 0.01 | 35.9 ± 0.2 | 4.8 ± 0.4 | 6.3 ± 0.3 |
| ABS E29 P | 1.44 ± 0.01 | 33.7 ± 0.5 | 4.5 ± 0.1 | 8.5 ± 0.1 |
| ABS E24 S P | 1.33 ± 0.01 | 30.3 ± 0.4 | 4.7 ± 0.2 | NB |
| ABS E29 S P | 1.34 ±0.01 | 32.4 ±0.8 | 4.3 ± 0.1 | NB |
| Samples | Impact Strength (kJ/m2) |
|---|---|
| ABS P | 3.3 ± 0.2 |
| ABS S P | 5.4 ± 0.1 |
| ABS E24 P | 5.9 ± 0.6 |
| ABS E29 P | 6.5 ± 0.3 |
| ABS E24 S P | 8.7 ± 0.4 |
| ABS E29 S P | 10.3 ± 0.4 |
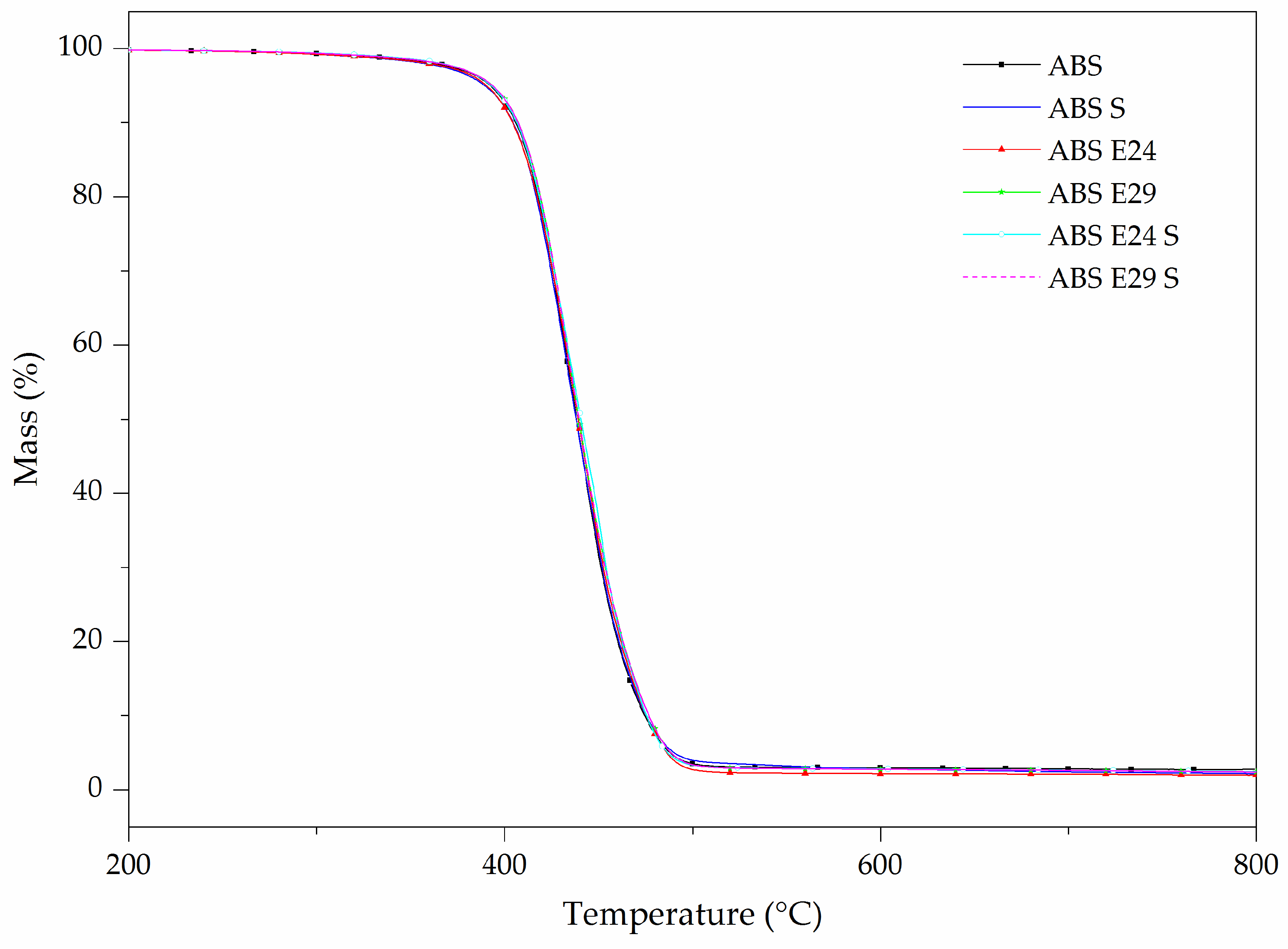
| Materials | TD Step | T5% (°C) | Tpeak (°C) | ML (%) | R800 °C (%) |
|---|---|---|---|---|---|
| ABS | 1 | 390 | 440 | 96.5 | 2.1 |
| E24 | 1 | 420 | 460 | 99.6 | 0.2 |
| E29 | 1 | 420 | 460 | 99.7 | 0.2 |
| UHMW-SR | 1 | 455 | 580 | 62.9 | 35.8 |
| Materials | TD Step | T5% (°C) | Tpeak (°C) | ML (%) | R800 °C (%) |
|---|---|---|---|---|---|
| ABS S | 1 | 390 | 440 | 96.3 | 2.1 |
| ABS E24 | 1 | 390 | 440 | 97.5 | 1.9 |
| ABS E29 | 1 | 390 | 440 | 96.7 | 2.4 |
| ABS E24 S | 1 | 390 | 440 | 96.9 | 2.2 |
| ABS E29 S | 1 | 390 | 440 | 96.7 | 2.5 |
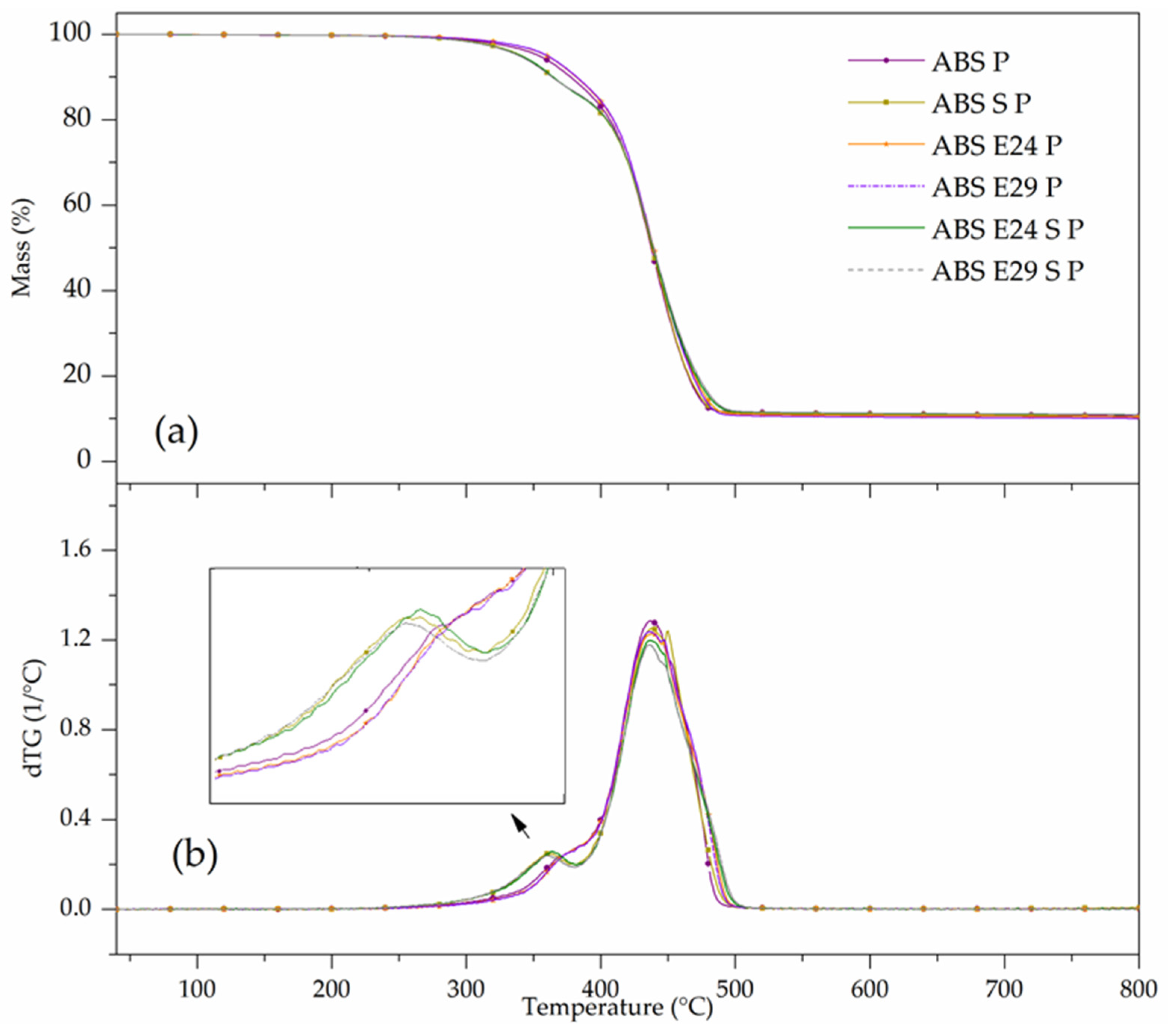
| Materials | TD Step | T5% (°C) | Tpeak (°C) | ML (%) | R800 °C (%) |
|---|---|---|---|---|---|
| ABS P | 1 | 365 | 370 | 12.6 | 10.5 |
| 2 | 440 | 75.1 | |||
| ABS S P | 1 | 340 | 360 | 13.0 | 10.7 |
| 2 | 440 | 75.5 | |||
| ABS E24 P | 1 | 365 | 370 | 12.1 | 10.5 |
| 2 | 440 | 76.7 | |||
| ABS E29 P | 1 | 365 | 370 | 12.2 | 10.4 |
| 2 | 440 | 76.8 | |||
| ABS E24 S P | 1 | 340 | 360 | 14.0 | 11.0 |
| 2 | 438 | 74.0 | |||
| ABS E29 S P | 1 | 340 | 360 | 13.2 | 11.0 |
| 2 | 435 | 75.1 |
| Samples | UL-94 | Dripping | t1 + t2 (s) |
|---|---|---|---|
| ABS | NC | Yes | - |
| ABS S | NC | Yes | - |
| ABS E24 | NC | Yes | - |
| ABS E29 | NC | Yes | - |
| ABS E24 S | NC | Yes | - |
| ABS E29 S | NC | Yes | - |
| ABS P | V-0 | No | 3 + 8 |
| ABS S P | V-0 | No | 1 + 7 |
| ABS E24 P | V-0 | No | 2 + 8 |
| ABS E29 P | V-0 | No | 2 + 4 |
| ABS E24 S P | V-0 | No | 3 + 7 |
| ABS E29 S P | V-0 | No | 2 + 3 |
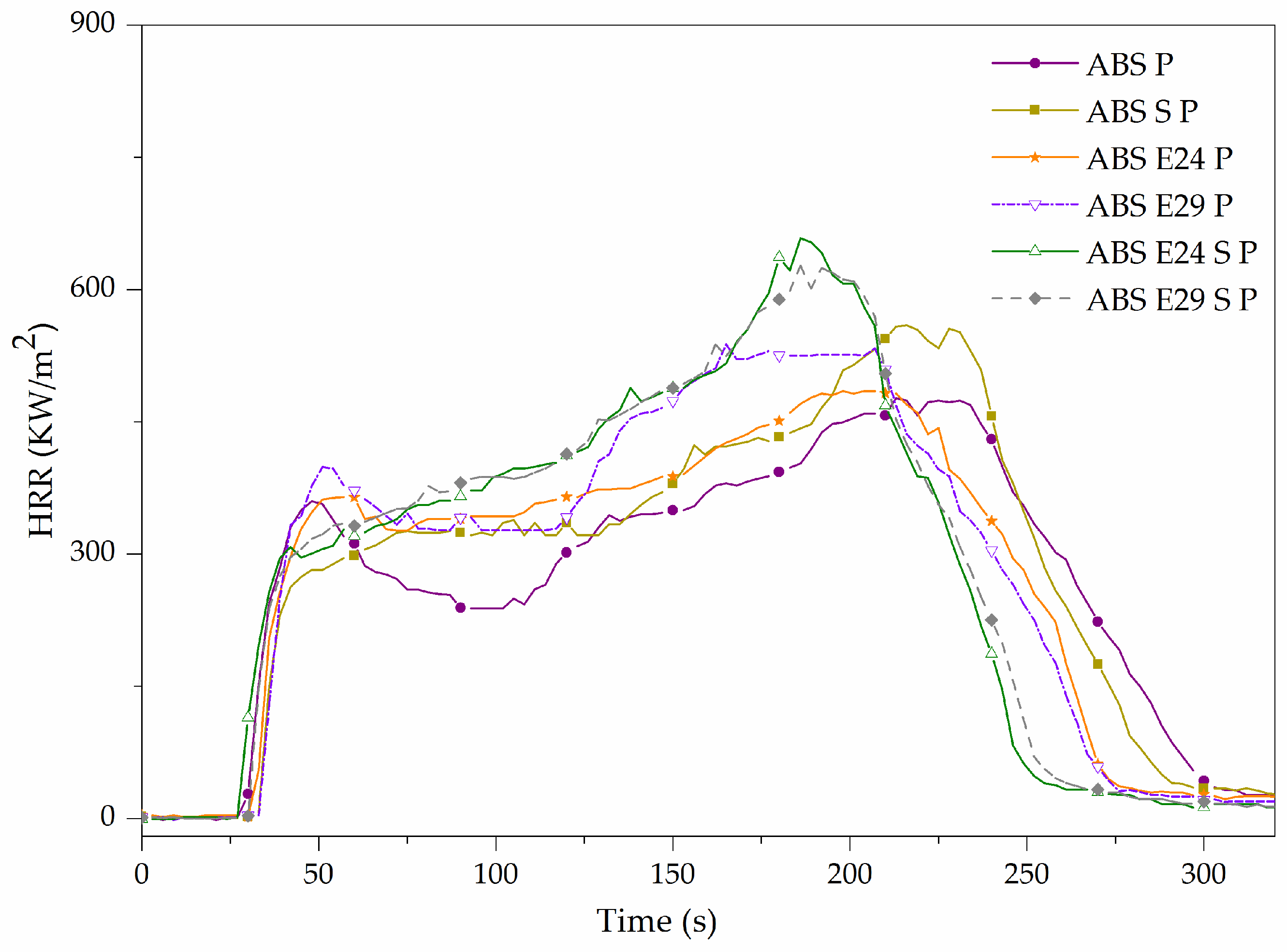
| Material Code | TTI (s) | pHRR (kW/m2) | tPHRR (s) | t combustion (s) | THE (MJ/m2) | EHC (MJ/Kg) | MARHE (kW/m2) | Residue (wt.%) |
|---|---|---|---|---|---|---|---|---|
| ABS | 35 ± 1 | 2767 ± 210 | 118 ± 2 | 142 ± 8 | 133 ± 9 | 32 ± 2 | 909 ± 51 | 0.26 ± 0.07 |
| ABS S | 40 ± 7 | 1815 ± 144 | 132 ± 4 | 174 ± 3 | 129 ± 10 | 32 ± 2 | 786 ± 45 | 1.02 ± 0.03 |
| ABS E24 | 32 ± 1 | 2379 ± 190 | 116 ± 2 | 135 ± 7 | 143 ± 1 | 35 ± 1 | 1037 ± 26 | 0.42 ± 0.03 |
| ABS E29 | 29 ± 3 | 2535 ± 179 | 108 ± 5 | 129 ± 6 | 133 ± 7 | 34 ± 2 | 1046 ± 52 | 0.24 ± 0.02 |
| ABS E24 S | 36 ± 5 | 1715 ± 153 | 135 ± 4 | 187 ± 3 | 134 ± 2 | 33 ± 1 | 765 ± 39 | 0.95 ± 0.05 |
| ABS E29 S | 29 ± 1 | 1747 ± 116 | 120 ± 8 | 156 ± 8 | 130 ± 6 | 33 ± 2 | 848 ± 27 | 0.93 ± 0.01 |
| Materials | TTI (s) | pHRR (kW/m2) | tPHRR (s) | t combustion (s) | THE (MJ/m2) | EHC (MJ/Kg) | MARHE (kW/m2) | Residue (wt.%) |
|---|---|---|---|---|---|---|---|---|
| ABS P | 24 ± 1 | 496 ± 27 | 209 ± 6 | 305 ± 8 | 91 ± 8 | 23 ± 2 | 336 ± 19 | 9.04 ± 0.46 |
| ABS S P | 27 ± 4 | 559 ± 1 | 211 ± 4 | 288 ± 16 | 94 ± 1 | 23 ± 1 | 338 ± 4 | 10.26 ± 0.15 |
| ABS E24 P | 30 ± 1 | 514 ± 42 | 189 ± 10 | 249 ± 15 | 93 ± 2 | 23 ± 1 | 350 ± 17 | 8.35 ± 0.17 |
| ABS E29 P | 28 ± 2 | 529 ± 13 | 177 ± 17 | 269 ± 8 | 94 ± 1 | 24 ± 1 | 364 ± 12 | 8.40 ± 0.01 |
| ABS E24 S P | 27 ± 1 | 643 ± 20 | 186 ± 1 | 264 ± 1 | 93 ± 1 | 24 ± 1 | 376 ± 1 | 10.23 ± 0.09 |
| ABS E29 S P | 29 ± 1 | 627 ± 1 | 189 ± 4 | 283 ± 4 | 94 ± 2 | 24 ± 1 | 372 ± 5 | 10.18 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghonjizade-Samani, F.; Haurie, L.; Malet, R.; Pérez, M.; Realinho, V. Phosphorus-Based Flame-Retardant Acrylonitrile Butadiene Styrene Copolymer with Enhanced Mechanical Properties by Combining Ultrahigh Molecular Weight Silicone Rubber and Ethylene Methyl Acrylate Copolymer. Polymers 2024, 16, 923. https://doi.org/10.3390/polym16070923
Ghonjizade-Samani F, Haurie L, Malet R, Pérez M, Realinho V. Phosphorus-Based Flame-Retardant Acrylonitrile Butadiene Styrene Copolymer with Enhanced Mechanical Properties by Combining Ultrahigh Molecular Weight Silicone Rubber and Ethylene Methyl Acrylate Copolymer. Polymers. 2024; 16(7):923. https://doi.org/10.3390/polym16070923
Chicago/Turabian StyleGhonjizade-Samani, Farnaz, Laia Haurie, Ramón Malet, Marc Pérez, and Vera Realinho. 2024. "Phosphorus-Based Flame-Retardant Acrylonitrile Butadiene Styrene Copolymer with Enhanced Mechanical Properties by Combining Ultrahigh Molecular Weight Silicone Rubber and Ethylene Methyl Acrylate Copolymer" Polymers 16, no. 7: 923. https://doi.org/10.3390/polym16070923
APA StyleGhonjizade-Samani, F., Haurie, L., Malet, R., Pérez, M., & Realinho, V. (2024). Phosphorus-Based Flame-Retardant Acrylonitrile Butadiene Styrene Copolymer with Enhanced Mechanical Properties by Combining Ultrahigh Molecular Weight Silicone Rubber and Ethylene Methyl Acrylate Copolymer. Polymers, 16(7), 923. https://doi.org/10.3390/polym16070923









