Effect of Environmental pH on the Mechanics of Chitin and Chitosan: A Single-Molecule Study
Abstract
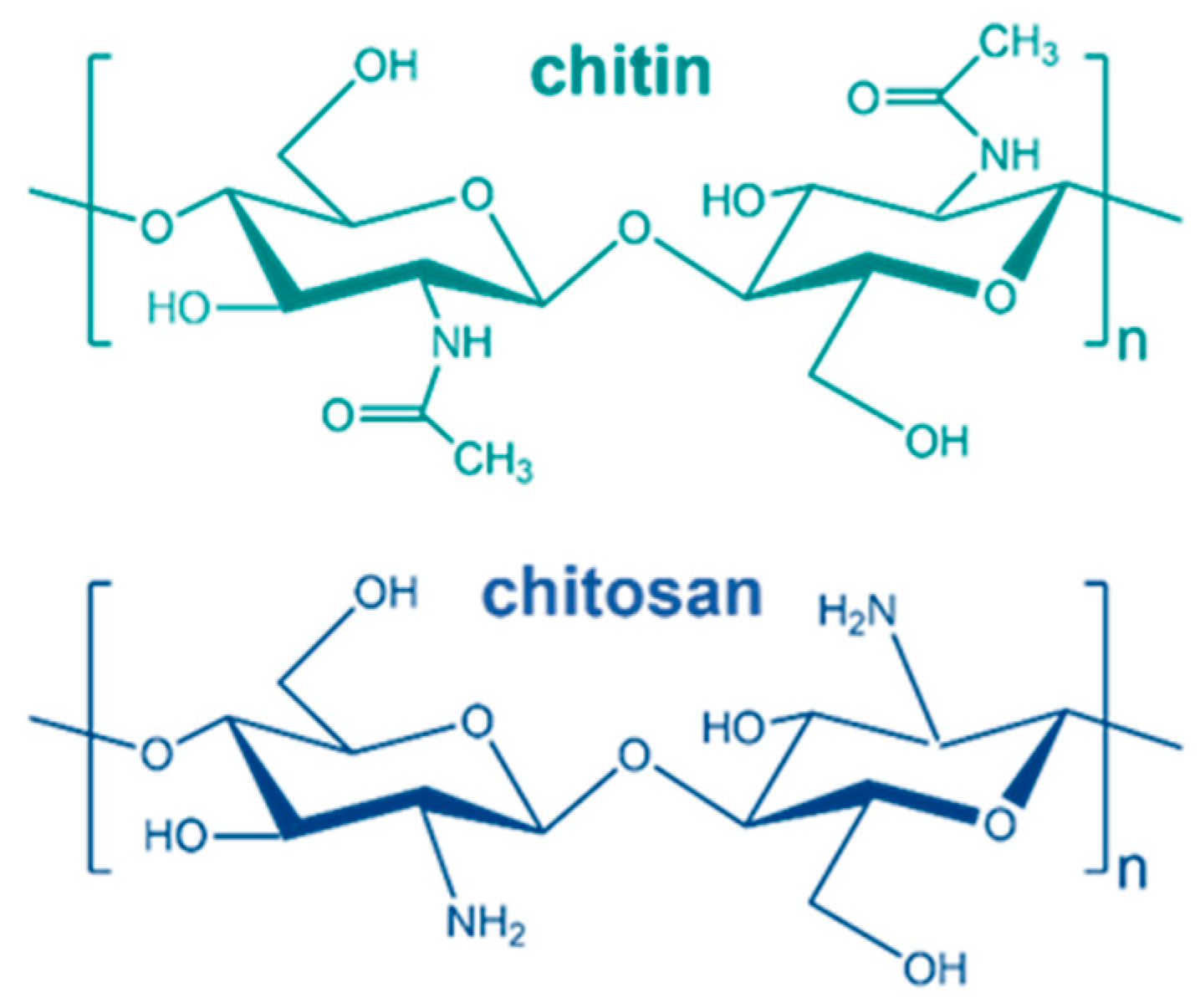
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Details of AFM-Based SMFS
3. Results and Discussion
3.1. The Inherent Elastic Behaviors of Chitin and Chitosan
3.2. The Elastic Behaviors of Chitin and Chitosan in DI Water and Acid Conditions
3.3. The Mechanical and Thermodynamic Properties of Chitin and Chitosan Determined by pH Value
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, R.; Grinstaff, M.W. Chemical synthesis of polysaccharides and polysaccharide mimetics. Prog. Polym. Sci. 2017, 74, 78–116. [Google Scholar] [CrossRef]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.-J. Food applications of chitin and chitosans. Trends Food Sci. Technol. 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Amiri, H.; Aghbashlo, M.; Sharma, M.; Gaffey, J.; Manning, L.; Moosavi Basri, S.M.; Kennedy, J.F.; Gupta, V.K.; Tabatabaei, M. Chitin and chitosan derived from crustacean waste valorization streams can support food systems and the UN Sustainable Development Goals. Nat. Food 2022, 3, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Ghormade, V.; Pathan, E.; Deshpande, M. Can fungi compete with marine sources for chitosan production? Int. J. Biol. Macromol. 2017, 104, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Pochanavanich, P.; Suntornsuk, W. Fungal chitosan production and its characterization. Lett. Appl. Microbiol. 2002, 35, 17–21. [Google Scholar] [CrossRef]
- Islam, S.; Bhuiyan, M.R.; Islam, M. Chitin and chitosan: Structure, properties and applications in biomedical engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Nair, S.; Tokura, S.; Tamura, H.; Selvamurugan, N. Novel carboxymethyl derivatives of chitin and chitosan materials and their biomedical applications. Prog. Mater. Sci. 2010, 55, 675–709. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Kumar, P.S.; Nair, S.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Parhi, R. Drug delivery applications of chitin and chitosan: A review. Environ. Chem. Lett. 2020, 18, 577–594. [Google Scholar] [CrossRef]
- Meyers, M.A.; Chen, P.-Y.; Lin, A.Y.-M.; Seki, Y. Biological materials: Structure and mechanical properties. Prog. Mater. Sci. 2008, 53, 1–206. [Google Scholar] [CrossRef]
- Cui, J.; Yu, Z.; Lau, D. Effect of acetyl group on mechanical properties of chitin/chitosan nanocrystal: A molecular dynamics study. Int. J. Mol. Sci. 2016, 17, 61. [Google Scholar] [CrossRef] [PubMed]
- Villar-Chavero, M.M.; Dominguez, J.C.; Alonso, M.V.; Oliet, M.; Rodriguez, F. Chitosan-reinforced cellulosic bionogels: Viscoelastic and antibacterial properties. Carbohydr. Polym. 2020, 229, 115569. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Heuzey, M.-C.; Bégin, A.; Carreau, P.J. Viscoelastic properties of chitosan solutions: Effect of concentration and ionic strength. J. Food Eng. 2006, 74, 500–515. [Google Scholar] [CrossRef]
- Calero, N.; Muñoz, J.; Ramírez, P.; Guerrero, A. Flow behaviour, linear viscoelasticity and surface properties of chitosan aqueous solutions. Food Hydrocoll. 2010, 24, 659–666. [Google Scholar] [CrossRef]
- Hamdi, M.; Nasri, R.; Hajji, S.; Nigen, M.; Li, S.; Nasri, M. Acetylation degree, a key parameter modulating chitosan rheological, thermal and film-forming properties. Food Hydrocoll. 2019, 87, 48–60. [Google Scholar] [CrossRef]
- Sacco, P.; Cok, M.; Asaro, F.; Paoletti, S.; Donati, I. The role played by the molecular weight and acetylation degree in modulating the stiffness and elasticity of chitosan gels. Carbohydr. Polym. 2018, 196, 405–413. [Google Scholar] [CrossRef]
- Wang, C.; Esker, A.R. Nanocrystalline chitin thin films. Carbohydr. Polym. 2014, 102, 151–158. [Google Scholar] [CrossRef]
- Ifuku, S.; Ikuta, A.; Izawa, H.; Morimoto, M.; Saimoto, H. Control of mechanical properties of chitin nanofiber film using glycerol without losing its characteristics. Carbohydr. Polym. 2014, 101, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, T.; Gan, X.; Yuan, R.; Li, Q.; Zhu, L.; Guo, N.; Zhu, J.; Li, Y.; Zhang, S. Schiff base reaction induced densification of chitosan-derived microporous carbon for compact capacitive energy storage. Chem. Eng. J. 2023, 470, 144257. [Google Scholar] [CrossRef]
- Guo, X.; Huang, W.; Tong, J.; Chen, L.; Shi, X. One-step programmable electrofabrication of chitosan asymmetric hydrogels with 3D shape deformation. Carbohydr. Polym. 2022, 277, 118888. [Google Scholar] [CrossRef]
- Maurstad, G.; Danielsen, S.; Stokke, B.T. The influence of charge density of chitosan in the compaction of the polyanions DNA and xanthan. Biomacromolecules 2007, 8, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Liu, C.; Wang, Z.; Zhang, X.; Strandman, S.; Tenhu, H.J.M. Single molecule force spectroscopy on polyelectrolytes: Effect of spacer on adhesion force and linear charge density on rigidity. Macromolecules 2004, 37, 946–953. [Google Scholar] [CrossRef]
- Köhler, M.; Karner, A.; Leitner, M.; Hytönen, V.P.; Kulomaa, M.; Hinterdorfer, P.; Ebner, A. pH-dependent deformations of the energy landscape of avidin-like proteins investigated by single molecule force spectroscopy. Molecules 2014, 19, 12531–12546. [Google Scholar] [CrossRef]
- Luna, R.; Touhami, F.; Uddin, M.; Touhami, A. Effect of temperature and pH on nanostructural and nanomechanical properties of chitosan films. Surf. Interfaces 2022, 29, 101706. [Google Scholar] [CrossRef]
- Wijesena, R.N.; Tissera, N.D.; Rathnayaka, V.; de Silva, R.M.; de Silva, K.N. Colloidal stability of chitin nanofibers in aqueous systems: Effect of pH, ionic strength, temperature & concentration. Carbohydr. Polym. 2020, 235, 116024. [Google Scholar]
- Zhang, W.; Zhang, X. Single molecule mechanochemistry of macromolecules. Prog. Polym. Sci. 2003, 28, 1271–1295. [Google Scholar] [CrossRef]
- Neuman, K.C.; Nagy, A. Single-molecule force spectroscopy: Optical tweezers, magnetic tweezers and atomic force microscopy. Nat. Methods 2008, 5, 491–505. [Google Scholar] [CrossRef]
- Lei, H.; He, C.; Hu, C.; Li, J.; Hu, X.; Hu, X.; Li, H. Single-molecule force spectroscopy trajectories of a single protein and its polyproteins are equivalent: A direct experimental validation based on a small protein NuG2. Angew. Chem. Int. 2017, 56, 6117–6121. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Di, W.; Li, Y.; Huang, W.; Wang, X.; Qin, M.; Wang, W.; Cao, Y. Mg2+-dependent high mechanical anisotropy of three-way-junction pRNA as revealed by single-molecule force spectroscopy. Angew. Chem. Int. Ed. 2017, 56, 9376–9380. [Google Scholar] [CrossRef]
- Cai, W.; Bullerjahn, J.T.; Lallemang, M.; Kroy, K.; Balzer, B.N.; Hugel, T. Angle-dependent strength of a single chemical bond by stereographic force spectroscopy. Chem. Sci. 2022, 13, 5734–5740. [Google Scholar] [CrossRef]
- Yu, M.; Qian, L.; Cui, S. Reentrant variation of single-chain elasticity of polyelectrolyte induced by monovalent salt. J. Phys. Chem. B 2017, 121, 4257–4264. [Google Scholar] [CrossRef]
- Qian, L.; Bao, Y.; Duan, W.; Cui, S. Effects of water content of the mixed solvent on the single-molecule mechanics of amylose. ACS Macro Lett. 2018, 7, 672–676. [Google Scholar] [CrossRef]
- Cai, W.; Lu, S.; Wei, J.; Cui, S. Single-chain polymer models incorporating the effects of side groups: An approach to general polymer models. Macromolecules 2019, 52, 7324–7330. [Google Scholar] [CrossRef]
- Cai, W.; Jäger, M.; Bullerjahn, J.T.; Hugel, T.; Wolf, S.; Balzer, B.N. Anisotropic friction in a ligand-protein complex. Nano Lett. 2023, 23, 4111–4119. [Google Scholar] [CrossRef]
- Qian, L.; Cai, W.; Xu, D.; Bao, Y.; Lu, Z.-Y.; Cui, S. Single-molecule studies reveal that water is a special solvent for amylose and natural cellulose. Macromolecules 2019, 52, 5006–5013. [Google Scholar] [CrossRef]
- Zhang, S.; Qian, H.J.; Liu, Z.; Ju, H.; Lu, Z.; Zhang, H.; Chi, L.; Cui, S. Towards unveiling the exact molecular structure of amorphous red phosphorus by single-molecule studies. Angew. Chem. Int. Ed. 2019, 58, 1659–1663. [Google Scholar] [CrossRef]
- Bao, Y.; Luo, Z.; Cui, S. Environment-dependent single-chain mechanics of synthetic polymers and biomacromolecules by atomic force microscopy-based single-molecule force spectroscopy and the implications for advanced polymer materials. Chem. Soc. Rev. 2020, 49, 2799–2827. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Trefs, J.L.; Hugel, T.; Balzer, B.N. Anisotropy of π–π stacking as basis for superlubricity. ACS Mater. Lett. 2022, 5, 172–179. [Google Scholar] [CrossRef]
- Bao, Y.; Qian, H.-J.; Lu, Z.-Y.; Cui, S. The unexpected flexibility of natural cellulose at a single-chain level and its implications to the design of nano materials. Nanoscale 2014, 6, 13421–13424. [Google Scholar] [CrossRef]
- Cai, W.; Xu, D.; Qian, L.; Wei, J.; Xiao, C.; Qian, L.; Lu, Z.-Y.; Cui, S. Force-induced transition of π–π stacking in a single polystyrene chain. J. Am. Chem. Soc. 2019, 141, 9500–9503. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, M.; Zhang, G.; He, G.; Ji, Y.; Dong, J.; Zheng, H.; Qian, L. Revealing the control mechanisms of pH on the solution properties of chitin via single-molecule studies. Molecules 2023, 28, 6769. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zhang, K.; Guo, X.; Qian, L. Effects of the degree of deacetylation on the single-molecule mechanics of chitosans. J. Phys. Chem. B 2023, 127, 4261–4267. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Zhang, K.; Guo, X.; Yu, M. What happens when chitin becomes chitosan? A single-molecule study. RSC Adv. 2023, 13, 2294–2300. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Z.; Bao, Y.; Lu, S.; Gong, Z.; Qian, H.-J.; Lu, Z.-Y.; Cui, S. Nanoscopic characterization reveals that bulk amorphous elementary boron is composed of a ladder-like polymer with B4 as the structural unit. ACS Nano 2023, 17, 10958–10964. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Guo, X.; Zhang, K.; Yu, M. Effects of hydrogen bonds on the single-chain mechanics of chitin. Phys. Chem. Chem. Phys. 2022, 24, 24535–24541. [Google Scholar] [CrossRef]
- Zhang, F.; Gong, Z.; Cai, W.; Qian, H.-J.; Lu, Z.-Y.; Cui, S. Single-chain mechanics of cis-1, 4-polyisoprene and polysulfide. Polymer 2022, 240, 124473. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, H.; Miao, X.; Zhang, G.; Song, Y.; Kang, X.; Qian, L. Surprising nanomechanical and conformational transition of neutral polyacrylamide in monovalent saline solutions. J. Phys. Chem. B 2023, 127, 10088–10096. [Google Scholar] [CrossRef]
- Cao, N.; Zhao, Y.; Chen, H.; Huang, J.; Yu, M.; Bao, Y.; Wang, D.; Cui, S. Poly (ethylene glycol) becomes a supra-polyelectrolyte by capturing hydronium ions in water. Macromolecules 2022, 55, 4656–4664. [Google Scholar] [CrossRef]
- Gornischeff, A.; Kruve, A.; Rebane, R. Characterization of wines with liquid chromatography electrospray ionization mass spectrometry: Quantification of amino acids via ionization efficiency values. J. Chromatogr. A 2020, 1620, 461012. [Google Scholar] [CrossRef]
- Lim, C.; Lee, D.W.; Israelachvili, J.N.; Jho, Y.; Hwang, D.S. Contact time-and pH-dependent adhesion and cohesion of low molecular weight chitosan coated surfaces. Carbohydr. Polym. 2015, 117, 887–894. [Google Scholar] [CrossRef]
- Lee, D.W.; Lim, C.; Israelachvili, J.N.; Hwang, D.S. Strong adhesion and cohesion of chitosan in aqueous solutions. Langmuir 2013, 29, 14222–14229. [Google Scholar] [CrossRef]
- Deacon, M.P.; McGURK, S.; Roberts, C.J.; Williams, P.M.; Tendler, S.J.; Davies, M.C.; Davis, S.; Harding, S.E. Atomic force microscopy of gastric mucin and chitosan mucoadhesive systems. Biochem. J. 2000, 348, 557–563. [Google Scholar] [CrossRef]
- Liu, T.; Li, B.; Huang, W.; Lv, B.; Chen, J.; Zhang, J.; Zhu, L. Effects and kinetics of a novel temperature cycling treatment on the N-deacetylation of chitin in alkaline solution. Carbohydr. Polym. 2009, 77, 110–117. [Google Scholar] [CrossRef]
- Duan, B.; Huang, Y.; Lu, A.; Zhang, L. Recent advances in chitin based materials constructed via physical methods. Prog. Polym. Sci. 2018, 82, 1–33. [Google Scholar] [CrossRef]
- Bao, Y.; Qian, H.J.; Lu, Z.Y.; Cui, S. Revealing the Hydrophobicity of Natural Cellulose by Single-Molecule Experiments. Macromolecules 2015, 48, 3685–3690. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Ji, Y.; He, Y.; Dong, J.; Li, H.; Yu, S. Effect of Environmental pH on the Mechanics of Chitin and Chitosan: A Single-Molecule Study. Polymers 2024, 16, 995. https://doi.org/10.3390/polym16070995
Zhang S, Ji Y, He Y, Dong J, Li H, Yu S. Effect of Environmental pH on the Mechanics of Chitin and Chitosan: A Single-Molecule Study. Polymers. 2024; 16(7):995. https://doi.org/10.3390/polym16070995
Chicago/Turabian StyleZhang, Song, Yunxu Ji, Yiwei He, Juan Dong, Haohang Li, and Shirui Yu. 2024. "Effect of Environmental pH on the Mechanics of Chitin and Chitosan: A Single-Molecule Study" Polymers 16, no. 7: 995. https://doi.org/10.3390/polym16070995



