Determining the Relationship between Delivery Parameters and Ablation Distribution for Novel Gel Ethanol Percutaneous Therapy in Ex Vivo Swine Liver
Abstract
1. Introduction
2. Materials and Methods
2.1. Formulation of ECE
2.2. Assessment of Distribution Volume of ECE In Ex Vivo and In Vivo Swine Liver
2.3. Experimental Design
2.4. Experimental Procedure
2.5. Computed Tomography Image Acquisition
2.6. Image Segmentation in 3D Slicer
2.7. Force Measurements
2.8. Selection of Injection Parameters
2.9. Statistical Analyses
3. Results
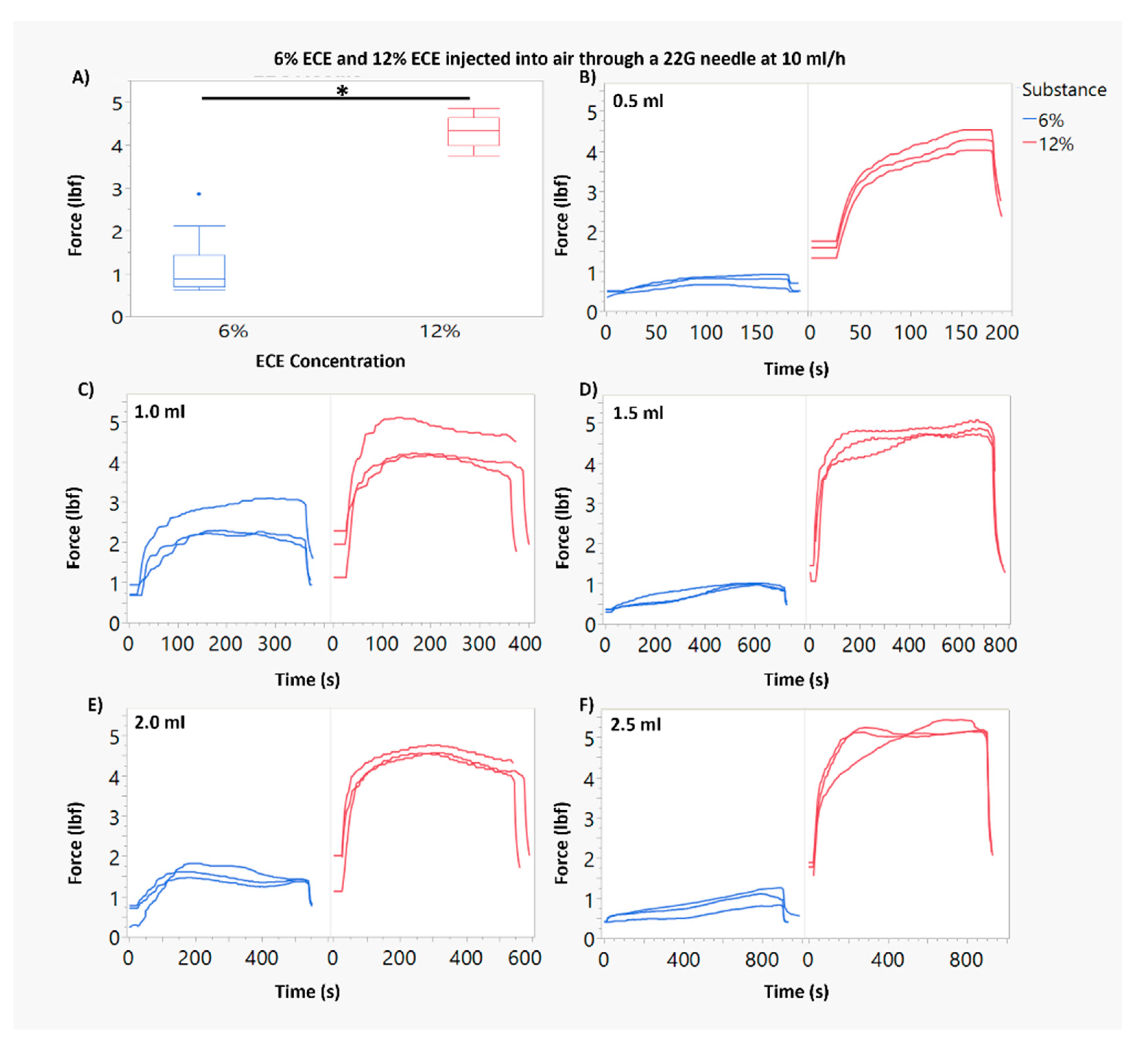
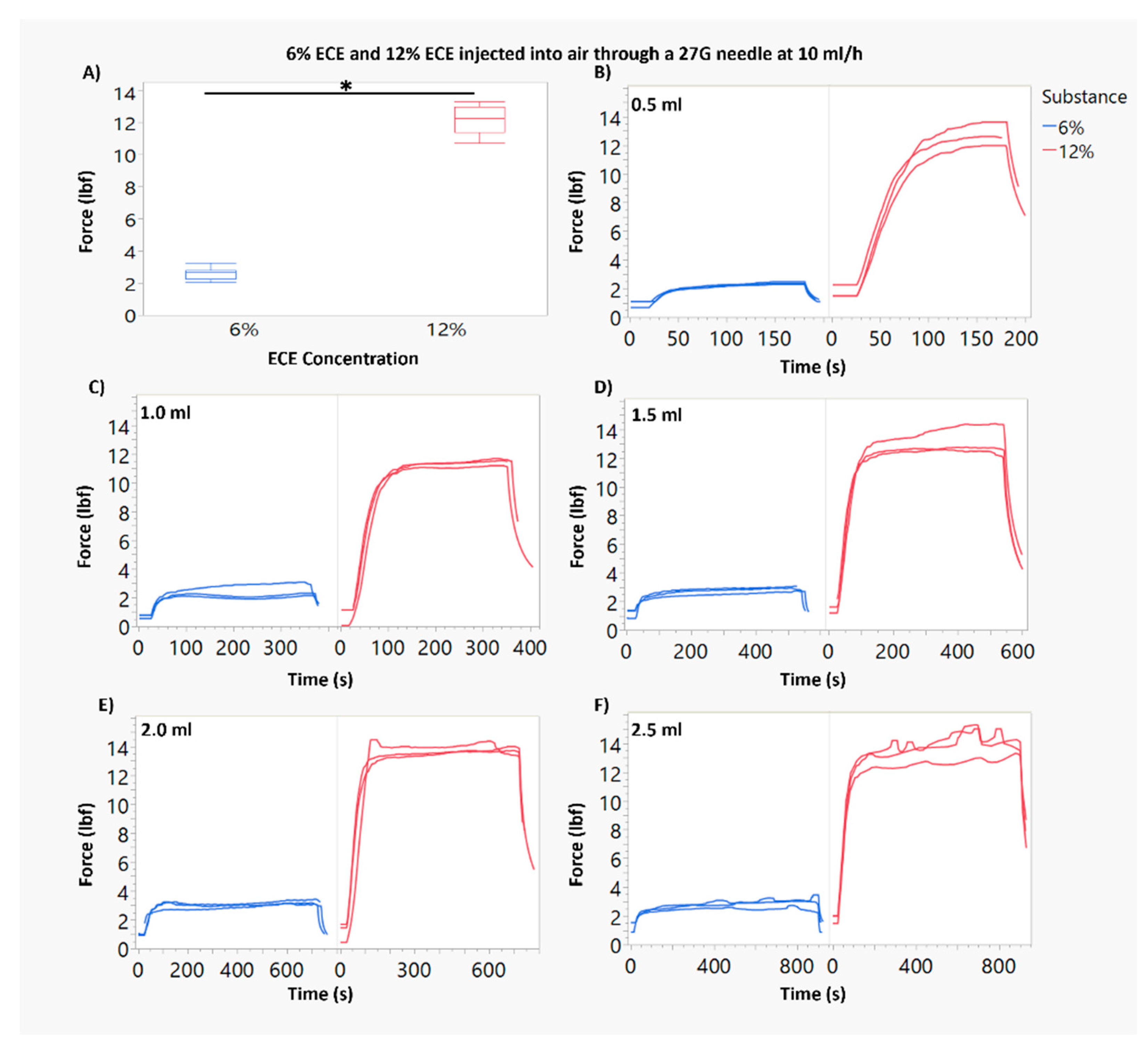
3.1. Force of Injection Increases with Higher ECE Concentration and Smaller Needle Gauge
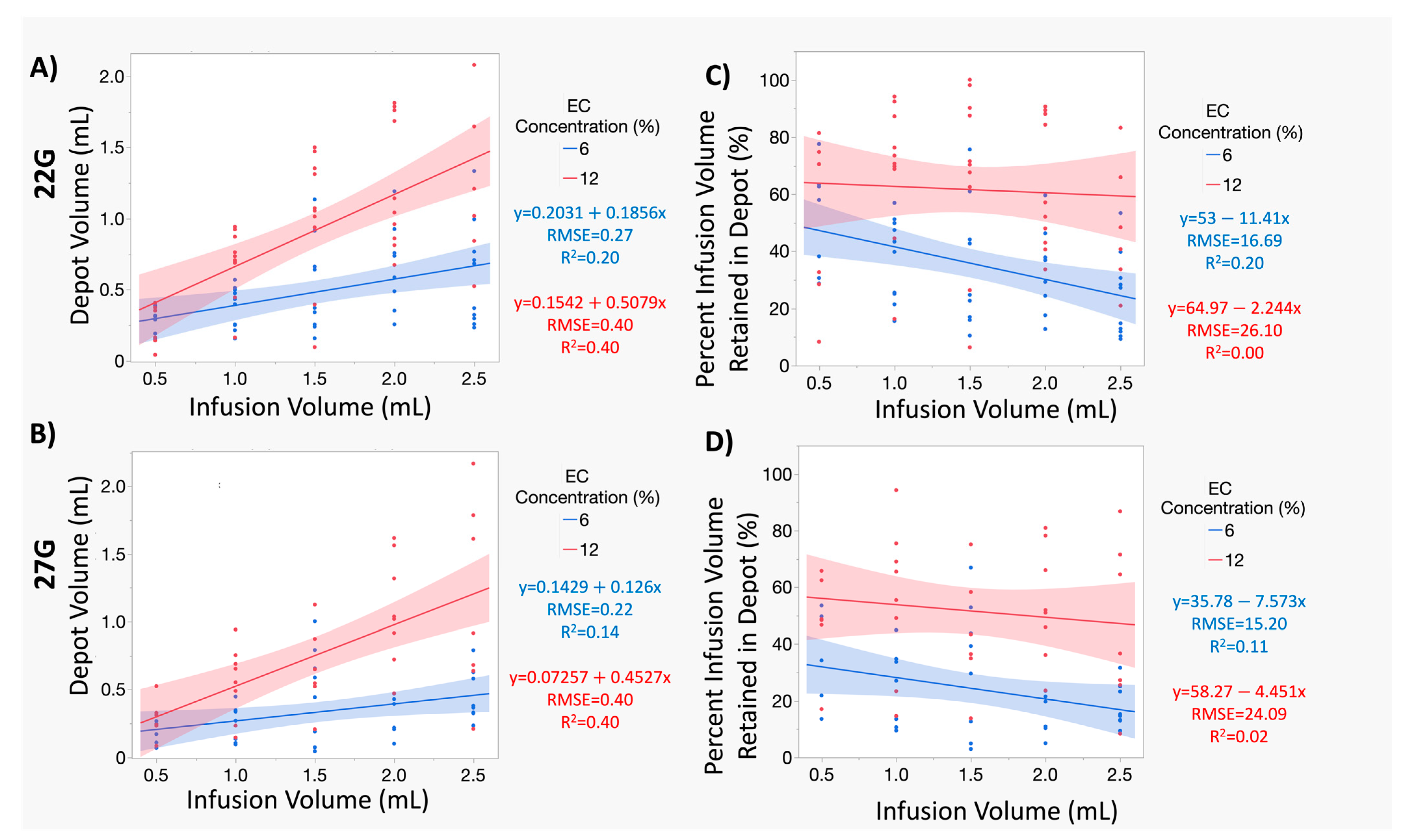
3.2. Increasing Infusion Volume of 12% ECE Achieves Significantly Greater Treatment Volumes, While Increasing the Infusion Volume 6% ECE Does Not Yield a Significant Increase in Treatment Volume, for Both 22 G and 27 G Needles
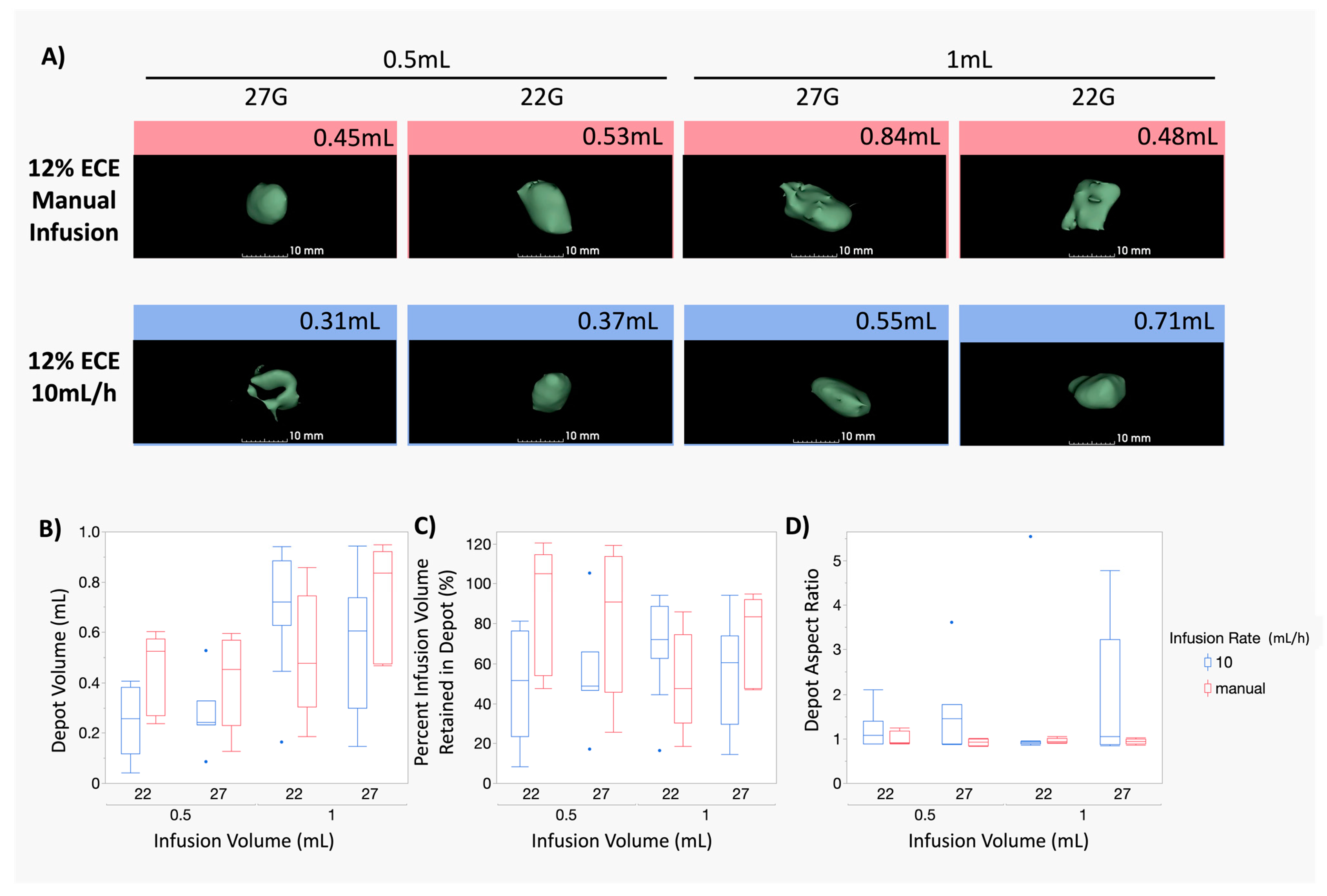
3.3. At Smaller Infusion Volumes, 12% ECE and 6% ECE Achieve Similar Treatment Volumes, but 12% Achieves Superior Treatment Volumes and Higher Percent Retained at the Target Site as Infusion Volume Increases Compared to 6% ECE, Regardless of Infusion Volume or Needle Gauge
3.4. The Impact of the Infusion Rate Is Not Significant for Smaller (0.5–1 mL) Infusion Volumes of 12% ECE for Both 22 G and 27 G Needles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sullivan, R.; Alatise, O.I.; Anderson, B.O.; Audisio, R.; Autier, P.; Aggarwal, A.; Balch, C.; Brennan, M.F.; Dare, A.; D’Cruz, A.; et al. Global cancer surgery: Delivering safe, affordable, and timely cancer surgery. Lancet Oncol. 2015, 16, 1193–1224. [Google Scholar] [CrossRef] [PubMed]
- Van Dyk, J.; Zubizarreta, E.; Lievens, Y. Cost evaluation to optimise radiation therapy implementation in different income settings: A time-driven activity-based analysis. Radiother. Oncol. 2017, 125, 178–185. [Google Scholar] [CrossRef]
- Bilchik, A.J.; Rose, D.M.; Allegra, D.P.; Bostick, P.J.; Hsueh, E.; Morton, D.L. Radiofrequency ablation: A minimally invasive technique with multiple applications. Cancer J. Sci. Am. 1999, 5, 356–361. (In English) [Google Scholar] [PubMed]
- Susini, T.; Nori, J.; Olivieri, S.; Livi, L.; Bianchi, S.; Mangialavori, G.; Branconi, F.; Scarselli, G. Radiofrequency ablation for minimally invasive treatment of breast carcinoma. A pilot study in elderly inoperable patients. Gynecol. Oncol. 2007, 104, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Gharib, H.; Hegedüs, L.; Pacella, C.M.; Baek, J.H.; Papini, E. Nonsurgical, Image-Guided, Minimally Invasive Therapy for Thyroid Nodules. J. Clin. Endocrinol. Metab. 2013, 98, 3949–3957. [Google Scholar] [CrossRef] [PubMed]
- Kanotra, S.P.; Lateef, M.; Kirmani, O. Non-surgical management of benign thyroid cysts: Use of ultrasound-guided ethanol ablation. Postgrad. Med. J. 2008, 84, 639. [Google Scholar] [CrossRef] [PubMed]
- Lü, M.D.; Kuang, M.; Liang, L.J.; Xie, X.Y.; Peng, B.G.; Liu, G.J.; Li, D.M.; Lai, J.M.; Li, S.Q. Surgical resection versus percutaneous thermal ablation for early-stage hepatocellular carcinoma: A randomized clinical trial. Zhonghua Yi Xue Za Zhi 2006, 86, 801. [Google Scholar]
- Otto, G.; Düber, C.; Hoppe-Lotichius, M.; König, J.; Heise, M.; Pitton, M.B. Radiofrequency ablation as first-line treatment in patients with early colorectal liver metastases amenable to surgery. Ann. Surg. 2010, 251, 796–803. (In English) [Google Scholar] [CrossRef]
- Ansari, D.; Andersson, R. Radiofrequency ablation or percutaneous ethanol injection for the treatment of liver tumors. World J. Gastroenterol. 2012, 18, 1003–1008. (In English) [Google Scholar] [CrossRef]
- Chua, T.; Faigel, D.O. Endoscopic Ultrasound-Guided Ablation of Liver Tumors. Gastrointest. Endosc. Clin. N. Am. 2019, 29, 369–379. [Google Scholar] [CrossRef]
- Erinjeri, J.P.; Thomas, C.T.; Samoilia, A.; Fleisher, M.; Gonen, M.; Sofocleous, C.T.; Thornton, R.H.; Siegelbaum, R.H.; Covey, A.M.; Brody, L.A.; et al. Image-guided thermal ablation of tumors increases the plasma level of interleukin-6 and interleukin-10. J. Vasc. Interv. Radiol. 2013, 24, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Baust, J.G.; Gage, A.A. The molecular basis of cryosurgery. BJU Int. 2005, 95, 1187–1191. (In English) [Google Scholar] [CrossRef]
- Giorgio, A.; Di Sarno, A.; De Stefano, G.; Scognamiglio, U.; Farella, N.; Mariniello, A.; Esposito, V.; Coppola, C.; Giorgio, V. Percutaneous radiofrequency ablation of hepatocellular carcinoma compared to percutaneous ethanol injection in treatment of cirrhotic patients: An Italian randomized controlled trial. Anticancer Res. 2011, 31, 2291–2295. (In English) [Google Scholar]
- Ishii, H.; Okada, S.; Nose, H.; Okusaka, T.; Yoshimori, M.; Takayama, T.; Kosuge, T.; Yamasaki, S.; Sakamoto, M.; Hirohashi, S. Local recurrence of hepatocellular carcinoma after percutaneous ethanol injection. Cancer 1996, 77, 1792–1796. (In English) [Google Scholar] [CrossRef]
- Ryu, M.; Shimamura, Y.; Kinoshita, T.; Konishi, M.; Kawano, N.; Iwasaki, M.; Furuse, J.; Yoshino, M.; Moriyama, N.; Sugita, M. Therapeutic results of resection, transcatheter arterial embolization and percutaneous transhepatic ethanol injection in 3225 patients with hepatocellular carcinoma: A retrospective multicenter study. Jpn. J. Clin. Oncol. 1997, 27, 251–257. (In English) [Google Scholar] [CrossRef]
- Livraghi, T.; Goldberg, S.N.; Lazzaroni, S.; Meloni, F.; Solbiati, L.; Gazelle, G.S. Small hepatocellular carcinoma: Treatment with radio-frequency ablation versus ethanol injection. Radiology 1999, 210, 655–661. (In English) [Google Scholar] [CrossRef]
- Lencioni, R.A.; Allgaier, H.P.; Cioni, D.; Olschewski, M.; Deibert, P.; Crocetti, L.; Frings, H.; Laubenberger, J.; Zuber, I.; Blum, H.E.; et al. Small hepatocellular carcinoma in cirrhosis: Randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology 2003, 228, 235–240. (In English) [Google Scholar] [CrossRef]
- Huang, G.; Lin, M.; Xie, X.; Liu, B.; Xu, Z.; Lencioni, R.; Lu, M.; Kuang, M. Combined radiofrequency ablation and ethanol injection with a multipronged needle for the treatment of medium and large hepatocellular carcinoma. Eur. Radiol. 2014, 24, 1565–1571. [Google Scholar] [CrossRef]
- Vilana, R.; Bruix, J.; Bru, C.; Ayuso, C.; Solé, M.; Rodés, J. Tumor size determines the efficacy of percutaneous ethanol injection for the treatment of small hepatocellular carcinoma. Hepatology 1992, 16, 353–357. (In English) [Google Scholar] [CrossRef] [PubMed]
- Shiina, S.; Tagawa, K.; Unuma, T.; Takanashi, R.; Yoshiura, K.; Komatsu, Y.; Hata, Y.; Niwa, Y.; Shiratori, Y.; Terano, A.; et al. Percutaneous ethanol injection therapy for hepatocellular carcinoma. A histopathologic study. Cancer 1991, 68, 1524–1530. [Google Scholar] [CrossRef] [PubMed]
- Arii, S.; Yamaoka, Y.; Futagawa, S.; Inoue, K.; Kobayashi, K.; Kojiro, M.; Makuuchi, M.; Nakamura, Y.; Okita, K.; Yamada, R. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: A retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology 2000, 32, 1224–1229. (In English) [Google Scholar] [CrossRef] [PubMed]
- Castells, A.; Bruix, J.; Bru, C.; Fuster, J.; Vilana, R.; Navasa, M.; Ayuso, C.; Boix, L.; Visa, J.; Rodés, J. Treatment of small hepatocellular carcinoma in cirrhotic patients: A cohort study comparing surgical resection and percutaneous ethanol injection. Hepatology 1993, 18, 1121–1126. (In English) [Google Scholar] [PubMed]
- Lencioni, R.; Pinto, F.; Armillotta, N.; Bassi, A.M.; Moretti, M.; Di Giulio, M.; Marchi, S.; Uliana, M.; Della Capanna, S.; Lencioni, M.; et al. Long-term results of percutaneous ethanol injection therapy for hepatocellular carcinoma in cirrhosis: A European experience. Eur. Radiol. 1997, 7, 514–519. (In English) [Google Scholar] [CrossRef] [PubMed]
- Livraghi, T.; Giorgio, A.; Marin, G.; Salmi, A.; de Sio, I.; Bolondi, L.; Pompili, M.; Brunello, F.; Lazzaroni, S.; Torzilli, G.; et al. Hepatocellular carcinoma and cirrhosis in 746 patients: Long-term results of percutaneous ethanol injection. Radiology 1995, 197, 101–108. (In English) [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Bartolozzi, C.; Caramella, D.; Paolicchi, A.; Carrai, M.; Maltinti, G.; Capria, A.; Tafi, A.; Conte, P.F.; Bevilacqua, G. Treatment of small hepatocellular carcinoma with percutaneous ethanol injection. Analysis of prognostic factors in 105 Western patients. Cancer 1995, 76, 1737–1746. (In English) [Google Scholar] [CrossRef] [PubMed]
- Shiina, S.; Tagawa, K.; Niwa, Y.; Unuma, T.; Komatsu, Y.; Yoshiura, K.; Hamada, E.; Takahashi, M.; Shiratori, Y.; Terano, A.; et al. Percutaneous ethanol injection therapy for hepatocellular carcinoma: Results in 146 patients. AJR Am. J. Roentgenol. 1993, 160, 1023–1028. (In English) [Google Scholar] [CrossRef] [PubMed]
- Livraghi, T.; Bolondi, L.; Lazzaroni, S.; Marin, G.; Morabito, A.; Rapaccini, G.L.; Salmi, A.; Torzilli, G. Percutaneous ethanol injection in the treatment of hepatocellular carcinoma in cirrhosis. A study on 207 patients. Cancer 1992, 69, 925–929. (In English) [Google Scholar] [CrossRef]
- Kuang, M.; Lu, M.D.; Xie, X.Y.; Xu, H.X.; Xu, Z.F.; Liu, G.J.; Yin, X.Y.; Huang, J.F.; Lencioni, R. Ethanol ablation of hepatocellular carcinoma Up to 5.0 cm by using a multipronged injection needle with high-dose strategy. Radiology 2009, 253, 552–561. [Google Scholar] [CrossRef]
- Lencioni, R.; Crocetti, L.; Cioni, D.; Pina, C.D.; Oliveri, F.; De Simone, P.; Brunetto, M.; Filipponi, F. Single-session percutaneous ethanol ablation of early-stage hepatocellular carcinoma with a multipronged injection needle: Results of a pilot clinical study. J. Vasc. Interv. Radiol. 2010, 21, 1533–1538. (In English) [Google Scholar] [CrossRef]
- Meara, J.G.; Leather, A.J.; Hagander, L.; Alkire, B.C.; Alonso, N.; Ameh, E.A.; Bickler, S.W.; Conteh, L.; Dare, A.J.; Davies, J.; et al. Global Surgery 2030: Evidence and solutions for achieving health, welfare, and economic development. Lancet 2015, 386, 569–624. (In English) [Google Scholar] [CrossRef]
- McGuire, S.; Yuan, F. Quantitative analysis of intratumoral infusion of color molecules. Am. J. Physiol.-Heart Circ. Physiol. 2001, 281, H715–H721. [Google Scholar] [CrossRef] [PubMed]
- Tapani, E.; Vehmas, T.; Taavitsainen, M. Ultrasound monitoring of experimental ethanol injection into pig liver. Acad. Radiol. 1994, 1, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Koda, M.; Okamoto, K.; Miyoshi, Y.; Kawasaki, H. Hepatic vascular and bile duct injury after ethanol injection therapy for hepatocellular carcinoma. Gastrointest. Radiol. 1992, 17, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Luck, J.; Dewhirst, M.W.; Yuan, F. Interstitial hydraulic conductivity in a fibrosarcoma. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H2726–H2734. (In English) [Google Scholar] [CrossRef] [PubMed]
- Morhard, R.; Nief, C.; Castedo, C.B.; Hu, F.; Madonna, M.; Mueller, J.L.; Dewhirst, M.W.; Katz, D.F.; Ramanujam, N. Development of enhanced ethanol ablation as an alternative to surgery in treatment of superficial solid tumors. Sci. Rep. 2017, 7, 8750. (In English) [Google Scholar] [CrossRef]
- Agrawal, A.M.; Manek, R.V.; Kolling, W.M.; Neau, S.H. Studies on the interaction of water with ethylcellulose: Effect of polymer particle size. AAPS PharmSciTech 2003, 4, E60. (In English) [Google Scholar] [CrossRef] [PubMed]
- Morhard, R.; Mueller, J.L.; Tang, Q.; Nief, C.; Chelales, E.; Lam, C.; Adrianzen Alvarez, D.; Rubinstein, M.; Katz, D.; Ramanujam, N. Understanding factors governing distribution volume of ethyl cellulose-ethanol to optimize ablative therapy in the liver. IEEE Trans. Biomed. Eng. 2019, 67, 2337–2348. [Google Scholar] [CrossRef]
- Chelales, E.; Morhard, R.; Nief, C.; Crouch, B.; Everitt, J.I.; Sag, A.A.; Ramanujam, N. Radiologic-pathologic analysis of increased ethanol localization and ablative extent achieved by ethyl cellulose. Sci. Rep. 2021, 11, 20700. (In English) [Google Scholar] [CrossRef]
- Wasilewska, K.; Winnicka, K. Ethylcellulose-A Pharmaceutical Excipient with Multidriectional Application in Drug Dosage Forms Development. Materials 2019, 12, 3386. [Google Scholar] [CrossRef]
- Lukova, P.; Katsarov, P.; Pilicheva, B. Application of Starch, Cellulose, and their Derivatives in the Development of Microparticle Drug-Delivery Systems. Polymers 2023, 15, 3615. [Google Scholar] [CrossRef]
- Nief, C.; Morhard, R.; Chelales, E.; Adrianzen Alvarez, D.; Bourla Bs, I.; Lam, C.T.; Sag, A.A.; Crouch, B.T.; Mueller, J.L.; Katz, D.; et al. Polymer-assisted intratumoral delivery of ethanol: Preclinical investigation of safety and efficacy in a murine breast cancer model. PLoS ONE 2021, 16, e0234535. (In English) [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.E.; Morhard, R.; Ramanujam, N.; Nolan, M.W. Minimally invasive ethyl cellulose ethanol ablation in domesticated cats with naturally occurring head and neck cancers: Six cats. Vet. Comp. Oncol. 2021, 19, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H. Is percutaneous ethanol injection therapy still effective for hepatocellular carcinoma in the era of radiofrequency ablation? Gut Liver 2010, 4 (Suppl. S1), S105–S112. [Google Scholar] [CrossRef] [PubMed]
- Allmendinger, A.; Mueller, R.; Schwarb, E.; Chipperfield, M.; Huwyler, J.; Mahler, H.-C.; Fischer, S. Measuring tissue back-pressure—In vivo injection forces during subcutaneous injection. Pharm. Res. 2015, 32, 2229–2240. [Google Scholar] [CrossRef]
- Rekhi, G.S.; Jambhekar, S.S. Ethylcellulose—A Polymer Review. Drug Dev. Ind. Pharm. 1995, 21, 61–77. [Google Scholar] [CrossRef]
- Davidovich-Pinhas, M.; Barbut, S.; Marangoni, A.G. Physical structure and thermal behavior of ethylcellulose. Cellulose 2014, 21, 3243–3255. [Google Scholar] [CrossRef]
- Court, F.G.; Wemyss-Holden, S.A.; Morrison, C.P.; Teague, B.D.; Laws, P.E.; Kew, J.; Dennison, A.R.; Maddern, G.J. Segmental nature of the porcine liver and its potential as a model for experimental partial hepatectomy. J. Br. Surg. 2003, 90, 440–444. [Google Scholar] [CrossRef]
- Vehmas, T.; Taari, K.; Kivisaari, L.; Laurila, P.; Taavitsainen, M. Intra- and Peri-Hepatic Effects of Ethanol Injections in the Pig Liver. Scand. J. Gastroenterol. 1993, 28, 791–794. [Google Scholar] [CrossRef]
- Hines-Peralta, A.U.; Pirani, N.; Clegg, P.; Cronin, N.; Ryan, T.P.; Liu, Z.; Goldberg, S.N. Microwave ablation: Results with a 2.45-GHz applicator in ex vivo bovine and in vivo porcine liver. Radiology 2006, 239, 94–102. (In English) [Google Scholar] [CrossRef]
- Lubner, M.G.; Hinshaw, J.L.; Andreano, A.; Sampson, L.; Lee, F.T., Jr.; Brace, C.L. High-powered microwave ablation with a small-gauge, gas-cooled antenna: Initial ex vivo and in vivo results. J. Vasc. Interv. Radiol. 2012, 23, 405–411. (In English) [Google Scholar] [CrossRef]
- Mulier, S.; Possebon, R.; Jiang, Y.; Jamart, J.; Wang, C.; Miao, Y.; Yu, T.; Jiang, K.; Feng, Y.; Marchal, G.; et al. Radiofrequency ablation with four electrodes as a building block for matrix radiofrequency ablation: Ex vivo liver experiments and finite element method modelling. Influence of electric and activation mode on coagulation size and geometry. Surg. Oncol. 2020, 33, 145–157. (In English) [Google Scholar] [CrossRef] [PubMed]
- Denys, A.L.; De Baere, T.; Kuoch, V.; Dupas, B.; Chevallier, P.; Madoff, D.C.; Schnyder, P.; Doenz, F. Radio-frequency tissue ablation of the liver: In vivo and ex vivo experiments with four different systems. Eur. Radiol. 2003, 13, 2346–2352. (In English) [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Geng, X.; Yang, Y.; Cai, X.; Hu, P.; Xia, L.; Zhang, B.; Wu, P. Dynamic imaging and pathological changes in pig liver after MR-guided microwave ablation. BMC Cancer 2018, 18, 397. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.D.; Rogers, S.; Corless, C.L.; Swanstrom, L.L.; Siperstien, A.E. Radiofrequency Ablation Lesions in a Pig Liver Model. J. Surg. Res. 1999, 87, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Hui, T.C.H.; Brace, C.L.; Hinshaw, J.L.; Quek, L.H.H.; Huang, I.K.H.; Kwan, J.; Lim, G.H.T.; Lee, F.T.; Pua, U. Microwave ablation of the liver in a live porcine model: The impact of power, time and total energy on ablation zone size and shape. Int. J. Hyperth. 2020, 37, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Dewall, R.J.; Varghese, T.; Brace, C.L. Visualizing ex vivo radiofrequency and microwave ablation zones using electrode vibration elastography. Med. Phys. 2012, 39, 6692–6700. (In English) [Google Scholar] [CrossRef]
- Lu, D.S.; Sinha, S.; Lucas, J.; Farahani, K.; Lufkin, R.; Lewin, K. MR-guided percutaneous ethanol ablation of liver tissue in a.2-T open MR system: Preliminary study in porcine model. J. Magn. Reson. Imaging 1997, 7, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Oyama, Y.; Nakamura, K.; Matsuoka, T.; Toyoshima, M.; Yamamoto, A.; Okuma, T.; Ikura, Y.; Ueda, M.; Inoue, Y. Radiofrequency ablated lesion in the normal porcine lung: Long-term follow-up with MRI and pathology. Cardiovasc. Interv. Radiol. 2005, 28, 346–353. (In English) [Google Scholar] [CrossRef] [PubMed]
- Brace, C.L.; Laeseke, P.F.; Sampson, L.A.; Frey, T.M.; van der Weide, D.W.; Lee, F.T., Jr. Microwave ablation with a single small-gauge triaxial antenna: In vivo porcine liver model. Radiology 2007, 242, 435–440. (In English) [Google Scholar] [CrossRef]
- Brace, C.L.; Laeseke, P.F.; Sampson, L.A.; Frey, T.M.; van der Weide, D.W.; Lee, F.T., Jr. Microwave ablation with multiple simultaneously powered small-gauge triaxial antennas: Results from an in vivo swine liver model. Radiology 2007, 244, 151–156. (In English) [Google Scholar] [CrossRef]
- Yu, N.C.; Raman, S.S.; Kim, Y.J.; Lassman, C.; Chang, X.; Lu, D.S. Microwave liver ablation: Influence of hepatic vein size on heat-sink effect in a porcine model. J. Vasc. Interv. Radiol. 2008, 19, 1087–1092. (In English) [Google Scholar] [CrossRef]
- Namur, J.; Wassef, M.; Millot, J.M.; Lewis, A.L.; Manfait, M.; Laurent, A. Drug-eluting beads for liver embolization: Concentration of doxorubicin in tissue and in beads in a pig model. J. Vasc. Interv. Radiol. 2010, 21, 259–267. (In English) [Google Scholar] [CrossRef] [PubMed]
- Arefiev, A.; Prat, F.; Chapelon, J.Y.; Tavakkoli, J.; Cathignol, D. Ultrasound-induced tissue ablation: Studies on isolated, perfused porcine liver. Ultrasound Med. Biol. 1998, 24, 1033–1043. (In English) [Google Scholar] [CrossRef] [PubMed]
- Tapani, E.; Vehmas, T.; Voutilainen, P. Effect of injection speed on the spread of ethanol during experimental liver ethanol injections. Acad. Radiol. 1996, 3, 1025–1029. (In English) [Google Scholar] [CrossRef]
- Fukuda, H.; Numata, K.; Moriya, S.; Shimoyama, Y.; Ishii, T.; Nozaki, A.; Kondo, M.; Morimoto, M.; Maeda, S.; Sakamaki, K.; et al. Hepatocellular carcinoma: Concomitant sorafenib promotes necrosis after radiofrequency ablation–propensity score matching analysis. Radiology 2014, 272, 598–604. (In English) [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.W.; Fan, X.X.; Mao, D.F.; Ding, Q.H.; Zhuang, L.H.; Lv, S.Y. Drug-eluting beads transarterial chemoembolization sequentially combined with radiofrequency ablation in the treatment of untreated and recurrent hepatocellular carcinoma. World J. Gastrointest. Surg. 2020, 12, 355–368. (In English) [Google Scholar] [CrossRef] [PubMed]
- Kikinis, R.; Pieper, S.D.; Vosburgh, K.G. 3D Slicer: A platform for subject-specific image analysis, visualization, and clinical support. In Intraoperative Imaging and Image-Guided Therapy; Springer: New York, NY, USA, 2014; pp. 277–289. [Google Scholar]
- Tapani, E.; Taavitsainen, M.; Lindros, K.; Vehmas, T.; Lehtonen, E. Toxicity of ethanol in low concentrations. Experimental evaluation in cell culture. Acta Radiol. 1996, 37, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Nief, C.A.; Gonzales, A.; Chelales, E.; Agudogo, J.S.; Crouch, B.T.; Nair, S.K.; Ramanujam, N. Targeting Tumor Acidosis and Regulatory T Cells Unmasks Anti-Metastatic Potential of Local Tumor Ablation in Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2022, 23, 8479. [Google Scholar] [CrossRef]
- Nief, C.A.; Swartz, A.M.; Chelales, E.; Sheu, L.Y.; Crouch, B.T.; Ramanujam, N.; Nair, S.K. Ethanol Ablation Therapy Drives Immune-Mediated Antitumor Effects in Murine Breast Cancer Models. Cancers 2022, 14, 4669. (In English) [Google Scholar] [CrossRef]
- Schwagmeier, R.; Schmidt, A.; Nolte, H. The effect of injection speed and needle gauge on the spread of sensory blockade in spinal anesthesia. Reg. Anaesth. 1990, 13, 148–152. (In German) [Google Scholar]
- Sim, W.S.; Park, H.J.; Kwon, J.H.; Oh, M.S.; Jung, H.J.; Cho, M.K.; Lee, J.Y. Fluoroscopic evaluation of the influence of needle gauge on epidural spread in caudal block. Medicine 2019, 98, e15896. (In English) [Google Scholar] [CrossRef] [PubMed]
- Weiss, L.; Haydock, K.; Pickren, J.W.; Lane, W.W. Organ vascularity and metastatic frequency. Am. J. Pathol. 1980, 101, 101. [Google Scholar] [PubMed]
- Guimarães, C.F.; Gasperini, L.; Marques, A.P.; Reis, R.L. The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater. 2020, 5, 351–370. [Google Scholar] [CrossRef]


| Experiment | Parameters Assessed (n > 6 for Each Group) | ||||
|---|---|---|---|---|---|
| Infusion Volume (mL) | EC Concentration (%) | Needle Gauge (G) | Needle Length * (inches) | Infusion Rate (mL/h) | |
| 1 | 0.5, 1, 1.5, 2, 2.5 | 6 | 27 | 0.5 | 10 |
| 12 | |||||
| 2 | 0.5, 1, 1.5, 2, 2.5 | 6 | 22 | 0.75 | 10 |
| 12 | |||||
| 3 | 0.5, 1 | 12 | 27 | 0.5 | Manual Infusions |
| 22 | 0.75 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chelales, E.; von Windheim, K.; Banipal, A.S.; Siebeneck, E.; Benham, C.; Nief, C.A.; Crouch, B.; Everitt, J.I.; Sag, A.A.; Katz, D.F.; et al. Determining the Relationship between Delivery Parameters and Ablation Distribution for Novel Gel Ethanol Percutaneous Therapy in Ex Vivo Swine Liver. Polymers 2024, 16, 997. https://doi.org/10.3390/polym16070997
Chelales E, von Windheim K, Banipal AS, Siebeneck E, Benham C, Nief CA, Crouch B, Everitt JI, Sag AA, Katz DF, et al. Determining the Relationship between Delivery Parameters and Ablation Distribution for Novel Gel Ethanol Percutaneous Therapy in Ex Vivo Swine Liver. Polymers. 2024; 16(7):997. https://doi.org/10.3390/polym16070997
Chicago/Turabian StyleChelales, Erika, Katriana von Windheim, Arshbir Singh Banipal, Elizabeth Siebeneck, Claire Benham, Corrine A. Nief, Brian Crouch, Jeffrey I. Everitt, Alan Alper Sag, David F. Katz, and et al. 2024. "Determining the Relationship between Delivery Parameters and Ablation Distribution for Novel Gel Ethanol Percutaneous Therapy in Ex Vivo Swine Liver" Polymers 16, no. 7: 997. https://doi.org/10.3390/polym16070997
APA StyleChelales, E., von Windheim, K., Banipal, A. S., Siebeneck, E., Benham, C., Nief, C. A., Crouch, B., Everitt, J. I., Sag, A. A., Katz, D. F., & Ramanujam, N. (2024). Determining the Relationship between Delivery Parameters and Ablation Distribution for Novel Gel Ethanol Percutaneous Therapy in Ex Vivo Swine Liver. Polymers, 16(7), 997. https://doi.org/10.3390/polym16070997







