Evaluation of Various Types of Alginate Inks for Light-Mediated Extrusion 3D Printing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methacrylation of Alginate
2.2.1. Alginate Methacrylation
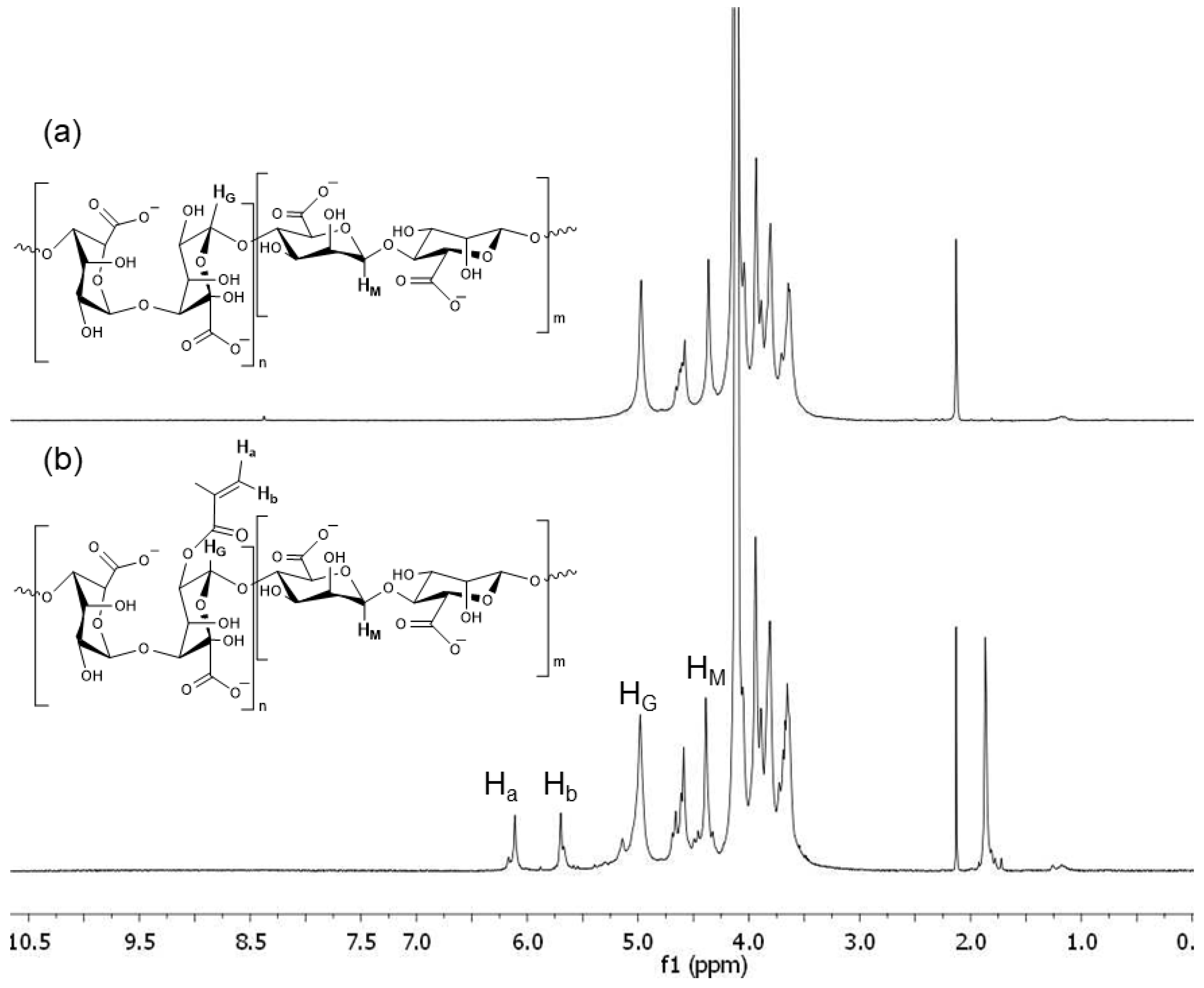
H-NMR
2.3. Printing of the Hydrogels
2.3.1. Photo-DSC Analysis
2.3.2. Optimization of the Printing
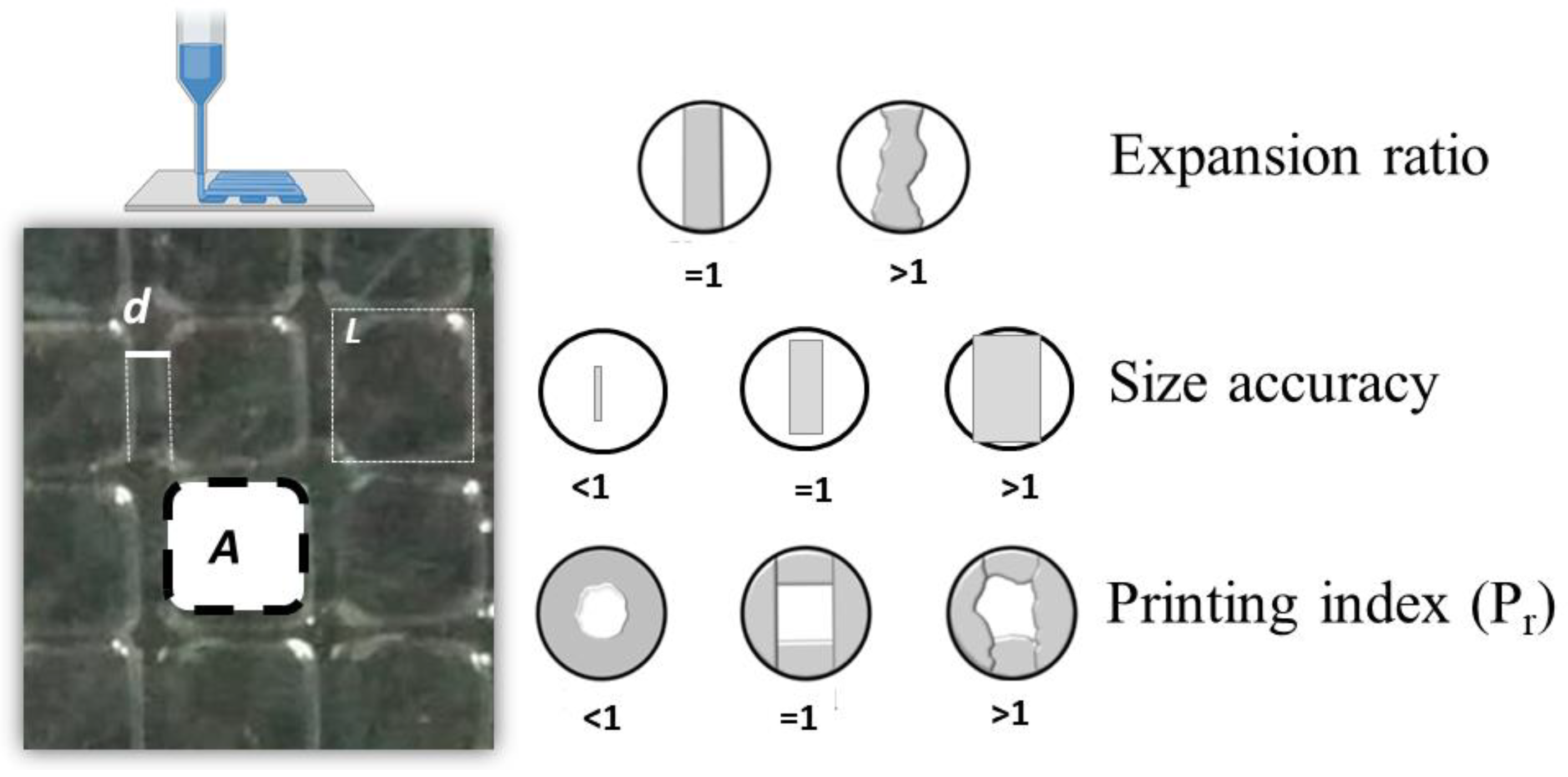
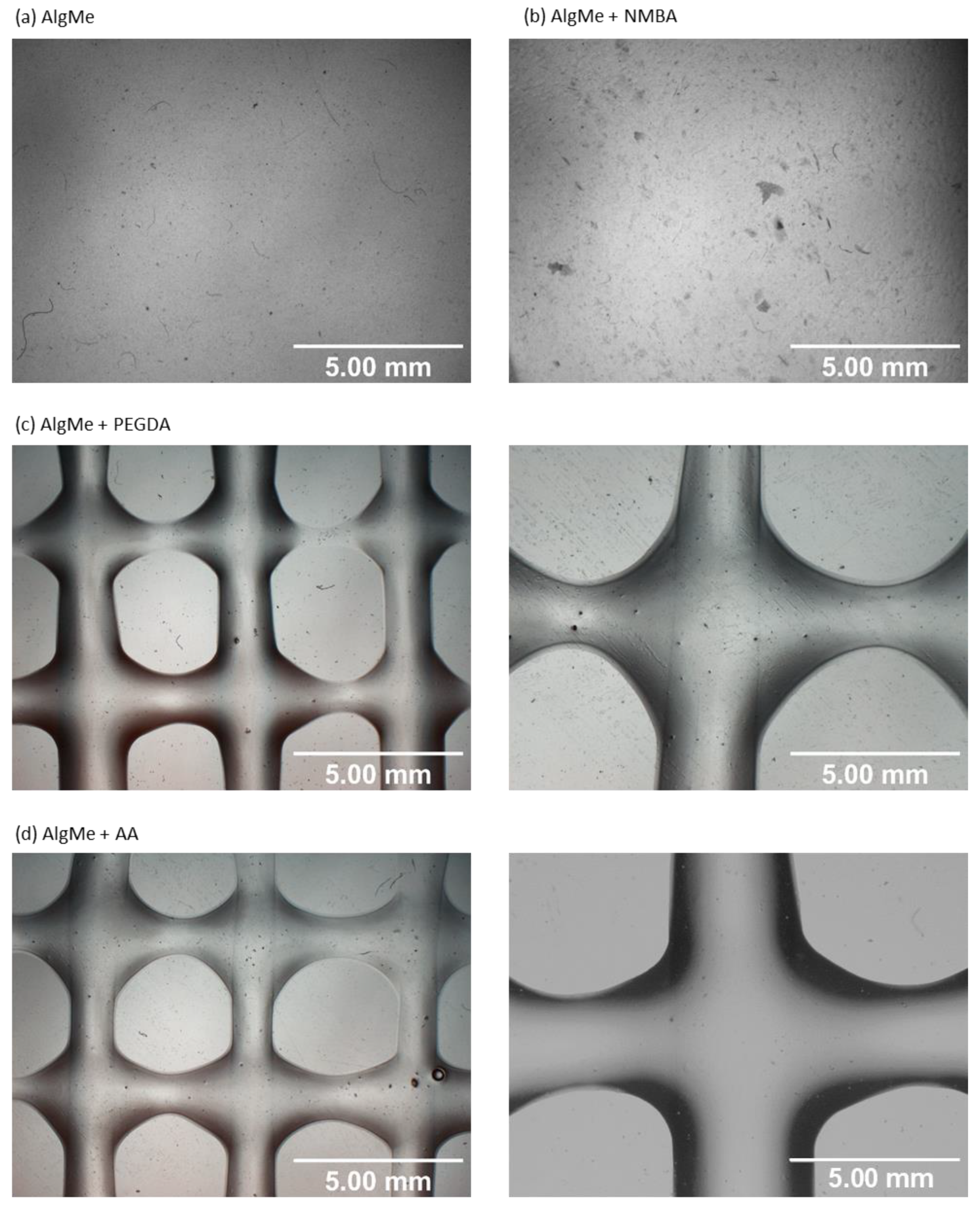
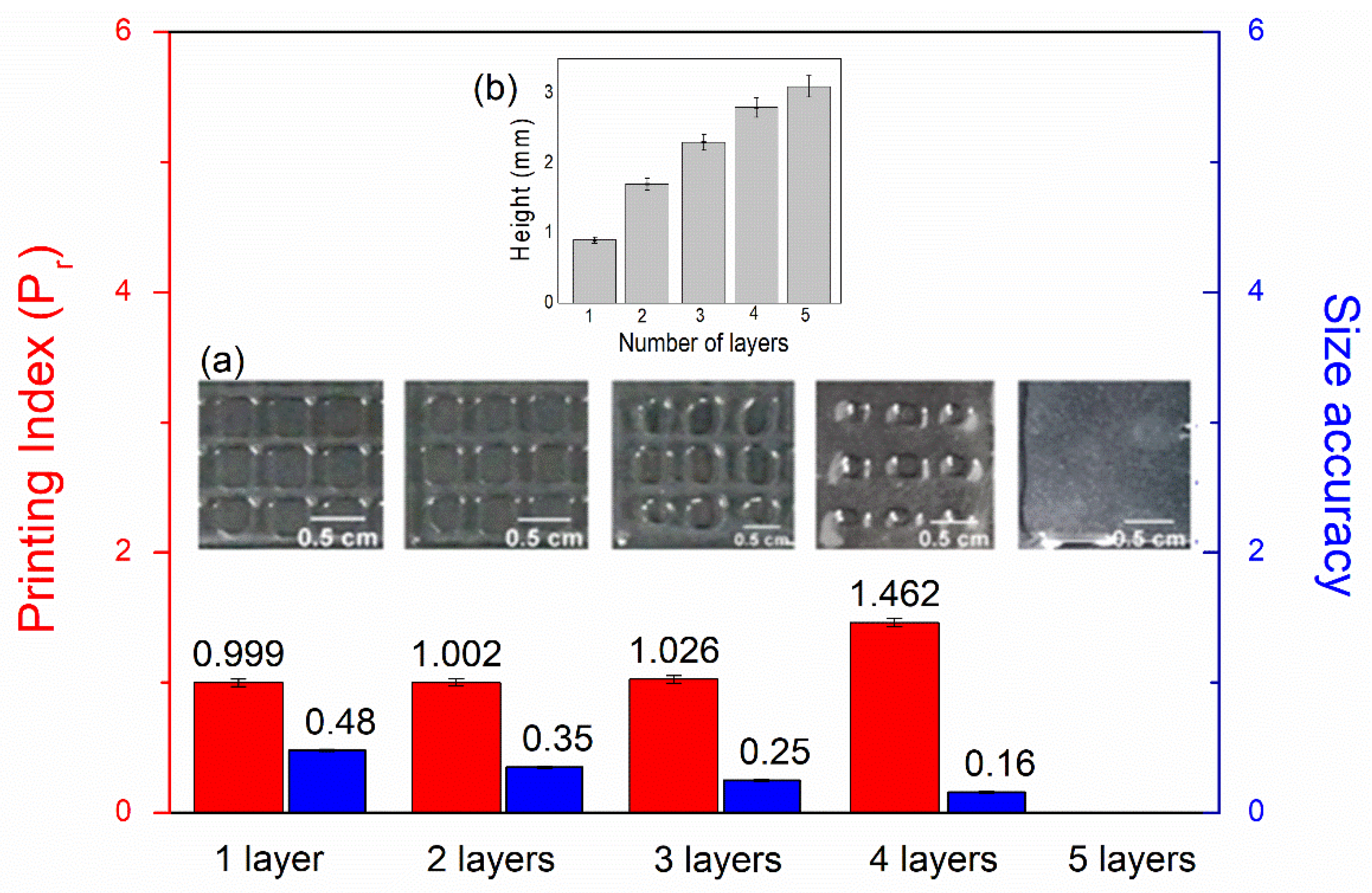
2.3.3. Printing Quality
2.4. Hydrogel Characterization
2.4.1. Mechanical Properties
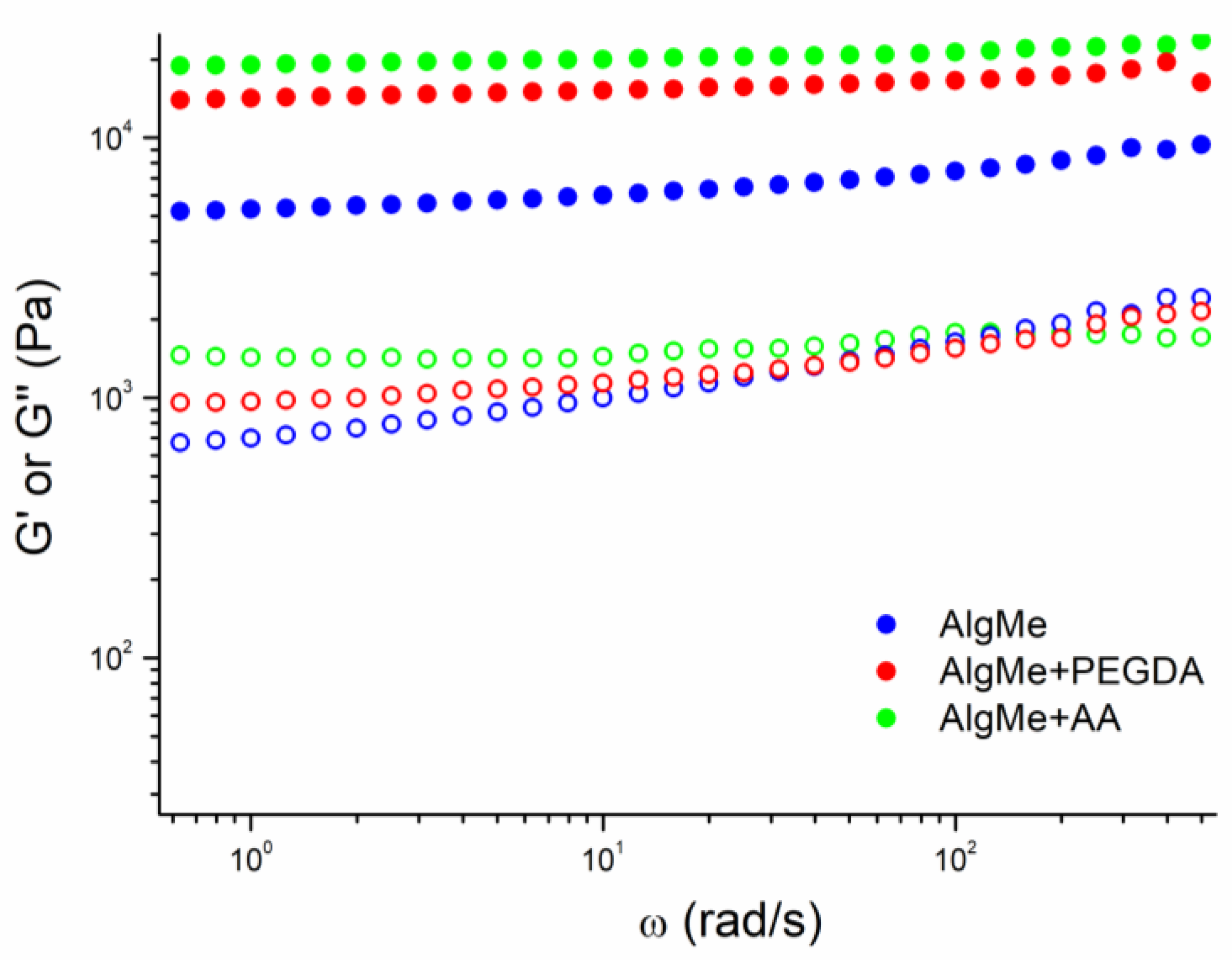
2.4.2. Rheology
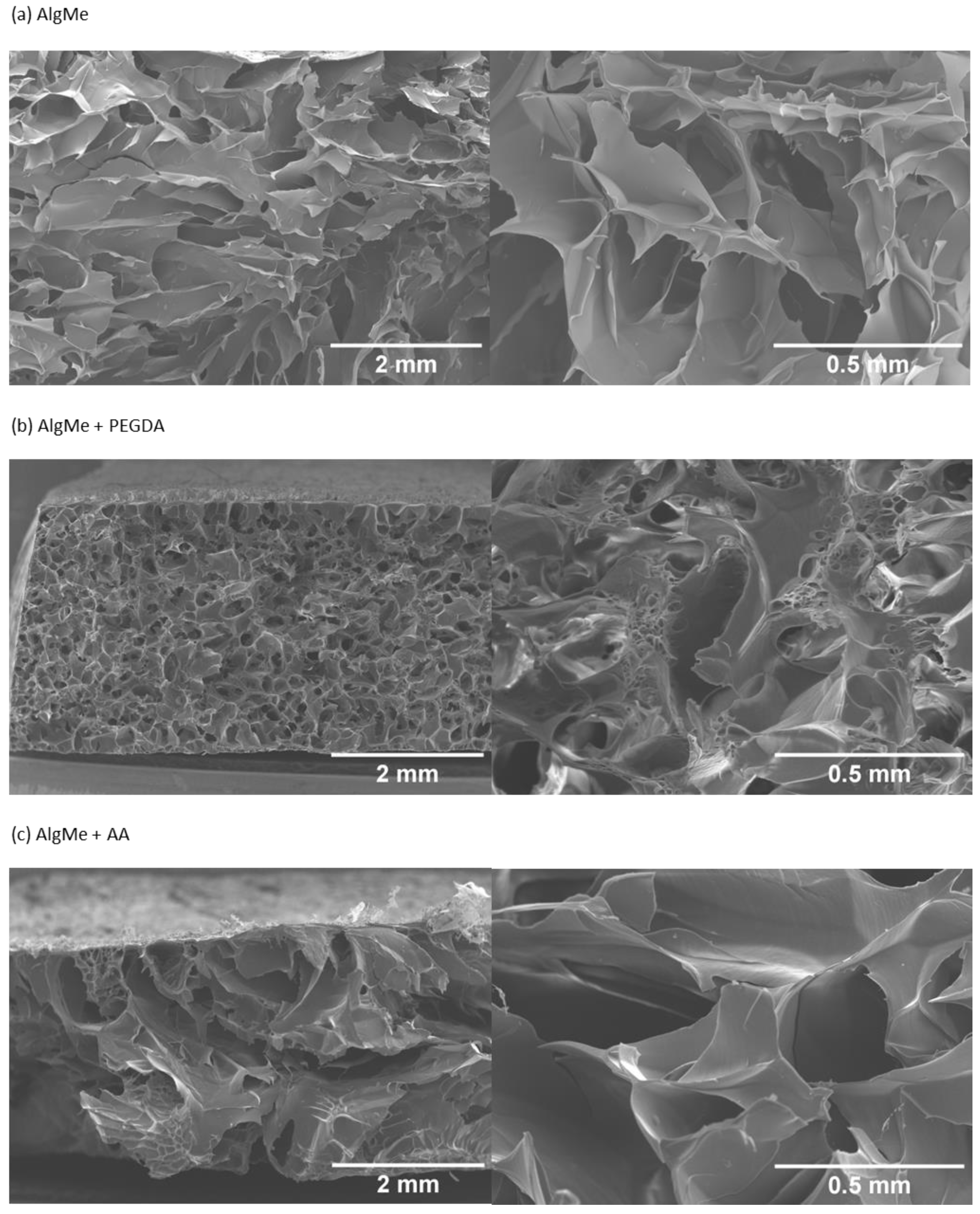
2.4.3. Morphology
2.4.4. Swelling Behavior
2.5. In Vitro Degradation
2.6. In Vitro Cytotoxicity Essay
3. Results
3.1. Alginate Methacrylation
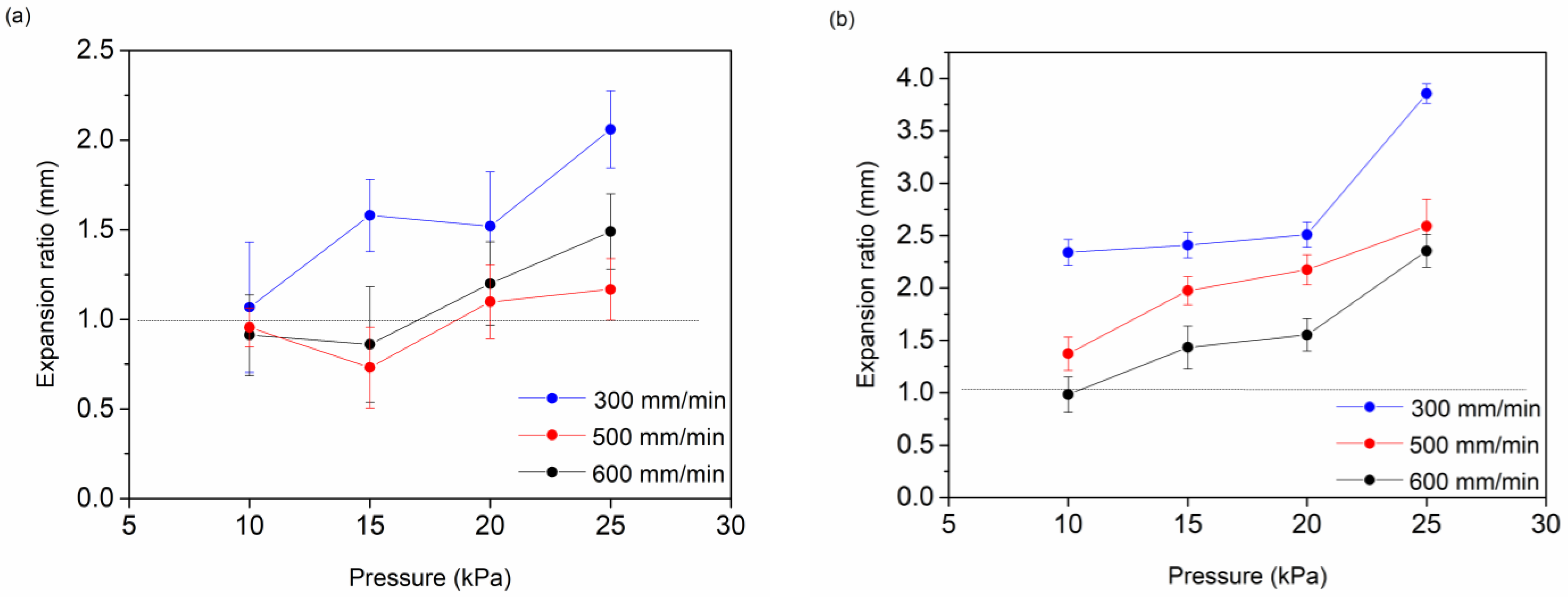
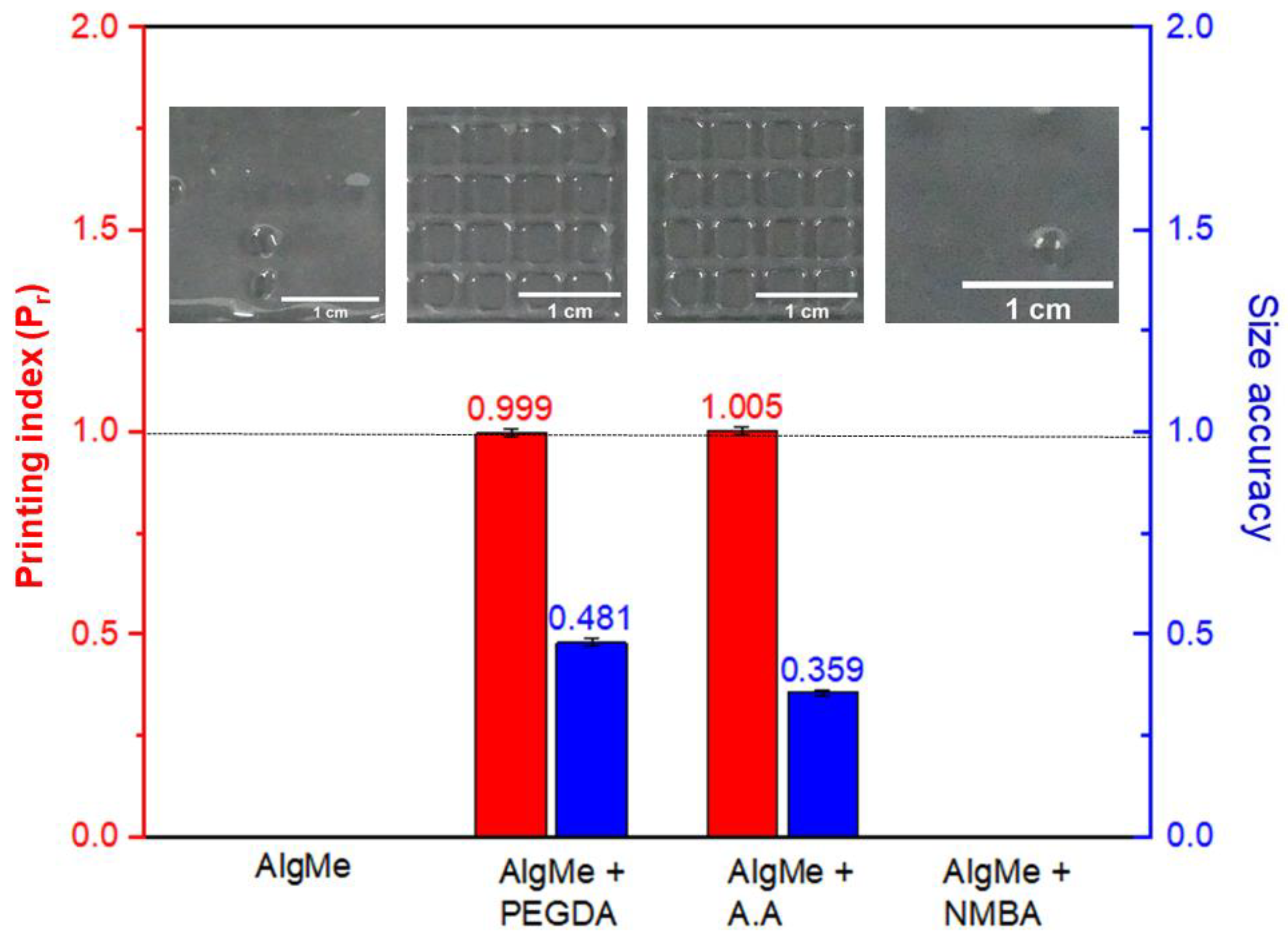
3.2. Extrusion-Mediated Photoprinting of the Hydrogels
3.3. Hydrogel Characterization
3.4. In Vitro Degradation
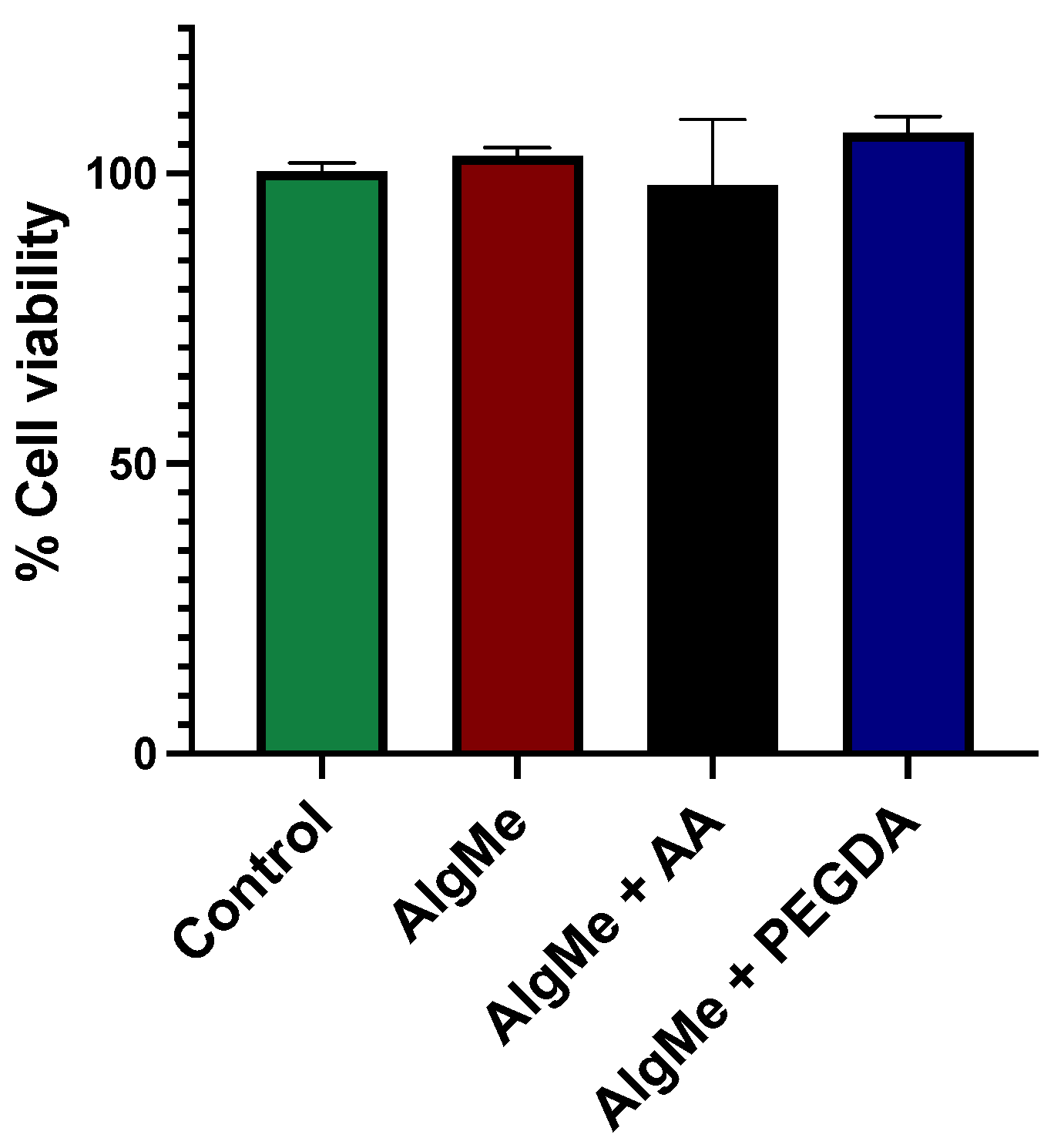
3.5. Hydrogel Biocompatibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D Bioprinting for Biomedical Devices and Tissue Engineering: A Review of Recent Trends and Advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. State-of-the-Art of 3D Printing Technology of Alginate-Based Hydrogels—An Emerging Technique for Industrial Applications. Adv. Colloid Interface Sci. 2021, 293, 102436. [Google Scholar] [CrossRef] [PubMed]
- Nachal, N.; Moses, J.A.; Karthik, P.; Anandharamakrishnan, C. Applications of 3D Printing in Food Processing. Food Eng. Rev. 2019, 11, 123–141. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Peng, W.; Ozbolat, V. Application Areas of 3D Bioprinting. Drug Discov. Today 2016, 21, 1257–1271. [Google Scholar] [CrossRef] [PubMed]
- Vanderploeg, A.; Lee, S.E.; Mamp, M. The Application of 3D Printing Technology in the Fashion Industry. Int. J. Fash. Des. Technol. Educ. 2017, 10, 170–179. [Google Scholar] [CrossRef]
- Zhang, X.N.; Zheng, Q.; Wu, Z.L. Recent Advances in 3D Printing of Tough Hydrogels: A Review. Compos. Part B Eng. 2022, 238, 109895. [Google Scholar] [CrossRef]
- Li, J.; Wu, C.; Chu, P.K.; Gelinsky, M. 3D Printing of Hydrogels: Rational Design Strategies and Emerging Biomedical Applications. Mater. Sci. Eng. R Rep. 2020, 140, 100543. [Google Scholar] [CrossRef]
- Xie, M.; Su, J.; Zhou, S.; Li, J.; Zhang, K. Application of Hydrogels as Three-Dimensional Bioprinting Ink for Tissue Engineering. Gels 2023, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, F.; Yuan, R. Applications of Natural Polymer-Based Hydrogels in the Food Industry; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128164211. [Google Scholar]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Axpe, E.; Oyen, M.L. Applications of Alginate-Based Bioinks in 3D Bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef]
- Coates, E.E.; Riggin, C.N.; Fisher, J.P. Photocrosslinked Alginate with Hyaluronic Acid Hydrogels as Vehicles for Mesenchymal Stem Cell Encapsulation and Chondrogenesis. J. Biomed. Mater. Res.-Part A 2013, 101, 1962–1970. [Google Scholar] [CrossRef] [PubMed]
- Chiulan, I.; Heggset, E.B.; Voicu, Ş.I.; Chinga-Carrasco, G. Photopolymerization of Bio-Based Polymers in a Biomedical Engineering Perspective. Biomacromolecules 2021, 22, 1795–1814. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Jin, X. Dual Crosslinked Methacrylated Alginate Hydrogel Micron Fibers and Tissue Constructs for Cell Biology. Mar. Drugs 2019, 17, 557. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.I.; Jeon, O.; Krebs, M.D.; Hill, M.C.; Alsberg, E. Biodegradable Photo-Crosslinked Alginate Nanofibre Scaffolds with Tuneable Physical Properties, Cell Adhesivity and Growth Factor Release. Eur. Cells Mater. 2013, 24, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Mignon, A.; Devisscher, D.; Graulus, G.J.; Stubbe, B.; Martins, J.; Dubruel, P.; De Belie, N.; Van Vlierberghe, S. Combinatory Approach of Methacrylated Alginate and Acid Monomers for Concrete Applications. Carbohydr. Polym. 2017, 155, 448–455. [Google Scholar] [CrossRef]
- Mishbak, H.H.; Cooper, G.; Bartolo, P.J. Development and Characterization of a Photocurable Alginate Bioink for Three-Dimensional Bioprinting. Int. J. Bioprint. 2019, 5, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Mistry, P.; Aied, A.; Alexander, M.; Shakesheff, K.; Bennett, A.; Yang, J. Bioprinting Using Mechanically Robust Core–Shell Cell-Laden Hydrogel Strands. Macromol. Biosci. 2017, 17, 1600472. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Ma, P.X. Stimuli-Responsive Supramolecular Hydrogels with High Extensibility and Fast Self-Healing via Precoordinated Mussel-Inspired Chemistry. Chem. Mater. 2015, 27, 7627–7635. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Hou, Y.; Park, H.; Choi, B.; Hou, S.; Chung, A.; Lee, M. Visible Light Crosslinkable Chitosan Hydrogels for Tissue Engineering. Acta Biomater. 2012, 8, 1730–1738. [Google Scholar] [CrossRef]
- Kunwar, P.; Jannini, A.V.S.; Xiong, Z.; Ransbottom, M.J.; Perkins, J.S.; Henderson, J.H.; Hasenwinkel, J.M.; Soman, P. High-Resolution 3D Printing of Stretchable Hydrogel Structures Using Optical Projection Lithography. ACS Appl. Mater. Interfaces 2020, 12, 1640–1649. [Google Scholar] [CrossRef]
- Monteiro, N.; Thrivikraman, G.; Athirasala, A.; Tahayeri, A.; França, C.M.; Ferracane, J.L.; Bertassoni, L.E. Photopolymerization of Cell-Laden Gelatin Methacryloyl Hydrogels Using a Dental Curing Light for Regenerative Dentistry. Dent. Mater. 2018, 34, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Ooi, H.W.; Mota, C.; Tessa Ten Cate, A.; Calore, A.; Moroni, L.; Baker, M.B. Thiol-Ene Alginate Hydrogels as Versatile Bioinks for Bioprinting. Biomacromolecules 2018, 19, 3390–3400. [Google Scholar] [CrossRef] [PubMed]
- Rouillard, A.D.; Berglund, C.M.; Lee, J.Y.; Polacheck, W.J.; Tsui, Y.; Bonassar, L.J.; Kirby, B.J. Methods for Photocrosslinking Alginate Hydrogel Scaffolds with High Cell Viability. Tissue Eng.-Part C Methods 2011, 17, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Tie, C.; Cheng, B.; Yang, S.; Wang, X.; Sun, Z.; Yin, M.; Zhu, H.; Yin, M. Effect of Altering Photocrosslinking Conditions on the Physical Properties of Alginate Gels and the Survival of Photoencapsulated Cells. Polym. Degrad. Stab. 2020, 179, 109297. [Google Scholar] [CrossRef]
- Lewandowska-Łańcucka, J.; Mystek, K.; Mignon, A.; Van Vlierberghe, S.; Łatkiewicz, A.; Nowakowska, M. Alginate- and Gelatin-Based Bioactive Photocross-Linkable Hybrid Materials for Bone Tissue Engineering. Carbohydr. Polym. 2017, 157, 1714–1722. [Google Scholar] [CrossRef] [PubMed]
- Hasany, M.; Talebian, S.; Sadat, S.; Ranjbar, N.; Mehrali, M.; Wallace, G.G.; Mehrali, M. Synthesis, Properties, and Biomedical Applications of Alginate Methacrylate (ALMA)-Based Hydrogels: Current Advances and Challenges. Appl. Mater. Today 2021, 24, 101150. [Google Scholar] [CrossRef]
- Jeon, O.; Bouhadir, K.H.; Mansour, J.M.; Alsberg, E. Photocrosslinked Alginate Hydrogels with Tunable Biodegradation Rates and Mechanical Properties. Biomaterials 2009, 30, 2724–2734. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, E.; Vahedi, N.; Sigaroodi, F.; Parandakh, A.; Hosseinzadeh, S.; Zeinali, F.; Khani, M.M. Recent Progress of Bio-Printed PEGDA-Based Bioinks for Tissue Regeneration. Polym. Adv. Technol. 2023, 34, 3505–3517. [Google Scholar] [CrossRef]
- Cha, C.; Kim, S.Y.; Cao, L.; Kong, H. Decoupled Control of Stiffness and Permeability with a Cell-Encapsulating Poly(Ethylene Glycol) Dimethacrylate Hydrogel. Biomaterials 2010, 31, 4864–4871. [Google Scholar] [CrossRef] [PubMed]
- Bekin, S.; Sarmad, S.; Gürkan, K.; Keçeli, G.; Gürdaǧ, G. Synthesis, characterization and bending behavior of electroresponsive sodium alginate/poly(acrylic acid) interpenetrating network films under an electric field stimulus. Sens. Actuators B 2014, 202, 878–892. [Google Scholar] [CrossRef]
- Chou, A.I.; Nicoll, S.B. Characterization of Photocrosslinked Alginate Hydrogels for Nucleus Pulposus Cell Encapsulation. J. Biomed. Mater. Res.-Part A 2009, 91, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Araiza-verduzco, F.; Rodr, E.; Cruz, H.; Alatorre-meda, M. Photocrosslinked Alginate-Methacrylate Hydrogels with Modulable Mechanical Properties: Effect of the Molecular Conformation and Electron Density of the Methacrylate Reactive Group. Materials 2019, 13, 534. [Google Scholar] [CrossRef] [PubMed]
- Mignona, A.; Devisscherb, D.; Graulusb, G.J.; Stubbeb, B.; Martins, J.; Dubruelb, P.; De Beliea, N.; Van Vlierbergheb, S. Combinatory approach of methacrylated alginate and acid monomersfor concrete applications. Carbohydr. Polym. 2017, 155, 448–455. [Google Scholar] [CrossRef]
- Ouyang, L.; Yao, R.; Zhao, Y.; Sun, W. Effect of Bioink Properties on Printability and Cell Viability for 3D Bioplotting of Embryonic Stem Cells. Biofabrication 2016, 8, 035020. [Google Scholar] [CrossRef] [PubMed]
- Rusu, M.C.; Block, C.; Van Assche, G.; Van Mele, B. Influence of Temperature and UV Intensity on Photo-Polymerization Reaction Studied by Photo-DSC. J. Therm. Anal. Calorim. 2012, 110, 287–294. [Google Scholar] [CrossRef]
- Poursamar, S.A.; Lehner, A.N.; Azami, M.; Ebrahimi-Barough, S.; Samadikuchaksaraei, A.; Antunes, A.P.M. The Effects of Crosslinkers on Physical, Mechanical, and Cytotoxic Properties of Gelatin Sponge Prepared via in-Situ Gas Foaming Method as a Tissue Engineering Scaffold. Mater. Sci. Eng. C 2016, 63, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Maitra, J.; Shukla, V.K. Cross-Linking in Hydrogels—A Review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar] [CrossRef]
- Wang, H.; Brown, H.R. Self-Initiated Photopolymerization and Photografting of Acrylic Monomers. Macromol. Rapid Commun. 2004, 25, 1095–1099. [Google Scholar] [CrossRef]
- Jakus, A.E.; Rutz, A.L.; Shah, R.N. Advancing the Field of 3D Biomaterial Printing. Biomed. Mater. 2016, 11, 014102. [Google Scholar] [CrossRef]
- Sridharan, R.; Ryan, E.J.; Kearney, C.J.; Kelly, D.J.; O’Brien, F.J. Macrophage Polarization in Response to Collagen Scaffold Stiffness Is Dependent on Cross-Linking Agent Used to Modulate the Stiffness. ACS Biomater. Sci. Eng. 2019, 5, 544–552. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zoco de la Fuente, A.; García-García, A.; Pérez-Álvarez, L.; Moreno-Benítez, I.; Larrea-Sebal, A.; Martin, C.; Vilas-Vilela, J.L. Evaluation of Various Types of Alginate Inks for Light-Mediated Extrusion 3D Printing. Polymers 2024, 16, 986. https://doi.org/10.3390/polym16070986
Zoco de la Fuente A, García-García A, Pérez-Álvarez L, Moreno-Benítez I, Larrea-Sebal A, Martin C, Vilas-Vilela JL. Evaluation of Various Types of Alginate Inks for Light-Mediated Extrusion 3D Printing. Polymers. 2024; 16(7):986. https://doi.org/10.3390/polym16070986
Chicago/Turabian StyleZoco de la Fuente, Aitana, Ane García-García, Leyre Pérez-Álvarez, Isabel Moreno-Benítez, Asier Larrea-Sebal, Cesar Martin, and Jose Luis Vilas-Vilela. 2024. "Evaluation of Various Types of Alginate Inks for Light-Mediated Extrusion 3D Printing" Polymers 16, no. 7: 986. https://doi.org/10.3390/polym16070986




