Synthesis, Structure and Properties of Polyester Polyureas via a Non-Isocyanate Route with Good Combined Properties
Abstract
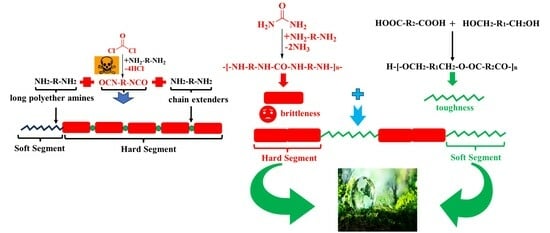
1. Introduction
2. Materials and Methods
2.1. Materials
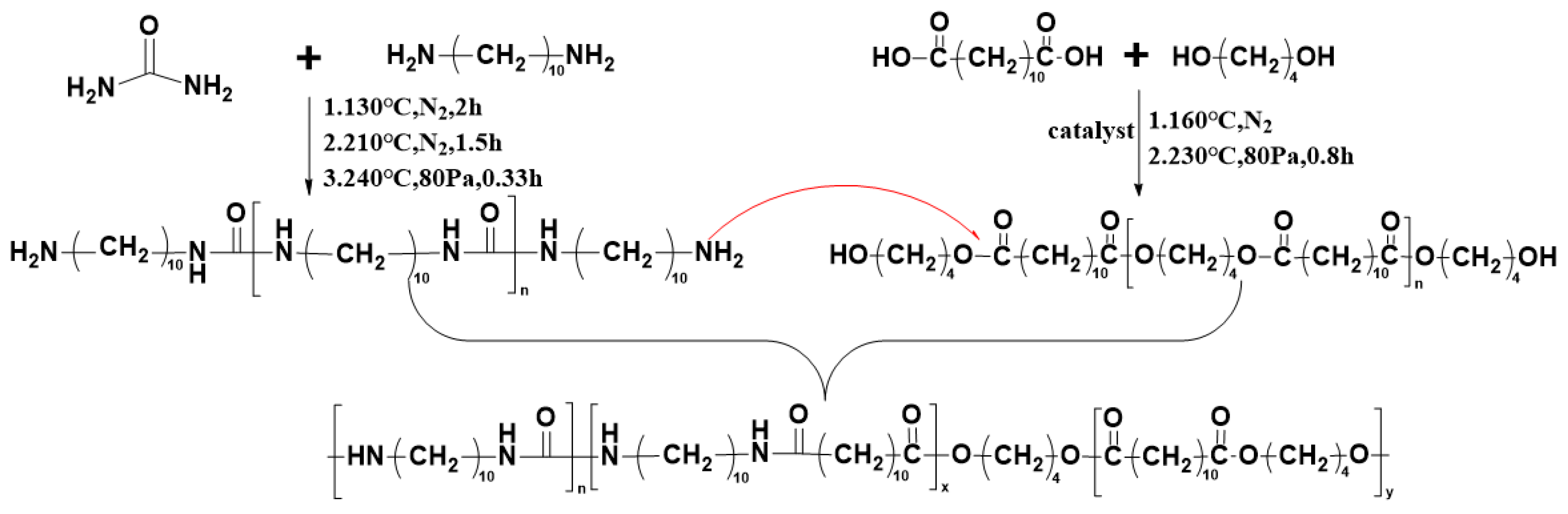
2.2. Synthesis of Polyureas (PBDxPUy)
2.2.1. Synthesis of Pre-PU
2.2.2. Synthesis of Pre-PBD
2.2.3. Synthesis of PBDxPUy
2.3. Characterizations
3. Results and Discussion
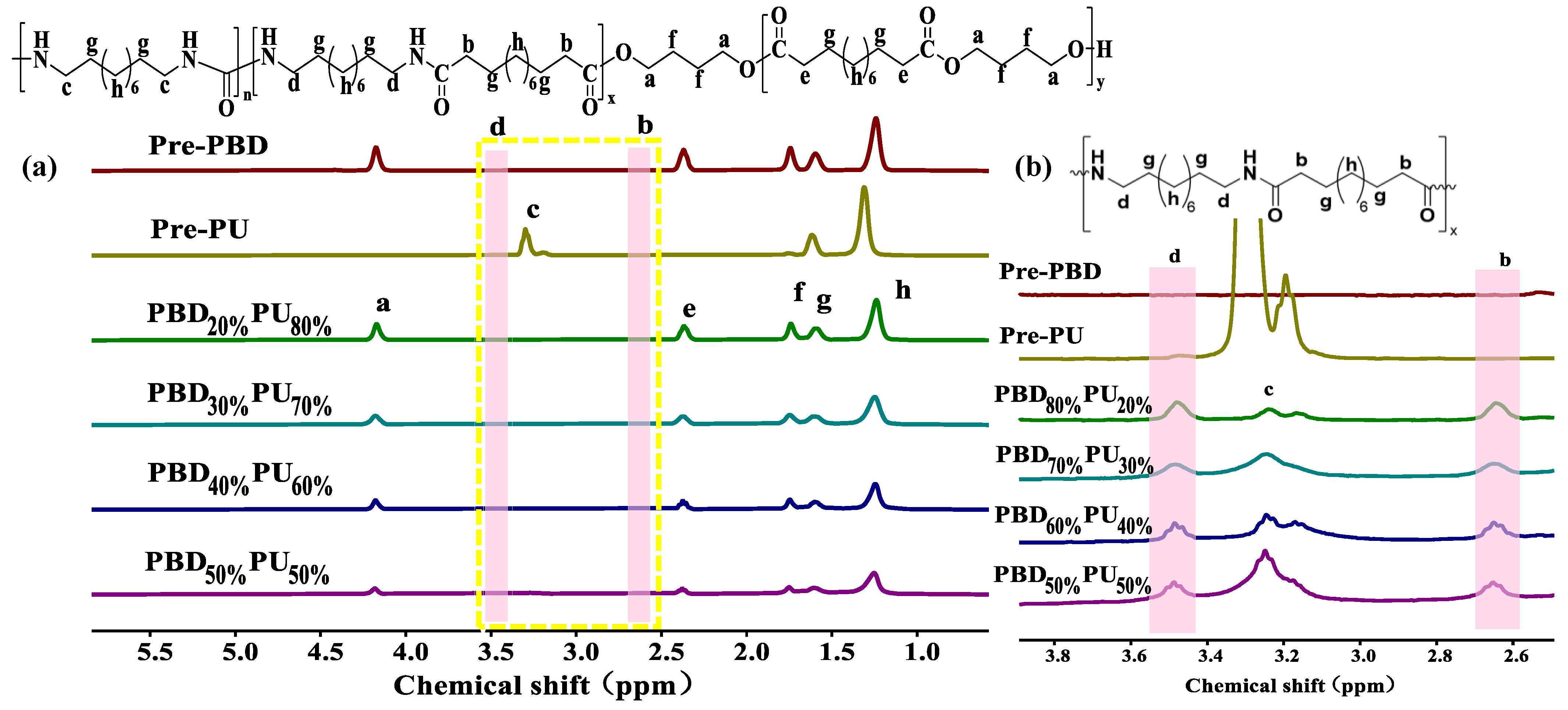
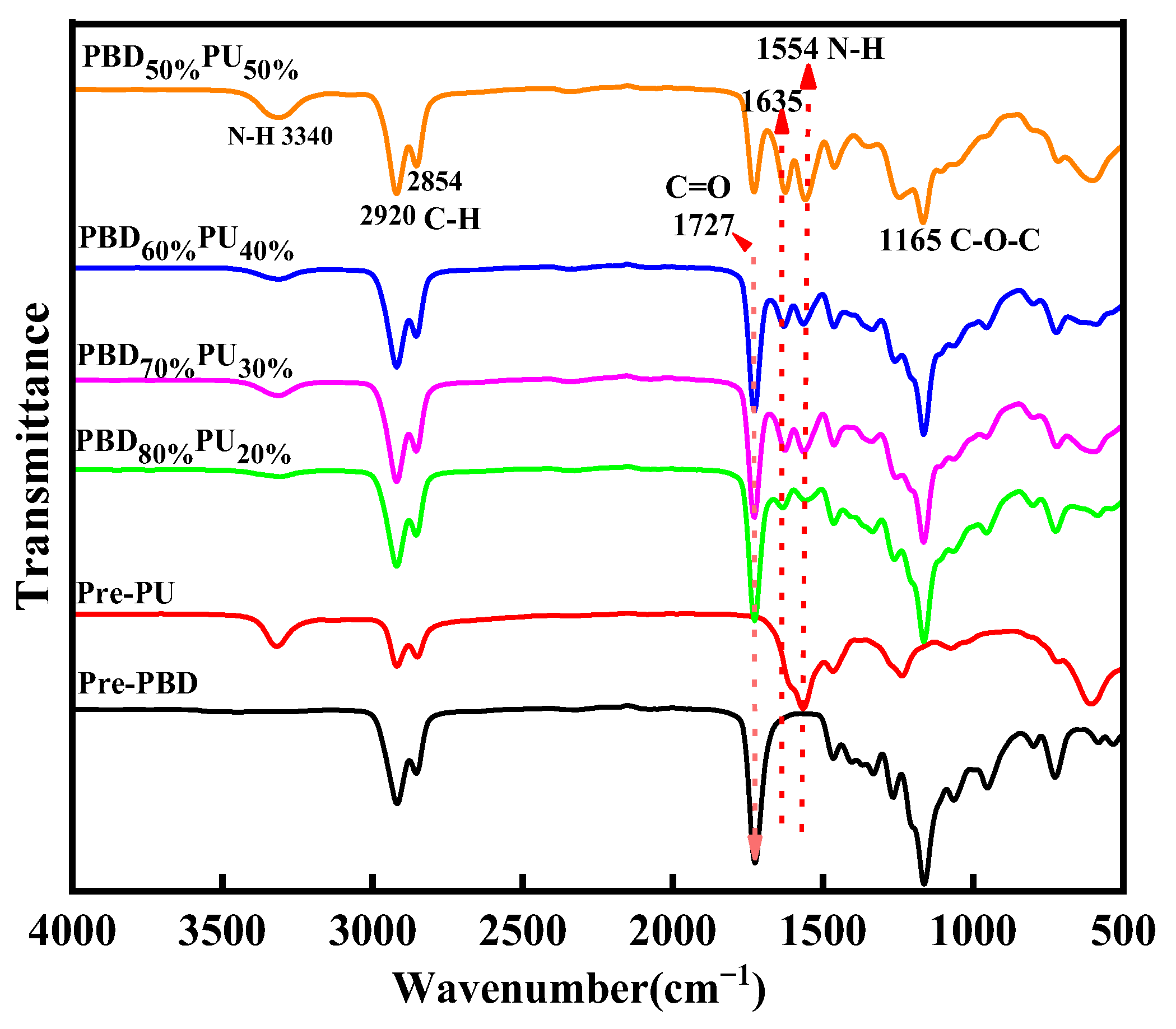
3.1. Chemical Structure Characterization of PBDxPUy
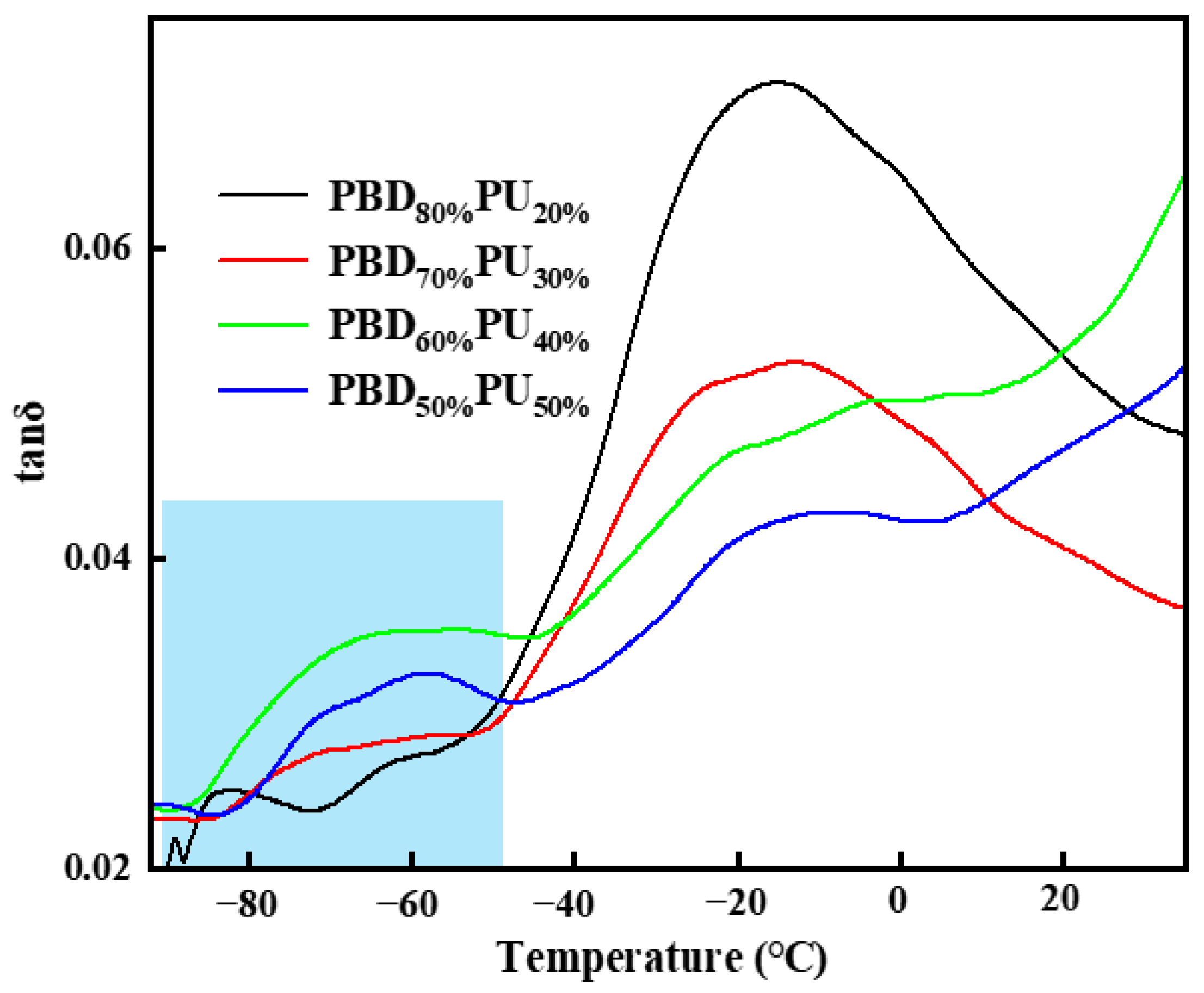
3.2. Thermal Properties of PBDxPUy
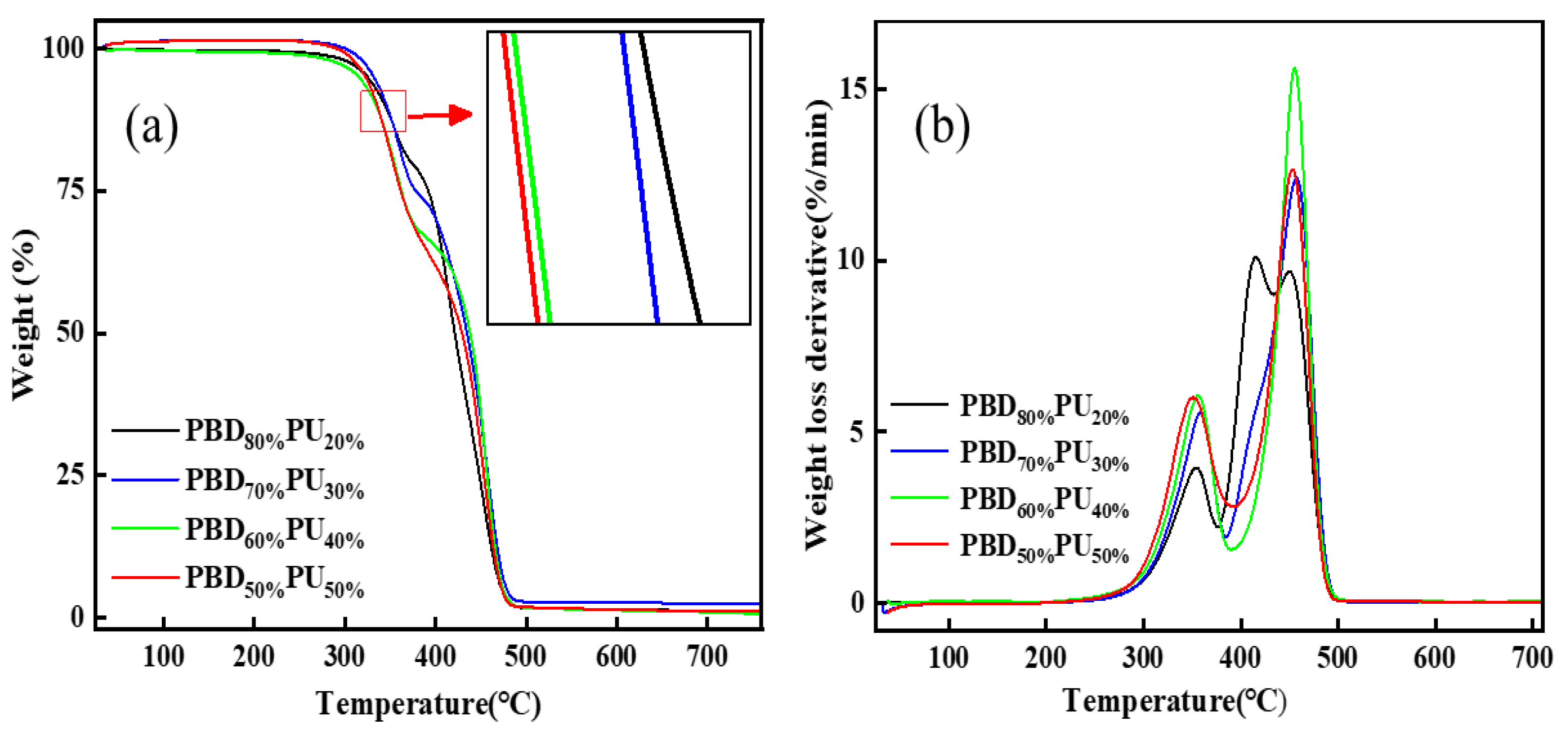
3.3. Thermal Stability of PBDxPUy
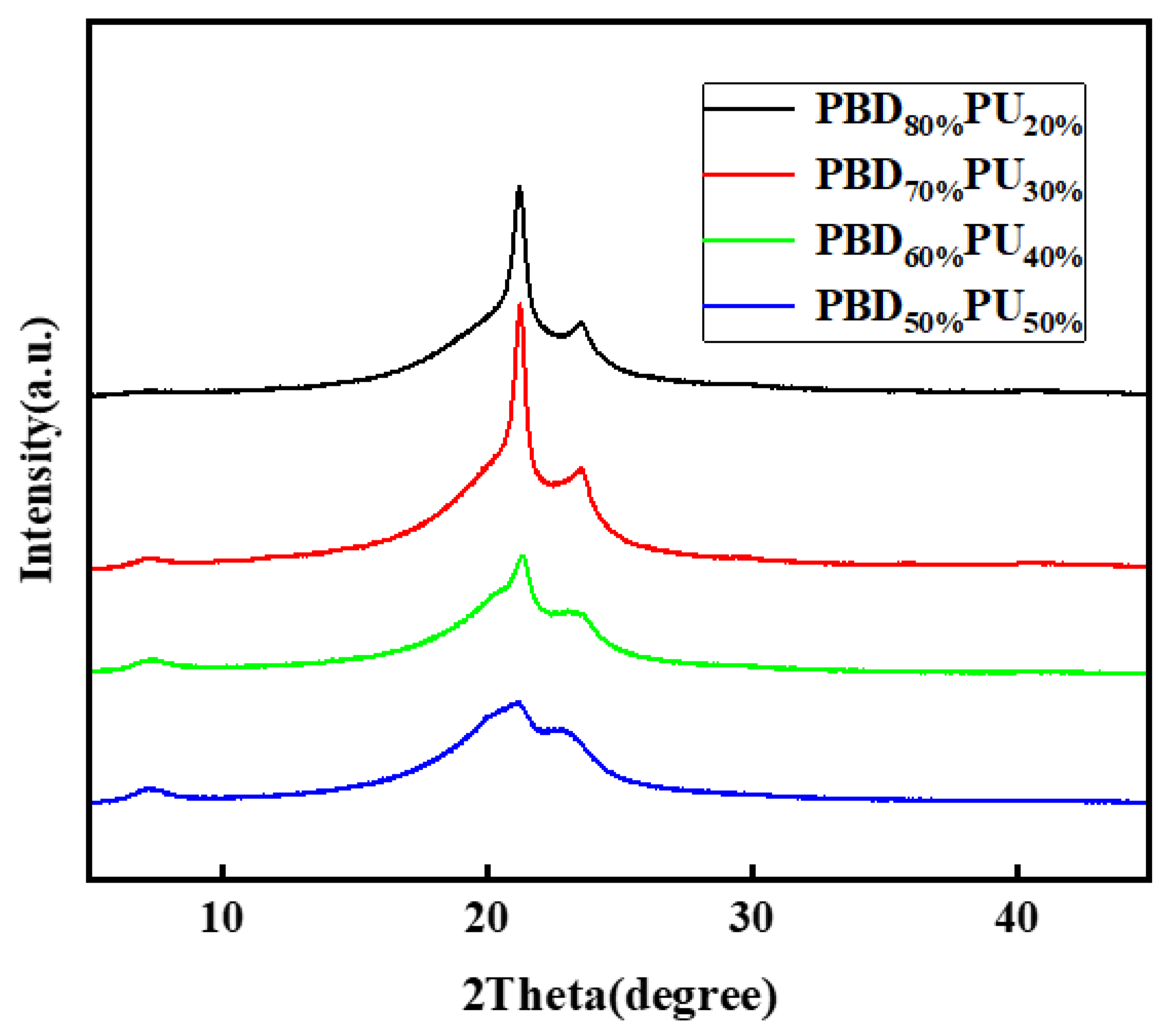
3.4. Crystallization Properties of PBDxPUy Films
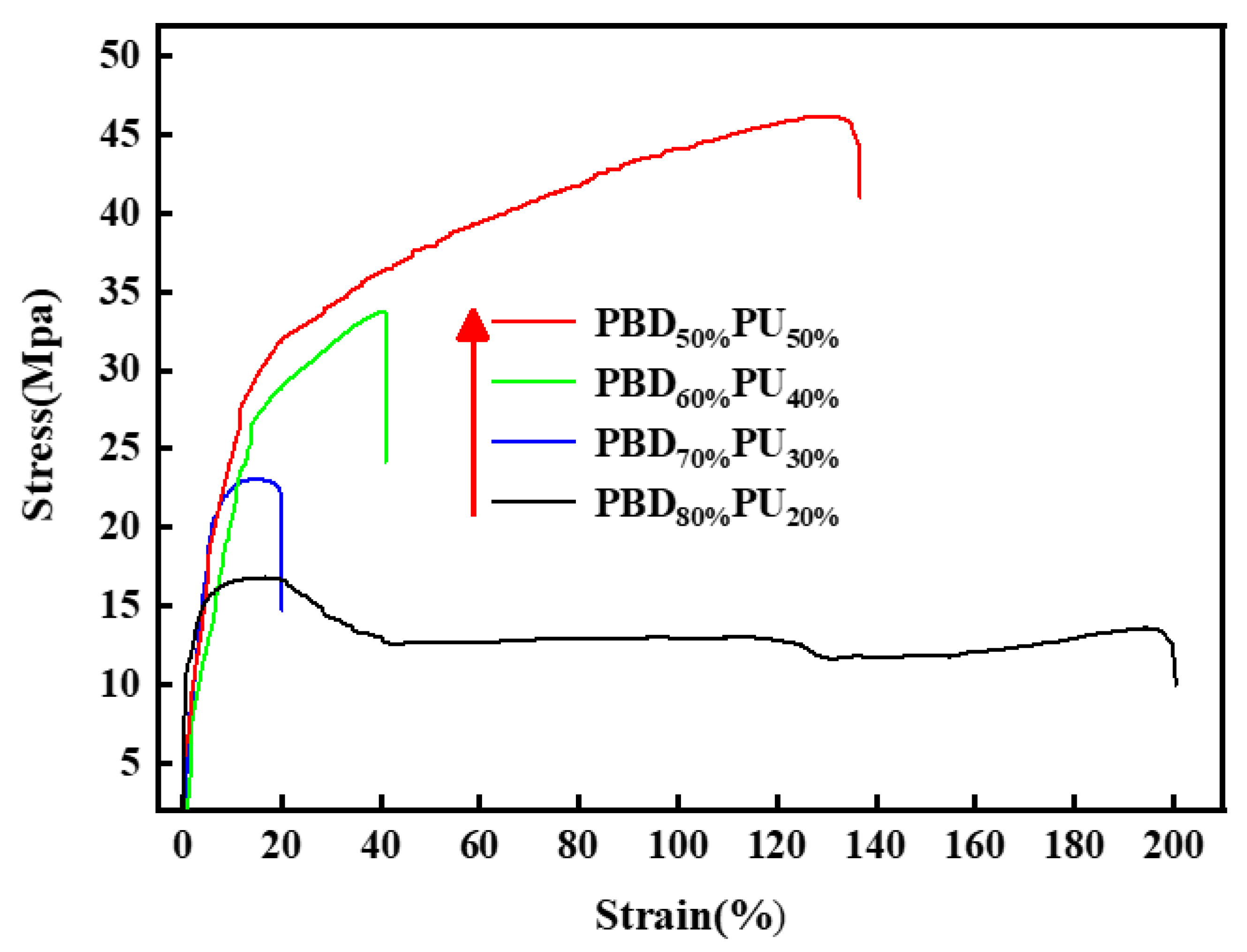
3.5. Mechanical Properties of PBDxPUy
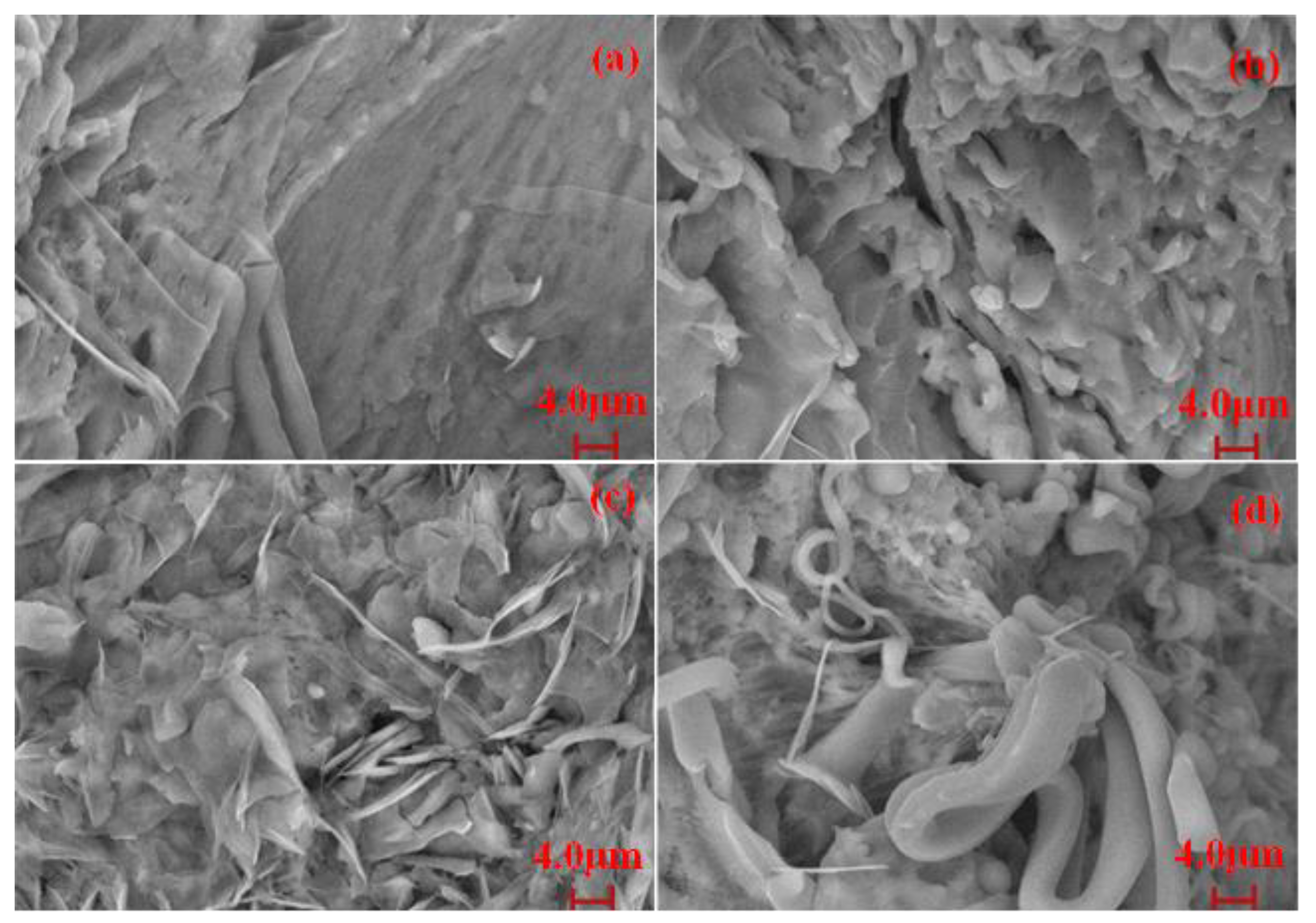
3.6. Morphology of PBDxPUy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sirrine, J.M.; Schexnayder, S.A.; Dennis, J.M.; Long, T.E. Urea as a monomer for isocyanate-free synthesis of segmented poly (dimethyl siloxane) polyureas. Polymer 2018, 154, 225–232. [Google Scholar] [CrossRef]
- Wu, C.; Wang, J.; Chang, P.; Cheng, H.; Yu, Y.; Wu, Z.; Dong, D.; Zhao, F. Polyureas from diamines and carbon dioxide: Synthesis, structures and properties. Phys. Chem. Chem. Phys. 2012, 14, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Armstrong, D.; Peters, G.; Nagala, M.; Shirran, S. Direct synthesis of polyureas from the dehydrogenative coupling of diamines and methanol. Chem. Commun. 2021, 57, 6153–6156. [Google Scholar] [CrossRef] [PubMed]
- Wolosz, D.; Parzuchowski, P.G.; Rolinska, K. Environmentally friendly synthesis of urea-free poly(carbonate-urethane) elastomers. Macromolecules. 2022, 55, 4995–5008. [Google Scholar] [CrossRef]
- Lin, C.; Xie, K.; Tang, D. High-performance thermoplastic polyureas via a non-isocyanate route from urea and aliphatic diamines. J. Appl. Polym. Sci. 2022, 139, 52513. [Google Scholar] [CrossRef]
- Ritter, B.S.; Mülhaupt, R. Isocyanate- and solvent-free route to thermoplastic poly(amide-urea) derived from renewable resources. Macromol. Mater. Eng. 2017, 302, 1600338. [Google Scholar] [CrossRef]
- Jiang, S.; Cheng, H.Y.; Shi, R.H.; Wu, P.X.; Lin, W.W.; Zhang, C.; Arai, M.; Zhao, F.-Y. Direct synthesis of polyurea thermoplastics from CO2 and diamines. ACS Appl. Mater. Interfaces 2019, 11, 47413–47421. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Jiang, S.; Cheng, H.; Wu, P.; Zhang, C.; Arai, M.; Zhao, F. Synthesis of polyurea thermoplastics through a nonisocyanate route using CO2 and aliphatic diamines. ACS Sustain. Chem. Eng. 2020, 8, 18626–18635. [Google Scholar]
- Wang, P.; Ma, X.; Li, Q.; Yang, B.; Shang, J.; Deng, Y. Green synthesis of polyureas from CO2 and diamines with a functional ionic liquid as the catalyst. RSC Adv. 2016, 6, 54013–54019. [Google Scholar] [CrossRef]
- Wu, P.; Cheng, H.; Shi, R.; Jiang, S.; Wu, Q.; Zhang, C.; Arai, M.; Zhao, F. Synthesis of polyurea via the addition of carbon dioxide to a diamine catalyzed by organic and inorganic bases. Adv. Synth. Catal. 2019, 361, 317–325. [Google Scholar] [CrossRef]
- Wu, P.; Cheng, H.; Wang, Y.; Shi, R.; Wu, Z.; Arai, M.; Zhao, F. New kind of thermoplastic polyurea elastomers synthesized from CO2 and with self-healing properties. ACS Sustain. Chem. Eng. 2020, 8, 12677–12685. [Google Scholar] [CrossRef]
- Ying, Z.; Wu, C.; Zhang, C.; Jiang, S.; Shi, R.; Cheng, H.; Zhang, B.; Li, Y.; Zhao, F. Synthesis of polyureas with CO2 as carbonyl building block and their high performances. J. CO2 Util. 2017, 19, 209–213. [Google Scholar] [CrossRef]
- Ying, Z.; Zhao, L.; Zhang, C.; Yu, Y.; Liu, T.; Cheng, H.; Zhao, F. Utilization of carbon dioxide to build a basic block for polymeric materials: An isocyanate-free route to synthesize a soluble oligourea. RSC Adv. 2015, 5, 42095–42100. [Google Scholar] [CrossRef]
- Kébir, N.; Benoit, M.; Burel, F. Elaboration of AA-BB and AB-type non-isocyanate polyurethanes (NIPUs) using a cross metathesis polymerization between methyl carbamate and methyl carbonate groups. Eur. Polym. J. 2018, 107, 155–163. [Google Scholar] [CrossRef]
- Kébir, N.; Benoit, M.; Legrand, C.; Burel, F. Non-isocyanate thermoplastic polyureas (NIPUreas) through a methyl carbamate metathesis polymerization. Eur. Polym. J. 2017, 96, 87–96. [Google Scholar] [CrossRef]
- Li, S.; Sang, Z.; Zhao, J.; Zhang, Z.; Zhang, J.; Yang, W. Crystallizable and tough aliphatic thermoplastic polyureas synthesized through a nonisocyanate route. Ind. Eng. Chem. Res. 2016, 55, 1902–1911. [Google Scholar] [CrossRef]
- Ma, S.; van Heeswijk, E.P.A.; Noordover, B.A.J.; Sablong, R.J.; van Benthem, R.; Koning, C.E. Isocyanate-free approach to water-borne polyurea dispersions and coatings. ChemSusChem 2018, 11, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Lecamp, L.; Labib, H.; Aloui, F.; Kébir, N.; Burel, F. Synthesis and properties of allyl terminated renewable non-isocyanate polyurethanes (NIPUs) and polyureas (NIPUreas) and study of their photo-crosslinking. Eur. Polym. J. 2016, 84, 828–836. [Google Scholar] [CrossRef]
- Pan, W.C.; Lin, C.H.; Dai, S.H.A. High-performance segmented polyurea by transesterification of diphenyl carbonates with aliphatic diamines. J. Polym. Sci. Pol. Chem. 2014, 52, 2781–2790. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, S.; Pan, X. Biomass-based polyureas derived from rigid furfurylamine and isomannide. ACS Appl. Polym. Mater. 2022, 4, 2197–2204. [Google Scholar] [CrossRef]
- Li, S.; Zhao, J.; Zhang, Z.; Zhang, J.; Yang, W. Aliphatic thermoplastic polyurethane-ureas and polyureas synthesized through a non-isocyanate route. RSC Adv. 2015, 5, 6843–6852. [Google Scholar] [CrossRef]
- Li, H.; Cheng, H.; Zhao, F. A review on CO2-based polyureas and polyurea hybrids. Asian J. Org. Chem. 2022, 11, e202200338. [Google Scholar] [CrossRef]
- Dennis, J.M.; Steinberg, L.I.; Pekkanen, A.M.; Maiz, J.; Hegde, M.; Müller, A.J.; Long, T.E. Synthesis and characterization of isocyanate-free polyureas. Green Chem. 2018, 20, 243–249. [Google Scholar] [CrossRef]
- Tang, D.; Chen, Z.; Correa-Netto, F.; Macosko, C.W.; Hillmyer, M.A.; Zhang, G. Poly(urea ester): A family of biodegradable polymers with high melting temperatures. J. Polym. Sci. Pol. Chem. 2016, 54, 3795–3799. [Google Scholar] [CrossRef]
- White, B.T.; Migliore, J.M.; Mapesa, E.U.; Wolfgang, J.D.; Sangoro, J.; Long, T.E. Isocyanate- and solvent-free synthesis of melt processible polyurea elastomers derived from urea as a monomer. RSC Adv. 2020, 10, 18760–18768. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lin, C.; Hou, R.; Tang, D. Construction of polyallophanate vitrimers from poly(urea carbonate) via group revival induced crosslinking. Eur. Polym. J. 2021, 161, 110819. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, W.B.; Lyu, P.; Yan, S.; Wang, X.; Ju, J.H. Polyurea for blast and impact protection: A review. Polymers 2022, 14, 2670. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Tripathi, M.; Parthasarathy, S.; Kumar, D.; Roy, P.K. Tuning the properties of segmented polyurea by regulating soft-segment length. J. Appl. Polym. Sci. 2018, 135, 46284. [Google Scholar] [CrossRef]
- Lyu, P.; Fang, Z.; Wang, X.; Huang, W.; Zhang, R.; Sang, Y.; Sun, P. Explosion test and numerical simulation of coated reinforced concrete slab based on blast mitigation polyurea coating performance. Materials 2022, 15, 2607. [Google Scholar] [CrossRef]
- ISO 527-2:2012; Plastics-Determination of Tensile Properties-Part 2: Test Conditions for Moulding and Etrusion Plastics. International Organization for Standardization: Geneva, Switzerland, 2012.
- ISO 178:2010; Plastics-Determination of Flexural Properties. International Organization for Standardization: Geneva, Switzerland, 2012.
- ISO 179:2010; Plastics-Determination of Charpy Impact Properties-Part 1: Non-Instrumented Impact Test. International Organization for Standardization: Geneva, Switzerland, 2010.
- Qi, J.; Wu, J.; Chen, J.; Wang, H. An investigation of the thermal and (bio)degradability of PBS copolyesters based on isosorbide. Polym. Degrad. Stab. 2019, 160, 229–241. [Google Scholar] [CrossRef]
- Ai, T.; Zou, G.; Feng, W.; Ren, Z.; Li, F.; Wang, P.; Lu, B.; Ji, J. Synthesis and properties of biobased copolyamides based on polyamide 10T and polyamide 56 through one-pot polymerization. New J. Chem. 2021, 45, 14677–14686. [Google Scholar] [CrossRef]
- Mattia, J.; Painter, P. A comparison of hydrogen bonding and order in a polyurethane and poly(urethane-urea) and their blends with poly(ethylene glycol). Macromolecules 2007, 40, 1546–1554. [Google Scholar] [CrossRef]
- Liu, X.; Desilles, N.; Jiang, B.; Chappey, C.; Lebrun, L. High barrier semi-crystalline polyesters involving nature occurring pyridine structure towards sustainable food packaging. Polymer 2022, 247, 124790. [Google Scholar] [CrossRef]
- Chen, M.; Saada, N.A.H.; Liu, F.; Na, H.; Zhu, J. Synthesis of copolyesters with bio-based lauric diacid: Structure and physico-mechanical studies. RSC Adv. 2017, 7, 55418–55426. [Google Scholar] [CrossRef]
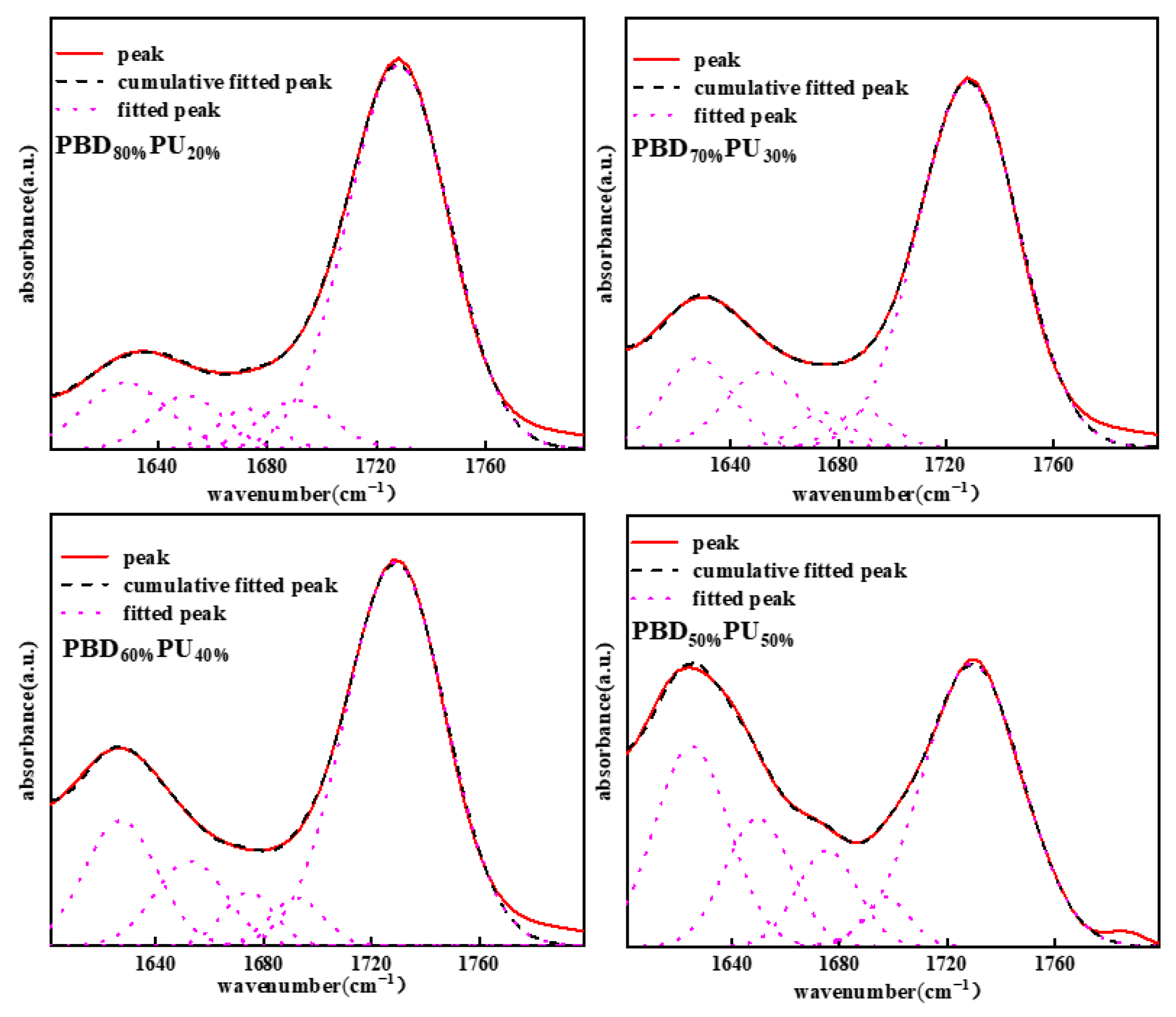


| PBD80% PU20% | PBD70% PU30% | PBD60% PU40% | PBD50% PU50% | |
|---|---|---|---|---|
| 1626–1628 urea (bonded, ordered) A1 | 10.34% | 11.51% | 15.48% | 25.03% |
| 1652–1654 urea (bonded disordered) A2 | 7.00% | 11.11% | 10.25% | 14.34% |
| 1690–1691 urea (free) A3 | 6.54% | 3.89% | 4.33% | 3.76% |
| 1672–1674 ester (bonded) A4 | 4.10% | 3.6% | 4.63% | 9.70% |
| 1728–1729 ester (free) A5 | 72.05% | 79.90% | 65.31% | 47.16% |
| (A1 + A2)/(A1 + A2 + A3) | 72.57% | 85.34% | 85.59% | 91.28% |
| A4/(A4 + A5) | 5.38% | 4.90% | 6.61% | 17.06% |
| Tm1 (°C) | ΔHm (J/g) | Tm2 (°C) | ΔHm (J/g) | Tc1 (°C) | Tc2 (°C) | Tg (°C) | ||
|---|---|---|---|---|---|---|---|---|
| β | α | |||||||
| PBD80%PU20% | 46.5 | 25.7 | 179.3 | 3.4 | 24.1 | 74.5 | −60.5 | −15.3 |
| PBD70%PU30% | 47.4 | 17.7 | 198.2 | 9.7 | 16.9 | 102.8 | −64.9 | −13.7 |
| PBD60% PU40% | 36.7 | 3.3 | 188.2 | 17.5 | - | 95.9 | −59.2 | −3.6 |
| PBD50%PU50% | - | - | 180.0, 194.7 | 21.0 | - | 102.6 | −62.7 | −11.2 |
| T5% (°C) | Tmax1 (°C) | Tmax2 (°C) | Tmax3 (°C) | |
|---|---|---|---|---|
| PBD80%PU20% | 320 | 354 | 415 | 450 |
| PBD70%PU30% | 316 | 358 | 416 | 457 |
| PBD60%PU40% | 331 | 356 | - | 455 |
| PBD50%PU50% | 325 | 351 | - | 453 |
| Property | PBD80% PU20% | PBD70% PU30% | PBD60% PU40% | PBD50% PU50% |
|---|---|---|---|---|
| Crystallinity (%) | 15.70 | 18.55 | 12.33 | 9.29 |
| Sample | Impact Strength kJ/m2 | Tensile Strength MPa | Elongation at Breaking% | Flexural Modulus MPa | Flexural Strength MPa |
|---|---|---|---|---|---|
| PBD80%PU20% | 7.5 | 16.5 | 197.8 | 269.0 | 9.8 |
| PBD70%PU30% | 24.5 | 23.0 | 18.2 | 423.1 | 14.8 |
| PBD60%PU40% | 31.1 | 33.6 | 41.4 | 456.4 | 15.6 |
| PBD50%PU50% | 37.2 | 46.1 | 135.0 | 524.6 | 18.6 |
| PBD | 30.0 | 13.0 | 380.1 | 500.4 | 16.2 |
| PU-10 | 6.8 | 70.0 | 24.0 | 1402.7 | 48.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, L.; Xie, Q.; Hu, G.; Wang, B.; Song, D.; Zhang, Y.; Liu, Y. Synthesis, Structure and Properties of Polyester Polyureas via a Non-Isocyanate Route with Good Combined Properties. Polymers 2024, 16, 993. https://doi.org/10.3390/polym16070993
Zheng L, Xie Q, Hu G, Wang B, Song D, Zhang Y, Liu Y. Synthesis, Structure and Properties of Polyester Polyureas via a Non-Isocyanate Route with Good Combined Properties. Polymers. 2024; 16(7):993. https://doi.org/10.3390/polym16070993
Chicago/Turabian StyleZheng, Liuchun, Qiqi Xie, Guangjun Hu, Bing Wang, Danqing Song, Yunchuan Zhang, and Yi Liu. 2024. "Synthesis, Structure and Properties of Polyester Polyureas via a Non-Isocyanate Route with Good Combined Properties" Polymers 16, no. 7: 993. https://doi.org/10.3390/polym16070993
APA StyleZheng, L., Xie, Q., Hu, G., Wang, B., Song, D., Zhang, Y., & Liu, Y. (2024). Synthesis, Structure and Properties of Polyester Polyureas via a Non-Isocyanate Route with Good Combined Properties. Polymers, 16(7), 993. https://doi.org/10.3390/polym16070993