Progress in Wound-Healing Products Based on Natural Compounds, Stem Cells, and MicroRNA-Based Biopolymers in the European, USA, and Asian Markets: Opportunities, Barriers, and Regulatory Issues
Abstract
1. Introduction
2. Wound-Healing Biopolymers, Based on Natural Compounds
2.1. Natural Compound-Based Regenerative Biopolymers for Wound Healing in Research
2.2. Natural Compound-Based Regenerative Biopolymers for Wound Healing in the Market
3. Stem Cell-Based Wound-Healing Biopolymers
3.1. Stem Cell-Based Regenerative Biopolymers for Wound Healing in Research
3.2. Stem Cell-Based Regenerative Biopolymers for Wound Healing in the Market
4. MicroRNA-Based Wound-Healing Biopolymers
4.1. MicroRNA-Based Regenerative Biopolymers for Wound Healing in Research
| Phase | miRNA | Function | Associated Wound Type | Reference |
|---|---|---|---|---|
| Inflammation | miR-23b | Reduces pro-inflammatory cytokines | Acute | [98] |
| Inflammation | miR-27b | Downregulation of miR-27b promotes fibroblast proliferation | Acute (burn) | [99] |
| Inflammation | miR-34a/b | Promotes production of chemokines and cytokines prolonging inflammation | Chronic (venous ulcers) | [100] |
| Inflammation | miR-146a | miR-146a deficiency is associated with enhanced inflammatory response in diabetic wounds | Diabetic foot ulcers | [101] |
| Inflammation | miR-203 | Inhibits proliferation and migration of keratinocytes | Diabetic foot ulcers | [102] |
| Inflammation | miR-223 | Enhances clearance of S. aureus through neutrophil activation | Acute (Bacterial infection) | [103] |
| Inflammation/Proliferation | miR-21 | Down-regulates PTEN/RECK and activates MAPK/ERK cascade, inhibiting inflammation | Acute | [104] |
| Inflammation/Proliferation | miR-31 | Enhances keratinocyte proliferation and migration | Acute | [105] |
| Inflammation/Proliferation | miR-125b | Interacts with TP53INP1 promoting cell migration and proliferation | Acute | [106] |
| Inflammation/Proliferation | miR-132 | Promotes endothelial cell proliferation, migration, and angiogenesis | Acute (burn) | [94] |
| Inflammation/Proliferation | miR-139-5p | Suppresses miR-139-5p expression enhancing neutrophil migration and proliferation in S. aureus wounds | Acute (Bacterial infection) | [96] |
| Inflammation/Proliferation | miR-126 | Promotes endothelial cell proliferation, migration, angiogenesis and inhibits apoptosis | Acute (burn) | [107] |
| Inflammation/Proliferation | miR-155 | Promotes keratinocyte migration and cellular proliferation | Acute | [108] |
| Proliferation | miR-99a/b | Suppresses keratinocyte migration and cellular proliferation | Acute (slow healing) | [109] |
| Remodeling | miR-29a/b/c | Represses extracellular matrix expression and fibroplasia, preventing fibrotic scars | Acute (scar prevention) | [110] |
| Remodeling | miR-192 | Enhances collagen expression targeting SMAD-interacting protein 1 (SIP1) | Acute | [111] |
4.2. MicroRNA-Based Regenerative Biopolymers for Wound Healing in the Market
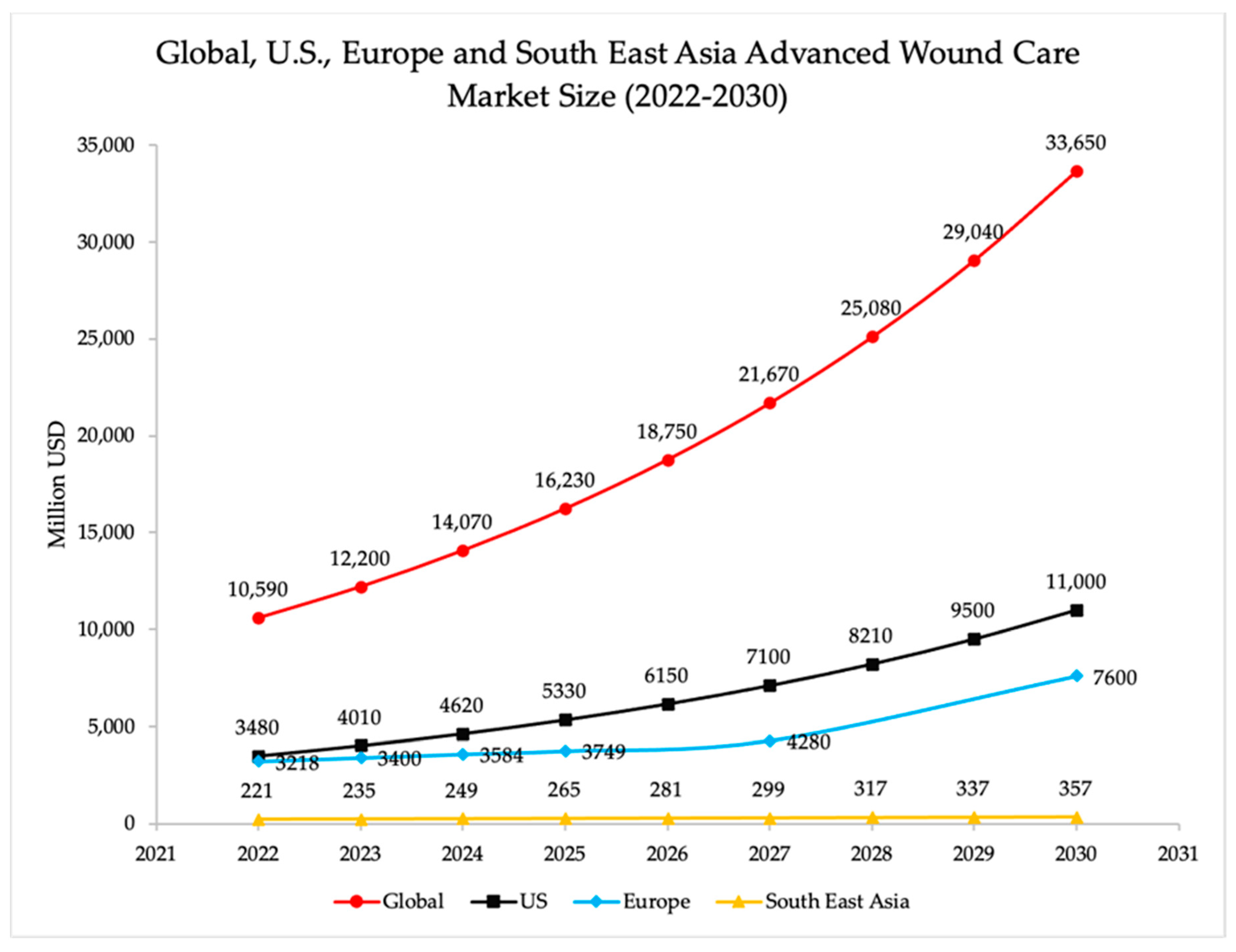
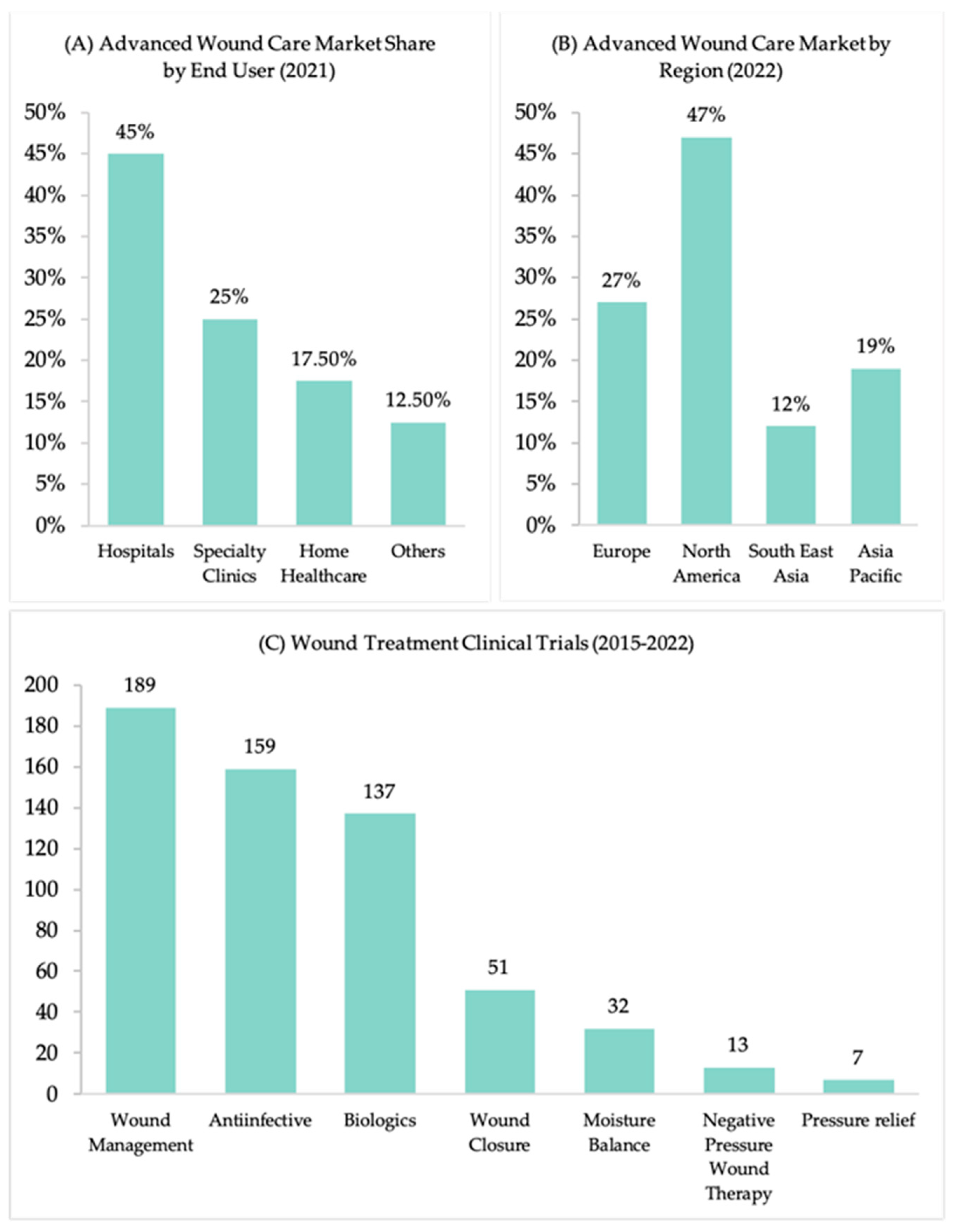
5. Market Growth of Wound-Healing Products
5.1. USA, Europe, and Asia’s Potential to Meet the Growing Demand for Biomaterials
| Forecasting Period (Years) | CAGR (%) | Reference Value (USD Billions) | Forecasted Value (USD Billions) | Reference |
|---|---|---|---|---|
| 2020–2027 | 12.2 | 110.0 | 245.6 | [117] |
| 2020–2027 | 15.2 | 109.4 | 390.9 | [120] |
| 2021–2030 | 12.7 | 65.0 | 212.4 | [118] |
| 2022–2030 | 15.4 | 135.4 | 488.7 | [116] |
| 2022–2030 | 12.2 | 121.4 | 343.7 | [119] |
5.2. Barriers in the Process to Meet the Growing Demand for Biomaterials
6. Global Regulatory Issues for Wound-Healing Products
6.1. USA Regulatory Issues
6.2. European Regulatory Issues
6.3. Asian Regulatory Issues
- Product Classification: Medical products are categorized according to their intended purpose, level of risk, and mechanism of action. Depending on its mechanism of action and intended usage, wound-healing products are often classed as Class III or IV medical devices. However, the classification system ranges from Class I (low risk) to Class IV (high-risk). Each class has specific regulatory requirements and evaluation processes.
- Premarket Approval: Before a wound-healing product may be sold in Asia, it must be authorized by each country’s regulatory agency. The approval procedure requires the submission of a dossier covering all pertinent information regarding the product, such as its safety, effectiveness, and quality.
- ○
- Clinical Trials and Evaluation: Depending on the risk classification, wound-healing products may be subject to clinical trials and evaluation to assess their safety and efficacy. Clinical data and evidence are required to demonstrate the product’s performance and benefits in promoting wound healing.
- ○
- Quality Management System: Compliance with quality management system requirements, such as Good Manufacturing Practice (GMP) and ISO certification, is necessary for wound-healing product manufacturers. These standards ensure consistent product quality and safety throughout the manufacturing process. Harmonization with international regulations and standards with global guidelines is necessary to facilitate international trade and ensure product quality and safety.
- Postmarket Surveillance: Once a wound-healing product has been approved and sold, the regulatory authority performs postmarket monitoring to ensure that the product continues to fulfill safety, effectiveness, and quality criteria. Adverse event reporting, postmarket studies, and periodic safety updates are required to identify and address any potential safety concerns.
- Labeling and Advertising: The labeling and advertising of wound-healing products must adhere to the norms and guidelines established by each country’s regulatory agency. The labeling and advertising must be precise, honest, and not deceptive.
7. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound Dressings—A Review. BioMedicine 2015, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound Dressings: Current Advances and Future Directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef]
- Heyer, K.; Augustin, M.; Protz, K.; Herberger, K.; Spehr, C.; Rustenbach, S.J. Effectiveness of Advanced versus Conventional Wound Dressings on Healing of Chronic Wounds: Systematic Review and Meta-Analysis. Dermatology 2013, 226, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Denny, K.; Lawand, C.; Perry, S. Compromised Wounds in Canada. Healthc. Q. 2014, 17, 7–10. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Ageing and Health. World Health Organization. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health#:~:text=At%20this%20time%20the%20share,2050%20to%20reach%20426%20million (accessed on 22 March 2023).
- Ageing Europe—Looking at the Lives of Older People in the EU—Eurostat 2019 Report. Available online: https://www.age-platform.eu/publications/ageing-europe-looking-lives-older-people-eu-eurostat-2019-report (accessed on 23 June 2023).
- Office for National Statistics. Overview of the UK Population; Office for National Statistics: London, UK. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/articles/overviewoftheukpopulation/august2019 (accessed on 29 March 2023).
- Mark, M.; Paola, S.; Lillian, K. Fact Sheet: Aging in the United States. Available online: https://www.prb.org/resources/fact-sheet-aging-in-the-united-states/ (accessed on 6 June 2023).
- He, W.; Gookind, D.; Kowal, P.; Almasarweh, W.I.S.; Giang, T.L.; Islam, M.M.; Lee, S.; Teerawichitchainan, B.; Tey, N.P. Asia Aging: Demographic, Economic, and Health Transitions; U.S. Department of Health and Human Services: Washington, DC, USA, 2022.
- Malaysia Attained Ageing Nation Status. Department of Statistics Malaysia Official Portal. Available online: https://www.dosm.gov.my/v1/index.php?r=column/cthemeByCat&cat=155&bul_id=OVByWjg5YkQ3MWFZRTN5bDJiaEVhZz09&menu_id=L0pheU43NWJwRWVSZklWdzQ4TlhUUT09 (accessed on 29 March 2023).
- Cross, K.; Harding, K. Risk Profiling in the Prevention and Treatment of Chronic Wounds Using Artificial Intelligence. Int. Wound J. 2022, 19, 1283–1285. [Google Scholar] [CrossRef] [PubMed]
- Guest, J.F.; Fuller, G.W.; Vowden, P. Cohort Study Evaluating the Burden of Wounds to the UK’s National Health Service in 2017/2018: Update from 2012/2013. BMJ Open 2020, 10, e045253. [Google Scholar] [CrossRef] [PubMed]
- Pokorná, A.; Leaper, D. Assessment and Documentation of Non-healing, Chronic Wounds in Inpatient Health Care Facilities in the Czech Republic: An Evaluation Study. Int. Wound J. 2014, 12, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Pragadheeswaran, M.; Sankar lingam, P.; Balan, Y.; Pyati, A.K. A Comparative Study Between Vacuum Dressing and Normal Saline Dressing for Chronic Non-Healing Ulcers. Cureus 2022, 14, e23870. [Google Scholar] [CrossRef]
- LinkedIn. Is Malaysian Market Ripe for Advanced Wound Care Products? Available online: https://www.linkedin.com/pulse/malaysian-market-ripe-advanced-wound-care-products-ariyanchira/ (accessed on 22 March 2023).
- Advanced Wound Care Market Size to Hit USD 45.33 Bn by 2032. Available online: https://www.precedenceresearch.com/advanced-wound-care-market (accessed on 22 March 2023).
- Advanced Wound Care Market Share, Growth. Forecast [2030]. Available online: https://www.fortunebusinessinsights.com/industry-reports/advanced-wound-care-market-100060 (accessed on 22 March 2023).
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef]
- South East Asia Market Study on Disposable Medical Supplies: Cardiovascular Procedures Account for Over a Quarter of Overall Demand. Available online: https://www.persistencemarketresearch.com/market-research/south-east-asia-disposable-medical-supplies-market.asp (accessed on 22 March 2023).
- Freedman, B.R.; Hwang, C.; Talbot, S.; Hibler, B.; Matoori, S.; Mooney, D.J. Breakthrough treatments for accelerated wound healing. Sci. Adv. 2023, 9, eade7007. [Google Scholar] [CrossRef] [PubMed]
- Wound Healing Market. Available online: https://www.transparencymarketresearch.com/wound-healing-market.html (accessed on 22 March 2023).
- Sahana, T.G.; Rekha, P.D. Biopolymers: Applications in Wound Healing and Skin Tissue Engineering. Mol. Biol. Rep. 2018, 45, 2857–2867. [Google Scholar] [CrossRef] [PubMed]
- Neha, R.; Radha, R.; Rakesh, P.; Madhu, G. Biopolymers and Treatment Strategies for Wound Healing: An Insight View. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 359–375. [Google Scholar] [CrossRef]
- Gardikiotis, I.; Cojocaru, F.-D.; Mihai, C.-T.; Balan, V.; Dodi, G. Borrowing the Features of Biopolymers for Emerging Wound Healing Dressings: A Review. Int. J. Mol. Sci. 2022, 23, 8778. [Google Scholar] [CrossRef] [PubMed]
- Postolović, K.; Ljujić, B.; Kovačević, M.M.; Đorđević, S.; Nikolić, S.; Živanović, S.; Stanić, Z. Optimization, Characterization, and Evaluation of Carrageenan/Alginate/Poloxamer/Curcumin Hydrogel Film as a Functional Wound Dressing Material. Mater. Today Commun. 2022, 31, 103528. [Google Scholar] [CrossRef]
- Diaz-Gomez, L.; Gonzalez-Prada, I.; Millan, R.; Da Silva-Candal, A.; Bugallo-Casal, A.; Campos, F.; Concheiro, A.; Alvarez-Lorenzo, C. 3D Printed Carboxymethyl Cellulose Scaffolds for Autologous Growth Factors Delivery in Wound Healing. Carbohydr. Polym. 2022, 278, 118924. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Wang, W.; Wang, M.-K.; Song, X.-S. Acceleration of Healing in Full-Thickness Wound by Chitosan-Binding BFGF and Antimicrobial Peptide Modification Chitosan Membrane. Front. Bioeng. Biotechnol. 2022, 10, 878588. [Google Scholar] [CrossRef] [PubMed]
- Mh Busra, F.; Rajab, N.F.; Tabata, Y.; Saim, A.B.; Idrus, R.B.H.; Chowdhury, S.R. Rapid Treatment of Full-Thickness Skin Loss Using Ovine Tendon Collagen Type I Scaffold with Skin Cells. J. Tissue Eng. Regen. Med. 2019, 13, 874–891. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Pandey, M.; Thu, H.E.; Kaur, T.; Jia, G.W.; Ying, P.C.; Xian, T.M.; Abourehab, M.A.S. Hyaluronic Acid Functionalization Improves Dermal Targeting of Polymeric Nanoparticles for Management of Burn Wounds: In Vitro, Ex Vivo and in Vivo Evaluations. Biomed. Pharmacother. 2022, 150, 112992. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, Y.; Shu, F.; Zhou, R.; Bao, B.; Xiao, S.; Li, K.; Lin, Q.; Zhu, L.; Xia, Z. In Situ-Formed Adhesive Hyaluronic Acid Hydrogel with Prolonged Amnion-Derived Conditioned Medium Release for Diabetic Wound Repair. Carbohydr. Polym. 2022, 276, 118752. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, L.; Wang, J.; Meng, Q.; Zhong, S.; Gao, Y.; Cui, X. Recent Advances in Polysaccharide-Based Self-Healing Hydrogels for Biomedical Applications. Carbohydr. Polym. 2022, 283, 119161. [Google Scholar] [CrossRef] [PubMed]
- Böhm, S.; Strauß, C.; Stoiber, S.; Kasper, C.; Charwat, V. Impact of Source and Manufacturing of Collagen Matrices on Fibroblast Cell Growth and Platelet Aggregation. Materials 2017, 10, 1086. [Google Scholar] [CrossRef]
- Busra, F.M.; Lokanathan, Y.; Nadzir, M.M.; Saim, A.; Idrus, R.B.H.; Chowdhury, S.R. Attachment, Proliferation, and Morphological Properties of Human Dermal Fibroblasts on Ovine Tendon Collagen Scaffolds: A Comparative Study. Malays. J. Med. Sci. 2017, 24, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Sultan, M.T.; Lee, O.J.; Kim, S.H.; Ju, H.W.; Park, C.H. Silk Fibroin in Wound Healing Process. Adv. Exp. Med. Biol. 2018, 1077, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Mao, Y.; Zhao, Z.; Zhang, J.; Zhu, L.; Chen, L.; Cao, L. Novel Fabrication of Antibiotic Containing Multifunctional Silk Fibroin Injectable Hydrogel Dressing to Enhance Bactericidal Action and Wound Healing Efficiency on Burn Wound: In Vitro and in Vivo Evaluations. Int. Wound J. 2022, 19, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Neamtu, B.; Barbu, A.; Negrea, M.O.; Berghea-Neamțu, C.Ș.; Popescu, D.; Zăhan, M.; Mireșan, V. Carrageenan-Based Compounds as Wound Healing Materials. Int. J. Mol. Sci. 2022, 23, 9117. [Google Scholar] [CrossRef] [PubMed]
- Algisite M Calcium Alginate Dressing. Medical Dressings. Available online: https://www.smith-nephew.com/es-es/health-care-professionals/products/advanced-wound-management/algisite-ppl (accessed on 18 March 2023).
- Suprasorb C Collagen Wound Dressing. Available online: https://www.lohmann-rauscher.com/en/products/wound-care/modern-wound-care/suprasorb-c/ (accessed on 18 March 2023).
- Kito Activator Chitosan Wound Healing Hydrogel Barrier. Available online: http://eng.endovision.co.kr/portfolio-item/kito-activator/ (accessed on 18 March 2023).
- Medihoney HoneyColloid Leptospermum Hydrocolloid Dressings. Available online: https://www.products.integralife.com/outpatient-clinic-private-office/category/wound-reconstruction-care-outpatient-clinic-private-office (accessed on 18 March 2023).
- Hyperoil. Hyperoil—For Any Wound, Any Time. Available online: https://hyperoil.com/ (accessed on 18 March 2023).
- Fibracol Plus Collagen Wound Dressing with Alginate. Available online: https://www.vitalitymedical.com/johnson-and-johnson-fibracol-plus-collagen-wound-dressing-with-alginate.html (accessed on 18 March 2023).
- ACTICOAT. Available online: https://www.smith-nephew.com/es-es/health-care-professionals/products/advanced-wound-management/acticoat-global#overview (accessed on 18 March 2023).
- 3MTM ActisorbTM Silver 220 Activated Charcoal Dressing with Silver. Available online: https://www.3m.co.za/3M/en_ZA/p/d/b5005265071/ (accessed on 18 March 2023).
- AmnioExcel®. Available online: https://www.integralife.com/es/amnioexcel-amniotic-allograft-membrane/product/wound-reconstruction-care-outpatient-clinic-private-office-treat-amnioexcel-amniotic-allograft-membrane (accessed on 18 March 2023).
- AQUACEL®/Hydrofiber®. Available online: https://www.convatec.com/es-es/productos/cuidado-avanzados-de-heridas/tipo-de-herida/pc-wound-burns/aquacel-extra/ (accessed on 18 March 2023).
- BIATAIN® Silicone. Available online: https://productos.coloplast.com.ar/coloplast/heridas/biatain-silicone/biatain-silicone/ (accessed on 18 March 2023).
- CalciCareTM Calcium Alginate Dressing. Calcium Alginate Dressings. Hollister US. Available online: https://www.hollister.com/en/products/wound-care-products/wound-dressings/calcium-alginate-dressings/calcicare-calcium-alginate-dressing# (accessed on 18 March 2023).
- Cutimed. Available online: https://medical.essity.de/marken/cutimed.html (accessed on 18 March 2023).
- Triage Meditech. Advanced Wound Dressings. Available online: https://www.triagemeditech.com/advance-wound-dressings (accessed on 18 March 2023).
- Granulox®. Available online: https://www.molnlycke.es/productos-soluciones/granulox/ (accessed on 18 March 2023).
- Kaltostat®. Available online: https://www.convatec.com/es-es/productos/cuidado-avanzados-de-heridas/tipo-de-herida/pc-wound-diabetic-foot-ulcers/b68c3a76-bd08-404d-a982-efeb45b1879d/ (accessed on 18 March 2023).
- Mepilex® Ag. Available online: https://www.molnlycke.es/productos-soluciones/mepilex-ag/ (accessed on 18 March 2023).
- NeutroPhase. Available online: https://novabay.com/products/neutrophase/ (accessed on 18 March 2023).
- Omnigraft. Available online: https://www.integralife.com/es/omnigraft-dermal-regeneration-matrix/product/wound-reconstruction-care-outpatient-clinic-private-office-treat-omnigraft-dermal-regeneration-matrix (accessed on 18 March 2023).
- PriMatrix® Dermal Repair Scaffold. Available online: https://www.integralife.com/primatrix-dermal-repair-scaffold/product/wound-reconstruction-care-inpatient-acute-or-primatrix-dermal-repair-scaffold (accessed on 18 March 2023).
- 3MTM Promogran PrismaTM Collagen Matrix with ORC and Silver. Available online: https://www.3m.com/3M/en_US/p/d/b5005265080/ (accessed on 18 March 2023).
- REGRANEX. Available online: https://www.smith-nephew.com/en-us/health-care-professionals/products/advanced-wound-management/regranex#productfeatures (accessed on 18 March 2023).
- Hollister US. RestoreTM Hydrocolloid Dressing with Foam Backing. Wound Dressings. Available online: https://www.hollister.com/en/products/wound-care-products/wound-dressings/hydrocolloid-dressings/restore-hydrocolloid-dressing-with-foam-backing (accessed on 18 March 2023).
- SILVERCELTM. Available online: https://www.acelity.com/healthcare-professionals/global-product-catalog/catalog/silvercel-dressing (accessed on 18 March 2023).
- V.A.C.® Therapy. Available online: https://www.acelity.com/healthcare-professionals/global-product-catalog/catalog/vac-freedom (accessed on 18 March 2023).
- Insticare VTG2901 Manufacturer & Supplier. Triage Meditech. Available online: https://www.triagemeditech.com/vtg-2901-new-advanced-solution (accessed on 18 March 2023).
- Ayavoo, T.; Murugesan, K.; Gnanasekaran, A. Roles and Mechanisms of Stem Cell in Wound Healing. Stem Cell Investig. 2021, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, G.K.; Rodriguez-Crespo, D.; Fernandez-Bueno, I.; Pastor, J.C. Factors Influencing Mesenchymal Stromal Cells in in Vitro Cellular Models to Study Retinal Pigment Epithelial Cell Rescue. Hum. Cell 2022, 35, 1005–1015. [Google Scholar] [CrossRef]
- Di Lauro, S.; Garcia-Gutierrez, M.T.; Fernandez-Bueno, I. Quantification of Pigment Epithelium-Derived Factor (PEDF) in an Ex Vivo Coculture of Retinal Pigment Epithelium Cells and Neuroretina. J. Allbiosolution 2020, 2, 81. [Google Scholar]
- Alonso-Alonso, M.L.; Srivastava, G.K.; Usategui-Martín, R.; García-Gutierrez, M.T.; Pastor, J.C.; Fernandez-Bueno, I. Mesenchymal Stem Cell Secretome Enhancement by Nicotinamide and Vasoactive Intestinal Peptide: A New Therapeutic Approach for Retinal Degenerative Diseases. Stem Cells Int. 2020, 2020, 9463548. [Google Scholar] [CrossRef]
- Trzyna, A.; Banaś-Ząbczyk, A. Adipose-Derived Stem Cells Secretome and Its Potential Application in “Stem Cell-Free Therapy”. Biomolecules 2021, 11, 878. [Google Scholar] [CrossRef] [PubMed]
- Gorecka, J.; Kostiuk, V.; Fereydooni, A.; Gonzalez, L.; Luo, J.; Dash, B.; Isaji, T.; Ono, S.; Liu, S.; Lee, S.R.; et al. The Potential and Limitations of Induced Pluripotent Stem Cells to Achieve Wound Healing. Stem Cell Res. Ther. 2019, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Paik, D.T.; Chandy, M.; Wu, J.C. Patient and Disease–Specific Induced Pluripotent Stem Cells for Discovery of Personalized Cardiovascular Drugs and Therapeutics. Pharmacol. Rev. 2020, 72, 320–342. [Google Scholar] [CrossRef] [PubMed]
- Nourian Dehkordi, A.; Mirahmadi Babaheydari, F.; Chehelgerdi, M.; Raeisi Dehkordi, S. Skin Tissue Engineering: Wound Healing Based on Stem-Cell-Based Therapeutic Strategies. Stem Cell Res. Ther. 2019, 10, 111. [Google Scholar] [CrossRef] [PubMed]
- Maarof, M.; Mohd Nadzir, M.; Sin Mun, L.; Fauzi, M.B.; Chowdhury, S.R.; Idrus, R.B.H.; Lokanathan, Y. Hybrid Collagen Hydrogel/Chondroitin-4-Sulphate Fortified with Dermal Fibroblast Conditioned Medium for Skin Therapeutic Application. Polymers 2021, 13, 508. [Google Scholar] [CrossRef]
- Ling-Chun, C.; Shyr-Yi, L.; Ming-Thau, S.; Ching-Hua, S.; Hong-Liang, L.; Chien-Ming, H. Fabrication and Characterization of Rhizochitosan and Its Incorporation with Platelet Concentrates to Promote Wound Healing. Carbohydr. Polym. 2021, 268, 118239. [Google Scholar] [CrossRef]
- Md Fadilah, N.I.; Mohd Abdul Kader Jailani, M.S.; Badrul Hisham, M.A.I.; Sunthar Raj, N.; Shamsuddin, S.A.; Ng, M.H.; Fauzi, M.B.; Maarof, M. Cell Secretomes for Wound Healing and Tissue Regeneration: Next Generation Acellular Based Tissue Engineered Products. J. Tissue Eng. 2022, 13, 20417314221114273. [Google Scholar] [CrossRef] [PubMed]
- Catanzano, O.; Quaglia, F.; Boateng, J.S. Wound Dressings as Growth Factor Delivery Platforms for Chronic Wound Healing. Expert Opin. Drug Deliv. 2021, 18, 737–759. [Google Scholar] [CrossRef]
- Mashiko, T.; Takada, H.; Wu, S.-H.; Kanayama, K.; Feng, J.; Tashiro, K.; Asahi, R.; Sunaga, A.; Hoshi, K.; Kurisaki, A.; et al. Therapeutic Effects of a Recombinant Human Collagen Peptide Bioscaffold with Human Adipose-Derived Stem Cells on Impaired Wound Healing after Radiotherapy. J. Tissue Eng. Regen. Med. 2018, 12, 1186–1194. [Google Scholar] [CrossRef]
- Sezer, A.D.; Cevher, E.; Sezer, A.D.; Cevher, E. Biopolymers as Wound Healing Materials: Challenges and New Strategies; IntechOpen: Rijeka, Croatia, 2011; ISBN 978-953-307-661-4. [Google Scholar]
- Growth Factors in Wound Healing—A Review. Available online: https://biomedpharmajournal.org/vol14no3/growth-factors-in-wound-healing-a-review/ (accessed on 18 March 2023).
- Aguiar Koga, B.A.; Fernandes, L.A.; Fratini, P.; Sogayar, M.C.; Carreira, A.C.O. Role of MSC-derived Small Extracellular Vesicles in Tissue Repair and Regeneration. Front. Cell Dev. Biol. 2023, 10, 1047094. [Google Scholar] [CrossRef]
- RHEACELL >< Technology 2023. Available online: https://www.rheacell.com/ (accessed on 18 March 2023).
- Allo-APZ2. 2019. Available online: https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu3192160 (accessed on 18 March 2023).
- Home of TruStem Cell Therapy—Top U.S. Stem Cell Therapy & Treatment Center TruStem Cell TherapyTM. 2023. Available online: https://trustemcell.com/ (accessed on 18 March 2023).
- Xintela XSTEM. 2022. Available online: https://www.xintela.se/en/press-release?slug=xintela-starts-clinical-study-of-xstem-r-in-knee-osteoarthritis (accessed on 18 March 2023).
- Avita Medical. A Feasibility Study of the ReGenerCellTM Autologous Cell Harvesting Device for Diabetic Foot Ulcer. Available online: https://clinicaltrials.gov/study/NCT02799121 (accessed on 18 March 2023).
- Kimmel, H.; Gittleman, H. Retrospective Observational Analysis of the Use of an Architecturally Unique Dermal Regeneration Template (Derma Pure®) for the Treatment of Hard-to-Heal Wounds. Int. Wound J. 2017, 14, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Lavery, L.A.; Fulmer, J.; Shebetka, K.A.; Regulski, M.; Vayser, D.; Fried, D.; Kashefsky, H.; Owings, T.M.; Nadarajah, J.; Grafix Diabetic Foot Ulcer Study Group. The Efficacy and Safety of Grafix® for the Treatment of Chronic Diabetic Foot Ulcers: Results of a Multi-Centre, Controlled, Randomised, Blinded, Clinical Trial. Int. Wound J. 2014, 11, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cañada, C.; Bernabé-García, Á.; Liarte, S.; Rodríguez-Valiente, M.; Nicolás, F.J. Chronic Wound Healing by Amniotic Membrane: TGF-β and EGF Signaling Modulation in Re-Epithelialization. Front. Bioeng. Biotechnol. 2021, 9, 689328. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Meng, Z.; Zhou, D.; Gao, Y.; Zeng, M.; Wang, W. MiRNA Delivery for Skin Wound Healing. Adv. Drug Deliv. Rev. 2018, 129, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Weiss, C.N.; Ito, K. A Macro View of MicroRNAs: The Discovery of MicroRNAs and Their Role in Hematopoiesis and Hematologic Disease. Int. Rev. Cell Mol. Biol. 2017, 334, 99–175. [Google Scholar] [CrossRef]
- Banerjee, J.; Sen, C.K. MicroRNA and Wound Healing. Adv. Exp. Med. Biol. 2015, 888, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Shitiz, K.; Singh, A. Chitin and Chitosan: Biopolymers for Wound Management. Int. Wound J. 2017, 14, 1276–1289. [Google Scholar] [CrossRef]
- Ramamoorth, M.; Narvekar, A. Non Viral Vectors in Gene Therapy—An Overview. J. Clin. Diagn. Res. 2015, 9, GE01–GE06. [Google Scholar] [CrossRef]
- Kolimi, P.; Narala, S.; Nyavanandi, D.; Youssef, A.A.A.; Dudhipala, N. Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements. Cells 2022, 11, 2439. [Google Scholar] [CrossRef]
- Zeng, X.; Chen, B.; Wang, L.; Sun, Y.; Jin, Z.; Liu, X.; Ouyang, L.; Liao, Y. Chitosan@Puerarin Hydrogel for Accelerated Wound Healing in Diabetic Subjects by MiR-29ab1 Mediated Inflammatory Axis Suppression. Bioact. Mater. 2023, 19, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Qu, X.; Zhu, Z.; Wang, L.; Qi, Q.; Zhou, P.; Wang, X.; Li, W. Inhibition of MiR-139-5p by Topical JTXK Gel Promotes Healing of Staphylococcus Aureus-Infected Skin Wounds. Cells Dev. 2021, 166, 203658. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, L.; Toma, M.A.; Li, D.; Bian, X.; Pastar, I.; Tomic-Canic, M.; Sommar, P.; Xu Landén, N. Integrative Small and Long RNA Omics Analysis of Human Healing and Nonhealing Wounds Discovers Cooperating MicroRNAs as Therapeutic Targets. eLife 2022, 11, e80322. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Han, X.; Zuo, K.; Li, L.; Liu, J.; Yuan, X.; Shen, Y.; Shao, M.; Pang, D.; Chu, Y.; et al. MiR-23b Promotes Cutaneous Wound Healing through Inhibition of the Inflammatory Responses by Targeting ASK1. Acta Biochim. Biophys. Sin. 2018, 50, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Bi, Q.; Liu, J.; Wang, X.; Sun, F. Downregulation of MiR-27b Promotes Skin Wound Healing in a Rat Model of Scald Burn by Promoting Fibroblast Proliferation. Exp. Ther. Med. 2020, 20, 63. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, X.; Li, D.; Ren, X.; Li, Y.; Herter, E.K.; Qian, M.; Toma, M.-A.; Wintler, A.-M.; Sérézal, I.G.; et al. MicroRNA-34 Family Enhances Wound Inflammation by Targeting LGR4. J. Investig. Dermatol. 2020, 140, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Zhou, L.; Liu, Y.; Gu, J.; Mi, Q.-S. MicroRNA-146a Deficiency Delays Wound Healing in Normal and Diabetic Mice. Adv. Wound Care 2022, 11, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, R.; Jia, Z.; Li, X.; Tang, Y.; Zhao, X.; Zhang, S.; Luo, L.; Fang, Z.; Zhang, Y.; et al. Downregulation of Hsa-MiR-203 in Peripheral Blood and Wound Margin Tissue by Negative Pressure Wound Therapy Contributes to Wound Healing of Diabetic Foot Ulcers. Microvasc. Res. 2022, 139, 104275. [Google Scholar] [CrossRef] [PubMed]
- De Kerckhove, M.; Tanaka, K.; Umehara, T.; Okamoto, M.; Kanematsu, S.; Hayashi, H.; Yano, H.; Nishiura, S.; Tooyama, S.; Matsubayashi, Y.; et al. Targeting MiR-223 in Neutrophils Enhances the Clearance of Staphylococcus aureus in Infected Wounds. EMBO Mol. Med. 2018, 10, e9024. [Google Scholar] [CrossRef]
- Xie, J.; Wu, W.; Zheng, L.; Lin, X.; Tai, Y.; Wang, Y.; Wang, L. Roles of MicroRNA-21 in Skin Wound Healing: A Comprehensive Review. Front. Pharmacol. 2022, 13, 828627. [Google Scholar] [CrossRef]
- Li, D.; Li, X.I.; Wang, A.; Meisgen, F.; Pivarcsi, A.; Sonkoly, E.; Ståhle, M.; Landén, N.X. MicroRNA-31 Promotes Skin Wound Healing by Enhancing Keratinocyte Proliferation and Migration. J. Investig. Dermatol. 2015, 135, 1676–1685. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Wang, T.; Wang, Z.-X.; Huang, K.-P.; Zhang, Y.-W.; Wang, G.-L.; Zhang, H.-J.; Chen, Z.-H.; Wang, C.-Y.; Zhang, J.-X.; et al. Hypoxic UcMSC-Secreted Exosomal MiR-125b Promotes Endothelial Cell Survival and Migration during Wound Healing by Targeting TP53INP1. Mol. Ther.—Nucleic Acids 2021, 26, 347–359. [Google Scholar] [CrossRef]
- Jiang, B.; Tang, Y.; Wang, H.; Chen, C.; Yu, W.; Sun, H.; Duan, M.; Lin, X.; Liang, P. Down-Regulation of Long Non-Coding RNA HOTAIR Promotes Angiogenesis via Regulating MiR-126/SCEL Pathways in Burn Wound Healing. Cell Death Dis. 2020, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zheng, Z.; Zhou, Q.; Bai, X.; Fan, L.; Yang, C.; Su, L.; Hu, D. MiR-155 Promotes Cutaneous Wound Healing through Enhanced Keratinocytes Migration by MMP-2. J. Mol. Histol. 2017, 48, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Tymen, S.D.; Chen, D.; Fang, Z.J.; Zhao, Y.; Dragas, D.; Dai, Y.; Marucha, P.T.; Zhou, X. MicroRNA-99 Family Targets AKT/MTOR Signaling Pathway in Dermal Wound Healing. PLoS ONE 2013, 8, e64434. [Google Scholar] [CrossRef]
- Gallant-Behm, C.L.; Piper, J.; Lynch, J.M.; Seto, A.G.; Hong, S.J.; Mustoe, T.A.; Maari, C.; Pestano, L.A.; Dalby, C.M.; Jackson, A.L.; et al. A MicroRNA-29 Mimic (Remlarsen) Represses Extracellular Matrix Expression and Fibroplasia in the Skin. J. Investig. Dermatol. 2019, 139, 1073–1081. [Google Scholar] [CrossRef]
- Mulholland, E.J.; Dunne, N.; McCarthy, H.O. MicroRNA as Therapeutic Targets for Chronic Wound Healing. Mol. Ther. Nucleic Acids 2017, 8, 46–55. [Google Scholar] [CrossRef]
- Hanna, J.; Hossain, G.S.; Kocerha, J. The Potential for MicroRNA Therapeutics and Clinical Research. Front. Genet. 2019, 10, 478. [Google Scholar] [CrossRef] [PubMed]
- Zomer, H.D.; Trentin, A.G. Skin Wound Healing in Humans and Mice: Challenges in Translational Research. J. Dermatol. Sci. 2018, 90, 3–12. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.-S. Therapeutic Advances of MiRNAs: A Preclinical and Clinical Update. J. Adv. Res. 2021, 28, 127–138. [Google Scholar] [CrossRef]
- Hoversten, K.P.; Kiemele, L.J.; Stolp, A.M.; Takahashi, P.Y.; Verdoorn, B.P. Prevention, Diagnosis, and Management of Chronic Wounds in Older Adults. Mayo Clin. Proc. 2020, 95, 2021–2034. [Google Scholar] [CrossRef] [PubMed]
- Global Biomaterials Market Size & Share Report, 2022–2030. 2021. Available online: https://www.grandviewresearch.com/industry-analysis/biomaterials-industry (accessed on 18 March 2023).
- Biomaterials Market Size, Share and Industry Analysis, by Material (Metallic, Ceramic, Polymers, and Natural), by Application (Cardiovascular, Dental, Orthopedic, Plastic Surgery, Urology, Gastroenterology, and Others), and Regional Forecast, 2020–2027. Biomaterials Market Size, Growth | Global Industry Analysis, 2017. Available online: https://www.fortunebusinessinsights.com/biomaterials-market-102770 (accessed on 18 March 2023).
- Allied Market Research. Biomaterials Market Size: Key Analysis: Forecast—2030. Available online: https://www.alliedmarketresearch.com/biomaterials-market (accessed on 18 March 2023).
- Biomaterials Market. Biomaterials Market Register a Revenue CAGR of 12.2%. 2022. Available online: https://www.reportsanddata.com/report-detail/biomaterials-market (accessed on 18 March 2023).
- Precedence Research. Biomaterials Market Size, Share, and Growth Analysis Report by Product (Metallic, Ceramics, Natural, and Polymers) by Application (Ophthalmology, Cardiovascular, Dental, Wound Healing, Orthopedic, Plastic Surgery, Tissue Engineering, Neurology, and Others)—Global Industry Analysis, Trends, Regional Outlook and Forecasts 2020–2027. Available online: https://www.precedenceresearch.com/biomaterials-market#:~:text=As%20per%20Precedence%20Research%2C%20the,USD%20390.92%20billion%20by%202027 (accessed on 18 March 2023).
- North America Biomaterials Market Analysis: 2022 to 2027. (2022). Market Data Forecast. Available online: https://www.marketdataforecast.com/market-reports/north-america-biomaterials-market (accessed on 18 March 2023).
- EMA. Medical Devices. European Medicine Agency. 2018. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/medical-devices (accessed on 18 March 2023).
- Fergal, D. European Perspectives on Biomaterials for Health. Eur. Wound Manag. Assoc. J. 2015, 15, 54–58. [Google Scholar]
- APAC Biomaterials Market. Available online: http://www.marketdataforecast.com/ (accessed on 22 March 2023).
- Rômulo RN, A.; Ierecê ML, R. Biodiversity, Traditional Medicine and Public Health: Where Do They Meet? J. Ethnobiol. Ethnomed. 2007, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound Healing and the Use of Medicinal Plants. Evid.-Based Complement. Altern. Med. 2019, 2019, 2684108. [Google Scholar] [CrossRef] [PubMed]
- ASEAN. ASEAN Investment Report 2020–2021; ASEAN: Jakarta, Indonesia, 2021. [Google Scholar]
- Worldometer. Life expectancy of the World Population. Available online: https://www.worldometers.info/demographics/life-expectancy/ (accessed on 22 March 2023).
- Uludağ, H. Grand Challenges in Biomaterials. Front. Bioeng. Biotechnol. 2014, 2, 43. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. Challenges with the Development of Biomaterials for Sustainable Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M. Chapter 12—Standardization and Regulation of Biomaterials. In Handbook of Biomaterials Biocompatibility; Mozafari, M., Ed.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Cambridge, UK, 2020; pp. 251–265. ISBN 978-0-08-102967-1. [Google Scholar]
- Ma, C.; Kuzma, M.L.; Bai, X.; Yang, J. Biomaterial-Based Metabolic Regulation in Regenerative Engineering. Adv. Sci. 2019, 6, 1900819. [Google Scholar] [CrossRef] [PubMed]
- CDC Overweight & Obesity. Available online: https://www.cdc.gov/obesity/index.html (accessed on 22 March 2023).
- Diabetes Quick Facts | Basics | Diabetes | CDC. Available online: https://www.cdc.gov/diabetes/basics/quick-facts.html (accessed on 22 March 2023).
- Zainuddin, L.R.M.; Isa, N.; Muda, W.M.W.; Mohamed, H.J. The Prevalence of Metabolic Syndrome According to Various Definitions and Hypertriglyceridemic-Waist in Malaysian Adults. Int. J. Prev. Med. 2011, 2, 229–237. [Google Scholar] [PubMed]
- Ranasinghe, P.; Mathangasinghe, Y.; Jayawardena, R.; Hills, A.P.; Misra, A. Prevalence and Trends of Metabolic Syndrome among Adults in the Asia-Pacific Region: A Systematic Review. BMC Public Health 2017, 17, 101. [Google Scholar] [CrossRef]
- Januschowski, K.; Irigoyen, C.; Pastor, J.C.; Srivastava, G.K.; Romano, M.R.; Heimann, H.; Stalmans, P.; Van Keer, K.; Boden, K.; Szurman, P.; et al. Retinal Toxicity of Medical Devices Used during Vitreoretinal Surgery: A Critical Overview. Ophthalmologica 2018, 240, 236–243. [Google Scholar] [CrossRef]
- Kalaiselvan, V.; Shubhang, A.; Rajeev Singh, R. A Systematic Review on Research Documentation of Ocular Medical Device Focused on Perfluorocarbon Liquid (PFCL). J. Allbiosolution 2023, 5, 221. [Google Scholar]
- Iglesias-Lopez, C.; Agustí, A.; Obach, M.; Vallano, A. Regulatory Framework for Advanced Therapy Medicinal Products in Europe and United States. Front. Pharmacol. 2019, 10, 471441. [Google Scholar] [CrossRef] [PubMed]
- Jarow, J.P.; Baxley, J.H. Medical Devices: US Medical Device Regulation. Urol. Oncol. 2015, 33, 128–132. [Google Scholar] [CrossRef]
- Sweet, B.V.; Schwemm, A.K.; Parsons, D.M. Review of the Processes for FDA Oversight of Drugs, Medical Devices, and Combination Products. J. Manag. Care Pharm. JMCP 2011, 17, 40–50. [Google Scholar] [CrossRef]
- Bliznakov, Z.; Mitalas, G.; Pallikarakis, N. Analysis and Classification of Medical Device Recalls. In Proceedings of the World Congress on Medical Physics and Biomedical Engineering 2006, Seoul, Republic of Korea, 27 August–1 September 2006; Magjarevic, R., Nagel, J.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 3782–3785. [Google Scholar]
- Oberweis, C.V.; Marchal, J.A.; López-Ruiz, E.; Gálvez-Martín, P. A Worldwide Overview of Regulatory Frameworks for Tissue-Based Products. Tissue Eng. Part B. Rev. 2020, 26, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Kramer, D.B.; Xu, S.; Kesselheim, A.S. Regulation of Medical Devices in the United States and European Union. N. Engl. J. Med. 2012, 366, 848–855. [Google Scholar] [CrossRef]
- Lisa, M.S.; Steven, W. Medical Marketing in the United States, 1997–2016. JAMA 2019, 321, 80–96. [Google Scholar] [CrossRef]
- Radley-Gardner, O.; Beale, H.; Zimmerman, R. (Eds.) Fundamental Texts on European Private Law; Hart Publishing: Portland, OR, USA, 2016; ISBN 978-1-78225-864-3/978-1-78225-865-0/978-1-78225-866-7/978-1-78225-867-4. [Google Scholar]
- Yonesi, M.; Garcia-Nieto, M.; Guinea, G.V.; Panetsos, F.; Pérez-Rigueiro, J.; González-Nieto, D. Silk Fibroin: An Ancient Material for Repairing the Injured Nervous System. Pharmaceutics 2021, 13, 429. [Google Scholar] [CrossRef] [PubMed]
- Yonesi, M.; Ramos, M.; Ramirez-Castillejo, C.; Fernández-Serra, R.; Panetsos, F.; Belarra, A.; Chevalier, M.; Rojo, F.J.; Pérez-Rigueiro, J.; Guinea, G.V.; et al. Resistance to Degradation of Silk Fibroin Hydrogels Exposed to Neuroinflammatory Environments. Polymers 2023, 15, 2491. [Google Scholar] [CrossRef]
- Martín-Martín, Y.; Fernández-García, L.; Sanchez-Rebato, M.H.; Marí-Buyé, N.; Rojo, F.J.; Pérez-Rigueiro, J.; Ramos, M.; Guinea, G.V.; Panetsos, F.; González-Nieto, D. Evaluation of Neurosecretome from Mesenchymal Stem Cells Encapsulated in Silk Fibroin Hydrogels. Sci. Rep. 2019, 9, 8801. [Google Scholar] [CrossRef]
- Jakovljevic, M.; Wu, W.; Merrick, J.; Cerda, A.; Varjacic, M.; Sugahara, T. Asian Innovation in Pharmaceutical and Medical Device Industry—Beyond Tomorrow. J. Med. Econ. 2021, 24, 42–50. [Google Scholar] [CrossRef] [PubMed]
- George, B. Registration of Medical Devices. Perspect. Clin. Res. 2010, 1, 90–93. [Google Scholar] [PubMed]
- Mohit; Deep, A.; Khurana, G.; Kumar, J.; Monga, A. Comparison of Regulatory Requirements for Registration of Pharmaceutical Drugs in Asean and GCC Regions. Appl. Clin. Res. Clin. Trials Regul. Aff. 2019, 6, 62–70. [Google Scholar]
- Aprile, P.; Letourneur, D.; Simon-Yarza, T. Membranes for Guided Bone Regeneration: A Road from Bench to Bedside. Adv. Healthc. Mater. 2020, 9, 2000707. [Google Scholar] [CrossRef] [PubMed]
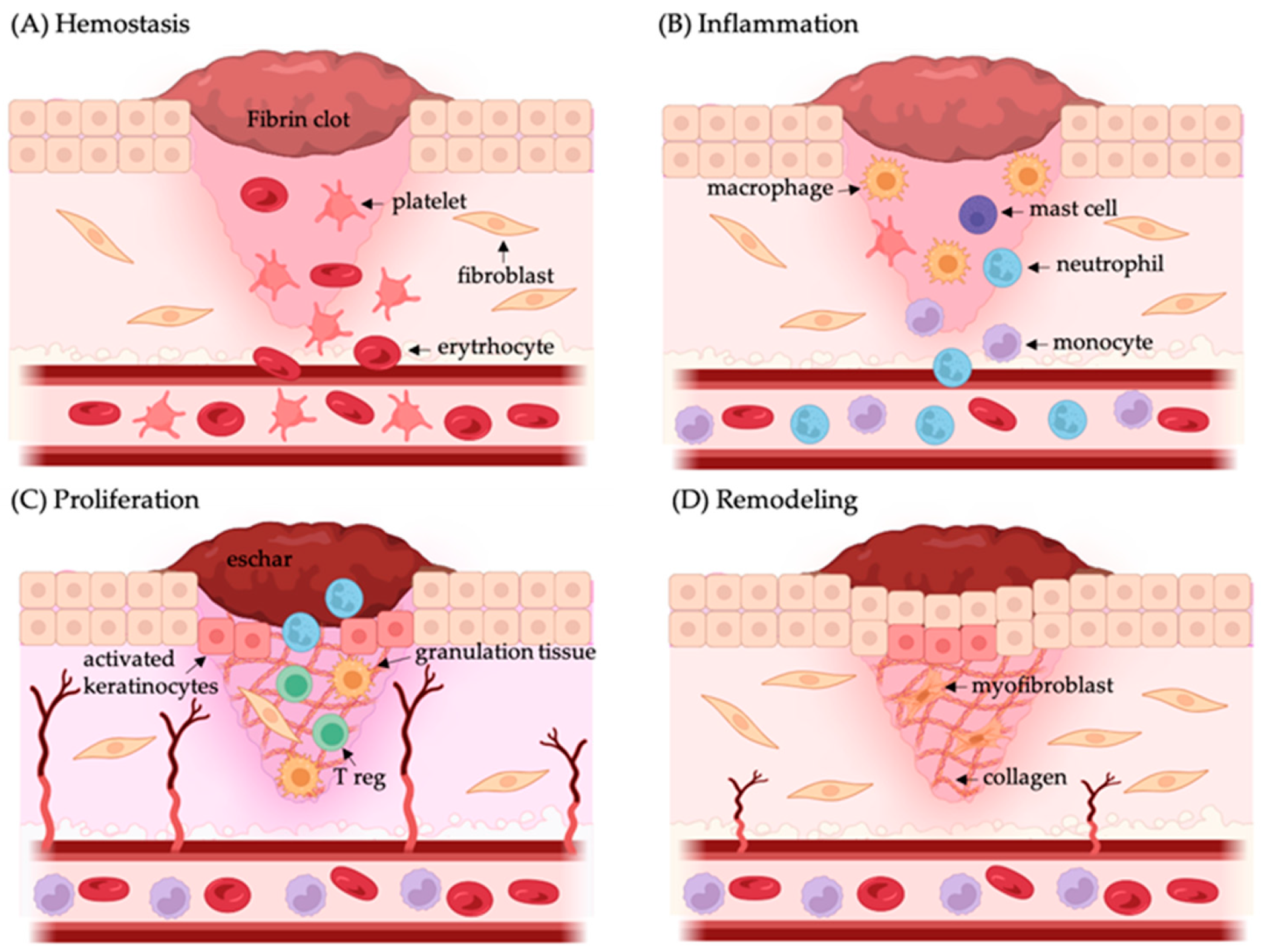
| Biopolymer | Components | Sources | Benefits | Limitations | Study | Reference |
|---|---|---|---|---|---|---|
| Alginate | β-D-mannuronic acid and α-ʟ-guluronic acid linked by α-1,4 glycosidic linkages | Brown algae | Promote wound healing by activating macrophages to produce cytokines. High absorbance | High viscosity, nonhomogeneous, and nontransparent formulations | in vitro in vivo | [26] |
| Carboxymethyl cellulose | β-D-Glucose linked by β-1,4-glycosidic linkage | Modified from wood and cotton | Exudate absorbing capacity Retain the moisture | Weak antibacterial and antimicrobial properties, low mechanical strength | in vitro in vivo | [27] |
| Chitosan | N-acetyl glucosamine linked by β-1,4 glycosidic linkages | Shrimp and crabs | Antimicrobial, antibacterial, analgesic, hemostatic. Promotes neovascularization and dermis regeneration | Limited ability to certain antibacterial | in vitro in vivo | [28] |
| Collagen | Amino acid linked by amide linkage | Goat and ovine (sheep) | Enrichment of new collagen deposition Hemostatic ability. Control of proteolytic activity | Collagen of porcine and bovine sources. Risk of transmitting diseases, e.g., bovine spongiform encephalopathy (BSE), caused by prions | in vitro in vivo | [29] |
| Hyaluronic acid | D-glucuronic acid and N-acetyl-D-glu-cosamine linked by β-1,4 and β-1,3 glycosidic linkages | Bovine vitreous humor | Exudate absorption capacity. Anti-inflammatory. Induce cell adhesion | Weak mechanical properties, poor adhesion, and rapid degradation | in vitro in vivo ex vivo | [30,31] |
| Products | Components | Benefits | Limitations | Company | Reference |
|---|---|---|---|---|---|
| ACTICOAT | Dressings with nanocrystalline silver technology | Sustained silver release into the wound exudate to help promote and retain a moist environment, when used with an appropriate secondary dressing. Bactericide. | Antimicrobial properties but helps the healing/healing process. It only prevents infections. | Smith & Nephew (UK) | [44] |
| Actisorb™ | Activated carbon bandage with silver. | Activated carbon traps odor in the dressing and traps bacteria and toxins that impair the healing process. Bactericide. | Limited regenerative effects. | 3M (USA) | [45] |
| Algisite M Calcium Alginate Dressing | Alginate Calcium | Fast gelling, high mannuronic acid fibers. Low fiber shed construction; it conforms to wound contours. It moisturizes wound environment, highly absorbent, biodegradable. | Calcium divalent cations can be released and exchange with monovalent cations in the surrounding media and dissolve the alginate gel. | Smith & Nephew (UK) | [38] |
| AmnioExcel® | Human placental amniotic fluid membrane. | Wound protection provided extracellular matrix proteins, growth factors and cytokines, which provides structural tissue and an environment for soft tissue reconstruction and regeneration. | Compositional differences between batches from different donors. Moderate effects, especially in chronic wounds. | Derma Sciences, Inc (USA) | [46] |
| AQUACEL®/Hydrofiber® | Sodium carboxymethyl-cellulose and regenerated cellulose fiber. | Adaptable and highly absorbent. In contact with the exudate, it creates a soft gel, maintaining a moist environment that facilitates progress in the healing process and autolytic debridement. It can be inserted into cavities or place on superficial lesions. | Bacteriostatic only. Healing improvement. Limited to simple and small wounds. | ConvaTec Group Plc (UK) | [47] |
| BIATAIN | Foam dressing with delicate silicone adhesive | Exudate absorption, even under compression. The 3D foam structure absorbs exudate, maintaining a moist environment. | Moderate effects especially on chronic and complex wounds. | Coloplast A/S (Denmark) | [48] |
| CalciCare™ | Alginate, Calcium and Guluronic Acid and Silver Bandage | Absorbent. Hemostatic properties may assist in supporting the control of minor bleeding in superficial wounds. It helps maintaining a moist environment. Aids autolytic debridement. | Moderate effects especially on chronic and complex wounds. | Hollister Incorporated (USA) | [49] |
| Cutimed® Epiona featuring 3D Matrix | Collagen and calcium alginate structure bandage | It can be easily molded to the surface of the wound. It does not contain chemical crosslinkers. | Recreates new extracellular matrix for regeneration: No regenerative cells or factors. Modest results. | Bsn Medical GmbH (Germany) | [50] |
| FD3101 (Wound Dressing) | Polyurethane foam and silver | High absorbency. Protection against water and bacteria. Nonadherent to the wound, no pain while removing the dressing. | Bacteriostatic only. It does not regenerate chronic skin wounds. | Triage Meditech Pvt (India) | [51] |
| Fibracol Plus Collagen Wound Dressing with Alginate | 90% collagen. 10% calcium alginate | Can be cut to fit any size wound. Nonadherent and easily removable. Biodegradable. Low immunogenicity, noncarcinogenic, collagen synthesis and reepithelization, promotes cell proliferation, provides support for cell attachments. During granulation and at the beginning of the epithelization, it supports collagen fibrils and fibers’ formation. | Ca2+ divalent cations can be released and exchange with other monovalent cations in the surrounding media, resulting in the dissolving of the alginate gel | Johnson & Johnson (USA) | [43] |
| Granulox® | Purified hemoglobin | Highly purified hemoglobin takes oxygen molecules from the environment. The hemoglobin is distributed by the exudate of the wound and helps its healing. | Indicated for diabetic leg ulcers and venous ulcers. Moderate results. | Mölnlycke Health Care AB (Sweden) | [52] |
| HyperOil | Neem (Azadirachtin). Hypericum (Hyper forin). | Infection prevention, re-epithelialization, fibrinolytic activity, cleansing activity. Skin regeneration and elasticity promoter. For all kind of wounds (acute, chronic, infected). Biodegradable, nontoxic, noncarcinogenic. Both, gel, and oil formulation | Low antimicrobial properties. | RI.MOS. srl (Italy) | [42] |
| KALTOSTAT® | Calcium/sodium and alginate dressings | For ulcers and traumatic wounds. It improves healing. On contact with exudate, it forms a moist, firm, absorbent gel. | Moderate effects especially on deep and complex wounds | ConvaTec Group Plc (UK) | [53] |
| Kito Activator Chitosan Hydrogel Barrier | Chitosan Hydrogel | Synergy effect between kito activator and HR-chitosan depressing. Hemostatic, quick coagulation by strengthening ionic bonds with red blood cell and platelet. Antimicrobial, anti-inflammatory, deodorant. Nonpreservative, nonbinding, nonantibiotic. Biodegradable, nontoxic, noncarcinogenic. | Nothing found. | Endovision (Korea) | [40] |
| Medihoney HoneyColloid Dressing | Active leptospermum honey. Hydrocolloidal gelling agent | Helps reduce overall wound pH. Natural and safe. Effective in all wound-healing stages. High osmolarity helps cleansing-debriding. Moisturizing, biodegradable, nontoxic, noncarcinogenic. | May be increase level of exudate upon initial use. Only suitable for moderately exuding wounds. | Derma Sciences (USA) | [41] |
| Mepilex® Ag | Foam bandage containing silver | Antibacterial, antifungal. Improves healing time. Atraumatic during dressing changes. Rapid and sustained activity. | Moderate effects, limited to certain types of wounds (e.g., burns) | Mölnlycke Health Care AB (Sweden) | [54] |
| NeutroPhase | Hypochlorous acid | Cleaning and debridement, from wounds and neutralization of toxins. | Bacteriostatic only. Not substantial speed up of chronic wounds healing. | Novabay Pharmaceutical, Inc. (USA) | [55] |
| Omnigraft | Bilayer matrix enriched in C6S collagen and silicone | Reduces inflammation, maintains moisture, and promotes cell and vascular growth in the wound. | Limited repairing effects, especially in chronic and complex wounds. | Derma Sciences, Inc (USA) | [56] |
| PriMatrix | Acellular dermal matrix enriched in type III collagen | Derived from fetal bovine dermis. It supports cell repopulation and revascularization critical in wound healing. Type III collagen helps tissue development and healing. | Limited repairing effects, especially in chronic and complex wounds. | Derma Sciences, Inc (USA) | [57] |
| Promogran PrismaTM | Collagen, oxidized regenerated cellulose (ORC) and silver-ORC bandage | In presence of exudate, it transforms into a soft biodegradable gel. It promotes granulation. It starts wounds that have been stalled in the inflammatory stage. Antimicrobial. | Moderate restorative effects | 3M (USA) | [58] |
| REGRANEX | Recombinant platelet-derived growth factor | The only FDA-approved PDGF for diabetic neuropathic ulcers treatment. It increases tissue growth, re-epithelialization and revascularization rate. | Moderate cure rates in diabetic neuropathic ulcers | Smith & Nephew (UK) | [59] |
| Restore™ | Hydrocolloid dressing | Occlusive dressing, impermeable to microorganisms, urine, and feces. With a disposable wound measuring guide. Heat-activated, self-adhesive inner layer maintains moist while absorbing wound exudates. | Barrier for bacterial and viral infections. Limited healing effects. Moderate exudate prevention. | Hollister Incorporated (USA) | [60] |
| SILVERCEL™ | Alginate bandage, methylcellulose and silver | Antimicrobial barrier to reduce the risk of infection. Improves healing. | Limited effects on large and complex wounds. | Acelity L.P. Inc (USA) | [61] |
| Suprasorb C Collagen Wound Dressing | Collagen | Porous structure, absorbs fluids, debris and proinflammatory proteases and cytokines. It accelerates granulation tissue formation, induces fibroblasts migration and collagen synthesis. It supports proliferation and migration of epidermal cells. Biodegradable, nontoxic, noncarcinogenic. | Low machinal strength. Low antiseptic properties. | Lohmann & Rauscher (Germany) | [39] |
| V.A.C.® Therapy | Programmable device | Negative compression therapy. Accelerates the healing process (reducing edema and promoting blood perfusion). | Very modest results; it prevents further worsening of wound. Very long-term treatments. | Acelity L.P. Inc (USA) | [62] |
| VTG2901 | Programmable device for compression therapy (negative pressure). | Negative compression therapy. Accelerates the healing process (reducing edema and promoting blood perfusion). Removes excess fluid and reduces edema. Protects wound from microbes. | Very modest results, although it prevents further worsening of the wound. Very long-lasting treatment. | Triage Meditech Pvt (India) | [63] |
| Product | Components | Problem, Deficiencies, Limitation | Company | Reference |
|---|---|---|---|---|
| allo-APZ2 | ABCB5- mesenchymal stem cells | Limitations inherent to the use of stem cells (contamination, allogeneic transplantation, modest healing) | Rheacell (Germany) | [80] |
| TruStem | Hematopoietic and mesenchymal stem cells | Limitations inherent to the use of stem cells (contamination, allogeneic transplantation, modest healing). Indefinite response time (weeks-to-months to notice therapeutic effects) | TruStem Cell Therapy (USA) | [82] |
| XSTEM | Human Stem Cells. Integrin α10β1 | Inherent limitations of stem cell use (cell line contamination, allogeneic transplant compatibility, modest healing) | Xintela (Sweden) | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivastava, G.K.; Martinez-Rodriguez, S.; Md Fadilah, N.I.; Looi Qi Hao, D.; Markey, G.; Shukla, P.; Fauzi, M.B.; Panetsos, F. Progress in Wound-Healing Products Based on Natural Compounds, Stem Cells, and MicroRNA-Based Biopolymers in the European, USA, and Asian Markets: Opportunities, Barriers, and Regulatory Issues. Polymers 2024, 16, 1280. https://doi.org/10.3390/polym16091280
Srivastava GK, Martinez-Rodriguez S, Md Fadilah NI, Looi Qi Hao D, Markey G, Shukla P, Fauzi MB, Panetsos F. Progress in Wound-Healing Products Based on Natural Compounds, Stem Cells, and MicroRNA-Based Biopolymers in the European, USA, and Asian Markets: Opportunities, Barriers, and Regulatory Issues. Polymers. 2024; 16(9):1280. https://doi.org/10.3390/polym16091280
Chicago/Turabian StyleSrivastava, Girish K., Sofia Martinez-Rodriguez, Nur Izzah Md Fadilah, Daniel Looi Qi Hao, Gavin Markey, Priyank Shukla, Mh Busra Fauzi, and Fivos Panetsos. 2024. "Progress in Wound-Healing Products Based on Natural Compounds, Stem Cells, and MicroRNA-Based Biopolymers in the European, USA, and Asian Markets: Opportunities, Barriers, and Regulatory Issues" Polymers 16, no. 9: 1280. https://doi.org/10.3390/polym16091280
APA StyleSrivastava, G. K., Martinez-Rodriguez, S., Md Fadilah, N. I., Looi Qi Hao, D., Markey, G., Shukla, P., Fauzi, M. B., & Panetsos, F. (2024). Progress in Wound-Healing Products Based on Natural Compounds, Stem Cells, and MicroRNA-Based Biopolymers in the European, USA, and Asian Markets: Opportunities, Barriers, and Regulatory Issues. Polymers, 16(9), 1280. https://doi.org/10.3390/polym16091280









