Effect of Operational Variables on Supercritical Foaming of Caffeic Acid-Loaded Poly(lactic acid)/Poly(butylene adipate-co-terephthalate) Blends for the Development of Sustainable Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extrusion of PLA and PLA/PBAT Blends
2.3. Sequential Supercritical Impregnation and Foaming
2.3.1. Supercritical Impregnation of CA in PLA and PLA Polymeric Blends
2.3.2. Supercritical Foaming of Obtained Biocomposites
2.4. Impregnated Films and Foams Characterization
2.4.1. Determination of the CA Content of Impregnated Films and Polymer Foams
2.4.2. Thermogravimetric Analysis (TGA)
2.4.3. Differential Scanning Calorimetry (DSC)
2.4.4. Attenuated Total Reflectance–Fourier-Transform Infrared (ATR-FTIR) Spectroscopy
2.4.5. Scanning Electronic Microscopy (SEM) Analysis
2.5. Antioxidant Activity of the Obtained Films and Foams
3. Results and Discussion
3.1. Quantification of Caffeic Acid in Impregnated Films
3.2. Quantification of CA in Developed Foams
3.3. Thermal Properties of Obtained Impregnated Films and Foams
3.3.1. Thermogravimetric Analysis (TGA)
3.3.2. Differential Scanning Calorimetry (DSC)
3.4. Structural Properties of Developed Films and Foams
3.5. Morphological Analysis of the Obtained Materials
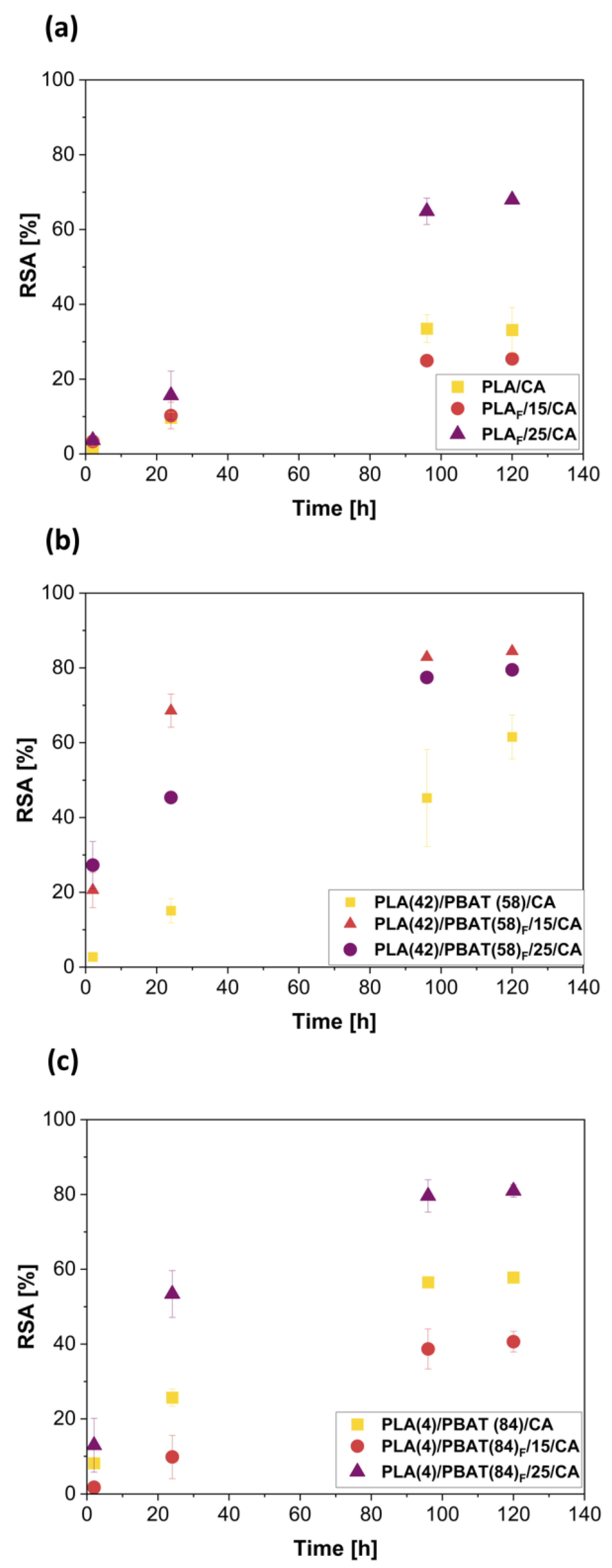
3.6. Antioxidant Activity of Obtained Films and Foams
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- PlasticsEurope AISBL Plastics—The Facts. 2022. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/ (accessed on 1 January 2024).
- Tapia-Blácido, D.R.; Aguilar, G.J.; de Andrade, M.T.; Rodrigues-Júnior, M.F.; Guareschi-Martins, F.C. Trends and Challenges of Starch-Based Foams for Use as Food Packaging and Food Container. Trends Food Sci. Technol. 2022, 119, 257–271. [Google Scholar] [CrossRef]
- Parker, K.; Garancher, J.-P.; Shah, S.; Fernyhough, A. Expanded Polylactic Acid—An Eco-Friendly Alternative to Polystyrene Foam. J. Cell. Plast. 2011, 47, 233–243. [Google Scholar] [CrossRef]
- Rai, P.; Mehrotra, S.; Priya, S.; Gnansounou, E.; Sharma, S.K. Recent Advances in the Sustainable Design and Applications of Biodegradable Polymers. Bioresour. Technol. 2021, 325, 124739. [Google Scholar] [CrossRef] [PubMed]
- Nofar, M.; Park, C.B. Poly (Lactic Acid) Foaming: A Review. Prog. Polym. Sci. 2014, 39, 1721–1741. [Google Scholar] [CrossRef]
- Peng, K.; Mubarak, S.; Diao, X.; Cai, Z.; Zhang, C.; Wang, J.; Wu, L. Progress in the Preparation, Properties, and Applications of PLA and Its Composite Microporous Materials by Supercritical CO2: A Review from 2020 to 2022. Polymers 2022, 14, 4320. [Google Scholar] [CrossRef] [PubMed]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(Lactic Acid) Modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Navarro-Baena, I.; Arrieta, M.P.; Sonseca, A.; Torre, L.; López, D.; Giménez, E.; Kenny, J.M.; Peponi, L. Biodegradable Nanocomposites Based on Poly(Ester-Urethane) and Nanosized Hydroxyapatite: Plastificant and Reinforcement Effects. Polym. Degrad. Stab. 2015, 121, 171–179. [Google Scholar] [CrossRef]
- Faba, S.; Arrieta, M.P.; Romero, J.; Agüero, Á.; Torres, A.; Martínez, S.; Rayón, E.; Galotto, M.J. Biodegradable Nanocomposite Poly(Lactic Acid) Foams Containing Carvacrol-Based Cocrystal Prepared by Supercritical CO2 Processing for Controlled Release in Active Food Packaging. Int. J. Biol. Macromol. 2024, 254, 127793. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.W.; Park, H.M.; Ha, C.S. Bio-Nanocomposites for Food Packaging Applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.C. Poly (Lactic Acid) Blends: Processing, Properties and Applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef]
- Boufarguine, M.; Guinault, A.; Miquelard-Garnier, G.; Sollogoub, C. PLA/PHBV Films with Improved Mechanical and Gas Barrier Properties. Macromol. Mater. Eng. 2013, 298, 1065–1073. [Google Scholar] [CrossRef]
- Arrieta, M.; Samper, M.; Aldas, M.; López, J. On the Use of PLA-PHB Blends for Sustainable Food Packaging Applications. Materials 2017, 10, 1008. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Mariano, M.; Lepesqueur, L.S.S.; Pinheiro, I.F.; Santos, L.G.; Burga-Sánchez, J.; Souza, D.H.S.; Koga-Ito, C.Y.; Teixeira-Neto, A.A.; Mei, L.H.I.; et al. Silver Nanoparticles Coated with Dodecanethiol Used as Fillers in Non-Cytotoxic and Antifungal PBAT Surface Based on Nanocomposites. Mater. Sci. Eng. C 2019, 98, 800–807. [Google Scholar] [CrossRef]
- Jian, J.; Xiangbin, Z.; Xianbo, H. An Overview on Synthesis, Properties and Applications of Poly(Butylene-Adipate-Co-Terephthalate)–PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26. [Google Scholar] [CrossRef]
- Jiang, L.; Wolcott, M.P.; Zhang, J. Study of Biodegradable Polylactide/Poly(Butylene Adipate- Co -Terephthalate) Blends. Biomacromolecules 2006, 7, 199–207. [Google Scholar] [CrossRef]
- Hernández-López, M.; Correa-Pacheco, Z.N.; Bautista-Baños, S.; Zavaleta-Avejar, L.; Benítez-Jiménez, J.J.; Sabino-Gutiérrez, M.A.; Ortega-Gudiño, P. Bio-Based Composite Fibers from Pine Essential Oil and PLA/PBAT Polymer Blend. Morphological, Physicochemical, Thermal and Mechanical Characterization. Mater. Chem. Phys. 2019, 234, 345–353. [Google Scholar] [CrossRef]
- Deng, Y.; Yu, C.; Wongwiwattana, P.; Thomas, N.L. Optimising Ductility of Poly(Lactic Acid)/Poly(Butylene Adipate-Co-Terephthalate) Blends Through Co-Continuous Phase Morphology. J. Polym. Environ. 2018, 26, 3802–3816. [Google Scholar] [CrossRef]
- Nofar, M.; Salehiyan, R.; Ciftci, U.; Jalali, A.; Durmuş, A. Ductility Improvements of PLA-Based Binary and Ternary Blends with Controlled Morphology Using PBAT, PBSA, and Nanoclay. Compos. B Eng. 2020, 182, 107661. [Google Scholar] [CrossRef]
- Sanches-Silva, A.; Costa, D.; Albuquerque, T.G.; Buonocore, G.G.; Ramos, F.; Castilho, M.C.; Machado, A.V.; Costa, H.S. Trends in the Use of Natural Antioxidants in Active Food Packaging: A Review. Food Addit. Contam. Part A 2014, 31, 374–395. [Google Scholar] [CrossRef]
- Wang, Y.; Du, H.; Xie, M.; Ma, G.; Yang, W.; Hu, Q.; Pei, F. Characterization of the Physical Properties and Biological Activity of Chitosan Films Grafted with Gallic Acid and Caffeic Acid: A Comparison Study. Food Packag. Shelf Life 2019, 22, 100401. [Google Scholar] [CrossRef]
- Luzi, F.; Torre, L.; Puglia, D. Antioxidant Packaging Films Based on Ethylene Vinyl Alcohol Copolymer (EVOH) and Caffeic Acid. Molecules 2020, 25, 3953. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Q.; Song, Q.-Y.; Su, J.-C.; Tang, W.; Song, J.-G.; Huang, X.-J.; An, J.; Li, Y.-L.; Ye, W.-C.; Wang, Y. Caffeic Acid Oligomers from Mesona Chinensis and Their In Vitro Antiviral Activities. Fitoterapia 2020, 144, 104603. [Google Scholar] [CrossRef] [PubMed]
- Cejudo Bastante, C.; Casas Cardoso, L.; Mantell Serrano, C.; Martínez de la Ossa, E.J. Supercritical Impregnation of Food Packaging Films to Provide Antioxidant Properties. J. Supercrit. Fluids 2017, 128, 200–207. [Google Scholar] [CrossRef]
- De Zordi, N.; Cortesi, A.; Kikic, I.; Moneghini, M.; Solinas, D.; Innocenti, G.; Portolan, A.; Baratto, G.; Dall’Acqua, S. The Supercritical Carbon Dioxide Extraction of Polyphenols from Propolis: A Central Composite Design Approach. J. Supercrit. Fluids 2014, 95, 491–498. [Google Scholar] [CrossRef]
- Pyne, S.; Paria, K. Optimization of Extraction Process Parameters of Caffeic Acid from Microalgae by Supercritical Carbon Dioxide Green Technology. BMC Chem. 2022, 16, 31. [Google Scholar] [CrossRef] [PubMed]
- García-Casas, I.; Montes, A.; Valor, D.; Pereyra, C.; Martínez de la Ossa, E.J. Foaming of Polycaprolactone and Its Impregnation with Quercetin Using Supercritical CO2. Polymers 2019, 11, 1390. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.A.; Virgilio, N.; Rouabhia, M.; Mighri, F. Polylactic Acid (PLA) Foaming: Design of Experiments for Cell Size Control. Mater. Sci. Appl. 2022, 13, 63–77. [Google Scholar] [CrossRef]
- Hrnčič, M.K.; Cör, D.; Verboten, M.T.; Knez, Ž. Application of Supercritical and Subcritical Fluids in Food Processing. Food Qual. Saf. 2018, 2, 59–67. [Google Scholar] [CrossRef]
- Gaglio, R.; Botta, L.; Garofalo, G.; Miceli, A.; Settanni, L.; Lopresti, F. Carvacrol Activated Biopolymeric Foam: An Effective Packaging System to Control the Development of Spoilage and Pathogenic Bacteria on Sliced Pumpkin and Melon. Food Packag. Shelf Life 2021, 28, 100633. [Google Scholar] [CrossRef]
- Ameri, A.; Sodeifian, G.; Sajadian, S.A. Lansoprazole Loading of Polymers by Supercritical Carbon Dioxide Impregnation: Impacts of Process Parameters. J. Supercrit. Fluids 2020, 164, 104892. [Google Scholar] [CrossRef]
- Bouledjouidja, A.; Masmoudi, Y.; Sergent, M.; Badens, E. Effect of Operational Conditions on the Supercritical Carbon Dioxide Impregnation of Anti-Inflammatory and Antibiotic Drugs in Rigid Commercial Intraocular Lenses. J. Supercrit. Fluids 2017, 130, 63–75. [Google Scholar] [CrossRef]
- Rivera, P.; Villegas, C.; Cabezas, R.; Pérez, B.; Torres, A.; de Dicastillo, C.L.; Garrido, L.; Galvez, P.; Araya, C.; Romero, J. Development of PLA Suture Materials by Extrusion, Electrospinning and Supercritical CO2 Impregnation of Ibuprofen and Naproxen. J. Supercrit. Fluids 2023, 194, 105854. [Google Scholar] [CrossRef]
- Goñi, M.L.; Gañán, N.A.; Strumia, M.C.; Martini, R.E. Eugenol-Loaded LLDPE Films with Antioxidant Activity by Supercritical Carbon Dioxide Impregnation. J. Supercrit. Fluids 2016, 111, 28–35. [Google Scholar] [CrossRef]
- Torres, A.; Ilabaca, E.; Rojas, A.; Rodríguez, F.; Galotto, M.J.; Guarda, A.; Villegas, C.; Romero, J. Effect of Processing Conditions on the Physical, Chemical and Transport Properties of Polylactic Acid Films Containing Thymol Incorporated by Supercritical Impregnation. Eur. Polym. J. 2017, 89, 195–210. [Google Scholar] [CrossRef]
- Torres, A.; Romero, J.; Macan, A.; Guarda, A.; Galotto, M.J. Near Critical and Supercritical Impregnation and Kinetic Release of Thymol in LLDPE Films Used for Food Packaging. J. Supercrit. Fluids 2014, 85, 41–48. [Google Scholar] [CrossRef]
- Faba, S.; Arrieta, M.P.; Agüero, Á.; Torres, A.; Romero, J.; Rojas, A.; Galotto, M.J. Processing Compostable PLA/Organoclay Bionanocomposite Foams by Supercritical CO2 Foaming for Sustainable Food Packaging. Polymers 2022, 14, 4394. [Google Scholar] [CrossRef]
- Okolieocha, C.; Raps, D.; Subramaniam, K.; Altstädt, V. Microcellular to Nanocellular Polymer Foams: Progress (2004–2015) and Future Directions—A Review. Eur. Polym. J. 2015, 73, 500–519. [Google Scholar] [CrossRef]
- Chauvet, M.; Sauceau, M.; Baillon, F.; Fages, J. Mastering the Structure of PLA Foams Made with Extrusion Assisted by Supercritical CO2. J. Appl. Polym. Sci. 2017, 134, 45067. [Google Scholar] [CrossRef]
- Tsivintzelis, I.; Sanxaridou, G.; Pavlidou, E.; Panayiotou, C. Foaming of Polymers with Supercritical Fluids: A Thermodynamic Investigation. J. Supercrit. Fluids 2016, 110, 240–250. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, B.; Wang, Y.; Liu, C.; Shen, C. Effect of Different Sterilization Methods on the Properties of Commercial Biodegradable Polyesters for Single-Use, Disposable Medical Devices. Mater. Sci. Eng. C 2019, 105, 110041. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Gammino, M.; La Mantia, F.P. Effect of an Organoclay on the Photochemical Transformations of a PBAT/PLA Blend and Morpho-Chemical Features of Crosslinked Networks. Polym. Degrad. Stab. 2021, 187, 109549. [Google Scholar] [CrossRef]
- Mistretta, M.C.; La Mantia, F.P.; Titone, V.; Botta, L.; Pedeferri, M.; Morreale, M. Effect of Ultraviolet and Moisture Action on Biodegradable Polymers and Their Blend. J. Appl. Biomater. Funct. Mater. 2020, 18, 2280800020926653. [Google Scholar] [CrossRef]
- Nofar, M.; Tabatabaei, A.; Sojoudiasli, H.; Park, C.B.; Carreau, P.J.; Heuzey, M.C.; Kamal, M.R. Mechanical and Bead Foaming Behavior of PLA-PBAT and PLA-PBSA Blends with Different Morphologies. Eur. Polym. J. 2017, 90, 231–244. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Arrua, D.; Strumia, M.C.; Nazareno, M.A. Immobilization of Caffeic Acid on a Polypropylene Film: Synthesis and Antioxidant Properties. J. Agric. Food Chem. 2010, 58, 9228–9234. [Google Scholar] [CrossRef]
- Pilla, S.; Kim, S.G.; Auer, G.K.; Gong, S.; Park, C.B. Microcellular Extrusion Foaming of Poly(Lactide)/Poly(Butylene Adipate-Co-Terephthalate) Blends. Mater. Sci. Eng. C 2010, 30, 255–262. [Google Scholar] [CrossRef]
- Morlin, B.; Litauszki, K.; Petrény, R.; Kmetty, Á.; Mészáros, L. Characterization of Polylactic Acid-Based Nanocomposite Foams with Supercritical CO2. Measurement 2021, 178, 109385. [Google Scholar] [CrossRef]
- Kuska, R.; Milovanovic, S.; Frerich, S.; Ivanovic, J. Thermal Analysis of Polylactic Acid under High CO2 Pressure Applied in Supercritical Impregnation and Foaming Process Design. J. Supercrit. Fluids 2018, 144, 71–80. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Ray, S.S.; Okamoto, M.; Ogami, A.; Yamada, K.; Ueda, K. Well-Controlled Biodegradable Nanocomposite Foams: From Microcellular to Nanocellular. Macromol. Rapid Commun. 2003, 24, 457–461. [Google Scholar] [CrossRef]
- Costa, V.P.; Braga, M.E.M.; Guerra, J.P.; Duarte, A.R.C.; Duarte, C.M.M.; Leite, E.O.B.; Gil, M.H.; de Sousa, H.C. Development of Therapeutic Contact Lenses Using a Supercritical Solvent Impregnation Method. J. Supercrit. Fluids 2010, 52, 306–316. [Google Scholar] [CrossRef]
- Masmoudi, Y.; Ben Azzouk, L.; Forzano, O.; Andre, J.-M.; Badens, E. Supercritical Impregnation of Intraocular Lenses. J. Supercrit. Fluids 2011, 60, 98–105. [Google Scholar] [CrossRef]
- de Souza, A.C.; Dias, A.M.A.; Sousa, H.C.; Tadini, C.C. Impregnation of Cinnamaldehyde into Cassava Starch Biocomposite Films Using Supercritical Fluid Technology for the Development of Food Active Packaging. Carbohydr. Polym. 2014, 102, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Villegas, C.; Torres, A.; Bruna, J.; Bustos, M.I.; Díaz-Barrera, A.; Romero, J.; Rojas, A.; Guarda, A. Obtaining Active Polylactide (PLA) and Polyhydroxybutyrate (PHB) Blends Based Bionanocomposites Modified with Graphene Oxide and Supercritical Carbon Dioxide (ScCO2)-Assisted Cinnamaldehyde: Effect on Thermal-Mechanical, Disintegration and Mass Transport. Polymers 2021, 13, 3968. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.M.D.; Leite, M.C.A.M.; de Sousa, A.M.F.; Furtado, C.R.G.; Escócio, V.A.; da Silva, A.L.N. Improvement in Toughness of Polylactide/Poly(Butylene Adipate-Co-Terephthalate) Blend by Adding Nitrile Rubber. Polym. Bull. 2017, 74, 1713–1726. [Google Scholar] [CrossRef]
- Ma, F.; Wang, B.; Leng, X.; Wang, Y.; Sun, Z.; Wang, P.; Sang, L.; Wei, Z. Biodegradable PBAT/PLA/CaCO3 Blowing Films with Enhanced Mechanical and Barrier Properties: Investigation of Size and Content of CaCO3 Particles. Macromol. Mater. Eng. 2022, 307. [Google Scholar] [CrossRef]
- Rocha, D.B.; Souza de Carvalho, J.; de Oliveira, S.A.; dos Santos Rosa, D. A New Approach for Flexible PBAT/PLA/CaCO3 Films into Agriculture. J. Appl. Polym. Sci. 2018, 135, 1–9. [Google Scholar] [CrossRef]
- Teamsinsungvon, A.; Jarapanyacheep, R.; Ruksakulpiwat, Y.; Jarukumjorn, K. Melt Processing of Maleic Anhydride Grafted Poly(Lactic Acid) and Its Compatibilizing Effect on Poly(Lactic Acid)/Poly(Butylene Adipate-Co-Terephthalate) Blend and Their Composite. Polym. Sci. Ser. A 2017, 59, 384–396. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Ayyoob, M.; Joo, J.; Deri, F. Polylactic Acid Blends: The Future of Green, Light and Tough. Prog. Polym. Sci. 2018, 85, 83–127. [Google Scholar] [CrossRef]
- La Mantia, F.; Ascione, L.; Mistretta, M.; Rapisarda, M.; Rizzarelli, P. Comparative Investigation on the Soil Burial Degradation Behaviour of Polymer Films for Agriculture before and after Photo-Oxidation. Polymers 2020, 12, 753. [Google Scholar] [CrossRef]
- Cejudo Bastante, C.; Casas Cardoso, L.; Fernández-Ponce, M.T.; Mantell Serrano, C.; Martínez de la Ossa, E.J. Supercritical Impregnation of Olive Leaf Extract to Obtain Bioactive Films Effective in Cherry Tomato Preservation. Food Packag. Shelf Life 2019, 21, 100338. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, G.; Liu, Y.; Ma, Z.; Jing, Z.; Fan, X. Microcellular Foaming of Polylactide and Poly(Butylene Adipate-Co-Terphathalate) Blends and Their CaCO3 Reinforced Nanocomposites Using Supercritical Carbon Dioxide. Polym. Adv. Technol. 2016, 27, 550–560. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization Behavior of Poly(ε-Caprolactone)/Layered Double Hydroxide Nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; Arrigo, R.; Baiamonte, M.; Rizzarelli, P.; Curcuruto, G. Concentration-Dependent Anti-/pro-Oxidant Activity of Natural Phenolic Compounds in Bio-Polyesters. Polym. Degrad. Stab. 2017, 142, 21–28. [Google Scholar] [CrossRef]
- Liu, W.; Chen, P.; Wang, X.; Wang, F.; Wu, Y. Effects of Poly(Butyleneadipate-Co-Terephthalate) as a Macromolecular Nucleating Agent on the Crystallization and Foaming Behavior of Biodegradable Poly(Lactic Acid). Cell. Polym. 2017, 36, 75–96. [Google Scholar] [CrossRef]
- Hao, A.; Geng, Y.; Xu, Q.; Lu, Z.; Yu, L. Study of Different Effects on Foaming Process of Biodegradable PLA/Starch Composites in Supercritical/Compressed Carbon Dioxide. J. Appl. Polym. Sci. 2008, 109, 2679–2686. [Google Scholar] [CrossRef]
- Carrasco, F.; Pagès, P.; Gámez-Pérez, J.; Santana, O.O.; Maspoch, M.L. Processing of Poly(Lactic Acid): Characterization of Chemical Structure, Thermal Stability and Mechanical Properties. Polym. Degrad. Stab. 2010, 95, 116–125. [Google Scholar] [CrossRef]
- Ni, J.; Yu, K.; Zhou, H.; Mi, J.; Chen, S.; Wang, X. Morphological Evolution of PLA Foam from Microcellular to Nanocellular Induced by Cold Crystallization Assisted by Supercritical CO2. J. Supercrit. Fluids 2020, 158, 104719. [Google Scholar] [CrossRef]
- Pai, A.J.; Sarojini, B.K.; Harshitha, K.R.; Shivarama Holla, B.; Lobo, A.G. Spectral, Morphological and Optical Studies on Bischalcone Doped Polylactic Acid (PLA) Thin Films as Luminescent and UV Radiation Blocking Materials. Opt. Mater. 2019, 90, 145–151. [Google Scholar] [CrossRef]
- Moliner, C.; Finocchio, E.; Arato, E.; Ramis, G.; Lagazzo, A. Influence of the Degradation Medium on Water Uptake, Morphology, and Chemical Structure of Poly(Lactic Acid)-Sisal Bio-Composites. Materials 2020, 13, 3974. [Google Scholar] [CrossRef]
- Ramezani, M.; Amoozegar, M.A.; Ventosa, A. Screening and Comparative Assay of Poly-Hydroxyalkanoates Produced by Bacteria Isolated from the Gavkhooni Wetland in Iran and Evaluation of Poly-β-Hydroxybutyrate Production by Halotolerant Bacterium Oceanimonas Sp. GK1. Ann. Microbiol. 2015, 65, 517–526. [Google Scholar] [CrossRef]
- Villegas, C.; Torres, A.; Rios, M.; Rojas, A.; Romero, J.; de Dicastillo, C.L.; Valenzuela, X.; Galotto, M.J.; Guarda, A. Supercritical Impregnation of Cinnamaldehyde into Polylactic Acid as a Route to Develop Antibacterial Food Packaging Materials. Food Res. Int. 2017, 99, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Nunes, F.C.; Ribeiro, K.C.; Martini, F.A.; Barrioni, B.R.; Santos, J.P.F.; Melo Carvalho, B. PBAT/PLA/Cellulose Nanocrystals Biocomposites Compatibilized with Polyethylene Grafted Maleic Anhydride (PE-g-MA). J. Appl. Polym. Sci. 2021, 138, 1–11. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, R.; Shao, C.; Wang, Y.; Shen, C. Cold Crystallization Behavior of Glassy Poly(Lactic Acid) Prepared by Rapid Compression. Polym. Eng. Sci. 2015, 55, 359–366. [Google Scholar] [CrossRef]
- Pérez Davila, S.; González Rodríguez, L.; Chiussi, S.; Serra, J.; González, P. How to Sterilize Polylactic Acid Based Medical Devices? Polymers 2021, 13, 2115. [Google Scholar] [CrossRef] [PubMed]
- Cejudo Bastante, C.; Cran, M.J.; Casas Cardoso, L.; Mantell Serrano, C.; Martínez de la Ossa, E.J.; Bigger, S.W. Effect of Supercritical CO2 and Olive Leaf Extract on the Structural, Thermal and Mechanical Properties of an Impregnated Food Packaging Film. J. Supercrit. Fluids 2019, 145, 181–191. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Balyklova, K.S.; Gegechkori, V.; Morton, D.W. HPTLC and ATR/FTIR Characterization of Antioxidants in Different Rosemary Extracts. Molecules 2021, 26, 6064. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Hernández, I.; Afseth, N.K.; López-Luke, T.; Contreras-Torres, F.F.; Wold, J.P.; Ornelas-Soto, N. Surface Enhanced Raman Spectroscopy of Phenolic Antioxidants: A Systematic Evaluation of Ferulic Acid, p -Coumaric Acid, Caffeic Acid and Sinapic Acid. Vib. Spectrosc. 2017, 89, 113–122. [Google Scholar] [CrossRef]
- García-Arroyo, P.; Arrieta, M.P.; Garcia-Garcia, D.; Cuervo-Rodríguez, R.; Fombuena, V.; Mancheño, M.J.; Segura, J.L. Plasticized Poly(Lactic Acid) Reinforced with Antioxidant Covalent Organic Frameworks (COFs) as Novel Nanofillers Designed for Non-Migrating Active Packaging Applications. Polymer 2020, 196, 122466. [Google Scholar] [CrossRef]
- Chen, R.; Abdelwahab, M.A.; Misra, M.; Mohanty, A.K. Biobased Ternary Blends of Lignin, Poly(Lactic Acid), and Poly(Butylene Adipate-Co-Terephthalate): The Effect of Lignin Heterogeneity on Blend Morphology and Compatibility. J. Polym. Environ. 2014, 22, 439–448. [Google Scholar] [CrossRef]
- Correa-Pacheco, Z.N.; Black-Solís, J.D.; Ortega-Gudiño, P.; Sabino-Gutiérrez, M.A.; Benítez-Jiménez, J.J.; Barajas-Cervantes, A.; Bautista-Baños, S.; Hurtado-Colmenares, L.B. Preparation and Characterization of Bio-Based PLA/PBAT and Cinnamon Essential Oil Polymer Fibers and Life-Cycle Assessment from Hydrolytic Degradation. Polymers 2019, 12, 38. [Google Scholar] [CrossRef]
- Hutchinson, A.R.; Iglauer, S. Adhesion of Construction Sealants to Polymer Foam Backer Rod Used in Building Construction. Int. J. Adhes. Adhes. 2006, 26, 555–566. [Google Scholar] [CrossRef]
- Valor, D.; Montes, A.; Monteiro, M.; García-Casas, I.; Pereyra, C.; de la Ossa, E.M. Determining the Optimal Conditions for the Production by Supercritical Co2 of Biodegradable Plga Foams for the Controlled Release of Rutin as a Medical Treatment. Polymers 2021, 13, 1645. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Xue, K.; Liu, Z.; Xu, Z.; Zhao, L. The Essential Role of PBS on PBAT Foaming under Supercritical CO2 toward Green Engineering. J. CO2 Util. 2022, 60, 101965. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, K.; Wu, J.; Muhunthan, B.; Shi, X. Evaluation of Mechanical Performance and Modification Mechanism of Asphalt Modified with Graphene Oxide and Warm Mix Additives. J. Clean. Prod. 2018, 193, 87–96. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Rayón, E.; López, J.; Arrieta, M.P. Enhancing the Thermal Stability of Polypropylene by Blending with Low Amounts of Natural Antioxidants. Macromol. Mater. Eng. 2019, 304, 1900379. [Google Scholar] [CrossRef]
- Ignatova, M.; Manolova, N.; Rashkov, I.; Markova, N. Quaternized Chitosan/κ-Carrageenan/Caffeic Acid–Coated Poly(3-Hydroxybutyrate) Fibrous Materials: Preparation, Antibacterial and Antioxidant Activity. Int. J. Pharm. 2016, 513, 528–537. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Castro-López, M.D.M.; Rayón, E.; Barral-Losada, L.F.; López-Vilariño, J.M.; López, J.; González-Rodríguez, M.V. Plasticized Poly(Lactic Acid)-Poly(Hydroxybutyrate) (PLA-PHB) Blends Incorporated with Catechin Intended for Active Food-Packaging Applications. J. Agric. Food. Chem. 2014, 62, 10170–10180. [Google Scholar] [CrossRef]

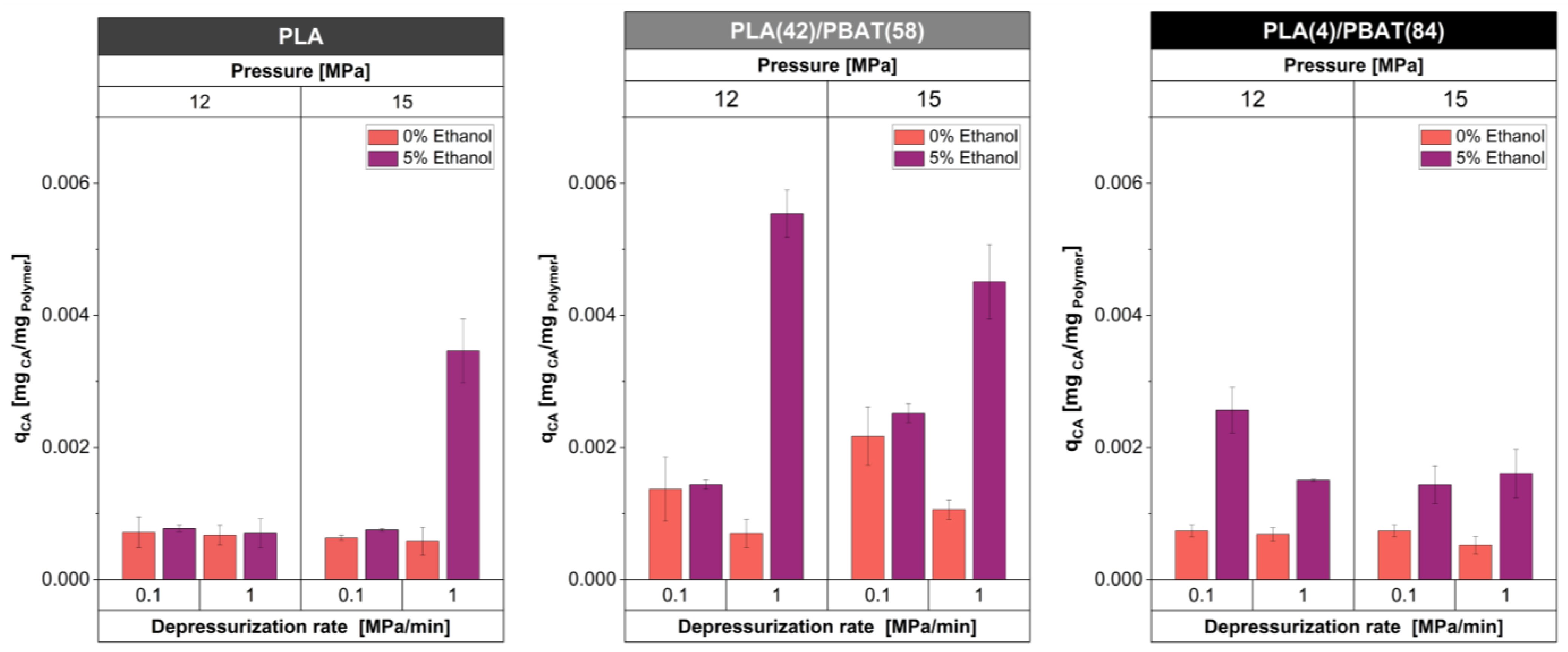
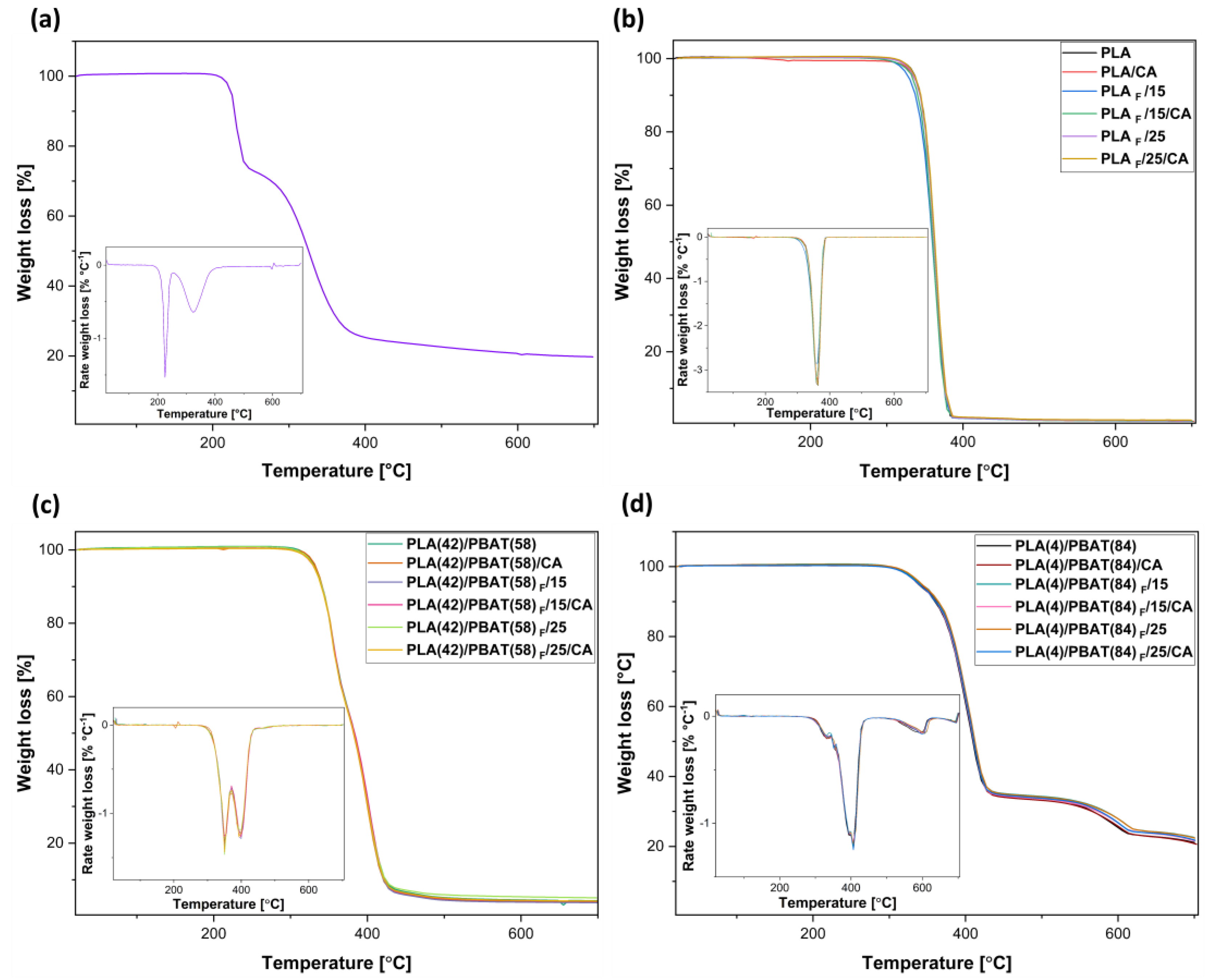
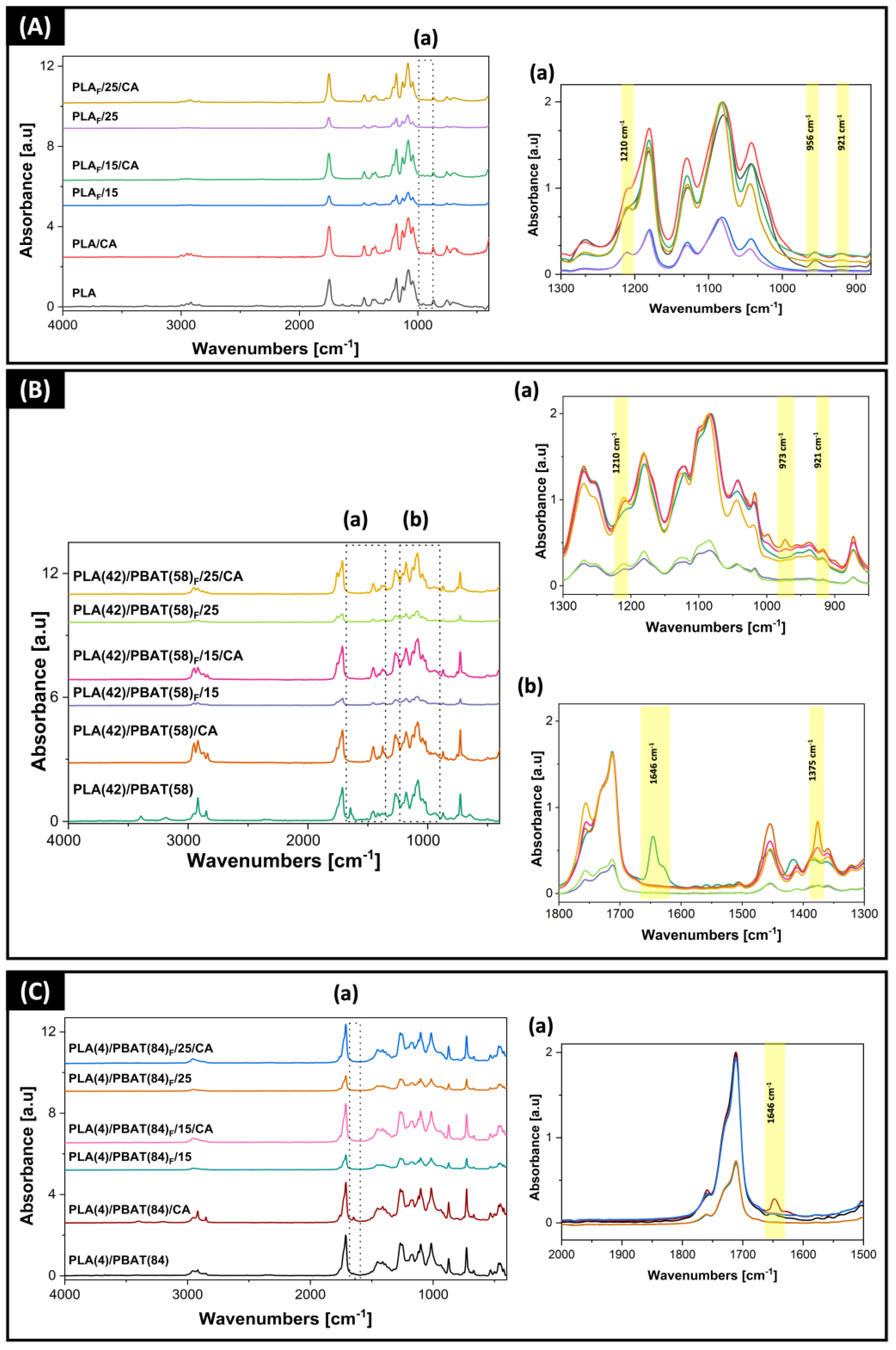

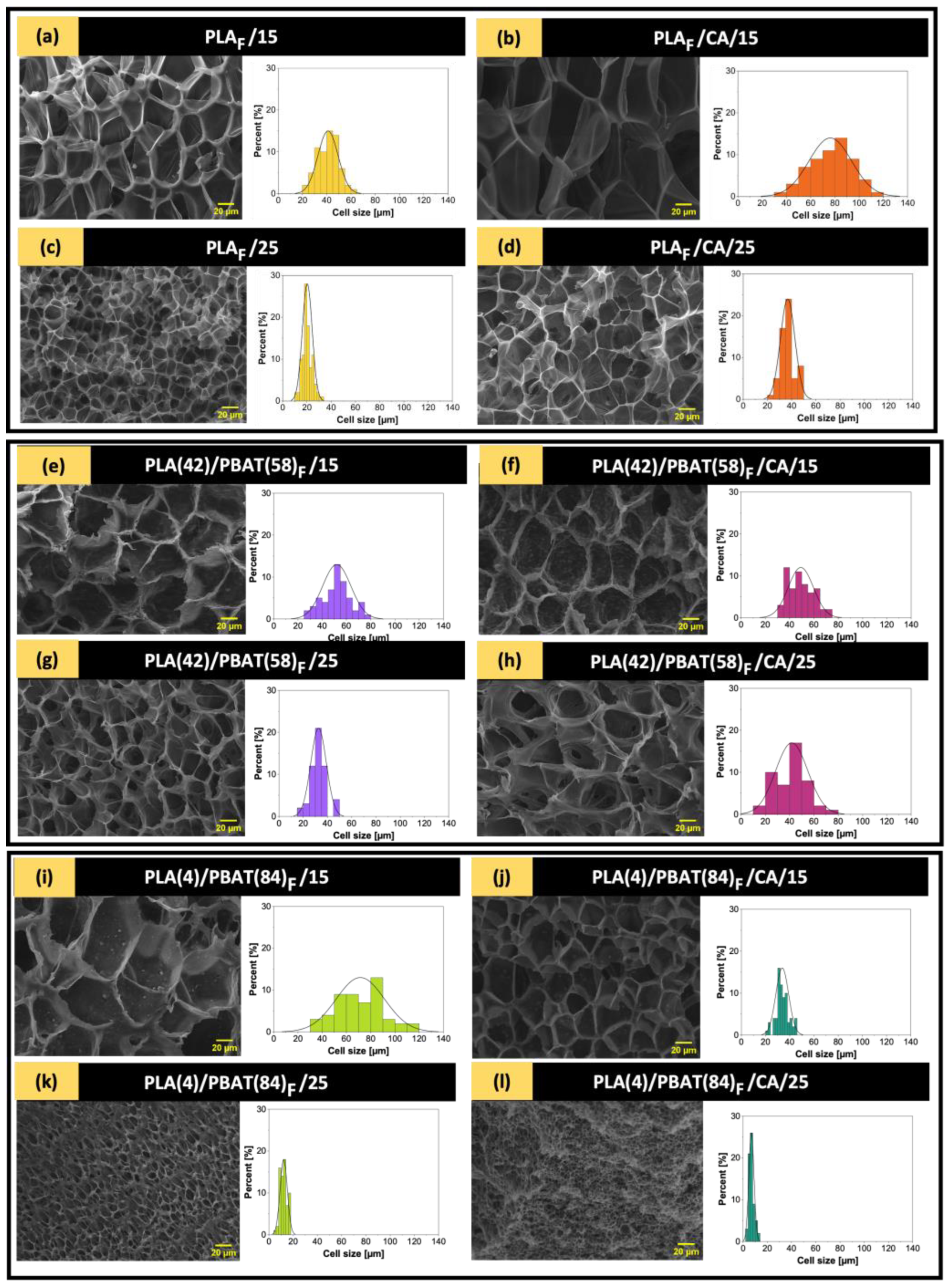
| Sample | Pressure [MPa] | Depressurization Rate [MPa/min] | Co-Solvent [wt%] |
|---|---|---|---|
| PLA/CA | 15 | 1 | 5 |
| PLA(42)/PBAT(58)/CA | 12 | 1 | 5 |
| PLA(4)/PBAT(84)/CA | 12 | 0.1 | 5 |
| Sample | Type | Pressure [MPa] | qCA [mg Caffeic Acid/mg Polymer] |
|---|---|---|---|
| PLA/CA | Film | - | 0.0035 ± 0.0005 |
| PLAF/CA | Foam | 15 | 0.0007 ± 0.0001 |
| PLAF/CA | Foam | 25 | 0.0007 ± 0.0003 |
| PLA(42)/PBAT(58)/CA | Film | - | 0.0055 ± 0.0004 |
| PLA(42)/PBAT(58)F/CA | Foam | 15 | 0.0032 ± 0.0001 |
| PLA(42)/PBAT(58)F/CA | Foam | 25 | 0.0021 ± 0.0003 |
| PLA(4)/PBAT(84)/CA | Film | - | 0.0026 ± 0.0003 |
| PLA(4)/PBAT(84)F/CA | Foam | 15 | 0.0016 ± 0.0002 |
| PLA(4)/PBAT(84)F/CA | Foam | 25 | 0.0011 ± 0.0001 |
| Sample | Tg PBAT [°C] | Tg PLA [°C] | Tm PBAT [°C] | ΔHm PBAT [J/g] | Tm PLA [°C] | ΔHm PLA [J/g] | %Xc PLA |
|---|---|---|---|---|---|---|---|
| PLA | N.D. | 58.1 ± 0.2 d | N.D. | N.D. | 148.2 ± 0.2 b | 29.2 ± 0.4 a | 4.1 ± 0.2 a |
| PLA(42)/PBAT(58) | −31.3 ± 0.4 c | 57.4 ± 1.1 c | 115.1 ± 0.1 a | 7.1 ± 0.4 a | 148.9 ± 0.2 a,b | 2.3 ± 0.1 b | 5.7 ± 0.2 a |
| PLA(4)/PBAT(84) | −30.8 ± 2.5 d | N.D. | 122.7 ± 0.1 b | 4.4 ± 0.2 b | 145.1 ± 0.7 c | 1.3 ± 0.1 c | 35.8 ± 1.6 b |
| PLA/CA | N.D. | 59.7 ± 0.4 b | N.D. | N.D. | 149.6 ± 0.1 a | 29.4 ± 0.5 a | 4.8 ± 0.4 a |
| PLA(42)/PBAT(58)/CA | −30.2 ± 0.7 a | 59.1 ± 0.1 a | 114.5 ± 0.1 c | 6.2 ± 0.2 c | 149.7 ± 0.1 a | 2.9 ± 0.1 b | 7.5 ± 0.3 c |
| PLA(4)/PBAT(84)/CA | −29.6 ± 1.4 b | N.D. | 123.4 ± 0.6 d | 4.5 ± 0.1 d | 145.3 ± 0.5 c | 1.4 ± 0.1 c | 36.2 ± 0.2 b |
| Sample | Pressure [MPa] | Tm PLA [°C] | ΔHm [J/g] PLA | %Xc |
|---|---|---|---|---|
| PLAF | 15 | 153.54 ± 0.61 c,d,e | 46.05 ± 4.28 a | 49.19 ± 4.57 b |
| PLA(42)/PBAT(58)F | 152.45 ± 0.00 a,b | 9.87 ± 0.71 f | 25.09 ± 1.82 g | |
| PLA(4)/PBAT(84)F | 150.90 ± 0.24 b,c | 1.95 ± 0.07 h | 52.08 ± 2.27 b | |
| PLAF | 25 | 148.85 ± 0.41 c,d,e,f,g | 32.96 ± 0.35 b | 35.21 ± 0.37 c,d,e |
| PLA(42)/PBAT(58)F | 147.23 ± 0.53 g,h | 15.23 ± 2.30 e | 38.73 ± 5.85 c | |
| PLA(4)/PBAT(84)F | 147.30 ± 0.20 g,h | 0.97 ± 0.00 h | 27.78 ± 2.64 f,g | |
| PLAF/CA | 15 | 153.41 ± 0.34 b,c,d | 39.00 ± 1.65 b | 41.67 ± 1.77 c,d |
| PLA(42)/PBAT(58)F/CA | 153.30 ± 0.12 a | 11.45 ± 0.73 f | 29.11 ± 1.85 e,f,g | |
| PLA(4)/PBAT(84)F/CA | 149.94 ± 0.32 c,d,e,f | 2.41 ± 0.03 h | 64.37 ± 1.13 a | |
| PLAF/CA | 25 | 150.42 ± 1.59 c,d,e,f,g | 33.94 ± 1.55 b | 36.26 ± 1.65 c,d,e |
| PLA(42)/PBAT(58)F/CA | 147.12 ± 0.10 g,h | 15.59 ± 1.06 e | 39.66 ± 2.70 c | |
| PLA(4)/PBAT(84)F/CA | 153.13 ± 0.38 a | 1.04 ± 0.01 h | 27.64 ± 0.19 f,g |
| Sample | Pressure (MPa) | d (µm) | ρf (kg/m3) | NC (×1011 cell/cm3) | ER |
|---|---|---|---|---|---|
| PLAF | 15 | 41.05 ± 8.37 | 104.7 ± 4.77 | 1.29 | 9.13 |
| PLA(42)/PBAT(58)F | 52.00 ± 11.64 | 269.63 ± 1.49 | 0.50 | 3.32 | |
| PLA(4)/PBAT(84)F | 72.87 ± 19.87 | 203.64 ± 8.67 | 0.21 | 5.00 | |
| PLAF | 25 | 20.26 ± 4.10 | 120.39 ± 46.19 | 10.5 | 7.94 |
| PLA(42)/PBAT(58)F | 32.65 ± 6.44 | 124.23 ± 38.03 | 2.52 | 7.21 | |
| PLA(4)/PBAT(84) F | 12.21 ± 2.99 | 295.53 ± 46.66 | 39 | 3.44 | |
| PLAF/CA | 15 | 76.22 ± 17.62 | 125.24 ± 34.33 | 0.20 | 7.57 |
| PLA(42)/PBAT(58)F/CA | 49.17 ± 10.37 | 302.59 ± 39.57 | 0.57 | 3.08 | |
| PLA(4)/PBAT(84)F/CA | 33.77 ± 5.32 | 237.84 ± 14.12 | 1.95 | 4.00 | |
| PLAF/CA | 25 | 36.72 ± 6.01 | 83.83 ± 4.26 | 1.84 | 11.31 |
| PLA(42)/PBAT(58)F/CA | 41.69 ± 13.32 | 96.53 ± 8.29 | 1.24 | 9.66 | |
| PLA(4)/PBAT(84)F/CA | 6.91 ± 2.13 | 327.86 ± 8.54 | 198 | 2.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera, P.; Torres, A.; Romero, J.; Alarcón, Á.; Martínez, S.; Arrieta, M.P.; Rodríguez-Mercado, F.; Galotto, M.J. Effect of Operational Variables on Supercritical Foaming of Caffeic Acid-Loaded Poly(lactic acid)/Poly(butylene adipate-co-terephthalate) Blends for the Development of Sustainable Materials. Polymers 2024, 16, 948. https://doi.org/10.3390/polym16070948
Rivera P, Torres A, Romero J, Alarcón Á, Martínez S, Arrieta MP, Rodríguez-Mercado F, Galotto MJ. Effect of Operational Variables on Supercritical Foaming of Caffeic Acid-Loaded Poly(lactic acid)/Poly(butylene adipate-co-terephthalate) Blends for the Development of Sustainable Materials. Polymers. 2024; 16(7):948. https://doi.org/10.3390/polym16070948
Chicago/Turabian StyleRivera, Patricia, Alejandra Torres, Julio Romero, Álvaro Alarcón, Sara Martínez, Marina P. Arrieta, Francisco Rodríguez-Mercado, and María José Galotto. 2024. "Effect of Operational Variables on Supercritical Foaming of Caffeic Acid-Loaded Poly(lactic acid)/Poly(butylene adipate-co-terephthalate) Blends for the Development of Sustainable Materials" Polymers 16, no. 7: 948. https://doi.org/10.3390/polym16070948
APA StyleRivera, P., Torres, A., Romero, J., Alarcón, Á., Martínez, S., Arrieta, M. P., Rodríguez-Mercado, F., & Galotto, M. J. (2024). Effect of Operational Variables on Supercritical Foaming of Caffeic Acid-Loaded Poly(lactic acid)/Poly(butylene adipate-co-terephthalate) Blends for the Development of Sustainable Materials. Polymers, 16(7), 948. https://doi.org/10.3390/polym16070948








