Unfolding Protein-Based Hapten Coupling via Thiol–Maleimide Click Chemistry: Enhanced Immunogenicity in Anti-Nicotine Vaccines Based on a Novel Conjugation Method and MPL/QS-21 Adjuvants
Abstract
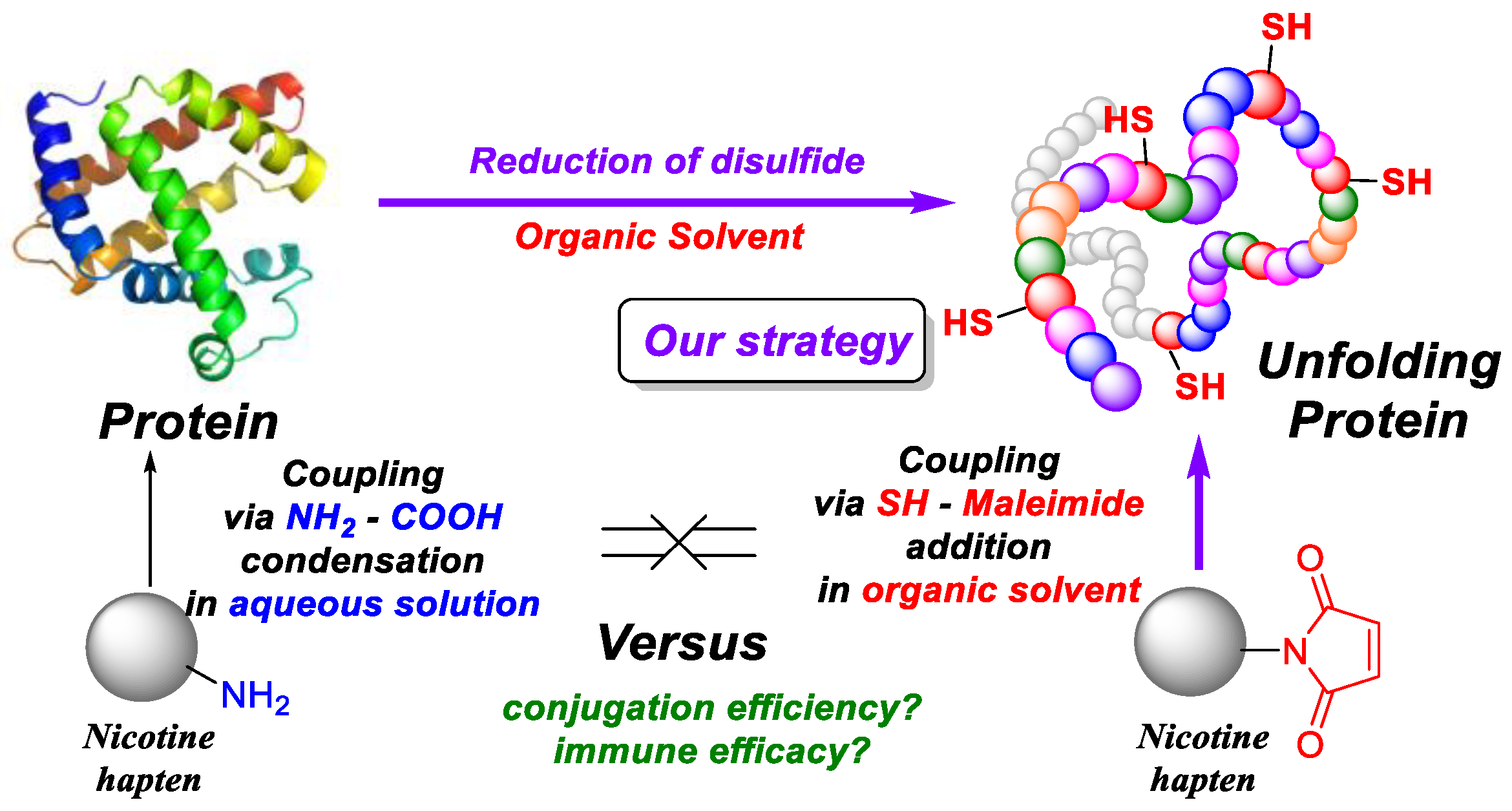
1. Introduction
2. Materials and Methods
2.1. Materials, Reagents, and Animals
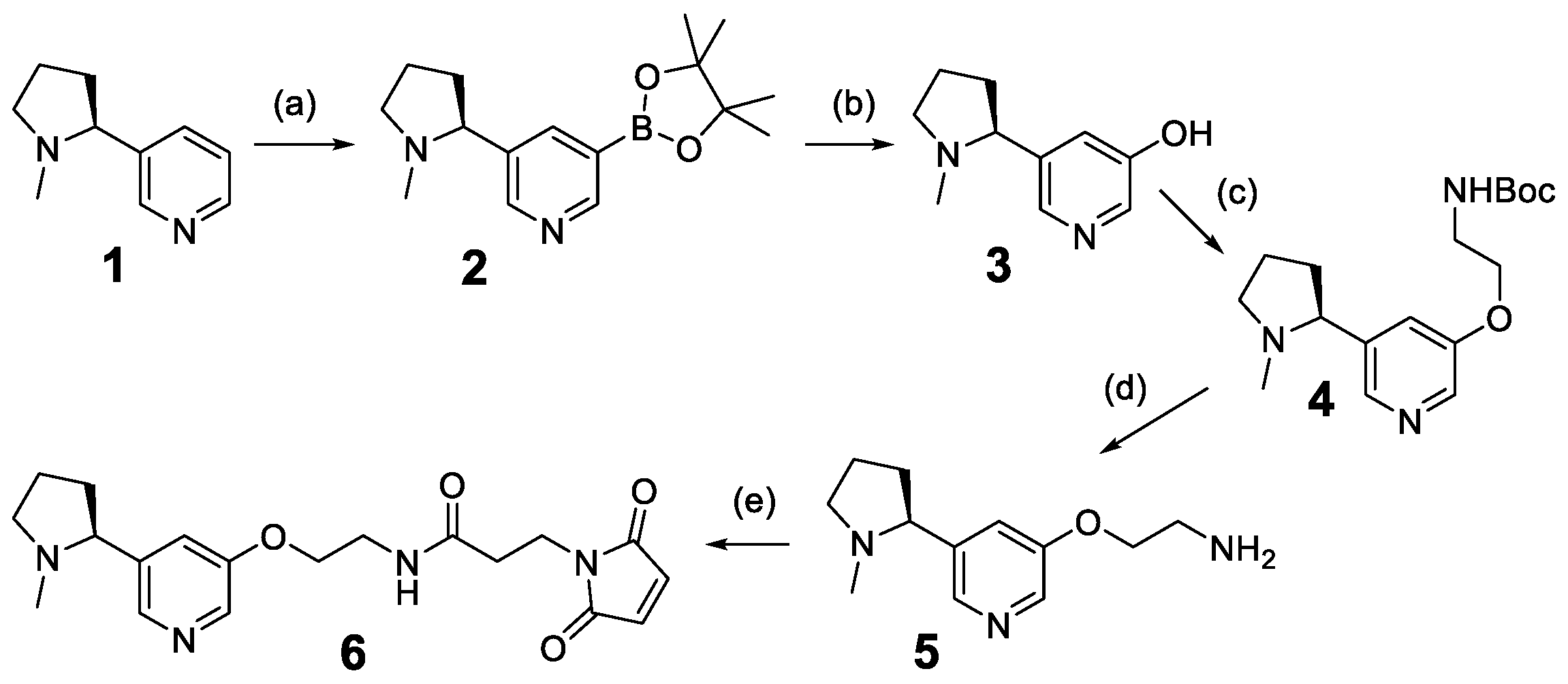
2.2. Chemical Synthesis
2.3. Protein Formulation
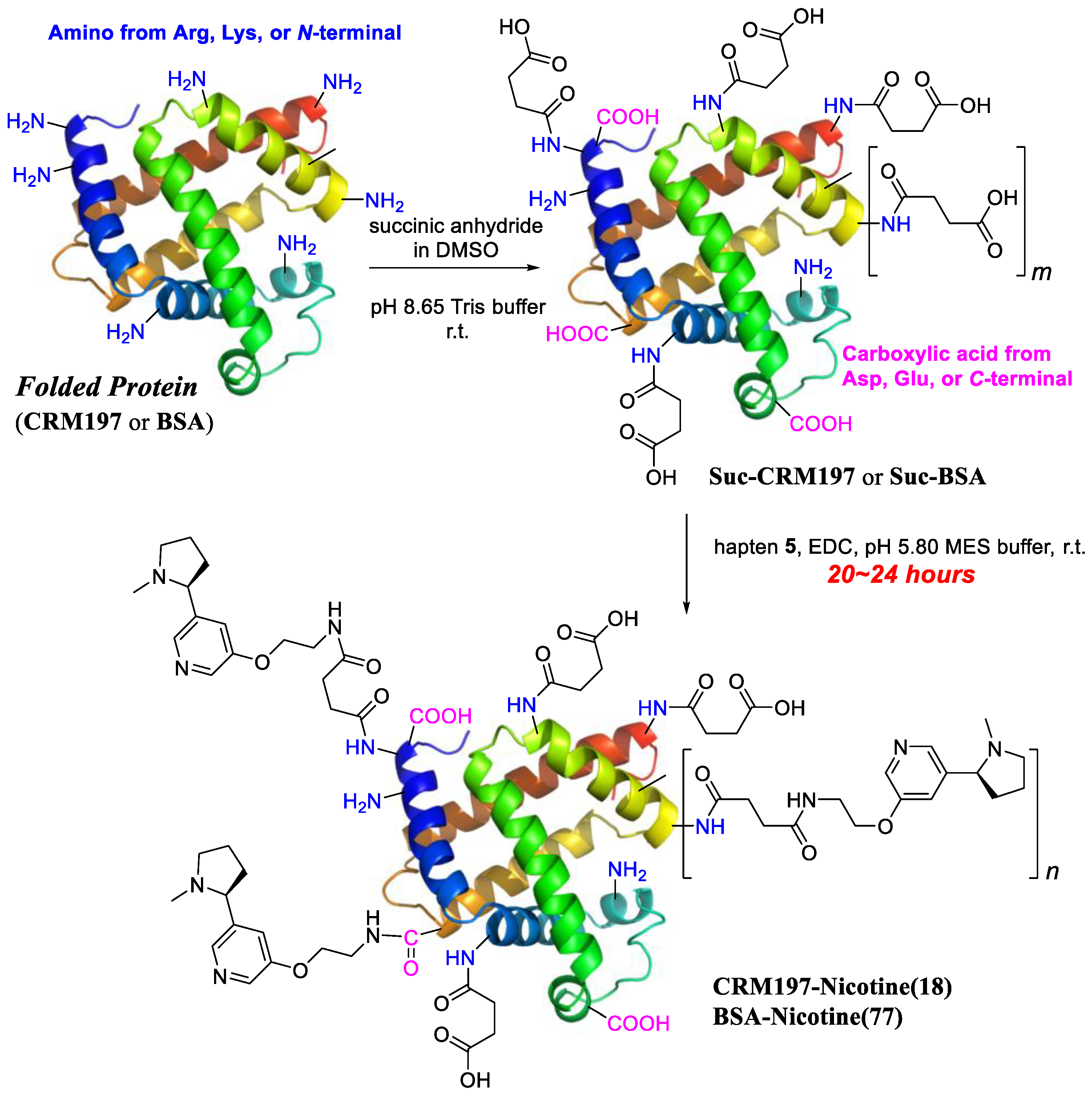
2.4. Conjugation
2.4.1. Conjugation in Buffer
2.4.2. Conjugation in DMF
2.5. Adjuvants
2.6. Immunological Test
2.6.1. Vaccine Formulation
2.6.2. Immunization
2.6.3. IgG Titer Determination
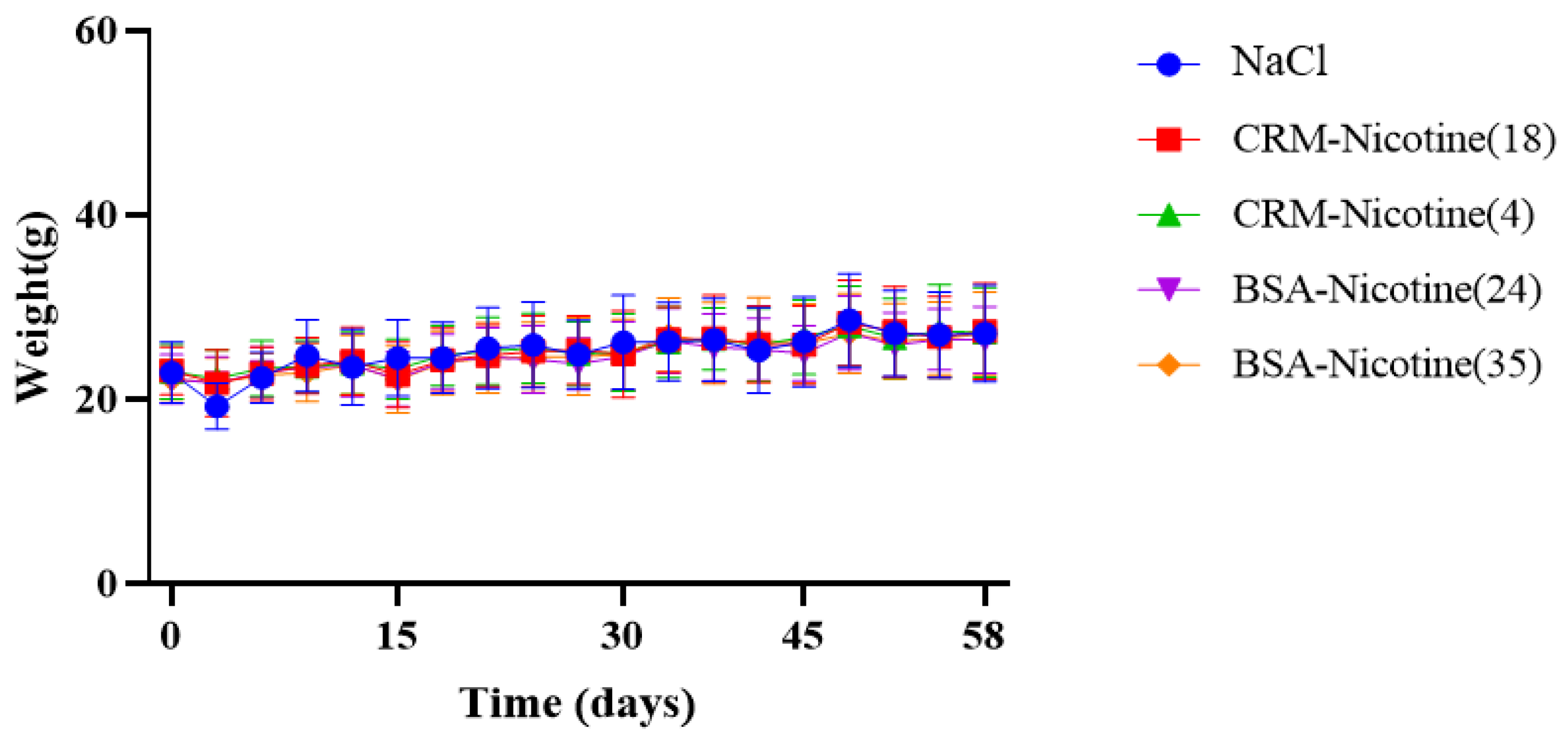
2.7. Clinical Observations
3. Results
3.1. Preparation of Anti-Nicotine Vaccines
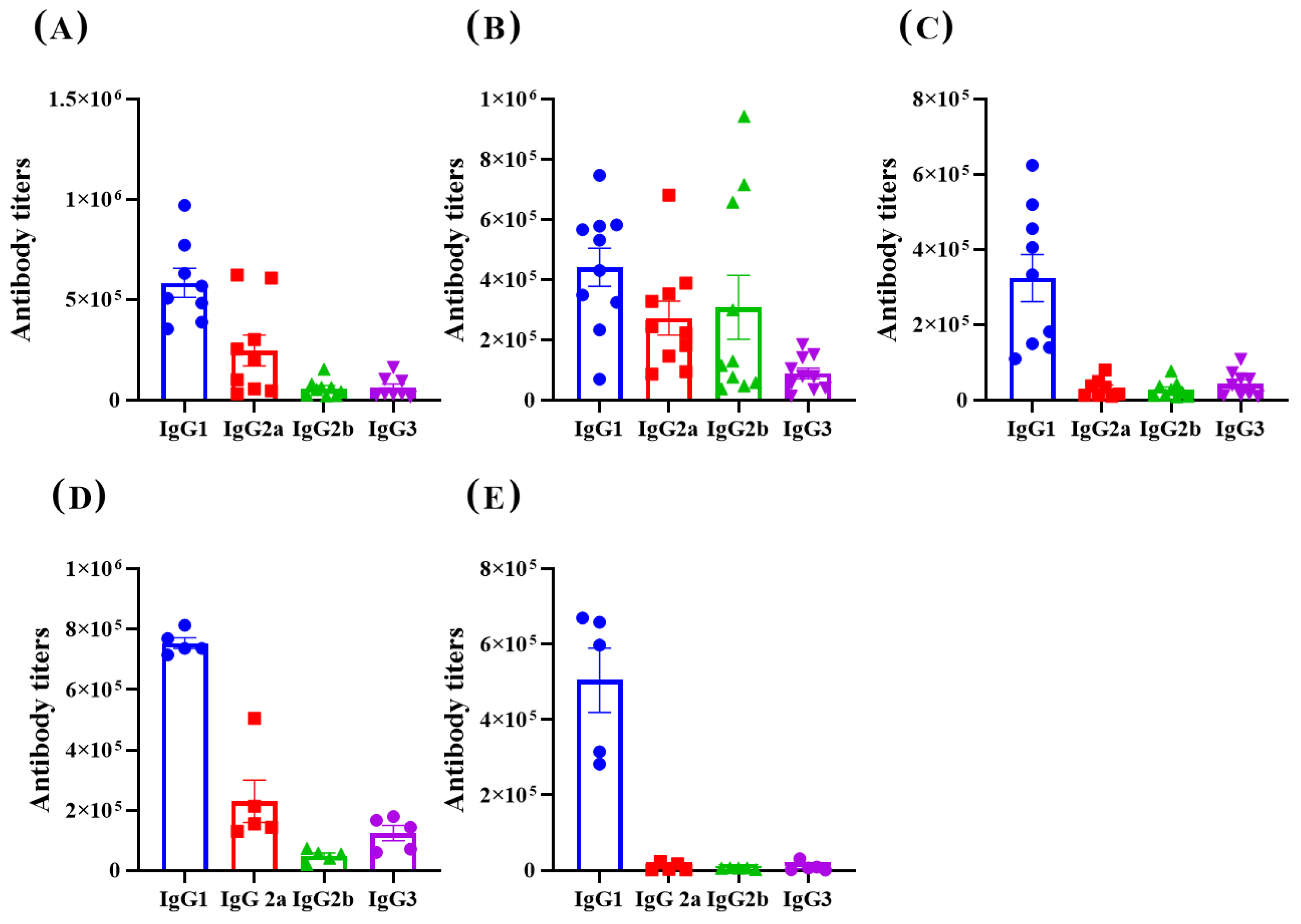
3.2. Immunological Effect
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Smith, L.C.; George, O. Advances in smoking cessation pharmacotherapy: Non-nicotinic approaches in animal models. Neuropharmacology 2020, 178, 108225–108247. [Google Scholar] [CrossRef] [PubMed]
- Goniewicz, M.L.; Delijewski, M. Nicotine vaccines to treat tobacco dependence. Hum. Vaccin. Immunother. 2013, 9, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Alzhrani, R.F.; Xu, H.; Valdes, S.A.; Cui, Z. Intranasal delivery of a nicotine vaccine candidate induces antibodies in mouse blood and lung mucosal secretions that specifically neutralize nicotine. Drug Dev. Ind. Pharm. 2020, 46, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- McCluskie, M.J.; Thorn, J.; Mehelic, P.R.; Kolhe, P.; Bhattacharya, K.; Finneman, J.I.; Stead, D.R.; Piatchek, M.B.; Zhang, N.; Chikh, G.; et al. Molecular attributes of conjugate antigen influence function of antibodies induced by anti-nicotine vaccine in mice and non-human primates. Int. Immunopharmacol. 2015, 25, 518–527. [Google Scholar] [CrossRef] [PubMed]
- McCluskie, M.J.; Thorn, J.; Gervais, D.P.; Stead, D.R.; Zhang, N.; Benoit, M.; Cartier, J.; Kim, I.-J.; Bhattacharya, K.; Finneman, J.I.; et al. Anti-nicotine vaccines: Comparison of adjuvanted CRM197 and Qb-VLP conjugate formulations for immunogenicity and function in non-human primates. Int. Immunopharmacol. 2015, 29, 663–671. [Google Scholar] [CrossRef]
- Lockner, J.W.; Lively, J.M.; Collins, K.C.; Vendruscolo, J.C.M.; Azar, M.R.; Janda, K.D. A Conjugate Vaccine Using Enantiopure Hapten Imparts Superior Nicotine-Binding Capacity. J. Med. Chem. 2015, 58, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Pryde, D.C.; Jones, L.H.; Gervais, D.P.; Stead, D.R.; Blakemore, D.C.; Selby, M.D.; Brown, A.D.; Coe, J.W.; Badland, M.; Beal, D.M.; et al. Selection of a Novel Anti-Nicotine Vaccine: Influence of Antigen Design on Antibody Function in Mice. PLoS ONE 2013, 8, e76557. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Pravetoni, M.; Bhayana, B.; Pentel, P.R.; Wu, M.X. High immunogenicity of nicotine vaccines obtained by intradermal delivery with safe adjuvants. Vaccine 2012, 31, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-Z.; Zhang, R.-Y.; Wang, X.-F.; Yin, X.-G.; Wang, J.; Wang, Y.-C.; Liu, X.; Du, J.-J.; Liu, Z.; Guo, J. Peptide-free Synthetic Nicotine Vaccine Candidates with α-Galactosylceramide as Adjuvant. Mol. Pharm. 2019, 16, 1467–1476. [Google Scholar] [CrossRef]
- Nouri-Shirazi, M.; Guinet, E. TLR3 and TLR7/8 agonists improve immunization outcome in nicotine exposed mice through different mechanisms. Immunol. Lett. 2022, 246, 18–26. [Google Scholar] [CrossRef]
- Heekin, R.D.; Shorter, D.; Kosten, T.R. Current status and future prospects for the development of substance abuse vaccines. Expert Rev. Vaccines 2017, 16, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Smith, D.; Zhao, Z.; Harmon, T.; Pentel, P.R.; Ehrich, M.; Zhang, C. Alum as an adjuvant for nanoparticle based vaccines: A case study with a hybrid nanoparticle-based nicotine vaccine. Nanomedicine 2019, 20, 102023. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Hu, Y.; Hoerle, R.; Devine, M.; Raleigh, M.; Pentel, P.; Zhang, C. A nanoparticle-based nicotine vaccine and the influence of particle size on its immunogenicity and efficacy. Nanomedicine 2017, 13, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Hu, Y.; Harmon, T.; Pentel, P.; Ehrich, M.; Zhang, C. Rationalization of a nanoparticle-based nicotine nanovaccine as an effective next-generation nicotine vaccine: A focus on hapten localization. Biomaterials 2017, 138, 46–56. [Google Scholar] [CrossRef]
- Hu, Y.; Smith, D.; Frazier, E.; Hoerle, R.; Ehrich, M.; Zhang, C. The next-generation nicotine vaccine: A novel and potent hybrid nanoparticle-based nicotine vaccine. Biomaterials 2016, 106, 228–239. [Google Scholar] [CrossRef]
- Hu, Y.; Zheng, H.; Huang, W.; Zhang, C. A novel and efficient nicotine vaccine using nano-lipoplex as a delivery vehicle. Hum. Vaccin. Immunother. 2014, 10, 64–72. [Google Scholar] [CrossRef]
- Lockner, J.W.; Ho, S.O.; McCague, K.C.; Chiang, S.M.; Do, T.Q.; Fujii, G.; Janda, K.D. Enhancing nicotine vaccine immunogenicity with liposomes. Bioorg. Med. Chem. Lett. 2013, 23, 975–978. [Google Scholar] [CrossRef]
- Zeigler, D.F.; Roque, R.; Clegg, C.H. Optimization of a multivalent peptide vaccine for nicotine addiction. Vaccine 2019, 37, 1584–1590. [Google Scholar] [CrossRef]
- Sun, T.; Mai, S.; Mao, H.; Li, H.; Duan, Y.; Meng, S.; Bao, J.; Ding, N.; Zong, C. Conjugate of structurally reassigned pneumococcal serotype 31 polysaccharide with CRM197 elicited potent immune response. Carbohydr. Polym. 2022, 289, 119414. [Google Scholar] [CrossRef]
- Didierlaurent, A.M.; Laupèze, B.; Di Pasquale, A.; Hergli, N.; Collignon, C.; Garçon, N. Adjuvant system AS01: Helping to overcome the challenges of modern vaccines. Expert Rev. Vaccines 2017, 16, 55–63. [Google Scholar] [CrossRef]
- de Jesus, R.; Hiesinger, K.; van Gemmeren, M. Preparative Scale Applications of C−H Activation in Medicinal Chemistry. Angew. Chem. Int. Ed. 2023, 62, e202306659. [Google Scholar] [CrossRef] [PubMed]
- Slack, E.D.; Colacot, T.J. Understanding the Activation of Air-Stable Ir(COD)(Phen)Cl Precatalyst for C–H Borylation of Aromatics and Heteroaromatics. Org. Lett. 2021, 23, 1561–1565. [Google Scholar] [CrossRef] [PubMed]
- Northrop, B.H.; Frayne, S.H.; Choudhary, U. Thiol–maleimide “click” chemistry: Evaluating the influence of solvent, initiator, and thiol on the reaction mechanism, kinetics, and selectivity. Polym. Chem. 2015, 6, 3415–3430. [Google Scholar] [CrossRef]
- Huang, W.; Wu, X.; Gao, X.; Yu, Y.; Lei, H.; Zhu, Z.; Shi, Y.; Chen, Y.; Qin, M.; Wang, W.; et al. Maleimide–thiol adducts stabilized through stretching. Nat. Chem. 2019, 11, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.P.; Smith, M.E.; Schumacher, F.F.; Grohmann, D.; Papaioannou, D.; Waksman, G.; Werner, F.; Baker, J.R.; Caddick, S. Tunable reagents for multi-functional bioconjugation: Reversible or permanent chemical modification of proteins and peptides by control of maleimide hydrolysis. Chem. Commun. 2011, 47, 5452–5454. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.D.; Kiick, K.L. Tunable Degradation of Maleimide–Thiol Adducts in Reducing Environments. Bioconjug. Chem. 2011, 22, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Alley, S.C.; Benjamin, D.R.; Jeffrey, S.C.; Okeley, N.M.; Meyer, D.L.; Sanderson, R.J.; Senter, P.D. Contribution of linker stability to the activities of anticancer immunoconjugates. Bioconjug. Chem. 2008, 19, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Betancourt, A.A.; González-Delgado, C.A.; Cinza-Estévez, Z.; Martínez-Cabrera, J.; Véliz-Ríos, G.; Alemán-Zaldívar, R.; Alonso-Martínez, M.I.; Lago-Baños, M.; Alvarez, N.P.; Fernandez, A.D.; et al. Safety and immunogenicity of a combined hepatitis B virus-Haemophilus influenzae type B vaccine comprising a synthetic antigen in healthy adults. Human Vaccines 2008, 4, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Verez-Bencomo, V.; Fernández-Santana, V.; Hardy, E.; Toledo, M.E.; Rodríguez, M.C.; Heynngnezz, L.; Rodriguez, A.; Baly, A.; Herrera, L.; Izquierdo, M.; et al. A Synthetic Conjugate Polysaccharide Vaccine Against Haemophilus influenzae Type b. Science 2004, 305, 522–525. [Google Scholar] [CrossRef]
- Pifferi, C.; Fuentes, R.; Fernández-Tejada, A. Natural and synthetic carbohydrate-based vaccine adjuvants and their mechanisms of action. Nat. Rev. Chem. 2021, 5, 197–216. [Google Scholar] [CrossRef]

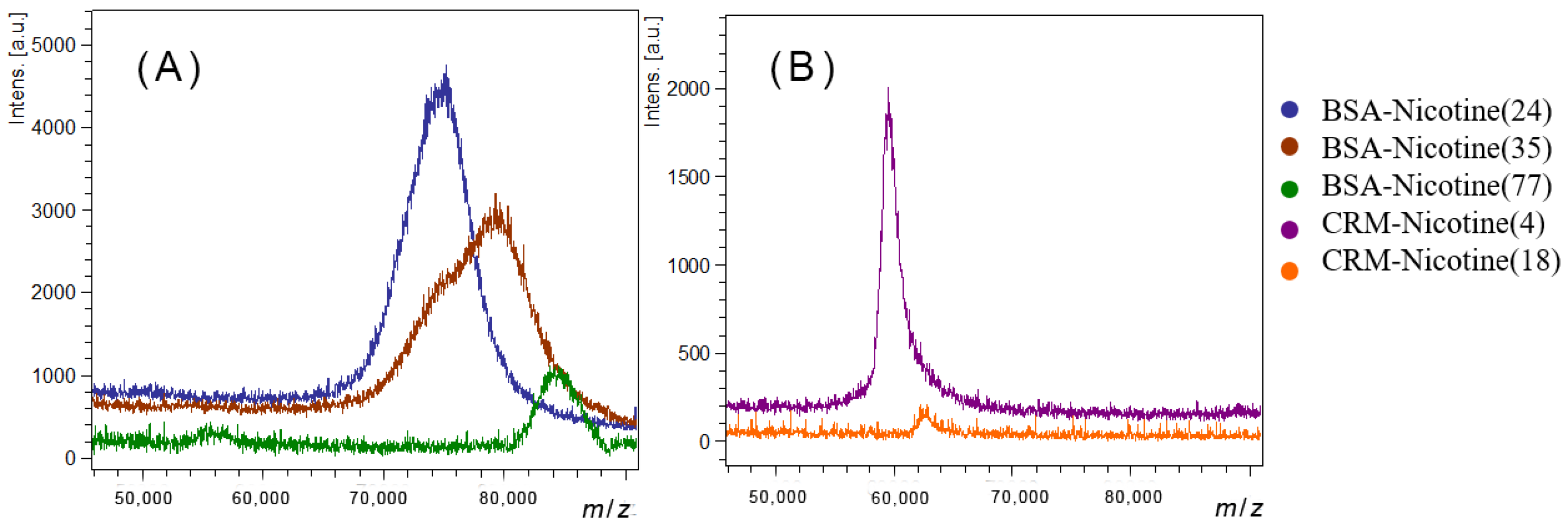


| Conjugate Name | Mass (m/z) | Nicotine Loading Number |
|---|---|---|
| CRM197-Nicotine(18) 1 | 62793 | 18 |
| CRM197-Nicotine(4) 2 | 59594 | 4 |
| BSA-Nicotine(24) 2 | 75020 | 24 |
| BSA-Nicotine(35) 2 | 79282 | 35 |
| BSA-Nicotine(77) 1 | 84341 | 77 |
| Vaccine Candidates | Adjuvant Content (μg/mL) | Nicotine Content (μg/mL) |
|---|---|---|
| CRM197-Nicotine(18) | MPL 100 + QS-21 50 | 20.0 |
| CRM197-Nicotine(4) | MPL 100 + QS-21 50 | 20.0 |
| BSA-Nicotine(24) | MPL 100 + QS-21 50 | 20.0 |
| BSA-Nicotine(35) | MPL 100 + QS-21 50 | 20.0 |
| BSA-Nicotine(77) | MPL 100 + QS-21 50 | 20.0 |
| BSA-Nicotine(77)/Al | Al(OH)3 1000 | 20.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Li, H.; Meng, X.; Yang, J.; Xue, Y.; Teng, C.; Lv, W.; Wang, Z.; Li, X.; Sun, T.; et al. Unfolding Protein-Based Hapten Coupling via Thiol–Maleimide Click Chemistry: Enhanced Immunogenicity in Anti-Nicotine Vaccines Based on a Novel Conjugation Method and MPL/QS-21 Adjuvants. Polymers 2024, 16, 931. https://doi.org/10.3390/polym16070931
Xu Y, Li H, Meng X, Yang J, Xue Y, Teng C, Lv W, Wang Z, Li X, Sun T, et al. Unfolding Protein-Based Hapten Coupling via Thiol–Maleimide Click Chemistry: Enhanced Immunogenicity in Anti-Nicotine Vaccines Based on a Novel Conjugation Method and MPL/QS-21 Adjuvants. Polymers. 2024; 16(7):931. https://doi.org/10.3390/polym16070931
Chicago/Turabian StyleXu, Ying, Huiting Li, Xiongyan Meng, Jing Yang, Yannan Xue, Changcai Teng, Wenxin Lv, Zhen Wang, Xiaodan Li, Tiantian Sun, and et al. 2024. "Unfolding Protein-Based Hapten Coupling via Thiol–Maleimide Click Chemistry: Enhanced Immunogenicity in Anti-Nicotine Vaccines Based on a Novel Conjugation Method and MPL/QS-21 Adjuvants" Polymers 16, no. 7: 931. https://doi.org/10.3390/polym16070931
APA StyleXu, Y., Li, H., Meng, X., Yang, J., Xue, Y., Teng, C., Lv, W., Wang, Z., Li, X., Sun, T., Meng, S., & Zong, C. (2024). Unfolding Protein-Based Hapten Coupling via Thiol–Maleimide Click Chemistry: Enhanced Immunogenicity in Anti-Nicotine Vaccines Based on a Novel Conjugation Method and MPL/QS-21 Adjuvants. Polymers, 16(7), 931. https://doi.org/10.3390/polym16070931







