Organic–Inorganic Hybrid Materials: Tailoring Carbon Dioxide-Based Polycarbonate with POSS-SH Crosslinking
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Measurements
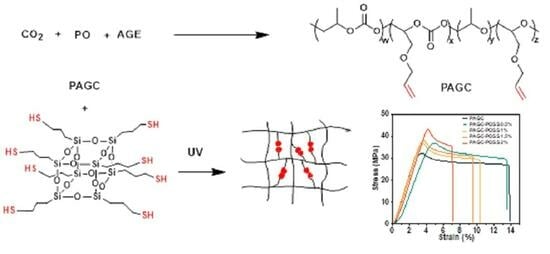
2.3. Synthesis of Carbon Dioxide-Based Polycarbonate Using PO and AGE
2.4. Synthesis of POSS-SH
2.5. Preparation of PAGC-POSS x% Thin Films
3. Results and Discussion
3.1. Characterization of Carbon Dioxide-Based Polycarbonate Using PO and AGE as Raw Materials
3.2. Preparation and Characterization of the PAGC Nanocomposite Films
3.3. Mechanical Properties of PAGC Composites
3.4. Thermomechanical Properties of PAGC Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Alagi, P.; Ghorpade, R.; Choi, Y.J.; Patil, U.; Kim, I.; Baik, J.H.; Hong, S.C. Carbon Dioxide-Based Polyols as Sustainable Feedstock of Thermoplastic Polyurethane for Corrosion-Resistant Metal Coating. ACS Sustain. Chem. Eng. 2017, 5, 3871–3881. [Google Scholar] [CrossRef]
- Poland, S.J.; Darensbourg, D.J. A quest for polycarbonates provided via sustainable epoxide/CO2 copolymerization processes. Green Chem. 2017, 19, 4990–5011. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Grignard, B.; Gennen, S.; Jerome, C.; Kleij, A.W.; Detrembleur, C. Advances in the use of CO2 as a renewable feedstock for the synthesis of polymers. Chem. Soc. Rev. 2019, 48, 4466–4514. [Google Scholar] [CrossRef]
- Muthuraj, R.; Mekonnen, T. Recent progress in carbon dioxide(CO2) as feedstock for sustainable materials development: Co-polymers and polymer blends. Polymer 2018, 145, 348–373. [Google Scholar] [CrossRef]
- Brege, A.; Grignard, B.; Méreau, R.; Detrembleur, C.; Jerome, C.; Tassaing, T. En Route to CO2-Based (a) Cyclic Carbonates and Polycarbonates from Alcohols Substrates by Direct and Indirect Approaches. Catalysts 2022, 12, 124. [Google Scholar] [CrossRef]
- Hertzberg, M.; Schreuder, H. Role of atmospheric carbon dioxide in climate change. Energy Environ. 2016, 27, 785–797. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, X. Carbon dioxide-based copolymers: Environmental benefits of PPC, an industrially viable catalyst. Biotechnol. J. 2010, 5, 1164–1180. [Google Scholar] [CrossRef]
- Yang, G.-W.; Zhang, Y.-Y.; Xie, R.; Wu, G.-P. Scalable Bifunctional Organoboron Catalysts for Copolymerization of CO2 and Epoxides with Unprecedented Efficiency. J. Am. Chem. Soc. 2020, 142, 12245–12255. [Google Scholar] [CrossRef]
- Zhang, X.H.; Wei, R.J.; Zhang, Y.Y.; Du, B.Y.; Fan, Z.Q. Carbon Dioxide/Epoxide Copolymerization via a Nanosized Zinc-Cobalt (III) Double Metal Cyanide Complex: Substituent Effects of Epoxides on Polycarbonate Selectivity, Regioselectivity and Glass Transition Temperatures. Macromolecules 2015, 48, 536–544. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, J.; Darensbourg, D.J. Construction of Versatile and Functional Nanostructures Derived from CO2-based Polycarbonates. Angew. Chem. Int. Ed. Engl. 2015, 54, 10206–10210. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lin, L.; Xiao, M.; Wang, S.; Smith, A.T.; Sun, L.; Meng, Y. Synthesis and properties of CO2-based plastics: Environmentally-friendly, energy-saving and biomedical polymeric materials. Prog. Polym. Sci. 2018, 80, 163–182. [Google Scholar] [CrossRef]
- Szelest-Lewandowska, A.; Masiulanis, B.; Szymonowicz, M.; Pielka, S.; Paluch, D. Modified polycarbonate urethane: Synthesis, properties and biological investigation in vitro. J. Biomed. Mater. Res. A 2007, 82, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Paek, K.H.; Im, S.G. Synthesis of a series of biodegradable poly(butylene carbonate-co-isophthalate) random copolymers derived from CO2-based comonomers for sustainable packaging. Green Chem. 2020, 22, 4570–4580. [Google Scholar] [CrossRef]
- Jia, M.; Zhang, D.; de Kort, G.W.; Wilsens, C.; Rastogi, S.; Hadjichristidis, N.; Gnanou, Y.; Feng, X. All-Polycarbonate Thermoplastic Elastomers Based on Triblock Copolymers Derived from Triethylborane-Mediated Sequential Copolymerization of CO2 with Various Epoxides. Macromolecules 2020, 53, 5297–5307. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, P.; Ogonczyk, D.; Kosinski, A.; Lisowski, W.; Garstecki, P. Hydrophobic modification of polycarbonate for reproducible and stable formation of biocompatible microparticles. Lab. Chip 2011, 11, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Gao, L.; Feng, J.; Huang, X.; Li, Z.; Huang, Z.; Wang, L. Cross-Linked Networks in Poly(propylene carbonate) by Incorporating (Maleic Anhydride/cis-1,2,3,6-Tetrahydrophthalic Anhydride) Oligomer in CO2/Propylene Oxide Copolymerization: Improving and Tailoring Thermal, Mechanical, and Dimensional Properties. ACS Omega 2020, 5, 17808–17817. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Ebihara, T.; Ohkawa, T.; Sugimoto, H. Alternating terpolymerization of carbon dioxide, propylene oxide, and various epoxides with bulky side groups for the tuning of thermal properties. Polym. J. 2020, 53, 121–127. [Google Scholar] [CrossRef]
- Geschwind, J.; Wurm, F.; Frey, H. From CO2-Based Multifunctional Polycarbonates With a Controlled Number of Functional Groups to Graft Polymers. Macromol. Chem. Phys. 2013, 214, 892–901. [Google Scholar] [CrossRef]
- Geschwind, J.; Frey, H. Poly(1,2-glycerol carbonate): A Fundamental Polymer Structure Synthesized from CO2 and Glycidyl Ethers. Macromolecules 2013, 46, 3280–3287. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Tsai, F.-T. Postpolymerization Functionalization of Copolymers Produced from Carbon Dioxide and 2-Vinyloxirane: Amphiphilic/Water-Soluble CO2-Based Polycarbonates. Macromolecules 2014, 47, 3806–3813. [Google Scholar] [CrossRef]
- Alagi, P.; Zapsas, G.; Hadjichristidis, N.; Hong, S.C.; Gnanou, Y.; Feng, X. All-Polycarbonate Graft Copolymers with Tunable Morphologies by Metal-Free Copolymerization of CO2 with Epoxides. Macromolecules 2021, 54, 6144–6152. [Google Scholar] [CrossRef]
- Belon, C.; Chemtob, A.; Croutxé-Barghorn, C.; Rigolet, S.; Le Houérou, V.; Gauthier, C. Combination of radical and cationic photoprocesses for the single-step synthesis of organic-inorganic hybrid films. J. Polym. Sci. Pol. Chem. 2010, 48, 4150–4158. [Google Scholar] [CrossRef]
- Daglar, O.; Çakmakçi, E.; Gunay, U.S.; Hizal, G.; Tunca, U.; Durmaz, H. All in one: The preparation of polyester/silica hybrid nanocomposites via three different metal-free click reactions. Eur. Polym. J. 2021, 154, 110532. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Vu, C.M.; Choi, H.J.; Kien, B.X. Nanosilica Extracted from Hexafluorosilicic Acid of Waste Fertilizer as Reinforcement Material for Natural Rubber: Preparation and Mechanical Characteristics. Materials 2019, 12, 2707. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.Y.; Zhang, X.M.; Chen, W.X.; Feng, L.F. Synthesis, characterization, and properties of polystyrene/SiO2 hybrid materials via sol–gel process. Polym. Compos. 2014, 36, 482–488. [Google Scholar] [CrossRef]
- Kuo, S.-W.; Chang, F.-C. POSS related polymer nanocomposites. Prog. Polym. Sci. 2011, 36, 1649–1696. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; Morici, E.; Arrigo, R.; La Mantia, F.P. Interaction in POSS-poly(ethylene-co-acrylic acid) nanocomposites. Polym. J. 2013, 46, 160–166. [Google Scholar] [CrossRef]
- Chen, D.; Yi, S.; Wu, W.; Zhong, Y.; Liao, J.; Huang, C.; Shi, W. Synthesis and characterization of novel room temperature vulcanized (RTV) silicone rubbers using Vinyl-POSS derivatives as cross linking agents. Polymer 2010, 51, 3867–3878. [Google Scholar] [CrossRef]
- Zhao, B.; Mei, H.; Liu, N.; Zheng, S. Organic–Inorganic Polycyclooctadienes with Double-Decker Silsesquioxanes in the Main Chains: Synthesis, Self-Healing, and Shape Memory Properties Regulated with Quadruple Hydrogen Bonds. Macromolecules 2020, 53, 7119–7131. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Qin, Z.; Wu, Y.; Zhang, W.; Yang, R. High-transparency polysilsesquioxane/glycidyl-azide-polymer resin and its fiberglass-reinforced composites with excellent fire resistance, mechanical properties, and water resistance. Compos. Part. B-Eng. 2021, 219, 108913. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Guo, R.; Zhou, H.; Li, Z.; Chen, G.; Zhou, Z.; Li, Q. Preparation of novel UV-cured methacrylate hybrid materials with high thermal stability via thiol–ene photopolymerization. J. Mater. Sci. 2018, 54, 5877–5897. [Google Scholar] [CrossRef]
- Li, Z.; Hao, X.; Cheng, G.; Huang, S.; Han, D.; Xiao, M.; Wang, S.; Meng, Y. In situ implantation of cross-linked functional POSS blocks in Nafion® for high performance direct methanol fuel cells. J. Membr. Sci. 2021, 640, 119798. [Google Scholar] [CrossRef]
- Fortman, D.J.; Brutman, J.P.; De Hoe, G.X.; Snyder, R.L.; Dichtel, W.R.; Hillmyer, M.A. Approaches to Sustainable and Continually Recyclable Cross-Linked Polymers. ACS Sustain. Chem. Eng. 2018, 6, 11145–11159. [Google Scholar] [CrossRef]
- Ree, M.; Hwang, Y.; Kim, J.-S.; Kim, H.; Kim, G.; Kim, H. New findings in the catalytic activity of zinc glutarate and its application in the chemical fixation of CO2 into polycarbonates and their derivatives. Catal. Today 2006, 115, 134–145. [Google Scholar] [CrossRef]
- Ervithayasuporn, V.; Wang, X.; Kawakami, Y. Synthesis and characterization of highly pure azido-functionalized polyhedral oligomeric silsesquioxanes (POSS). Chem. Commun. 2009, 34, 5130–5132. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Ding, S.; Liu, Y.; Qi, Z. Facile Synthesis and Self-Assembly of Amphiphilic Polyether-Octafunctionalized Polyhedral Oligomeric Silsesquioxane via Thiol-Ene Click Reaction. Polymers 2017, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Yao, H.; Cui, M.; Ma, Y.; Kong, Z.; Wu, B.; Qi, Z.; Sun, Y. Theoretical and experimental investigations on mono-substituted and multi-substituted functional polyhedral oligomeric silsesquioxanes. RSC Adv. 2015, 5, 80339–80345. [Google Scholar] [CrossRef]
- Deng, K.; Wang, S.; Ren, S.; Han, D.; Xiao, M.; Meng, Y. A Novel Single-Ion-Conducting Polymer Electrolyte Derived from CO2-Based Multifunctional Polycarbonate. ACS Appl. Mater. Interfaces 2016, 8, 33642–33648. [Google Scholar] [CrossRef]
- Iyer, S.; Abu-Ali, A.; Detwiler, A.; Schiraldi, D.A. Transparent Polymer-Polyhedral Oligomeric Silsesquioxane Composites. Science and Technology of Silicones and Silicone-Modified Materials. Am. Chem. Soc. 2007, 964, 313–325. [Google Scholar] [CrossRef]
- Cui, S.; Qin, Y.; Li, Y. Sustainable Approach for the Synthesis of Biopolycarbonates from Carbon Dioxide and Soybean Oil. ACS Sustain. Chem. Eng. 2017, 5, 9014–9022. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liu, J.; Qu, R.; Suo, H.; Sun, M.; Qin, Y. Organic–Inorganic Hybrid Materials: Tailoring Carbon Dioxide-Based Polycarbonate with POSS-SH Crosslinking. Polymers 2024, 16, 983. https://doi.org/10.3390/polym16070983
Li Y, Liu J, Qu R, Suo H, Sun M, Qin Y. Organic–Inorganic Hybrid Materials: Tailoring Carbon Dioxide-Based Polycarbonate with POSS-SH Crosslinking. Polymers. 2024; 16(7):983. https://doi.org/10.3390/polym16070983
Chicago/Turabian StyleLi, Yue, Jianyu Liu, Rui Qu, Hongyi Suo, Miao Sun, and Yusheng Qin. 2024. "Organic–Inorganic Hybrid Materials: Tailoring Carbon Dioxide-Based Polycarbonate with POSS-SH Crosslinking" Polymers 16, no. 7: 983. https://doi.org/10.3390/polym16070983
APA StyleLi, Y., Liu, J., Qu, R., Suo, H., Sun, M., & Qin, Y. (2024). Organic–Inorganic Hybrid Materials: Tailoring Carbon Dioxide-Based Polycarbonate with POSS-SH Crosslinking. Polymers, 16(7), 983. https://doi.org/10.3390/polym16070983