Solution-Phase Synthesis of KCl Nanocrystals Templated by PEO-PPO-PEO Triblock Copolymers Micelles
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials and Reagents
2.2. Synthesis of Inorganic Nanocrystals
2.3. Characterization of Inorganic Nanocrystals
3. Results and Discussion
3.1. Determination of Crystallographic Structure and Morphology
3.2. The Effect of the Concentration of Pluronic and pH
3.3. The Effect of the Type of Alkali Metal Ion
- (1)
- Reduction of metal ions by block copolymers in solution and formation of gold clusters:
- (2)
- Adsorption of block copolymers on gold clusters and reduction of metal ions on the surfaces of those gold clusters:
- (3)
- Stabilization of gold particles by block copolymers
3.4. The Mechanism of Crystallization
- (1)
- (2)
- Chloride anions are released with the deformation of hydrogen tetrachloroaurate(III) trihydrate.
- (3)
- Potassium chloride nanocrystals are formed.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peng, W.-C.; Wang, S.-B.; Li, X.-Y. Shape-controlled synthesis of one-dimensional α-MnO2 nanocrystals for organic detection and pollutant degradation. Sep. Purif. Technol. 2016, 163, 15–22. [Google Scholar] [CrossRef]
- Gao, W.; Hood, Z.D.; Chi, M. Interfaces in Heterogeneous Catalysts: Advancing Mechanistic Understanding through Atomic-Scale Measurements. Acc. Chem. Res. 2017, 50, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-C.; Xu, B.; Wang, X. Engineering nanointerfaces for nanocatalysis. Chem. Soc. Rev. 2014, 43, 7870–7886. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Chein, H. Generation and Evaluation of Monodisperse Sodium Chloride and Oleic Acid Nanoparticles. Aerosol Air Qual. Res. 2006, 6, 305–321. [Google Scholar] [CrossRef]
- Chen, Q.; Hood, Z.D.; Qiu, J.; Guan, B.; Xia, Y. Continuous Production of Water-Soluble Nanocrystals through Anti-Solvent Precipitation in a Fluidic Device. ChemNanoMat 2019, 5, 1131–1136. [Google Scholar] [CrossRef]
- Wang, B.; Jin, P.; Yue, Y.; Ji, S.; Li, Y.; Luo, H. Synthesis of NaCl single crystals with defined morphologies as templates for fabricating hollow nano/micro-structures. RSC Adv. 2015, 5, 5072–5076. [Google Scholar] [CrossRef]
- Wang, F.; Sun, L.-D.; Gu, J.; Wang, Y.-F.; Feng, W.; Yang, Y.; Wang, J.; Yan, C.-H. Selective Heteroepitaxial Nanocrystal Growth of Rare Earth Fluorides on Sodium Chloride: Synthesis and Density Functional Calculations. Angew. Chem. Int. Ed. 2012, 51, 8796–8799. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, J.; Song, X.; Luo, K.; Nan, Z.-A.; Fan, F.R.; Tian, Z.-Q. Utilization of charged microdroplets for the controlled rapid synthesis of hollow sodium chloride single crystals. Chem. Commun. 2024, 60, 980–983. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Wang, D.; Fang, H.; Wang, H.; Wang, M.; Huang, K.; Chen, H.; Feng, S. Controlled Crystallization of Sodium Chloride Nanocrystals in Microdrop lets Produced by Electrospray from an Ultra-Dilute Solution. Eur. J. Inorg. Chem. 2016, 2016, 1860–1865. [Google Scholar] [CrossRef]
- Park, K.; Kim, J.-S.; Miller, A.L. A study on effects of size and structure on hygroscopicity of nanoparticles using a tandem differential mobility analyzer and TEM. J. Nanopart. Res. 2009, 11, 175–183. [Google Scholar] [CrossRef]
- Annen, T.; Epple, M. A facile synthesis of dispersable NaCl nanocrystals. Dalton Trans. 2009, 44, 9731–9734. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zou, B.; Rondinone, A.J.; Zhang, Z.J. Reverse Micelle Synthesis and Characterization of Superparamagnetic MnFe2O4 Spinel Ferrite Nanocrystallites. J. Phys. Chem. B 2000, 104, 1141–1145. [Google Scholar] [CrossRef]
- Qi, L. Colloidal chemical approaches to inorganic micro- and nanostructures with controlled morphologies and patterns. Coord. Chem. Rev. 2010, 254, 1054–1071. [Google Scholar] [CrossRef]
- Huang, T.; Qi, L. Solution-phase synthesis of inorganic nanostructures by chemical transformation from reactive templates. Sci. China Chem. 2010, 53, 365–371. [Google Scholar] [CrossRef]
- Nakashima, K.; Takeuchi, K. Water content in micelles of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymers in aqueous solutions as studied by fluorescence spectroscopy. Appl. Spectmsc. 2001, 55, 1237–1244. [Google Scholar] [CrossRef]
- Zheng, L.; Guo, C.; Wang, J.; Liang, X.; Bahadur, P.; Chen, S.; Ma, J.; Liu, H. Micellization of Pluronic L64 in salt solution by FTIR spectroscopy. Vib. Spectrosc. 2005, 39, 157–162. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.-H.; Huang, J.S. Small-Angle Neutron Scattering Analysis of the Structure and Interaction of Triblock Copolymer Micelles in Aqueous Solution. Macromolecules 1998, 31, 2236–2244. [Google Scholar] [CrossRef]
- Khullar, P.; Singh, V.; Mahal, A.; Kaur, H.; Singh, V.; Banipal, T.S.; Kaur, G.; Bakshi, M.S. Tuning the Shape and Size of Gold Nanoparticles with Triblock Polymer Micelle Structure Transitions and Environments. J. Phys. Chem. C 2011, 115, 10442–10454. [Google Scholar] [CrossRef]
- Shou, Q.; Guo, C.; Yang, L.; Jia, L.; Liu, C.; Liu, H. Effect of pH on the single-step synthesis of gold nanoparticles using PEO–PPO–PEO triblock copolymers in aqueous media. J. Colloid Interface Sci. 2011, 363, 481–489. [Google Scholar] [CrossRef]
- Khullar, P.; Singh, V.; Mahal, A.; Kumar, H.; Kaur, G.; Bakshi, M.S. Block Copolymer Micelles as Nanoreactors for Self-Assembled Morphologies of Gold Nanoparticles. J. Phys. Chem. B 2013, 117, 3028–3039. [Google Scholar] [CrossRef]
- Khullar, P.; Mahal, A.; Singh, V.; Banipal, T.S.; Kaur, G.; Bakshi, M.S. How PEO-PPO-PEO Triblock Polymer Micelles Control the Synthesis of Gold Nanoparticles: Temperature and Hydrophobic Effects. Langmuir 2010, 26, 11363–11371. [Google Scholar] [CrossRef]
- Sabir, T.S.; Rowland, L.K.; Milligan, J.R.; Yan, D.; Aruni, A.W.; Chen, Q.; Boskovic, D.S.; Kurti, R.S.; Perry, C.C. Mechanistic Investigation of Seeded Growth in Triblock Copolymer Stabilized Gold Nanoparticles. Langmuir 2013, 29, 3903–3911. [Google Scholar] [CrossRef]
- Sidorov, S.N.; Bronstein, L.M.; Kabachii, Y.A.; Valetsky, P.M.; Soo, P.L.; Maysinger, D.; Eisenberg, A. Influence of Metalation on the Morphologies of Poly(ethylene oxide)-block-poly(4-vinylpyridine) Block Copolymer Micelles. Langmuir 2004, 20, 3543–3550. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bai, S.B.; Nie, M.; Wang, Q. Effect of blend composition on crystallization behavior of polyoxymethylene/poly(ethylene oxide) crystalline/crystalline blends. J. Polym. Res. 2012, 19, 9787. [Google Scholar] [CrossRef]
- Ghazali, N.M.; Yasui, K.; Hashim, A.M. Synthesis of gallium nitride nanostructures by nitridation of electrochemically deposited gallium oxide on silicon substrate. Nanoscale Res. Lett. 2014, 9, 685. [Google Scholar] [CrossRef] [PubMed]
- Ci, T.; Li, T.; Chen, L.; Chang, G.; Yu, L.; Ding, J. Effects of “mature micelle” formation of Pluronic P123 on equilibrium between lactone and carboxylate forms of 10-hydrocamptothecin in water. Polym. Chem. 2013, 4, 3245–3255. [Google Scholar] [CrossRef]
- Li, C.; Tho, C.C.; Galaktionova, D.; Chen, X.; Kral, P.; Mirsaidov, U. Dynamics of Amphiphilic Block Copolymers in an Aqueous Solution: Direct Imaging of Micelle Formation and Nanoparticle Encapsulation. Nanoscale 2019, 11, 2299–2305. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.J.; Aswal, V.K.; Goyal, P.S.; Bahadur, P. Salt induced micellization and micelle structures of’ PEO/PPO/PEO block copolymers in aqueous solution. Colloids Surf. Physicochem. Eng. Aspects 2000, 173, 85–94. [Google Scholar] [CrossRef]
- Jain, N.J.; George, A.; Bahadur, P. Effect of salt on the micellization of pluronic P65 in aqueous solution. Colloids Surf. Physicochem. Eng. Aspects 1999, 157, 275–283. [Google Scholar] [CrossRef]
- Lam, Y.M.; Grigorieff, N.; Goldbeck-Wood, G. Direct visualisation of micelles of Pluronic block copolymers in aqueous solution by cryo-TEM. Phys. Chem. Chem. Phys. 1999, 1, 3331–3334. [Google Scholar] [CrossRef]
- Terashima, T.; Kawabe, M.; Miyabara, Y.; Yoda, H.; Sawamoto, M. Polymeric pseudo-crown ether for cation recognition via cation template-assisted cyclopolymerization. Nat. Commun. 2013, 4, 2321. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Guo, C.; Chen, S.; Ma, J.H.; Wang, J.; Liang, X.F.; Zheng, L.; Liu, H.Z. Effect of acid on the aggregation of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) block copolymers. J. Phys. Chem. B 2006, 110, 23068–23074. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Alexandridis, P. Single-step synthesis and stabilization of metal nanoparticles in aqueous pluronic block copolymer solutions at ambient temperature. Langmuir 2004, 20, 8426–8430. [Google Scholar] [CrossRef] [PubMed]
- Gomes, D.S.B.; Paterno, L.G.; Santos, A.B.S.; Garay, A.V.; Mertz, D.; Freitas, S.M.; Soler, M.A.G. New insights on the formation of gold nanoparticles and Pluronic nanocomposites: Kinetics and thermodynamics parameters. J. Mol. Liq. 2018, 268, 181–189. [Google Scholar] [CrossRef]
- Sokolsky-Papkov, M.; Kabanov, A. Synthesis of Well-Defined Gold Nanoparticles Using Pluronic: The Role of Radicals and Surfactants in Nanoparticles Formation. Polymers 2019, 11, 1553. [Google Scholar] [CrossRef] [PubMed]
- Yasui, K. The Reducing Agents in Sonochemical Reactions without Any Additives. Molecules 2023, 28, 4198. [Google Scholar] [CrossRef]
- Chatterjee, P.; Hazra, S. pH-dependent size and structural transition in P123 micelle induced gold nanoparticles. RSC Adv. 2015, 5, 69765–69775. [Google Scholar] [CrossRef]
- Yang, S.; Yuan, S.; Zhang, X.; Yan, Y. Phase behavior of tri-block copolymers in solution: Mesoscopic simulation study. Colloids Surf. Physicochem. Eng. Aspects 2008, 322, 87–96. [Google Scholar] [CrossRef]
- Su, Y.L.; Liu, H.Z.; Wang, J.; Chen, J.Y. Study of salt effects on the micellization of PEO-PPO-PEO block copolymer in aqueous solution by FTIR spectroscopy. Langmuir 2002, 18, 865–871. [Google Scholar] [CrossRef]
- da Silva, L.H.M.; Hespanhol da Silva, M.C.; de Aquino, R.A.N.; Francisco, K.R.; Cardoso, M.V.C.; Minim, L.A.; Coimbra, J.S.R. Nitroprusside−PEO Enthalpic Interaction as a Driving Force for Partitioning of the [Fe(CN)5NO]2− Anion in Aqueous Two-Phase Systems Formed by Poly(ethylene oxide) and Sulfate Salts. J. Phys. Chem. B 2006, 110, 23540–23546. [Google Scholar] [CrossRef]
- Li, X.; Huang, K.; Xu, Y.; Liu, H. Interaction of sodium and potassium ions with PEO-PPO copolymer investigated by FTIR, Raman and NMR. Vib. Spectrosc 2014, 75, 59–64. [Google Scholar] [CrossRef]
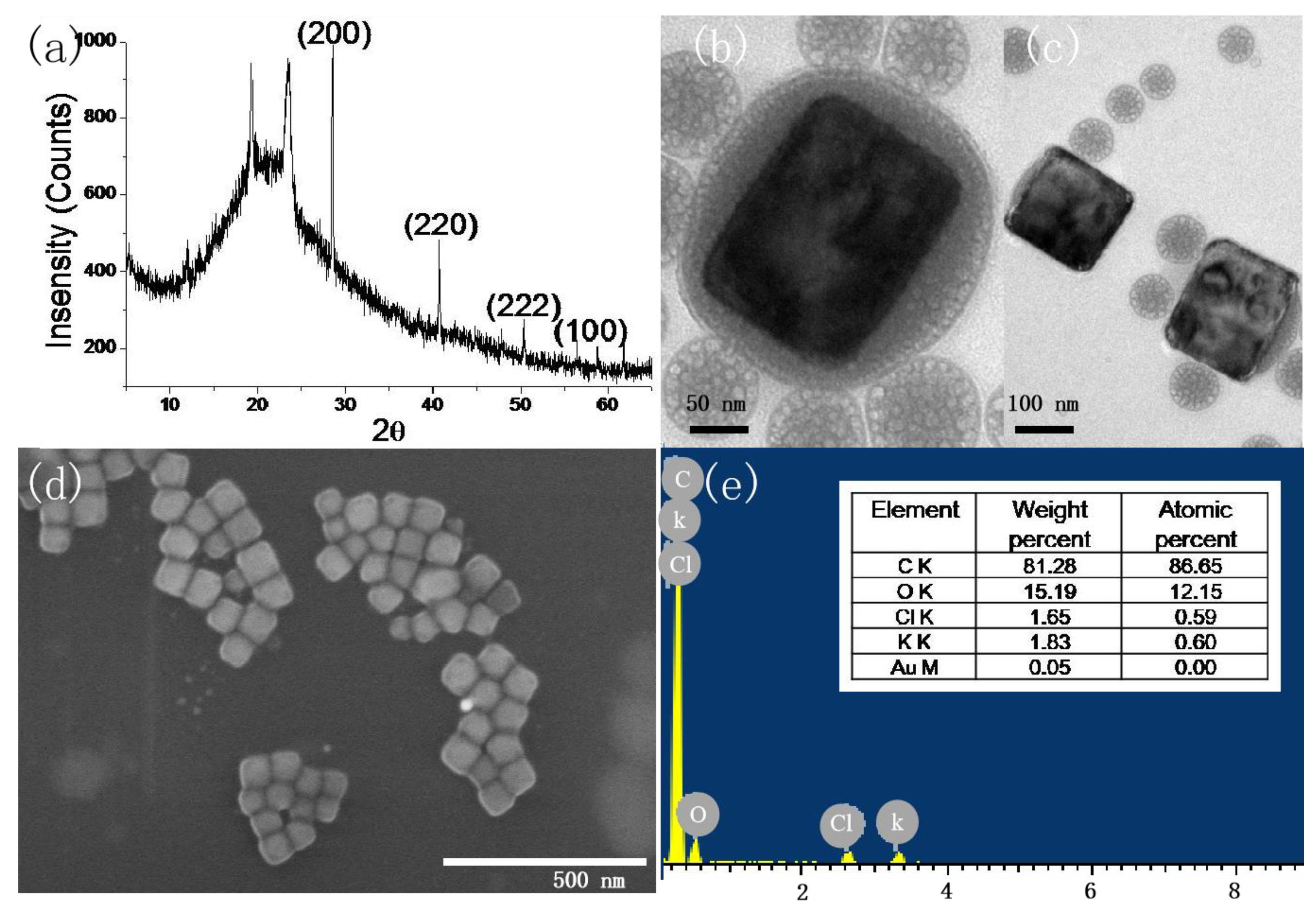

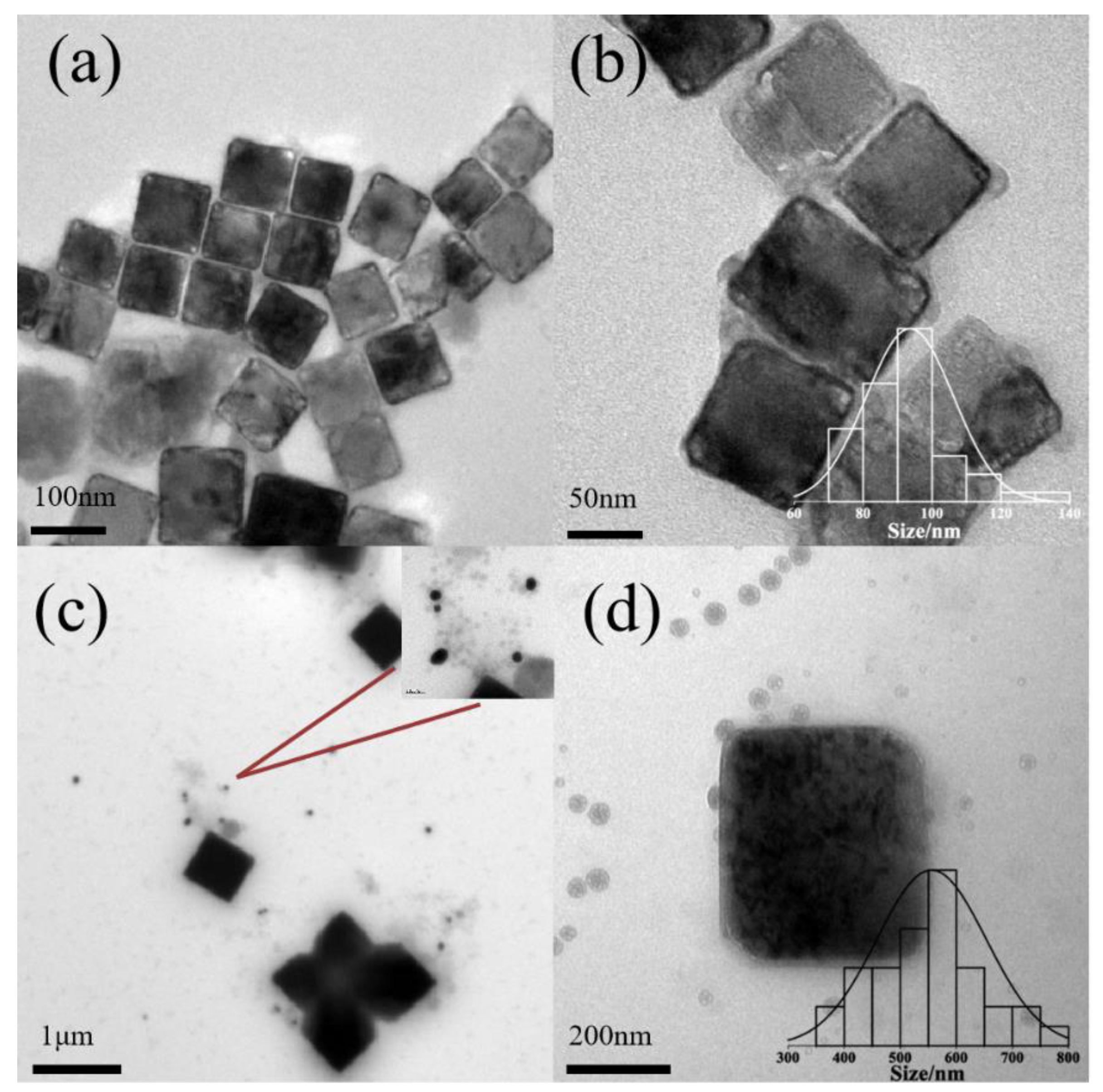
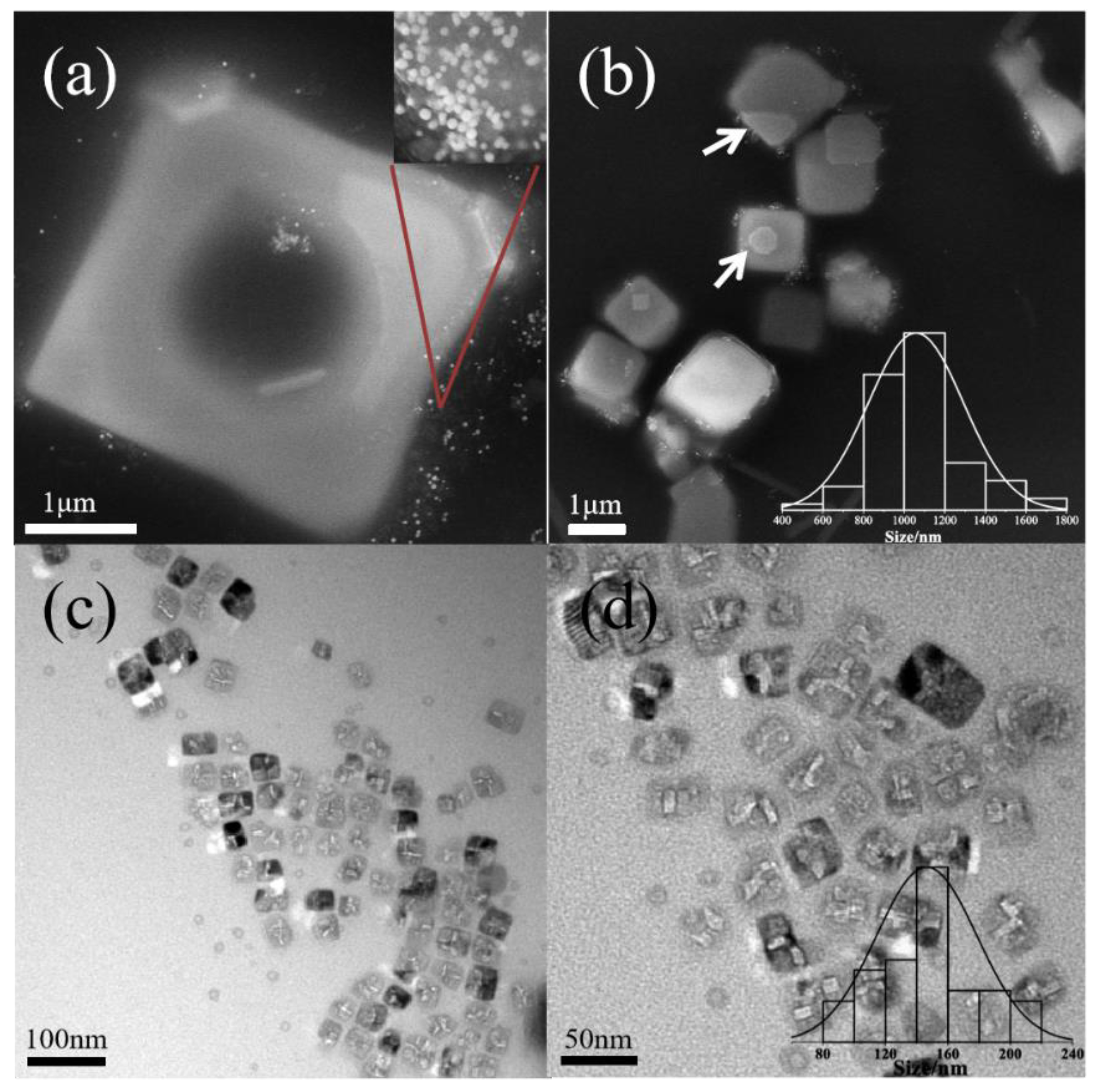
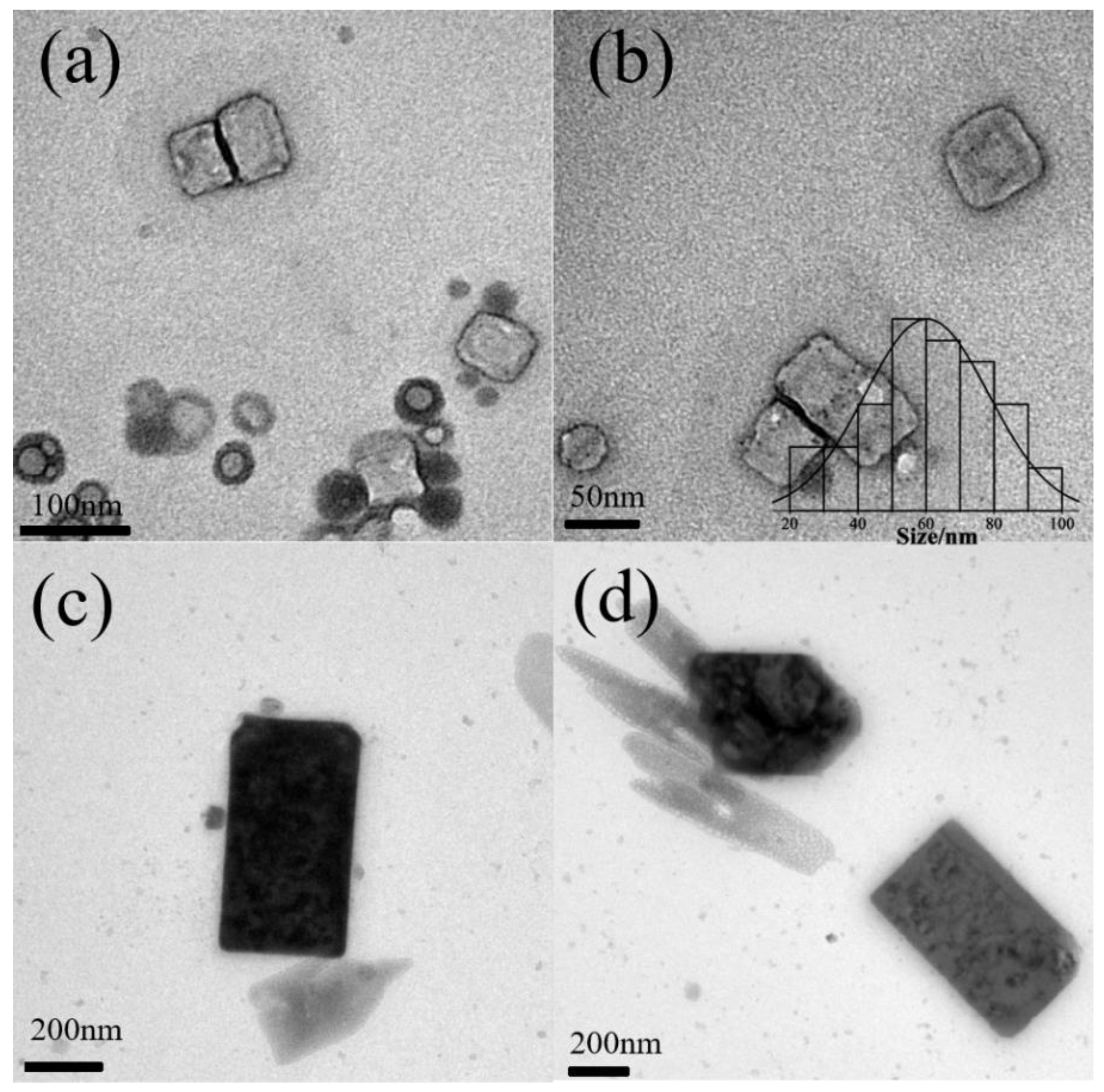


| P123 | pH 6.9 | pH 7.3 | pH 8.3 | pH 9.5 |
|---|---|---|---|---|
| Concentration | ||||
| 5 g/L | - | - | - | - |
| 10 g/L | Nanocubes | Nanocubes | - | - |
| 50 g/L | Nanocubes | Nanocubes | Nanocubes | - |
| 100 g/L | Nanocubes | Nanocubes | - | - |
| 200 g/L | Nanocubes | Nanocubes | - | - |
| 250 g/L | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Li, M.; Li, F.; Wang, F.; Liang, X.; Shou, Q. Solution-Phase Synthesis of KCl Nanocrystals Templated by PEO-PPO-PEO Triblock Copolymers Micelles. Polymers 2024, 16, 982. https://doi.org/10.3390/polym16070982
Sun L, Li M, Li F, Wang F, Liang X, Shou Q. Solution-Phase Synthesis of KCl Nanocrystals Templated by PEO-PPO-PEO Triblock Copolymers Micelles. Polymers. 2024; 16(7):982. https://doi.org/10.3390/polym16070982
Chicago/Turabian StyleSun, Lingling, Min Li, Fei Li, Fuchun Wang, Xiangfeng Liang, and Qinghui Shou. 2024. "Solution-Phase Synthesis of KCl Nanocrystals Templated by PEO-PPO-PEO Triblock Copolymers Micelles" Polymers 16, no. 7: 982. https://doi.org/10.3390/polym16070982






