Abstract
Meat quality and shelf life are important parameters affecting consumer perception and safety. Several factors contribute to the deterioration and spoilage of meat products, including microbial growth, chemical reactions in the food’s constituents, protein denaturation, lipid oxidation, and discoloration. This study reviewed the development of functional packaging biomaterials that interact with food and the environment to improve food’s sensory properties and consumer safety. Bioactive packaging incorporates additive compounds such as essential oils, natural extracts, and chemical substances to produce composite polymers and polymer blends. The findings showed that the incorporation of additive compounds enhanced the packaging’s functionality and improved the compatibility of the polymer–polymer matrices and that between the polymers and active compounds. Food preservatives are alternative substances for food packaging that prevent food spoilage and preserve quality. The safety of food contact materials, especially the flavor/odor contamination from the packaging to the food and the mass transfer from the food to the packaging, was also assessed. Flavor is a key factor in consumer purchasing decisions and also determines the quality and safety of meat products. Novel functional packaging can be used to preserve the quality and safety of packaged meat products.
1. Introduction
Petroleum-based plastic packaging is environmentally unfriendly because petroleum plastics produce high carbon emissions during their extraction and refinement processes [1]. Petroleum-based plastic packaging is poorly biodegradable and can take more than a hundred years to degrade. Environmentally friendly bioplastic packaging is now widely used in the food industry [2,3], and the biocircular green (BCG) economic model has promoted increasing consumer demand for biodegradable plastics [1,2,3]. In recent years, the packaging industry has undergone a transformative shift towards sustainability and eco-conscious solutions. With growing concerns about environmental degradation and plastic pollution, there is a pressing need for innovative packaging technologies that preserve the quality and safety of food products and minimize their environmental footprint [3,4,5,6]. In this context, the emergence of functional biodegradable packaging represents a groundbreaking advancement.
Bioplastic materials represent a significant stride toward sustainability in packaging and material production. These materials are derived from renewable biomass sources such as plant starches, cellulose, or even agricultural waste, offering an eco-friendly alternative to traditional plastics and also reducing carbon emissions. Bioplastics can be categorized based on their composition, which includes biobased, biodegradable, or both [3,5,7,8]. Bioplastic materials present a viable alternative to traditional petroleum-based plastics. Bioplastics represent a burgeoning field within the broader realm of sustainable materials science. The state of the art in bioplastic materials is characterized by ongoing advancements in feedstock utilization, biodegradability, performance properties, and processing technologies [9,10]. These developments have contributed to the growing adoption of bioplastics across various industries, fostering a more sustainable approach to plastic production and consumption. Functional bio-material packaging offers a holistic approach to addressing the dual challenges of food preservation and environmental sustainability. When engineered with functional properties tailored to meat products, such as barrier enhancement, antimicrobial efficacy, and active packaging features, biodegradable packaging can revolutionize the way meat is packaged, distributed, and consumed [8,11,12,13].
The critical factors at different stages of the meat supply chain that effect the shelf life of packaged fresh meat and potential strategies to improve its quality have been reviewed [14]. Furthermore, the utilization of active functional packaging materials in the meat industry has garnered significant attention due to their potential to enhance food safety, extend shelf life, and maintain product quality. The potential of applying essential oils, nanoparticles, and natural antioxidants in functional meat packaging has been reviewed [15,16,17]. However, a comprehensive review of the meat quality standards and packaging necessities needs to be thoroughly evaluated. This review evaluates the application of alternative active packaging materials, particularly in the realm of meat packaging, including chemical additives and natural extracts. We also focus on evaluating the utilization of biodegradable packaging materials such as PBAT, PLA, and starch, whether used singly or in blends. Moreover, this review investigates the factors contributing to meat spoilage and flavor/odor contamination of meat products by packaging components, offering insights into the strategies for improving packaging performance and ensuring consumer safety.
2. Major Challenges for Meat Products
Meat products are an important source of protein and rich in key nutrients, minerals, vitamins, and water, thereby supporting human health. Fresh meat is a highly perishable food due to the composition of meat, while its high water activity (aw > 0.95) promotes the propagation of spoilage and pathogenic microorganisms. The quality and shelf life of meat products are determined by their color, odor, texture, and flavor. The factors impacting meat’s deterioration include holding time, temperature, storage conditions, moisture, pH, relative humidity, water activity, and atmosphere. Both intrinsic and extrinsic factors play critical roles in meat quality changes [18].
2.1. Meat Composition
Meat is composed of macromolecules including protein, fat, minerals, and vitamins. Its protein content ranges from 16 to 22% and averages 18.5% of the weight of muscle. Meat is very low in carbohydrates at 0.5–1.5%, with its lipid and fat contents ranging from 1 to 13% depending on the muscle type and animal age. Meat also contains non-protein nitrogenous substances (phosphate, peptides) and other non-protein substances (minerals, vitamins) at 1.7% and 0.85%, respectively. The percentage of water (approximately 75%) in the muscle plays a key role in supporting microorganism growth [19,20].
2.2. Quality and Safety of Meat Products
Meat quality is described according to its appearance, texture, flavor, odor, and color, with its nutritional value resulting from chemical/biochemical, microbiological, and physical reactions. Interactions between both intrinsic and extrinsic factors impact the occurrence of reactions that affect the quality, shelf life, and safety of food products [21].
2.2.1. Discoloration
Meat color is an important factor in purchasing decisions because consumers use color to discern the freshness of meat. Color preservation of meat in the food industry can be achieved by introducing additives such as salt and nitrite and using modified atmosphere packaging (MAP) and vacuum packaging [20,22]. Myoglobin is a complex muscle protein and gives meat its red color. The color of myoglobin is determined by oxidation–reduction (redox) reactions, with a reduced ferrous form (Fe2+) or an oxidized ferric form (Fe3+). Myoglobin can exist in four redox states, namely (i) oxymyoglobin (Fe2+), (ii) metmyoglobin (Fe3+), (iii) deoxymyoglobin (Fe2+), and (iv) carboxymyoglobin (Fe2+), which cause the meat color to be bright red, brown, purple, or cherry red, respectively [21,23].
The interrelationships of myoglobin as primarily responsible for meat color are shown in Figure 1. The reversible reaction between oxymyoglobin and deoxymyoglobin is explained by oxygenation and deoxygenation, attributed to the amount of oxygen present. Deoxymyoglobin is detectable as purple on meat’s surface, typically associated with vacuum-packaged products because of their rapid oxygen change to a low oxygen concentration (0.5–1.0%), while oxygenation occurs when myoglobin is rapidly exposed to oxygen, generating a red color.
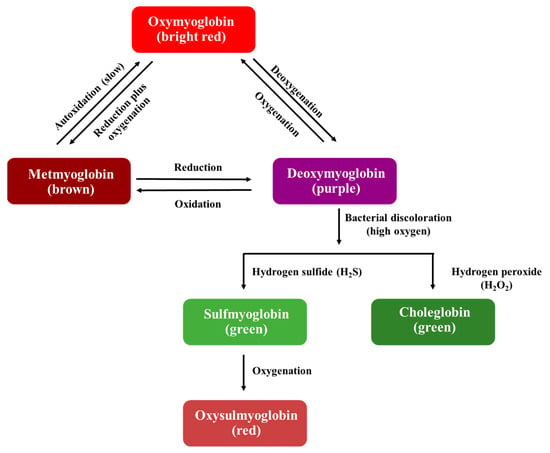
Figure 1.
The interrelationships between meat pigments according to oxidation–reduction myoglobin reactions. Modified from Kerry [20], Robertson [21], and Mancini and Hunt [23].
Oxidation occurs when heme iron is converted from the ferrous into ferric form. Metmyoglobin is a stable form that can exist either as oxymyoglobin or deoxymyoglobin. The formation of metmyoglobin from oxymyoglobin results from the lipid oxidation rate, which depends on oxygen partial pressure, temperature, pH, meat activity, and microbial growth. The reduction reaction works in the opposite way to oxidation. All the reactions in this cycle are reversible, with different discoloration according to the storage conditions, which depend on intrinsic factors, including pH, type of muscle, and meat activity, and extrinsic factors, including temperature, oxygen content, and the environment [20,21,22,23]. Deoxymyoglobin undergoes a wide variety of reactions, leading to discoloration in meat. Bacterial discoloration occurs in the log phase of microbial growth, when a high oxygen content affects the growth of aerobic bacteria and causes the formation of myoglobin. Some bacteria produce hydrogen sulfide (H2S) and hydrogen peroxide (H2O2), which react with deoxymyoglobin to produce sulfmyoglobin and choleglobin, respectively. The resulting pigment gives meat a green color. Oxysulfmyoglobin is generated when meat is exposed to high oxygen levels (oxygenation) and causes a red appearance [21,24]. The packaging types used to preserve a red color in meat are shown in Table 1.

Table 1.
Active packaging used to maintain or improve the color stability of meat.
Color stability in meat products is mostly achieved using modified atmosphere packaging to alter the concentrations of nitrogen, oxygen, carbon dioxide, and carbon monoxide. Meat is also immersed in various solutions such as tea catechins, Vitamin E, thymol, carvacrol, and grapefruit seed extract to reduce oxidation and preserve color stabilization. However, scant research has addressed the use of additives to develop functional packaging and improve the color stability of meat and poultry products. Copious research is now focused on novel functional packaging for extending the shelf life and preserving the quality of meat products.
2.2.2. Texture
Texture is one important indicator of the quality of meat products influencing consumer acceptability and satisfaction. Texture describes meat’s firmness, tenderness, and juiciness depending on its physicochemical and biochemical changes [20]. Firmness and tenderness are complex quality parameters, described as the mechanical strength of muscle and connective tissue, which is affected by both antemortem and postmortem factors. Intrinsic antemortem factors affecting meat tenderness include the animal species, connective tissue, muscle fiber cells, and fat, while postmortem factors impacting meat’s texture include the temperature and pH of the muscle, proteolysis, and its water-holding capacity [19,20,30]. Protein oxidation is associated with both physical and chemical changes in meat quality and is linked to meat tenderness. Protein oxidation occurs through a loss of enzyme activity and the formation of amino acids, leading to a loss of amino acid structure, a reduced water-holding capacity, and decreased protein solubility [20].
Texture can be improved using salt marination, high-pressure processing, irradiation, and a modified atmosphere to reduce water loss and increase products’ shelf life [31]. Seyfert et al. [32] investigated the impact of modified atmosphere packaging on the oxidative and sensory properties of beef. The results showed that a low oxygen content decreased oxidation and improved its tenderness, while Kim et al. [33] found that modified atmosphere packaging (70% O2/30% CO2) reduced the cross-linking of myosin chains through disulfide bonding and the content of protein thiols, indicating protein oxidation, with reduced tenderness and juiciness of the meat. Protein oxidation or deformation modifies the texture of meat.
2.2.3. Flavor and Odor
Flavor and odor are factors used to determine the quality of meat and impact consumer perception. Flavor and odor are important quality attributes of muscle foods and comprise the two sensations of taste and aroma or smell. The characteristics of flavor and odor depend on the animal species, temperature, and method of cooking. Beef, chicken, and pork contain many organic compounds, such as hydrocarbons, alcohols, aldehydes, ketones, carboxylic acids, esters, lactones, ethers, furans, pyridines, pyrazines, pyrroles, oxazoles and oxazolines, thiazoles and thiazolines, thiophenes, and other sulfur- and halogen-containing substances [34]. In fresh meat, flavor and odor denaturation occur as an off flavor/odor and rancidity during storage as a result of lipid oxidation [20,34,35].
Lipid oxidation causes quality deterioration in flavor and odor, as well as in color and texture, as described previously. Lipid oxidation occurs due to fatty acid and phospholipid exposure to oxygen and is accelerated by light and catalysts such as free iron. Free iron is an important catalyst because it is abundant in meat muscle. Lipid oxidation consists of three steps: (i) initiation, (ii) propagation, and (iii) termination [36,37].
(i) Initiation step: Free radicals such as the hydroxy radical (•OH), abstracted from an unsaturated fatty acid, are accelerated by free iron to form the lipid peroxyl radical, which then undergoes molecular rearrangement to form conjugated dienes or trienes.
(ii) Propagation step: The lipid peroxyl radicals from the initiation step react with molecular oxygen to form peroxyl radicals (•OO), which then abstract hydrogen from adjacent lipid molecules, resulting in lipid hydroperoxide (OOH). This then reacts with molecular oxygen to form new peroxyl radicals, and the reaction continues.
(iii) Termination step: Peroxyl radicals (•OO) react with radicals or other non-radical compounds (antioxidants) to form a non-radical product.
The use of natural antioxidants to increase meat’s oxidative stability is a topic of great interest, whereby an antioxidant compound transfers a hydrogen atom to the radical derived from lipid oxidation. This reaction neutralizes the lipid radical and creates a new radical from the antioxidant compound [36].
2.2.4. Microorganisms
Microorganisms play an important role in meat quality and safety because microbial contamination or microbial growth is of the highest concern for consumers. Meat and poultry have a high water activity (aw > 0.95) and are highly perishable products. Microbes including bacteria, yeast, and mold lead to spoilage and pathogens. Microorganisms break down the fats, carbohydrates, and proteins in the meat muscle, causing oxidation and chemical deformation, resulting in off flavors, off odors, slime formation, texture change, gas production, and discoloration, which impact consumer acceptability [38,39].
The microorganisms mostly found in meat include Arthrobacter spp., Acinetobacter spp., Aeromonas spp., Staphylococcus spp., Enterococcus spp., Moraxella spp., Psychrobacter spp., Pseudomonas spp., Cladosporium spp., Geotrichum spp., Mucor spp., Rhizopus spp., Sporotrichum spp., Thamnidium spp., Candida spp., and Torulopsis spp. [38,39,40]. Indications of the microbial population are expressed as organisms per square meter or as organisms per gram. Generally, the initial microbial counts in meat range from 102 to 105 CFU/cm2, and meat starts to spoil at a total viable count of 106 CFU/cm2, resulting in the production of an off flavor, while at 108 CFU/cm2, the meat shows slime on its surface and discoloration [41,42].
3. Bioplastic Materials
Bioplastics are sustainable materials produced from renewable resources or obtained from biomass that are used to produce environmentally friendly packaging with reduced carbon emissions. Bioplastic, biobased, or biodegradable materials can be classified according to the type of plastic, as shown in Figure 2. The bioplastic materials that are fossil-based or made according to chemical synthesis through polymerization include polybutylene adipate-co-terephthalate (PBAT), polycaprolactone (PCL), and polyvinyl alcohol (PVOH). Bioplastics are also made from natural resources such as sugar cane, starch, cellulose, and seaweed. Cellulose-based material can be prepared using 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO)-oxidized bacterial cellulose powder [43]. The bioplastics produced from microorganism fermentation include polylactic acid (PLA) and polyhydroxyalkanoate (PHA). However, not all bioplastics made from biobased natural resources are biodegradable. Biodegradable materials undergo polymer transformation by biological organisms and can be easily cleaved through hydrolysis or enzymatic activity [5,6,13].
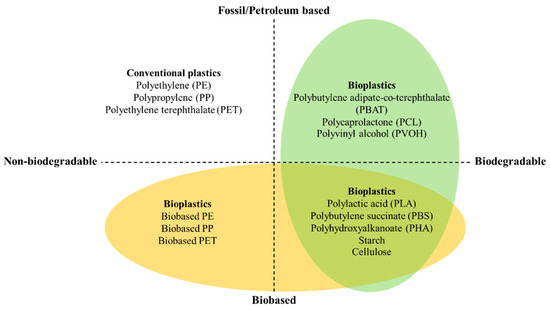
Figure 2.
Type of plastic classified by resource (fossil-based or biobased) and biodegradable characteristics. Modified from Bioplastics (2021) [44].
Bioplastic materials have diverse properties. This study focused on PBAT, starch, PLA, cellulose, and PBS as the biodegradable materials mostly used in the plastic industry and produced as flexible and rigid materials to support market demand [4]. The current market studies forecast the global production capacity of biodegradable materials of 1.14 million tons to reach 4.61 million tons between 2023 and 2028. This growth is stimulated by demand from Asia and the United States [4].
3.1. Polybutylene Adipate-Co-Terephthalate (PBAT)
PBAT is a random co-polyester produced according to the polycondensation reaction of adipic acid, 1,4-butanediol, and dimethyl terephthalate. PBAT is biodegradable and made from a synthetic petroleum base including two segments of (i) polybutylene adipate (PBA) and (ii) polybutylene terephthalate (PBT), containing both aliphatic and aromatic units, as shown in Figure 3. The fractions of the aliphatic and aromatic units affect the biodegradable rate and properties [45,46]. According to data from European Bioplastics [4], in 2023, the global production capacities of PBAT represented 4.6% of the overall bioplastic production, at more than 100 thousand tons [4]. PBAT has excellent mechanical properties compared to polyesters such as polylactic acid and polybutylene succinate, while the mechanical properties of PBAT show a high flexibility, similar to low-density polyethylene (LDPE). PBAT-based products have been widely used in many applications, such as shopping bags, garbage bags, and mulch films. However, the limitations of PBAT include high production costs and low transparency [3,46]. The improvement and development of PBAT properties will be discussed later.
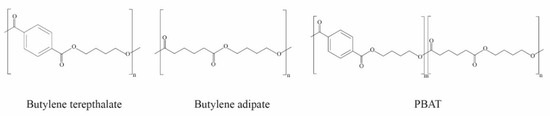
Figure 3.
Structures of polybutylene terephthalate (PBT), polybutylene adipate (PBA), and polybutylene adipate-co-terephthalate (PBAT). (Reproduced with permission from Harnkarnsujarit et al. (2021) [5]).
3.2. Starch
Starch is a source of carbohydrates found in plants as energy reserve materials. This polymeric mixture consists of two main polymers, amylose (liner polymer) (Figure 4a) and amylopectin (branch structure) (Figure 4b). The structure of starch comprises D-glucopyranose units joined together by glycosidic bonds between α-1,4-glycosidic linkages and α-1,6-glycosidic linkages, as shown in Figure 4. The starch granules in semi-crystalline starch consist of crystalline and amorphous regions, which are derived from amylose and amylopectin, respectively. The amylose and amylopectin proportions depend on the source type, molecular size, chain length distribution, and degree of polymerization [47,48,49]. The proportions of amylose and amylopectin influence the fundamental and physicochemical properties of starch, with variations in composition and structures related to the diverse genotypic sources of starch.
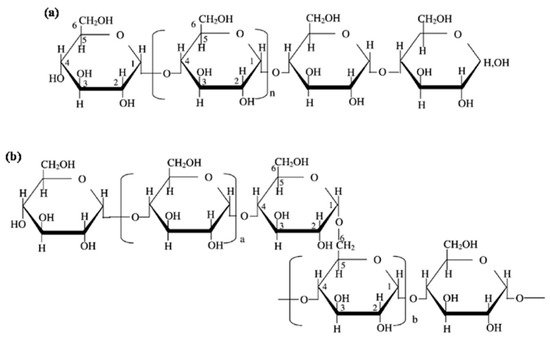
Figure 4.
Structure of (a) amylose and (b) amylopectin. (Reproduced with permission from Amagliani, O’Regan, Kelly, and O’Mahony (2016) [50]).
Starch is used in the food industry as a thickener and stabilizer and also for the preparation of plastic materials because it is an abundant, low-cost, and biodegradable renewable resource. Starch films can be prepared from plasticized starch as thermoplastic starch (TPS) using plasticizers such as water, glycerol, and sorbitol to reduce their melting point because the melting temperature of starch granules is higher than the decomposition temperature (230 °C). Starch transforms from granules into TPS when heated at high temperatures, with high shear forces and added plasticizers contributing to the de-structure of the starch granules via extrusion processing [5,51,52].
A plasticizer is incorporated into the material to increase the flexibility and processability of starch and also reduce the glass transition temperature [53]. Molecules of the plasticizer penetrate the starch granules (amylose and amylopectin) to break the inner hydrogen bonding under high-temperature, high-pressure, and shearing conditions. Substitution through the starch–plasticizer interaction allows the starch network to be easily deformed and eliminates the starch–starch reaction because the plasticizer molecules are smaller and have higher molecular mobility than the starch molecules [54]. The most common plasticizers used in the processing of TPS are water and glycerol. The type and amount of plasticizer influence the films’ mechanical and barrier properties, thermal stability, and transparency.
3.3. Polybutylene Succinate (PBS)
PBS is an aliphatic co-polyester produced through the polycondensation of succinic acid and 1,4-butanediol, as shown in Figure 5. Monomers of PBS can be produced from fossil-based or renewable resources according to bacterial fermentation. PBS is a semi-crystalline polymer that influences the stiffness or mechanical strength, transparency, and flexibility of materials. The properties of PBS depend on its degree of crystallinity. PBS has similar properties and processability to polyolefins such as polyethylene (PE) and polypropylene (PP) with a low glass transition temperature and high elongation at break (more than 500%). PBS is widely used to produce packaging films, agriculture mulch films, and compost bags [13,55,56,57].
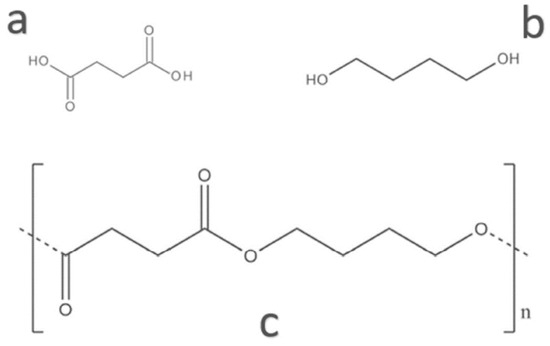
Figure 5.
Chemical structure of (a) succinic acid, (b) 1,4-butanediol, and (c) polybutylene succinate (PBS) (Reproduced with permission from Aliotta et al. (2022) [55]).
3.4. Polylactic Acid (PLA)
Figure 6 shows the chemical structure of PLA. PLA is an aliphatic polyester that is synthesized through different polymerization processes, including (i) the polycondensation of lactic acid and (ii) ring-opening polymerization of lactide [58]. Direct condensation of lactic acid is easier in its synthesis and commercialization, but this process produces low-molecular-weight products. Ring-opening polymerization is mostly used for PLA synthesis to produce high-molecular-weight products [59]. Ring-opening polymerization of lactide involves enantiomers such as L-lactide and D-lactide, to produce poly(L-lactide) (PLLA) and poly(D-lactide) (PDLA), respectively. Both PLA forms have semi-crystalline structures with glass transition and melting temperatures at around 55 and 175 °C, respectively. PLA is highly brittle at room temperature and has poor thermal stability; therefore, it needs to be modified and blended with other polymers to improve these limitations [5,13].
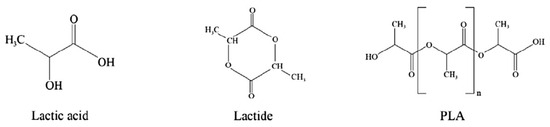
Figure 6.
Chemical structure of lactic acid, lactide, and polylactic acid (PLA). (Reproduced with permission from Harnkarnsujarit et al. (2021) [5]).
3.5. Bioplastic Blends
Blending is an important process for modifying and improving the properties of bioplastic polymers. Pure biopolymers have limitations during processing and poor properties compared to fossil-based or conventional polymers [3]. The properties of bioplastic packaging, including PBAT, TPS, PBS, and PLA, are shown in Table 2.
Bioplastic limitations have led to the blending of various bioplastics to improve their properties and increase economic competitiveness. Different blending ratios influence the physical, chemical, morphological, and thermal properties of films. Zhai et al. [60] demonstrated the effect of the TPS/PBAT ratios on films’ chemical and physical properties. The compatibility of starch and PBAT improved by increasing the PBAT content from 10% to 50%, while the film strength and flexibility improved through blending with PBAT, which modified the barrier properties by improving the hydrophobic surface of the films. Zhang et al. [61] prepared bioplastic blends of PLA and PBS that improved the properties of pure PLA or PBS. They found that adding PBS increased the elongation at break and decreased the tensile strength by enhancing the distinctive PBS features of flexibility, while PLA acted as a rigid filler, with stiffness improvement. Garalde et al. [62] investigated the impact of TPS/PBAT film ratios of 20/80, 40/60, and 60/40 on the films’ morphological, mechanical, and thermal properties. The results showed that increasing the TPS/PBAT ratio to 40/60 led to an improved polymeric component distribution and an increased PBAT crystallization temperature, while the tensile properties of the TPS/PBAT films were reduced by increasing the proportion of TPS. Bumbudsanpharoke, Wongphan, Promhuad, Leelaphiwat, and Harnkarnsujarit [2] studied different PBAT/PBS ratios of 20/80, 40/60, 60/40, and 80/20 on microstructural modification, the degree of crystallinity, and relaxation temperatures. The bioplastic blended polymer had smooth and compact microstructures, causing compatibility and adhesion at the polymer interface. The barrier properties of the bioplastic blended film increased with an increasing PBS content because the degree of crystallinity followed a more tortuous path, with reduced permeation.

Table 2.
Properties and applications of bioplastic packaging.
Table 2.
Properties and applications of bioplastic packaging.
| Bioplastic | Properties | Advantages | Disadvantages | Packaging Applications | References |
|---|---|---|---|---|---|
| PBAT | Tm~110–125 °C EB > 500% TS~15–20 MPa WVP~3 g·mm/m2·d·kPa OP~60 cm2·mm/m2·d·atm | High flexibility Good biodegradability Thermal stability Good processing stability | Low transparency High production costs | Blowing film application Mulch film Cutlery Bags | [2,3,46] |
| TPS | EB < 100% TS < 5 MPa WVP~7–11 10−10·g/s·m·Pa Water solubility > 20% | Abundant renewable resources Biodegradable Cheap biopolymer | Poor thermal processability Low water vapor barrier Moisture sensitivity Retrogradation processes | Compost bags Food packaging Edible film Coating | [6,52,53,63] |
| PBS | EB~100–200%. TS~25 MPa. WVP~1.5 g·mm/m2·d·kPa OP~30 cm2·mm/m2·d·atm | High flexibility Excellent thermal stability | High stiffness High melt viscosity for processing Low transparency | Film (polymer blends) Film coating | [2,57,63] |
| PLA | Tm~130–210 °C EB < 15% TS~20–60 MPa WVP~3 g·mm/m2·d·kPa OP~60 cm2·mm/m2·d·atm | High strength High transparency High processability | High brittleness Low heat distortion temperature Slow crystallization rate | Tray Bag Metallized and shrink films | [3,57,58] |
Tm is melting temperature, EB is elongation at break, TS is tensile strength, WVP is water vapor permeability, and OP is oxygen permeability.
However, polymer blends have limits due to the incompatibility between polymers, giving poor mechanical and barrier properties. The properties of polymer blends depend strongly on the miscibility (or immiscibility) of the polymers. Compatibility is necessary to enhance the interfacial adhesion of the polymer blend, leading to a homogeneous structure with strong mechanical properties and high barrier and thermal resistance properties [3,64]. A compatibilizer is a compound used to reduce the interfacial adhesion energy and improve the adhesion between polymers. These compatibilizers have different responsibilities and functionalities, such as crosslink agent, plasticizer, and hydrolytic agent, leading to strong mechanical properties, a smooth surface structure, high water vapor and oxygen barriers, and high thermal stability [65]. Commonly used compatibilizers for TPS/polyester films are maleic anhydride, citric acid, itaconic acid, tartaric acid, and organic acid [66,67,68]. The compatibilizers used in PLA/PBS films include diphenyl diisocyanate, lysine triisocyanate, lysine diisocyanate, glycidyl methacrylate, benzoyl peroxide, organoclays, and epoxy functionality [57]. Compatibilizers can be extracted using essential oils such as carvacrol, citral, and α-terpineol to enhance the compatibility of PBAT/PLA and PBAT/PBS packaging [69,70].
4. Bio-Functional Packaging
Packaging plays an important role in the food supply chain. Four primary functions of packaging have been identified: (i) containment to move or transport something from one place to another place; (ii) protection from physical and chemical damage such as water, gases, microorganisms, dust, shocks, and force; (iii) convenience in responding to consumer demand and promoting products; and (iv) communication as a silent salesman to convey information to consumers [21].
Food packaging is used to protect food products from physical and chemical changes including heat, light, oxygen, moisture, pressure, and microorganisms while also preventing biological activity. Packaging prevents the spoilage and contamination of food products during storage, transport, and distribution [71]. Food marketing requires that the quality of the food products must be preserved, and food packaging plays a key role in reducing the impact of external factors and avoiding or delaying the deterioration of food quality. The role of food packaging, along with marketing needs, has led to the development of active or functional packaging that maintains or improves the quality of food products.
Active packaging is defined in European Regulation (EC) No. 450/2009 as “active materials mean materials and articles that are intended to extend the shelf life or to maintain or improve the condition of packaged food; they are designed to deliberately incorporate components that would release or absorb substances into or from the packaged food or the environment surrounding the food” [72]. Active packaging can be classified into release and scavenger/absorber systems, including antimicrobial, antioxidant-, or carbon-dioxide-releasing systems and systems absorbing oxygen, moisture, or ethylene, which prolong the shelf life or enhance the quality and safety of products [73]. Functional packaging is enhanced in its ability to maintain, improve, or modify the quality and safety of food products and also has modified or improved properties itself [71,74]. Functional packaging can be produced using functional compounds including natural extracts such as essential oil, herbs, and spices and synthetic chemical compounds such as potassium sorbate, citric acid, and nano-oxide compounds. Functional compounds are widely used to preserve food quality and agricultural products. Some properties of polymeric bioplastic packaging are listed in Table 3.

Table 3.
Related research on the development of functional bioplastic films for food products.
5. Functional Packaging for Meat Products
5.1. Antioxidant Packaging
Antioxidant packaging uses antioxidants to delay oxidation or maintain oxidation stability, leading to a reduction in quality deterioration in flavor and odor, as well as color and texture deterioration. Food additive antioxidants such as butylated hydroxytoluene (BHT) or butylated hydroxyanisole (BHA) incorporated into polyolefin film [84,85] may influence health hazards for consumers. An alternative approach now being widely studied is the use of natural extracts, synthetic chemicals, and nanoparticles as antioxidants. Many studies have demonstrated that antioxidant agents incorporated into bioplastic packaging effectively extend the shelf life of meat.
Chollakup et al. [86] studied the incorporation of rambutan peel extract and cinnamon oil as antioxidants into cassava starch and whey protein isolate films to preserve the quality of salami. The release of polyphenols from the rambutan peel extract and cinnamon oil gave strong antioxidant functions to the films, as confirmed using DPPH assay. Moreno et al. [87] investigated potato starch films containing the bioactive proteins lactoferrin and lysozyme, which acted as antioxidants. These films effectively reduced the lard oxidation of minced pork after long storage times (14 days) due to the strong chelation capacity of the transition metals in the bioactives, which inhibited the oxidation reaction, used as natural antioxidant preservatives. Fiore et al. [88] showed that rosemary essential oil incorporated into a chitosan–caseinate coating with a polylactic acid film prolonged the shelf life of fresh minced chicken, and 2% rosemary essential oil demonstrated the greatest radical scavenging activity. Panrong et al. [89] demonstrated that a bioplastic film containing green tea extract acted as an antioxidant. Green tea is a rich source of polyphenols, especially catechin, epicatechin, and epigallocatechin, which accelerated the formation of oxymyoglobin (redness) with its free radical scavenging capacity and reduced the lipid oxidation of bacon after storage for 20 days. Ribeiro Sanches et al. [90] examined the influence of the concentration of red cabbage extract and sweet whey as antioxidants due to their significant contents of phenolic compounds and anthocyanins. The results showed greater stabilization of oxymyoglobin after storing ground beef for 4 days. The interaction of the antioxidants from natural extraction was mainly due to their content of polyphenols, which can reduce oxidation and delay the quality deterioration of meat. Packaging can also incorporate sodium nitrite [74], ferulic acid [91], ethylenediaminetetraacetic acid [29], zinc oxide (ZnO) nanoparticles [92,93], pyrogallol [94], and gallic acid [95].
5.2. Antimicrobial Packaging
The growth of microorganisms influences product quality, shelf life, and safety. Thus, technology has been developed to maintain food quality and extend shelf life through the addition of antimicrobial agents that reduce or inhibit microorganism growth [11]. The basic mechanism involves the penetration of active compounds into the lipid structure of bacterial walls, leading to protein denaturation, cell membrane destruction, bacterial membrane leakage, and ultimately cell lysis. Furthermore, the efficiency of antimicrobial agents is highly dependent on their release ability, according to factors such as the concentration gradient and solubility with the food product [69].
Antimicrobial agents such as organic acids, essential oils, enzymes, plant extracts, and nanoparticles can be incorporated into bioplastic polymers to increase food packaging’s functional properties. Various types of antimicrobial packaging for extending the shelf life of meat products are presented in Table 4.

Table 4.
Antimicrobial packaging produced using bioplastic polymers to extend the shelf life of meat products.
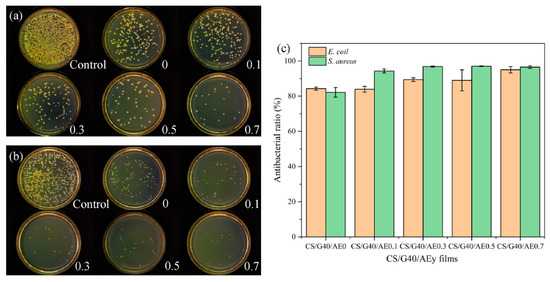
Figure 7.
Antibacterial activity of chitosan, glycerol, and aloe emodin films against E. coli (a) and S. aureus (b); antibacterial ratio of chitosan, glycerol, and aloe emodin films (c). (Reproduced with permission from Yang, Ning, Ren, Xu, Li, and Wang [101]).
Table 4.
Antimicrobial packaging produced using bioplastic polymers to extend the shelf life of meat products.
| Packaging Material | Antimicrobial Agent | Product | Findings | Reference |
|---|---|---|---|---|
| PLA/PBAT | Cinnamaldehyde and tea polyphenols | Meat analogue | Films protective against E. coli and S. aureus during 10 days of storage. | [96] |
| PBAT/TPS | Nisin and ethylenediaminetetraacetic acid | Pork | Ethylenediaminetetraacetic acid and nisin protective against L. monocytogenes, C. perfringens, S. aureus, and L. innocua. | [29] |
| TPS/whey protein isolate | Rambutan peel extract and cinnamon oil | Salami | Rambutan peel extract and cinnamon oil displayed antibacterial activity against B. cereus, E. coli, and S. aureus. | [86] |
| PLA | Carvacrol | Ground beef | Carvacrol-loaded PLA film reduced TVC and extended shelf life at 1.1 ± 1.5 days. | [97] |
| PLA | Lauric arginate ester, sodium lactate, and sorbic acid | Meat | - Lauric arginate ester reduced L. innocua, L. monocytogenes, and S. typhimurium growth for 3–5 weeks - Sorbic acid reduced the growth of L. innocua but not Salmonella. | [98] |
| TPS | Gallic acid, chitosan, and carvacrol | Ham | - Chitosan and carvacrol acted synergistically in L. monocytogenes inhibition. | [99] |
| TPS/PBAT | Sodium nitrite | Pork | - Nitrite reduced TVC up to 1.5 log CFU/g. - Nitrite against Pseudomonas aeruginosa. | [74] |
| Chitosan | Thyme essential oil | Meat | - Thyme essential oil reduced yeast populations but did not affect aerobic mesophilic bacteria, lactic acid bacteria, and enterobacteria. | [100] |
| Chitosan | Aloe emodin | Pork | - Chitosan is a natural antibacterial agent with positively charged amino groups that interacted with microbial cell membranes. - Aloe emodin inhibited bacterial formation, bringing about bacterial metabolism disorder and death and protecting against E. coli and S. aureus as shown in Figure 7. | [101] |
| Sodium caseinate | Pomegranate peel extract | Ground beef | - Pomegranate peel extract protecting against S. aureus and E. coli enhanced the germicidal activity of sodium caseinate. | [102] |
| Carboxymethyl cellulose (CMC) | Encapsulated pomegranate extract | Fresh beef and chicken meat | - CMC film and encapsulated pomegranate extract interacted via hydrogen bonding at 3320 cm−1, which shifted to a lower wavenumber. - The film system was more effective in inhibiting L. monocytogenes than C. jejuni, S. typhimurium, and S. aureus. - The classical CMC could not retard the growth of bacteria until the end of storage, while CMC with encapsulated pomegranate extract could delay microbial growth in beef and chicken meat. | [103] |
| Cassava starch and sodium carboxymethyl cellulose | Caffeic acid and silica nanoparticles (C@SNPs) | Fresh beef and chicken meat | - Reduction in hydrogen bonds and hydroxyl groups of the film formed by the nanoparticles, reduced the hydrophilicity of the film matrix. - Film containing C@SNPs 5:1 could inhibit E. coli and S. aureus with percent reductions up to 65.43% and 61.90%, respectively. - The active packaging delayed the microbial growth and TBARS in the fresh meat. The TBARS in the meat packed in film were 0.130 mg MDA/kg, while in the control group, they were 0.611 mg MDA/kg. | [104] |
| PBAT/TPS | Zinc oxide (ZnO) nanoparticles | Pork | - ZnO delayed microbial growth and extended shelf life to more than 12 days. - ZnO against S. aureus, E. coli, Enterobacteriaceae, and Pseudomonas spp. | [105] |
| Bacterial nanocellulose (BNC) | Postbiotics of Lactobacillus sakei | Buffalo patty | - A slight shift in the stretching vibration of the carboxylic groups of the BNC (1627 cm−1) indicates the successful incorporation of postbiotics into the BNC matrix. - The growth of L. monocytogenes in the buffalo patty was significantly reduced by the BNC film containing postbiotics of Lactobacillus sakei. | [106] |
Antimicrobial agents inhibit the growth of microorganisms, namely Gram-positive bacteria, Gram-negative bacteria, and fungi, through their incorporation with bioplastic matrices using coating, extruding, and casting. Novel functional packaging technologies can control and reduce microbial spoilage to better preserve the quality of and prolong the shelf life of meat.
5.3. Other Functional Packaging
Furthermore, functional packaging can also enhance other aspects of quality, such as tenderness, texture, and color. Wongphan et al. [107] developed a novel edible film made of starch incorporated with papain that improved the tenderness of beef, with reduced Warner–Bratzler shear values and hardness. Papain has proteolytic activities that hydrolyze collagen into myofibrillar proteins, leading to the degradation of the myofibrillar proteins. Enzymatic catalysis (papain) influenced the transformation and chemistry of myoglobin and thereby gave a bright red color. Moreover, the papain interacted with the starch film matrices, delaying the water dissolution of the starch films. The interaction between the starch and papain via hydrogen bonding reduced the binding between the starch and water, resulting in reduced dissolution, as shown in Figure 8 and Figure 9.
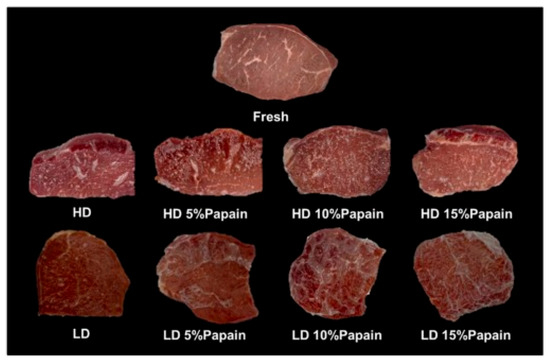
Figure 8.
Appearance of packaged beef in starch films containing different papain concentrations (5%, 10%, and 15%) after incubation for 30 min. (Reproduced with permission from Wongphan, Khowthong, Supatrawiporn, and Harnkarnsujarit [107]).
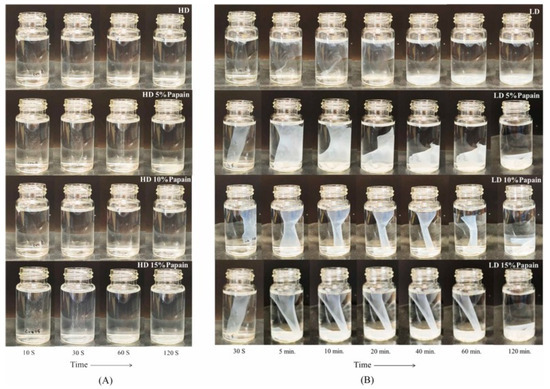
Figure 9.
Water solubility as a function of time in starch films containing different papain concentrations (5%, 10%, and 15%). (Reproduced with permission from Wongphan, Khowthong, Supatrawiporn, and Harnkarnsujarit [107]). (A) high dissolution (HD)(B) low dissolution (LD).
Chatkitanan and Harnkarnsujarit [28] reported that the incorporation of 1% and 2% sodium nitrite into LLPDE/TPS retained the hardness of the pork because the nitrite prevented protein aggregation and retained the protein structure. Therefore, incorporating active compounds into packaging improves the tenderness, maintains the protein structure, and extends the shelf life of meat.
6. Food Preservatives as Alternative Functional Compounds
Food preservatives are added to prevent food spoilage due to microorganisms (bacteria, mold, yeast, and fungi) and slow or prevent discoloration, flavor, or texture changes by delaying oxidation to maintain product freshness [108]. The food preservatives on product labels include ascorbic acid, butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), calcium propionate, calcium sorbate, citric acid, ethylenediaminetetraacetic acid (EDTA), potassium sorbate, sodium benzoate, sodium erythorbate, sodium nitrite, and tocopherols (Vitamin E). These can be classified into chemical or synthetic preservatives and natural preservatives [109]. Both types of preservatives are commonly used in meat products, acting as antimicrobials and antioxidants. Natural preservatives are plant-based bioactive phenolic compounds found in plants, fruits, herbs, and spices. Essential oils or plant extracts, including rosemary, sage, thyme, oregano, cinnamon, clove extracts, green tea, and eugenol [110,111,112,113], are natural bioactive compounds that do not require a labeled additive (e-number) for their application in food products. Conversely, the amounts of chemical or synthetic preservatives used are limited by safety and toxicological requirements and regulated by legislation [108,114]. The functionality of some organic acids and their salts, commonly used in industry, is shown in Table 5, acting as antioxidant, antimicrobial, emulsifying, and stabilizing agents to maintain the freshness of appearance and consistency of products [115,116].

Table 5.
Food preservative functionalities commonly used in industry.
However, the direct addition of food preservatives to products impacts consumer health due to the residuals in the products, with the amount of residual food preservative being a significant factor to consider. Nevertheless, studies have shown that incorporating additives into packaging reduces the amount of additives remaining in the food compared to with their direct addition to food [28,74,105]. This serves as another option for maintaining food quality and enhancing consumer safety. Additionally, it helps address packaging-related issues, as shown in Table 6. The selection of active compounds must adhere to the standards for food contact materials, generally recognized as safe (GRAS) designation, and the specific regulations set by each country. When adding active compounds added to active packaging, their concentrations must align with the acceptable daily intake to ensure food safety. However, this should not compromise their ability to effectively preserve food products. Ideally, active compounds should be used at the minimum concentration that maximizes their efficiency while maintaining human safety and the desired packaging properties.

Table 6.
Functional bioplastic packaging incorporating food preservatives.
From these studies, it was found that research on incorporating food preservatives into food packaging through compression processes to enhance the efficiency of food packaging and increase consumer satisfaction and the development of packaging properties incorporating food preservatives is a new method for maintaining the quality and safety of meat products. This can be considered a new challenge emerging in the food and packaging industries. The current research on bioplastic meat packaging is primarily centered on scaling up production to the industrial level using extrusion technology. However, there is a need to more deeply investigate the stability of active packaging for meat products under various storage conditions (i.e., temperature, pressure, vibration) for specific durations of time. The storage conditions and duration can be directly linked to the meat supply chain (production, distribution, retail, and consumer behavior). Therefore, a comprehensive approach involving collaboration between marketing, supply chain stakeholders, and packaging developers is essential to address these challenges effectively.
7. Flavor/Odor Contamination
Contamination or migration are major issues in food packaging. Contamination or migration is defined as the transfer of compounds from packaging to food, which can affect its quality and safety in accordance with legislation or regulations. Contamination can alter the sensory and quality properties of food or cause harm to consumers’ health [126].
Flavor is an important sensory aspect of the overall acceptability of meat products [127]. The flavor of meat is attributed to a complex mixture of both volatile and non-volatile compounds. Organic natural volatile components include pyrazines, aldehydes, acids, ketones, hydrocarbons, esters, alcohols, nitrogen, and sulfur-containing compounds that are formed due to lipid oxidation and bacterial metabolism, leading to odorous sensations [128,129]. Non-volatile compounds produce a sense of taste by interacting with the taste buds on the surface of the tongue and in the mucous membranes of the palate and throat area [129]. Flavor can determine the quality of meat and poultry products. The volatile components derived from the degradation of proteins and lipids impact the sensory quality of meat depending on the degree of meat degradation [128].
Table 7 lists the volatile compounds in meat that impart specific product flavors or odors. The product packaging and conditions between storage and distribution also influence the formation of volatile compounds [130]. Some aromatic hydrocarbons in meat are derived from packaging due to migration. Song, Canellas, and Nerin [128] analyzed the volatile compounds in minced pork during storage in an active film (rosemary essential oil coated PET sheet) and identified 41 compounds using HS-SPME–GC–MS. These included alcohols, aldehydes, ketones, and hydrocarbons. Aromatic hydrocarbons such as toluene, ethylbenzene, m-xylene, o-xylene, isopropylbenzene, and p-cymene came from the packaging, indicating that the aromatic hydrocarbons in food contact material can be transferred into food and consequently pose a risk to consumers. Rivas-Canedo et al. [131] investigated how high-pressure processing (HPP) significantly changed the levels of some volatile compounds. The amount of alcohols and aldehydes decreased while 2,3-butanedione and 2-butanone were more abundant in the high-pressure-processed meats, with the migration of compounds from the plastic material such as branched-chain alkanes and benzene. Ref. [132] studied the volatile profile of Spanish salchichón when subjected to HPP using a multilayer plastic as the packaging material. The results showed high levels of compounds emanating from the plastic material, especially branched-chain alkanes and benzene. Wrona et al. [133] demonstrated that the incorporation of green tea extract into polyethylene using extrusion technology extended the shelf life of fresh meat, with epigallocatechin gallate, gallocatechin gallate, epicatechin gallate, gallocatechin, epigallocatechin gallate, and catechin gallate migrating from the packaging. Therefore, using active packaging as food contact materials should be studied in relation to the flavor (mass) transfer from packaging into products. The flavor of both volatile and non-volatile compounds affects the senses and can also impact food quality and product safety.

Table 7.
Volatile compounds in beef, pork, and poultry and their odor data, modified from [128,134].
8. The Prospective Future of Functional Packaging Technology for Meat Products
Functional packaging technology for meat products is continuously evolving to address various challenges in the industry, including extending shelf life, preserving freshness, enhancing food safety, and meeting consumer demand for convenience.
Shelf life extension: One of the main challenges in meat packaging is extending shelf life while maintaining product quality and safety. Emerging technologies such as active packaging with natural compounds, extracted or synthesis compounds, and food preservatives such as antioxidant and antimicrobial agents can help extend shelf life.
Sustainability: With increasing environmental concerns and the aim of reducing food loss and waste, the demand for sustainable packaging solutions in the meat industry is therefore increasing. Future directions may include the development of biodegradable and compostable packaging materials derived from renewable sources, such as bioplastics.
Active packaging: Active packaging systems that include active ingredients such as antimicrobials, antioxidants, and oxygen scavengers directly added to the packaging materials are gaining attention. Future advances may involve the use of nanotechnology to increase the efficacy of the active ingredients and improve the kinetics of their release.
Convenience and consumer interaction: Packaging technology is also evolving to meet consumer demands for convenience and an enhanced user experience.
Food safety and quality assurance: Ensuring food safety and quality remains a paramount concern in the meat industry. Advanced packaging technologies such as barrier films, active coatings, and nanocomposites can help prevent contamination and maintain product freshness during storage and transportation.
Regulatory compliance: As the regulations governing food packaging become more stringent, manufacturers need to ensure that safety and labeling requirements are followed. Future directions may involve developing packaging materials that are compliant with emerging regulatory standards and guidelines.
9. Conclusions and Challenges
This study reviewed the recent advances in functional bioplastic meat packaging, focusing on enhancing the ability to maintain, improve, or modify the quality and safety of meat products. Meat products are crucial sources of protein and essential nutrients and play a significant role in supporting human health. However, their perishable nature poses challenges in terms of maintaining their quality and safety. Maintaining the optimal conditions during their production, storage, and distribution is essential for preserving the quality and safety of meat products to meet consumer expectations and regulatory standards. Advanced packaging technologies, proper handling practices, and stringent quality control measures play vital roles in mitigating spoilage and ensuring food safety throughout the meat supply chain. Further research is required to scale up polymeric-based packaging to industrial market production and promote a circular economy.
Author Contributions
Conceptualization, P.W. and N.H.; methodology, P.W. and N.H.; formal analysis, P.W. and N.H.; investigation, P.W. and N.H.; writing—original draft preparation, P.W. and N.H.; writing—review and editing, P.W., K.P., A.S., Y.L., C.O. and N.H.; supervision, N.H.; funding acquisition, N.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research project was supported by the National Research Council of Thailand (NRCT), Contract No. N41A640190 (The Royal Golden Jubilee Ph.D. Program) and Contract No. N41A640082, and Kasetsart University Research and Development Institute (KURDI) FF(KU) 5.67.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author (due to privacy).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nachod, B.; Keller, E.; Hassanein, A.; Lansing, S. Assessment of petroleum-based plastic and bioplastics degradation using anaerobic digestion. Sustainability 2021, 13, 13295. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Wongphan, P.; Promhuad, K.; Leelaphiwat, P.; Harnkarnsujarit, N. Morphology and permeability of bio-based poly(butylene adipate-co-terephthalate) (PBAT), poly(butylene succinate) (PBS) and linear low-density polyethylene (LLDPE) blend films control shelf-life of packaged bread. Food Control 2022, 132, 108541. [Google Scholar] [CrossRef]
- Jariyasakoolroj, P.; Leelaphiwat, P.; Harnkarnsujarit, N. Advances in research and development of bioplastic for food packaging. J. Sci. Food Agric. 2020, 100, 5032–5045. [Google Scholar] [CrossRef] [PubMed]
- European, B. Global Production Capacities of Biodegradable Plastic 2023 (by Material Type). Available online: https://www.european-bioplastics.org/bioplastics/materials/biodegradable/ (accessed on 23 January 2024).
- Harnkarnsujarit, N.; Wongphan, P.; Chatkitanan, T.; Laorenza, Y.; Srisa, A. Bioplastic for Sustainable Food Packaging. In Sustainable Food Processing and Engineering Challenges; Academic Press: Amsterdam, The Netherlands, 2021; pp. 203–277. [Google Scholar]
- Narancic, T.; Cerrone, F.; Beagan, N.; O’Connor, K.E. Recent Advances in Bioplastics: Application and Biodegradation. Polymers 2020, 12, 920. [Google Scholar] [CrossRef] [PubMed]
- Dammak, M.; Fourati, Y.; Tarrés, Q.; Delgado-Aguilar, M.; Mutjé, P.; Boufi, S. Blends of PBAT with plasticized starch for packaging applications: Mechanical properties, rheological behaviour and biodegradability. Ind. Crops Prod. 2020, 144, 112061. [Google Scholar] [CrossRef]
- de Castro, L.; Silva, L.G.L.; Abreu, I.R.; Braz, C.J.F.; Rodrigues, S.C.S.; Moreira-Araujo, R.; Folkersma, R.; de Carvalho, L.H.; Barbosa, R.; Alves, T.S. Biodegradable PBAT/PLA blend films incorporated with turmeric and cinnamomum powder: A potential alternative for active food packaging. Food Chem. 2024, 439, 138146. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Ghosh, T.; Zhang, W.; Rhim, J.-W. Recent progress in PBAT-based films and food packaging applications: A mini-review. Food Chem. 2024, 437, 137822. [Google Scholar] [CrossRef]
- RameshKumar, S.; Shaiju, P.; O’Connor, K.E. Bio-based and biodegradable polymers-State-of-the-art, challenges and emerging trends. Curr. Opin. Green Sustain. Chem. 2020, 21, 75–81. [Google Scholar] [CrossRef]
- Zhang, D.; Ivane, N.M.A.; Haruna, S.A.; Zekrumah, M.; Elyse, F.K.R.; Tahir, H.E.; Wang, G.; Wang, C.; Zou, X. Recent trends in the micro-encapsulation of plant-derived compounds and their specific application in meat as antioxidants and antimicrobials. Meat Sci. 2022, 191, 108842. [Google Scholar] [CrossRef]
- Wongphan, P.; Nerin, C.; Harnkarnsujarit, N. Enhanced compatibility and functionality of thermoplastic cassava starch blended PBAT blown films with erythorbate and nitrite. Food Chem. 2023, 420, 136107. [Google Scholar] [CrossRef]
- Rosenboom, J.G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Nethra, P.V.; Sunooj, K.V.; Aaliya, B.; Navaf, M.; Akhila, P.P.; Sudheesh, C.; Mir, S.A.; Shijin, A.; George, J. Critical factors affecting the shelf life of packaged fresh red meat–A review. Meas. Food 2023, 10, 100086. [Google Scholar] [CrossRef]
- Li, X.L.; Shen, Y.; Hu, F.; Zhang, X.X.; Thakur, K.; Rengasamy, K.R.; Khan, M.R.; Busquets, R.; Wei, Z.J. Fortification of polysaccharide-based packaging films and coatings with essential oils: A review of their preparation and use in meat preservation. Int. J. Biol. Macromol. 2023, 242, 124767. [Google Scholar] [CrossRef] [PubMed]
- Smaoui, S.; Chérif, I.; Hlima, H.B.; Khan, M.U.; Rebezov, M.; Thiruvengadam, M.; Sarkar, T.; Shariati, M.A.; Lorenzo, J.M. Zinc oxide nanoparticles in meat packaging: A systematic review of recent literature. Food Packag. Shelf Life 2023, 36, 101045. [Google Scholar] [CrossRef]
- Domínguez, R.; Barba, F.J.; Gómez, B.; Putnik, P.; Kovačević, D.B.; Pateiro, M.; Santos, E.M.; Lorenzo, J.M. Active packaging films with natural antioxidants to be used in meat industry: A review. Food Res. Int. 2018, 113, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.S.; Nair, D.V.; Johny, A.K.; Venkitanarayanan, K. Use of food preservatives and additives in meat and their detection techniques. In Meat Quality Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 187–213. [Google Scholar]
- Toldrá, F. Handbook of Meat Processing; John Wiley & Sons: Ames, IA, USA, 2010. [Google Scholar]
- Kerry, J.P. Advances in Meat, Poultry and Seafood Packaging; Woodhead Publishing: Sawston, UK, 2012. [Google Scholar]
- Robertson, G.L. Food Packaging—Principles and Practice, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Holman, B.W.; Kerry, J.P.; Hopkins, D.L. Meat packaging solutions to current industry challenges: A review. Meat Sci. 2018, 144, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Mancini, R.A.; Hunt, M.C. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef]
- Brewer, S. Irradiation effects on meat color—A review. Meat Sci. 2004, 68, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Luo, X.; Zhang, Y.; Hopkins, D.L.; Liang, R.; Dong, P.; Zhu, L. Effects of microbiota dynamics on the color stability of chilled beef steaks stored in high oxygen and carbon monoxide packaging. Food Res. Int. 2020, 134, 109215. [Google Scholar] [CrossRef]
- Sánchez-Escalante, A.; Djenane, D.; Torrescano, G.; Beltrán, J.A.; Roncalés, P. The effects of ascorbic acid, taurine, carnosine and rosemary powder on colour and lipid stability of beef patties packaged in modified atmosphere. Meat Sci. 2001, 58, 421–429. [Google Scholar] [CrossRef]
- Seydim, A.C.; Guzel-Seydim, Z.B.; Acton, J.C.; Dawson, P.L. Effects of Rosemary extract and sodium lactate on quality of vacuum-packaged ground ostrich meat. J. Food Sci. 2006, 71, S71–S76. [Google Scholar] [CrossRef]
- Chatkitanan, T.; Harnkarnsujarit, N. Effects of nitrite incorporated active films on quality of pork. Meat Sci. 2021, 172, 108367. [Google Scholar] [CrossRef] [PubMed]
- Leelaphiwat, P.; Pechprankan, C.; Siripho, P.; Bumbudsanpharoke, N.; Harnkarnsujarit, N. Effects of nisin and EDTA on morphology and properties of thermoplastic starch and PBAT biodegradable films for meat packaging. Food Chem. 2022, 369, 130956. [Google Scholar] [CrossRef]
- Guerrero-Legarreta, I. Handbook of Poultry Science and Technology, Volume 2: Secondary Processing; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Juárez, M.; Aldai, N.; López-Campos, Ó.; Dugan, M.; Uttaro, B.; Aalhus, J. Beef texture and juiciness. Handb. Meat Meat Process. 2012, 9, 177–206. [Google Scholar]
- Seyfert, M.; Hunt, M.; Mancini, R.; Hachmeister, K.; Kropf, D.; Unruh, J.; Loughin, T. Beef quadriceps hot boning and modified-atmosphere packaging influence properties of injection-enhanced beef round muscles. J. Anim. Sci. 2005, 83, 686–693. [Google Scholar] [CrossRef][Green Version]
- Kim, Y.H.; Huff-Lonergan, E.; Sebranek, J.G.; Lonergan, S.M. High-oxygen modified atmosphere packaging system induces lipid and myoglobin oxidation and protein polymerization. Meat Sci. 2010, 85, 759–767. [Google Scholar] [CrossRef]
- Shahidi, F. Flavor of meat and meat products—An overview. In Flavor of Meat and Meat Products; Springer: New York, NY, USA, 1994; pp. 1–3. [Google Scholar]
- Rhee, K.S. Chemistry of meat flavor. In Flavor Chemistry of Lipid Foods; The American Oil Chemists Society: Urbana, IL, USA, 1989; pp. 166–189. [Google Scholar]
- Dominguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.; Pearson, A. Lipid-derived off-flavours in meat—Formation and inhibition. In Flavor of Meat and Meat Products; Springer: New York, NY, USA, 1994; pp. 116–143. [Google Scholar]
- de W Blackburn, C. Food Spoilage Microorganisms; Woodhead Publishing: Boca Raton, FL, USA, 2006. [Google Scholar]
- Pellissery, A.J.; Vinayamohan, P.G.; Amalaradjou, M.A.R.; Venkitanarayanan, K. Spoilage bacteria and meat quality. In Meat Quality Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 307–334. [Google Scholar]
- Jay, J.M.; Loessner, M.J.; Golden, D.A. Fresh meats and poultry. In Modern Food Microbiology; Springer Science & Business Media: New York, NY, USA, 2005; pp. 63–99. [Google Scholar]
- Doyle, M.P. Compendium of the Microbiological Spoilage of Foods and Beverages; Springer: New York, NY, USA, 2009. [Google Scholar]
- Barros-Velazquez, J. Antimicrobial Food Packaging; Academic Press: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Rahmadiawan, D.; Shi, S.C.; Abral, H.; Ilham, M.K.; Sugiarti, E.; Muslimin, A.N.; Putra, N.S.D. Comparative Analysis of the Influence of Different Preparation Methods on the Properties of TEMPO-Oxidized Bacterial Cellulose Powder Films. J. Nat. Fibers 2024, 21, 2301386. [Google Scholar] [CrossRef]
- Bioplastics, E. Global Production Capacities of Bioplastics 2021 (by Material Type). Available online: https://www.european-bioplastics.org/bioplastics/materials/ (accessed on 20 May 2021).
- Herrera, R.; Franco, L.; Rodríguez-Galán, A.; Puiggalí, J. Characterization and degradation behavior of poly(butylene adipate-co-terephthalate)s. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 4141–4157. [Google Scholar] [CrossRef]
- Jiao, J.; Zeng, X.; Huang, X. An overview on synthesis, properties and applications of poly(butylene-adipate-co-terephthalate)–PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26. [Google Scholar] [CrossRef]
- Dominici, F.; Gigli, M.; Armentano, I.; Genovese, L.; Luzi, F.; Torre, L.; Munari, A.; Lotti, N. Improving the flexibility and compostability of starch/poly(butylene cyclohexanedicarboxylate)-based blends. Carbohydr. Polym. 2020, 246, 116631. [Google Scholar] [CrossRef] [PubMed]
- Menzel, C. Improvement of starch films for food packaging through a three-principle approach: Antioxidants, cross-linking and reinforcement. Carbohydr. Polym. 2020, 250, 116828. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Composition, structure, physicochemical properties, and modifications of cassava starch. Carbohydr. Polym. 2015, 122, 456–480. [Google Scholar] [CrossRef] [PubMed]
- Amagliani, L.; O’Regan, J.; Kelly, A.L.; O’Mahony, J.A. Chemistry, structure, functionality and applications of rice starch. J. Cereal Sci. 2016, 70, 291–300. [Google Scholar] [CrossRef]
- Colivet, J.; Carvalho, R.A. Hydrophilicity and physicochemical properties of chemically modified cassava starch films. Ind. Crops Prod. 2017, 95, 599–607. [Google Scholar] [CrossRef]
- Zhang, Y.; Rempel, C.; Liu, Q. Thermoplastic starch processing and characteristics-a review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1353–1370. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi Nafchi, A.; Moradpour, M.; Saeidi, M.; Alias, A.K. Thermoplastic starches: Properties, challenges, and prospects. Starch-Stärke 2013, 65, 61–72. [Google Scholar] [CrossRef]
- Khan, B.; Bilal Khan Niazi, M.; Samin, G.; Jahan, Z. Thermoplastic Starch: A Possible Biodegradable Food Packaging Material-A Review. J. Food Process. Eng. 2017, 40, e12447. [Google Scholar] [CrossRef]
- Aliotta, L.; Seggiani, M.; Lazzeri, A.; Gigante, V.; Cinelli, P. A Brief Review of Poly (Butylene Succinate) (PBS) and Its Main Copolymers: Synthesis, Blends, Composites, Biodegradability, and Applications. Polymers 2022, 14, 844. [Google Scholar] [CrossRef]
- Mochane, M.J.; Magagula, S.I.; Sefadi, J.S.; Mokhena, T.C. A Review on Green Composites Based on Natural Fiber-Reinforced Polybutylene Succinate (PBS). Polymers 2021, 13, 1200. [Google Scholar] [CrossRef]
- Su, S.; Kopitzky, R.; Tolga, S.; Kabasci, S. Polylactide (PLA) and Its Blends with Poly(butylene succinate) (PBS): A Brief Review. Polymers 2019, 11, 1193. [Google Scholar] [CrossRef] [PubMed]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Deng, S.; Chen, P.; Ruan, R. Polylactic acid (PLA) synthesis and modifications: A review. Front. Chem. China 2009, 4, 259–264. [Google Scholar] [CrossRef]
- Zhai, X.; Wang, W.; Zhang, H.; Dai, Y.; Dong, H.; Hou, H. Effects of high starch content on the physicochemical properties of starch/PBAT nanocomposite films prepared by extrusion blowing. Carbohydr. Polym. 2020, 239, 116231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Q.; Shi, J.; Ye, H.; Zhou, Q. Distinctive Tensile Properties of the Blends of Poly(l-lactic acid) (PLLA) and Poly(butylene succinate) (PBS). J. Polym. Environ. 2017, 26, 1737–1744. [Google Scholar] [CrossRef]
- Garalde, R.A.; Thipmanee, R.; Jariyasakoolroj, P.; Sane, A. The effects of blend ratio and storage time on thermoplastic starch/poly(butylene adipate-co-terephthalate) films. Heliyon 2019, 5, e01251. [Google Scholar] [CrossRef] [PubMed]
- Bartolucci, L.; Cordiner, S.; De Maina, E.; Kumar, G.; Mele, P.; Mulone, V.; Igliński, B.; Piechota, G. Sustainable valorization of bioplastic waste: A review on effective recycling routes for the most widely used biopolymers. Int. J. Mol. Sci. 2023, 24, 7696. [Google Scholar] [CrossRef]
- Qiu, S.; Zhou, Y.; Waterhouse, G.I.N.; Gong, R.; Xie, J.; Zhang, K.; Xu, J. Optimizing interfacial adhesion in PBAT/PLA nanocomposite for biodegradable packaging films. Food Chem. 2021, 334, 127487. [Google Scholar] [CrossRef] [PubMed]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Studies on mechanical, thermal, and morphological characteristics of biocomposites from biodegradable polymer blends and natural fibers. In Biocomposites; Woodhead Publishing: Cambridge, UK, 2015; pp. 93–140. [Google Scholar]
- Garcia, P.S.; Grossmann, M.V.E.; Shirai, M.A.; Lazaretti, M.M.; Yamashita, F.; Muller, C.M.O.; Mali, S. Improving action of citric acid as compatibiliser in starch/polyester blown films. Ind. Crops Prod. 2014, 52, 305–312. [Google Scholar] [CrossRef]
- Olivato, J.B.; Grossmann, M.V.; Bilck, A.P.; Yamashita, F. Effect of organic acids as additives on the performance of thermoplastic starch/polyester blown films. Carbohydr. Polym. 2012, 90, 159–164. [Google Scholar] [CrossRef]
- Olivato, J.B.; Grossmann, M.V.E.; Yamashita, F.; Nobrega, M.M.; Scapin, M.R.S.; Eiras, D.; Pessan, L.A. Compatibilisation of starch/poly(butylene adipate co-terephthalate) blends in blown films. Int. J. Food Sci. Technol. 2011, 46, 1934–1939. [Google Scholar] [CrossRef]
- Laorenza, Y.; Harnkarnsujarit, N. Carvacrol, citral and alpha-terpineol essential oil incorporated biodegradable films for functional active packaging of Pacific white shrimp. Food Chem. 2021, 363, 130252. [Google Scholar] [CrossRef] [PubMed]
- Klinmalai, P.; Srisa, A.; Laorenza, Y.; Katekhong, W.; Harnkarnsujarit, N. Antifungal and plasticization effects of carvacrol in biodegradable poly(lactic acid) and poly(butylene adipate terephthalate) blend films for bakery packaging. LWT 2021, 152, 112356. [Google Scholar] [CrossRef]
- Basavegowda, N.; Baek, K.H. Advances in Functional Biopolymer-Based Nanocomposites for Active Food Packaging Applications. Polymers 2021, 13, 4198. [Google Scholar] [CrossRef]
- European, C. Commission Regulation (EC) No 450/2009 of 29 May 2009 on Active and Intelligent Materials and Articles Intended to Come into Contact with Food. Available online: https://eur-lex.europa.eu/eli/reg/2009/450/oj (accessed on 5 July 2022).
- Suppakul, P.; Miltz, J.; Sonneveld, K.; Bigger, S.W. Active packaging technologies with an emphasis on antimicrobial packaging and its applications. J. Food Sci. 2003, 68, 408–420. [Google Scholar] [CrossRef]
- Katekhong, W.; Wongphan, P.; Klinmalai, P.; Harnkarnsujarit, N. Thermoplastic starch blown films functionalized by plasticized nitrite blended with PBAT for superior oxygen barrier and active biodegradable meat packaging. Food Chem. 2022, 374, 131709. [Google Scholar] [CrossRef] [PubMed]
- Jha, P. Effect of plasticizer and antimicrobial agents on functional properties of bionanocomposite films based on corn starch-chitosan for food packaging applications. Int. J. Biol. Macromol. 2020, 160, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Vilarinho, F.; Andrade, M.; Buonocore, G.G.; Stanzione, M.; Vaz, M.F.; Sanches Silva, A. Monitoring lipid oxidation in a processed meat product packaged with nanocomposite poly(lactic acid) film. Eur. Polym. J. 2018, 98, 362–367. [Google Scholar] [CrossRef]
- Tsironi, M.; Kosma, I.S.; Badeka, A.V. The Effect of Whey Protein Films with Ginger and Rosemary Essential Oils on Microbiological Quality and Physicochemical Properties of Minced Lamb Meat. Sustainability 2022, 14, 3434. [Google Scholar] [CrossRef]
- Kongkaoroptham, P.; Piroonpan, T.; Pasanphan, W. Chitosan nanoparticles based on their derivatives as antioxidant and antibacterial additives for active bioplastic packaging. Carbohydr. Polym. 2021, 257, 117610. [Google Scholar] [CrossRef]
- Leal, I.L.; Silva Rosa, Y.C.; Silva Penha, J.; Cruz Correia, P.R.; Silva Melo, P.; Guimarães, D.H.; Barbosa, J.D.V.; Druzian, J.I.; Machado, B.A.S. Development and application starch films: PBAT with additives for evaluating the shelf life of Tommy Atkins mango in the fresh-cut state. J. Appl. Polym. Sci. 2019, 136, 48150. [Google Scholar] [CrossRef]
- Balaguer, M.P.; Fajardo, P.; Gartner, H.; Gomez-Estaca, J.; Gavara, R.; Almenar, E.; Hernandez-Munoz, P. Functional properties and antifungal activity of films based on gliadins containing cinnamaldehyde and natamycin. Int. J. Food Microbiol. 2014, 173, 62–71. [Google Scholar] [CrossRef]
- Pires, J.R.A.; de Souza, V.G.L.; Fernando, A.L. Chitosan/montmorillonite bionanocomposites incorporated with rosemary and ginger essential oil as packaging for fresh poultry meat. Food Packag. Shelf Life 2018, 17, 142–149. [Google Scholar] [CrossRef]
- Phothisarattana, D.; Wongphan, P.; Promhuad, K.; Promsorn, J.; Harnkarnsujarit, N. Biodegradable Poly(Butylene Adipate-Co-Terephthalate) and Thermoplastic Starch-Blended TiO2 Nanocomposite Blown Films as Functional Active Packaging of Fresh Fruit. Polymers 2021, 13, 4192. [Google Scholar] [CrossRef]
- Wangprasertkul, J.; Siriwattanapong, R.; Harnkarnsujarit, N. Antifungal packaging of sorbate and benzoate incorporated biodegradable films for fresh noodles. Food Control 2021, 123, 107763. [Google Scholar] [CrossRef]
- Sebranek, J.; Sewalt, V.; Robbins, K.; Houser, T. Comparison of a natural rosemary extract and BHA/BHT for relative antioxidant effectiveness in pork sausage. Meat Sci. 2005, 69, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.; Han, I.; Acton, J.; Ogale, A.; Barmore, C.; Dawson, P. Effects of antioxidants in polyethylene film on fresh beef color. J. Food Sci. 2003, 68, 99–104. [Google Scholar] [CrossRef]
- Chollakup, R.; Pongburoos, S.; Boonsong, W.; Khanoonkon, N.; Kongsin, K.; Sothornvit, R.; Sukyai, P.; Sukatta, U.; Harnkarnsujarit, N. Antioxidant and antibacterial activities of cassava starch and whey protein blend films containing rambutan peel extract and cinnamon oil for active packaging. LWT 2020, 130, 109573. [Google Scholar] [CrossRef]
- Moreno, O.; Atares, L.; Chiralt, A. Effect of the incorporation of antimicrobial/antioxidant proteins on the properties of potato starch films. Carbohydr. Polym. 2015, 133, 353–364. [Google Scholar] [CrossRef]
- Fiore, A.; Park, S.; Volpe, S.; Torrieri, E.; Masi, P. Active packaging based on PLA and chitosan-caseinate enriched rosemary essential oil coating for fresh minced chicken breast application. Food Packag. Shelf Life 2021, 29, 100708. [Google Scholar] [CrossRef]
- Panrong, T.; Karbowiak, T.; Harnkarnsujarit, N. Thermoplastic starch and green tea blends with LLDPE films for active packaging of meat and oil-based products. Food Packag. Shelf Life 2019, 21, 100331. [Google Scholar] [CrossRef]
- Ribeiro Sanches, M.A.; Camelo-Silva, C.; da Silva Carvalho, C.; Rafael de Mello, J.; Barroso, N.G.; Lopes da Silva Barros, E.; Silva, P.P.; Pertuzatti, P.B. Active packaging with starch, red cabbage extract and sweet whey: Characterization and application in meat. LWT 2021, 135, 110275. [Google Scholar] [CrossRef]
- Li, T.; Xia, N.; Xu, L.; Zhang, H.; Zhang, H.; Chi, Y.; Zhang, Y.; Li, L.; Li, H. Preparation, characterization and application of SPI-based blend film with antioxidant activity. Food Packag. Shelf Life 2021, 27, 100614. [Google Scholar] [CrossRef]
- Mohammadi, H.; Kamkar, A.; Misaghi, A.; Zunabovic-Pichler, M.; Fatehi, S. Nanocomposite films with CMC, okra mucilage, and ZnO nanoparticles: Extending the shelf-life of chicken breast meat. Food Packag. Shelf Life 2019, 21, 100330. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Kim, S.-M.; Rhim, J.-W. Carboxymethyl cellulose-based multifunctional film combined with zinc oxide nanoparticles and grape seed extract for the preservation of high-fat meat products. Sustain. Mater. Technol. 2021, 29, e00325. [Google Scholar] [CrossRef]
- Promsorn, J.; Harnkarnsujarit, N. Pyrogallol loaded thermoplastic cassava starch based films as bio-based oxygen scavengers. Ind. Crops Prod. 2022, 186, 115226. [Google Scholar] [CrossRef]
- Promsorn, J.; Harnkarnsujarit, N. Oxygen absorbing food packaging made by extrusion compounding of thermoplastic cassava starch with gallic acid. Food Control 2022, 142, 109273. [Google Scholar] [CrossRef]
- Wang, L.; Xu, J.; Zhang, M.; Zheng, H.; Li, L. Preservation of soy protein-based meat analogues by using PLA/PBAT antimicrobial packaging film. Food Chem. 2022, 380, 132022. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Heising, J.; Fogliano, V.; Dekker, M. Fat content and storage conditions are key factors on the partitioning and activity of carvacrol in antimicrobial packaging. Food Packag. Shelf Life 2020, 24, 100500. [Google Scholar] [CrossRef]
- Guo, M.; Jin, T.Z.; Yang, R. Antimicrobial Polylactic Acid Packaging Films against Listeria and Salmonella in Culture Medium and on Ready-to-Eat Meat. Food Bioprocess. Technol. 2014, 7, 3293–3307. [Google Scholar] [CrossRef]
- Zhao, Y.; Teixeira, J.S.; Saldana, M.D.A.; Ganzle, M.G. Antimicrobial activity of bioactive starch packaging films against Listeria monocytogenes and reconstituted meat microbiota on ham. Int. J. Food Microbiol. 2019, 305, 108253. [Google Scholar] [CrossRef] [PubMed]
- Quesada, J.; Sendra, E.; Navarro, C.; Sayas-Barbera, E. Antimicrobial Active Packaging including Chitosan Films with Thymus vulgaris L. Essential Oil for Ready-to-Eat Meat. Foods 2016, 5, 57. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ning, Y.; Ren, Z.; Xu, S.; Li, J.; Wang, L. Fabricating anti-ultraviolet and antibacterial films with lower water solubility from chitosan, glycerol and aloe emodin for pork preservation. Food Hydrocoll. 2024, 153, 110030. [Google Scholar] [CrossRef]
- Emam-Djomeh, Z.; Moghaddam, A.; Yasini Ardakani, S.A. Antimicrobial Activity of Pomegranate (Punica granatum L.) Peel Extract, Physical, Mechanical, Barrier and Antimicrobial Properties of Pomegranate Peel Extract-incorporated Sodium Caseinate Film and Application in Packaging for Ground Beef. Packag. Technol. Sci. 2015, 28, 869–881. [Google Scholar] [CrossRef]
- Khalid, S.A.; Ghanem, A.F.; Abd-El-Malek, A.; Ammar, M.A.; El-Khateib, T.; El-Sherbiny, I.M. Free-standing carboxymethyl cellulose film incorporating nanoformulated pomegranate extract for meat packaging. Carbohydr. Polym. 2024, 332, 121915. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Peng, S.; Chen, X.; Li, C.; Cui, H. Silica nanoparticles loaded with caffeic acid to optimize the performance of cassava starch/sodium carboxymethyl cellulose film for meat packaging. Int. J. Biol. Macromol. 2023, 241, 124591. [Google Scholar] [CrossRef] [PubMed]
- Phothisarattana, D.; Wongphan, P.; Promhuad, K.; Promsorn, J.; Harnkarnsujarit, N. Blown film extrusion of PBAT/TPS/ZnO nanocomposites for shelf-life extension of meat packaging. Colloids Surf. B Biointerfaces 2022, 214, 112472. [Google Scholar] [CrossRef]
- Rasouli, Y.; Moradi, M.; Tajik, H.; Molaei, R. Fabrication of anti-Listeria film based on bacterial cellulose and Lactobacillus sakei-derived bioactive metabolites; application in meat packaging. Food Biosci. 2021, 42, 101218. [Google Scholar] [CrossRef]
- Wongphan, P.; Khowthong, M.; Supatrawiporn, T.; Harnkarnsujarit, N. Novel edible starch films incorporating papain for meat tenderization. Food Packag. Shelf Life 2022, 31, 100787. [Google Scholar] [CrossRef]
- FDA. Overview of Food Ingredients, Additives & Colors. Available online: https://www.fda.gov/food/food-ingredients-packaging/overview-food-ingredients-additives-colors (accessed on 14 July 2022).
- Kumari, P.; Akhila, S.; Rao, Y.S.; Devi, B.R. Alternative to artificial preservatives. Syst. Rev. Pharm. 2019, 10, 99–102. [Google Scholar]
- Martí-Quijal, F.J.; Khubber, S.; Remize, F.; Tomasevic, I.; Roselló-Soto, E.; Barba, F.J. Obtaining Antioxidants and Natural Preservatives from Food By-Products through Fermentation: A Review. Fermentation 2021, 7, 106. [Google Scholar] [CrossRef]
- Falleh, H.; Ben Jemaa, M.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef] [PubMed]
- Al-Maqtari, Q.A.; Rehman, A.; Mahdi, A.A.; Al-Ansi, W.; Wei, M.; Yanyu, Z.; Phyo, H.M.; Galeboe, O.; Yao, W. Application of essential oils as preservatives in food systems: Challenges and future prospectives—A review. Phytochem. Rev. 2021, 21, 1209–1246. [Google Scholar] [CrossRef]
- Yu, H.H.; Chin, Y.W.; Paik, H.D. Application of Natural Preservatives for Meat and Meat Products against Food-Borne Pathogens and Spoilage Bacteria: A Review. Foods 2021, 10, 2418. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations; World Health Organization. FOOD ADDITIVE INDEX, GSFA Online. Available online: https://www.fao.org/gsfaonline/additives/index.html (accessed on 14 July 2022).
- Ben Braïek, O.; Smaoui, S.; Wan, C. Chemistry, Safety, and Challenges of the Use of Organic Acids and Their Derivative Salts in Meat Preservation. J. Food Qual. 2021, 2021, 6653190. [Google Scholar] [CrossRef]
- Theron, M.M.; Lues, J.F.R. Organic Acids and Meat Preservation: A Review. Food Rev. Int. 2007, 23, 141–158. [Google Scholar] [CrossRef]
- Pirnia, M.; Shirani, K.; Tabatabaee Yazdi, F.; Moratazavi, S.A.; Mohebbi, M. Characterization of antioxidant active biopolymer bilayer film based on gelatin-frankincense incorporated with ascorbic acid and Hyssopus officinalis essential oil. Food Chem. X 2022, 14, 100300. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, G.M.; Sibaja, J.C.; Espitia, P.J.; Otoni, C.G. Antioxidant active packaging based on papaya edible films incorporated with Moringa oleifera and ascorbic acid for food preservation. Food Hydrocoll. 2020, 103, 105630. [Google Scholar] [CrossRef]
- Almasi, L.; Radi, M.; Amiri, S.; McClements, D.J. Fabrication and characterization of antimicrobial biopolymer films containing essential oil-loaded microemulsions or nanoemulsions. Food Hydrocoll. 2021, 117, 106733. [Google Scholar] [CrossRef]
- Sayanjali, S.; Ghanbarzadeh, B.; Ghiassifar, S. Evaluation of antimicrobial and physical properties of edible film based on carboxymethyl cellulose containing potassium sorbate on some mycotoxigenic Aspergillus species in fresh pistachios. LWT—Food Sci. Technol. 2011, 44, 1133–1138. [Google Scholar] [CrossRef]
- Brink, I.; Šipailienė, A.; Leskauskaitė, D. Antimicrobial properties of chitosan and whey protein films applied on fresh cut turkey pieces. Int. J. Biol. Macromol. 2019, 130, 810–817. [Google Scholar] [CrossRef] [PubMed]
- da Rocha, M.; Loiko, M.R.; Tondo, E.C.; Prentice, C. Physical, mechanical and antimicrobial properties of Argentine anchovy (Engraulis anchoita) protein films incorporated with organic acids. Food Hydrocoll. 2014, 37, 213–220. [Google Scholar] [CrossRef]
- Flores, S.; Haedo, A.S.; Campos, C.; Gerschenson, L. Antimicrobial performance of potassium sorbate supported in tapioca starch edible films. Eur. Food Res. Technol. 2006, 225, 375–384. [Google Scholar] [CrossRef]
- Mondal, D.; Bhowmick, B.; Maity, D.; Mollick, M.M.; Rana, D.; Rangarajan, V.; Sen, R.; Chattopadhyay, D. Investigation on sodium benzoate release from poly(butylene adipate-co-terephthalate)/organoclay/sodium benzoate based nanocomposite film and their antimicrobial activity. J. Food Sci. 2015, 80, E602–E609. [Google Scholar] [CrossRef] [PubMed]
- San, H.; Harnkarnsujarit, N. Sulfite incorporated thermoplastic cassava starch blended PBAT blown films as antimicrobial and antibrowning packaging. Ind. Crops Prod. 2023, 206, 117610. [Google Scholar] [CrossRef]
- Aznar, M.; Domeno, C.; Osorio, J.; Nerin, C. Release of volatile compounds from cooking plastic bags under different heating sources. Food Packag. Shelf Life 2020, 26, 100552. [Google Scholar] [CrossRef]
- Khan, M.I.; Jo, C.; Tariq, M.R. Meat flavor precursors and factors influencing flavor precursors—A systematic review. Meat Sci. 2015, 110, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Canellas, E.; Nerin, C. Screening of volatile decay markers of minced pork by headspace-solid phase microextraction-gas chromatography-mass spectrometry and chemometrics. Food Chem. 2021, 342, 128341. [Google Scholar] [CrossRef] [PubMed]
- Kerry, J.P.; Kerry, J.F.; Ledward, D.A. Meat Processing: Improving Quality; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Argyri, A.A.; Mallouchos, A.; Panagou, E.Z.; Nychas, G.J. The dynamics of the HS/SPME-GC/MS as a tool to assess the spoilage of minced beef stored under different packaging and temperature conditions. Int. J. Food Microbiol. 2015, 193, 51–58. [Google Scholar] [CrossRef]
- Rivas-Canedo, A.; Fernandez-Garcia, E.; Nunez, M. Volatile compounds in fresh meats subjected to high pressure processing: Effect of the packaging material. Meat Sci. 2009, 81, 321–328. [Google Scholar] [CrossRef]
- Rivas-Canedo, A.; Nunez, M.; Fernandez-Garcia, E. Volatile compounds in Spanish dry-fermented sausage s‘alchichon’ subjected to high pressure processing. Effect of the packaging material. Meat Sci. 2009, 83, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Wrona, M.; Nerín, C.; Alfonso, M.J.; Caballero, M.Á. Antioxidant packaging with encapsulated green tea for fresh minced meat. Innov. Food Sci. Emerg. Technol. 2017, 41, 307–313. [Google Scholar] [CrossRef]
- Calkins, C.R.; Hodgen, J.M. A fresh look at meat flavor. Meat Sci. 2007, 77, 63–80. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).









